Abstract
BACKGROUND:
In deep brain stimulation (DBS) for essential tremor, the primary target ventrointermedius (VIM) nucleus cannot be clearly visualized with structural imaging. As such, there has been much interest in the dentatorubrothalamic tract (DRTT) for target localization, but evidence for the DRTT as a putative stimulation target in tremor suppression is lacking. We evaluated proximity of the DRTT in relation to DBS stimulation parameters.
METHODS:
This is a retrospective analysis of 26 consecutive patients who underwent DBS with microelectrode recordings (46 leads). Fiber tracking was performed with a published deterministic technique. Clinically optimized stimulation parameters were obtained in all patients at the time of most recent follow-up (6.2 months). Volume of tissue activated (VTA) around contacts was calculated from a published model.
RESULTS:
Tremor severity was reduced in all treated hemispheres, with 70% improvement in the treated hand score of the Clinical Rating Scale for Tremor. At the level of the active contact (2.9 ± 2.0 mm superior to the commissural plane), the center of the DRTT was lateral to the contacts (5.1 ± 2.1 mm). The nearest fibers of the DRTT were 2.4 ± 1.7 mm from the contacts, whereas the radius of the VTA was 2.9 ± 0.7 mm. The VTA overlapped with the DRTT in 77% of active contacts. The distance from active contact to the DRTT was positively correlated with stimulation voltage requirements (Kendall s [τ = 0.33, P = 0.006), whereas distance to the atlas-based VIM coordinates was not.
CONCLUSIONS:
Active contacts in proximity to the DRTT had lower voltage requirements. Data from a large cohort provide support for the DRTT as an effective stimulation target for tremor control.
Keywords: Deep brain stimulation, Dentatorubrothalamic tract, Deterministic tractography, Diffusion tensor imaging, Essential tremor, Neurosurgical targeting, Tractography-based targeting
INTRODUCTION
Thalamic deep brain stimulation (DBS) is an effective therapeutic modality for medication-refractory essential tremor (ET).1–3 Because the traditionally targeted ventrointermedius nucleus (VIM) is not clearly delineated with structural magnetic resonance imaging (MRI), even at field strengths higher than 3 T, surgical targeting is primarily guided by intraoperative neurophysiology.4 Neurophysiology is not available, however, for techniques such as asleep DBS and noninvasive ablative techniques (e.g., focused ultrasound).5,6 Consequently, there has been much interest in the dentatorubrothalamic tract (DRTT) as a putative target because it can be readily reconstructed from diffusion magnetic resonance data.6,7
The VIM is one node within the pathologic tremor network, serving as the thalamic relay for information from the cerebellum to the cortex. Other more proximal nodes within the tremor network that have been targeted include the posterior subthalamic area, prelemniscal radiations, and caudal zona incerta.8–10 The DRTT originates in the contralateral dentate nucleus and de-cussates in the tegmentum toward the region of the ipsilateral red nucleus, where it intercepts these more proximal targets, before reaching the thalamus in the vicinity of the VIM.11,12 More recently, nondecussating fibers of the DRTT projecting to the ipsilateral red nucleus and thalamus have been described, which may serve as the neural substrate for the known involvement of each cerebellar hemisphere in bilateral limb movements.13
The DRTT has been proposed as the underlying neural substrate for stimulation-induced tremor reduction.7,14–18 At our center, tractography of the DRTT is performed during the presurgical workup of patients with refractory ET, using a deterministic tracking method implemented on widely used surgical planning software. In this study, we present data from a large cohort of patients that support the notion of the DRTT as an effective stimulation target for tremor alleviation.
METHODS
Patient Cohort and DBS Surgery
This is a retrospective review of 26 consecutive patients (14 women) with ET who underwent thalamic DBS. Before surgery, all patients had undergone a trial of pharmacotherapy, most commonly with primidone (n = 17), propranolol (n = 11), and topiramate (n = 7), inadequate with symptom control and/or unacceptable side effects.
All procedures were performed by a single surgeon (G. H. B.) at a single institution (August 2018 to January 2019). Patients underwent stereotactic placement of DBS leads with a Leksell frame (Elekta, Stockholm, Sweden). The VIM was targeted indirectly as follows: 14 mm lateral to the midline, 25% of the anterior commissure (AC) to posterior commissure (PC) distance from the PC, and in the commissural plane. The final implant position was determined with microelectrode recordings (MERs), specifically targeting regions containing neurons with hand and/or distal upper extremity kinesthetic receptive fields. Macrostimulation was subsequently performed to confirm tremor reduction and to evaluate for side effects, in particular orofacial paresthesias.19,20 Although tractography was performed prospectively, the data were not used to guide lead placement. All patients were implanted with Medtronic DBS lead Model 3389 (Dublin, Ireland): 4 electrodes (height 1.5 mm) with an interelectrode spacing of 0.5 mm.21
The study protocol was approved by our local institutional review board. Patient consent was waived because the only risk was a breach of confidentiality.
Imaging Protocol
Prior to the procedure, MRI was obtained with a 3-T scanner (19-channel; Discovery MR750w [GE Healthcare, Waukesha Wisconsin, USA]). Sequences obtained for preoperative planning included the following: axial 3-dimensional T1 spoiled gradient recalled acquisition, axial 2-dimensional T2 (2-mm slice thickness), sagittal/coronal 3-dimensional T2 fast spin echo, axial susceptibility-weighted angiography, and diffusion tensor imaging (33 gradient directions, one B0 scan, field of view 26 cm, 128 × 128 acquisition matrix, slice thickness 3 mm, b value = 1000–2000s/mm2).
