Abstract
The understanding of anesthetic side-effects on the heart have been hindered by the lack of sophisticated clinical models. Using micropatterned human induced pluripotent stem cell derived cardiomyocytes (hiPSC-CMs), we obtained cardiac musle depressant profiles for propofol, etomidate, and our newly identified anesthetic compound KSEB01-S2. Propofol was the strongest depressant among the three compounds tested exhibiting the largest decrease in contraction velocity, depression rate, and beating frequency. Interestingly, KSEB01-S2 behaved similarly to etomidate suggesting a better cardiac safety profile. Our results provide a proof of concept for using hiPSC-CMs as an in vitro platform for future drug design.
Keywords: Propofol, Etomidate, hiPSC-CM, Cardiotoxicity
Introduction
Although their mechanism of action remains to be elucidated, anesthetics have been used widely around the world to safely relieve surgical suffering for over 170 years. Propofol (PFL, 1-hydroxyl-2,6-diisopropylbenzene) is currently one of the most well-known intravenous anesthetics used but has known cardiovascular side-effects. Etomidate, a more hemodynamically stable anesthetic acting through the γ-aminobutyric acid type A receptors (GABAAR), poses a toxicity profile that suppresses corticosteroid synthesis in the adrenal cortex. Using in silico docking algorithms and our validated model of the GABAAR, we have previously identified a novel anesthetic compound, compound KSEB01-S21–3, that is a highly potent anesthetic, but has a predicted cardiovascular depressant profile that is similar to etomidate and which has been chemically modified to abolish corticosteroid deficiencies. Prior to induced pluripotent stem cell technology, it was impossible to profile cardiovascular depressant levels in a human cardiomyocyte. Recent advances in bioengineering have allowed testing of small compounds on geometrically more mature human induced pluripotent stem cell derived cardiomyocytes (hiPSC-CMs)4. Using micropatterned hiPSC-CMs, we tested cardiac side-effects of the aforementioned compounds. Our results show that we can identify the level of anesthetic side-effects at physiological concentrations. Our findings strongly support the use of this hiPSC-CM platform for the future characterization of anesthetics.
Methods
Culture of hiPSCs and hiPSC-CM.
hiPSC lines were generated as part of the Stanford Cardiovascular Institute Biobank initiative. The culturing of the established hiPSC lines in our laboratory were reviewed and approved by the Stanford Stem Cell Research Oversight (SCRO) committee (#602). hiPSCs derived from two male (aged 22 and 45) and two female patients (aged 42 and 48) peripheral blood mononuclear cells were grown on Matrigel (Corning) coated plates using chemically defined Nutristem (Stemgent) medium as previously described (Figure 1A)5,6. The medium was changed daily, and cells were passaged every 4 days using Accutase (Thermo Scientific) with the addition of rock inhibitor 5μM Y-27632 2HCl (Selleck Chem). hiPSCs were grown to 70–90% confluence and subsequently differentiated into beating cardiomyocytes (Figure 1A)5,6. On day 1 and 3, 4–6μM CHIR-99021(Wnt activator, Selleck Chem) and 5μM IWR-1 (Wnt inhibitor, Sigma) was added to RPMI-1640 medium supplemented with insulin-free B27 (Life Technologies), respectively. On day 5, the differentiating hiPSCs were treated with RPMI-1640 insulin-free B27 alone. On day 7 and 9, the medium was changed to RPMI-1640 medium supplemented with insulin-containing B27. It has been shown that glucose deprivation allows removal of fibroblasts that arise during differentiation while lactate supplementation enhances metabolic maturation of hiPSC-CMs6. Beating hiPSC-CMs were purified and maintained in glucose-free conditions using RPMI-1640 glucose free medium supplemented with B27 supplement and lactate from day 11 onward until day 25 for seeding.
Figure 1. Cardiotoxicity profiling of KSEB01-S2 using biopatterned hiPSC-CMs.
