Abstract
Preoperative testing and evaluation for coronavirus disease 2019 (COVID-19) have been an enigmatic challenge for the neurosurgical community during the pandemic. Since the beginning of the pandemic, laboratory diagnostic methods have evolved substantially, and with them has been the necessity for readily available, fast, and accurate preoperative testing methods. In this article, we provide an overview of the various laboratory testing methods that are presently available and a comprehensive literature review how various institutes and neurosurgical communities across the globe are employing them to ensure safe and effective delivery of surgical care to patients. Through this review, we highlight the guiding principles for preoperative testing, which may serve as a road map for other medical institutions to follow. In addition, we provide an Indian perspective of preoperative testing and share our experience in this regard.
Key words: COVID-19, Diagnostic assay, Neurosurgery, Preoperative testing
Abbreviations and Acronyms: CB-NAAT, Cartridge-based nucleic acid amplification test; COVID-19, Coronavirus disease 2019; CT, Computed tomography; ICMR, Indian Council of Medical Research; POC, Point of care; RT-PCR, Reverse-transcription polymerase chain reaction; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2
Introduction
As the coronavirus disease 2019 (COVID-19) pandemic continues to affect lives across the globe, one of the most discussed issues in the management of the pandemic remains safe and effective delivery of surgical care. While the exponentially rising toll of confirmed COVID-19 cases has stretched the medical resources to the brink, there has been a mounting rise in the unmet need for surgical care among patients. Sound preparedness and planning is quintessential to provide safe and robust surgical care in these unprecedented times. Since the inception of the pandemic in December 2019, neurosurgical societies, associations, and groups of prominent neurosurgeons around the world have put forth guiding strategies for preoperative evaluation and testing of patients in the COVID-19 era. Through this manuscript, we provide a comprehensive literature review of these guidelines and also discuss the Indian viewpoint pertaining to preoperative testing. We would first like to discuss the various available testing strategies, emphasizing their strengths, weaknesses, and utility in the present scenario (Table 1 ).
Table 1.
Comparison of Methods of Laboratory Diagnosis of SARS-CoV-2
| Testing Method | Preferred Specimen | Sensitivity | Specificity | Turnover Time | Utility | Limitations |
|---|---|---|---|---|---|---|
| Laboratory-based RT-PCR | Paired nasopharyngeal and throat swab in ambulatory patients; bronchoalveolar lavage in patients on mechanical ventilator | 71%–98%∗ | 98–100% | 6–18 hours | Current infection with virus; viral detection in acute illness; gold standard diagnostic test | Sophisticated equipment; longer turnaround time; need for efficient cold-chain transport system and storage of specimen; trained laboratory staff |
| CBNAAT | Paired nasopharyngeal and throat swab in ambulatory patients; bronchoalveolar lavage in patients on mechanical ventilator | 96%–100% compared with RT-PCR† | 96%–100% compared with RT-PCR† | 30–45 minutes | On-demand, rapid, easy-to-use diagnostic test | Need for continuous power supply; expensive in India; cartridge waste disposal required; trained laboratory staff |
| TrueNAT | Paired nasopharyngeal and throat swab in ambulatory patients; bronchoalveolar lavage in patients on mechanical ventilator | 85%–92% compared with culture in studies of tuberculosis‡ | 98%–99% compared with culture in studies of tuberculosis‡ | 35–50 minutes | Point of care, portable, cost effective rapid diagnostic test | Low throughput (tests a maximum of 4 samples at a time) |
| Rapid Antigen Test | Nasopharyngeal swab | 23.9%–93.9% compared with RT-PCR | ~100% compared with RT-PCR | 15–30 minutes | Useful as a screening test in health care settings in conjunction with nucleic acid tests (e.g., before emergency surgery while nucleic acid test results are awaited) | Cannot be used as a standalone diagnostic or screening test; limited sensitivity |
| Antibody-based test | Blood | 40%–86% during second week of illness§ | 78%–100% | 1–12 hours | Serosurveys; past infection; return-to-work decision; plasma donation | Not useful as a diagnostic test; potential for cross-reactivity |
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; RT-PCR, reverse transcription-polymerase chain reaction; CB-NAAT, cartridge-based nucleic acid amplification test.
Performance depends on the type of specimen.
Data regarding performance of cartridge-based tests for detection of SARS-CoV-2 are still scarce.
No studies have been conducted regarding the performance of TrueNAT for SARS-CoV-2 detection.
Performance is time-dependent.
Methods for Laboratory Diagnosis
Real-time Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
Real-time RT-PCR tests are the most widely used laboratory tests for detection of RNA viruses.1 , 2 RT-PCR involves viral RNA isolation and purification, RT to complementary DNA, followed by amplification of specific regions of complementary DNA with RT-PCR equipment and fluorescent signal detection using specific probes.3 , 4 RT-PCR is currently considered the gold standard for diagnosing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection because of its easy methodology, extensively validated standard operating procedure, and high sensitivity and specificity.2 , 5 , 6 It is especially useful in detecting the virus in the setting of an acute illness.7 , 8 A positive test can inform the individual of a current infection with the virus so that they can anticipate the course of illness and take measures to prevent further transmission.7 However, a negative test does not rule out infection and needs to be interpreted with caution, considering clinical features, radiologic findings, and even repeat testing in cases with strong clinical suspicion of COVID-19.1 , 5 , 9
According to World Health Organization and U.S. Centers for Disease Control and Prevention, the recommended sample for initial COVID-19 testing is an upper respiratory specimen (nasopharyngeal swab, oropharyngeal swab, or wash in ambulatory patients) and/or lower respiratory specimen in patients with more severe respiratory disease (endotracheal aspirate, bronchoalveolar lavage for patients on mechanical ventilation or sputum, if produced).10 , 11 Wang et al.12 in their paper investigating the biodistribution of SARS-CoV-2 among different tissues of inpatients with COVID-19 recently reported that bronchoalveolar lavage has the greatest positive viral detection rates (93%), followed by sputum (72%), nasal swabs (63%), bronchoscope brush biopsy (46%), throat swabs (32%), feces (29%), and blood (1%). Based on these and similar findings, many other authors have recommended collecting preferably nasopharyngeal swabs with or without oropharyngeal swabs for upper respiratory sampling.3, 4, 5 , 7 , 13, 14, 15 Standard RT-PCR assays rely on sophisticated equipment and consumable reagents, which maybe unavailable in all resource-limited settings. In addition, high-throughput instruments use batched testing to make it cost-effective, which prolongs turnaround times.6 , 16 These limitations can be addressed by isothermal amplification methods and other point-of-care (POC) tests, which can be performed outside of centralized facilities at the community level.2 , 4 , 16, 17, 18
