Abstract
Multicomponent Petasis reaction has been widely applied for the synthesis of functionalized amine building blocks and biologically active compounds. Employing primary aromatic amines that are not typical reactive substrates contributes to expand the application scope of the Petasis reaction. In this study, we demonstrated the synthesis of functionalized 2-aminothiophenes using Gewald-reaction-derived 2-aminothiophenes as the amine substrates, whose low reactivity in the Petasis reaction was overcome using hexafluoro-2-propanol as the solvent in a mild condition. The obtained Petasis products are amenable for further transformations owing to the presence of multiple functional handles. A following intramolecular cyclization of selected Petasis products afforded substituted tricyclic heterocycles that incorporate a pharmaceutically interesting thienodiazepine moiety.
Keywords: multicomponent reaction, Petasis reaction, 2-aminothiophenes, thienodiazepines, small molecules
Petasis borono-Mannich reaction, or Petasis reaction (PR), is a powerful multicomponent transformation of an amine, an aldehyde, and a boronic acid to afford functionalized amines via the in situ formation of a tetraboronate intermediate (Figure 1A).1−3 Although a wide selection of carbonyl compounds and boronic acids or esters have been successfully applied in PRs, reactive amine substrates in PRs are still mainly restricted to secondary nonaromatic amines,1−5 as shown in recent applications in peptide modification, selective bioconjugation, and DNA-encoded library synthesis.6−9 Primary aromatic amines are typically not reactive substrates in PRs, although anilines, pyridine-2-amines, and naphthalen-2-amines have been used in a few catalyzed variants or at the expense of microwave irradiation conditions (Figure 1B).3,10 Overall, the PR provides a versatile strategy to access highly functionalized amines that are of both synthetic and biological interest.
Figure 1.
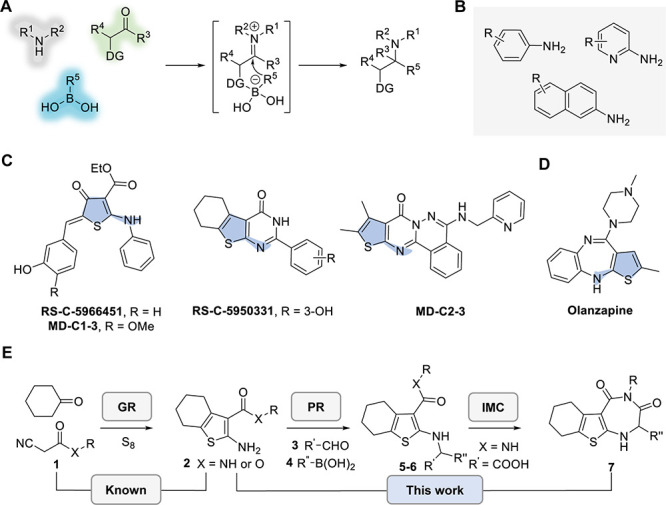
Background and overview. (A) Petasis reaction (PR) of an amine, an aldehyde, and a boronic acid. DG: Directing group. (B) Primary aromatic amines that have been applied in the PR under demanding conditions. (C) 2-Aminothiophene-containing molecules that were reported as activators of ribonuclease L. (D) Olanzapine is a 2-aminothiophene-containing antipsychotic drug. (E) Gewald reaction (GR)–PR–intramolecular cyclization (IMC) in this study to synthesize highly functionalized 2-aminothiophenes and thienodiazepines.
Aminothiophene is a common moiety in biologically active compounds and FDA-approved drugs.11−15 For example, diverse 2-aminothiophene-containing compounds, such as RS-C-5966451/-5950331 and MD-C1-3/-C2-3, were reported as activators of latent ribonuclease and have been evaluated for their antiviral activity and applied to recruit ribonuclease to cleave an oncogenic microRNA (Figure 1C).16,17 Olanzapine is a thienodiazepine that is used as an antipsychotic drug (Figure 1D). Furthermore, 2-aminothiophene-containing compounds have been evaluated for their anticancer activities by inhibiting kinases or bromodomain-containing protein 4.18−20 One of the most robust methods to synthesize 2-aminothiophene is via the three-component Gewald reaction (GR) of a ketone, an α-cyanoester, and sulfur, as well as a few optimized variants.21−24 As a part of our current efforts to evaluate scaffold-diverse small molecules as potential modulators of RNA-binding proteins, we report herein the synthesis of a series of functionalized 2-aminothiophenes via a GR–PR route, followed by an intramolecular cyclization (IMC) to afford thienodiazepines (Figure 1E).
