Abstract
Despite recent rapid advances in medical knowledge that have improved survival, conventional medical science’s understanding of human health and disease relies heavily on people of European descent living in contemporary urban industrialized environments. Given that modern conditions in high-income countries differ widely in terms of lifestyle and exposures compared to those experienced by billions of people and all our ancestors over several hundred thousand years, this narrow approach to the human body and health is very limiting. We argue that preventing and treating chronic diseases of aging and other mismatch diseases will require both expanding study design to sample diverse populations and contexts, and fully incorporating evolutionary perspectives. In this paper, we first assess the extent of biased representation of industrialized populations in high profile, international biomedical journals, then compare patterns of morbidity and health across world regions. We also compare demographic rates and the force of selection between subsistence and industrialized populations to reflect on the changes in how selection operates on fertility and survivorship across the lifespan. We argue that, contrary to simplistic misguided solutions like the PaleoDiet, the hypothesis of evolutionary mismatch needs critical consideration of population history, evolutionary biology and evolved reaction norms to prevent and treat diseases. We highlight the critical value of broader sampling by considering the effects of three key exposures that have radically changed over the past century in many parts of the world—pathogen burden, reproductive effort and physical activity—on autoimmune, cardiometabolic and other mismatch diseases.
Keywords: mismatch, evolutionary medicine, diversity, WEIRD
1. Introduction
How are you using your body? Chances are you’re reading this bespectacled in a temperature-controlled room under artificial light, resting in a comfortable chair. We suspect you are wearing cushioned shoes, your skin is soap-cleaned and scented with deodorant, and perhaps you just ate a pizza slice or a tuna-fish sandwich washed down with (diet) Coke using teeth you brush twice a day and have deep-cleaned maybe once a year by a dentist. To get to and from work, you drove or took a bus, an elevator whisked you to your office floor, you got water from a tap and used myriad other labor-saving devices that allowed you to barely elevate your heart rate as you moved from one ergonomically designed chair to the next. You even sat when going to the bathroom. If you did sweat, it was to exercise, perhaps on a treadmill in specially designed shoes and high-tech clothes.
The way you use your body may seem typical, but from an evolutionary perspective it is anything but. Over the past two hundred years the industrial and post-industrial revolutions have rapidly transformed how we use and treat our bodies. We see even greater change if we go further back towards the origin of our species. Plant and animal domestication spread only in the last 5 to 12 thousand years, a tiny blip in the two million or so years our ancestors were hunter-gatherers, and the 150–250 thousand years that Homo sapiens has been around. The way you use your body is also different from the way many other humans today still use theirs. Although the industrial and post-industrial revolutions are continuing to sweep in succession across the globe, billions of people, especially in rural areas of low- and middle-income countries, continue to depend on daily manual labor, eat mostly non-processed food, and otherwise lack access to the myriad goods and services that affect how their bodies function.
Recognizing and considering the evolutionarily novel nature of human health in Western, Educated, Industrialized, Rich Democracies, i.e. WEIRD countries has many ramifications, perhaps the most important being the mismatch hypothesis (Lieberman, 2013; Nesse & Williams, 1994). This hypothesis, a central tenet of evolutionary medicine, posits that many of the recent and profound changes in how WEIRD people use their bodies have led to mismatches―conditions that are more common or severe when an individual’s genes are inadequately or imperfectly adapted to novel environmental conditions. According to the mismatch hypothesis, the accelerating pace and scope of culturally-driven environmental change has increased the prevalence of conditions and diseases like myopia, cavities, type 2 diabetes and heart disease that used to be much less common (Eaton et al., 1988; Pollard, 2008). Put differently, many people in WEIRD societies use their bodies in evolutionarily weird ways. In invoking the WEIRD acronym, we recognize that any simple classification like WEIRD, non-WEIRD, “high-income country” or “developed country” glosses over important internal variation, and that any such label is used to reflect either idealized circumstances, or statistical summaries. Obviously, many in the US or Sweden are not physically inactive, and many in urban areas of countries like Kenya or Nepal eat highly processed food. Despite the problems with any binary classification, we use these terms as shorthand to identify broad-scale differences in representation, experience and exposure.
Our main argument is that the restricted focus on WEIRD bodies limits the potential of modern biomedicine by paying insufficient attention to the diversity of human phenotypes and how some of these may be mismatched in modern environments. This is not to say that being WEIRD doesn’t have many benefits. Without doubt, many recent environmental changes have improved rather than harmed human health. Over the last century, life expectancy has doubled, and age-specific survivorship rates have improved considerably at all ages (Oeppen & Vaupel, 2003). Such improvements came largely from clean drinking water and public sanitation reducing infectious disease in urban areas; antibiotics and other medical advances further reduced morbidity and mortality (McKeown, 1976). In addition, just because human bodies were selected under non-WEIRD conditions, it doesn’t follow that ancestral environments (or attempts to mimic them with lifestyle alterations such as the PaleoDiet) necessarily promote health. The problem with this sort of thinking is that natural selection favors health only to the extent that health increases reproductive success. And natural selection did not stop with the advent of agriculture and population explosions. Rather, during the past ten millennia, the pace of genetic change has increased (Cochran & Harpending, 2009; Hawks et al., 2007), particularly with respect to diet and immune function. Local and recent adaptations to specific environments require us to think critically about how to apply one-size-fits-all remedies to improving health. Addressing health needs globally requires broader attention and expanded sampling due to unappreciated variation in needs, conditions and bodies.
There is abundant evidence that many common diseases today are mismatch conditions that we can help prevent and possibly improve treatment by incorporating an evolutionary perspective (Gluckman et al., 2009; Lieberman, 2013; Nesse & Stearns, 2008; Stearns & Medzhiyov, 2016; Trevathan et al., 2008). Accordingly, here we critically review some of the ways in which a dominant focus on data from WEIRD countries and bodies can lead to an incomplete, if not sometimes incorrect, understanding of human physiology, health and disease. To this end, we first report inclusion of study participants from WEIRD versus non-WEIRD countries in research published in the top three international medical journals, to consider biased coverage in study sampling. Are study participants recruited in proportion to population size? And to health needs? Similar exercises have showcased the WEIRD bias in psychology (Arnett, 2008), and more recently in genetics (Sirugo et al., 2019). Given the potential for biased representation, we then summarize key differences in mortality and morbidity between WEIRD and non-WEIRD countries, and explore how the force of natural selection may operate differently in contemporary WEIRD populations. Details about the force of selection with age are critical in debates about the evolution of senescence, and for estimating rates at which late-age deleterious alleles might accumulate in the genome (Baudisch, 2005). Next we discuss evolutionary mismatch, reaction norms and gene-by-environment interactions to justify why broader sampling is necessary to best improve global health. We also outline ways to test mismatch hypotheses using data from non-WEIRD societies. We illustrate through several case studies how consideration of exposures less common to WEIRD countries (i.e. greater pathogen exposure, high fertility and greater physical activity) can alter how we think about the landscape of human health. Through this, we hope to show how evolutionary perspectives combined with broader sampling of human variation can inspire productive new research directions.
2. How WEIRD is study participation in the biomedical literature?
A first step towards evaluating the effects of ignoring the full range of human variation when studying health and disease is to assess the extent to which the medical literature is biased towards WEIRD populations. This is an important question given that the vast majority of the world is not WEIRD, and study findings might depend on the details particular to population, locality and culture, making the “where” and “who” of sampling critical for diagnosing, preventing and treating disease. Though there are some similarities in the range of conditions that cause morbidity and mortality in people everywhere (Table 1), effective diagnosis and treatment can vary for the same ailment in different regions (see section 6).
Table 1.
Global Daily Adjusted Life Years (DALYs) (or WEIRD and non-WEIRD countries, 2016.
| Source of mortality/morbidity | WEIRD | Nan-WEIRD | ||
|---|---|---|---|---|
| DALYs | % | DALYs | % | |
| I. Communicable, maternal, perinatal and nutritional conditions | 11.573 | 5.0 | 763,974 | 31.3 |
| A. Infectious and parasitic diseases | 3434 | 1.5 | 328,275 | 13.5 |
| 9. Parasitic and vector diseases | 18 | 0.0 | 51,820 | 2.1 |
| B. Respiratory Infectious | 4280 | 1.8 | 135,103 | 5.5 |
| C. Maternal conditions | 86 | 0.0 | 19,131 | 0.8 |
| D. Neonatal conditions | 2967 | 1.3 | 216,407 | 8.9 |
| E. Nutritional deficiencies | 805 | 0.3 | 65,058 | 2.7 |
| II. Noncommunicable diseases | 199,114 | 86.0 | 1,396,420 | 57.3 |
| A. Malignant neoplasms | 40,943 | 17.7 | 203,631 | 8.4 |
| B. Other neoplasms | 1124 | 0.5 | 5063 | 0.2 |
| C. Diabetes mellitus | 7601 | 3.3 | 58,065 | 2.4 |
| D. Endocrine, blood, immune disorders | 2875 | 1.2 | 22,768 | 0.9 |
| E. Mental and substance use disorders | 25,870 | 11.2 | 145,632 | 6.0 |
| 1. Depressive disorders | 6193 | 2.7 | 37,981 | 1.6 |
| F. Neurological conditions | 18,536 | 8.0 | 80,459 | 3.3 |
| 1. Alzheimer disease and other dementias | 8878 | 3.8 | 25,446 | 1.0 |
| G. Sense organ diseases | 9882 | 4.3 | 91,680 | 3.8 |
| H. Cardiovascular diseases | 40,139 | 17.3 | 373,013 | 15.3 |
| 1. Rheumatic heart disease | 256 | 0.1 | 10,142 | 0.4 |
| 3. Ischaemic heart disease | 19,955 | 8.6 | 183,746 | 7.5 |
| 4. Stroke | 8648 | 3.7 | 129,294 | 5.3 |
| I. Respiratory diseases | 12,751 | 5.5 | 93,005 | 3.8 |
| J. Digestive diseases | 7886 | 3.4 | 80,963 | 3.3 |
| K. Genitourinary diseases | 5912 | 2.6 | 56,151 | 2.3 |
| L. Skin diseases | 2446 | 1.1 | 20,992 | 0.9 |
| M. Musculoskeletal diseases | 16,860 | 7.3 | 85,978 | 3.5 |
| N. Congenital anomalies | 2916 | 1.3 | 60,064 | 2.5 |
| O. Oral conditions | 3184 | 1.4 | 15,849 | 0.7 |
| III. Injuries | 20,776 | 9.0 | 276,618 | 11.4 |
| A. Unintentional Injuries | 14,404 | 6.2 | 200,754 | 8.2 |
| B. Intentional injuries | 6373 | 2.8 | 75,863 | 3.1 |
| ALL CAUSES | 231,463 | 100.0 | 2,437,012 | 100.0 |
Therefore, we investigated which world regions participants come from in biomedical studies published in the top three medical journals in the world: #1) New England Journal of Medicine (NEJM, Impact factor (IF)=70.67, 2018), #2) Lancet (IF=59.10), and #3) Journal of the American Medical Association (JAMA, IF=51.27). Though there are many other medical journals, these three are the most influential, widely read, and cited. Of 160 general and internal medicine journals tracked by Clarivate Analytics’ Journal Citation Reports in 2018, these three alone have a combined IF equal to the sum of the bottom 130 journals. Despite being based in the U.S. (NEJM, JAMA) or U.K. (Lancet), these three journals serve an international audience, have international editorial boards, and make it their explicit mission to serve the global medical community. For example, JAMA’s mission as stated on their journal website is “to promote the science and art of medicine and the betterment of the public health”, with one of its critical objectives “To improve health and health care internationally by elevating the quality of medical care, disease prevention, and research”.