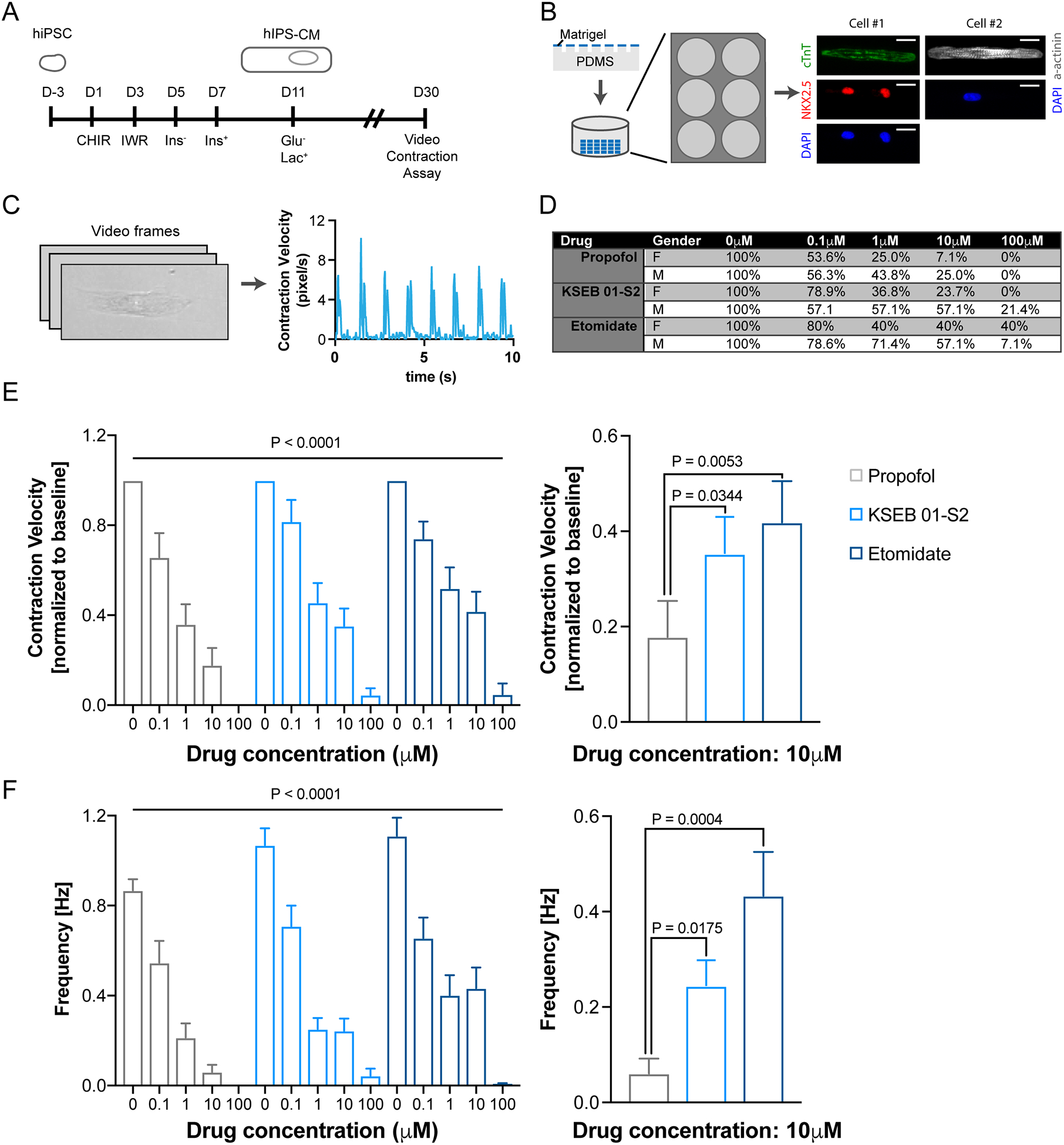
(A) Patient hiPSC lines were used to differentiate into hiPSC-CMs. (B) Single hiPSC-CMs were seeded onto microprinted matrigel patterned plates. Representative hiPSC-CMs stained with cardiac markers Troponin-T, NKX2.5, α-actinin and nuclear DAPI (blue) (scale bars, 10 μm). (C) hiPSC-CM contraction video analysis and representative tracing of the average contraction speed versus time for a single patterned hiPSC-CM. (D) Dropoff rate, (E) average contraction velocity, and (F) beating frequency of propofol (n=43), KSEB01-S2 (n=52), and etomidate (n=39) treated hiPSC-CMs and plotted as mean ± SEM.