The 2-aminothiophene-3-carboxamide 2a was obtained by a straightforward Gewald condensation of α-cyanoamide 1a derived from ethyl cyanoacetate and ethyl amine, cyclohexanone, and sulfur.20 Using ethyl cyanoacetate 1b in the Gewald condensation gave the 2-aminothiophene-3-carboxylate 2b.25 Then the three-component Petasis reaction of the Gewald condensation product 2a, glyoxylic acid 3a, and phenylboronic acid 4a was performed to test the optimal condition to form the target phenylacetic acid 5a. Our initial test of the reaction in dichloromethane did yield the Petasis product 5a, although in low conversion of only 24%. Solvents of different acidity were then tested in the presence of molecular sieves (MS) for the Petasis reaction (Table 1). Consistent with the reactivity enhancement using hexafluoroisopropanol (HFIP) in the Petasis reaction,26−28 the use of HFIP accelerated the reaction and led to the improved conversion to the expected product 5a. In monitoring the reaction in a duration up to 24 h, the maximum conversion was observed after 1 h. The formation of two byproducts with a combined conversion of less than 10% judged by LC–MS was also observed. It is noteworthy that sequential addition of amine 2a and aldehyde 3a first before adding boronic acid 4a showed a lower conversion to 5a in comparison with simultaneous addition of all three components (entries 2 and 7, Table 1)—an indication that both the direct migration of the phenyl group to the iminium intermediate and the migration with the formation of a tetra-coordinated boronate intermediate are likely involved, with the latter being the favored pathway.29 HFIP presumably promotes the formation of the iminium species and stabilizes ionic transition states involved in the Petasis reaction owing to its ionizing property.26−28,30 The condition with the best conversion of 63% in HFIP led to an isolated yield of 53% for product 5a and was used to synthesize 2-amino-3-carbonylhydrobenzothiophene derivatives 5 and 6.
Table 1. Reaction Conditions for the Three-Component Petasis Reaction Using the Gewald Reaction Product 2a.
| entry | solvent | time (h) | conversion (%)a |
|---|---|---|---|
| 1b | CH2Cl2 | 24 | 24 |
| 2b | HFIP | 1 | 37 |
| 3b | MeCN | 6 | 13 |
| 4b | EtOH | 12 | trace |
| 5b | THF | 24 | trace |
| 6c | CH2Cl2 | 1 | 48 |
| 7c | HFIP | 1 | 63 |
Monitored by LC–MS.
Amine 2a and aldehyde 3a were stirred for 10 min before boronic acid 4a was added .
Simultaneous addition of all three components.