Seven UCSB undergraduates surveyed original research articles of these weekly journals for the year 2018, covering 99,039,173 participants from 534 original research articles. World region of residence for study participants and authors (first and last three when more than six authors) were recorded for each article (see Supplement for additional methodological details).
Overall study inclusion for WEIRD and non-WEIRD regions is 80.2% and 19.8%, respectively. Yet WEIRD and non-WEIRD countries respectively account for 11.1% and 88.9% of the total global census population in 2018, and 8.9% and 91.1% of the global disability-adjusted life years (DALYs) (Table S2). DALYs reflect the total number of years lost due to illness, disability or premature death, and thus conveniently combine effects of both morbidity and mortality in one metric (World Health Organization, 2018). Overall, study participation relative to population is 7.2 for WEIRD, and 0.2 for non-WEIRD countries; someone from a WEIRD country is therefore 37 times more likely to appear in a medical study from these top journals than someone from a non-WEIRD country. The equivalent bias is even stronger, at 47 times, when inclusion is assessed relative to DALYs. Figure 1 shows the relative representation in terms of total study participants, and by individual studies (irrespective of number of participants in each study) for each geographic region (see Table S2 for further breakdown). Author representation is similar to study participants: 89% of lead and senior study authors are from WEIRD countries.
FIGURE 1. Study inclusion across world regions for top three medical journals in 2018.
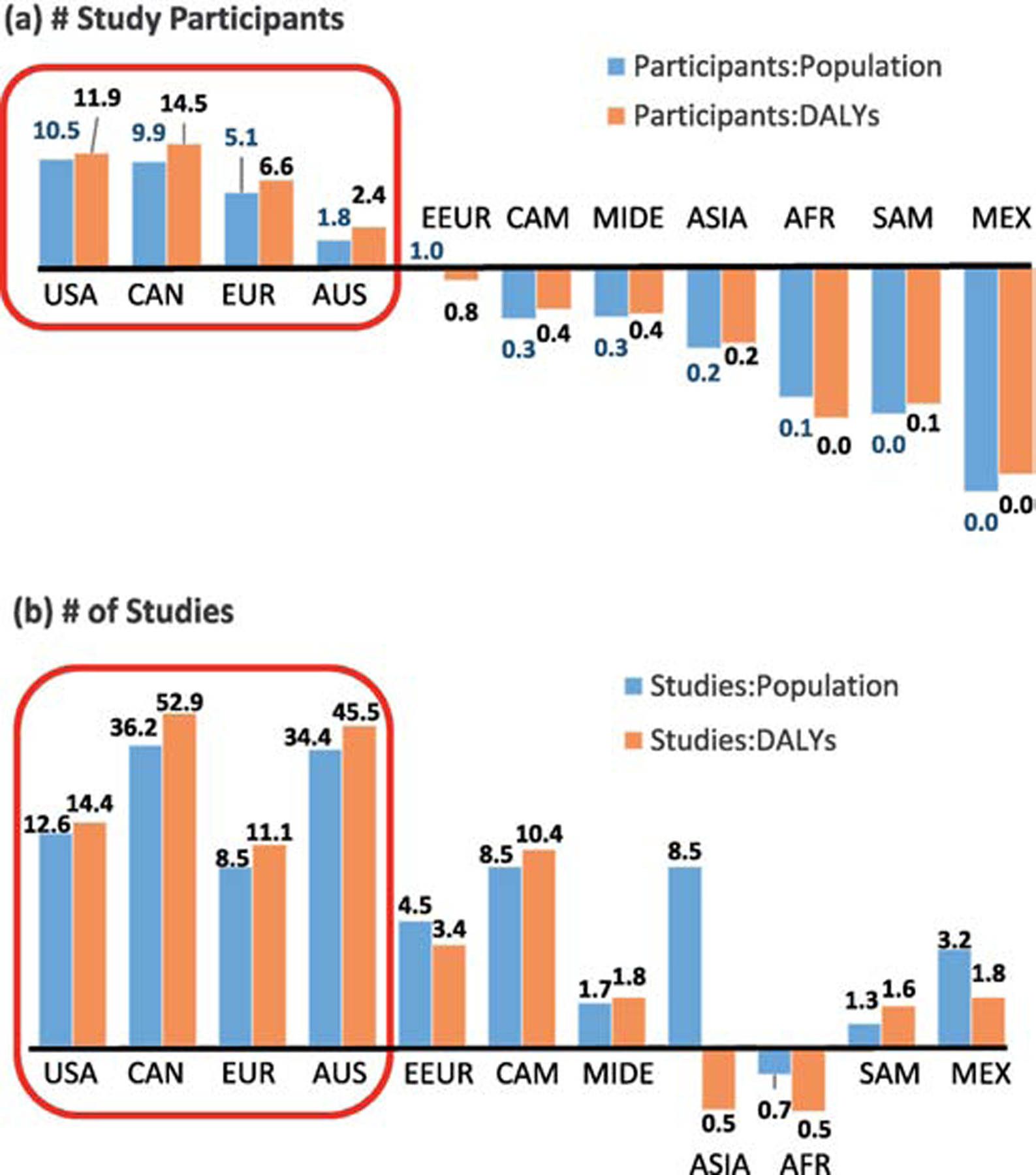
Inclusion in terms of (a) proportion of total study participants, or (b) proportion of total studies, relative to their global population proportion and relative to proportion of global DALYs. World region legend: CAN=Canada, EUR=northern, western and southern Europe, AUS=Australia, New Zealand & Pacific, EEUR=eastern Europe, CAM=Central America & Caribbean, MIDE=Middle East, AFR=Africa, SAM=South America, MEX=Mexico.
3. Morbidity and mortality in global perspective
To what extent does medical science’s disproportionate focus on WEIRD populations affect our understanding of health and human vulnerabilities to disease? A practical way of first thinking about the human body is studying when it “fails.” Death is universal and inescapable, escalating at an exponential rate over most of adulthood no matter the cause (Carnes et al., 1996). Certain age-dependent diseases, like cancers and osteoarthritis, are believed to be human universals, expressed more visibly in low mortality populations, albeit often at lower age-specific prevalence than in many WEIRD populations (e.g. Wallace et al., 2017). But based on verbal autopsies (interviews of symptoms and circumstances designed to diagnose cause of death), the largest share of deaths among seven contemporary subsistence-level hunter-gatherer and horticulturalist populations is from infection or “illness” (72%), 11% from violence, and 5% from accidents, while <10% can be construed as “degenerative” (Gurven & Gomes, 2017). How does the human body suffer around the world today?
Table 1 compares select disease burden by world regions classified loosely as WEIRD (North America, Western, Northern & Southern Europe, Australia & New Zealand, n=800,185 people) and non-WEIRD (Africa, Central & South America, Asia, Middle East, Eastern Europe, n=6,661,699 people) (see Table S1 for full comparison of diseases). Disease burden is again assessed using DALYs for the year 2016.
Overall, non-WEIRD regions account for 91% of global DALYs, supporting the overall health benefits of living in a WEIRD country. However, after accounting for the Epidemiological Transition (Omran, 1971) — i.e. the shift from non-infectious to chronic disease that has accompanied increases in longevity from improvements in public health and medicine— a different picture emerges. Infectious disease accounts for a six-fold greater proportion of DALYs in non-WEIRD than WEIRD regions, whereas non-communicable diseases account for 1.5 times greater share of DALYs among WEIRD compared to non-WEIRD regions. More specifically, diarrheal, childhood-cluster infections and other parasitic/vector diseases account for twenty-fold greater relative burden in non-WEIRD (5.9%) than WEIRD (0.3%) regions (Table S1). Nutritional diseases (e.g., protein-energy malnutrition, iodine deficiency) carry a nine-fold greater relative burden in non-WEIRD regions. Given the rapid pace of socioeconomic change and urbanization in non-WEIRD countries, DALYs of many sources of mortality/morbidity differ mainly by degree. In WEIRD regions, cancers carry 2.1 times larger relative burden, Alzheimer’s Disease and other dementias incur almost four times greater burden, while mental health and substance use problems account for 1.9 times greater relative burden. Cardiovascular disease is responsible for a similar overall share of DALYs (17.3% vs. 15.3%, respectively for WEIRD and non-WEIRD regions), though rheumatic heart disease and stroke cause greater relative burden in non-WEIRD regions. From a public health standpoint, the eight-fold greater population share of non-WEIRD countries means that they carry more aggregate DALY burden in all areas of health, including categories where the relative individual burden is much lower than in WEIRD countries (e.g., Alzheimer’s Disease).
As mentioned above, our use of the WEIRD acronym is as a shorthand for residence in particular countries, and it is important to recognize that there is much heterogeneity within and among countries in health, lifestyle, socioeconomic status and other conditions (e.g., Amish populations in the U.S.) that defies any simple classification. For example, average U.S. life expectancy (e0) was 79 years in 2013, but varied by seven years among states (75.3 in West Virginia to 82.0 in Hawaii). Recent analysis of U.S. census tracts reveals for the first time that e0 is much more variable at finer resolution: there is a two to three decade difference in e0 across census tracts within most U.S. states (Arias et al., 2018). Overall, neighborhood-level e0 just within the U.S. (56.3 to 97.5 years) spans almost the entire range in average e0 of world countries. Census tracts in the lowest quartile of e0 (56.3 to 75.7) are areas of low educational attainment, low median income, are located largely in the U.S. South, and have large non-Hispanic black populations (Arias et al., 2018: Table 8).