Cartridge-Based Nucleic Acid Amplification Test (CB-NAAT)
Many laboratories all over the world now offer automated nucleic acid amplification tests to reduce turnaround times and overcome other shortcomings of traditional RT-PCR tests.1 , 4 , 15 , 17 This has been made possible by the use of self-contained cartridge-based nucleic acid testing platform—CB-NAAT. Various cartridge-based testing systems have received Emergency Use Authorization by the U.S. Food and Drug Administration—The True Sample-to-Answer Solution ePlex by GenMark DX (Carlsbad, California, USA), BioFire COVID-19 test (BioFire, Salt Lake City, Utah, USA), Accula SARS-CoV-2 by Mesa Biotech (San Diego, California, USA), ID NOW by Abbott (Abbott Park, Illinois, USA), and Xpert Xpress SARS-CoV-2 test by Cepheid (Sunnyvale, California, USA), to name a few.1
The CB-NAAT testing system used in India, and authorized by the Indian Council of Medical Research (ICMR), is based on the Xpert Xpress SARS-CoV-2 test (Cepheid Inc.).19 CB-NAAT has been widely employed as a rapid diagnostic test for tuberculosis and for diagnosing rifampin resistance in positive patients using the Xpert MTB/RIF (Cepheid Inc.).20, 21, 22 The test has proven especially useful in initiation of treatment of tuberculosis in resource-limited, high-burden Indian rural settings.23 Xpert Xpress SARS-CoV-2 uses upper respiratory specimens and involves transfer of the transport medium harboring the sample/swab into a testing cartridge, which is loaded into a testing module within the Gene-Xpert equipment. This is followed by an automated purification, extraction, and amplification of viral genetic material. The test has a fast turnaround time of 30–45 minutes.24 Literature pertaining to the performance of cartridge-based tests is still scarce. In 2 multi-institutional studies that evaluated the performance of Cepheid Xpert Xpress SARS-CoV-2 assay, the test demonstrated concordance of 96%–100% compared with RT-PCR tests.25 , 26 CB-NAAT is an on-demand, rapid, easy-to-use testing system that can test up to 80 samples at a time, depending on the number of test modules available on the machine. Limitations of this testing system include need for continuous power supply, waste disposal system for cartridges, minimum biosafety 2 level conditions, and trained laboratory staff.19
TrueNAT
TrueNAT is an improvement over the CB-NAAT test as it overcomes the shortcomings of Gene-Xpert, which limits its decentralization to peripheral laboratories. TrueNAT Beta CoV and SARS-CoV-2 (Molbio Diagnostics/Bigtec Labs, Goa/Bengaluru, India) are chip-based real-time PCR tests for semiquantitative detection of beta coronavirus (genus) and SARS-CoV-2 virus (strain), respectively. The TrueNAT system has been previously used as a rapid, portable, POC, and cost-effective test for the diagnosis of tuberculosis within primary health care facilities and has proven more cost effective than smear microscopy or Xpert.27, 28, 29 The ICMR recommends TrueNAT test as a 2-step test: step 1 comprises an E gene (which helps build the envelope) screening assay (TrueNAT Beta CoV) for all suspected COVID-19 samples followed by step 2 for the RdRp gene (RNA-dependent RNA polymerase)-based confirmatory test (TrueNAT SARS-CoV-2) in all samples testing positive for E gene. The samples testing negative from step 1 and those testing positive from step 2 are to be considered as true negatives and true positives respectively and no further RT-PCR based confirmation is required in such a scenario.19 No studies have been conducted so far evaluating the performance of TrueNAT test for detection of SARS-CoV-2 virus. Lee et al.,27 in their model-based analysis, compared the performance of TrueNAT test with other diagnostic tests for POC diagnosis of tuberculosis. They reported a sensitivity of 89% (85–92%) and a specificity of about 99% (98–99%) compared with culture.
This test involves the use of a battery-operated TrueNAT system where the RNA is first extracted from the specimen (oropharyngeal/nasopharyngeal swab) using Trueprep Auto sample prep device. Purified RNA is subsequently mixed with freeze dried PCR reagents and dispensed into the reaction well of the TrueNAT Beta CoV chip, which is inserted in the Truelab real-time micro PCR analyzer, which carries out automated amplification and RNA detection.30 As the sample is collected in a viral lysis medium, requirements for biosafety are minimal. The test has a fast turnaround time of 35–50 minutes for a single assay and can test 1–4 samples at a time depending on the module configuration of the machine. Portability, no need for special infrastructure, easy to use, rapid, and minimal biosafety requirements are features that make TrueNAT suitable for use at the periphery level.
Rapid Antigen Test
The rapid antigen test further reduces turnaround time by simplifying the test to an immunochromatographic assay that detects the SARS-CoV-2 antigen in the sample. The preferred specimen for this test is a nasopharyngeal swab. The test involves insertion of the collected nasopharyngeal swab into an extraction buffer followed by application of the extracted solution to a specimen well on the test device. Following this, the test result is read in 15–30 minutes (not after 30 minutes, as it may give false results).31 This test has a very high specificity (~100%) and it may have potential utility as a rapid, POC test for early diagnosis of COVID-19 in resource-limited settings.32 , 33 Although scarce, literature pertaining to use of this test for detection of SARS-CoV-2 suggests that the performance of rapid antigen tests is limited by poor sensitivity. Overall sensitivity ranges from 23.9% to 93.9% for the use of rapid antigen tests.33, 34, 35, 36, 37 In the study by Porte et al.33 reporting exceptionally high 93.9% sensitivity (compared with the rest of literature), the authors observed that they collected majority of samples (93.7%) from patients during the first week of clinical disease. This could have positively affected the reported sensitivity in their experience, and hence justifies further scientific exploration for the temporal association of diagnostic assay and disease progression, as an important confounder. Concurrently, the literature suggests the use of this test as an adjunct and not as a standalone frontline test for triage purposes.
The ICMR recommends the use of Standard Q COVID-19 antigen kit (SD Biosensor, based in South Korea) as a rapid POC diagnostic test for use in health care settings and containment zones in combination with the gold standard RT-PCR test.32 Hence, suspected patients testing negative for COVID-19 by rapid antigen test need to be tested using RT-PCR and patients testing positive do not require further testing.
