Abstract
Background
Little is known about the practice of ventilation management in patients with COVID-19. We aimed to describe the practice of ventilation management and to establish outcomes in invasively ventilated patients with COVID-19 in a single country during the first month of the outbreak.
Methods
PRoVENT-COVID is a national, multicentre, retrospective observational study done at 18 intensive care units (ICUs) in the Netherlands. Consecutive patients aged at least 18 years were eligible for participation if they had received invasive ventilation for COVID-19 at a participating ICU during the first month of the national outbreak in the Netherlands. The primary outcome was a combination of ventilator variables and parameters over the first 4 calendar days of ventilation: tidal volume, positive end-expiratory pressure (PEEP), respiratory system compliance, and driving pressure. Secondary outcomes included the use of adjunctive treatments for refractory hypoxaemia and ICU complications. Patient-centred outcomes were ventilator-free days at day 28, duration of ventilation, duration of ICU and hospital stay, and mortality. PRoVENT-COVID is registered at ClinicalTrials.gov (NCT04346342).
Findings
Between March 1 and April 1, 2020, 553 patients were included in the study. Median tidal volume was 6·3 mL/kg predicted bodyweight (IQR 5·7–7·1), PEEP was 14·0 cm H2O (IQR 11·0–15·0), and driving pressure was 14·0 cm H2O (11·2–16·0). Median respiratory system compliance was 31·9 mL/cm H2O (26·0–39·9). Of the adjunctive treatments for refractory hypoxaemia, prone positioning was most often used in the first 4 days of ventilation (283 [53%] of 530 patients). The median number of ventilator-free days at day 28 was 0 (IQR 0–15); 186 (35%) of 530 patients had died by day 28. Predictors of 28-day mortality were gender, age, tidal volume, respiratory system compliance, arterial pH, and heart rate on the first day of invasive ventilation.
Interpretation
In patients with COVID-19 who were invasively ventilated during the first month of the outbreak in the Netherlands, lung-protective ventilation with low tidal volume and low driving pressure was broadly applied and prone positioning was often used. The applied PEEP varied widely, despite an invariably low respiratory system compliance. The findings of this national study provide a basis for new hypotheses and sample size calculations for future trials of invasive ventilation for COVID-19. These data could also help in the interpretation of findings from other studies of ventilation practice and outcomes in invasively ventilated patients with COVID-19.
Funding
Amsterdam University Medical Centers, location Academic Medical Center.
Introduction
COVID-19 is caused by the highly contagious severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the first outbreak of which was reported in Wuhan, China, at the beginning of December, 2019.1 Since then, it has spread rapidly across the world, with hundreds of thousands of new cases each day as of late June, 2020.2 Worldwide, health-care workers have faced surges of infected patients who need hospital care and who are eventually admitted to an intensive care unit (ICU) to receive invasive ventilation.3
Large differences in outcomes for invasively ventilated patients with COVID-19 have been reported for different countries—eg, mortality rates for these patients in China1 were reported to be two-times higher than those in Italy4, 5 and the USA6, 7—and even within a single country, such as the UK.8 Several ventilatory interventions, such as lung-protective ventilation with a low tidal volume9 and a low driving pressure,10 high positive end-expiratory pressure (PEEP) with recruitment manoeuvers,11, 12 prone positioning,13 and extracorporeal membrane oxygenation (ECMO)14, 15 affect case fatality in patients with acute respiratory distress syndrome (ARDS). It is not clear how these interventions are applied in routine practice in patients with ARDS related to COVID-19. Differences in outcomes motivate urgent comparative research to characterise between-country differences to inform best practice in the context of a surge of cases.
We did the PRactice of VENTilation in COVID-19 study (PRoVENT-COVID) to describe ventilation management, epidemiological characteristics, and outcomes in invasively ventilated patients with COVID-19 in the Netherlands. The primary objective was to compare invasive ventilation settings and parameters over the first 4 days of ventilation in the ICUs of hospitals across the country. We also aimed to establish whether some ventilation settings and parameters have an independent association with the duration of ventilation and clinical outcomes.
Research in context.
Evidence before this study
We searched MEDLINE, Embase, CINAHL, and Web of Science on Aug 26, 2020, with the terms “mechanical ventilation” AND (”coronavirus” OR ”COVID-19”), with no date or language restrictions. Studies including patients not receiving ventilation were excluded, as were those reporting on paediatric and single-centre populations. Only two studies reported detailed ventilator settings and outcomes, which were multicentre observational studies in ventilated patients with COVID-19, including one in Italy with 1150 patients and one in Spain with 742 patients. In the Italian study, reporting was restricted to a single timepoint and contained only information on positive end-expiratory pressure (PEEP), adjunctive treatments for refractory hypoxaemia, and oxygenation parameters. The Spanish study reported values during the whole period of ventilatory support, but restricted data collection to worst values on each ventilation day. The search did not identify studies that used regression models to identify factors independently associated with outcome, or studies assessing outcomes after 28 days.
Added value of this study
Our study provides a detailed description of various important ventilation variables and parameters, adjunctive treatments for refractory hypoxaemia, and patient characteristics and outcomes in a large set of hospitals in the Netherlands. Furthermore, we report on these variables and parameters over 4 consecutive calendar days, which allows insight into ventilation practice over time. Our study was retrospective but included consecutive patients in the first month of the outbreak in the Netherlands. In contrast to several studies in patients with COVID-19, ours included mortality at day 90.
Implications of all the available evidence
Most patients receiving invasive ventilation for respiratory failure due to COVID-19 had moderate or severe acute respiratory distress syndrome. Respiratory system compliance was low in all patients. Protective ventilation was used often, especially with regard to the use of lower tidal volumes and the use of prone positioning as an adjunctive treatment for refractory hypoxaemia. The level of PEEP varied widely and did not change over the first days of ventilation. Of the various ventilatory variables, higher tidal volume and lower compliance in the first day of ventilation were associated with higher 28-day mortality. Mortality was high, but similar to that reported for other cohorts. Our results add to existing knowledge about epidemiological characteristics and outcomes and could be useful in planning future studies and understanding previous findings about invasive ventilation in patients with COVID-19.
Methods
Study design and participants
PRoVENT-COVID is an investigator-initiated, national, multicentre, observational cohort study done at 18 ICUs in the Netherlands (appendix p 2). The study protocol, including the statistical analysis plan, is provided in the appendix (pp 4–20).16 Study sites were recruited through direct contact by members of the PRoVENT-COVID steering committee. The study coordinators (MB and AMT) contacted local doctors, who sought approval from their respective institutional review boards or research ethics committees. Need for individual informed consent was waived because of the observational nature of this investigation. The study coordinators and trained data collectors assisted local doctors, and monitored the study according to the International Conference on Harmonization's Good Clinical Practice Guideline. The study coordinators ensured the integrity and timely completion of data collection.
Consecutive patients aged 18 years or older were eligible for participation in PRoVENT-COVID if they were admitted to one of the participating ICUs and had received invasive ventilation for respiratory failure related to COVID-19. COVID-19 had to be confirmed by RT-PCR, or highly suspected on the basis of the presence of typical abnormalities on chest CT17 in the absence of an alternative diagnosis. We excluded patients who received only non-invasive ventilation, and patients who were transferred to a non-participating ICU within 1 h after intubation and the start of invasive ventilation. Here, we report on patients admitted in the first month of the COVID-19 outbreak in the Netherlands, from March 1 to April 1, 2020.
