Abstract
An infant with transposition of the great arteries was paced for postoperative heart block (single-site, RV epicardial). She developed severe LV dysfunction and septal dyskinesis. Resynchronization was performed at age 4 with an LV epicardial lead and an RV septal endocardial lead. The endocardial lead was affixed to the interventricular septum, then tunneled through the RV free wall and attached to an abdominal pulse generator. QRS duration decreased (176 to 122 ms) and LVEF improved (26% to 61%) and remained stable for 8 years. We present a case of successful resynchronization in congenital heart disease using a transmural RV septal lead.
Keywords: CRT, pacing, heart block, congenital heart disease, pediatrics
Introduction:
Transvenous pacing systems are routine in adults, but children often receive epicardial pacemakers. Patient size, vascular access issues, and complex postsurgical anatomy complicate pacemaker placement in pediatric and congenital heart disease.1 In some children, single-site right ventricular (RV) pacing causes dyssynchronous left ventricular (LV) activation, pathologic LV remodeling, and heart failure.2–5 The success of resynchronization in adults has prompted attempts at resynchronization in congenital heart disease.6–13 A common approach is to place leads on one epicardial location and then choose a second location based on an anatomic prediction or electrical mapping.10 One lead is usually placed on the RV epicardial surface. We report a case of a child who was too small for transvenous resynchronization and in whom we were concerned that RV conduction delay would prevent effective LV resynchronization (severe septal dyskinesis and a pre-existing RV-to-PA conduit). Our theory was that RV delay might cause latency between the stimulation impulse and onset of LV activation, potentially decreasing the effectiveness of resynchronization on LV function. No consensus approach exists to provide resynchronization in pediatric and congenital heart disease patients with RV conduction delay and severe septal dyskinesis. Individualized solutions are required. This case demonstrates a novel solution to the problem of LV resynchronization in the setting of RV conduction delay secondary to a prior ventriculotomy for congenital heart disease. While other epicardial approaches have been used more recently, we report over 8 years of follow-up using this novel approach.
Case:
A child with {S,D,D} transposition of the great arteries, pulmonary artery (PA) stenosis, and a ventricular septal defect (VSD) underwent a Blalock-Taussig shunt at 5 days of life, followed by a Nikaidoh operation 6 months later (aortic translocation, RV-PA conduit, and VSD closure). The Nikaidoh operation was complicated by complete heart block, for which a dual chamber pacemaker was implanted with an epicardial lead on the RV free wall. Pre-operatively, she had a narrow QRS duration (QRSd, 58 ms) with normal QRS morphology. Postoperatively, she had no ventricular escape, and so her intrinsic conduction morphology could not be determined, but in our experience, an RV-PA conduit produces right bundle branch block. As expected, RV free wall pacing produced a left bundle branch block pattern (QRSd 176ms). By age 4, she had severe LV dysfunction, moderate LV dilation, septal dyskinesis, mild-moderate aortic regurgitation, and mild-moderate mitral regurgitation. She required RV-PA conduit revision, aortic annuloplasty, and mitral valve repair. Due to patient size (12.6 kg), she was not eligible for transvenous resynchronization. At the time in 2011, we considered resynchronization with epicardial biventricular pacing. However, we were concerned that the RV conduction delay from RV-PA conduit placement and revision might exceed the programmable RV-LV timing characteristics on the pulse generator. Additionally, we hypothesized that direct activation of the interventricular septum would lead to more improved mechanical synchrony compared to RV free wall activation. Therefore, we attempted surgical placement of a pacing lead on the RV septum to provide LV resynchronization without including RV conduction delay in the electrical circuit. Electroanatomic mapping was not performed prior to surgery due to her small size. A Medtronic 5076 CapSureFix Novus extendable helix lead was affixed to the midpoint of the RV septal endocardium under direct visualization (Figure 1). The lead was exteriorized through the RV, sparing the tricuspid valve, and a purse string suture was placed around the exit site of the ventricular lead. An additional epicardial lead was implanted on the posterior-lateral LV free wall. The leads were connected to a Syncra CRT-P device in an abdominal generator pocket.