Antibody-Based Tests
These tests evaluate the presence of IgM/IgG antibodies against SARS-CoV-2 viral antigen proteins. The specimen for testing is serum, plasma, or whole blood.3 These tests are extremely useful for epidemiologic surveillance and indicate a past exposure to the virus even in asymptomatic individuals.15 , 16 , 32 The level of seroprevalence in a population can allow appropriate public health measures to be instituted to contain disease spread. In addition, in health care workers, serologic tests can guide return to work decisions and have a utility in risk assessment.15 , 16 Antibody tests are also being used to screen donors for convalescent plasma donation, which is currently under investigation as a possible therapeutic option for COVID-19 in clinical trials.38 , 39 Antibody production is host dependent and generally begins 1 week after the onset of clinical symptoms and lasts for several weeks (IgM) or months (IgG).7 , 8 , 40 Hence, these tests are not useful for making an early diagnosis or the diagnosis of an acute infection.1 , 2 Moreover, serologic tests have a potential problem of cross reactivity with other coronaviruses proteins leading to false positive results.1 , 15 They should not be employed for triage purposes in view of their moderate, time-dependent sensitivity (40%–86%) and specificity (78%–100%) compared to RT-PCR based tests.1 , 6 , 41 , 42 ICMR recommends use of IgG antibody test for conducting serosurveys and not for diagnosis of COVID-19.32
Preoperative Testing for COVID-19—Rationale and Significance
Research evidence regarding spread of COVID-19 by asymptomatic carriers is still limited. However, studies suggest that the potential of asymptomatic and pre-symptomatic individuals infected with SARS-CoV-2 virus to further transmit infection is significant.43, 44, 45, 46 Arons et al.,44 in their study describing an outbreak of COVID-19 in a skilled nursing facility, evaluated the adequacy of symptom-based screening amongst residents. They observed that more than one half of the residents (27/48, 56.2%) who had tested positive during a facility-wide point-prevalence screening were asymptomatic at testing. In addition, they identified large quantities of viral RNA, and isolated viable SARS-CoV-2 virus from specimens of asymptomatic and pre-symptomatic residents. The authors concluded that these asymptomatic residents most likely contributed to transmission and infection control strategies focused entirely on symptomatic residents were inadequate.44 This recommendation may be extended to include individuals living in closed, congregated facilities that are at high risk of asymptomatic transmission, viz. prisons, nursing homes, hospitals, etc. Revision of protocols to include these individuals under the umbrella of laboratory screening is the need of the hour.45
In addition to the risk of transmission to contacts, mortality and morbidity associated with perioperative COVID-19 infection can be pronounced. In an international, multicenter cohort study conducted at 235 hospitals across 24 countries, the authors assessed postoperative mortality and the rate of pulmonary complications in 1128 patients with COVID-19. They observed an alarming 30-day mortality of 23.8% and pulmonary complications in 51.2% patients.47 In a similar matched-cohort study involving 123 patients from a hospital in Brescia, Italy, the authors observed 30-day mortality and postoperative complications significantly greater in patients with SARS-CoV-2 infection than the control group.48 Similar findings were noted in some other studies.49 , 50 Findings from all these studies impress upon the hazardous outcomes of surgery in patients with COVID-19 and the need for postponing non-urgent surgical procedures in such patients. This also underlines the importance of mandatory preoperative testing for comprehensive risk assessment, prognosis and decision making in surgical patients.
COVID-19, which primarily spreads through virus aerosolized from the upper airway,51 , 52 places the health care provider involved in aerosol-generating procedures under tremendous risk. This means that the neurosurgeon might be under an additional risk of contracting infection during surgery as this surgery routinely involves bone drilling and use of other aerosol generating instruments, exposure of mucosa in the paranasal sinuses during craniotomy and most importantly, transnasal procedures.53 This accentuates the need for preoperative testing before neurosurgical procedures to ensure safety and allay anxiety among operating room staff.
Preoperative Testing for COVID-19—Guidelines in Neurosurgery
A review of the articles on PubMed was carried out using key words “COVID-19” and “neurosurgery” on June 15, 2020. The search yielded 351 articles, which were reviewed for relevance. References from relevant articles were reviewed to locate other articles of interest. From these data, we were able to compile a review of preoperative testing protocols of 19 different institutes in 11 different countries around the world (Table 2 ).54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74 In addition, documents published on webpages of various international neurosurgical societies and consensus statements from various neurosurgical institutes also were noted (Table 3 ).75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85
Table 2.
Literature Review of Recommendations on Preoperative Testing: Institutional Protocols
| No. | Country/Region | Institute | Confirmed Cases54,55 (Region or Country) | Recommendation on Preoperative Testing |
|---|---|---|---|---|
| 1 | USA/Connecticut (CT)56 | Yale | 47,209 (CT) | Patients tested for COVID-19 within 24 hours leading up to surgery. Screening for symptoms and temperature measurement on the morning of surgery. |
| 2 | USA/New York (NY)57 | Mount Sinai Hospital | 404,997 (NY) | Patients generally considered to be COVID-19 positive until proven otherwise and multiple rounds of testing sent as soon as possible, when available. |
| 3 | USA/Massachusetts (MA)58 | UMass Memorial Health Care, Worcester | 110,897 (MA) | All patients undergoing surgical procedure to be tested preoperatively. |
| 4 | USA/Florida (FL)59 | Jackson Memorial, Miami | 244,143 (FL) | COVID-19 testing all surgical cases preoperatively. Fourteen-day delay imposed for cases if testing not available. |
| 5 | USA/California (CA)60 | Stanford University School of Medicine | 304,558 (CA) | All inpatients tested 48 hours before procedures. Patient undergoing emergent and urgent cases received the same day test (without waiting for test results). Patients undergoing high-risk aerosol-generating procedures (endonasal, transsphenoidal procedures, FESS) received dual testing. (RT-PCR <72 hours and rapid test on the day of surgery). |
| 6 | USA/Alabama (AL)61 | University of Alabama at Birmingham | 50,508 (AL) | All patients tested within 72 hours before using RT-PCR and emergency procedures tested on the same day using Cepheid nucleic acid test. |
| 7 | Italy/Lombardy62 | University of Insubria, Varese | 94,905 (Lombardy) | Mandatorily define the COVID-19 status of patients irrespective of symptoms: Nasopharyngeal swabs complemented with chest imaging, immunologic antibodies determination and saliva viral load quantification. |