On the day of surgery, MRI was obtained at 1.5 T (12-channel; Optima 450w [GE Healthcare]) after placement of the Leksell frame. T1 images were obtained in axial/sagittal planes after administration of contrast. Immediate postoperative imaging during the same admission consisted of axial/sagittal T1, axial (3-mm slice thickness) and coronal T2, and axial diffusion-weighted imaging, also at 1.5 T, in addition to a computed tomography (CT) scan (1-mm thickness).
Fiber Tracking Technique
We implemented a published deterministic tracking method proposed by Sammartino et al.,22 which has been widely evaluated at multiple centers.6,23–25 Fibers reconstructed with this technique have been validated with intraoperative neurophysiology.22,24 In brief, the DRTT was identified using the corticospinal tract (CST) and medial lemniscus as landmarks to determine a seed region of interest (ROI) at the level of the thalamus. The generation of the medial lemniscus required placement of a seed ROI in the dorsal brainstem and a target ROI in the ipsilateral primary sensory cortex, whereas for the CST a seed ROI was placed in the ipsilateral cerebral peduncle and a target ROI in the ipsilateral primary motor cortex. The DRTT was then delineated using a circular seed ROI (3-mm radius) within the angle of the CST and medial lemniscus at the level of the AC-PC line.
Fiber tracking was performed in all patients during their preoperative evaluation under the supervision of a neuroradiologist (R. L. W.) using DynaSuite Neuro (InVivo Corp., Pewaukee, Wisconsin, USA). Motion artifact was corrected for prior to tracking. Termination criteria were a minimum fractional anisotropy of 0.15 and maximum curvature of 30°. All reconstructed tracts were then overlaid on the coregistered preoperative T2-weighted images. Post hoc imaging analysis was conducted with iPlan NET 3.0 (Brainlab AG, Munich, Germany).
Clinical Outcomes
DBS stimulation settings at the time of the most recent follow-up were acquired in all patients, and were used as the primary clinical end point to correlate with imaging data. DBS programming was conducted empirically based on clinical response by neurologists specializing in movement disorders. In a subset of patients (n = 16, 29 leads), tremor severity evaluated with the Clinical Rating Scale for Tremor (CRST) was available from both before and after DBS surgery. The treated hand score, derived from parts A (items 5 and 6) and B (items 11e15) of the CRST, was calculated for all treated hands.26 Both for CRST and DBS programming, the involved clinicians were unaware of the proximity of lead contacts to DRTT.
Analysis of Proximity of DBS Contacts to the DRTT
To measure coordinates of the DRTT and DBS leads, the preimplant T2-weighted images overlaid with the DRTT were coregistered with the postimplant T2 images and postimplant CT scan after all sequences were realigned in the AC-PC plane.
The coordinates of the radial centers of DBS leads and active contacts were determined from the centers of the artifacts induced on the postimplant images. In cases of interleaved stimulation, the active contact was determined at the center of the 2 electrodes in the craniocaudal axis. In cases of bipolar stimulation, the cathode electrode was designated as the active contact. The coordinates of the center of the DRTT were also measured at the level of the AC-PC line and in the plane of the active contact. To account for the irregularity of the DRTT, its center was defined as its geometric center in each axial plane. The 2-dimensional Euclidean distance between the centers of the DRTT and lead were also calculated.
The volume of tissue activated (VTA) was calculated based on data from a published finite element model of monopolar stimulation.27,28 Because stimulation with a bipolar configuration elicits a distinct activation pattern, VTA analysis was constrained to only the leads in which monopolar or interleaved monopolar stimulation was used.29 In brief, the radius of the VTA is a function of stimulation voltage and tissue impedance. The overlap between the VTA and the nearest fibers of the DRTT was determined across both the axial and coronal planes.
Differences in coordinates were tested with the Wilcoxon signed-rank test. A P value of 0.05 was considered statistically significant. All analysis was conducted with MATLAB (MathWorks, Natick, Massachusetts, USA). Values are reported as mean ± SD (range).
RESULTS
A total of 26 patients with ET who underwent thalamic DBS (46 leads) were included in this study. Patient age at surgery was 66 ± 11 years (range, 39–83 years). There were no intra- or peri-operative complications. Twenty patients had bilateral implants, and the remaining 6 patients had unilateral implants (2 right). DBS stimulation parameters for all patients from the most recent follow-up (6.2 ± 2.3 months) are shown in Table 1. Stimulation modes were primarily monopolar (n = 31), with 11 leads programmed in bipolar mode and 4 leads in interleaved mode. Stimulation-induced adverse effects included gait disturbance (n = 2), dysarthria (n = 2), paresthesias (n = 2), and dysgeusia (n = 1) in 4 patients. None of the adverse effects were severe enough to require repositioning of leads.
Table 1.
Thalamic Deep Brain Stimulation for Essential Tremor: Stimulation Parameters
| Pulse Width (ms) | Frequency (Hz) | Voltage (V) |
|---|---|---|
| 80 ± 34 (60–180) | 142 ± 21 (100–185) | 2.5 ± 1.0 (0.5–6.5) |
Values are mean ± SD (range).