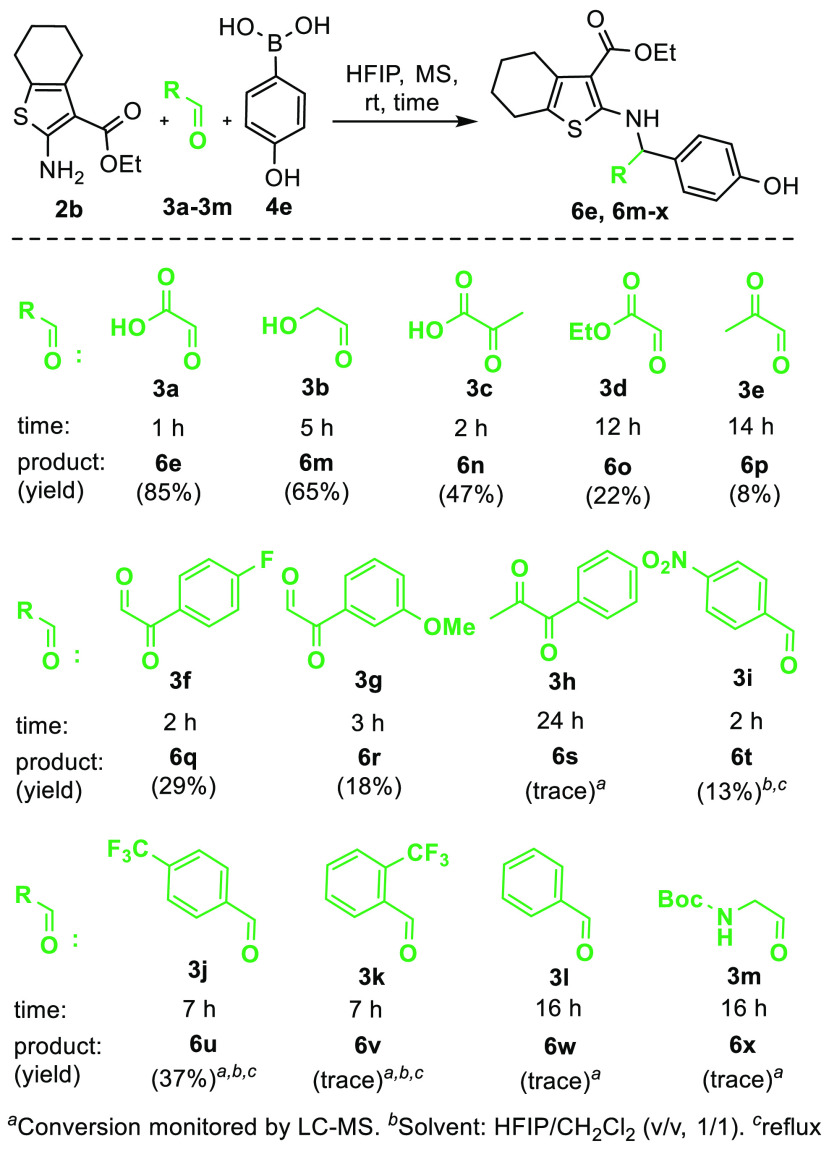
We then investigated the scope for the Petasis reaction in terms of both boronic acids and aldehydes under the HFIP condition (Scheme 1). The Petasis reaction using carboxamide 2a as the amine substrate tolerates a range of boronic acids, such as phenyl (5a) and substituted phenyl boronic acids. Most phenyl boronic acids substituted by electron-donating groups at the 4-position led to isolation of the Petasis products in good yields (5b–d), whereas the 3-hydroxyphenyl boronic acid only led to the Petasis product 5f in poor yield in comparison with that of 5a. Phenylboronic acids substituted by electron-withdrawing groups at the 3-position, such as fluoro-, trimethylfluoro-, and a carboxylic acid group, only led to less than 10% conversion, as monitored by LC–MS, even with prolonged reaction time (5g–i). The electron-rich 2-furanylboronic acid, which usually showed high reactivity in Petasis reactions, did not lead to 5j in synthetically useful conversion, whereas product 5k from 2-thienylboronic acid was isolated in good yield as did product 5l from (E)-styrylboronic acid. Use of the carboxylate 2b as the amine substrate showed the same tolerable profile among the same group of boronic acids, with the 4-hydroxyphenyl boronic acid affording product 6e in the highest yield (85%).
Scheme 1. Scope of the Petasis Reaction Testing Boronic Acids.
Conversion monitored by LC–MS.