| 8 | Italy/Lombardy63,64 | C. Besta Neurological Institute, Milan | 94,905 (Lombardy) | Deep screening of all admitted patients with body temperature, O2 saturation, C-reactive protein, transaminases levels, complete cell blood count, chest radiography, pharyngeal SARS-CoV-2 swab and a thorough history. Emphasis on ensuring a “COVID-19–free” hospital following the “hub-and-spoke” policy. |
| 9 | Switzerland/Geneva65 | Geneva University medical Centre | 32,586 (Switzerland) 5229 (Geneva) |
Emergent cases deemed as positive. Urgent cases taken for surgery after COVID-19 testing. |
| 10 | China/Hubei66 | Tongji Hospital, Wuhan | 68,135 (Hubei) | Clinical screening in outpatient department. A pulmonary CT scan and nucleic acid sequencing of throat swab recommended. |
| 11 | China/Guangdong67 | Sun Yat-Sen University Cancer Center | 1645 (Guangdong) | All patients admitted for surgery underwent “COVID-19 screening,” including contact tracing, symptoms interrogation, novel coronavirus nucleic acid and antibody test, and chest CT scan. |
| 12 | France/Alsace68 | Strasbourg University Hospital, Alsace | 1,70,752 (France) | Emergency: no need for swab Deferrable: management after swab; surgical intervention deferrable at least 48 hours, and expedited within 7–15 days. Elective: management after swab; surgical intervention rescheduled within 2–4 months. |
| 13 | UK/Cambridge69 | Addenbrooke Hospital, Cambridge | 2,87,621 (UK) | Patient screened for symptoms 1 week prior and asked to self-isolate. Two nasopharyngeal swabs are performed preoperatively (first on day 4–5 preoperatively and second on day 2 preoperatively). Patients with 2 negative swabs are admitted on the day of surgery and are screened again for any COVID-19 symptoms. |
| 14 | UK/London70 | National Hospital for Neurology and Neurosurgery | 2,87,621 (UK) | All patients undergo RT-PCR based testing before non-emergency surgery. |
| 15 | Ireland/Dublin71 | Beaumont Hospital, Dublin | 25,589 (Ireland) | All elective surgical patients tested using RT-PCR 1 day prior. |
| 16 | South Korea/Seoul72 | Yonsei University College of Medicine | 13,338 (South Korea) | All patients screened for respiratory symptoms and tested for COVID-19 before surgery. |
| 17 | Morocco, Rabat73 | WFNS Rabat Reference Centre ONO Hospital |
14,771 (Morocco) | Initial assessment by pulmonary CT scan reinforced by COVID-19 testing of suspected cases. |
| 18 | Singapore74 | Singapore General Hospital | 45,614 (Singapore) | Clinical screening and exposure history questionnaire used for all preoperative patients. Routine preoperative chest radiography for all patients. Routine preoperative swab for all patients requiring elective high-risk surgery (transgressing upper airways). |
| 19 | India/New Delhi | All India Institute of Medical Sciences, New Delhi | 1,07,051 (Delhi) | Mandatory testing of all neurosurgical patients planned for diagnostic or therapeutic procedures. Hospitalized patients for semiemergent/elective surgery (Category I): RT-PCR. Outpatients for diagnostic or therapeutic day care procedures (Category II): Schedule procedure for 1week prior. Schedule RT-PCR for 72–96 hours before procedure. Emergent diagnostic or therapeutic procedures (Category III): CB-NAAT/TrueNAT rapid assays. CT of the chest: reserved for symptomatic patients. |
COVID-19, coronavirus disease 2019; FESS, functional endoscopic sinus surgery; RT-PCR, reverse transcription-polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; CT, computed tomography; CB-NAAT, cartridge-based nucleic acid amplification test.
Table 3.
Literature Review of Recommendations on Preoperative Testing: Professional Bodies
| No. | Society/Professional Body | Recommendation on Preoperative Testing |
|---|---|---|
| 1 | Society of British Neurological Surgeons75 | Preoperative COVID-19 testing should be employed when available. |
| 2 | AANS/CNS Tumour Section and Society for Neuro-Oncology76 | To the extent possible, patients should receive COVID-19 testing on the day of surgery. |
| 3 | Royal College of Surgeons of England77 | COVID-19 should be sought in all patients before surgery either directly via testing or through proxy indicators. |
| 4 | Hong Kong Neurosurgical Society78 | Before surgery, it is prudent to ask for FTOCC (fever, travel, occupation, contact, clustering) histories and upper respiratory and gastrointestinal symptoms. Body temperature checked and chest radiograph should be done. SARS-CoV-2 status should be checked by nasopharyngeal and throat swab, whenever possible. |
| 5 | American Society of Anesthesiologists and Anesthesia Patient Safety Foundation79 | All patients should be screened for symptoms before presenting to the health care facility. Patients reporting symptoms should be referred for additional evaluation. All other patients should undergo nucleic acid amplification testing (including PCR tests) before undergoing nonemergent surgery. |
| 6 | Professional Education Committee of the Pituitary Society80 | Screening for cough, fever, and other symptoms and, if suspected, swab for testing. Consider (depending on local guidance): Isolation up to 2 weeks before surgery; paired swabs for testing and/or serological tests; chest radiograph and/or chest CT. |
| 7 | Italian Skull Base Society81 | It is mandatory to test for COVID-19 in all patients who are candidates for surgery (except for emergency procedures), with at least 2 tests, repeated at a distance of 2–4 days, to minimize the possibility of false negatives. The last test must be performed within 48 hours before surgery. |
| 8 | International consensus guidelines for head and neck oncology (39 societies and professional bodies)82 | Strong agreement for “COVID-19 status of a patient should be considered before surgery” and “positive laboratory test would be sufficient as a minimum criterion for diagnosis.” |
| 9 | Consensus statement from India for practice of Neurosurgery and Neurology83 | Acute cases: Initial screening – Thermal screening and Rapid COVID-19 diagnostic Kit. Subacute/Chronic Cases: Initial screening – Thermal screening and Rapid COVID-19 diagnostic Kit followed by pulmonary CT scan (if available) and nucleic acid testing by RT-PCR. |
| 10 | Recommendations based on expert opinion of 4 worldwide-known neurosurgeons from 3 different continents (USA/Europe/Asia)84 | Management based on preoperative COVID-19 testing, 2 times within 24 hours or CT of the chest. |
| 11 | Multicentre recommendation based on expert opinion85 | Emergent: Assume COVID-19 positive. Urgent: Preoperative testing if available to be done as close as possible to surgery. Quarantine until result negative. If testing unavailable assume COVID-19 positive. Semi-urgent: test if available. If unavailable, self-quarantine for 14 days. |
COVID-19, coronavirus disease; AANS/CNS, American Association or Neurological Surgeons/Congress of Neurological Surgeons; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; CT, computed tomography; RT-PCR, reverse transcription-polymerase chain reaction.