The preoperative clinical severity of tremor as measured with the treated hand score derived from the CRST was 12.9 out of a maximum score of 32 in the dominant hand (n = 16) and 11.5 out of 28 in the nondominant hand (n = 13) (Table 2). After implant (3.4 ± 2.9 months), treated hand scores improved to 3.0 for both dominant- and nondominant-treated hands, representing a 70% improvement (paired 2-sample t test, P < 10−8). None of the treated hands had worse tremor postoperatively. In the remaining 10 patients (17 leads) in whom pre- and postoperative CRST data were not available, none of the patients were noted to be non-responders on clinical examination.
Table 2.
Pre- and Postimplant Clinical Rating Scale for Tremor: Treated Hand Score
| Treated Hand Score | Preoperative | Postoperative | Absolute Change* | Relative Change (%)† |
|---|---|---|---|---|
| Dominant hand‡ | 12.9 ± 6.8 (3–26) | 3.0 ± 3.0 (0–9) | −10.0 ± 5.9 | −72 ± 26 |
| Nondominant hand§ | 11.5 ± 5.9 (3–22) | 3.0 ± 3.4 (0–13) | −8.0 ± 5.0 | −71 ± 22 |
Values are mean ± SD or mean ± SD (range).
Pre-post.
(Pre − post)/pre × 100.
Maximum score 32.
Maximum score 28.
In all hemispheres, the DRTT was successfully reconstructed, demonstrating ipsilateral or bilateral cerebellar input fibers traversing the superior cerebellar peduncle, and ipsilateral motor cortical output fibers. The decussating fibers of the DRTT were visualized in 18 hemispheres (39%). At the level of the intercommissural line, leads were located 12.1 ± 1.3 mm lateral to the midline and 5.4 ± 1.1 mm anterior to the PC (Table 3). The DRTT was located 14.2 ± 2.1 mm lateral to the midline and 6.9 ± 2.0 mm anterior to the PC. The DRTT was hence lateral to the lead, with a distance of 2.1 ± 1.9 mm (P < 10−5) (Figure 1), and anterior to the lead by 1.5 ± 2.1 mm (P < 10−7). The 2-dimensional Euclidean distance from the DRTT to the lead was 3.4 ± 1.8 mm.
Table 3.
Deep Brain Stimulation Lead and Dentatorubrothalamic Tract Coordinates
| Distance Anterior from PC (mm) | Distance Anterior from PC (% of AC-PC Length*) | Distance Posterior from MCP (mm) | |
|---|---|---|---|
| DBS lead | |||
| At the level of AC-PC line | 5.4 ± 1.1 | 21.3 ± 4.2 | 7.3 ± 1.2 |
| At the level of active contact | 9.3 ± 3.0 | 35.5 ± 13.9 | 3.7 ± 3.5 |
| Lateral Distance from AC-PC Line (mm) | Lateral Distance from Wall of Third Ventricle† (mm) | ||
| DBS lead | |||
| At the level of AC-PC line | 12.1 ± 1.3 | 9.0 ± 1.4 | |
| At the level of active contact | 13.3 ± 2.1 | ||
| Distance Anterior from PC (mm) | Distance Anterior from PC (% of AC-PC Length) | Distance Posterior from MCP (mm) | |
| DRTT | |||
| At the level of AC-PC line | 6.9 ± 2.0 | 27.1 ± 7.8 | 5.8 ± 2.0 |
| At the level of active contact | 9.0 ± 3.7 | 36.6 ± 11.3 | 3.4 ± 2.9 |
| Lateral Distance from AC-PC Line (mm) | Lateral Distance from Wall of Third Ventricle (mm) | ||
| DRTT | |||
| At the level of AC-PC line | 14.2 ± 2.1 | 11.1 ± 2.0 | |
| At the level of active contact | 18.4 ± 2.9 | ||
PC, posterior commissure; AC, anterior commissure; MCP, midcommissural point; DBS, deep brain stimulation; DRTT, dentatorubrothalamic tract.
AC-PC length, 25.5 ± 1.9 mm.
Third ventricle width, 6.3 ± 1.9 mm.
Figure 1.