4. Demographic differences and force of selection
Beyond providing a skewed perspective on patterns of morbidity and mortality, the bias towards WEIRD populations also affects efforts to assess the effects of recent selection. It is sometimes assumed that natural selection has become either weak or ceased to operate in WEIRD populations (Gould, 2000). This cannot be true since natural selection is the emergent property of three factors that are just as present in WEIRD as non-WEIRD populations: variation, heritability and differential reproductive success. Further, as noted above, empirical evidence shows that natural selection did not cease to winnow variation following the agricultural and industrial revolutions (Hawks et al., 2007), and is still operating today in countries like the U.S and Canada (e.g. Byars et al., 2009; Milot et al., 2011). But how might the force of selection operate in a more traditional high mortality and fertility context versus a WEIRD low mortality-fertility context? Differences in the magnitude and shape of the ‘force of selection’ are critical for testing evolutionary theories underlying aging, such as antagonistic pleiotropy - where early life fertility advantages may outweigh any late-life deleterious effects of certain alleles (see section 7.1).
The two main determinants of fitness (i.e., population growth) are fertility and survivorship. The average total fertility of a woman surviving over her reproductive years (TFR) ranges from four to ten children in natural fertility populations (Bentley et al., 1993). Hunter-gatherer women, on average, birth six children. Life expectancy at birth (e0) is about 30 years (range: 21–37 y), though hunter-gatherers can expect to live to age ~65 if surviving up to age 40 (Gurven & Davison, 2019). In contrast, e0 in the United States in 2017 was 78.6 years and TFR was 1.8; e0 is slightly higher and TFR even lower among many European Union countries.
The force of selection itself is usually proxied with fitness sensitivities. These describe the static effects of small perturbations in survivorship (ss) or fertility (sf) on population fitness (Hamilton, 1966). We show in Figure 2 ss and sf with age among Hadza hunter-gatherers, Tsimane horticulturalists and the United States in 2017. The force of selection has a similar shape with age despite large differences in fertility and mortality across these three populations. In particular, fitness sensitivity to survival (ss) peaks before reproduction, then declines thereafter and ceases by menopause (Fig. 2a). Fitness sensitivities to perturbations in age-specific fertility (sf) decline with age, and are generally lower than ss (Fig. 2b). For a high-income, WEIRD country like the U.S., ss is comparably low before the onset of reproduction, and higher over ages 20–30. This pattern is consistent with Jones (2009), who showed that early life ss increases with TFR and declines with e0. Note that Sf is higher at all ages in the U.S. than for Hadza or Tsimane. Given the larger effects of fertility change on U.S. population growth rates, fertility perturbations have greater effects on fitness than survival perturbations by age 23 in the U.S., versus mid-late 30’s for Hadza and Tsimane. Thus, the unique demographic structure of WEIRD populations suggests that the pace at which a somatic mutation could spread by selection ceteris paribus (and relative to high fertility-mortality populations) will largely be impacted more by changes in adult fertility, and to a lesser degree by changes in survival among young adults. As a result, and contrary to some arguments (e.g. Gould, 2000), natural selection is still operating in WEIRD environments (Byars et al., 2009), but possibly more on genes that affect variation in fertility. However, because natural selection becomes weaker with age, the extent to which selection can act against alleles with harmful expression in old age in WEIRD as well as non-WEIRD contexts is probably small. This diminishing of selection has especially important implications for diseases that occur at late ages. Higher adult survivorship in WEIRD populations alone should increase the health burden of late age diseases, especially if such diseases are mismatches.
Figure 2. Force of selection in three ecological contexts.
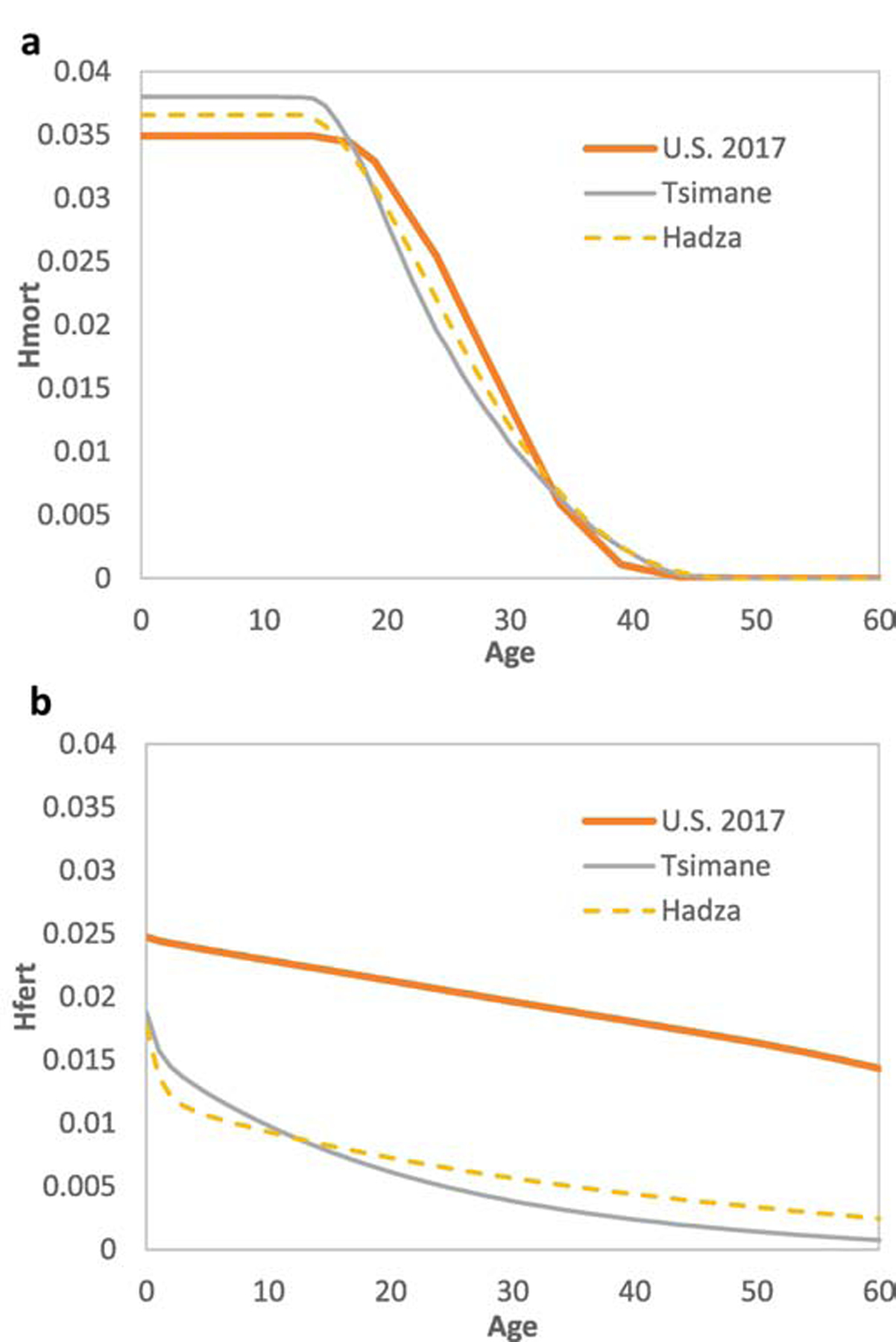
Fitness sensitivity in response to perturbations to (a) survival, and (b) fertility. Lines reflect hunter-gatherers (Hadza, life expectancy at birth (e0) 35, Total fertility rate (TFR) =6.2), horticulturalists (Tsimane, e0=43, TFR=9.0) and urban industrialized population (United States, 2017, e0=79, TFR=1.8).
5. Evolutionary mismatch as explanation and inspiration
Evidence for selection over the last few thousand years on disparate populations around the world highlights additional important drawbacks of insufficiently sampling human variation from a broad range of non-WEIRD environments and genetic backgrounds. From a practical standpoint, considering bodies to be more or less the same everywhere reinforces strong evidence that health inequalities stem mostly from differences in exposures and in access to amenities. It is indisputable that better daily living conditions like access to clean water, high-quality food, safe neighborhoods, preventive medical care, and attention to other social-structural factors would vastly improve health in most populations in low and middle income countries that are overwhelmingly non-WEIRD- arguably greater than improved precision medicine or more intense clinical practice (Bayer & Galea, 2015). Nonetheless, aside from the crude differences in morbidity across world regions highlighted in Table 1, multiple lines of additional evidence suggest that bodies do not respond similarly to all exposures, or even to the same treatments. For example, people of African descent respond better to calcium blockers and diuretics than to other effective treatments for hypertension commonly used in practice, such as ACE inhibitors and β-adrenergic blockers (Brewster & Seedat, 2013). Other well-documented examples in the field of pharmacogenetics of interethnic differences in drug-metabolizing enzymes and receptor systems suggest that a one-size-fits-all approach is not only sometimes ineffective for clinical treatment, but can be lethal (Meyer, 1999). A reasonable hypothesis is that the evolutionary basis for at least some of these differences arises from mismatches due to past selection.
Mismatches are not just a phenomenon of the past century, but instead arise whenever organisms are inadequately or imperfectly adapted to novel environmental conditions. Mismatches caused by changes in climate and dispersal into new habitats have been a longstanding driver of selection. Humans are no exception to this process and arguably have been more subject to mismatch than most mammals. It is now well established that modern humans originated in Africa, itself a diverse continent, and then dispersed across the globe over the last 80,000 years into an astonishing array of tropical, temperate and even arctic habitats (Reich, 2018). By 40,000 years ago, human hunter-gatherers were present in almost every part of the globe. The novel pathogens, foods and climatic conditions that people encountered on every continent save Antarctica, coupled with dramatic, rapid climate change during the end of the Pleistocene and the beginning of the Holocene, must have resulted in a wide range of mismatches. Cultural innovations helped overcome some of these challenges (Henrich, 2017), but they also helped enable natural selection to operate in these populations, promoting adaptations to novel pathogens, foods and climactic conditions. Although 85–90% of human genetic variation occurs within populations (Barbujani et al., 1997; Witherspoon et al., 2007), the 10–15% of genetic variance that differs between populations is evidence that there was ample time for local adaptations to many of these diverse environments.
Agricultural and industrial revolutions brought substantial changes in livelihoods, environments and exposures (Trumble & Finch, 2019). The shift from nomadic hunting and gathering to include plant and animal domestication and greater sedentism occurred between 4–12 kya in different locations. The Neolithic period during the Holocene witnessed domestication of animals like goats, cattle and pigs, and narrowed dietary diversity by increasing reliance on a limited number of carbohydrate-rich but nutrient-poor staple crops with increased susceptibility to famines (Cohen & Armelagos, 1984). Settlements became more permanent, with intensive irrigation and economic specialization, along with dense populations that could support virulent contagious infections, many arising from contact with domesticated animals. This package of traits varied in composition, sequence and timing in different world areas, from earliest timing in the Levant ~12 kya, Southwest Asia ~9–10 kya, to later appearances like in the Americas between 4–8 kya (Rindos, 2013). Although these transitions led to rapid population growth, they also led to a broad set of mismatches between genetic ancestry and the combination of altered ecological niches and lifestyles. Substantial research has documented that these mismatches included numerous infectious diseases such as measles, smallpox and influenza, nutritional diseases such as pellagra and rickets, and some chronic health conditions such as dental caries and osteoporosis (reviewed in Lieberman, 2013) (Table 2). Among the many consequences of these mismatches, farmers became shorter and life expectancy likely declined relative to ancestral hunter-gatherer populations (Gage & DeWitte, 2009).