Procedures
Local doctors and data collectors obtained baseline and demographic variables, including the available disease severity scores, which could be the Acute Physiology and Chronic Health Evaluation II or IV, the Simplified Acute Physiology Score II, or the Sequential Organ Failure Assessment (SOFA) score. Chest CT scan and chest radiography images were scored by trained data collectors for extent of lung involvement: chest CT scans were scored as having 0%, 25%, 50%, 75%, or 100% involvement; chest radiographs were scored as having opacities in one, two, three, or four quadrants. ARDS severity was classified according to the current Berlin definition for ARDS.18
We defined day 0 as the first calendar day that a patient received invasive ventilation in a participating ICU, irrespective of hospital or ICU admission date. For each day until day 28, hospital discharge, or death, an assessment was made as to whether a patient was under invasive ventilation. We counted a ventilation day as any day that a patient was under invasive ventilation, irrespective of the duration of invasive ventilation for that day and whether or not it was done through an orotracheal tube or a tracheostomy.
Local doctors and data collectors captured detailed information regarding ventilation management up to day 3 (ie, the first 4 days of invasive ventilation), and pulmonary and extrapulmonary events until hospital discharge, up to a maximum of 28 days. In the first hour of invasive ventilation and every 8 h thereafter, at fixed timepoints until day 3, ventilator settings and parameters, use of muscle paralysis, vital signs, and arterial lactate levels were captured. Once a day until day 3, SOFA score (if available), use of sedation, use of vasopressors, cumulative fluid balance, urine output, and highest plasma creatinine were collected. Use of adjunctive treatments for refractory hypoxaemia, including recruitment manoeuvres, prone positioning, and ECMO, was recorded until day 3.
Additionally, for each day until day 28, hospital discharge, or death, an assessment was made as to whether a patient was under invasive ventilation and whether they had developed complications such as pneumothorax or thromboembolic events, including deep venous thrombosis and pulmonary embolism, and kidney injury and the need for renal replacement therapy. Dates of ICU discharge and hospital discharge were captured, as was the date of death.
All data were entered into a password-secured, internet-based, electronic case report form (Castor EDC). Before analysis, the study coordinators screened all data for potentially erroneous or incomplete recordings and verified and corrected information, as appropriate, with the help of local doctors and data collectors. After cleaning, the database was closed for analysis.
Outcomes
The primary outcome was a combination of the main ventilator settings during the first 4 calendar days of invasive ventilation, including tidal volume, PEEP, respiratory system compliance, and driving pressure. Secondary outcomes included other ventilation variables and parameters (appendix p 13), the use of adjunctive treatments for refractory hypoxaemia, and ICU complications. Patient-centred outcomes were the number of ventilator-free days at day 28, duration of ventilation, ICU and hospital length of stay, and 28-day and 90-day mortality rates. Ventilator-free days were defined as calendar days of unassisted breathing for at least 24 consecutive hours and considering the last date of successful extubation; all patients who had died by day 28 were considered to have had no ventilator-free days.19
Statistical analysis
We have presented ventilation settings for all patients and focused on the first 4 calendar days in the first month of the national outbreak. A convenience sample was considered for this analysis, with consecutively included patients. We did not adjust for multiplicity across analyses; therefore, we do not claim confirmatory statistical evidence. Thus, these findings should be interpreted as exploratory.
Continuous variables are presented as medians (IQR) and categorical variables as number and percentages. Ventilatory variables and parameters over the first 4 calendar days are shown in cumulative distribution plots and in line graphs. To compare ventilatory variables over time, a mixed-effects model considering centres as a random effect was used, with random intercept and slopes for participants and with an unstructured covariance matrix.
Time to extubation within first 28 days was presented in cumulative incidence plots with death before extubation accounted for as a competing risk. In addition, 28-day survival was plotted in a Kaplan-Meier curve. Patients discharged from the hospital to home before day 28 were considered to be alive and without ventilation at day 28.
A mixed-effects multivariable logistic or linear regression model was used to identify factors independently associated with 28-day mortality and ventilator-free days. A list of candidate baseline predictors was established a priori and considered only variables with a known or suspected relationship with these outcomes. The following variables were included in the multivariable model based on clinical relevance only: (1) ventilatory and oxygenation variables in the first day aggregated as the median from a maximum of six assessments (PEEP, tidal volume, respiratory system compliance, and the ratio of partial pressure of arterial oxygen to fractional concentration of oxygen in inspired air [PaO2/FiO2]); (2) laboratory tests and vital signs in the first day aggregated as the median from a maximum of six assessments (arterial pH, lactate, creatinine, heart rate, and mean arterial pressure); (3) organ support at the first day (use of vasopressor and fluid balance); and (4) demographic characteristics (age, gender, body-mass index, hypertension, heart failure, diabetes, chronic kidney disease, chronic obstructive pulmonary disease, use of angiotensin-converting enzyme inhibitor, and use of angiotensin II receptor blockers). To assess multicollinearity, first the correlation between the continuous variables was assessed in a correlation matrix (appendix p 29). Peak pressure and driving pressure were excluded because of collinearity with respiratory compliance (which was judged to be more important in the model) and FiO2 was excluded because of collinearity with PaO2/FiO2. In addition, multicollinearity in the final models was assessed using variance-inflation factors. The linearity assumption of continuous variables in the logistic model was assessed through the Box-Tidwell transformation considering the full model, testing the log-odds and the predictor variable in the 28-day mortality model (appendix p 21). In the linear model, the linearity was assessed by plotting the ventilator-free days at day 28 against the predictor and comparing a locally estimated scatterplot smoothing curve against a linear regression curve (appendix p 30). Variables not satisfying this criterion were entered as restricted cubic splines in the final model. The full model is provided in the appendix (pp 25–26). However, to improve interpretation of the model, we report the odds ratio and the mean difference of the variables included as restricted cubic splines determined over the quartile range observed for the variable (estimated effect of an IQR increase in the predictor variable) and the p value reported is for the first spline. The final logistic model was assessed for discrimination using C statistics, and for calibration using calibration belt and the Brier score. The final linear model was assessed using the conditional R2 (coefficient of determination). The normality of the residuals for the model for ventilator-free days at day 28 was assessed using quantile–quantile plots. All continuous variables were entered after standardisation to improve convergence of the model, and all effect estimates show the increase in one SD of the variable. Missing data in continuous predictors considered in the models was present in less than 5% of the patients; thus, these values were imputed by the median. As a post-hoc analysis, the clinical outcomes were presented according to ARDS severity at the start of invasive ventilation.
All analyses were done in R version 4.0.2 and significance level was set at 0·05. The study is registered at ClinicalTrials.gov (NCT04346342).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The writing and steering committee members had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
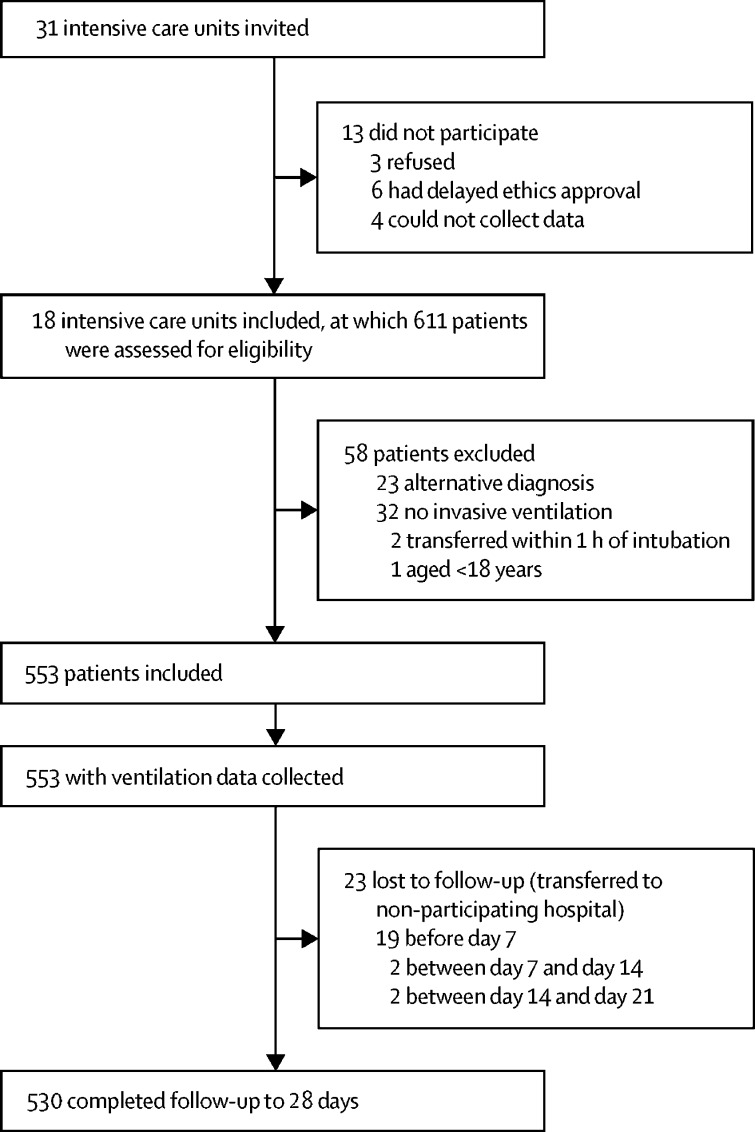
Between March 1 and April 1, 2020, 31 ICUs were invited to participate in PRoVENT-COVID, and 18 met inclusion criteria (appendix p 2; figure 1 ). Of 611 individuals screened for the study, 553 patients were included; the main reasons for exclusion were that they did not receive invasive ventilation or had an alternative diagnosis. We recruited fewer patients than the planned 1000 because we specifically assessed the first month of the pandemic in the Netherlands because there was an urgent need for information. All patients had ARDS according to the Berlin definition, and all patients had a positive RT-PCR for SARS-CoV-2 during their hospital stay. Patients were followed up for a median of 28·0 days (IQR 15·4–28·0; 9·4 days [IQR 5·4–17·4] in non-survivors). 417 (75%) patients were men and 136 (25%) were women, median age was 67 years (IQR 59–73), and common comorbidities were hypertension (200 [36%] patients) and diabetes (111 [20%] patients; table 1 ). 106 (19%) patients were using an angiotensin-converting enzyme inhibitor and 113 (20%) were taking a β blocker. 43% of patients had extensive lung involvement on chest images (136 of 318 patients who had an x-ray), and most were classified as having moderate or severe ARDS (406 [75%] of 541). The amount of missing data was low for most variables (appendix pp 22–23).
Figure 1.
Study profile
Follow-up to 90 days was completed in 495 patients.
Table 1.
Baseline patient characteristics
| All participants (n=553) | |||
|---|---|---|---|
| Age, years | 67·0 (59·0–73·0) | ||
| Gender | |||
| Men | 417/553 (75%) | ||
| Women | 136/553 (25%) | ||
| Body-mass index, kg/m2 | 27·7 (25·1–30·4) | ||
| Transferred from another intensive care unit under invasive ventilation | 104/553 (19%) | ||
| Duration of invasive ventilation before admission, days | 0·0 (0·0–2·0) | ||
| Use of non-invasive ventilation | 51/489 (10%) | ||
| Duration of non-invasive ventilation, h | 8·0 (3·8–13·9) | ||
| Chest CT scan performed | 146/553 (26%) | ||
| Lung parenchyma affected | |||
| 0% | 8/146 (5%) | ||
| 25% | 46/146 (32%) | ||
| 50% | 39/146 (27%) | ||
| 75% | 46/146 (32%) | ||
| 100% | 7/146 (5%) | ||
| Chest x-ray performed | 321/553 (87%) | ||
| Number of quadrants affected* | |||
| 1 | 16/318 (5%) | ||
| 2 | 69/318 (22%) | ||
| 3 | 97/318 (31%) | ||
| 4 | 136/318 (43%) | ||
| Pneumothorax† | 1/129 (1%) | ||
| Severity of illness | |||
| SAPS II (n=198) | 38·0 (31·0–45·0) | ||
| APACHE II (n=146) | 16·0 (12·0–20·0) | ||
| APACHE IV (n=271) | 57·0 (45·0–70·5) | ||
| SOFA (n=253) | 8·0 (6·0–11·0) | ||
| Severity of acute respiratory distress syndrome‡ | |||
| Mild | 135/541 (25%) | ||
| Moderate | 360/541 (67%) | ||
| Severe | 46/541 (9%) | ||
| Co-existing disorders | |||
| Hypertension | 200/553 (36%) | ||
| Heart failure | 25/553 (5%) | ||
| Diabetes | 111/553 (20%) | ||
| Chronic kidney disease | 23/553 (4%) | ||
| Baseline creatinine, μmol/L§ | 79·0 (64·0–98·0) | ||
| Liver cirrhosis | 2/553 (<1%) | ||
| Chronic obstructive pulmonary disease | 52/553 (9%) | ||
| Active haematological neoplasia | 8/553 (1%) | ||
| Active solid neoplasia | 13/553 (2%) | ||
| Neuromuscular disease | 8/553 (1%) | ||
| Immunosuppression | 12/553 (2%) | ||
| Previous medication | |||
| Systemic steroids | 27/553 (5%) | ||
| Inhalation steroids | 67/553 (12%) | ||
| Angiotensin-converting enzyme inhibitor | 106/553 (19%) | ||
| Angiotensin II receptor blocker | 64/553 (12%) | ||
| β blockers | 113/553 (20%) | ||
| Insulin | 31/553 (6%) | ||
| Metformin | 74/553 (13%) | ||
| Statins | 174/553 (31%) | ||
| Calcium channel blockers | 104/553 (19%) | ||
Data are median (IQR) or n/N (%). APACHE=Acute Physiology and Chronic Health Evaluation. SAPS=Simplified Acute Physiology Score. SOFA=Sequential Organ Failure Assessment.
321 patients had a chest x-ray, but three are not included here because original images could not be accessed for analysis.
Data was available for 129 patients.
Baseline ratio of partial pressure of arterial oxygen to fractional concentration of oxygen in inspired air was missing in 12 patients.
Most recent measurement in the 24 h before intubation or at ICU admission under invasive ventilation.
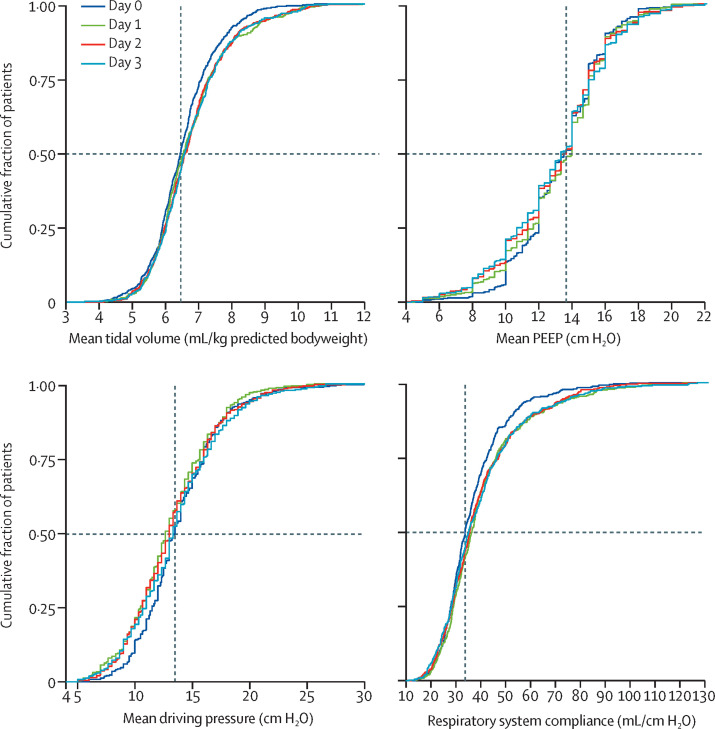
The most common ventilation mode in the first day of invasive ventilation was pressure-controlled ventilation, followed by volume-controlled ventilation (table 2 ). Median tidal volume was 6·3 mL/kg predicted bodyweight (IQR 5·7–7·1), and 289 (58%) of 501 patients had a tidal volume of 6 mL/kg predicted bodyweight or less (figure 2 ; appendix pp 31–36). Median PEEP was 14·0 cm H2O (IQR 11·0–15·0), driving pressure was 14·0 cm H2O (IQR 11·2–16·0), and mechanical power of ventilation was 17·7 J/min (IQR 14·2–22·3). Median respiratory system compliance was 31·9 mL/cm H2O (IQR 26·0–39·9), with a near normal distribution (appendix p 31). Median PaO2/FiO2 was 158·8 (IQR 128·6–200·5), with 46 (9%) of 541 patients having PaO2/FiO2 less than 100. Of all variables and parameters, only blood gas analysis results and FiO2 changed over the first 2 days of ventilation (appendix pp 33–34).
Table 2.
Characteristics of advanced life support in the first day of ventilation
| All participants (n=553) | ||
|---|---|---|
| Ventilation support | ||
| Mode of ventilation | ||
| Volume-controlled ventilation | 104/551 (19%) | |
| Pressure-controlled ventilation | 284/551 (52%) | |
| Pressure-support ventilation | 21/551 (4%) | |
| Synchronised intermittent mandatory ventilation | 36/551 (7%) | |
| Airway pressure release ventilation | 18/551 (3%) | |
| INTELLiVENT adaptive support ventilation | 32/551 (6%) | |
| Other | 56/551 (10%) | |
| Tidal volume, mL/kg predicted bodyweight | 6·3 (5·7–7·1) | |
| Positive end-expiratory pressure, cm H2O | 14·0 (11·0–15·0) | |
| Peak pressure, cm H2O | 27·0 (24·0–31·0) | |
| Driving pressure, cm H2O | 14·0 (11·2–16·0) | |
| Mechanical power, J/min | 17·7 (14·2–22·3) | |
| Respiratory system compliance, mL/cm H2O | 31·9 (26·0–39·9) | |
| Total respiratory rate, breaths per min | 20·0 (18·0–24·0) | |
| FiO2 | 0·60 (0·50–0·80) | |
| SpO2/FiO2 | 152·9 (118·7–190·0) | |
| End tidal CO2, mm Hg | 36·8 (32·0–42·8) | |
| Rescue treatments for refractory hypoxaemia | ||
| Prone positioning | 135/544 (25%) | |
| Duration of prone positioning, h | 8·0 (4·0–12·0) | |
| Recruitment manoeuver | 5/444 (1%) | |
| Use of neuromuscular blockade | 126/532 (24%) | |
| Duration of neuromuscular blockade, h | 8·0 (8·0–16·0) | |
| Extracorporeal membrane oxygenation | 1/554 (<1%) | |
| Vital signs | ||
| Mean arterial pressure, mm Hg | 84·0 (74·0–98·9) | |
| Heart rate, beats per min | 89·0 (76·0–102·0) | |
| Laboratory tests | ||
| pH | 7·36 (7·30–7·42) | |
| PaO2, mm Hg | 83·3 (71·1–101·3) | |
| PaO2/FiO2 | 158·8 (128·6–200·5) | |
| Partial pressure of CO2, mm Hg | 43·5 (37·5–51·0) | |
| Lactate, mmol/L | 1·1 (0·9–1·4) | |
| Creatinine, μmol/L | 74·0 (62·0–98·0) | |
| Other | ||
| Continuous sedation | 532/551 (97%) | |
| Use of neuromuscular blockade | 126/532 (24%) | |
| Duration of neuromuscular blockade, h* | 8·0 (8·0–16·0) | |
| Vasopressor use | 430/551 (78%) | |
| Fluid balance, mL | 584·0 (32·7–1327·5) | |
| Urine output, mL | 635·0 (335·0–1130·0) | |
Data are median (IQR) or n/N (%). FiO2=fractional concentration of oxygen in inspired air. PaO2=partial pressure of arterial oxygen. SpO2=oxygen saturation.
In patients who received continuous infusion of a neuromuscular blocking agent.
Figure 2.
Ventilation parameters
Cumulative frequency distribution of tidal volume, PEEP, driving pressure, and respiratory system compliance. Vertical dotted lines represent the median on the first calendar day of ventilation for each variable, and horizontal dotted lines show the respective proportion of patients reaching each cutoff. PEEP=positive end-expiratory pressure.