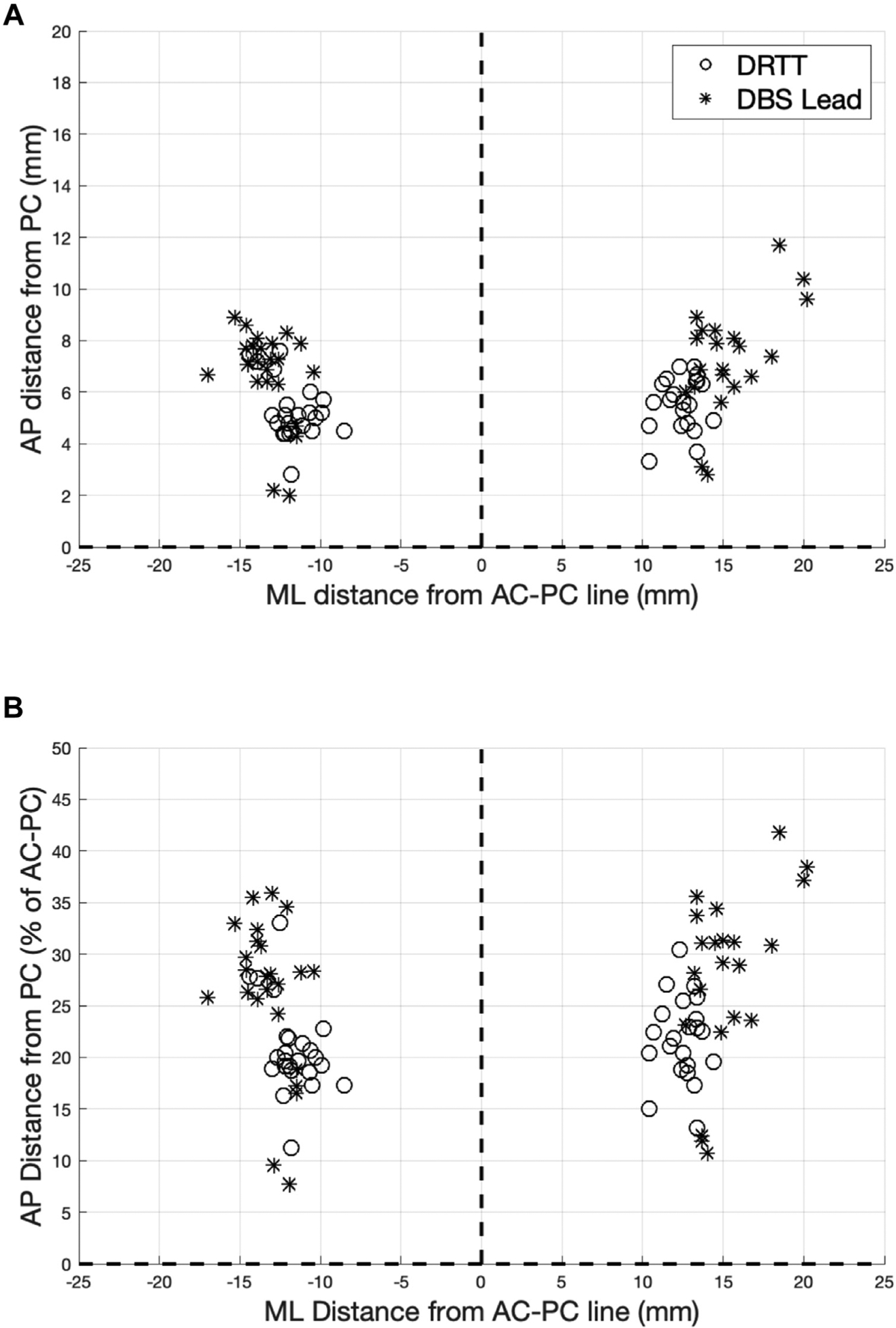
Coordinates of the centers of deep brain stimulation lead and dentatorubrothalamic tract in the commissural plane. Mediolateral coordinates expressed as absolute distance (millimeters) from the intercommissural line. (A) Anteroposterior coordinates expressed as absolute distance (millimeters) from the posterior commissure. (B) Anteroposterior coordinates expressed as percentage of anterior to posterior commissure length. AC-PC, anterior to posterior commissure; AP, anteroposterior; DBS, deep brain stimulation; DRTT, dentatorubrothalamic tract; ML, mediolateral; PC, posterior commissure.
In our cohort, the stereotactic VIM targeting formula from Benabid et al.1 resulted in 14.7 ± 0.9 mm lateral from the midline and 5.3 ± 0.4 mm anterior to the PC, which was lateral to the lead (P < 10−8) and without a difference in the anteroposterior (AP) dimension (P = 0.8). Euclidean distance from the atlas-based VIM coordinates to the lead was 2.8 ± 1.2 mm. The VIM coordinates were posterior to the DRTT (P < 10−4), and without a difference in the mediolateral dimension (P = 0.2).
The active contact was located superior to the commissural plane (2.9 ± 2.0 mm). At the level of the active contact, the DRTT was lateral to the contact (P < 10−8) by 5.1 ± 2.1 mm (Figure 2). The DRTT was more widely dispersed in the AP dimension across patients, without an overall difference from the active contact (−0.2 ± 2.7 mm; P = 0.5). The Euclidean distance from the radial center of the active contact to the geometric center of the DRTT was 5.8 ± 1.8 mm (Figure 3A). The distance from the center of the active contact to the nearest fibers of the DRTT was 2.4 ± 1.7 mm.
Figure 2.

Coordinates of the centers of active contact and dentatorubrothalamic tract at the level of the active contact. AP, anteroposterior; DRT, dentatorubrothalamic tract; ML, mediolateral.
Figure 3.