Evaluation of the aldehyde scope revealed varied results (Scheme 2). Glyceraldehyde (dimer) 3b led to the Petasis product 6m in good yield but a reduced yield in comparison with that of 6e, presumably due to the reduced electrophilicity of glyceraldehyde in comparison to that with glyoxylic acid. The fact that pyruvic acid also afforded the Petasis product 6n showed that this reaction is tolerable to certain ketones, albeit with reduced reactivity. Ethyl glyoxylate 3d and pyruvaldehyde 3e only led to the corresponding products 6o and 6p in poor yields, which may be explained by the lack of a strong directing effect by an α-hydroxy group that can facilitate the formation of the tetraboronate intermediate. For the same assumption, phenylglyoxals 3f and 3g afforded the products in low yield, whereas acetylbenzoyl 3h did not proceed with significant conversion even with prolonged reaction time. For the benzaldehydes, unless substituted by the strong electron-withdrawing nitro or trifluoromethyl group at the para-position (3i and 3j), benzaldehydes did not lead to isolatable products (6v and 6w). Additionally, N-Boc-2-aminoacetaldehyde 3m did not lead to isolatable product 6x. In summary, the results of evaluating aldehydes revealed that the low nucleophilicity of the 2-aminothiophene requires the use of an activated carbonyl component to render the 2-amine sufficiently reactive toward in situ formation of the tetraboronate intermediate. The obtained 2-amino-3-carbonylhydrobenzothiophenes 5 and 6 share several common structural moieties with reported RNase L activators, such as the tetrahydrobenzo[4,5]thieno moiety of C-5950331 and the 3-carboxylate-2-aminothiophen-3-one moiety of C-5966451 and MD-C1-3 (Figure 1C), and are thus currently being evaluated in-house for their ribonuclease-activating activity.
Scheme 2. Scope of the Petasis Reaction Testing Aldehydes and Ketones.
Conversion monitored by LC–MS.
Solvent: HFIP/CH2Cl2 (v/v, 1/1).
Reflux.
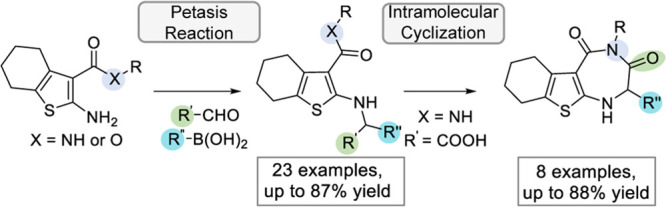
In addition to being potential biologically active compounds, the obtained 2-amino-3-carbonylhydrobenzothiophenes 5 and 6 feature several functional handles that are amenable for further transformations to novel scaffolds. One such scaffold could be a corresponding tricyclic thienodiazepine core after a further intramolecular cyclization step. Indeed, screening of different coupling conditions revealed EDC as the optimal coupling reagent that led to the intramolecularly cyclized thienodiazepine-3,5-diones 7a–h from the Petasis products 5a–j in overall good yields (56–88%). It is noteworthy to mention that a condition using DCC as the coupling reagent led to the intermolecular cyclization to afford the corresponding dimerized compound 8 (Scheme 3).
Scheme 3. Cyclization of the Petasis Products 5.
In conclusion, a synthesis method to access highly functionalized 2-aminothiophenes has been developed using a three-component Petasis reaction employing Gewald reaction products as the amine substrates. This method converts low reactive primary aromatic amines into corresponding Petasis products under a mild and easily operational condition (no complex catalysts, microwave irradiation, or photoredox conditions) using HFIP. We obtained a collection of functionalized 2-aminothiophenes by testing the scope of the Petasis reaction, which is tolerable toward a wide range of boronic acids with yields up to 87%, although a limited selection of aldehydes led to satisfactory yields. The obtained 2-aminothiophene products are amenable for further transformations to construct biologically interesting scaffolds, such as the formation of the new thienodiazepines 7 through an intramolecular cyclization in good yield up to 88%. This is the first report of applying 2-aminothiophenes as the amine substrate in Petasis reactions. The highly functional 2-aminothiophenes 5 and 6 and the tricyclic thienodiazepines 7 are being evaluated for their modulating activities against RNA-cleaving and -binding proteins.
Acknowledgments
Financial support provided by AstraZeneca, Merck KGaA, Pfizer Inc., and the Max Planck Society is gratefully acknowledged. The authors thank Pascal Hommen for proofreading.