TABLE 2. Suspected mismatch diseases.
Adapted from Lieberman (2013: Table 3). Diseases in italics have been observed in contemporary subsistence populations.
| Acid reflux/chronic heartburn | Depression (Major) | Irritable bowel syndrome |
|---|---|---|
| Acne vulgaris | Diabetes (Type 2) | Lactose intolerance |
| Alzheimer’s Disease | Diaper rash | Lower back pain |
| Anxiety | Eating disorders | Malocclusion |
| Apnea | Emphysema | Metabolic syndrome |
| Atherosclerosis | Endometriosis | Multiple sclerosis |
| Athlete’s foot | Fatty liver syndrome | Myopia |
| Atopy (allergies, asthma) | Fibromyalgia | Obsessive-compulsive disorder |
| Attention deficit hyperactivity disorder | Flat feet | Osteoporosis |
| Bunions | Glaucoma | Plantar fasciitis |
| Cancers (ovarian, prostate) | Gout | Polycystic ovarian syndrome |
| Carpal tunnel syndrome | Hammer toes | Preeclampsia |
| Cavities | Hemorrhoids | Rickets |
| Chronic fatigue syndrome | Hypertension | Scurvy |
| Cirrhosis | Iodine deficiency | Stomach ulcers |
| Constipation (chronic) | Impacted wisdom teeth | |
| Crohn’s disease | Insomnia (chronic) |
While there was selection in the last 10,000 years on some mismatches triggered by the agricultural revolution (well-studied examples include lactase persistence and malarial resistance), there has been almost no time for significant selection to cope with the arguably even more profound mismatches caused by the last few centuries of industrialization, large-scale urbanization, and the post-industrial revolution. These still ongoing processes have further transformed diets, physical activity levels, social and residence structures, and more. Recent innovations like sanitation systems, refrigeration, antibiotics, and modern dentistry have ameliorated many of the mismatches caused by the agricultural revolution, but others have opened a Pandora’s Box of new mismatch conditions from environmental exposures (Table 2) (Lieberman, 2013). Some of these mismatches are caused by too little of formerly important exposures like non-lethal pathogens or diverse microbial communities (see Section 7.1), extended breastfeeding, number of pregnancies (see Section 7.2), endurance physical activities (see Section 7.3), and dietary fiber. Other mismatches result from too much of formerly rare resources like sugar, saturated fat, and dietary salt, whose consumption has increased by several-fold in many WEIRD populations (Adrogué & Madias, 2007). Yet other mismatches arise from environmental exposures that are too new, like nicotine and high heels to which the body is poorly or inadequately adapted. As a consequence, recent trends towards urbanization and development have been accompanied by an explosion of new forms of morbidity including myopia and flat feet, as well as obesity- and energy-related chronic conditions such as type 2 diabetes, atherosclerosis, hypertension, and certain cancers (Tables 1,2).
Despite broad changes in exposures and lifestyle across these transitions, not all changes result in morbidity or increased risk of mortality. Most phenotypes are influenced by evolved reaction norms in which a broad continuum of structural or functional responses occur in response to variations in environmental stimuli. While mismatches are often framed as the consequence of drastic historical changes, as with the Anthropocene or Industrial Revolution, mismatches are themselves a fundamental cause of evolutionary change. It follows that the pace and scope of environmental change often influences the degree of mismatch, and many mismatches manifest as continuous not discrete variations in health. That said, rapidly accelerating cultural evolution and population growth have expedited the prevalence and severity of many mismatches. As examples, obesity prevalence in the U.S. and UK grew rapidly since the 1970’s, and has tripled worldwide over the past four decades, increasing the risk of metabolic disease, heart disease, stroke, some cancers, and numerous other causes of morbidity and mortality (Gregg & Shaw, 2017) (Table 1). To a large extent, the rapid increase in these causes of morbidity and mortality has coincided with the declining cost and greater availability of cheap, sugar- and fat-rich processed foods (Headey & Alderman, 2019).
In short, while recent cultural change has increased the prevalence and severity of mismatches in WEIRD populations, the range of conditions experienced by modern humans has always varied over space and time causing mismatches of various degrees. For hundreds of thousands of years, health and well-being have been affected by variation in factors such as diet, activity levels, pathogen burden, reproductive behavior and breastfeeding, technology, risk buffering and the degree of social isolation. Genetic differences reflect some long-term exposures, and plasticity over different time scales can adjust physiological responses to some degree, but opportunities for mismatch to result in health problems lurk everywhere there is rapid, large-scale environmental change.
6. Local adaptations and gene-environment interactions
Another benefit of assessing diversity beyond WEIRD populations is to better evaluate gene-environment interactions that affect individual and population-level vulnerabilities to disease. Founder effects (the loss of genetic diversity when a small new population forms from a larger population), in-group endogamy (mating within cultural group boundaries), and selection can lead to greater genetic health risks in some groups, like greater Tay-Sachs and breast cancer risk among Ashkenazi Jews, cystic fibrosis risk among white Europeans, susceptibility to and severity of systemic lupus erythematosus among those of African and South Asian ancestry (Lewis & Jawad, 2016), and prostate cancer among men of West African ancestry (Lachance et al., 2018). Sickle cell anemia risk is a textbook example of a health risk arising as a consequence of past genetic adaptations against another disease - in this case, malaria throughout large areas of Africa. Malaria remains a major killer today, especially of children, with 219 million estimated cases across 87 countries and 435,000 deaths just in 2017 (WHO, 2018). Selection has shaped red blood cells by favoring ‘loss of function’ gene variants in malaria-rich regions, resulting in health consequences such as thalassemia, glucose-6-phosphate dehydrogenase (G6PD) deficiency, pyruvate kinase deficiency, and others (Kwiatkowski, 2005). While these represent cases of strong selection over the last 5–10kya, much evidence confirms that pathogens have been the most important selective force shaping the genetics of the human immune system (Fumagalli et al., 2011). Many genetic polymorphisms show geographic gradients, affecting inflammation-related cytokine expression in ways that could make certain populations today more susceptible to inflammatory disorders (Brinkworth & Barreiro, 2014). Recent ancestry from tropical Africa, where infectious burden has arguably been strongest, has selected for a number of inflammation-related genes; mismatch due to recent migration to a less pathogenic environment could contribute, for example, to differences in autoimmune and chronic disease rates between tropical Africans and African-Americans (Pennington et al., 2009; Yao et al., 2018).
Other genetic differences among populations stem from when agriculture was first adopted and from previous history of having a carbohydrate-rich diet. These varying dietary backgrounds could help partly explain some group differences in the propensity for obesity, diabetes and metabolic syndrome. For example, it has been known for some time that more gene copy number variants of the salivary amylase (AMY1) gene increase amylase proteins that help digest starches (but see Fernández & Wiley, 2017), and are associated with lower postprandial glucose levels and lower insulin resistance. More copy number variants are evident in farmers and hunter-gatherers from arid regions consuming high-starch diets than among low-starch pastoralists and rain forest or Arctic hunter-gatherers (Perry et al., 2007). More recently, the CLTCL1 gene was identified as directing production of a protein that regulates glucose levels and glucose storage in muscle and fat, with different variants found in hunter-gatherers than farming populations (Fumagalli et al., 2019).
Other genetic differences of relevance to current health risks have been documented (e.g. Mortensen et al., 2011), and many others await discovery, though progress is slow because of the lack of diversity in genomics studies. As of 2018, 78% of individuals included in genome-wide association studies (GWAS) were European, 10% Asian, 2% African, 1% Hispanic and <1% for all other ethnicities (Sirugo et al., 2019). Limited sampling can have real consequences. For example, one-fourth of GWAS-identified variants related to obesity, diabetes and lipid levels had substantially different effect sizes in non-European ancestry populations (Carlson et al., 2013). The 1000 Genomes Project, sampling 2,500+ people from 26 populations and completed in 2015, was the first to intentionally sample broadly across world regions, and other initiatives are underway (e.g. Population Architecture using Genomics and Epidemiology, and the Human Heredity & Health in Africa Initiative), though these are exceptions, not the rule. The recent release of UK Biobank data on genomics and rich health phenotype for 500,000 British adults (ongoing until 2020) has seen a vast flurry of important discoveries, but 94% of the sample are white and of Northern European ancestry.
7. Case studies: Why it’s worth including non-WEIRD populations
As reviewed above, WEIRD people typically carry low infectious burden, have low fertility, eat more processed energy-rich diets, are more sedentary, and differ from non-WEIRD people in how they dress, rest, sit, sleep, and countless other ways from people in many rural contexts today and from most of human history. Given that many high profile medical studies involve WEIRD participants (Figure 1), current biomedical understanding of human bodies and health is not always generalizable by virtue of being largely limited to this reduced range of exposures. Below we briefly review health consequences of three key differences in exposure between WEIRD and non-WEIRD contexts: pathogen burden, fertility, and physical activity. These examples also help illustrate how consideration of human evolutionary biology, with its emphasis on functional design, evolved reaction norms, ancestral history and gene-by-environment interactions, is a necessary addition to traditional biomedical approaches.
7.1. Infection, immune regulation and chronic disease
Since the 1950’s, declines in numerous infectious diseases like measles and tuberculosis coincided with rapid increases in the incidence of autoimmune and allergic diseases, including multiple sclerosis, Crohn’s disease, asthma, hay fever and allergies (Bach, 2002). Antibiotics, vaccines and better hygiene helped reduce those infections, and the past three decades have seen attempts to understand the mechanisms underlying linkages between pathogen- and autoimmune-related diseases. The leading hypothesis in this complex field is that early and regular exposure to diverse “friendly” microbes, rather than harmful infectious pathogens, helps train the immune system to learn what to attack and tolerate to insure appropriate immune responses. Indeed, infections like measles may exacerbate autoimmune diseases, not decrease them, whereas commensals like Prevotella and Escherichia coli may help gauge appropriate T-helper type 1 responses in ways that are protective against autoimmunity (Vatanen et al., 2016). The human immune system coevolved with a rich diversity of microbial and parasitic species, often dubbed “old friends” that our bodies anticipate to be present.