Microprinting.
Elastomeric microstamps of polydimethylsiloxane-182 (Sylgard) containing rectangles of 17×119 μm2 were cleaned, pre-chilled, and coated with 100μL of diluted Matrigel (Matrigel:F10 medium 1:10) for at least 2hr at 4°C. Matrigel was aspirated and the patterns were air-dried. Patterns were carefully microprinted onto glass bottom 6-well plates (Cellvis; Figure 1B). Day 25 hiPSC-CMs were lifted and resuspended into single cells using Accutase, and were then seeded onto microprinted 6-wells at 10,000–50,000 cells/mL. The hiPSC-CMs were allowed to attach and recover for 5 days before contractility was assessed.
Measurement of hiPSC-CM contractility.
Videos of beating micropatterned single hiPSC-CMs were captured on a Keyence BZ-X710 microscope using a 40X/0.6 NA objective in oblique illumination/standard capture mode at 960×720 resolution at 29 fps for 10 seconds (Supplemental Video 1). hiPSC-CMs were maintained at 37°C and 5% CO2 (Tokai Hit Incubation System). Propofol, KSEB01-S2, and etomidate were dissolved in DMSO for 100μM stock solutions and diluted with lactate medium to generate working solutions (0.1μM, 1μM, and 10μM). Quantification of hiPSC-CM contractions was performed in a blinded fashion using Matlab-based motion tracking software as previously described (Figure 1C)7. Contraction velocities and frequencies of hiPSC-CMs were averaged over the duration of each recording normalized to untreated baseline. Dropout rates at various drug conditions were calculated as the percentage of beating hiPSC-CMs normalized to the number of beating hiPSC-CMs at baseline.
Sample size and statistical methods.
Statistical differences between the compound profiles were determined using a non-parametric Kruskal-Wallis test with multiple comparison with uncorrected Dunn’s tests. All data are shown as the mean±SEM. Significant differences were defined as a P value <0.05. Based on empirically determined acquisition times, a total of 50 cells per condition (two independent hiPSC-CM differentiation to minimize batch differences) were targeted. A priori statistical power was 94.2% when a two-tail test with a 50% decrease in signal, sample size of 50, SD of 1, and a 5% alpha error level was assumed.
Results
Previously we identified a novel anesthetic KSEB01-S2 using in silico flexible docking targeting GABAAR8. In tadpoles, KSEB01-S2 exhibit an EC50 of about 500nM8. We were interested in testing anesthetic side-effects in human cardiomyocytes. To determine the side-effects of propofol, etomidate and KSEB01-S2, we measured changes in contraction frequencies and velocities of micropatterned single hiPSC-CMs in response to incremental drug doses (0μM, 0.1μM, 1μM, 10μM, and 100μM; Figure 1B–C). With incrementing concentrations, the drugs’ negative inotropic and chronotropic effects on hiPSC-CMs increased (Figure 1D). Consistent with patient clinical data, the observed contraction suppression was most pronounced with propofol and least with etomidate. In accordance with dropoff rates as well, we observed a decrease in average contraction velocity by propofol (0.66±0.11, 0.36±0.09, 0.18±0.08, and 0.00±0.00), but to a lesser degree by KSEB01-S2 (0.82±0.10, 0.46±0.09, 0.35±0.08, and 0.05±0.02), and etomidate (0.74±0.08, 0.52±0.09, 0.42±0.09, and 0.05±0.05) treated hiPSC-CMs at 0.1μM, 1μM, 10μM, and 100μM, respectively (Figure 1E). Moreover, we observed a slower beating frequency in propofol (0.87±0.05, 0.55±0.10, 0.21±0.06, 0.06±0.03, and 0.00±0.00) compared to KSEB01-S2 (1.07±0.07, 0.71±0.07, 0.25±0.05, 0.24±0.05, and 0.04±0.03) or etomidate (1.11±0.08, 0.66±0.09, 0.40±0.09, 0.43±0.09, and 0.01±0.01) treated hiPSC-CMs at 0μM, 0.1μM, 1μM, 10μM, and 100μM, respectively (Figure 1F).