Figure 1:
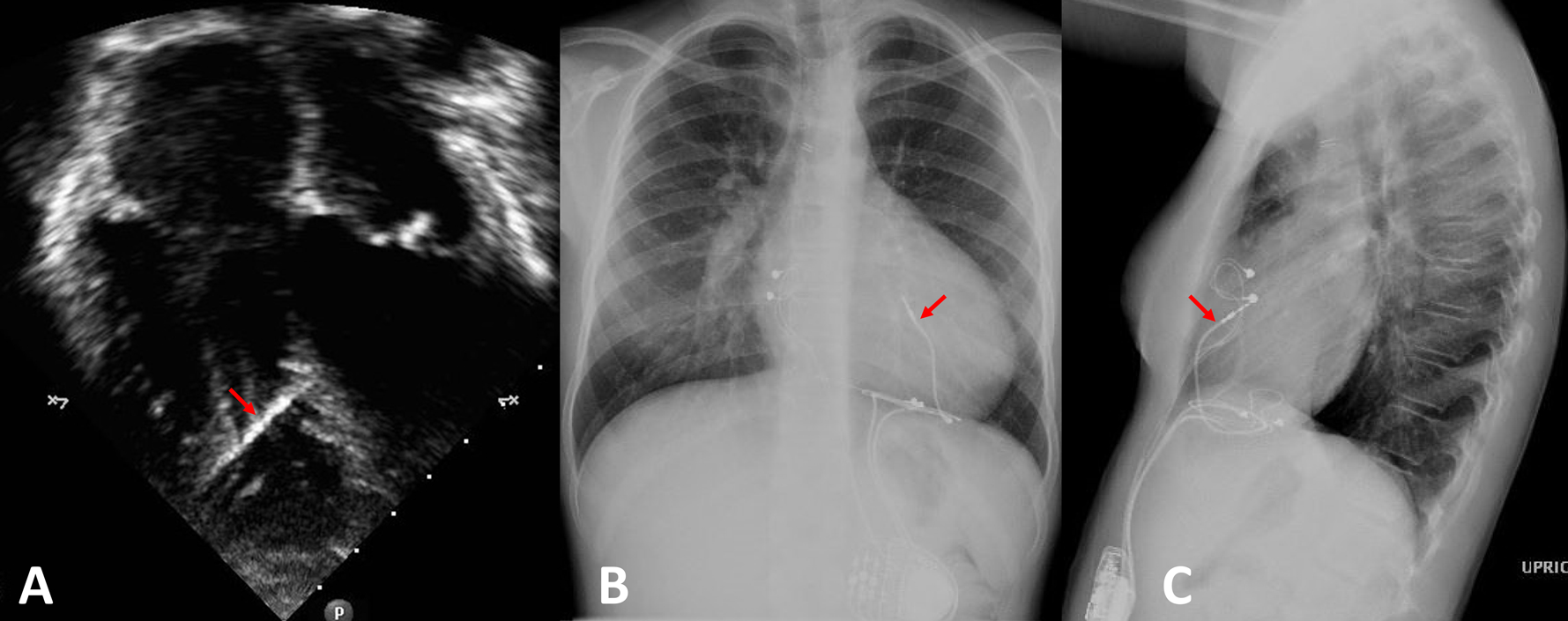
The position of the RV endocardial lead affixed to the interventricular septum is demonstrated in an echocardiographic apical 4 chamber view (panel A) and chest X-ray (posterior-anterior view in panel B, lateral view in panel C). The RV septal lead is indicated with a red arrow.
After implantation and recovery, the RV-LV timing was programmed to an offset of 0 ms after testing 20 ms increments from −80 ms to +80 ms offset using echocardiographic evaluation of LV function and synchrony. Simultaneous RV-LV timing resulted in the most significant improvement in mechanical synchrony and LV function, which remained stable on future echocardiograms. Systolic contraction of the interventricular septum improved, and the classic dyssynchrony pattern of paradoxical septal motion resolved (Figure 2). Basal septal hypokinesis persisted, related to the VSD patch. LV ejection fraction improved from 26% to 61% and has been stable during 8 years of follow-up with nearly 100% biventricular pacing (Figure 3). After resynchronization, the QRS duration decreased from 176 ms to 122 ms (31% improvement, Supplementary Figure). Her heart failure symptoms resolved after her valve repair and resynchronization; no signs or symptoms of heart failure have recurred since the operation. Capture thresholds and sensitivities have been stable.
