Abstract
Backgrounds and Purpose
Although new methods for genetic analyses are rapidly evolving, there are currently knowledge gaps in how to detect Mendelian forms of stroke.
Methods
We performed whole exome sequencing (WES) in 22 probands, under 56 years at their first ischemic stroke episode, from multi-incident stroke families. With the use of a comprehensive stroke-gene panel we searched for variants in stroke-related genes. The probands’ clinical stroke subtype was related to clinical characteristics previously associated with pathogenic variants in these genes. Relatives were genotyped in seven families to evaluate stroke-gene variants of unknown significance. In two larger families with embolic stroke of unknown source (ESUS), WES was performed in additional members to examine the possibility to identify new stroke-genes.
Results
Six of 22 probands carried pathogenic or possibly pathogenic variants in genes reported to be associated with their stroke subtype. A known pathogenic variant in NOTCH3 and a possibly pathogenic variant in ACAD9 gene were identified. A novel JAK2:c.3188G>A (p.Arg1063His) mutation was seen in a proband with ESUS and pro-thrombotic status. However, penetrance in the family was incomplete. COL4A2:c.3368A>G (p.Glu1123Gly) was detected in two probands but did not co-segregate with the disease in their families. WES in multiple members of two pedigrees with ESUS revealed possibly pathogenic variants in genes not previously associated with stroke, GPR142:c.148C>G (p.Leu50Val), and PTPRN2:c.2416A>G (p.Ile806Val); LRRC1 c.808A>G (p.Ile270Val), SLC7A10c.1294dupG (p.Val432fs), IKBKB: c.1070C>T (p.Ala357Val), and OXGR1 c.392G>A (p.Arg131His), respectively.
Conclusions
Screening with WES using a comprehensive stroke-gene panel may identify rare monogenic forms of stroke, but careful evaluation of clinical characteristics and potential pathogenicity of novel variants remain important. In our study, the majority of individuals with familial aggregation of stroke lacked any identified genetic causes.
Keywords: Stroke; Databases, genetic; Whole exome sequencing; Family history of stroke; Sequence analysis, DNA
Background and Purpose
Stroke is known to be partially genetically determined, although the genetic mechanisms leading to stroke are not yet fully elucidated.1–4 Stroke may occur in familial clusters5, 6 and a large number of single-gene defects associated with ischemic stroke (IS) have been reported.7 However, these variants are rare or very rare.8 To our knowledge, no previous study has systematically analyzed the frequency of all reported monogenic variants related to stroke among a group of patients with familial clustering for stroke.
Technological advances with, in particular, the availability of whole exome sequencing (WES), now offer opportunities for simultaneous investigations of a large number of genes.9 We previously described a method for comprehensive evaluation of monogenic causes of stroke and compiled stroke-gene panels that systematically include genes related to monogenic stroke and monogenic diseases related to stroke, also taking into account clinical stroke subtypes.7
We hypothesize that clustering of stroke in families may indicate a monogenic cause, particularly for index patients who have their first stroke episode at an earlier age. Here, we use WES to analyze a group of stroke patients under 56 years, with a family history compatible with monogenic hereditary patterns and focus on the evaluation of possible high impact genetic variants that could result in familial clustering of stroke and stroke-related disease.
Methods
The authors declare that all supporting data are available within the article and its online supplement.