Discussion
All currently used intravenous anesthetic agents are associated with an entire spectrum of undesirable side-effect, most notably cardiovascular instabilities. These side effects can be poorly tolerated in many surgical patients without proper intervention, but especially in very young children who possess immature physiologic compensatory mechanisms, as well as in the elderly with confounding comorbidities and otherwise exhausted compensatory mechanisms. However necessary such animal studies are in the pipeline of drug development, performing analyses in rats and higher mammals is both time consuming and expensive. hiPSC-CM technology has been successfully used for studying aberrant electrophysiological conditions such as long QT9 and used in disease model genetic cardiomyopathies10,11; it is also currently being developed under the Comprehensive In Vitro Proarrhythmia Assay (CiPA) initiative as the new cardiotoxicity standard12. In this study, we show the use of an efficient hiPSC-CM platform to assess and predict the potential for anesthetic side-effects within a class of intravenous general anesthetics. It has been shown that cell shape and aspect ratio can greatly influence hiPSC-CM contractile maturation13. Our microprinting13 coupled video contraction7 hiPSC-CM platform affords us the ability to track the side-effect responses of prescribed anesthetics on a single hiPSC-CM. Although we only tested a limited number of healthy hiPSC-CMs in this study, it is foreseeable that cohorts consisting of varying disease states, ethnicity, and ages could be tested for anesthetic side-effects. At physiological concentrations, our results demonstrate that propofol exhibits more undesirable side-effects compared to etomidate and KSEB01-S2. As demonstrated before, hiPSC-CM is a very powerful platform for disease modeling10,11 and cardiotoxicity testing5. Our results serve as a proof-of-concept which now affords the ability to interrogate the signaling pathway behind anesthetic side-effects, the unique opportunity to directly characterize specific cardiac physiologic responses of currently prescribed drugs using a purely in vitro methodology, as well as well as test new lead compounds in the drug development pipeline.
Supplementary Material
Video. Micropatterned hiPSC-CM. Healthy hiPSC-CM (day 30) biopatterned with 1:7 aspect ratio. Video captured with 40X objective at 29fps.
Acknowledgements
We would like to thank Noëlie Cayla for compound preparation. This research was supported by the Canadian Institutes of Health Research Fellowship (201411MFE-338745-169197 to A.C.Y.C.); the Baxter Foundation, and National Institutes of Health (AG044815 and AR063963 to H.M.B.); Stanford University Department of Anesthesia FIDL Grant, Stanford University SPARK Drug Discovery Program, Stanford University Children’s Health Research Initiative to M.F.D. and E.J.B.
Footnotes
Conflict of Interest
All authors declare no conflict of interest.
References
- 1.Bertaccini EJ, Yoluk O, Lindahl ER, Trudell JR. Assessment of homology templates and an anesthetic binding site within the γ-aminobutyric acid receptor. Anesthesiology 2013;119:1087–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertaccini EJ, Davies MF, The Board Of Trustees Of The Leland Stanford Junior University, Affairs TUSGARBTDOV. Novel methods, compounds, and compositions for anesthesia. 2016. [Google Scholar]
- 3.Cayla N, Dagne B, Davies M, Gross E, BacIver M, Bertaccini EJ. Teasing apart the desired effects of anesthetics from unwanted side effects at GABAA receptors. In: Boston, MA, 2017. Available at: https://www.isaponline.org/application/files/6015/0817/9040/ISAP_Syllabus_17.pdf. [Google Scholar]
- 4.Ribeiro AJS, Ang Y-S, Fu J-D, Rivas RN, Mohamed TMA, Higgs GC, Srivastava D, Pruitt BL. Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proc Natl Acad Sci U S A 2015;112:12705–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burridge PW, Li YF, Matsa E, Wu H, Ong S-G, Sharma A, Holmström A, Chang AC, Coronado MJ, Ebert AD, Knowles JW, Telli ML, Witteles RM, Blau HM, Bernstein D, Altman RB, Wu JC. Human induced pluripotent stem cell-derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity. Nat Med 2016;22:547–56. Available at: http://www.nature.com/doifinder/10.1038/nm.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tohyama S, Hattori F, Sano M, Hishiki T, Nagahata Y, Matsuura T, Hashimoto H, Suzuki T, Yamashita H, Satoh Y, Egashira T, Seki T, Muraoka N, Yamakawa H, Ohgino Y, Tanaka T, Yoichi M, Yuasa S, Murata M, Suematsu M, Fukuda K. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell 2013;12:127–37. [DOI] [PubMed] [Google Scholar]
- 7.Huebsch N, Loskill P, Mandegar MA, Marks NC, Sheehan AS, Ma Z, Mathur A, Nguyen TN, Yoo JC, Judge LM, Spencer CI, Chukka AC, Russell CR, So P-L, Conklin BR, Healy KE. Automated Video-Based Analysis of Contractility and Calcium Flux in Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes Cultured over Different Spatial Scales. Tissue Eng Part C Methods 2015;21:467–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertaccini EJ, Davies MF, Cayla N, Sunay M, Heifets B, Trudell JR, Maclver BM. Molecular Modeling Leads to a New Class of Intravenous Anesthetics. In: San Diego, CA, 2015. Available at: http://w.asaabstracts.com/strands/asaabstracts/abstract.htm?year=2015&index=2&absnum=3745. [Google Scholar]
- 9.Liang P, Lan F, Lee AS, Gong T, Sanchez-Freire V, Wang Y, Diecke S, Sallam K, Knowles JW, Wang PJ, Nguyen PK, Bers DM, Robbins RC, Wu JC. Drug screening using a library of human induced pluripotent stem cell-derived cardiomyocytes reveals disease-specific patterns of cardiotoxicity. Circulation 2013;127:1677–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun N, Yazawa M, Liu J, Han L, Sanchez-Freire V, Abilez OJ, Navarrete EG, Hu S, Wang L, Lee A, Pavlovic A, Lin S, Chen R, Hajjar RJ, Snyder MP, Dolmetsch RE, Butte MJ, Ashley EA, Longaker MT, Robbins RC, Wu JC. Patient-specific induced pluripotent stem cells as a model for familial dilated cardiomyopathy. Sci Transl Med 2012;4:130ra47–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lan F, Lee AS, Liang P, Sanchez-Freire V, Nguyen PK, Wang L, Han L, Yen M, Wang Y, Sun N, Abilez OJ, Hu S, Ebert AD, Navarrete EG, Simmons CS, Wheeler M, Pruitt B, Lewis R, Yamaguchi Y, Ashley EA, Bers DM, Robbins RC, Longaker MT, Wu JC. Abnormal calcium handling properties underlie familial hypertrophic cardiomyopathy pathology in patient-specific induced pluripotent stem cells. Cell Stem Cell 2013;12:101–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Millard D, Dang Q, Shi H, Zhang X, Strock C, Kraushaar U, Zeng H, Levesque P, Lu H-R, Guillon J-M, Wu JC, Li Y, Luerman G, Anson B, Guo L, Clements M, Abassi YA, Ross J, Pierson J, Gintant G. Cross-Site Reliability of Human Induced Pluripotent Stem-Cell Derived Cardiomyocyte Based Safety Assays using Microelectrode Arrays: Results from a Blinded CiPA Pilot Study. Toxicol Sci 2018;84:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ribeiro AJS, Schwab O, Mandegar MA, Ang Y-S, Conklin BR, Srivastava D, Pruitt BL. Multi-Imaging Method to Assay the Contractile Mechanical Output of Micropatterned Human iPSC-Derived Cardiac Myocytes. Circ Res 2017;120:1572–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video. Micropatterned hiPSC-CM. Healthy hiPSC-CM (day 30) biopatterned with 1:7 aspect ratio. Video captured with 40X objective at 29fps.