Figure 2:

Panel A: Pre-resynchronization contraction pattern. M-mode (top) demonstrated limited thickening of the interventricular septum. There was paradoxical motion towards the RV during LV free wall contraction. Longitudinal strain pattern (bottom) showed early pre-ejection septal contraction (red lines) with opposing early stretch in the LV free wall (green lines), followed by late free wall contraction and termination of septal contraction with associated stretch. The white arrow highlights the point of maximal early stretch. Panel B: Post-resynchronization contraction pattern. Improvement in mechanical synchrony by M-mode imaging. Longitudinal strain showed slightly earlier septal activation (red) than LV free wall activation (green), but reciprocal stretch was absent.
Figure 3:
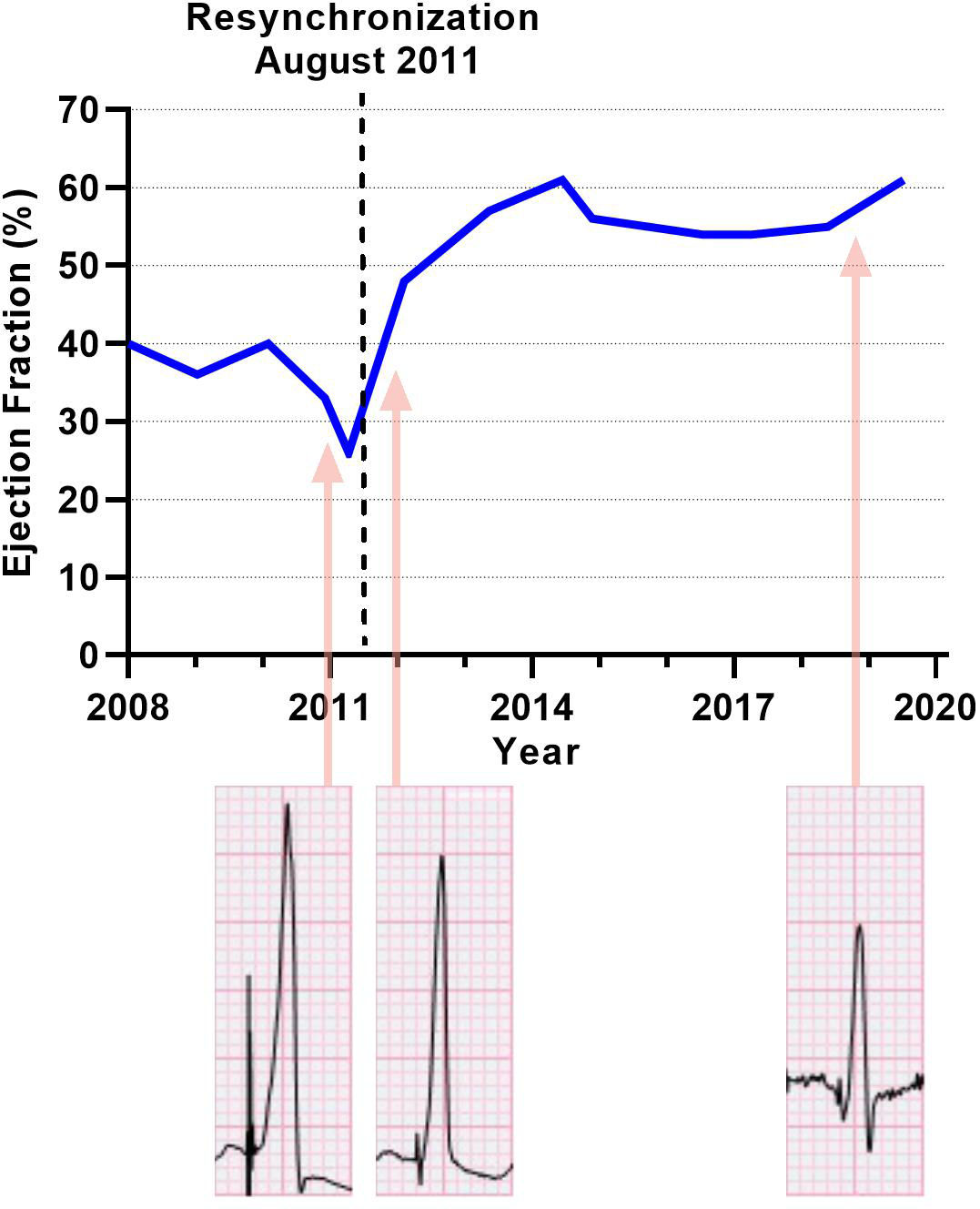
Ejection fraction plotted over time. The dashed vertical line marks the date of surgical resynchronization. QRS complexes: lead V6 with 5mm/mV amplitude settings.