Recruitment manoeuvres were seldom performed (appendix p 24). In the first 4 days of ventilation, 283 (53%) of 530 patients received at least one session of prone positioning, with a median duration of 13·0 h (IQR 10·5–18·0). Baseline PaO2/FiO2 was significantly lower in patients who received prone positioning (163·8 [131·4–192·1]) than in those who did not (181·0 [157·0–214·4]; p<0·0001). Worst PaO2/FiO2 in patients who received prone positioning was lower than that in patients who did not receive prone positioning (97·0 [80·6–124·7] vs 120·0 [100·2–142·9]; p<0·0001). Of 239 patients with PaO2/FiO2 less than 150 at baseline, 143 (60%) received prone positioning for refractory hypoxaemia; of 449 patients with PaO2/FiO2 less than 150 at any observation point within the first 4 days of invasive ventilation, 234 (52%) received prone positioning. ECMO was used in two patients; no patient was transferred to another hospital for ECMO.
Continuous sedation and muscle paralysis were used often and for most patients for the first 4 calendar days (table 2; appendix p 24). Vasopressors were used in most patients, and there was a progressive increase in cumulative fluid balance and in plasma creatinine levels over the 4 days (table 2; appendix pp 24, 37). One in five patients developed a thromboembolic complication, mainly pulmonary embolism; almost half of the patients developed acute kidney injury, and one in six needed renal replacement therapy (table 3 ).
Table 3.
Clinical outcomes
| All participants (n=553) | ||
|---|---|---|
| Ventilatory support | ||
| Ventilator-free days at day 28 | 0·0 (0·0–15·0) | |
| Mean (SD) | 6·8 (8·5) | |
| Successful extubation | 266/553 (48%) | |
| Duration of ventilation, days | 13·5 (7·5–22·5) | |
| In survivors at ICU discharge, days | 16·5 (10·5–26·5) | |
| Tracheostomy | 74/553 (13%) | |
| Reintubation | 70/546 (13%) | |
| Pneumothorax | 6/542 (1%) | |
| Complications | ||
| Thromboembolic complications | 118/552 (21%) | |
| Pulmonary embolism* | 75/552 (14%) | |
| Deep vein thrombosis | 25/552 (5%) | |
| Ischaemic stroke | 13/552 (2%) | |
| Myocardial infarction | 8/552 (1%) | |
| Systemic arterial embolism | 3/552 (1%) | |
| Acute kidney injury† | 259/553 (47%) | |
| Need for renal replacement therapy | 93/552 (17%) | |
| Clinical outcomes | ||
| ICU length of stay, days | 14·0 (8·0–24·0) | |
| In survivors at ICU discharge, days | 18·0 (10·0–30·0) | |
| Hospital length of stay, days | 21·0 (11·5–33·0) | |
| In survivors at hospital discharge, days | 29·0 (20·0–43·0) | |
| Mortality | ||
| Day 7 | 81/533 (16%) | |
| Day 28 | 186/530 (35%) | |
| Day 90 | 214/495 (43%) | |
| ICU | 203/530 (38%) | |
| Hospital | 210/496 (42%) | |
Data are median (IQR) or n/N (%). Outcomes were assessed up to day 28 when not indicated. ICU=intensive care unit.
Pulmonary embolism was defined when confirmed by chest CT angiography or when highly suspicious according to clinical assessment and treated accordingly by the attending physician.
Acute kidney injury was defined when at least one of the following criteria was met at any point within 28 days after intubation: (1) a 1·5-times increase in creatinine versus baseline; (2) an absolute creatinine increase of 26·5 μmol/L versus baseline; or (3) a urinary output of less than 0·5 mL/kg per h for more than 6 h.
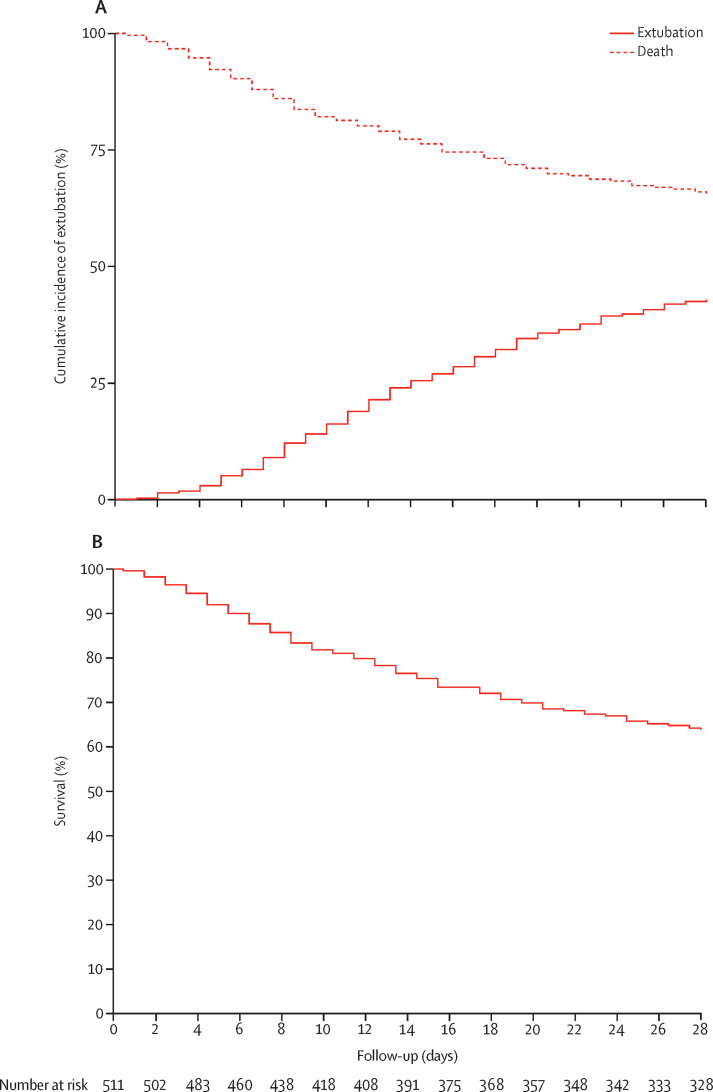
Reintubation was often required and tracheostomy were done in about one in six patients. Patients had a median number of ventilator-free days at day 28 of 0 (IQR 0–15), and duration of ventilation in survivors was 16·5 days (10·5–26·5) versus 13·5 days (7·5–22·5) in all participants (table 3). Mortality increased with increasing age and decreasing PaO2/FiO2 at the start of invasive ventilation (appendix pp 38–39). 186 (35%) of 530 patients had died by day 28 (figure 3 ; table 3). 214 (43%) of 495 patients died by day 90.
Figure 3.
Cumulative incidence of extubation with death before extubation as a competing risk (A) and 28-day survival (B) in the overall cohort (n=530)
After multivariable adjustment, higher age, male gender, lower arterial pH, higher heart rate, higher tidal volume, and lower respiratory system compliance in the first calendar day of ventilation were associated with increased risk of 28-day mortality (table 4 ; appendix pp 25–27, 40–41). Also, after multivariable adjustment, higher age, male gender, not using angiotensin-converting enzyme inhibitor, and lower PaO2/FiO2 were associated with a lower number of ventilator-free days at day 28 (table 4; appendix pp 42–43). Respiratory system compliance was associated with a higher number of ventilator-free days at day 28, but only in the first spline (compliance increasing from 10 mL/cm H2O to 40 mL/cm H2O).
Table 4.
Multivariable model assessing predictors of 28-day mortality and ventilator-free days at day 28
|
28-day mortality |
Ventilator-free days at day 28 |
|||
|---|---|---|---|---|
| Odds ratio (95% CI) | p value | Mean difference (95% CI) | p value | |
| Ventilatory variables on day 0* | ||||
| Positive end-expiratory pressure, cm H2O | 1·08 (0·85 to 1·39) | 0·51 | −0·73 (−1·52 to 0·06) | 0·069 |
| Tidal volume, mL/kg predicted bodyweight | 1·28 (1·00 to 1·64) | 0·049 | −0·35 (−1·15 to 0·45) | 0·39 |
| Respiratory system compliance, mL/cm H2O | 0·75 (0·57 to 0·98) | 0·037 | 0·60 (−1·27 to 2·47)† | 0·016 |
| Oxygenation variables on day 0* | ||||
| PaO2/FiO2 | 0·77 (0·43 to 1·38)† | 0·11 | 1·00 (0·27 to 1·72) | 0·0073 |
| Laboratory tests on day 0* | ||||
| pH | 0·71 (0·55 to 0·93) | 0·012 | 1·78 (−0·11 to 3·68)† | 0·42 |
| Lactate, mmol/L | 1·12 (0·88 to 1·43) | 0·37 | −2·68 (−4·44 to −0·90)† | 0·87 |
| Creatinine, μmol/L | 1·04 (0·82 to 1·32) | 0·76 | −1·09 (−2·99 to 0·82)† | 0·59 |
| Vital signs on day 0* | ||||
| Heart rate, beats per min | 1·02 (1·00 to 1·03) | 0·013 | −0·62 (−1·36 to 0·11) | 0·10 |
| Mean arterial pressure, mm Hg | 0·99 (0·96 to 1·02) | 0·46 | 1·22 (−0·59 to 3·03)† | 0·97 |
| Organ support on day 0 | ||||
| Use of vasopressor | 2·07 (0·76 to 5·66) | 0·16 | 0·80 (−1·80 to 3·40) | 0·54 |
| Fluid balance, mL | 1·07 (0·85 to 1·36) | 0·55 | −0·24 (−1·01 to 0·53) | 0·55 |
| Demographic characteristics | ||||
| Age, years | 2·19 (1·65 to 2·90) | <0·0001 | −2·13 (−2·90 to −1·35) | <0·0001 |
| Male gender | 2·16 (1·24 to 3·78) | 0·0069 | −2·38 (−4·24 to −0·52) | 0·013 |
| Body-mass index, kg/m2 | 0·85 (0·66 to 1·09) | 0·19 | 0·51 (−0·24 to 1·26) | 0·18 |
| Hypertension | 1·16 (0·72 to 1·88) | 0·54 | −0·01 (−1·58 to 1·57) | 0·99 |
| Heart failure | 0·73 (0·26 to 2·08) | 0·56 | 1·22 (−2·03 to 4·46) | 0·46 |
| Diabetes | 1·58 (0·93 to 2·67) | 0·087 | −1·12 (−2·88 to 0·64) | 0·21 |
| Chronic kidney disease | 0·89 (0·30 to 2·61) | 0·83 | −0·53 (−4·17 to 3·10) | 0·77 |
| Chronic obstructive pulmonary disease | 1·70 (0·86 to 3·36) | 0·13 | −0·71 (−3·00 to 1·58) | 0·54 |
| Use of angiotensin-converting enzyme inhibitor | 0·85 (0·47 to 1·53) | 0·59 | 2·75 (0·86 to 4·63) | 0·0044 |
| Use of angiotensin II receptor blocker | 0·60 (0·30 to 1·21) | 0·15 | 0·24 (−2·05 to 2·53) | 0·84 |
All models are mixed-effects models with centres as a random effect and considering a binomial distribution (28-day mortality) or a Gaussian distribution (ventilator-free days at day 28). All continuous variables were entered after standardisation to improve convergence of the model, and odds ratios show the increase in one SD of the variable. C statistic (area under the curve) is 0·797 (95% CI 0·757 to 0·836) and Brier score is 0·170 for the 28-day mortality model. Conditional R2 is 0·301 for the ventilator-free days at day 28 model. PaO2/FiO2=ratio of partial pressure of arterial oxygen to fractional concentration of oxygen in inspired air.
Median value from a maximum of six assessments during the first 24 h (day 0).
Variables included as restricted cubic splines; odds ratio or mean difference are determined over the IQR observed for the variable (estimated effect of an IQR increase in the predictor variable); reported p value is for the first spline (appendix pp 25–26).
Number of ventilator-free days at day 28 was lower, and ICU and hospital length of stay in survivors was longer in patients with severe ARDS (appendix pp 28, 44).
Discussion
This report describes ventilation practice in patients with COVID-19 who received invasive ventilation during the first month of the outbreak in the Netherlands. First, it provides information on ventilation practice in these patients, which can be used to improve local practices. Second, this information could aid the understanding of potential differences in ventilatory practices and different outcomes in reports of patients with COVID-19.20, 21 In our study, more than 50% of patients received protective ventilation with a low tidal volume. The applied PEEP varied substantially and respiratory system compliance was low, with a normal distribution in all patients. Of all adjunctive treatments for refractory hypoxaemia, prone positioning was used in about 50% of patients.
The findings of our study are very much in line with those from a recently published study assessing clinical features and ventilatory management in patients with COVID-19-related ARDS in Spain.22 Our study confirms that lung-protective ventilation is applied well in patients with COVID-19—eg, the use of a low tidal volume was more common than in previous service reviews of ventilation, and driving pressure was consistently lower than 15 cm H2O, even though most patients had moderate to severe ARDS.23, 24 One reason why invasive ventilation settings and parameters did not vary substantially between ICUs in hospitals in the Netherlands could be that COVID-19-related ARDS allows for better use of lung-protective ventilation than does ARDS due to other causes, although it has been suggested that COVID-19 ARDS is broadly similar to other forms of ARDS.25, 26, 27 Another reason could be that the ICUs were well prepared, using local guidelines designed especially for this outbreak. We focused on patients in the first month of the outbreak, which was at a time when little knowledge existed about the best way to ventilate these patients. It is also possible that because care for a surge of invasively ventilated patients with COVID-19 had to be provided by hospital personnel who had less experience or confidence with setting a ventilator, there was better compliance with existing guidelines for the ventilation of patients with ARDS.
One notable finding is that the applied PEEP varied substantially between patients, from 5 cm H2O to 20 cm H2O. This is also consistent with the study in patients with COVID-19 in Spain,22 although median PEEP was slightly higher in our study. It has been suggested that patients with COVID-19 might have two different phenotypes, in part based on the respiratory system compliance.28 However, in our cohort, compliance was low in nearly all patients, with quite a narrow distribution. This finding is in line with results from several recently published studies showing median respiratory compliance in patients with COVID-19 of about 35 mL/cm H2O.6, 7, 26, 29, 30, 31, 32 Data on whether caregivers titrated PEEP on the basis of respiratory system compliance, oxygenation, or extent of pulmonary involvement on lung images, and whether they used a low or high PEEP to FiO2 table (a common tool to titrate PEEP in critically ill patients) could not be collected in a reliable way. Notably, the level of PEEP in our study did not have an effect on patient-centred outcomes such as ventilator-free days or mortality at day 28. Our data do not yet support the suggestion that there are distinct phenotypes needing different approaches in the titration of PEEP.28
In our study, adjunctive treatments were frequently used, as would be expected for patients with moderate or severe ARDS20, 32 and in line with recent reports of COVID-19.5, 6, 22 Prone positioning was used in about 50% of patients, higher than in other large cohorts of patients with ARDS due to other causes.23, 33 It remains uncertain whether this reflects improvement in care over recent years, or whether prone positioning was applied more often because of the severe hypoxaemia typically present in patients with COVID-19.5, 34 Recruitment manoeuvres were seldom used, but it could be that not every recruitment manoeuvre was reported in the patient data management system. ECMO was also seldom used. This could reflect the policy of trying to treat as many patients as possible, thereby restricting the resources that could be used for such a highly complex intervention.
Duration of ventilation was long, especially in comparison with that reported in cohorts of patients with ARDS due to other causes.23 This longer duration of ventilation placed an enormous burden on ICUs and entire hospital systems. Mortality was high, but not different from that seen in other cohorts of patients with COVID-19.1, 4, 5, 6, 7, 8, 23 However, direct comparison between studies is difficult, because most studies also included patients who were not admitted to an ICU as well as patients who did not receive invasive ventilation, and because different mortality measures were reported.1, 5, 8
High tidal volume and low respiratory system compliance on the first day of ventilation were associated with a higher risk of 28-day mortality. These findings are in agreement with those of previous studies in patients with ARDS unrelated to COVID-19. However, PEEP showed no association with clinical outcomes in this study. Additionally, the design of the study precludes any conclusion regarding the effect of a ventilator strategy in this group of patients. Consistent with a recent report from the UK and other reports,8 age was one of the main predictors of outcome.
PRoVENT-COVID has several limitations. First, as in any observational study, the knowledge that ventilation data were being captured could have interfered with daily practice—eg, doctors and nurses could have been keener to use a lung-protective tidal volume. Second, selection of ICUs was based on personal contacts between steering committee members and ICUs that participated in recent research projects of ventilation, which could have resulted in an over-representation of units with more experience in lung-protective ventilation. Third, willingness of participating ICUs to join PRoVENT-COVID could have led to selection bias towards the inclusion of units with an interest in this topic. Similar to other epidemiological studies, access to patients' data was restricted to data collectors who were granted access only to patients who were labelled as eligible for participation by the local doctors—thus, we could not control whether all patients with COVID-19 receiving invasive ventilation in participating ICUs were enrolled. However, all patients included were treated as patients with COVID-19 with bilateral infiltrates and hypoxaemia. Fourth, the national nature of PRoVENT-COVID might mean that these results are not representative of other countries. Fifth, we did not collect data regarding secondary infections, or treatment with steroids or antiviral or antimalarial drugs, which in particular might have happened before admission to the ICU during the first months of the outbreak in the Netherlands. Sixth, information regarding ventilatory support after extubation was restricted to the need for reintubation. Thus, the effect on outcomes of the use of strategies such as non-invasive ventilation after unsuccessful extubation could not be assessed. Seventh, the collection of ventilation variables and adjunctive treatments was restricted to the first 4 calendar days of ventilation to keep the workload of the study at an acceptable level. We cannot exclude the possibility that ventilation practices and use of adjunctive treatments beyond day 4 also had an effect on outcome. Eighth, information regarding ventilatory support before intubation was limited to the use of non-invasive ventilation. Early in the pandemic in the Netherlands, use of high-flow nasal oxygen was advised against in patients with respiratory failure. Therefore, the effect of supportive treatments other than non-invasive ventilation could not be assessed in this cohort of patients. Ninth, this cohort represents the first month of the pandemic in the Netherlands, during which an understandable emphasis was put on patient care rather than on collecting data for severity of disease scores. Consequently, more data were missing than would normally be expected. Tenth, the models were not adjusted for laboratory test results such as D-dimers or troponin, which are not measured daily as part of standard care and were therefore not collected.
The findings of this study extend our knowledge of ventilation practice in patients with COVID-19. Furthermore, they provide important information about the outcomes of patients who received invasive ventilation for this disease. The study's design assured the completeness of data collection. The short timeframe within which data was gathered (ie, 1 month) avoided the effect of practice changes over time.
The data presented here could function as a basis for new hypotheses and sample size calculations for future trials of invasive ventilation in patients with COVID-19. In addition, these data could help to guide the adjustment of local practices and the interpretation of findings from other studies of COVID-19—eg, the findings of this study show that lung-protective ventilation is applicable in patients with COVID-19-associated ARDS, and not different from best practice for ARDS due to other causes. Additionally, our finding that respiratory system compliance and tidal volume affect major outcomes has implications for the understanding of differences in outcomes in the cohorts of patients with COVID-19 that have been reported and will be reported in the near future.4, 6, 7, 22
Data sharing
A deidentified dataset will be made available upon request to the corresponding author at least 1 year after the publication of this study. The request must include a statistical analysis plan.
Contributors
All writing and steering committee members designed the study and were involved in collecting the data with the help of the study collaborators. MB and AMT recruited the centres, instructed, coordinated, and monitored data collectors, and cleaned data. MB, AMT, FP, MJS, and ASN did the analyses and drafted the manuscript. LSB, JP, AGA, LDJB, DAD, MWH, JH, and APJV revised the initial draft. All committee members approved the final version of the manuscript, and subsequent versions after revisions.
Declaration of interests
LDJB reports grants from Health Holland and Dutch Lung Foundation, Dutch Lung Foundation, Amsterdam UMC, and Innovative Medicine Initiative, outside the submitted work. MWH reports non-financial support for his role as Executive Section Editor for Pharmacology with Anesthesia & Analgesia and as Section Editor for Anesthesiology with the Journal of Clinical Medicine; and grants from Edwards Life Sciences, Eurocept, ZonMW, and CSL Behring, outside the submitted work. ASN reports personal fees from Dräger, outside the submitted work. All other authors declare no competing interests.
Contributor Information
PRoVENT-COVID Collaborative Group:
Jesse P. van Akkeren, Anna Geke Algera, Cheetel K. Algoe, Rombout B. van Amstel, Onno L. Baur, Pablo van de Berg, Alida E. van den Berg, Dennis C.J.J. Bergmans, Dido I. van den Bersselaar, Freke A. Bertens, Alexander J.G.H. Bindels, Milou M. de Boer, Sylvia den Boer, Leonoor S. Boers, Margriet Bogerd, Lieuwe D.J. Bos, Michela Botta, Jennifer S. Breel, Hendrik de Bruin, Sanne de Bruin, Caro L. Bruna, Laura A. Buiteman-Kruizinga, Olaf L. Cremer, Rogier M. Determann, Willem Dieperink, Dave A. Dongelmans, Hildegard S. Franke, Michal S. Galek-Aldridge, Mart J. de Graaff, Laura A. Hagens, Jasper J. Haringman, Sebastiaan T. van der Heide, Pim L.J. van der Heiden, Nanon F.L. Heijnen, Stephan J.P. Hiel, Lotte L. Hoeijmakers, Liselotte Hol, Markus W. Hollmann, Marga E. Hoogendoorn, Janneke Horn, Robrecht van der Horst, Evy L.K. Ie, Dimitri P. Ivanov, Nicole Juffermans, Eline Kho, Eline S. de Klerk, Ankie W.M.M. Koopman-van Gemert, Matty Koopmans, Songul Kucukcelebi, Michael A. Kuiper, Dylan W. de Lange, Niels van Mourik, Sunny G.L.H. Nijbroek, Marisa Onrust, Evelien A.N. Oostdijk, Frederique Paulus, Charlotte J. Pennartz, Janesh Pillay, Luigi Pisani, Ilse M. Purmer, Thijs C.D. Rettig, Jan-Paul Roozeman, Michiel T.U. Schuijt, Marcus J. Schultz, Ary Serpa Neto, Mengalvio E. Sleeswijk, Marry R. Smit, Peter E. Spronk, Willemke Stilma, Aart C. Strang, Anissa M. Tsonas, Pieter R. Tuinman, Christel M.A. Valk, Felicia L. Veen-Schra, Lars I. Veldhuis, Patricia van Velzen, Ward H. van der Ven, Alexander P.J. Vlaar, Peter van Vliet, Peter H.J. van der Voort, Louis van Welie, Henrico J.F.T. Wesselink, Hermien H. van der Wier-Lubbers, Bas van Wijk, Tineke Winters, Wing Yi Wong, and Arthur R.H. van Zanten
Supplementary Material
References
- 1.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Center for Systems Science and Engineering COVID-19 dashboard. Johns Hopkins University and Medicine. 2020. https://coronavirus.jhu.edu/map.html
- 3.Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323:1545–1546. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 4.Zangrillo A, Beretta L, Scandroglio AM, et al. Characteristics, treatment, outcomes and cause of death of invasively ventilated patients with COVID-19 ARDS in Milan, Italy. Crit Care Resusc. 2020;22:200–211. doi: 10.1016/S1441-2772(23)00387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Auld SC, Caridi-Scheible M, Blum JM, et al. ICU and ventilator mortality among critically ill adults with coronavirus disease 2019. Crit Care Med. 2020;48:e799–e804. doi: 10.1097/CCM.0000000000004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qian Z, Alaa AM, van der Schaar M, Ercole A. Between-centre differences for COVID-19 ICU mortality from early data in England. Intensive Care Med. 2020;46:1779–1780. doi: 10.1007/s00134-020-06150-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 10.Amato MB, Meade MO, Slutsky AS, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372:747–755. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 11.Constantin JM, Jabaudon M, Lefrant JY, et al. Personalised mechanical ventilation tailored to lung morphology versus low positive end-expiratory pressure for patients with acute respiratory distress syndrome in France (the LIVE study): a multicentre, single-blind, randomised controlled trial. Lancet Respir Med. 2019;7:870–880. doi: 10.1016/S2213-2600(19)30138-9. [DOI] [PubMed] [Google Scholar]
- 12.Cavalcanti AB, Suzumura ÉA, Laranjeira LN, et al. Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2017;318:1335–1345. doi: 10.1001/jama.2017.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guérin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 14.Combes A, Hajage D, Capellier G, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 15.Goligher EC, Tomlinson G, Hajage D, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome and posterior probability of mortality benefit in a post hoc bayesian analysis of a randomized clinical trial. JAMA. 2018;320:2251–2259. doi: 10.1001/jama.2018.14276. [DOI] [PubMed] [Google Scholar]
- 16.Boers NS, Botta M, Tsonas AM, et al. PRactice of VENTilation in Patients with Novel Coronavirus Disease (PRoVENT-COVID): rationale and protocol for a national multicenter observational study in the Netherlands. Ann Transl Med. 2020;8 doi: 10.21037/atm-20-5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO . World Health Organization; Geneva: 2020. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance, 25 January 2020.https://apps.who.int/iris/handle/10665/330854 [Google Scholar]
- 18.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 19.Yehya N, Harhay MO, Curley MAQ, Schoenfeld DA, Reeder RW. Reappraisal of ventilator-free days in critical care research. Am J Respir Crit Care Med. 2019;200:828–836. doi: 10.1164/rccm.201810-2050CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salluh JIF, Ramos F, Chiche JD. Delivering evidence-based critical care for mechanically ventilated patients with COVID-19. Lancet Respir Med. 2020;8:756–758. doi: 10.1016/S2213-2600(20)30266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Camporota L, Vasques F, Sanderson B, Barrett NA, Gattinoni L. Identification of pathophysiological patterns for triage and respiratory support in COVID-19. Lancet Respir Med. 2020;8:752–754. doi: 10.1016/S2213-2600(20)30279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrando C, Suarez-Sipmann F, Mellado-Artigas R, et al. Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS. Intensive Care Med. 2020 doi: 10.1007/s00134-020-06192-2. published online July 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 24.Neto AS, Barbas CSV, Simonis FD, et al. Epidemiological characteristics, practice of ventilation, and clinical outcome in patients at risk of acute respiratory distress syndrome in intensive care units from 16 countries (PRoVENT): an international, multicentre, prospective study. Lancet Respir Med. 2016;4:882–893. doi: 10.1016/S2213-2600(16)30305-8. [DOI] [PubMed] [Google Scholar]
- 25.Bos LDJ. COVID-19-related acute respiratory distress syndrome: not so atypical. Am J Respir Crit Care Med. 2020;202:622–624. doi: 10.1164/rccm.202004-1423LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haudebourg AF, Perier F, Tuffet S, et al. Respiratory mechanics of COVID-19- versus non-COVID-19-associated acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;202:287–290. doi: 10.1164/rccm.202004-1226LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grasselli G, Tonetti T, Protti A, et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: a multicentre prospective observational study. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30370-2. published online Aug 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46:1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region—case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bos LDJ, Paulus F, Vlaar APJ, Beenen LFM, Schultz MJ. Subphenotyping acute respiratory distress syndrome in patients with COVID-19: consequences for ventilator management. Ann Am Thorac Soc. 2020;17:1161–1163. doi: 10.1513/AnnalsATS.202004-376RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ziehr DR, Alladina J, Petri CR, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respir Crit Care Med. 2020;201:1560–1564. doi: 10.1164/rccm.202004-1163LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schenck EJ, Hoffman K, Goyal P, et al. Respiratory mechanics and gas exchange in COVID-19-associated respiratory failure. Ann Am Thorac Soc. 2020;17:1158–1161. doi: 10.1513/AnnalsATS.202005-427RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guérin C, Beuret P, Constantin JM, et al. A prospective international observational prevalence study on prone positioning of ARDS patients: the APRONET (ARDS Prone Position Network) study. Intensive Care Med. 2018;44:22–37. doi: 10.1007/s00134-017-4996-5. [DOI] [PubMed] [Google Scholar]
- 34.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
A deidentified dataset will be made available upon request to the corresponding author at least 1 year after the publication of this study. The request must include a statistical analysis plan.