The implication of this hypothesis is that our contemporary “epidemic of absence” results in a variety of immune function mismatches (Velasquez-Manoff, 2012). Antibacterial soap and aerosols, Cesarean-section births, dishwashers, living in cement buildings rather than on farms, and other aspects of contemporary urban living have depleted the types and quantity of microbial exposures in ways leading to immune dysregulation. Despite the growing recognition of the role of microbial exposures on immune modulation and health, many studies of this phenomenon are epidemiological and cross-sectional, and few randomized controlled trials have tested interventions to regulate consistently and successfully the immune system in health-promoting ways. Yet some successes have come with intestinal helminth inoculation therapy for treatment of inflammatory bowel disease (Helmby, 2015). Intestinal helminths have coexisted with humans for millennia, and likely represent a major feature of early human disease ecology (Hurtado et al., 2008).
Many questions remain, and answering them will require consideration of evolutionary history, diversity within and among populations, and environmental mismatch. First, not everyone in WEIRD places suffer from hypersensitive allergic reactions and autoimmune diseases. What makes some more susceptible than others? Certainly diet affects the composition of host commensals (Lassalle et al., 2018). As described in section 6 above, geographic-specific pathogens are the strongest driver of local genetic adaptations, more so than climate or dietary differences. In addition, variation in these genes showing most enrichment from selection are also associated with autoimmune diseases like celiac and multiple sclerosis (Fumagalli et al., 2011). The way genetic and epigenetic factors interact with environmental exposures to shape immune regulation and chronic diseases is certainly complex.
It is also not clear why people tend to become allergic to certain antigens and not others. While air particulates from industrial processes are more novel stimuli for our immune system, why would 32 million Americans be allergic to nutritious foods like peanuts and seeds? Many common allergens share molecular markers resembling pathogen antigens, and so will bind to immunoglobulin-E (IgE), the primary antibody involved in allergic reactions that also provides protection from parasitic infections, especially helminths. Another mystery is that many autoimmune diseases do not manifest clinically until adulthood, even though much immune education occurs early in life. These and other problems are not yet resolved, but better ecological understanding of the immune system is needed if we are to help reduce the DALY burden from inflammatory diseases. Simple symptomatic treatments like using anti-inflammatories such as steroids to broadly dampen immune responses, are unlikely to be as helpful as more targeted approaches.
Inflammation is an innate immune response that acts as the first line of defense against host attack. Yet inflammation’s lack of precision results in collateral damage that, unchecked over time, can contribute to the initiation and progression of many diseases including atherosclerosis and type 2 diabetes (Libby et al., 2002). Inflammation-mediated insulin resistance might be beneficial for fueling immunity against an acute bacterial infection, but in the absence of pathogens, “sterile” inflammation derives from obesity, cigarette smoking, non-alcoholic fatty liver disease, and physical inactivity (Brestoff & Artis, 2015). Obesity leads to chronic, low-grade inflammation because of macrophage and other pro-inflammatory activity in white adipose tissue. New experimental and epidemiological research suggests that the absence of helminths in WEIRD countries may also contribute to adult-onset diabetes and atherosclerosis (reviewed in Gurven et al., 2016; Wiria et al., 2014). Long-evolved strategies of helminths include drawing metabolic resources from their host, including blood lipids and glucose, as well as modulating and regulating immune function towards greater T-helper type 2 (Th2, anti-inflammatory) polarization, glucose tolerance and insulin sensitivity.
Greater consideration of the role of parasites may also shed light on certain risk alleles that in WEIRD contexts only seem to harm health. Apolipoprotein-E4 (ApoE4) allele is the greatest genetic risk factor for Alzheimer’s Disease (AD), and its extensive variation across populations is a puzzle. Yet ApoE4 carriers have been shown to clear some infections like viral hepatitis C, giardia and cryptosporidium (Oria et al., 2010), especially in early childhood when the force of selection is greatest (Figure 1). Further, among Tsimane Amerindians with active helminth infection, ApoE4 carriers are more protected against cognitive decline than ApoE3 carriers (Trumble et al., 2017). ApoE4 is also unrelated to AD risk among the Yoruba of Nigeria (Hendrie et al., 2014). Indeed, the ApoE4-AD link is strongest among Caucasians and East Asians. Thus, consideration of gene-environment interactions across a greater range of environmental conditions typically encountered in WEIRD countries can provide new insight about risk alleles; to do so effectively requires sampling beyond WEIRD contexts.
7.2. Reproduction, autoimmune disease and cancer
Currently, female life expectancy exceeds male life expectancy in all countries (UNDP, 2019). Yet, prior to a century ago, maternal mortality and high fertility would have lowered female life expectancy considerably. As fertility declined over the 20th century, the sex gap in cohort life expectancies widened. For example, as the TFR declined from 9 to 4 births per woman in Utah, e0 went from male-biased by one year to female-biased by four years; fertility differences accounted for at least a six year difference in post-reproductive lifespan, accounting for the strong female-biased longevity we see today (Bolund et al., 2016).
Despite female-advantaged survivorship, higher morbidity among females is well documented (the “male-female morbidity-mortality paradox”). In WEIRD populations, men tend to suffer from cardiovascular disease and non-reproductive cancers at higher rates, and women at higher rates of inflammatory-related autoimmune diseases like systemic lupus erythematosus, Grave’s disease and rheumatoid arthritis (Crimmins et al., 2019; Ngo et al., 2014). Sex differences in exposures only partially explain the gender health gap. Instead, many sex differences in health may lie in adjustments to immune function that are unique to mothers. Mothers must strike a balance between upregulated responses to combat pathogens, and downregulated responses to tolerate a fetus through pregnancy (Natri et al., 2019). Such immune modulation favors a dampened, anti-inflammatory bias, but low fertility in WEIRD contexts combined with low pathogen exposure may jointly lead to sex-specific immune dysregulation in ways that augment autoimmune disease risk. Comorbidity can extend even to mental health. While women are more prone to depression in WEIRD countries, depression often co-occurs with elevated inflammatory immune activity, which puts depressed women at double the risk of developing later lupus (Roberts et al., 2018).
Reproductive effort interacts with other novel WEIRD conditions to affect cancer risk. Energetic stress, active lifestyle and heavier immune burden in subsistence populations are associated with lower levels of reproductive hormones like testosterone, estradiol and progesterone than those in WEIRD populations (Ellison et al., 2002; Nunez-De La Mora et al., 2007). A typical woman in low fertility settings will experience 3–4 times more menstrual cycles over her lifetime than a woman in high fertility settings with intensive breastfeeding, resulting in higher circulating estrogens (Eaton et al., 1994). In women, high levels of (unopposed) estrogens- from fewer pregnancies, earlier menarche and later first birth -- have been linked to greater risk of endometrial and breast cancer (especially estrogen receptor positive) (Key et al., 2001). These cancers are exacerbated further by obesity and lower physical activity. Among men, greater cumulative testosterone exposure elevates prostate cancer risk (Alvarado, 2013).
7.3. Physical activity and diseases of aging
Another major defining characteristic of WEIRD societies is the shift away from occupations that require manual labor and the replacement of human-powered locomotion, mostly walking, with mechanized transportation (Church et al., 2011). Hunter-gatherers and other subsistence populations walk 2–4 times more steps per day than in WEIRD countries like the US and UK (Althoff et al., 2017), and engage in at least 10 times more daily moderate-to-vigorous activity (Gurven et al., 2013; Raichlen et al., 2017). Aside from the direct effects of decreased physical activity on energy balance, weight gain and obesity-related metabolic diseases, physical inactivity —mostly sitting—has other profound effects on senescence and many diseases because varying doses of physical activity influence numerous processes that build, repair and maintain many anatomical and physiological capacities. Although there are too many of these diseases to review here, we briefly note how WEIRD levels of physical inactivity are causally associated with the prevalence of three mismatch diseases that have recently become much more common: hypertension, osteoarthritis, and Alzheimer’s Disease.
It is widely believed that blood pressure (BP) rises inevitably with age, and that the main reason for the epidemic of hypertension is the increased number of older individuals alive today. However, cross-sectional studies of numerous subsistence populations show little if any age-related differences in BP (Barnicot et al., 1972; Shave et al., 2019; Truswell et al., 1972), whereas longitudinal study among Tsimane horticulturalists showed only minimal age-based BP increases (Gurven et al., 2012). Although hypertension is influenced by numerous factors including diet and psychosocial stress, physical activity is known to prevent and sometimes partially reverse hypertension in a dose-dependent manner through at least three mechanisms (Diaz & Shimbo, 2013; Liu et al., 2017). First, greater blood flow during physical activity, especially endurance physical activities, stimulates the expansion of the peripheral circulatory system that helps reduce vascular resistance. Second, endurance physical activity increases or maintains the diameter and elasticity of arteries, with strong effects on blood pressure. Finally, physical activity can indirectly lower hypertension (and atherosclerosis) by reducing systemic inflammation, weight loss, and improved renal and endothelial function (Fiuza-Luces et al., 2018).
Physical activity also generates mechanical loads that impact muscle and bone mass, helping prevent sarcopenia and osteoporosis, which are both increasingly common mismatch diseases (Edwards et al., 2015). Osteoarthritis (OA), on the other hand, is often considered a wear-and-tear consequence of aging attributable to obesity and inflammation. Studies with non-WEIRD populations, however, contradict this view and show that OA is largely a preventable mismatch disease (Berenbaum et al., 2018). An analysis of over 2,500 knees from North American adults aged 50+ over the last 6,000 years shows that the age-related prevalence of knee OA more than doubled in the last two generations as activity levels declined (Wallace et al., 2017). Physical activity may be protective because it reduces inflammation, strengthens muscles that protect joints, and helps support cartilage volume (Urquhart et al., 2008). However, despite being physically active, subsistence farmers transitioning to modern lifestyles with high levels of visceral adiposity carry an elevated risk of OA (Wallace et al., 2019), presumably caused by greater obesity-induced inflammation.