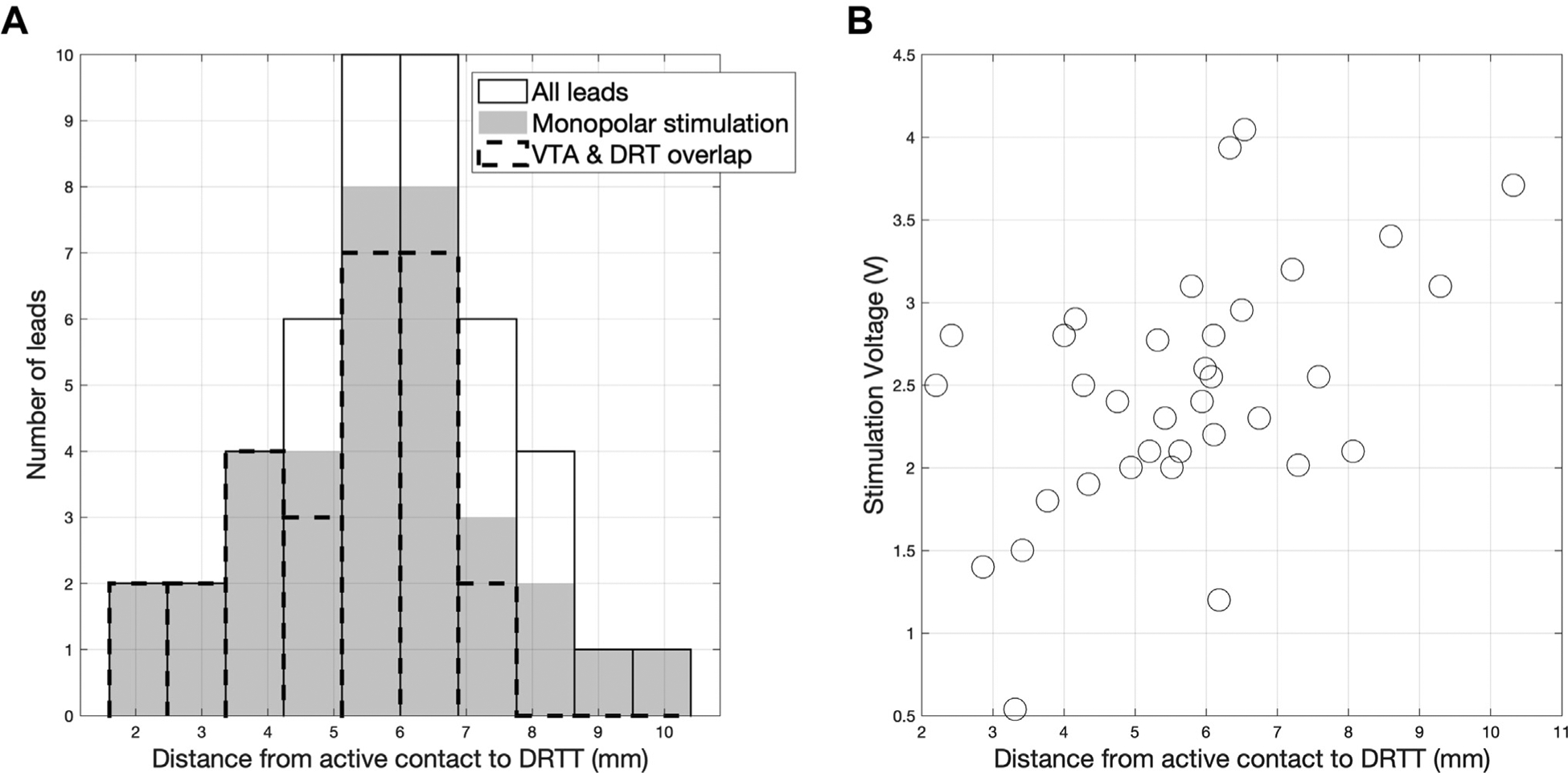
(A) Histogram of distance between the centers of active contact and dentatorubrothalamic tract (DRTT) at the level of the active contact. Among the leads programmed with monopolar stimulation settings (solid), the volume of tissue activated around active contacts overlapped with the fibers of the DRTT in 77% of contacts (dotted outline). (B) Scatterplot of active contact to DRTT distance and stimulation voltage (Kendall τ = 0.33, P = 0.006). DRT, dentatorubrothalamic; VTA, volume of tissue activated.
Among the leads programmed with monopolar or interleaved monopolar settings (35 leads in 18 patients), there was a positive correlation between active contact to DRTT distance and stimulation voltage (Kendall τ = 0.33, P = 0.006) (Figure 3B). In contrast, our data did not show a statistically significant correlation between the distance from active contact to atlas-based VIM coordinates and stimulation voltage (τ = 0.24, P = 0.2). Although bipolar stimulation elicits a distinct field distribution from monopolar, inclusion of all implanted leads gave congruent results: proximity to the DRTT was correlated with stimulation voltage (τ = 0.33, P = 0.001), whereas proximity atlas-based VIM was not to (τ = 0.18, P = 0.08). The radius of the VTA estimated with a finite element model of monopolar stimulation was 2.9 ± 0.7 mm. Hence, among the 35 leads with monopolar or interleaved monopolar modes, VTA overlapped with the nearest fibers of the DRTT in 77% of active contacts.
DISCUSSION
Given the nascency of tractography as an adjunct for surgical targeting, thalamic DBS leads for ET continue to be implanted at our center with the conventional MER-guided approach. Furthermore, stimulation parameters are optimized using standard programming strategies. In this retrospective analysis of 26 consecutive patients who underwent DBS (46 leads), we found that the VTA of most active contacts overlapped with the DRTT, and that stimulation voltage requirements were lower in contacts in spatial proximity to the DRTT. These results are consistent with the hypothesis that the DRTT may be the underlying neural substrate for stimulation-induced tremor reduction.
In the commissural plane, our DBS lead coordinates were highly concordant with the optimal coordinates determined in other modern series.2,3 The DRTT was anterolateral to the lead (1.5 and 2.1 mm), which is consistent with the findings of Sammartino et al.,22 who placed the DRTT 1.8 mm anterior and 1 mm lateral to their leads. When the location of the DRTT was compared with atlas-based VIM coordinates, there was a significant difference only in the AP dimension, with the DRTT located anteriorly by 1.6 mm. Similarly, Anthofer et al.30 reported that the DRTT was anterior by 2.8 mm, and Krishna et al.6 found the DRTT to be 0.9 mm anterior.