Glossary
Abbreviations
- DCC
N,N′-dicyclohexylcarbodiimide
- EDC
1-ethyl-3-(3-(dimethylamino)propyl)carbodiimide
- GR
Gewald condensation reaction
- HFIP
hexafluoroisopropanol
- IMC
intramolecular cyclization
- LC–MS
liquid chromatography–mass spectrometry
- PR
Petasis borono-Mannich reaction
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscombsci.0c00173.
Detailed synthetic procedures, compound characterization data, and NMR spectra of all isolated products (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Petasis N. A.; Akritopoulou I. The boronic acid Mannich reaction - A new method for the synthesis of geometrically pure allylamines. Tetrahedron Lett. 1993, 34 (4), 583–586. 10.1016/S0040-4039(00)61625-8. [DOI] [Google Scholar]
- Candeias N. R.; Montalbano F.; Cal P.; Gois P. M. P. Boronic acids and esters in the Petasis-borono Mannich multicomponent reaction. Chem. Rev. 2010, 110 (10), 6169–6193. 10.1021/cr100108k. [DOI] [PubMed] [Google Scholar]
- Wu P.; Givskov M.; Nielsen T. E. Reactivity and synthetic applications of multicomponent Petasis reactions. Chem. Rev. 2019, 119 (20), 11245–11290. 10.1021/acs.chemrev.9b00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.; Cui C.-x.; Zhang G.-h.; Li X.-q.; Yang J. Regioselective synthesis of functionalized dihydropyrones via the Petasis reaction. J. Org. Chem. 2020, 85 (2), 1285–1290. 10.1021/acs.joc.9b02651. [DOI] [PubMed] [Google Scholar]
- Lenci E.; Bellini Puglielli R.; Bucaletti E.; Innocenti R.; Trabocchi A. A glucose-derived α-hydroxy aldehyde for the Petasis reaction: facile access to polyfunctional δ-amino acids. Eur. J. Org. Chem. 2020, 2020 (27), 4227–4234. 10.1002/ejoc.202000600. [DOI] [Google Scholar]
- Ricardo M. G.; Llanes D.; Wessjohann L. A.; Rivera D. G. Introducing the Petasis reaction for late-stage multicomponent diversification, labeling, and stapling of peptides. Angew. Chem., Int. Ed. 2019, 58 (9), 2700–2704. 10.1002/anie.201812620. [DOI] [PubMed] [Google Scholar]
- Sim Y. E.; Nwajiobi O.; Mahesh S.; Cohen R. D.; Reibarkh M. Y.; Raj M. Secondary amine selective Petasis (SASP) bioconjugation. Chem. Sci. 2020, 11 (1), 53–61. 10.1039/C9SC04697F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potowski M.; Esken R.; Brunschweiger A. Translation of the copper/bipyridine-promoted Petasis reaction to solid phase-coupled DNA for encoded library synthesis. Bioorg. Med. Chem. 2020, 28 (9), 115441. 10.1016/j.bmc.2020.115441. [DOI] [PubMed] [Google Scholar]
- Zambaldo C.; Geigle S. N.; Satz A. L. High-throughput solid-phase building block synthesis for DNA-encoded libraries. Org. Lett. 2019, 21 (23), 9353–9357. 10.1021/acs.orglett.9b03553. [DOI] [PubMed] [Google Scholar]
- Yi J.; Badir S. O.; Alam R.; Molander G. A. Photoredox-catalyzed multicomponent Petasis reaction with alkyltrifluoroborates. Org. Lett. 2019, 21 (12), 4853–4858. 10.1021/acs.orglett.9b01747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozorov K.; Nie L. F.; Zhao J.; Aisa H. A. 2-Aminothiophene scaffolds: Diverse biological and pharmacological attributes in medicinal chemistry. Eur. J. Med. Chem. 2017, 140, 465–493. 10.1016/j.ejmech.2017.09.039. [DOI] [PubMed] [Google Scholar]
- Desantis J.; Nannetti G.; Massari S.; Barreca M. L.; Manfroni G.; Cecchetti V.; Palù G.; Goracci L.; Loregian A.; Tabarrini O. Exploring the cycloheptathiophene-3-carboxamide scaffold to disrupt the interactions of the influenza polymerase subunits and obtain potent anti-influenza activity. Eur. J. Med. Chem. 2017, 138, 128–139. 10.1016/j.ejmech.2017.06.015. [DOI] [PubMed] [Google Scholar]
- Thanna S.; Knudson S. E.; Grzegorzewicz A.; Kapil S.; Goins C. M.; Ronning D. R.; Jackson M.; Slayden R. A.; Sucheck S. J. Synthesis and evaluation of new 2-aminothiophenes against Mycobacterium tuberculosis. Org. Biomol. Chem. 2016, 14 (25), 6119–6133. 10.1039/C6OB00821F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheich C.; Puetter V.; Schade M. Novel small molecule inhibitors of MDR mycobacterium tuberculosis by NMR fragment screening of antigen 85C. J. Med. Chem. 2010, 53 (23), 8362–8367. 10.1021/jm100993z. [DOI] [PubMed] [Google Scholar]
- Oza V.; Ashwell S.; Almeida L.; Brassil P.; Breed J.; Deng C.; Gero T.; Grondine M.; Horn C.; Ioannidis S.; Liu D.; Lyne P.; Newcombe N.; Pass M.; Read J.; Ready S.; Rowsell S.; Su M.; Toader D.; Vasbinder M.; Yu D.; Yu Y.; Xue Y.; Zabludoff S.; Janetka J. Discovery of checkpoint kinase inhibitor (S)-5-(3-fluorophenyl)-N-(piperidin-3-yl)-3-ureidothiophene-2-carboxamide (AZD7762) by structure-based design and optimization of thiophenecarboxamide ureas. J. Med. Chem. 2012, 55 (11), 5130–5142. 10.1021/jm300025r. [DOI] [PubMed] [Google Scholar]
- Thakur C. S.; Jha B. K.; Dong B. H.; Das Gupta J.; Silverman K. M.; Mao H. X.; Sawai H.; Nakamura A. O.; Banerjee A. K.; Gudkov A.; Silverman R. H. Small-molecule activators of RNase L with broad-spectrum antiviral activity. Proc. Natl. Acad. Sci. U. S. A. 2007, 104 (23), 9585–9590. 10.1073/pnas.0700590104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costales M. G.; Aikawa H.; Li Y.; Childs-Disney J. L.; Abegg D.; Hoch D. G.; Pradeep Velagapudi S.; Nakai Y.; Khan T.; Wang K. W.; Yildirim I.; Adibekian A.; Wang E. T.; Disney M. D. Small-molecule targeted recruitment of a nuclease to cleave an oncogenic RNA in a mouse model of metastatic cancer. Proc. Natl. Acad. Sci. U. S. A. 2020, 117 (5), 2406–2411. 10.1073/pnas.1914286117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.-H.; Coumar M. S.; Chu C.-Y.; Lin W.-H.; Chen Y.-R.; Chen C.-T.; Shiao H.-Y.; Rafi S.; Wang S.-Y.; Hsu H.; Chen C.-H.; Chang C.-Y.; Chang T.-Y.; Lien T.-W.; Fang M.-Y.; Yeh K.-C.; Chen C.-P.; Yeh T.-K.; Hsieh S.-H.; Hsu J. T. A.; Liao C.-C.; Chao Y.-S.; Hsieh H.-P. Design and synthesis of tetrahydropyridothieno[2,3-d]pyrimidine scaffold based epidermal growth factor receptor (EGFR) kinase inhibitors: The role of side chain chirality and Michael acceptor group for maximal potency. J. Med. Chem. 2010, 53 (20), 7316–7326. 10.1021/jm100607r. [DOI] [PubMed] [Google Scholar]