Lastly, we briefly highlight the relevance of physical activity to Alzheimer’s Disease (AD), which is predicted to increase in prevalence four-fold worldwide by 2050 (Brookmeyer et al., 2007). Practically all clinical efforts to eliminate beta-amyloid or tau protein tangles, the primary target causes of AD according to the dominant amyloid beta cascade hypothesis, have been unsuccessful (Ricciarelli & Fedele, 2017). Alternative theories and approaches are needed. One recent contender addresses the role of infection, and suggests that AD may one day be classified as an autoimmune disease (see section 7.1). For example, AD may be caused by microglial-activated toxins perhaps influenced by gut and other microbes that cross a brain-blood barrier that is weakened by chronic inflammation (Osorio et al., 2019). According to this hypothesis, beta-amyloid is an antimicrobial peptide that helps protects the brain from infection (Kumar et al., 2016). Regardless of the precise mechanisms, physical activity is one of the most effective known forms of prevention and management for AD. A meta-analysis of 16 high-quality prospective studies sampling more than 160,000 individuals found that moderate levels of physical activity lowered AD risk by 45% (Hamer & Chida, 2009). To date, however, there is less consistent evidence that exercise improves cognition or neuropsychiatric symptoms for those already having dementia (Forbes et al., 2015). Proposed mechanisms for protective effects of physical activity on lower AD risk and AD progression include higher levels of brain derived neurotropic growth factor (BDNF), which helps maintain neuronal health (Wang & Holsinger, 2018), increase cerebral blood flow, suppress inflammation and counter oxidative damage (Paillard et al., 2015).
8. Toward the future
Our primary aim was to identify the WEIRD bias in biomedical research, justify why this bias limits our understanding of the human body, and demonstrate how this bias diminishes our ability to improve global health. A wider, more sophisticated view of mismatch can offer biomedicine new ways of thinking about risk factor relationships and genetic pleiotropies. Greater integration of non-WEIRD data with existing WEIRD-focused literature can also move the conversation away from extreme views based on caricatures of our ancestral past and erroneous evolutionary logic (e.g., newer is always better or always worse), and help evaluate fads like the PaleoDiet, PaleoFitness and RePOOPulation. Evolution-minded thinking combined with sampling across diverse environments will likely see an expansion of new interventions, such as those affecting immune regulation: e.g. probiotics, helminthic antigen inoculations, vaginal seeding for C-section births. Other evolution-inspired interventions based in part on non-WEIRD sampling include multigenerational structured living to improve well-being among older adults, and community-level Blue Zones Project (bluezonesproject.com) designed to help facilitate healthier eating, more physical activity, and less social isolation.
While many health needs overlap across countries, and individual countries show heterogeneity, we identified large differences in DALYs between WEIRD and non-WEIRD world regions. Yet high-profile medical research disproportionately favors those living in WEIRD countries. This major focus on WEIRD bodies is not only unrepresentative of humans today, but ignores how our species lived over most of its history. Human dispersals over many millennia have resulted in considerable local genetic and cultural differentiation. Overcoming medical science’s WEIRD bias will thus require diverse sampling in terms of ethnicity, geographic location, socioeconomic status, lifestyle, and other exposures. But it will be worth it because greater consideration of diversity can impact research at all levels: study questions and research design, analysis, interpretation and generalizability of findings. Greater diversity will also affect how findings are applied in practice: diagnosis, treatment, prevention and drug discovery, dosage and efficacy.
Because most medical research highlighted in Figure 1 is done in WEIRD countries, and often funded by federal or regional institutes, one could argue that a WEIRD bias is a reasonable and desirable way to best serve the health needs of WEIRD patients. This logic is misguided. First, citizens of many WEIRD countries are born elsewhere or have ancestry from other parts of the world. Studies of non-WEIRD populations from around the world can therefore have direct relevance to people living in WEIRD countries. Second, as we have argued, better understanding of risk alleles and mismatch diseases in WEIRD places requires attention to a broader environmental context that is ecologically relevant to the physiological system. That inflammation or ApoE4 may function differently in infected individuals is critically informative and worth further exploring to inspire new ways to both treat and prevent disease. Given that the morbidity from complex mismatch diseases listed in Table 2 limits healthspan in WEIRD countries, there is strong value to broadening our approach. Antibiotics won’t help cure complex chronic diseases, nor are single gene variants of large effect likely to be discovered. Complete mapping of the genetic architecture of complex diseases instead will require analyses of diverse populations.
Third, there is already a good precedent for medical discoveries in non-WEIRD regions having important beneficial effects in WEIRD countries. For example, studies of Burkitt’s lymphoma in Uganda and other sub-Saharan African countries helped identify the causal role of the Epstein-Barr virus, while also providing the impetus for suspecting infectious origins of other cancers (Burkitt, 1958; Rebbeck, 2020). Another example of the perils of ignoring diversity for both WEIRD and non-WEIRD populations are the multiple genetic variants that were initially misclassified as increasing the risk of hypertrophic cardiomyopathy (a major cause of heart failure) but turned out to be benign following yet broader genetic studies of African patient populations (Manrai et al., 2016). It is believed that the lack of diverse sampling leading to similar ascertainment biases will result in false positives and genetic misdiagnoses for other diseases. One recent deliberate genomic sampling of 49,839 non-Europeans found 27 new genetic variants related to cardiometabolic and renal health, and showed substantial effect size heterogeneity across ancestries for many established variants (Wojcik et al., 2019). These discoveries would never have been found if restricted to more homogenous datasets, even with huge sample sizes. Lastly, artificial intelligence is increasingly used in clinical medicine, such as to help diagnose skin cancer, diabetic retinopathy, and seizures, but algorithms are often trained using homogenous WEIRD datasets. A lack of diversity in training datasets leads to misdiagnoses, especially in under-represented populations, and could therefore further worsen health disparities within WEIRD countries (Khullar, 2019).
The obvious and most important reason to pay more attention to non-WEIRD bodies is to improve global health, poverty reduction and environmental sustainability as articulated by the UN Millennium Development Goals of 2000. The UN Sustainable Development Goals continue this vision with improved global partnership, stressing the principle of “leaving no one behind” (UN 2015). To this end, broader sampling and attention are vital to help serve the health needs in underserved regions (Figure 1) and to reduce global health inequalities. Diagnostic and treatment tools developed studying WEIRD people may not translate effectively when employed in under-studied populations. For example, genetic risk scores developed from sequencing white Europeans may poorly estimate disease risk in non-Europeans, leading to a failure to treat properly. Not only might other gene variants be involved, but gene modification due to interactions with other genes (epistasis) can vary in ways that are clinically relevant, as has been shown for sickle cell disease (Sirugo et al., 2019). There are also many health risks in non-WEIRD countries that are extremely rare in WEIRD countries and thus receive relatively little research attention or pharmacological innovation, such as the ‘neglected tropical diseases’ (e.g., leishmaniasis, Chagas disease, lymphatic filariasis), which affect over 1 billion people living in tropical or subtropical regions of 149 countries (WHO).
The mission of the U.S. National Institutes of Health, the largest public funder of biomedical research in the world, is to “seek fundamental knowledge about the nature and behavior of living systems… to enhance health, lengthen life and reduce illness and disability”. While U.S. health needs may be a priority for U.S. federal funding, just fulfilling this mission requires the kind of broad sampling we advocate here. Further, the U.S. has a diverse populace, a point that sometimes gets lost using the WEIRD label to refer to urban living, whiteness or student populations. Despite the large U.S. representation in Figure 1, sampling within the U.S. is also subject to biases. For example, a recent review of studies using genome and exome sequencing of U.S. cancer patients reports under-representation of U.S. minorities (Nugent et al., 2019). Even when attempts are made for broader sampling (e.g. All of Us, https://allofus.nih.gov), indigenous populations are often the most under-sampled. Despite having higher rates of cardiovascular disease, diabetes, cancer and infectious disease, indigenous North Americans remain grossly under-represented and under-studied in genetic and clinical health research (Claw et al., 2018).
Sampling beyond WEIRD faces similar obstacles as those raised when considering similar concerns in psychology and the social sciences (Hruschka et al., 2018; Medin et al., 2017). Remedies will be needed on many fronts. Identification of the WEIRD bias is growing, having recently been showcased in genomics (Popejoy & Fullerton, 2016; Sirugo et al., 2019), for mental health (Patel & Sumathipala, 2001) and cancer (Rebbeck, 2020). Exploring beyond WEIRD was the subject of a recent National Academy of Sciences/National Institute on Aging meeting addressing different social processes related to aging (National Academies of Science, 2018). Greater inclusion, especially of at-risk, vulnerable, illiterate or geographically remote populations requires deliberate effort and careful engagement to counter a history of distrust and exploitation. Improving recruitment of indigenous populations will require good-faith practices, such as treating research as a community-based collaboration, tailored benefits to local communities, incorporating capacity-building, greater transparency at all stages, and disseminating study findings to communities and other interest groups (Claw et al., 2018). A very inexpensive amendment to current studies would be to include information on sources of diversity, such as ancestry, and to report how effects might differ among subgroups. For example, genomic studies of cancer often under-report ethnic origin or do not explicitly perform analyses by ethnic categories (Nugent et al., 2019). Such was the case with sex before established guidelines made simultaneous study of males and females a requirement (e.g. NIH Policy on Sex as a Biological Variable). Women now account for about half of all study participants in NIH-supported clinical research.
9. Conclusion
Future improvements in life expectancy are unlikely to occur at the same impressive pace using the same tools we’ve used in the past. And with higher survivorship, medical science’s ability to improve healthspan requires tackling chronic diseases with new understandings and novel approaches. For most of our history as a species, we thrived by craving calorically-dense fatty foods, and obtaining them efficiently at minimal cost whenever possible. We live in a unique period of human history where much of our daily health regimen centers around choosing to do abnormal things like exercise or avoid tempting calorie-rich foods. Conscious attention to the consequences of our daily behavior on long-term health can be a constant struggle. Increasing our perspective on these challenges beyond WEIRD contexts, especially where many chronic mismatch diseases are minimal or absent, presents new opportunities for learning about mismatch and devising innovative strategies for reducing morbidity. However, as populations around the world witness varying degrees of socioeconomic change, the landscape of human disease is rapidly shifting. More knowledge about non-WEIRD bodies and their environments is necessary to both effectively reduce global DALY burdens, and preventing or mitigating rising waves of chronic non-communicable diseases.
Supplementary Material
Acknowledgements
We thank Fischer Basham, Halle Clark, Alyssa Cunningham, Liam Knox, Selin Lopez, Shani Tra and Julia Weber for recording participant information from the three biomedical journals. MDG was supported by NIH/National Institute on Aging #RF1AG054442. We thank Joe Henrich for inviting us to contribute to this special issue.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Works Cited
- Adrogué HJ, & Madias NE (2007). Sodium and potassium in the pathogenesis of hypertension. New England Journal of Medicine, 356(19), 1966–1978. [DOI] [PubMed] [Google Scholar]
- Althoff T, Hicks JL, King AC, Delp SL, & Leskovec J (2017). Large-scale physical activity data reveal worldwide activity inequality. Nature, 547(7663), 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado LC (2013). Do evolutionary life-history trade-offs influence prostate cancer risk? a review of population variation in testosterone levels and prostate cancer disparities. Evolutionary Applications, 6(1), 117–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias E, Escobedo LA, Kennedy J, Fu C, & Cisewski J (2018). U.S. small-area life expectancy estimates project: Methodology and results summary. National Center for Health Statistics. Vital Health Statistics, 2(181). [PubMed] [Google Scholar]
- Arnett JJ (2008). The neglected 95%: why American psychology needs to become less American. American Psychologist, 63(7), 602. [DOI] [PubMed] [Google Scholar]
- Bach J-F (2002). The effect of infections on susceptibility to autoimmune and allergic diseases. New England Journal of Medicine, 347(12), 911–920. [DOI] [PubMed] [Google Scholar]
- Barbujani G, Magagni A, Minch E, & Cavalli-Sforza LL (1997). An apportionment of human DNA diversity. Proceedings of the National Academy of Sciences, 94(9), 4516–4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnicot N, Bennett F, Woodburn J, Pilkington T, & Antonis A (1972). Blood pressure and serum cholesterol in the Hadza of Tanzania. Human Biology, 87–116. [PubMed] [Google Scholar]
- Baudisch A (2005). Hamilton’s indicators of the force of selection. Proceedings of the National Academy of Sciences of the United States of America, 102(23), 8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer R, & Galea S (2015). Public health in the precision-medicine era. New England Journal of Medicine, 373(6), 499–501. [DOI] [PubMed] [Google Scholar]
- Bentley GR, Goldberg T, & Jasieńska G. z. y. (1993). The fertility of agricultural and non-agricultural traditional societies. Population Studies, 47(2), 269–281. [Google Scholar]
- Berenbaum F, Wallace IJ, Lieberman DE, & Felson DT (2018). Modern-day environmental factors in the pathogenesis of osteoarthritis. Nature Reviews Rheumatology, 14(11), 674–681. [DOI] [PubMed] [Google Scholar]
- Bolund E, Lummaa V, Smith KR, Hanson HA, & Maklakov AA (2016). Reduced costs of reproduction in females mediate a shift from a male-biased to a female-biased lifespan in humans. Scientific Reports, 6, 24672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brestoff Jonathan R., & Artis D (2015). Immune Regulation of Metabolic Homeostasis in Health and Disease. Cell, 161(1), 146–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster LM, & Seedat YK (2013). Why do hypertensive patients of African ancestry respond better to calciumblockers and diuretics than to ACE inhibitors and β-adrenergic blockers? Asystematic review. BMC medicine, 11(1), 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkworth JF, & Barreiro LB (2014). The contribution of natural selection to present-day susceptibility to chronic inflammatory and autoimmune disease. Current Opinion in Immunology, 31, 66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookmeyer R, Johnson E, Ziegler-Graham K, & Arrighi HM (2007). Forecasting the global burden of Alzheimer’s disease. Alzheimer’s & dementia, 3(3), 186–191. [DOI] [PubMed] [Google Scholar]
- Burkitt D (1958). A sarcoma involving the jaws in African children. British Journal of Surgery, 46(197), 218–223. [DOI] [PubMed] [Google Scholar]
- Byars SG, Ewbank D, Govindaraju DR, & Stearns SC (2009). Natural selection in a contemporary human population. Proceedings of the National Academy of Sciences, 107(suppl 1), 1787–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson CS, Matise TC, North KE, Haiman CA, Fesinmeyer MD, Buyske S, … Ritchie MD (2013). Generalization and dilution of association results from European GWAS in populations of non-European ancestry: the PAGE study. PLoS biology, 11(9), e1001661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnes BA, Olshansky SJ, & Grahn D (1996). Continuing the search for a law of mortality. Population and Development Review, 22, 231–264. [Google Scholar]
- Church TS, Thomas DM, Tudor-Locke C, Katzmarzyk PT, Earnest CP, Rodarte RQ, … Bouchard C (2011). Trends over 5 decades in US occupation-related physical activity and their associations with obesity. PLoS ONE, 6(5), e19657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claw KG, Anderson MZ, Begay RL, Tsosie KS, Fox K, & Nanibaa’A G (2018). A framework for enhancing ethical genomic research with Indigenous communities. Nature communications, 9(1), 2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran G, & Harpending H (2009). The 10,000 year explosion: How civilization accelerated human evolution: Basic Books. [Google Scholar]
- Cohen MN, & Armelagos GJ (1984). Paleopathology at the origins of agriculture. Orlando, FL: Academic Press. [Google Scholar]
- Crimmins EM, Shim H, Zhang YS, & Kim JK (2019). Differences between men and women in mortality and the health dimensions of the morbidity process. Clinical chemistry, 65(1), 135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz KM, & Shimbo D (2013). Physical activity and the prevention of hypertension. Current hypertension reports, 15(6), 659–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton SB, Konner MJ, & Shostak M (1988). Stone agers in the fast lane: chronic degenerative diseases in evolutionary perspective. American Journal of Medicine, 84, 739–749. [DOI] [PubMed] [Google Scholar]
- Eaton SB, Pike MC, Short RV, Lee NC, Trussell J, Hatcher RA, … Hurtado AM (1994). Women’s reproductive cancers in evolutionary context. Quarterly Review of Biology, 69(3), 353–367. [DOI] [PubMed] [Google Scholar]
- Edwards M, Dennison E, Sayer AA, Fielding R, & Cooper C (2015). Osteoporosis and sarcopenia in older age. Bone, 80, 126–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison PT, Bribiescas RG, Bentley GR, Campbell BC, Lipson SF, Panter-Brick C, & Hill K (2002). Population variation in age-related decline in male salivary testosterone. Human Reproduction, 17, 3251–3253. [DOI] [PubMed] [Google Scholar]
- Fernández CI, & Wiley AS (2017). Rethinking the starch digestion hypothesis for AMY1 copy number variation in humans. American Journal of Physical Anthropology, 163(4), 645–657. [DOI] [PubMed] [Google Scholar]
- Fiuza-Luces C, Santos-Lozano A, Joyner M, Carrera-Bastos P, Picazo O, Zugaza JL, … Lucia A (2018). Exercise benefits in cardiovascular disease: beyond attenuation of traditional risk factors. Nature Reviews Cardiology, 15(12), 731–743. [DOI] [PubMed] [Google Scholar]
- Forbes D, Forbes SC, Blake CM, Thiessen EJ, & Forbes S (2015). Exercise programs for people with dementia. Cochrane Database of Systematic Reviews(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli M, Camus SM, Diekmann Y, Burke A, Camus MD, Norman PJ, … Rasteiro R (2019). Genetic diversity of CHC22 clathrin impacts its function in glucose metabolism. eLife, 8, e41517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli M, Sironi M, Pozzoli U, Ferrer-Admettla A, Pattini L, & Nielsen R (2011). Signatures of environmental genetic adaptation pinpoint pathogens as the main selective pressure through human evolution. PLoS Genet, 7(11), e1002355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage TB, & DeWitte S (2009). What do we know about the agricultural demographic transition? Current Anthropology, 50(5), 649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman P, Beedle A, & Hanson M (2009). Principles of Evolutionary Medicine. Oxford: Oxford University Press. [Google Scholar]
- Gould SJ (2000). The spice of life. Leader to Leader, 15, 14–19. [Google Scholar]
- Gregg EW, & Shaw JE (2017). Global health effects of overweight and obesity. Mass Medical Soc. [DOI] [PubMed]
- Gurven M, Blackwell AD, Rodríguez DE, Stieglitz J, & Kaplan H (2012). Does Blood Pressure Inevitably Rise With Age? Longitudinal Evidence Among Forager-Horticulturalists. Hypertension, 60(1), 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurven M, Jaeggi AV, Kaplan H, & Cummings D (2013). Physical Activity and Modernization among Bolivian Amerindians. PLoS ONE, 8(1), e55679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurven MD, & Davison RJ (2019). Periodic catastrophes over human evolutionary history are necessary to explain the forager population paradox. Proceedings of the National Academy of Sciences, 201902406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurven MD, & Gomes CM (2017). Mortality, senescence, and life span In Muller MN, Wrangham RW & Pilbeam D (Eds.), Chimpanzees and Human Evolution (pp. 181–216). Cambridge, MA: Harvard University Press [Google Scholar]
- Gurven MD, Trumble BC, Stieglitz J, Blackwell AD, Michalik DE, Finch CE, & Kaplan HS (2016). Cardiovascular disease and type 2 diabetes in evolutionary perspective: A critical role for helminths? Evolution, medicine, and public health, 2016(1), 338–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer M, & Chida Y (2009). Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychological medicine, 39(1), 3–11. [DOI] [PubMed] [Google Scholar]
- Hamilton WD (1966). The molding of senescence by natural selection. Journal of Theoretical Biology, 12, 12–45. [DOI] [PubMed] [Google Scholar]
- Hawks J, Wang ET, Cochran GM, Harpending HC, & Moyzis RK (2007). Recent acceleration of human adaptive evolution. Proceedings of the National Academy of Sciences, USA, 104(52), 20753–20758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headey DD, & Alderman HH (2019). The Relative Caloric Prices of Healthy and Unhealthy Foods Differ Systematically across Income Levels and Continents. The Journal of nutrition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmby H (2015). Human helminth therapy to treat inflammatory disorders-where do we stand? BMC immunology, 16(1), 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrie HC, Murrell J, Baiyewu O, Lane KA, Purnell C, Ogunniyi A, … Saykin AJ (2014). APOE ε4 and the risk for Alzheimer disease and cognitive decline in African Americans and Yoruba. International psychogeriatrics, 26(6), 977–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich J (2017). The secret of our success: How culture is driving human evolution, domesticating our species, and making us smarter: Princeton University Press. [Google Scholar]
- Hruschka DJ, Medin DL, Rogoff B, & Henrich J (2018). Pressing questions in the study of psychological and behavioral diversity. Proceedings of the National Academy of Sciences, 201814733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado AM, Anderson Frey M, Hurtado I, Hill K, & Baker J (2008). The role of helminthes in human evolution: implications for global health in the 21st century In Elton S & O’Higgins P (Eds.), Medicine and evolution: Current applications, future prospects (pp. 151–178). New York: Taylor and Francis [Google Scholar]
- Jones JH (2009). The force of selection on the human life cycle. Evolution and Human Behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key TJ, Verkasalo PK, & Banks E (2001). Epidemiology of breast cancer. The lancet oncology, 2(3), 133–140. [DOI] [PubMed] [Google Scholar]
- Khullar D (2019). AI Could Worsen Health Disparities. New York Times. [Google Scholar]
- Kumar DKV, Choi SH, Washicosky KJ, Eimer WA, Tucker S, Ghofrani J, … Moir RD (2016). Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Science Translational Medicine, 8(340), 340ra372–340ra372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski DP (2005). How malaria has affected the human genome and what human genetics can teach us about malaria. The American Journal of Human Genetics, 77(2), 171–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachance J, Berens AJ, Hansen ME, Teng AK, Tishkoff SA, & Rebbeck TR (2018). Genetic hitchhiking and population bottlenecks contribute to prostate cancer disparities in men of African descent. Cancer research, 78(9), 2432–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassalle F, Spagnoletti M, Fumagalli M, Shaw L, Dyble M, Walker C, … Balloux F (2018). Oral microbiomes from hunter-gatherers and traditional farmers reveal shifts in commensal balance and pathogen load linked to diet. Molecular ecology, 27(1), 182–195. [DOI] [PubMed] [Google Scholar]
- Lewis MJ, & Jawad AS (2016). The effect of ethnicity and genetic ancestry on the epidemiology, clinical features and outcome of systemic lupus erythematosus. Rheumatology, 56(suppl_1), i67–i77. [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM, & Maseri A (2002). Inflammation and atherosclerosis. Circulation, 105(9), 1135–1143. [DOI] [PubMed] [Google Scholar]
- Lieberman D (2013). The story of the human body: evolution, health, and disease. New York: Pantheon. [PubMed] [Google Scholar]
- Liu X, Zhang D, Liu Y, Sun X, Han C, Wang B, … Shi Y (2017). Dose-response association between physical activity and incident hypertension: a systematic review and meta-analysis of cohort studies. Hypertension, 69(5), 813–820. [DOI] [PubMed] [Google Scholar]
- Manrai AK, Funke BH, Rehm HL, Olesen MS, Maron BA, Szolovits P, … Kohane IS (2016). Genetic misdiagnoses and the potential for health disparities. New England Journal of Medicine, 375(7), 655–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown T (1976). The Modern Rise of Population. New York: Academic Press. [Google Scholar]
- Medin D, Ojalehto B, Marin A, & Bang M (2017). Systems of (non-) diversity. Nature Human Behaviour, 1(5), 0088. [Google Scholar]
- Meyer U (1999). Medically relevant genetic variation of drug effects Evolution in health and disease. Oxford Univ. Press, New York, 41–61. [Google Scholar]
- Milot E, Mayer FM, Nussey DH, Boisvert M, Pelletier F, & Réale D (2011). Evidence for evolution in response to natural selection in a contemporary human population. Proceedings of the National Academy of Sciences, 108(41), 17040–17045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen HM, Froment A, Lema G, Bodo J-M, Ibrahim M, Nyambo TB, … Tishkoff SA (2011). Characterization of genetic variation and natural selection at the arylamine N-acetyltransferase genes in global human populations. Pharmacogenomics, 12(11), 1545–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Science, E. a. M. (2018). Leveraging rarely-investigated populations for research on behavioral and social processes in an aging context Expert Meeting. In C. o. Population; (Ed.). [Google Scholar]
- Natri H, Garcia AR, Buetow KH, Trumble BC, & Wilson MA (2019). The Pregnancy Pickle: Evolved Immune Compensation Due to Pregnancy Underlies Sex Differences in Human Diseases. Trends in Genetics, 35(7), 478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesse RM, & Stearns SC (2008). The great opportunity: evolutionary applications to medicine and public health. Evolutionary Applications, 1(1), 28–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesse RM, & Williams GC (1994). Why we get sick: The new science of Darwinian medicine. Times Books, New York. [Google Scholar]
- Ngo ST, Steyn FJ, & McCombe PA (2014). Gender differences in autoimmune disease. Frontiers in neuroendocrinology, 35(3), 347–369. [DOI] [PubMed] [Google Scholar]
- Nugent A, Conatser KR, Turner LL, Nugent JT, Sarino EMB, & Ricks-Santi LJ (2019). Reporting of race in genome and exome sequencing studies of cancer: a scoping review of the literature. Genetics in Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez-De La Mora A, Chatterton RT, Choudhury OA, Napolitano DA, & Bentley GR (2007). Childhood conditions influence adult progesterone levels. PLoS Medicine, 4(5), e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeppen J, & Vaupel JW (2003). Broken limits to life expectancy. Science, 296, 1029–1031. [DOI] [PubMed] [Google Scholar]
- Omran AR (1971). The epidemiologic transition: A theory of the epidemiology of population change. Milbank Memorial Fund Quarterly, 49(4), 509–538. [PubMed] [Google Scholar]
- Oria R, Patrick P, Oriá M, Lorntz B, Thompson M, Azevedo O, … Lima A (2010). ApoE polymorphisms and diarrheal outcomes in Brazilian shanty town children. Brazilian Journal of Medical and Biological Research, 43(3), 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio C, Kanukuntla T, Diaz E, Jafri N, Cummings M, & Sfera A (2019). The Post-amyloid Era in Alzheimer’s Disease: Trust Your Gut Feeling. Frontiers in Aging Neuroscience, 11(143). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillard T, Rolland Y, & de Souto Barreto P (2015). Protective Effects of Physical Exercise in Alzheimer’s Disease and Parkinson’s Disease: A Narrative Review. J Clin Neurol, 11(3), 212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V, & Sumathipala A (2001). International representation in psychiatric literature: survey of six leading journals. The British Journal of Psychiatry, 178(5), 406–409. [DOI] [PubMed] [Google Scholar]
- Pennington R, Gatenbee C, Kennedy B, Harpending H, & Cochran G (2009). Group differences in proneness to inflammation. Infection, Genetics and Evolution, 9(6), 1371–1380. [DOI] [PubMed] [Google Scholar]
- Perry GH, Dominy NJ, Claw KG, Lee AS, Fiegler H, Redon R, … Misra R (2007). Diet and the evolution of human amylase gene copy number variation. Nature Genetics, 39(10), 1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard TM (2008). Western diseases: an evolutionary perspective: Cambridge University Press. [Google Scholar]
- Popejoy AB, & Fullerton SM (2016). Genomics is failing on diversity. Nature News, 538(7624), 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichlen DA, Pontzer H, Harris JA, Mabulla AZ, Marlowe FW, Josh Snodgrass J, … Wood BM (2017). Physical activity patterns and biomarkers of cardiovascular disease risk in hunter-gatherers. American Journal of Human Biology, 29(2). [DOI] [PubMed] [Google Scholar]
- Rebbeck TR (2020). Cancer in sub-Saharan Africa. Science, 367(6473), 27–28. [DOI] [PubMed] [Google Scholar]
- Ricciarelli R, & Fedele E (2017). The Amyloid Cascade Hypothesis in Alzheimer’s Disease: It’s Time to Change Our Mind. Current neuropharmacology, 15(6), 926–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rindos D (2013). The origins of agriculture: an evolutionary perspective: Academic Press. [Google Scholar]
- Roberts AL, Kubzansky LD, Malspeis S, Feldman CH, & Costenbader KH (2018). Association of depression with risk of incident systemic lupus erythematosus in women assessed across 2 decades. JAMA psychiatry, 75(12), 1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shave RE, Lieberman DE, Drane AL, Brown MG, Batterham AM, Worthington S, … Weiner RB (2019). Selection of endurance capabilities and the trade-off between pressure and volume in the evolution of the human heart. Proceedings of the National Academy of Sciences, 116(40), 19905–19910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirugo G, Williams SM, & Tishkoff SA (2019). The missing diversity in human genetic studies. Cell, 177(1), 26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns SC, & Medzhiyov R (2016). Evolutionary Medicine. Sunderland, MA: Sinauer and Associates. [Google Scholar]
- Trevathan W, Smith EO, & McKenna JJ (2008). Evolutionary medicine and health: new perspectives: Oxford University Press. [Google Scholar]
- Trumble BC, & Finch CE (2019). The Exposome in Human Evolution: From Dust to Diesel. The Quarterly Review of Biology, 94(4), 333–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumble BC, Stieglitz J, Blackwell AD, Allayee H, Beheim B, Finch CE, … Kaplan H (2017). Apolipoprotein E4 is associated with improved cognitive function in Amazonian forager-horticulturalists with a high parasite burden. The FASEB Journal, 31(4), 1508–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truswell AS, Kennelry BM, Hansen JDL, & Lee RB (1972). Blood pressure of !Kung bushmen in Northern Botswana. American Heart Journal, 84, 5–12. [DOI] [PubMed] [Google Scholar]
- UNDP. (2019). Human Development Report United Nations Development Report. New York: UN Development Programme. [Google Scholar]
- Urquhart DM, Soufan C, Teichtahl AJ, Wluka AE, Hanna F, & Cicuttini FM (2008). Factors that may mediate the relationship between physical activity and the risk for developing knee osteoarthritis. Arthritis research & therapy, 10(1), 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatanen T, Kostic AD, d’Hennezel E, Siljander H, Franzosa EA, Yassour M, … Hämäläinen A-M (2016). Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell, 165(4), 842–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasquez-Manoff M (2012). An epidemic of absence: a new way of understanding allergies and autoimmune diseases: Simon and Schuster. [Google Scholar]
- Wallace IJ, Felson DT, Worthington S, Duryea J, Clancy M, Aliabadi P, … Lieberman DE (2019). Knee osteoarthritis risk in non-industrial societies undergoing an energy balance transition: evidence from the indigenous Tarahumara of Mexico. Annals of the rheumatic diseases, annrheumdis-2019–215886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace IJ, Worthington S, Felson DT, Jurmain RD, Wren KT, Maijanen H, … Lieberman DE (2017). Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proceedings of the National Academy of Sciences, 114(35), 9332–9336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, & Holsinger RMD (2018). Exercise-induced brain-derived neurotrophic factor expression: Therapeutic implications for Alzheimer’s dementia. Ageing research reviews, 48, 109–121. [DOI] [PubMed] [Google Scholar]
- WHO. (2018). World Malaria Report 2018. Geneva: WHO. [Google Scholar]
- Wiria AE, Sartono E, Supali T, & Yazdanbakhsh M (2014). Helminth infections, type-2 immune response, and metabolic syndrome. PLoS Pathogens, 10(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witherspoon DJ, Wooding S, Rogers AR, Marchani EE, Watkins WS, Batzer MA, & Jorde LB (2007). Genetic similarities within and between human populations. Genetics, 176(1), 351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik GL, Graff M, Nishimura KK, Tao R, Haessler J, Gignoux CR, … Avery CL (2019). Genetic analyses of diverse populations improves discovery for complex traits. Nature, 570(7762), 514–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S, Hong C-C, Ruiz-Narváez EA, Evans SS, Zhu Q, Schaefer BA, … Sucheston-Campbell LE (2018). Genetic ancestry and population differences in levels of inflammatory cytokines in women: role for evolutionary selection and environmental factors. PLoS Genetics, 14(6), e1007368. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