Evidence to support the DRTT as a potential stimulation target has been scant. In a mixed cohort of 9 patients with movement disorder (3 patients with ET), Avecillas-Chasin et al.18 reported that active contacts were closer to the DRTT as it intersects with the thalamus than to the atlas-based VIM. Several studies have reported on the overlap between the VTA of active contacts and the DRTT. In a prospective study in which the DRTT was directly targeted in 20 patients, Fenoy and Schiess7 found overlap between the DRTT and VTA in all leads. Fiechter et al.17 projected VTAs on a stereotactic atlas, and demonstrated overlap with the DRTT in all 7 patients with ET. Notably, statistical testing was not conducted in this analysis type. In our series, the VTA of 77% of active contacts overlapped with the DRTT. Of note, because the model for VTA estimation was originally derived for monopolar stimulation, we limited the VTA analysis to hemispheres receiving monopolar or interleaved monopolar stimulation.27,28
Our data also demonstrated a statistically significant correlation between stimulation voltage and active contact to DRTT distance. Several prior studies have been unable to find correlational evidence for DRTT stimulation with similar proximity analyses. Anthofer et al.31 compared the distance from active contact to the DRTT, and found no difference between hemispheres demonstrating clinical habituation to stimulation versus those that had durable tremor reduction. Studies by Nowacki et al.32 and Coenen et al.16 likewise did not find any correlation between active contact to DRTT distance and clinical outcomes as measured with the CRST. Schlaier et al.33 evaluated the degree of tremor reduction with intraoperative microelectrode stimulation and found no correlation with the distance between stimulation site and the DRTT.
There are several reasons for these negative results, including a small sample size ranging between 5 and 11 patients in the aforementioned studies. Another reason of technical significance is the impact of which brain regions are chosen as ROIs for tractography. Nowacki et al.32 presented 4 different tractography results, each based on 2e3 ROIs, and found significant differences in the coordinates of the reconstructed DRTT at various axial planes. Indeed, tractography was performed in the aforementioned studies with variable ROIs. Anthofer et al.31 and Schlaier et al.33 used the contralateral dentate nucleus, superior cerebellar peduncle, and ipsilateral red nucleus. Coenen et al.16 used the entire midbrain at the level of the red nuclei, the ipsilateral dentate nucleus, and primary motor cortex. Avecillas-Chasin et al.18 chose the ipsilateral dentate and red nuclei.
Although the entirety of the DRTT is reconstructed with tractography, where along this tract the optimal stimulation target may be is unknown. Fenoy and Schiess,7 for instance, directly targeted the DRTT at 0e1 mm inferior to the commissural plane. In contrast, the active contact in our cohort was 2.9 mm superior to the plane of the AC-PC line. Other studies have reported clinical response with intraoperative stimulation at multiple locations along the DRTT.22,33 Just as there have been several conventional targets in DBS for ET, it is possible that more than one anatomic region along the DRTT may be effectively targeted.
Probabilistic algorithms are more sensitive in detecting the DRTT, and offer superior identification of its decussating fibers. Sammartino et al.22 reported that the DRTT was trackable in 72% of hemispheres (n = 36) with deterministic tracking versus in 100% of hemispheres (n = 12) with a probabilistic algorithm. Decussating fibers were present in 62% versus 100% of reconstructed tracts, respectively. Similarly, Schlaier et al.34 reported successful tracking of the DRTT in all hemispheres with probabilistic tracking and in only 17% with deterministic (n = 12). In the same study, sensitivity of deterministic tracking was increased to 75% when the number of gradient directions was reduced from 64 to 12, which the authors postulated may be secondary to lower fractional anisotropy values with more gradient directions. In our study, the DRTT was reconstructed in all hemispheres, with decussations visualized in 39% of tracts.
Studies on patients with ET using probabilistic tracking have demonstrated similar correlation evidence for DRTT stimulation.14,15 Although probabilistic methods likely offer more accurate depictions of the DRTT, whether this translates to clinical benefit in the use of tractography-based DBS is yet to be proven. In fact, Schlaier et al.34 compared the coordinates of the DRTT reconstructed with deterministic or probabilistic methods in the commissural plane, and did not find a significant difference. It is also important to note that the ultimate trade-off of enhanced sensitivity is that probabilistic tracking can suffer from excessive false positivies.35 Finally, even for a given seed ROI, probabilistic algorithms produce slightly different trajectories every time a tract is generated, further adding to ambiguity in surgical targeting.
Moreover, practical considerations such as additional software, technical expertise, scan time, and computational load mandate that any improvements to tractography are clinically validated before being widely adopted. In comparative studies of the DRTT, probabilistic tracking required longer processing time (4.5–33 hours) than deterministic techniques (11–18 minutes).22,34 Furthermore, high-quality probabilistic tracking generally requires a larger number of gradient directions and/or higher b-values that can be used for higher-order models of intravoxel fiber orientations, all of which can prolong scan time. Finally, at this time, only deterministic algorithms have been integrated into Food and Drug Administration–approved surgical planning platforms.