Discussion:
We describe the successful long-term use of an RV endocardial and LV epicardial lead for resynchronization in a pediatric patient with complex congenital heart disease and surgical complete heart block. Resynchronization was achieved with the novel approach of surgically tunneling the RV endocardial lead through a purse-string aperture in the RV free wall. This pacing system achieved a significant and durable improvement in systolic function in addition to improvements in mechanical synchrony and QRS duration without any significant complications. Other surgical interventions (RV-PA conduit replacement, mitral valve repair, aortic annuloplasty) likely contributed to improvement in LV function. However, mitral valve and aortic valve interventions are unlikely to have such a profound effect on mechanical synchrony and QRS duration. Therefore, we suspect that resynchronization was the primary driver of improved function.
We present long-term follow-up in this case report, which means that the original system was placed approximately ten years ago. Since that time, reports have been published of improved LV function with epicardial biventricular pacing in patients with repaired D-TGA and complete heart block.11 At the time of this procedure, our hypothesis was that scar from the RV-PA conduit would result in profound RV conduction delay, preventing us from improving LV/septal synchrony even after optimizing programmable LV-RV offset. Our goal was to use an RV endocardial lead on the interventricular septum to provide direct stimulation of the dyskinetic septum and bypass the RV conduction delay from the RV-PA conduit. Essentially, we attempted to duplicate the lead positions of a transvenous system via surgery. Multiple studies have assessed the optimal RV lead position in transvenous resynchronization systems, and some data suggest that RV septal pacing is superior to RV apical pacing.14–15 Although this system produced successful resynchronization without complications, we have not needed to repeat this approach in the intervening decade. Our institution has subsequently had success with biventricular epicardial leads, which do not include the risks of the small ventriculotomy to pass the lead. We present this case as quantitative, long-term support for the feasibility of a tunneled RV septal approach to resynchronization. Looking forward, we do not advocate this approach as a first-line therapy, but it can be considered if neither transvenous resynchronization nor biventricular epicardial resynchronization are possible or if they fail to achieve a durable result.
Supplementary Material
Supplementary Figure: 412-lead ECG before (top) and after (bottom) resynchronization demonstrating an improvement in QRS duration from 176ms to 122ms.
Funding:
Research reported in this publication was supported, in part, by the National Institutes of Health, National Heart, Lung and Blood Institute, grant number K23HL130554.
Footnotes
Disclosures: The authors have no conflicts of interest to report.