All of the centers and most of the prominent surgical societies around the world that we reviewed recommend mandatory preoperative testing in nonemergent cases for COVID-19 (Tables 2 and 3). RT-PCR using a nasopharyngeal or a throat swab was found to be the most widely used method for preoperative screening and diagnosis. As the testing capacity increases around the world, there is a mounting trend toward the routine use of a rapid nucleic acid test (e.g., Cepheid Xpert Xpress SARS-CoV-2 test) before emergency cases.60 , 61 It is recommended to have the diagnostic test performed as close as possible to the surgery. Many centers in the United States and around the world follow a protocol of testing in less than 72 hours before surgery,56 , 60 , 61 , 71 with some even recommend having 2 negative swab results (especially in high-risk cases) before proceeding for nonemergent surgery.60 , 69 , 84 For instance, the Italian Skull Base Society recommends that “it is mandatory to test for COVID-19 in all patients who are candidates for surgery (except for emergency procedures), with at least 2 tests, repeated with a temporal separation of 2–4 days, in order to minimize the possibility of false negatives. The last test must be performed within 48 hours prior to surgery.”81 Neurosurgeons from many countries have recommended the inclusion of chest radiography (Italy and Singapore) or pulmonary computed tomography (CT) scan (China and Morocco) in the armamentarium for diagnosis of COVID-19.63 , 64 , 66 , 67 , 73 , 74 A report from neurosurgery department at ONO Hospital in Rabat, Morocco, mentions the use of pulmonary CT scan for initial assessment of COVID-19 status in patients followed by confirmation using diagnostic laboratory testing.73 Neurosurgeons from China, Hong Kong, and Singapore impress upon the use of clinical screening and use of questionnaires for interrogation of exposure history and symptoms.66 , 67 , 74 , 78 Similarly, neurosurgeons from C. Besta Institute in Milan, Italy recommend a “deep” COVID-19 screening of all surgical patients using ancillary tools like oxygen saturation, C-reactive protein, and serum transaminases in addition to clinical history and pharyngeal SARS-CoV-2 swab.63
Preoperative Testing for COVID-19 – The Indian Viewpoint and Our Experience
ICMR, the apex medical research governing body in India, has periodically revised the guidelines governing the strategy for COVID-19 testing in India. While the first version of guidelines impressed upon testing of only symptomatic persons who were contacts of laboratory-confirmed cases or had a history of international travel,32 the latest version recommended the use of Standard Q COVID-19 Antigen assay for screening of asymptomatic patients undergoing aerosol generating surgical or nonsurgical procedures.32 This clause included patients undergoing elective or emergency surgical procedures like neurosurgery, ear–nose–throat surgery and dental procedures, and other nonsurgical procedures viz. bronchoscopy, upper gastrointestinal endoscopy and dialysis. In a multi-institutional consensus statement published from India for the practice of Neurology and Neurosurgery, Gupta et al.83 recommend initial screening of acute cases using body temperature measurement and rapid COVID-19 diagnostic kits while for other cases this initial screening should be followed by nucleic acid testing using RT-PCR and pulmonary CT, if available.
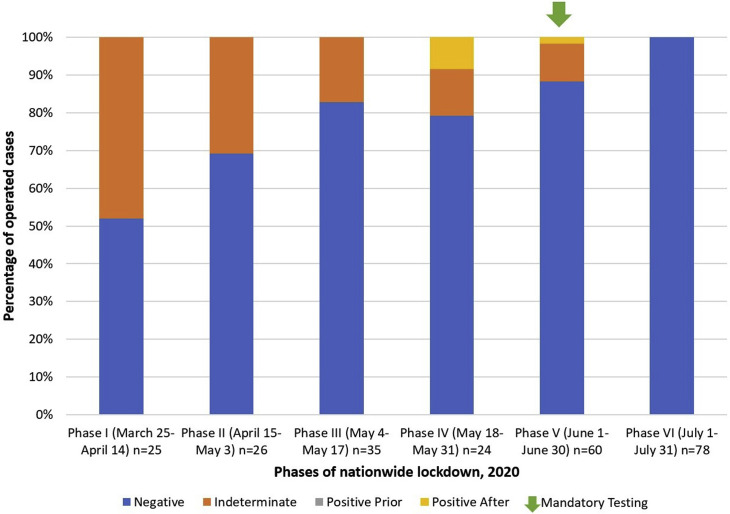
The pandemic has been an enigmatic challenge for centers around the globe. We have had our share of learnings from managing the same. Figure 1 summarizes our experience with preoperative testing for COVID-19 as the pandemic progressed. For the purpose of illustration of data, we have divided the COVID-19 period in our setting into 6 phases of nationwide lockdown in India (Figure 1).86 We analyzed the neurosurgical cases operated during this period to ascertain the fraction of cases which were negative, positive or indeterminate (suspect) for COVID-19 before surgery. During this period, all the patients that we operated (248 patients) were either negative or indeterminate prior to surgery. A total of 3 cases tested positive after surgery (2 of them were negative initially and one was indeterminate/suspect before surgery). During the initial phases of the pandemic, we relied primarily on gold standard RT-PCR tests (turnaround times of up to 18 hours), with emergent procedures taken for surgery as COVID-19 suspects unless proven otherwise. As the pandemic progressed, we were able to procure and use other rapid nucleic acid tests with shorter turn-around times (CB-NAAT and TrueNAT tests). As a result, we were able to use these for emergent surgery. More recently, we have also included rapid antigen tests in our testing armamentarium (turnaround times of less than 30 minutes). Hence, with the gradual step-up of testing capacity and periodic revision of national testing guidelines by ICMR, the fraction of cases with indeterminate/suspect COVID-19 status decreased. Eventually, we were able to emplace mandatory testing of COVID-19 status before all (emergent/elective/semi-emergent) surgical procedures by Phase V of national lockdown (Figure 1).
Figure 1.
Stacked-column chart showing percentages of neurosurgical cases operated at our center that tested negative, indeterminate, or positive for coronavirus disease 2019 (COVID-19) during various phases of nationwide lockdown. With the gradual step-up of testing capacity, we were able to emplace mandatory preoperative testing (green arrow) for COVID-19 by Phase V.
Currently, we conduct mandatory testing of all patients planned for diagnostic procedures or neurosurgical intervention at our center. For all in-patients for semiemergent or elective surgery (Category I), standard real-time RT-PCR assay is employed with a turnaround time of 6–18 hours depending on when the specimen is collected. For all outpatients requiring diagnostic or therapeutic day-care procedures (Category II), the procedure is scheduled for at least 1 week later. The patient is then provided an RT-PCR test appointment for 72–96 hours before the scheduled procedure date. Regardless of the symptom and clinical history, for emergency diagnostic and therapeutic procedures (Category III) we employ CB-NAAT or TrueNAT nucleic acid sequencing assays with a turnaround time of less than 3 hours. In view of low sensitivity of the rapid antigen test, we reserve its use before emergency procedures in suspected cases, while nucleic acid test results are awaited. For all nucleic acid tests, we employ use of paired (nasopharyngeal and oropharyngeal) swabs for specimen collection. Use of CT of the chest is generally reserved for symptomatic cases, after consultation with an infectious disease specialist.
Conclusions
The importance of preoperative testing for COVID-19 in surgical patients cannot be overemphasized. With the growing body of evidence in favor of this clause, rapid increase of asymptomatic and presymptomatic COVID-19 patients in the community, and possible hazardous postoperative outcomes of unexpected COVID-19 patients, mandatory preoperative testing of surgical patients is the need of the hour. However, it is also prudent to take into consideration the logistics, central and state governments' co-operation, merits, and demerits of the available testing methods and local surge of cases in the community before formulating protocols to safeguard health care workers and patients against further spread of this menacing illness.