- Wu P.; Hu Y. Small molecules targeting phosphoinositide 3-kinases. MedChemComm 2012, 3 (11), 1337–1355. 10.1039/c2md20044a. [DOI] [Google Scholar]
- Ouyang L.; Zhang L.; Liu J.; Fu L.; Yao D.; Zhao Y.; Zhang S.; Wang G.; He G.; Liu B. Discovery of a small-molecule bromodomain-containing protein 4 (BRD4) inhibitor that induces AMP-activated protein kinase-modulated autophagy-associated cell death in breast cancer. J. Med. Chem. 2017, 60 (24), 9990–10012. 10.1021/acs.jmedchem.7b00275. [DOI] [PubMed] [Google Scholar]
- Gewald K. Heterocyclen aus CH-aciden Nitrilen, VII. 2-Amino-thiophene aus α-Oxo-mercaptanen und methylenaktiven Nitrilen. Chem. Ber. 1965, 98 (11), 3571–3577. 10.1002/cber.19650981120. [DOI] [Google Scholar]
- Shao W.; Kaldas S. J.; Yudin A. K. 3-Cyanoallyl boronates are versatile building blocks in the synthesis of polysubstituted thiophenes. Chem. Sci. 2017, 8 (6), 4431–4436. 10.1039/C7SC00831G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T. T. T.; Le V. A.; Retailleau P.; Nguyen T. B. Access to 2-amino-3-arylthiophenes by base-catalyzed redox condensation reaction between arylacetonitriles, chalcones, and elemental sulfur. Adv. Synth. Catal. 2020, 362 (1), 160–165. 10.1002/adsc.201901235. [DOI] [Google Scholar]
- Thomas J.; Jana S.; Sonawane M.; Fiey B.; Balzarini J.; Liekens S.; Dehaen W. A new four-component reaction involving the Michael addition and the Gewald reaction, leading to diverse biologically active 2-aminothiophenes. Org. Biomol. Chem. 2017, 15 (18), 3892–3900. 10.1039/C7OB00707H. [DOI] [PubMed] [Google Scholar]
- Saravanan J.; Mohan S.; Roy J. J. Synthesis of some 3-substituted amino-4,5-tetramethylene thieno[2,3-d][ 1,2,3]-triazin-4(3H)-ones as potential antimicrobial agents. Eur. J. Med. Chem. 2010, 45 (9), 4365–4369. 10.1016/j.ejmech.2010.05.061. [DOI] [PubMed] [Google Scholar]
- Nanda K. K.; Wesley Trotter B. Diastereoselective Petasis Mannich reactions accelerated by hexafluoroisopropanol: a pyrrolidine-derived arylglycine synthesis. Tetrahedron Lett. 2005, 46 (12), 2025–2028. 10.1016/j.tetlet.2005.01.151. [DOI] [Google Scholar]
- Jourdan H.; Gouhier G.; Van Hijfte L.; Angibaud P.; Piettre S. R. On the use of boronates in the Petasis reaction. Tetrahedron Lett. 2005, 46 (46), 8027–8031. 10.1016/j.tetlet.2005.09.060. [DOI] [Google Scholar]
- Norsikian S.; Beretta M.; Cannillo A.; Martin A.; Retailleau P.; Beau J.-M. Synthesis of enantioenriched 1,2-trans-diamines using the borono-Mannich reaction with N-protected α-amino aldehydes. Chem. Commun. 2015, 51 (49), 9991–9994. 10.1039/C5CC01716E. [DOI] [PubMed] [Google Scholar]
- Wu P.; Nielsen T. E. Petasis three-component reactions for the synthesis of diverse heterocyclic scaffolds. Drug Discovery Today: Technol. 2018, 29, 27–33. 10.1016/j.ddtec.2018.06.010. [DOI] [PubMed] [Google Scholar]
- Shuklov I. A.; Dubrovina N. V.; Börner A. Fluorinated alcohols as solvents, cosolvents and additives in homogeneous catalysis. Synthesis 2007, 2007 (19), 2925–2943. 10.1055/s-2007-983902. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.