Our tractography was based on diffusion magnetic resonance acquisitions feasible in a clinical setting and deterministic fiber tracking implemented on commercial surgical planning software. The limitations of our acquisitions and technique are important to note in interpreting our findings. Currently, ongoing work at our institution will evaluate DRTT reconstructions with more advanced magnetic resonance acquisitions, viz. high angular resolution diffusion imaging based on a larger number of gradient directions at a higher b-value, or a range of higher b-values (multishell high angular resolution diffusion imaging). Diffusion magnetic resonance data at higher angular resolution enable more advanced tractography models that address the limitations of the diffusion tensor imaging model. In addition to probabilistic versus deterministic tracking, and different choices of ROIs, it is para-mount to demonstrate the clinical impact of more sophisticated tractography solutions, which will likely be specific to the tract of interest, the pathology, and the clinical indication for tractography.
CONCLUSIONS
This is a retrospective study of 26 consecutive patients with ET who underwent thalamic DBS (46 leads) using conventional MER-based techniques. The active contact was empirically chosen based on clinical examinations. We found that 77% of active contacts demonstrated overlap between its VTA and DRTT. Moreover, stimulation voltage requirements were significantly correlated with the distance from active contact to the DRTT. There was no such correlation for distance to the atlas-based VIM. Our results suggest that the DRTT may be an effective stimulation target in DBS for ET.
Abbreviations and Acronyms
- AC
Anterior commissure
- AP
Anteroposterior
- CRST
Clinical rating scale for tremor
- CST
Corticospinal tract
- CT
Computed tomography
- DBS
Deep brain stimulation
- DRTT
Dentatorubrothalamic tract
- ET
Essential tremor
- MER
Microelectrode recording
- ML
Mediolateral
- MRI
Magnetic resonance imaging
- PC
Posterior commissure
- ROI
Region of interest
- VIM
Ventrointermedius nucleus
- VTA
Volume of tissue activated
Footnotes
Conflict of interest statement: The authors declare that the article content was composed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
REFERENCES
- 1.Benabid AL, Pollak P, Hoffmann D, et al. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet. 1991;337:403–406. [DOI] [PubMed] [Google Scholar]
- 2.Papavassiliou E, Rau G, Heath S, et al. Thalamic deep brain stimulation for essential tremor: relation of lead location to outcome. Neurosurgery. 2004;54:1120–1129 [discussion: 1129–1130]. [DOI] [PubMed] [Google Scholar]
- 3.Pilitsis JG, Metman LV, Toleikis JR, Hughes LE, Sani SB, Bakay RAE. Factors involved in long-term efficacy of deep brain stimulation of the thalamus for essential tremor. J Neurosurg. 2008;109: 640–646. [DOI] [PubMed] [Google Scholar]
- 4.Forstmann BU, Isaacs BR, Temel Y. Ultra high field MRI-guided deep brain stimulation. Trends Biotechnol. 2017;35:904–907. [DOI] [PubMed] [Google Scholar]
- 5.Chen T, Mirzadeh Z, Chapple K, Lambert M, Dhall R, Ponce FA. “Asleep” deep brain stimulation for essential tremor. J Neurosurg. 2016;124: 1842–1849. [DOI] [PubMed] [Google Scholar]
- 6.Krishna V, Sammartino F, Agrawal P, et al. Prospective tractography-based targeting for improved safety of focused ultrasound thalamotomy. Neurosurgery. 2019;84:160–168. [DOI] [PubMed] [Google Scholar]
- 7.Fenoy AJ, Schiess MC. Deep brain stimulation of the dentato-rubro-thalamic tract: outcomes of direct targeting for tremor. Neuromodulation. 2017; 20:429–436. [DOI] [PubMed] [Google Scholar]
- 8.Plaha P, Javed S, Agombar D, et al. Bilateral caudal zona incerta nucleus stimulation for essential tremor: outcome and quality of life. J Neurol Neurosurg Psychiatry. 2011;82:899–904. [DOI] [PubMed] [Google Scholar]
- 9.Carrillo-Ruiz JD, Velasco F, Jimènez F, et al. Bilateral electrical stimulation of prelemniscal radiations in the treatment of advanced Parkinson’s disease. Neurosurgery. 2008;62:347–357 [discussion: 357–359]. [DOI] [PubMed] [Google Scholar]
- 10.Blomstedt P, Sandvik U, Tisch S. Deep brain stimulation in the posterior subthalamic area in the treatment of essential tremor. Mov Disord. 2010;25:1350–1356. [DOI] [PubMed] [Google Scholar]
- 11.Morel A, Magnin M, Jeanmonod D. Multi-architectonic and stereotactic atlas of the human thalamus. J Comp Neurol. 1997;387:588–630. [DOI] [PubMed] [Google Scholar]
- 12.Gallay MN, Jeanmonod D, Liu J, Morel A. Human pallidothalamic and cerebellothalamic tracts: anatomical basis for functional stereotactic neurosurgery. Brain Struct Funct. 2008;212:443–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meola A, Comert A, Yeh F-C, Sivakanthan S, Fernandez-Miranda JC. The nondecussating pathway of the dentatorubrothalamic tract in humans: human connectome-based tractographic study and microdissection validation. J Neurosurg. 2016;124:1406–1412. [DOI] [PubMed] [Google Scholar]
- 14.Calabrese E, Hickey P, Hulette C, et al. Postmortem diffusion MRI of the human brainstem and thalamus for deep brain stimulator electrode localization. Hum Brain Mapp. 2015;36:3167–3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Groppa S, Herzog J, Falk D, Riedel C, Deuschl G, Volkmann J. Physiological and anatomical decomposition of subthalamic neurostimulation effects in essential tremor. Brain. 2014;137:109–121. [DOI] [PubMed] [Google Scholar]
- 16.Coenen VA, Allert N, Paus S, Kronenbürger M, Urbach H, Mädler B. Modulation of the cerebello-thalamo-cortical network in thalamic deep brain stimulation for tremor: a diffusion tensor imaging study. Neurosurgery. 2014;75:657–669 [discussion: 669–670]. [DOI] [PubMed] [Google Scholar]
- 17.Fiechter M, Nowacki A, Oertel MF, et al. Deep brain stimulation for tremor: is there a common structure? Stereotact Funct Neurosurg. 2017;95: 243–250. [DOI] [PubMed] [Google Scholar]
- 18.Avecillas-Chasin JM, Alonso-Frech F, Parras O, Del Prado N, Barcia JA. Assessment of a method to determine deep brain stimulation targets using deterministic tractography in a navigation system. Neurosurg Rev. 2015;38:739–750 [discussion: 751]. [DOI] [PubMed] [Google Scholar]
- 19.Kramer DR, Halpern CH, Buonacore DL, et al. Best surgical practices: a stepwise approach to the University of Pennsylvania deep brain stimulation protocol. Neurosurg Focus. 2010;29:E3. [DOI] [PubMed] [Google Scholar]
- 20.Gross RE, Krack P, Rodriguez-Oroz MC, Rezai AR, Benabid A-L. Electrophysiological mapping for the implantation of deep brain stimulators for Parkinson’s disease and tremor. Mov Disord. 2006;21(suppl 14):S259–S283. [DOI] [PubMed] [Google Scholar]
- 21.Medtronic. DBS 3387 3389 Lead Kit for Deep Brain Stimulation-Implant Manual. Available at: http://manuals.medtronic.com/content/dam/emanuals/neuro/M927780A_a_035_view.pdf. Accessed January 31, 2020.
- 22.Sammartino F, Krishna V, King NKK, et al. Tractography-based ventral intermediate nucleus targeting: novel methodology and intraoperative validation. Mov Disord. 2016;31:1217–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chazen JL, Sarva H, Stieg PE, et al. Clinical improvement associated with targeted interruption of the cerebellothalamic tract following MR-guided focused ultrasound for essential tremor. J Neurosurg. 2018;129:315–323. [DOI] [PubMed] [Google Scholar]
- 24.King NKK, Krishna V, Basha D, et al. Microelectrode recording findings within the tractography-defined ventral intermediate nucleus. J Neurosurg. 2017;126:1669–1675. [DOI] [PubMed] [Google Scholar]
- 25.Yang AI, Chaibainou H, Wang S, et al. Focused ultrasound thalamotomy for essential tremor in the setting of a ventricular shunt: technical report. Oper Neurosurg (Hagerstown). 2019;17:376–381. [DOI] [PubMed] [Google Scholar]
- 26.Elias WJ, Lipsman N, Ondo WG, et al. A randomized trial of focused ultrasound thalamotomy for essential tremor. N Engl J Med. 2016; 375:730–739. [DOI] [PubMed] [Google Scholar]
- 27.Mädler B, Coenen VA. Explaining clinical effects of deep brain stimulation through simplified target-specific modeling of the volume of activated tissue. AJNR Am J Neuroradiol. 2012;33: 1072–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butson CR, Maks CB, McIntyre CC. Sources and effects of electrode impedance during deep brain stimulation. Clin Neurophysiol. 2006;117:447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hancu I, Boutet A, Fiveland E, et al. On the (Non-) equivalency of monopolar and bipolar settings for deep brain stimulation fMRI studies of Parkinson’s disease patients. J Magn Reson Imaging. 2019; 49:1736–1749. [DOI] [PubMed] [Google Scholar]
- 30.Anthofer J, Steib K, Fellner C, Lange M, Brawanski A, Schlaier J. The variability of atlas-based targets in relation to surrounding major fibre tracts in thalamic deep brain stimulation. Acta Neurochir (Wien). 2014;156:1497–1504 [discussion: 1504]. [DOI] [PubMed] [Google Scholar]
- 31.Anthofer JM, Steib K, Lange M, et al. Distance between active electrode contacts and dentatorubrothalamic tract in patients with habituation of stimulation effect of deep brain stimulation in essential tremor. J Neurol Surg A Cent Eur Neurosurg. 2017;78:350–357. [DOI] [PubMed] [Google Scholar]
- 32.Nowacki A, Schlaier J, Debove I, Pollo C. Validation of diffusion tensor imaging tractography to visualize the dentatorubrothalamic tract for surgical planning. J Neurosurg. 2018;130:99–108. [DOI] [PubMed] [Google Scholar]
- 33.Schlaier J, Anthofer J, Steib K, et al. Deep brain stimulation for essential tremor: targeting the dentato-rubro-thalamic tract? Neuromodulation. 2015;18:105–112. [DOI] [PubMed] [Google Scholar]
- 34.Schlaier JR, Beer AL, Faltermeier R, et al. Probabilistic vs. deterministic fiber tracking and the influence of different seed regions to delineate cerebellar-thalamic fibers in deep brain stimulation. Eur J Neurosci. 2017;45:1623–1633. [DOI] [PubMed] [Google Scholar]
- 35.Behrens TEJ, Berg HJ, Jbabdi S, Rushworth MFS, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage. 2007;34:144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]