Acknowledgments
We would like to acknowledge the efforts of the Hospital Infection Control Committee (HICC) and the hospital SARS-CoV-2 testing laboratories, All India Institute of Medical Sciences, New Delhi, India.
Footnotes
Conflict of interest statement: The authors declare that the article content was composed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Ward S., Lindsley A., Courter J., Assa'ad A. Clinical testing for COVID-19. J Allergy Clin Immunol. 2020;146:23–34. doi: 10.1016/j.jaci.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yan Y., Chang L., Wang L. Laboratory testing of SARS-CoV, MERS-CoV, and SARS-CoV-2 (2019-nCoV): current status, challenges, and countermeasures. Rev Med Virol. 2020;30:e2106. doi: 10.1002/rmv.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lippi G., Mattiuzzi C., Bovo C., Plebani M. Current laboratory diagnostics of coronavirus disease 2019 (COVID-19) Acta Biomed. 2020;91:137–145. doi: 10.23750/abm.v91i2.9548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Udugama B., Kadhiresan P., Kozlowski H.N., et al. Diagnosing COVID-19: the disease and tools for detection. ACS Nano. 2020;14:3822–3835. doi: 10.1021/acsnano.0c02624. [DOI] [PubMed] [Google Scholar]
- 5.Tahamtan A., Ardebili A. Real-time RT-PCR in COVID-19 detection: issues affecting the results. Expert Rev Mol Diagn. 2020;20:453–454. doi: 10.1080/14737159.2020.1757437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bachelet V.C. Do we know the diagnostic properties of the tests used in COVID-19? A rapid review of recently published literature. Medwave. 2020;20:e7890. doi: 10.5867/medwave.2020.03.7891. [DOI] [PubMed] [Google Scholar]
- 7.Patel R., Babady E., Theel E.S., et al. Report from the American Society for Microbiology COVID-19 International Summit, 23 March 2020: Value of Diagnostic Testing for SARS-CoV-2/COVID-19. mBio. 2020;11 doi: 10.1128/mBio.00722-20. :e00722-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venter M., Richter K. Towards effective diagnostic assays for COVID-19: a review. J Clin Pathol. 2020;73:370–377. doi: 10.1136/jclinpath-2020-206685. [DOI] [PubMed] [Google Scholar]
- 9.Zitek T. The appropriate use of testing for COVID-19. West J Emerg Med. 2020;21:470–472. doi: 10.5811/westjem.2020.4.47370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention Information for Laboratories about Coronavirus (COVID-19) https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html Available at: Published February 11, 2020. Accessed July 2, 2020.
- 11.World Health Organization . 2020. Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases: interim guidance, 2 March 2020.https://apps.who.int/iris/handle/10665/331329 Available at: [Google Scholar]
- 12.Wang W., Xu Y., Gao R., et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mawaddah A., Gendeh H.S., Lum S.G., Marina M.B. Upper respiratory tract sampling in COVID-19. Malays J Pathol. 2020;42:23–35. [PubMed] [Google Scholar]
- 14.Tang Y.W., Schmitz J.E., Persing D.H., Stratton C.W. Laboratory diagnosis of COVID-19: current issues and challenges. J Clin Microbiol. 2020;58:e00512–e00520. doi: 10.1128/JCM.00512-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng M.P., Papenburg J., Desjardins M., et al. Diagnostic testing for severe acute respiratory syndrome-related coronavirus 2: a narrative review. Ann Intern Med. 2020;172:726–734. doi: 10.7326/M20-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu H., Stratton C.W., Tang Y.W. An evolving approach to the laboratory assessment of COVID-19. https://doi.org/10.1002/jmv.25954 [e-pub ahead of print]. J Med Virol. [DOI] [PMC free article] [PubMed]
- 17.Loeffelholz M.J., Tang Y.W. Laboratory diagnosis of emerging human coronavirus infections - the state of the art. Emerg Microbes Infect. 2020;9:747–756. doi: 10.1080/22221751.2020.1745095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pang J., Wang M.X., Ang I.Y.H., et al. Potential rapid diagnostics, vaccine and therapeutics for 2019 novel coronavirus (2019-nCoV): a systematic review. J Clin Med. 2020;9:623. doi: 10.3390/jcm9030623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Information for COVID-19 Testing Labs. https://www.icmr.gov.in/ctestlab.html Available at:
- 20.Kashyap B., Goyal N., Hyanki P., Singh N.P., Khanna A. Cartridge-based nucleic acid amplification test: a novel rapid diagnostic tool to study the burden of tuberculosis from a tertiary care hospital. Trop Doct. 2019;49:274–281. doi: 10.1177/0049475519859958. [DOI] [PubMed] [Google Scholar]
- 21.Arora D., Jindal N., Bansal R., Arora S. Rapid detection of Mycobacterium tuberculosis in sputum samples by Cepheid Xpert assay: a clinical study. J Clin Diagn Res. 2015;9:DC03–DC05. doi: 10.7860/JCDR/2015/11352.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sachdeva K., Shrivastava T. CBNAAT: a boon for early diagnosis of tuberculosis-head and neck. Indian J Otolaryngol Head Neck Surg. 2018;70:572–577. doi: 10.1007/s12070-018-1364-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Youngs J., Patil S., Jain Y. A prospective study evaluating the impact of cartridge-based nucleic acid amplification test (CBNAAT) on the management of tuberculosis in a low-resource high-burden Indian rural setting. J Family Med Prim Care. 2018;7:982–992. doi: 10.4103/jfmpc.jfmpc_104_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xpert® Xpress SARS-CoV-2 has received FDA emergency use authorization. https://www.cepheid.com/coronavirus Available at:
- 25.Hou H., Chen J., Wang Y., et al. Multicenter evaluation of the Cepheid Xpert Xpress SARS-CoV-2 assay for the detection of SARS-CoV-2 in oropharyngeal swab specimens. J Clin Microbiol. 2020;58 doi: 10.1128/JCM.01288-20. :e01288-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolters F., van de Bovenkamp J., van den Bosch B., et al. Multi-center evaluation of cepheid xpert® xpress SARS-CoV-2 point-of-care test during the SARS-CoV-2 pandemic. J Clin Virol. 2020;128 doi: 10.1016/j.jcv.2020.104426. 104426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee D.J., Kumarasamy N., Resch S.C., et al. Rapid, point-of-care diagnosis of tuberculosis with novel Truenat assay: cost-effectiveness analysis for India's public sector. PLoS One. 2019;14:e0218890. doi: 10.1371/journal.pone.0218890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nikam C., Kazi M., Nair C., et al. Evaluation of the Indian TrueNAT micro RT-PCR device with GeneXpert for case detection of pulmonary tuberculosis. Int J Mycobacteriol. 2014;3:205–210. doi: 10.1016/j.ijmyco.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Nikam C., Jagannath M., Narayanan M.M., et al. Rapid diagnosis of Mycobacterium tuberculosis with Truenat MTB: a near-care approach. PLoS One. 2013;8:e51121. doi: 10.1371/journal.pone.0051121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Our Products Truenat™ SARS CoV-2. http://www.molbiodiagnostics.com/product_details.php?id=55 Available at:
- 31.Products - Standard Q Covid-19 Ag. http://sdbiosensor.com/xe/product/7672 Available at:
- 32.Testing Strategy. https://www.icmr.gov.in/cteststrat.html Available at:
- 33.Porte L., Legarraga P., Vollrath V., et al. Evaluation of novel antigen-based rapid detection test for the diagnosis of SARS-CoV-2 in respiratory samples. Int J Infect Dis. 2020;99:328–333. doi: 10.1016/j.ijid.2020.05.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mak G.C., Cheng P.K., Lau S.S., et al. Evaluation of rapid antigen test for detection of SARS-CoV-2 virus. J Clin Virol. 2020;129 doi: 10.1016/j.jcv.2020.104500. :104500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mertens P., De Vos N., Martiny D., et al. Development and potential usefulness of the COVID-19 Ag Respi-Strip diagnostic assay in a pandemic context. Front Med (Lausanne) 2020;7:225. doi: 10.3389/fmed.2020.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scohy A., Anantharajah A., Bodéus M., Kabamba-Mukadi B., Verroken A., Rodriguez-Villalobos H. Low performance of rapid antigen detection test as frontline testing for COVID-19 diagnosis. J Clin Virol. 2020;129 doi: 10.1016/j.jcv.2020.104455. :104455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blairon L., Wilmet A., Beukinga I., Tré-Hardy M. Implementation of rapid SARS-CoV-2 antigenic testing in a laboratory without access to molecular methods: experiences of a general hospital. J Clin Virol. 2020;129 doi: 10.1016/j.jcv.2020.104472. :104472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casadevall A., Pirofski L.A. The convalescent sera option for containing COVID-19. J Clin Invest. 2020;130:1545–1548. doi: 10.1172/JCI138003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duan K., Liu B., Li C., et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jin Y., Wang M., Zuo Z., et al. Diagnostic value and dynamic variance of serum antibody in coronavirus disease 2019. Int J Infect Dis. 2020;94:49–52. doi: 10.1016/j.ijid.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bastos M.L., Tavaziva G., Abidi S.K., et al. Diagnostic accuracy of serological tests for covid-19: systematic review and meta-analysis. https://doi.org/10.1136/bmj.m2516 [e-pub ahead of print]. BMJ. [DOI] [PMC free article] [PubMed]
- 42.La Marca A., Capuzzo M., Paglia T., et al. Testing for SARS-CoV-2 (COVID-19): a systematic review and clinical guide to molecular and serological in-vitro diagnostic assays. Reprod Biomed Online. 2020;41:483–499. doi: 10.1016/j.rbmo.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bai Y., Yao L., Wei T., et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323:1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arons M.M., Hatfield K.M., Reddy S.C., et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382:2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gandhi M., Yokoe D.S., Havlir D.V. Asymptomatic transmission, the Achilles' heel of current strategies to control Covid-19. N Engl J Med. 2020;382:2158–2160. doi: 10.1056/NEJMe2009758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mandal S., Bhatnagar T., Arinaminpathy N., et al. Prudent public health intervention strategies to control the coronavirus disease 2019 transmission in India: a mathematical model-based approach. Indian J Med Res. 2020;151:190–199. doi: 10.4103/ijmr.IJMR_504_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.COVIDSurg Collaborative Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;396:27–38. doi: 10.1016/S0140-6736(20)31182-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doglietto F., Vezzoli M., Gheza F., et al. Factors associated with surgical mortality and complications among patients with and without coronavirus disease 2019 (COVID-19) in Italy. JAMA Surg. 2020;155:1–14. doi: 10.1001/jamasurg.2020.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lei S., Jiang F., Su W., et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine. 2020;21 doi: 10.1016/j.eclinm.2020.100331. :100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nahshon C., Bitterman A., Haddad R., et al. Hazardous postoperative outcomes of unexpected COVID-19 infected patients: a call for global consideration of sampling all asymptomatic patients before surgical treatment. World J Surg. 2020;44:2477–2481. doi: 10.1007/s00268-020-05575-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Doremalen N., Bushmaker T., Morris D.H., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Transmission of SARS-CoV-2: implications for infection prevention precautions. https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions Available at:
- 53.Iorio-Morin C., Hodaie M., Sarica C., et al. Letter: The risk of COVID-19 infection during neurosurgical procedures: a review of severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2) modes of transmission and proposed neurosurgery-specific measures for mitigation. Neurosurgery. 2020;87:E178–E185. doi: 10.1093/neuros/nyaa157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.COVID-19 pandemic - Wikipedia. https://en.wikipedia.org/wiki/COVID-19_pandemic Available at:
- 55.Coronavirus in the U.S.: Latest Map and Case Count - The New York Times. https://www.nytimes.com/interactive/2020/us/coronavirus-us-cases.html Available at:
- 56.Boffa D.J., Judson B.L., Billingsley K.G., et al. Pandemic recovery using a COVID-minimal cancer surgery pathway. Ann Thorac Surg. 2020;110:718–724. doi: 10.1016/j.athoracsur.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kessler R.A., Zimering J., Gilligan J., et al. Neurosurgical management of brain and spine tumors in the COVID-19 era: an institutional experience from the epicenter of the pandemic. J Neurooncol. 2020;148:211–219. doi: 10.1007/s11060-020-03523-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Daci R., Natarajan S.K., Johnson M.D. Letter: safety considerations for neurosurgical procedures during the COVID-19 pandemic. Neurosurgery. 2020;87:E239–E240. doi: 10.1093/neuros/nyaa196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eichberg D.G., Shah A.H., Luther E.M., et al. Letter: academic neurosurgery department response to COVID-19 pandemic: the University of Miami/Jackson Memorial Hospital Model. Neurosurgery. 