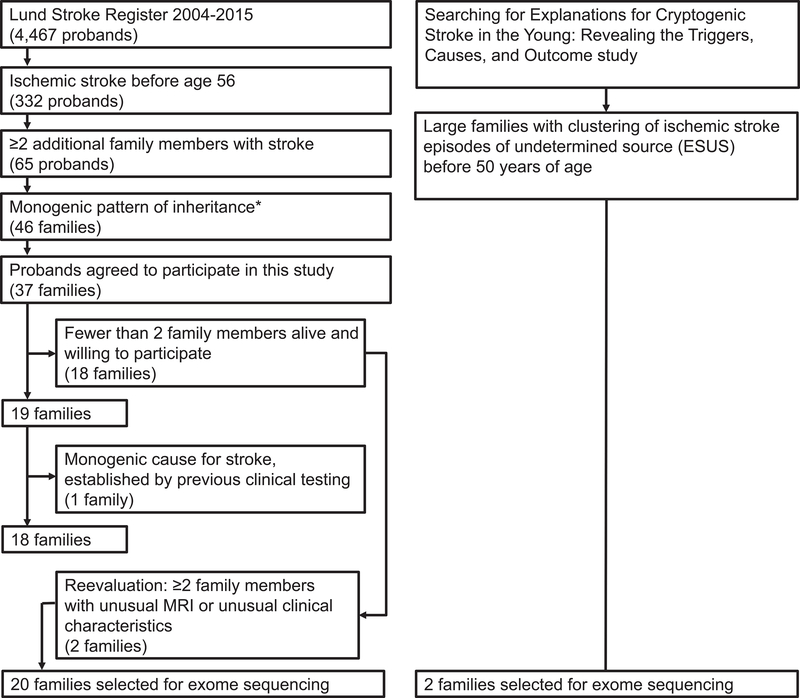
Twenty probands from Lund Stroke Register5 and two from the ongoing prospective Searching for Explanations for Cryptogenic Stroke in the Young: Revealing the Etiology, Triggers, and Outcome (SECRETO; NCT01934725) study10 younger than 56 years at their first IS-episode, with familial clustering of stroke irrespective of the age at which family members presented with a stroke episode presumed to be caused by the same genetic mechanism, and without a known stroke-related monogenic disorder, were selected for genetic analysis (figure 1).The probands were clinically examined by a neurologist for the purpose of this study. Pedigrees were drawn based on information provided by probands, relatives, medical records and additional information from publically available genealogical databases. Affected and unaffected family members were personally interviewed and clinically examined when feasible. Medical records for both living and deceased family members were reviewed whenever available. Brain MRIs were performed in selected affected and unaffected family members. Stroke subtype and health status was assessed and documented.
Figure 1:
Flowchart describing the selection of probands included in this study, based on initial data from probands and their family members. FM=family members; MRI=magnetic resonance imaging of the brain; *as defined in ref. 5.
The first-ever stroke episode was classified according to the Causative Classification System for Ischemic Stroke (CCS).11 Stroke episodes fulfilling the definition of embolic stroke of undetermined source (ESUS)12 were also identified. The two Finnish probands were specifically selected because they had ESUS and a large pedigree indicating monogenic pattern of IS.
WES of the 22 probands was performed on Ion Proton (Life Technologies). Libraries were generated with Ion AmpliSeq Exome RDY kit. Variant calling was performed by Torrent Suite and/or HaplotypeCaller, following Genome Analysis ToolKit (GATK, version 3.5.0)13 according to best practice recommendations. The following quality filter was used for SNPs: quality of depth (QD) <2.0, mapping quality (MQ) <40.0, Fisher Strand test (FS) >60.0, ReadPosRankSum <−8.0, MQRankSum <−12.5, and for indels: QD <2.0, FS> 200.0, ReadPosRankSum <−20.0. Duplicate reads were removed using Integrative Genomics Viewer version 2.3. Variants were annotated using ANNOVAR14 and filtered (Figure 2). We evaluated genes from previously reported comprehensive stroke-gene panels (SGP1 and SGP2)7 which include genes associated with stroke and information on associated stroke subtypes.
Figure 2:
Filtering process of the variants resulting from whole exome sequencing. GWAS=genome wide association studies; MAF=minor allele frequency; *for the 20 Swedish probands;**for the 2 Finnish probands SweGenes, ExAC Fin, ClinVar are population, disease-specific databases; GERP(http://mendel.stanford.edu/sidowlab/downloads/gerp/index.html); PhyloP(http://compgen.bscb.cornell.edu/phast/); SIFT(http://sift.jcvi.org); Polyphen2(http://genetics.bwh.harvard.edu/pph2); MutationTester(http://www.mutationtaster.org) are in silico predictive algorithms;
Variants were further filtered against 1000 Genomes15 for all probands, SweGene16 for Swedish and ExAC Fin17 for Finnish probands, removing variants with a minor allele frequency (MAF) >1%. However, we also included variants classified as “pathogenic” in the ClinVar browser18 with MAF >1%, to avoid elimination of more frequent possible stroke-associated variants. A lower cut-off, MAF<0,1% was used when filtering for very rare variants in new candidate genes in the two Finnish families. We excluded synonymous variants unless they were situated at a splice site. Variants identified in >10% of in-house data from 200 other non-stroke patients investigated on the same IonTorrent platform were eliminated and considered platform-specific artefacts.
For in silico functional predictions we used SIFT, PolyPhen2, MutationTaster, GERP++, PhyloP, and Alamut Analyses (Figure 2). We compared the clinical stroke subtype of the proband with the phenotype associated with the particular gene listed in the previously reported stroke-panels6.
In families where the proband carried a possibly pathogenic variant, DNA from the proband and family members was analyzed by Sanger sequencing to confirm WES reads and to evaluate the pathogenicity17 of detected variants by assessing co-segregation of phenotype and genotype. ControlFREEC was used to detect possible larger copy number variants in these individuals.
WES was also performed in 16 family members in the two Finnish families with ESUS18. The same filtering algorithm was used (Figure 2), this time with a cut-off at >0.1%MAF. Following evaluation of variants in genes from the stroke-gene panels, we performed exome-wide comparisons of affected and unaffected members. In both families, WES data from three affected family members with similar clinical picture were compared to data from one unaffected member. We then examined the resulting candidate variants in WES of the additional family members.
Indel variants identified in 6 or more probands from LSR presenting other types of stroke (small vessel disease or dissections) were considered artefacts and eliminated.
The study was approved by the ethics committees in Lund, and Helsinki and Uusimaa Hospital District. All participants gave their written informed consent prior to participation.
Results
Fifty persons from 20 Swedish families with clustering of stroke were included in the study and clinically examined. Eighteen persons from two Finnish families were analyzed. The 22 studied families had an average of 4.27 affected members/family cluster. The average sum of the coefficient of relatedness of affected individuals within one family cluster was 0.39. We identified twelve probands with first-ever stroke episode associated with small vessel disease (SVD). Two probands had large artery atherosclerosis and one proband (having dissection and intracerebral aneurysms) non-atherosclerotic large artery stroke.7 Two probands had atrial fibrillation, classified as a high-risk source of cardioembolism (CE). Three of the Swedish probands had ESUS,12 as did the two Finnish probands (Figure 3).
Figure 3:
Eight variants remaining after the filtering process, six of these were confirmed by Sanger sequencing analysis; CACNA1A c.6845delG and COL4A1c.4048G>A variants were not reproduced by Sanger sequencing and eliminated as artefact.; SAO=small artery occlusion; SVD=small vessel disease, LAA=supra-aortic large artery atherosclerotic, LAN=large artery non-atherosclerotic, CE= cardio-embolism.
Systematic filtering of WES data from the 20 Swedish probands (Figure 2) revealed eight possibly pathogenic variants in genes previously related to the respective individual stroke subtype. Six of these eight variants were confirmed by Sanger sequencing. Thirteen additional affected and unaffected family members were genotyped in five of these families.
No variant established as pathogenic in ClinVar, irrespective of allele frequency, was identified. No large copy number variants were detected by control-FREEC. Pedigree drawings of all families are provided (supplemental Figure I–VIII).
Familial clustering of stroke related to cerebral small vessel disease (families 1–12)
Four stroke-gene variants were identified by filtering and confirmed by Sanger sequencing in the twelve probands with SVD.
The proband of family1 carried a NOTCH3 c.1672C>T (p.Arg558Cys) variant, previously reported as pathogenic and associated with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL).19 The same individual also carried a previously undescribed COL4A1 c.161C>T(p.Pro54Leu) variant, and this variant segregated with the disease in two affected and two unaffected relatives (supplemental Figure I).
Probands from family2 and family3 carried the same variant in a stroke-gene, COL4A2 c.3368A>G (p.Glu1123Gly). However, genotyping of family members in these two pedigrees suggested that the variant does not segregate with the SVD (supplemental Figure II). In family2, two members with lacunar infarcts and deep intracerebral bleedings did not have the mutation. In family3, one member carried the mutation but was considered unaffected. This person had hypertension and depressive episodes but was otherwise healthy, without any clinical event that could associate with known Col4A1/Col4A2 clinical spectrum20 and with a brain image in the 6th decade without cerebral white matter hyperintensities.
One homozygous variant, ACAD9 c.976G>A (p.Ala326Pro), was identified in the proband of family4. According to information provided by the proband and medical records of one relative, two family members had developed stroke. Intracerebral bleeding was reported in two relatives and one reportedly had hemispastic cerebral palsy of unknown cause. Additional phenotypes in this family included progressive muscle weakness starting in early 40s, seizure episodes, scoliosis, and heart failure of unclear cause at adult age. Unfortunately, we were unable to confirm this information through direct contact with the affected family members or review of medical records of the other family members. Also, we could not obtain any DNA from additional family members. This family originates from a population group in the Balkan region of Europe where consanguinity is more frequent, which may make recessive inheritance conceivable, however the distribution of affected members in the pedigree is not easily compatible with recessive inheritance (supplemental Figure III).
Familial clustering of stroke related to cardio-embolic pattern (families 15–17)
The proband of family16 carried an intronic variant at GLA c.−30G>A which we examined further because Fabry disease may be caused by intronic mutations in GLA. The proband and a sibling, previously healthy individuals, experienced cardioembolic strokes at 53 and 55 years. They both had a history of paroxysmal atrial fibrillation but no other classical risk factors. They were treated with anticoagulant therapy. Alpha-galactosidase activity was normal, indicating that these patients did not have Fabry disease (supplemental Figure IV). No uncommon variant in a stroke-gene was detected for the other probands with CE/paroxysmal atrial fibrillation episodes phenotype. Stroke of cardioembolic subtype, according to CCS11 was considered to be present in three of the 22 investigated probands (families 15–17). The proband of family17 had a patent foramen ovale (PFO) resulting in a CE classification according to CCS but also fulfilling the ESUS definition.12
Familial clustering of stroke not related to a determined subtype (families 18–19)
The probands presented non-lacunar stroke episodes in normal, in territories apparently healthy large arteries, supposed to be of embolic nature (cryptogenic stroke). Consequently, the filtering process for this probands was broader, including both coagulation and possible cardioembolic pathomechanisms (Figure 3; supplemental Figure VIII).
Familial clustering of stroke related to coagulation disorders (families 20–21)
Two probands and their families developed stroke probably related to hyper-coagulation status. The stroke episodes in these two probands also fulfilled the ESUS definition.12 In family20, the affected individuals developed coagulation factor VII deficiency and IS-episodes.21 No specific genetic defect in a known stroke-gene was identified in this family. However, the proband in family21, was carrier of the JAK2 c.3188G>A (p.Arg1063His) mutation which has been related to venous thromboembolism.22 Two family members had experienced stroke and/or TIA episodes at age of 29 and 32 years. One of them also experienced deep vein thrombosis (DVT) episode. Spontaneous miscarriages in the second trimester of pregnancy were reported by four family members. Cardiac and large artery examinations of the proband were normal and classical risk factors for stroke were absent. The JAK2 variant was present in two affected and absent in one unaffected relatives, while one unaffected family member was a carrier (supplemental Figure V).
Expanded investigation of the two Finnish families with clustering of ESUS (families 17–18)
Two larger Finnish families with clustering of ESUS at age under 50 years, were analyzed in detail (supplemental Figure VI). No known stroke gene variant that matched the clinical stroke subtype was identified in these two probands.
The proband in family17 experienced a cardioembolic stroke (ESUS) with concurrent pulmonary embolism and PFO, and thus a high likelihood for PFO-mediated paradoxical embolism (RoPE score 8 points).23 At the time of his stroke episode the proband also had increased coagulation factor VIII level, that normalized four months later. The proband also had migraine with aura, but no known classical vascular risk factors. Among the family members, ESUS was identified in three, migraine with aura in seven and DVT in two individuals. Migraine with aura and/or ESUS were defining affected status. The unaffected status was difficult to assess in healthy individuals, considering that the condition might have an incomplete penetrance. Even though migraine with aura could possibly be part of the disease phenotype, members with migraine but not stroke were disregarded in the co-segregation analysis. Segregation of WES data from the three affected and one unaffected individuals in the family identified one rare variant: GPR142 c.148C>G (p.Leu50Val), rs139285923 (supplemental Figure VI).
The proband in family18 experienced ESUS in the context of status migrainosus lasting for six days. The proband had known migraine with visual aura, and did not have any classical risk factors for stroke. One parent had similar symptoms, and this parent’s parent had a clinical lacunar syndrome at 66 years but according to CCS an undetermined stroke subtype. Comparative analysis of WES data from four family members, two with ESUS, one presumed obligate carrier and one healthy individual, identified five rare variants: PTPRN2 c.2416A>G(p.Ile806Val), LRRC1 c.808A>G(p.Ile270Val), SLC7A10 c.1294dupG(p.Val432fs), IKBKB c.1070C>T(p.Ala357Val), and OXGR1 c.392G>A(p.Arg131His), (supplemental Figure VI).
Discussion
The present study focused on individuals with stroke for whom genetic testing for monogenic disease might be considered in clinical practice because of the young age and positive family history. By using WES and comprehensive stroke-gene panels7 we identified rare variants compatible with the clinical stroke subtype in only 27% (6/22) of the probands. In most families, genetic analysis of the probands’ family members did not confirm an association between suspected variants and stroke.
Our findings suggest that some of the initially suspected variants, in fact, are not related to monogenic stroke. One known disease-associated mutation in NOTCH3 in a family with the clinical picture of CADASIL, and one variant possibly relevant for stroke-causing coagulation abnormality (JAK2 c.3188G>A) were identified.
The absence of an identifiable genetic cause among all stroke-associated genes known today might suggest that a monogenic pattern of inheritance may be more often related to variants in other yet unrecognized genes. We expanded our analyses of the two larger Finnish pedigrees with the aim to find novel monogenic causes for stroke, performing WES in several members, and identified one variant, GPR142 c.148C>G in one family, and in the other family we were able to narrow down the list of candidate variants to five. Of them, two variants, IKBKB c.1070C>T, PTPRN2 c.2416A>G and OXGR1 c.392G>A, are the most infrequent in population databases, only PTPRN2 perfectly segregated with the presumed combined phenotype (ESUS and migraine), while SLC7A10 c.1294dupG is predicted to cause a terminal shift mutation with a premature stop codon. We consider each of these five variants a possible cause of the disorder.
The low rate of identifying reported monogenic variants related to stroke may also be explained by the proband selection in the study, focusing on familial clustering of stroke of yet genetically undiagnosed cause after comprehensive clinical workup at our tertiary centers. Prior to inclusion in our study, one proband with artery dissections had been investigated for mutations associated with arterial/aortic dissection, two probands for CADASIL and one for Fabry disease, with negative results.
We evaluated the pathogenicity of each of the identified genetic variants considering published criteria24 and stroke subtype7 (online supplement). The NOTCH3 c.1672C>T, variant has previously been reported in two families with small vessel disease19 and we considered it the cause of the clinical phenotype in family1. However, the possible contribution of COL4A1 c.161C>T, identified in the same proband and also segregating with disease in this family (supplemental Figure I) is difficult to interpret.
COL4A2 c.3368A>G detected in two probands (supplemental Figure II) initially appeared to be a plausible cause of monogenic stroke-associated disease in these families. Pathogenic mutations in COL4A1 or COL4A2 are well known for a highly variable phenotypic expression, except the presence of white matter intensities on brain images that are usually described.20 Population databases show a relatively high frequency (0.5%) of COL4A2 c.3368A>G. However, previous studies also showed that common COL4A1/COL4A2 variants are associated with cerebral small vessel disease and intracerebral bleeding.25, 26 A potentially pathogenic effect of COL4A2 p.Glu1123Gly has previously been shown through three different functional tests. Two sporadic cases of stroke, deep intracerebral bleeding type related to this specific variant and one familial case with porencephaly are reported in the literature,20, 27, 28 indicating that this relatively common variant may influence the risk for stroke, a common disease. The variant did not segregate with the diseases in the two investigated families. However, this variant may be a low penetrance factor playing a role for polygenic stroke risk.
ACAD9 c.976G>A was identified in family4 (supplemental Figure III). The ACAD9 gene was previously associated with lethal cardiomyopathy in early childhood, encephalopathy and lactic acidosis, and rarely with stroke of undetermined source.29 Pathogenic ACAD9 mutations affect mitochondrial function by severely reducing complex I activity.30 In one previous study, homozygous c.976G>A genotype was in 14 out of 470 controls (genotype frequency 0.0298) and was therefore interpreted as benign.30 However, no information on possible additional health problems of the 470 controls was provided, and in larger population databases, the frequency of homozygous carriers was considerably lower (0.00069, in gnomAD, supplemental Figure III c). The variant was not previously reported in the context of a milder mitochondrial disease phenotype that would for example cause stroke episodes later in adult life or myopathy, as observed among the individuals in family4. The variant might be of interest in the context of familial clustering of stroke because the clinical consequences of the associated condition might be treatable by daily riboflavin administration.30 No other family member has been available for genotyping, and we were unable to resolve the variants or genotypes potential pathogenic role.
A JAK2 c.3188G>A mutation was detected in a young proband with stroke and coagulation defects of unclear cause (family21). The variant was present in two affected and absent in two unaffected relatives, but also found in one relative who was clinically unaffected in the 5th decade, suggesting incomplete penetrance or that stroke may sometimes occur at a later age (supplemental Figure V). JAK2 germline mutations may be related to stroke as a result of thrombocytosis31, 32 or polycythemia, or may associate with prothrombotic tendency with normal platelet and erythrocyte count, through an as yet unknown mechanism.21
Functional studies of blood cells from a germline carrier of c.3188G>A mutation, have suggested this variant to be a weak activator of constitutive JAK2 kinase signaling, leading to erythrocytosis when present with other JAK2 variants,21, 23, 32, 33 and also to be the cause of venous thromboembolism in a patient with and normal blood cell count.21 Similarly, the affected individuals in family21 had normal blood cell counts. This suggests that this variant should be considered in further studies regarding ESUS or in the context of otherwise unexplained pro-thrombotic states.
One rare variant, GPR142 c.148C>G segregated with ESUS in family17. Affected family members also had migraine. GPR142 encodes a G protein-coupled receptor (previously named GPRg1) that is highly expressed in the brain.34 Activated by tryptophan, GPRg1 function is coupled to Gq/11, which mediates blood vessel constriction and platelet adhesion, suggesting that this might indicate a functional link to this family’s phenotype.35, 36 GPR142 also seems to stimulate insulin secretion from pancreatic islet cells, and GPR142 agonists are being studied as novel diabetes treatment.37
Five variants including IKBKB c.1070C>T, LRRC1 c.808A>G and PTPRN2 c.2416A>G, were identified in a family with individuals with ESUS (family18). IKBKB is known to be involved in nuclear factor kappa B signaling and inflammatory processes.38, 39 PTPRN2, tyrosine-protein phosphatase N2 (also known as ICAAR or phogrin) is a major autoantigen associated with insulin-dependent diabetes mellitus38, while LRRC1 has not been the subject of detailed studies.
Our study’s main limitations are that most of the intronic and other non-coding nuclear genome and the mitochondrial DNA were not examined. The sequential filtering steps used to narrow down the number of variants of interest may have been too strict, and more common pathogenic variants might have been missed.
While co-segregation between disease and variant, as well as familial clustering, might be a chance finding since some of the pedigrees are small and stroke is a common disease, we tried to minimize the risk of misinterpretations by carefully clinically defining specific subtype of stroke in our patients.
Functional studies of the newly identified genetic variants would be useful to investigate potential metabolic mechanisms or pathways related to stroke or underlying diseases. Further, ESUS or CE phenotypes are less straightforward for studying familial aggregation compared with SVD and have been less investigated, as ESUS lacks a well-defined prodromal or intermediate phenotype to determine which family members are affected or not affected.
Conclusions
We studied families where a monogenic cause of stroke appeared likely, and by WES and follow-up genetic examinations analyzed if they carry mutations in known or suspected stroke-associated genes from our comprehensive panels.
Screening for monogenic stroke by using stroke-gene panels may identify rare monogenic variants associated with stroke. However, in most of the families studied, we did not detect or confirm a pathogenic or possibly pathogenic variant in known stroke-genes. Our results suggest there are additional, yet unidentified causes of monogenic stroke. An alternative explanation is that familial clustering of stroke may result from oligogenic or environmental mechanisms. Whole genome analyses may increase the diagnostic yield.
Supplementary Material
Acknowledgements
The authors would like to thank the participating patients and families. We thank Trinette van Vliet, PhD, for producing high-resolution figures. We acknowledge Genome Aggregation Database (gnomAD) and the groups that provided exome and genome variant data to this resource. A full list of contributing groups can be found at https://gnomad.broadinstitute.org/about.
Sources of Funding: This work was supported by Region Skåne, Skåne University Hospital, Lund University, Swedish Government (under the “Avtal om Läkarutbildning och Medicinsk Forskning, ALF”), Swedish Heart and Lung Foundation, Sparbanksstiftelsen Färs och Frosta, Fremasons Lodge of Instruction Eos in Lund, Hans-Gabriel och Alice Trolle-Wachtmeisters Stiftelse för medicinsk forskning, Sahlgrenska University Hospital, University of Gothenburg and Sigrid Juselius Foundation, Region Västra Götaland, all Sweden, Academy of Finland, Helsinki and Uusimaa Hospital District, Finland. The effort of Drs. Kittner and Cole on this project was partly supported by National Institute of Neurological Disorders and Stroke (R01NS100178 and R01NS105150).
Disclosures: Outside the submitted work, Dr Tatlisumak reports personal fees and other from Bayer, Boehringer-Ingelheim, BrainsGate, Bristol Myers Squibb, Pfizer, Sanofi Aventis, Lumosa Pharma, Portola Pharma; Dr Lindgren reports personal fees from Bayer, Astra Zeneca, BMS Pfizer, Portola; Dr Putaala reports grants, personal fees and other from Pfizer, Boehringer-Ingelheim, Abbott, St. Jude Medical, Bayer, Vital Signum, Nokia Technologies, BcB Medical, Bittium, Portola, Terve Media, BusinessFinland; Dr Puschmann personal fees from Elsevier. The other authors report no conflicts.
References
- 1.Flossmann E, Schulz UG, Rothwell PM. Systematic review of methods and results of studies of the genetic epidemiology of ischemic stroke. Stroke. 2004;35:212–227 [DOI] [PubMed] [Google Scholar]
- 2.Lindgren A, Lovkvist H, Hallstrom B, Hoglund P, Jonsson AC, Kristoffersson U, et al. Prevalence of stroke and vascular risk factors among first-degree relatives of stroke patients and control subjects. A prospective consecutive study. Cerebrovasc Dis. 2005;20:381–387 [DOI] [PubMed] [Google Scholar]
- 3.Starby H, Delavaran H, Andsberg G, Lovkvist H, Norrving B, Lindgren A. Multiplicity of risk factors in ischemic stroke patients: Relations to age, sex, and subtype - a study of 2,505 patients from the Lund Stroke Register. Neuroepidemiology. 2014;42:161–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holliday EG, Traylor M, Malik R, Bevan S, Falcone G, Hopewell JC, et al. Genetic overlap between diagnostic subtypes of ischemic stroke. Stroke. 2015;46:615–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ilinca A, Kristoffersson U, Soller M, Lindgren AG. Familial aggregation of stroke amongst young patients in Lund Stroke Register. Eur J Neurol. 2016;23:401–407 [DOI] [PubMed] [Google Scholar]
- 6.Silventoinen K, Hjelmborg J, Moller S, Ripatti S, Skythe A, Tikkanen E, et al. Family aggregation of cardiovascular disease mortality: A register-based prospective study of pooled nordic twin cohorts. Int J Epidemiol. 2017;46:1223–1229 [DOI] [PubMed] [Google Scholar]
- 7.Ilinca A, Samuelsson S, Piccinelli P, Soller M, Kristoffersson U, Lindgren AG. A stroke gene panel for whole-exome sequencing. Eur J Hum Genet. 2019;27:317–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang T, Tan MS, Tan L, Yu JT. Application of next-generation sequencing technologies in neurology. Ann Transl Med. 2014;2:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Putaala J, Martinez-Majander N, Saeed S, Yesilot N, Jakala P, Nerg O, et al. Searching for explanations for cryptogenic stroke in the young: Revealing the triggers, causes, and outcome (SECRETO): Rationale and design. Eur Stroke J. 2017;2:116–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ay H, Benner T, Arsava EM, Furie KL, Singhal AB, Jensen MB, et al. A computerized algorithm for etiologic classification of ischemic stroke: The Causative Classification of Stroke system. Stroke. 2007;38:2979–2984 [DOI] [PubMed] [Google Scholar]
- 12.Hart RG, Diener HC, Coutts SB, Easton JD, Granger CB, O’Donnell MJ, et al. Embolic Strokes of Undetermined Source: The case for a new clinical construct. The Lancet. Neurology. 2014;13:429–438 [DOI] [PubMed] [Google Scholar]
- 13.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The Genome Analysis ToolKit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang K, Li M, Hakonarson H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.1000 Genomes Project Consortium, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, et al. A global reference for human genetic variation. Nature. 2015;526:68–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ameur A, Dahlberg J, Olason P, Vezzi F, Karlsson R, Martin M, et al. Swegen: A whole-genome data resource of genetic variability in a cross-section of the swedish population. Eur J Hum Genet. 2017;25:1253–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landrum MJ, Lee JM, Benson M, Brown G, Chao C, Chitipiralla S, et al. ClinVar: Public archive of interpretations of clinically relevant variants. Nucleic Acids Res. 2016;44:D862–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, et al. NOTCH3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383:707–710 [DOI] [PubMed] [Google Scholar]
- 20.Jeanne M, Gould DB. Genotype-phenotype correlations in pathology caused by collagen type IV alpha 1 and 2 mutations. Matrix Biol. 2017;57–58: 29–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Girolami A, Ferrari S, Cosi E, Santarossa C, Randi ML. Vitamin K-dependent coagulation factors that may be responsible for both bleeding and thrombosis (FII, FVII, and FIX). Clin Appl Thromb Hemost. 2018:1076029618811109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee EJ, Dykas DJ, Leavitt AD, Camire RM, Ebberink E, Garcia de Frutos P, et al. Whole-exome sequencing in evaluation of patients with venous thromboembolism. Blood Adv. 2017;1:1224–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiladjian JJ, Cervantes F, Leebeek FW, Marzac C, Cassinat B, Chevret S, et al. The impact of JAK2 and MPL mutations on diagnosis and prognosis of splanchnic vein thrombosis: A report on 241 cases. Blood. 2008;111:4922–4929 [DOI] [PubMed] [Google Scholar]
- 24.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rannikmae K, Davies G, Thomson PA, Bevan S, Devan WJ, Falcone GJ, et al. Common variation in COL41A/COL4A2 is associated with sporadic cerebral small vessel disease. Neurology. 2015;84:918–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rannikmae K, Sivakumaran V, Millar H, Malik R, Anderson CD, Chong M, et al. COL4A2 is associated with lacunar ischemic stroke and deep ICH: Meta-analyses among 21,500 cases and 40,600 controls. Neurology. 2017;89:1829–1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeanne M, Labelle-Dumais C, Jorgensen J, Kauffman WB, Mancini GM, Favor J, et al. COL4A2 mutations impair COL4A1 and COL4A2 secretion and cause hemorrhagic stroke. Am J Hum Genet. 2012;90:91–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meuwissen ME, Halley DJ, Smit LS, Lequin MH, Cobben JM, de Coo R, et al. The expanding phenotype of COL4A1 and COL4A2 mutations: Clinical data on 13 newly identified families and a review of the literature. Genet Med. 2015;17:843–853 [DOI] [PubMed] [Google Scholar]
- 29.He M, Rutledge SL, Kelly DR, Palmer CA, Murdoch G, Majumder N, et al. A new genetic disorder in mitochondrial fatty acid beta-oxidation: ACAD9 deficiency. Am J Hum Genet. 2007;81:87–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haack TB, Danhauser K, Haberberger B, Hoser J, Strecker V, Boehm D, et al. Exome sequencing identifies ACAD9 mutations as a cause of complex i deficiency. Nat Genet. 2010;42:1131–1134 [DOI] [PubMed] [Google Scholar]
- 31.Lanikova L, Babosova O, Swierczek S, Wang L, Wheeler DA, Divoky V, et al. Coexistence of gain-of-function jak2 germ line mutations with JAK2V617F in polycythemia vera. Blood. 2016;128:2266–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mead AJ, Rugless MJ, Jacobsen SE, Schuh A. Germline JAK2 mutation in a family with hereditary thrombocytosis. N Engl J Med. 2012;366:967–969 [DOI] [PubMed] [Google Scholar]
- 33.Kapralova K, Horvathova M, Pecquet C, Fialova Kucerova J, Pospisilova D, Leroy E, et al. Cooperation of germ line JAK2 mutations E846D and R1063H in hereditary erythrocytosis with megakaryocytic atypia. Blood. 2016;128:1418–1423 [DOI] [PubMed] [Google Scholar]
- 34.Matsuo A, Matsumoto S, Nagano M, Masumoto KH, Takasaki J, Matsumoto M, et al. Molecular cloning and characterization of a novel Gq-coupled orphan receptor GPRg1 exclusively expressed in the central nervous system. Biochem Biophys Res Commun. 2005;331:363–369 [DOI] [PubMed] [Google Scholar]
- 35.Mircic GM, Beleslin DB, Jankovic SM. [Hormones of the posterior region of the hypophyseal gland]. Srp Arh Celok Lek. 1998;126:111–118 [PubMed] [Google Scholar]
- 36.Murakoshi M, Kuwabara H, Nagasaki M, Xiong YM, Reagan JD, Maeda H, et al. Discovery and pharmacological effects of a novel GPR142 antagonist. J Recept Signal Transduct Res. 2017;37:290–296 [DOI] [PubMed] [Google Scholar]
- 37.Zhang G, Li J, Purkayastha S, Tang Y, Zhang H, Yin Y, et al. Hypothalamic programming of systemic ageing involving IKK-beta, KF-kappaB and GnRH. Nature. 2013;497:211–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu J, Li Q, Xie H, Chen ZJ, Borovitskaya AE, Maclaren NK, et al. Identification of a second transmembrane protein tyrosine phosphatase, IA-2beta, as an autoantigen in insulin-dependent diabetes mellitus: Precursor of the 37-kDa tryptic fragment. Proc Natl Acad Sci U S A. 1996;93:2307–2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pannicke U, Baumann B, Fuchs S, Henneke P, Rensing-Ehl A, Rizzi M, et al. Deficiency of innate and acquired immunity caused by an IKBKB mutation. N Engl J Med. 2013;369:2504–2514 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.