References:
- 1.Kelle AM, Backer CL, Tsao S, Stewart RD, Franklin WH, Deal BJ, Mavroudis C. Dual-chamber epicardial pacing in neonates with congenital heart block. The Journal of Thoracic and Cardiovascular Surgery 2007;134:1188–92. [DOI] [PubMed] [Google Scholar]
- 2.Gebauer RA, Tomek V, Salameh A, Marek J, Chaloupecky V, Gebauer R, Matejka T, et al. Predictors of left ventricular remodelling and failure in right ventricular pacing in the young. European Heart Journal 2009;30:1097–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song MK, Kim NY, Bae EJ, Kim GB, Kwak JG, Kim WH, Lee JR. Long-term follow-up of epicardial pacing and left ventricular dysfunction in children with congenital heart block. The Annals of Thoracic Surgery 2019. [DOI] [PubMed] [Google Scholar]
- 4.Thambo JB, Bordachar P, Garrigue S, Lafitte S, Sanders P, Reuter S, Girardot R, et al. Detrimental ventricular remodeling in patients with congenital complete heart block and chronic right ventricular apical pacing. Circulation 2004;110:3766–72. [DOI] [PubMed] [Google Scholar]
- 5.Vatasescu R, Shalganov T, Paprika D, Kornyei L, Prodan Z, Bodor G, Szatmari A, et al. Evolution of left ventricular function in paediatric patients with permanent right ventricular pacing for isolated congenital heart block: a medium term follow-up. Europace 2007;9:228–32. [DOI] [PubMed] [Google Scholar]
- 6.Khairy P, Van Hare GF, Balaji S, Berul CI, Cecchin F, Cohen MI, Daniels CJ, et al. PACES/HRS Expert Consensus Statement on the Recognition and Management of Arrhythmias in Adult Congenital Heart Disease: Developed in Partnership Between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the Governing Bodies of PACES, HRS, the American College of Cardiology (ACC), the American Heart Association (AHA), the European Heart Rhythm Association (EHRA), the Canadian Heart Rhythm Society (CHRS), and the International Society for Adult Congenital Heart Disease (ISACHD). Heart Rhythm 2014;11:e102–e165. [DOI] [PubMed] [Google Scholar]
- 7.Moak JP, Hasbani K, Ramwell C, Freedenberg V, Berger JT, Dirusso G, Callahan P. Dilated Cardiomyopathy Following Right Ventricular Pacing for AV Block in Young Patients: Resolution After Upgrading to Biventricular Pacing Systems. Journal of Cardiovascular Electrophysiology 2006;17:1068–71. [DOI] [PubMed] [Google Scholar]
- 8.Janousek J, Gebauer RA, Abdul-Khaliq H, Turner M, Kornyei L, Grollmuss O, Rosenthal E, et al. Cardiac resynchronisation therapy in paediatric and congenital heart disease: differential effects in various anatomical and functional substrates. Heart (British Cardiac Society) 2009;95:1165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubin AM, Janousek J, Rhee E, Strieper MJ, Cecchin F, Law IH, Shannon KM, et al. Resynchronization therapy in pediatric and congenital heart disease patients: an international multicenter study. Journal of the American College Cardiology 2005;46:2277–83. [DOI] [PubMed] [Google Scholar]
- 10.Cecchin F, Frangini PA, Brown DW, Fynn-Thompson F, Alexander ME, Triedman JK, Gauvreau K, et al. Cardiac resynchronization therapy (and multisite pacing) in pediatrics and congenital heart disease: five years experience in a single institution. Journal of Cardiovascular Electrophysiology 2009;20:58–65. [DOI] [PubMed] [Google Scholar]
- 11.Mah DY, Alexander ME, Banka P, Abrams DJ, Triedman JK, Walsh EP, Fynn-Thompson F, et al. The role of cardiac resynchronization therapy for arterial switch operations complicated by complete heart block. The Annals of Thoracic Surgery 2013;96:904–9 [DOI] [PubMed] [Google Scholar]
- 12.Cazeau S, Leclercq C, Lavergne T, Walker S, Varma C, Linde C, Garrigue S, et al. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. The New England Journal of Medicine 2001;344:873–80. [DOI] [PubMed] [Google Scholar]
- 13.Normand C, Linde C, Singh J, Dickstein K. Indications for Cardiac Resynchronization Therapy. A Comparison of the Major International Guidelines. Journal of the American College Cardiology: Heart Failure 2018;6:308–16. [DOI] [PubMed] [Google Scholar]
- 14.Members ATF, Brignole M, Auricchio A, et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: The Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). European heart journal 2013;34:2281–329. [DOI] [PubMed] [Google Scholar]
- 15.Shimony A, Eisenberg MJ, Filion KB, Amit G. Beneficial effects of right ventricular non-apical vs. apical pacing: a systematic reviewand meta-analysis of randomizedcontrolled trials. Europace 2012;14:81–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure: 412-lead ECG before (top) and after (bottom) resynchronization demonstrating an improvement in QRS duration from 176ms to 122ms.