2020;87:E63–E65. doi: 10.1093/neuros/nyaa118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lu A.C., Schmiesing C.A., Mahoney M., et al. COVID-19 preoperative assessment and testing: from surge to recovery. Ann Surg. 2020;272:e230–e235. doi: 10.1097/SLA.0000000000004124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morris M., Pierce A., Carlisle B., et al. Pre-operative COVID-19 testing and decolonization. Am J Surg. 2020;220:558–560. doi: 10.1016/j.amjsurg.2020.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Turri-Zanoni M., Battaglia P., Karligkiotis A., Locatelli D., Castelnuovo P. Managing care for patients with sinonasal and anterior skull base cancers during the COVID-19 pandemic. Head Neck. 2020;42:1503–1506. doi: 10.1002/hed.26257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perin A., Servadei F., DiMeco F., ‘Hub and Spoke’ Lombardy Neurosurgery Group May we deliver neuro-oncology in difficult times (e.g. COVID-19)? J Neurooncol. 2020;148:203–205. doi: 10.1007/s11060-020-03496-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cenzato M., DiMeco F., Fontanella M., et al. Editorial. Neurosurgery in the storm of COVID-19: suggestions from the Lombardy region, Italy (ex malo bonum) https://doi.org/10.3171/2020.3.JNS20960 [e-pub ahead of print]. J Neurosurg. [DOI] [PMC free article] [PubMed]
- 65.Molliqaj G., Schaller K. How neurosurgeons are coping with COVID-19 and how it impacts our neurosurgical practice: report from Geneva University Medical Center. World Neurosurg. 2020;139:624–627. doi: 10.1016/j.wneu.2020.04.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tan Y.T., Wang J.W., Zhao K., et al. Preliminary recommendations for surgical practice of neurosurgery department in the central epidemic area of 2019 coronavirus infection. Curr Med Sci. 2020;40:281–284. doi: 10.1007/s11596-020-2173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hu Y.J., Zhang J.M., Chen Z.P. Experiences of practicing surgical neuro-oncology during the COVID-19 pandemic. J Neurooncol. 2020;148:199–200. doi: 10.1007/s11060-020-03489-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chibbaro S., Ganau M., Todeschi J., Proust F., Cebula H. How SARS-CoV-2 is forcing us to reconsider and reorganize our daily neurosurgical practice. Neurochirurgie. 2020;66:189–191. doi: 10.1016/j.neuchi.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kolias A., Tysome J., Donnelly N., et al. A safe approach to surgery for pituitary and skull base lesions during the COVID-19 pandemic. Acta Neurochir (Wien) 2020;162:1509–1511. doi: 10.1007/s00701-020-04396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hill C.S., Muirhead W.R., Vakharia V.N., Marcus H.J., Choi D. An exit strategy for resuming nonemergency neurosurgery after severe acute respiratory syndrome coronavirus 2: a United Kingdom perspective. World Neurosurg. 2020;140:e395–e400. doi: 10.1016/j.wneu.2020.05.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.O' Connell K., Fitzpatrick F., Richmond A., Foley M., Martin F. Re: Testing recommendation for COVID-19 (SARS-CoV-2) in patients planned for surgery—continuing the service and 'suppressing' the pandemic. Br J Oral Maxillofac Surg. 2020;58:733. doi: 10.1016/j.bjoms.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.COVIDSurg Collaborative Global guidance for surgical care during the COVID-19 pandemic. https://doi.org/10.1002/bjs.11646 [e-pub ahead of print]. Br J Surg. [DOI] [PMC free article] [PubMed]
- 73.Oudrhiri M.Y., Bechri H., Hakkou E.M., Melhaoui A., Arkha Y., El Ouahabi A. Letter: neurosurgical patients' management during the COVID-19 pandemic—an institutional report from an African Neurosurgical Center. Neurosurgery. 2020;87:E230–E231. doi: 10.1093/neuros/nyaa182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lo Y.T., Yang Teo N.W., Ang B.T. Editorial. Endonasal neurosurgery during the COVID-19 pandemic: the Singapore perspective. https://doi.org/10.3171/2020.4.JNS201036 [e-pub ahead of print]. J Neurosurg. [DOI] [PMC free article] [PubMed]
- 75.SBNS COVID -19 Guidelines. https://www.sbns.org.uk/index.php/policies-and-publications/covid/ Available at:
- 76.Ramakrishna R., Zadeh G., Sheehan J.P., Aghi M.K. Inpatient and outpatient case prioritization for patients with neuro-oncologic disease amid the COVID-19 pandemic: general guidance for neuro-oncology practitioners from the AANS/CNS Tumor Section and Society for Neuro-Oncology. J Neurooncol. 2020;147:525–529. doi: 10.1007/s11060-020-03488-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Safety considerations and risk assessment. https://www.rcseng.ac.uk/coronavirus/recovery-of-surgical-services/tool-2/ Available at:
- 78.The Hong Kong Neurosurgical Society HKNS COVID-19 STATEMENT. https://www.ns.org.hk/post/hkns-covid-19-statement Available at:
- 79.The ASA and APSF Joint Statement on Perioperative Testing for the COVID-19 Virus. https://www.asahq.org/about-asa/newsroom/news-releases/2020/04/asa-and-apsf-joint-statement-on-perioperative-testing-for-the-covid-19-virus Available at:
- 80.Fleseriu M., Buchfelder M., Cetas J.S., et al. Pituitary society guidance: pituitary disease management and patient care recommendations during the COVID-19 pandemic—an international perspective. Pituitary. 2020;23:327–337. doi: 10.1007/s11102-020-01059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Castelnuovo P., Turri-Zanoni M., Karligkiotis A., et al. Skull-base surgery during the COVID-19 pandemic: the Italian Skull Base Society recommendations. Int Forum Allergy Rhinol. 2020;10:963–967. doi: 10.1002/alr.22596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mehanna H., Hardman J.C., Shenson J.A., et al. Recommendations for head and neck surgical oncology practice in a setting of acute severe resource constraint during the COVID-19 pandemic: an international consensus. Lancet Oncol. 2020;21:e350–e359. doi: 10.1016/S1470-2045(20)30334-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gupta P., Muthukumar N., Rajshekhar V., et al. Neurosurgery and neurology practices during the novel COVID-19 pandemic: a consensus statement from India. Neurol India. 2020;68:246–254. doi: 10.4103/0028-3886.283130. [DOI] [PubMed] [Google Scholar]
- 84.Muhammad S., Tanikawa R., Lawton M.T., et al. Letter: safety instructions for neurosurgeons during COVID-19 pandemic based on recent knowledge and experience. Neurosurgery. 2020;87:E220–E221. doi: 10.1093/neuros/nyaa184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zacharia B.E., Eichberg D.G., Ivan M.E., et al. Letter: surgical management of brain tumor patients in the COVID-19 era. Neurosurgery. 2020;87:E197–E200. doi: 10.1093/neuros/nyaa162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.COVID-19 pandemic lockdown in India. https://en.wikipedia.org/w/index.php?title=COVID-19_pandemic_lockdown_in_India&oldid=982699588 Wikipedia; 2020. Available at: