Abstract
Objectives
The aim of our study was to describe the incidence and predictive factors of secondary infections in patients with coronavirus disease 2019 (COVID-19).
Methods
This was a cohort study of patients hospitalized with COVID-19 at IRCCS San Raffaele Hospital between 25th February and 6th April 2020 (NCT04318366). We considered secondary bloodstream infections (BSIs) or possible lower respiratory tract infections (pLRTIs) occurring 48 hours after hospital admission until death or discharge. We calculated multivariable Fine–Gray models to assess factors associated with risk of secondary infections.
Results
Among 731 patients, a secondary infection was diagnosed in 68 patients (9.3%); 58/731 patients (7.9%) had at least one BSI and 22/731 patients (3.0%) at least one pLRTI. The overall 28-day cumulative incidence was 16.4% (95%CI 12.4–21.0%). Most of the BSIs were due to Gram-positive pathogens (76/106 isolates, 71.7%), specifically coagulase-negative staphylococci (53/76, 69.7%), while among Gram-negatives (23/106, 21.7%) Acinetobacter baumanii (7/23, 30.4%) and Escherichia coli (5/23, 21.7%) predominated. pLRTIs were caused mainly by Gram-negative pathogens (14/26, 53.8%). Eleven patients were diagnosed with putative invasive aspergillosis. At multivariable analysis, factors associated with secondary infections were low baseline lymphocyte count (≤0.7 versus >0.7 per 109/L, subdistribution hazard ratios (sdHRs) 1.93, 95%CI 1.11–3.35), baseline PaO2/FiO2 (per 100 points lower: sdHRs 1.56, 95%CI 1.21–2.04), and intensive-care unit (ICU) admission in the first 48 hours (sdHR 2.51, 95%CI 1.04–6.05).
Conclusions
Patients hospitalized with COVID-19 had a high incidence of secondary infections. At multivariable analysis, early need for ICU, respiratory failure, and severe lymphopenia were identified as risk factors for secondary infections.
Keywords: Bacterial infections, Bloodstream infections, COVID-19, Fungal infections, Lower respiratory tract infections, SARS-CoV-2, Secondary infections
Introduction
The pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has affected more than 32 million patients worldwide as of 27th September 2020 [1]. The clinical manifestations of this condition (coronavirus disease 2019, COVID-19) range from asymptomatic infection to severe viral pneumonia requiring treatment in an intensive care unit (ICU) [[2], [3], [4]].
SARS-CoV-2 can directly damage the lung epithelium and indirectly ignite an aberrant ‘cytokine storm’, eventually leading to multi-organ failure [5,6]. To reverse this dysregulated activation of the immune system, immunosuppressive drugs are widely used [6,7]. A combination of virus- and drug-induced immunosuppression likely increases the susceptibility to secondary infections.
Nevertheless, few reports of infectious complications in COVID-19 are available to date [[8], [9], [10]]. The primary objective of this study was to estimate the cumulative incidence of secondary infections since hospital admission in patients with COVID-19 admitted to our institution (study baseline). The secondary objective included the evaluation of risk factors for secondary infections.
Methods
Study population
Patients considered in this analysis are part of the COVID-19 prospective institutional cohort (COVID-BioB [[11], [12], [13]]) at the Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) San Raffaele Hospital, a 1350-bed tertiary-care hospital in Milan, Italy. We included all patients admitted with COVID-19 between 25th February 2020 and 6th April 2020 who gave written consent and whose outcome (discharge or death) was known up to 19th May 2020.
COVID-19 was defined as a positive real-time reverse transcriptase polymerase chain reaction (RT-PCR) for SARS-CoV-2 from a nasopharyngeal swab associated with suggestive signs, symptoms, and/or radiological findings.
Patient management and microbiological methods are described in the Supplementary Material.
Bloodstream infections (BSIs) were defined as a single positive blood culture for a likely pathogen or two or more positive blood cultures for common skin colonizers (i.e. coagulase-negative staphylococci, diphtheroids, Bacillus spp., Propionibacterium spp., viridans group streptococci), without a concomitant microbiologically documented lower respiratory tract infection due to the same pathogen. Patients who had more than one positive blood culture within 7 days from the first positive blood culture were considered to have a single episode of BSI with multiple isolates.
Positive cultures of potentially pathogenic organisms from the lower respiratory tract were defined as positive culture of a respiratory specimen obtained with invasive techniques (bronchoscopy-guided bronchoalveolar lavage (BAL) or, when not available, bronchial aspirate (BRASP)), excluding Candida spp. Putative aspergillosis was defined according to the criteria developed for critically ill patients by Blot et al. [14] (with the inclusion of serum or BAL galactomannan assay), in order to differentiate it from respiratory tract colonization.
Both blood and respiratory cultures were requested by the attending physicians in patients with suspected secondary infections because of clinical and/or respiratory deterioration associated with suggestive laboratory or radiological findings. BAL and BRASP were not routinely collected for surveillance. Galactomannan assay was requested only when invasive aspergillosis was considered as a possible diagnosis by the attending physician (99 patients tested). Therefore, we included as possible lower respiratory tract infections (pLRTIs) patients with a suspected secondary infection and with either positive cultures of potentially pathogenic organisms from the lower respiratory tract or putative aspergillosis.
Patients for whom no microbiology specimens were requested were considered not to have secondary infections.
Ethics
The study was approved by the hospital ethics committee (protocol No. 34/int/2020) and was registered on ClinicalTrials.gov (NCT04318366).
Statistical analysis
Results of continuous variables were described by median (quartiles) while categorical variables were described by frequency (%).
We compared characteristics and outcomes of patients who had at least one secondary infection during hospitalization and those who did not using the χ-square test or Fisher's exact test for categorical variables and the Mann–Whitney U test for continuous variables.
In the analysis we used three scores (the cytolysis score, the coagulation score, and the inflammation score), defined as the number of laboratory parameters with markedly elevated values (values at or above the 75th percentile). Absolute lymphocyte counts were stratified on the 25th percentile (at or below).
Incidence rates of secondary infections were defined as cases occurring at least 48 hours after hospital admission; they were calculated by univariable Poisson regression and reported as number of secondary infections per 1000 person-days of follow-up (PDFUs).
Incidence rate calculation according to patients' stay in the ICU during hospitalization used a time-dependent approach accounting for the person-time during follow-up. Only secondary infections occurring at least 48 hours after ICU admission were considered as ICU-acquired.
The cumulative incidence function (CIF) of one or more secondary infections was calculated in the overall cohort, according to absolute lymphocyte count and PaO2/FiO2 with Gray's method [15]; 95% confidence intervals (CIs) for survival probabilities and cumulative incidence were calculated accounting for competing risks of death.
We subsequently performed inverse probability-weighted (IPW) competing risks multivariable analyses to simultaneously account for indication bias associated with the treatment with biological immunosuppressive drugs and competing death for the estimation of the cumulative incidence of patients with secondary infections, to provide a more accurate estimate of the secondary infections burden [15,16].
A logistic regression analysis was applied to estimate the propensity of biological immunosuppressive drug use, conditioned on a prespecified list of baseline covariates; the predicted probabilities of biological immunosuppressive drug treatment (propensity-score) were used to calculate the stabilized IPW in order to account for non-randomization to biological drugs in this observational study.
The inverses of these propensities were used as weights in multivariable Fine–Gray models assessing the association between demographic and other clinical or laboratory factors and the risk of secondary infections.
All statistical tests were two-sided at 5% level and were performed using SAS 9.4 (Statistical Analyses System Inc, Cary, NC, USA).
Further details on the cytolysis score, the coagulation score, and the inflammation score definitions and on the statistical analyses are provided in the Supplementary Material.
Results
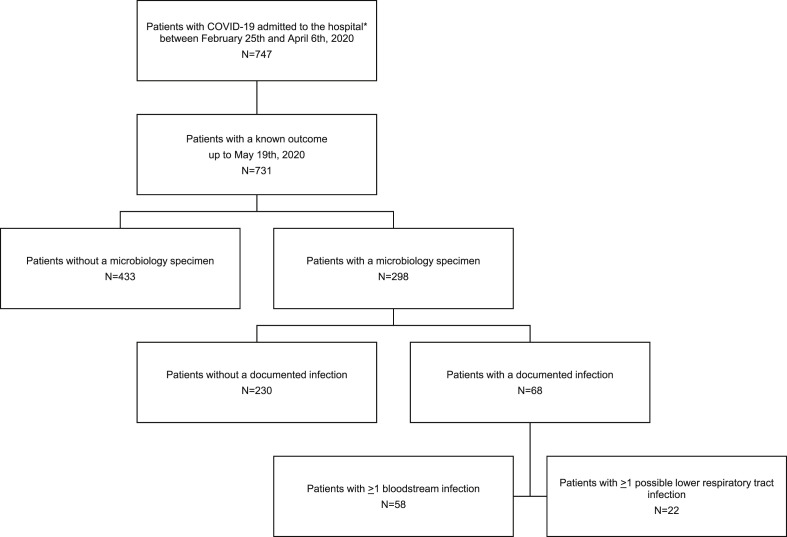
The characteristics of 731 patients are shown in Table 1, Table 2 . Blood or lower respiratory tract cultures were performed in 298/731 patients (40.8%), and a microbiologically documented infection was diagnosed in 68/731 patients (9.3%) (Fig. 1 ); 58/731 patients (7.9%) had at least one BSI and 22/731 patients (3.0%) had at least one pLRTI. Overall, 74 cases of BSIs and 24 cases of pLRTIs were documented. Eleven patients had more than one BSI, while two patients had two pLRTIs. Median time to the first secondary infection from hospital admission was 12 days (8.5–16.5).
Table 1.
Characteristics of patients hospitalized with coronavirus disease 19 (COVID-19)
| Characteristic | Category | Overall (n = 731) |
With ≥1 secondary infection (n = 68) |
Without secondary infection (n = 663) |
pa |
|---|---|---|---|---|---|
| Demographic characteristics: | |||||
| Age, years | 64 (55–76) | 63 (56–70) | 64 (54–76) | 0.504 | |
| Sex, male | 496 (67.9%) | 53 (77.9%) | 443 (66.8%) | 0.076 | |
| Comorbidities: | |||||
| Hypertension | 0.231 | ||||
| No | 383 (53.2%) | 37 (60.7%) | 346 (52.5%) | ||
| Yes | 337 (46.8%) | 24 (39.3%) | 313 (47.5%) | ||
| Coronary heart disease | 0.277 | ||||
| No | 547 (76%) | 50 (82%) | 497 (75.4%) | ||
| Yes | 173 (24%) | 11 (18%) | 162 (24.6%) | ||
| Diabetes mellitus | 0.225 | ||||
| No | 591 (82%) | 47 (75.8%) | 544 (82.5%) | ||
| Yes | 130 (18%) | 15 (24.2%) | 115 (17.5%) | ||
| Chronic obstructive pulmonary disease | 0.789 | ||||
| No | 673 (93.5%) | 59 (95.2%) | 614 (93.3%) | ||
| Yes | 47 (6.5%) | 3 (4.8%) | 44 (6.7%) | ||
| Chronic kidney disease | 0.666 | ||||
| No | 646 (89.5%) | 57 (91.9%) | 589 (89.2%) | ||
| Yes | 76 (10.5%) | 5 (8.1%) | 71 (10.8%) | ||
| Malignancies | 0.084 | ||||
| No | 618 (85.8%) | 57 (93.4%) | 561 (85.1%) | ||
| Yes | 102 (14.2%) | 4 (6.6%) | 98 (14.9%) | ||
| Liver disease | 0.461 | ||||
| No | 412 (97.6%) | 24 (96%) | 388 (97.7%) | ||
| Yes | 10 (2.4%) | 1 (4%) | 9 (2.3%) | ||
| Comorbidities | 0.037 | ||||
| Yes | 442 (60.5%) | 33 (48.5%) | 409 (61.7%) | ||
| Body mass index, kg/m2 | 26.54 (24.17–29.41) | 26.83 (24.69–29.38) | 26.53 (24.02–29.64) | 0.474 | |
| Clinical characteristics and management: | |||||
| Days from symptoms to hospital admission | 6.5 (3–10) n = 400 |
5.5 (3–10) n = 22 |
6.5 (3–10) n = 378 |
0.617 | |
| Fever, Celsius degrees | 37.7 (36.8–38.5) n = 643 |
38 (37.1–38.7) n = 54 |
37.7 (36.8–38.4) n = 589 |
0.099 | |
| PaO2/FiO2 | 271 (191–325) | 181 (88–248) | 281 (210–328) | <0.0001 | |
| PaO2/FiO2 | <0.0001 | ||||
| >300 | 209 (28.6%) | 6 (8.8%) | 203 (30.6%) | ||
| 201-300 | 220 (30.1%) | 21 (30.9%) | 199 (30%) | ||
| 101–200 | 78 (10.7%) | 16 (23.5%) | 62 (9.4%) | ||
| <100 | 78 (10.7%) | 18 (26.5%) | 60 (9%) | ||
| Missing | 146 (20%) | 7 (10.3%) | 139 (21%) | ||
| ICU admission <48 hours from hospital admission | 45 (6.2%) | 20 (29.4%) | 25 (3.8%) | <0.0001 | |
| Use of biological immunosuppressive drugs | 129 (17.6%) | 18 (26.5%) | 111 (16.7%) | 0.045 | |
| Type of biological immunosuppressive drug | 0.677 | ||||
| Anakinra | 54 (41.9%) | 8 (44.4%) | 46 (41.4%) | ||
| Mavrilimumab | 13 (10.1%) | 1 (5.6%) | 12 (10.8%) | ||
| Sarilumab | 25 (19.4%) | 5 (27.8%) | 20 (18%) | ||
| Tocilizumab | 37 (28.7%) | 4 (22.2%) | 33 (29.7%) | ||
| Days to biological immunosuppressive drugs start from hospital admission | 3 (2–5) | 3 (2–5) | 3 (2–5) | 0.733 | |
Results reported as median (IQR) or frequency (%). For variables with more than 10% of missing values actual number of observations were reported.
ICU, intensive care unit; NA, not applicable.
By χ-square or Fisher's exact test (categorical variables) or Wilcoxon rank-sum test (continuous variables).
Table 2.
Baseline laboratory parameters of patients hospitalized with coronavirus disease 19 (COVID-19)
| Laboratory parameter | Category | Overall (n = 731) |
With ≥1 secondary infection (n = 68) |
Without secondary infection (n = 663) |
pa |
|---|---|---|---|---|---|
| White blood cells, per 109/L | 6.85 (5–9.9) | 7.7 (5.05–12.05) | 6.8 (5–9.7) | 0.027 | |
| Lymphocytes, per 109/L | 0.9 (0.7–1.3) n = 638 |
0.8 (0.6–1.1) n = 57 |
1 (0.7–1.3) n = 581 |
0.009 | |
| Lymphocytes, per 109/L | 0.024 | ||||
| ≤0.7 | 202 (31.7%) | 26 (45.6%) | 176 (30.3%) | ||
| >0.7 | 436 (68.3%) | 31 (54.4%) | 405 (69.7%) | ||
| Neutrophils, per 109/L | 5.1 (3.4–7.8) n = 638 |
6 (4.0–9.7) n = 57 |
4.9 (3.3–7.5) n = 581 |
0.005 | |
| Haemoglobin, g/dL | 13.6 (12.2–14.7) | 13.75 (12.2–14.95) | 13.6 (12.2–14.7) | 0.533 | |
| Platelets, per 109/L | 203.5 (157–264) | 213.5 (153.5–314.5) | 203 (158–262) | 0.388 | |
| Creatinine, mg/dL | 0.98 (0.80–1.24) | 1 (0.85–1.27) | 0.98 (0.80–1.23) | 0.485 | |
| Alanine aminotransferase, U/L | 35 (23–56) | 41 (28–66) | 34 (23–56) | 0.039 | |
| Aspartate aminotransferase, U/L | 45 (31–65) | 54 (38–90) | 44 (31–63) | 0.005 | |
| Lactate dehydrogenase, U/L | 371 (278–483) | 440 (352–597) | 362 (275–466) | <0.0001 | |
| Ferritin, ng/mL | 1111 (618–2198) n = 389 |
1876 (1045–2972) n = 41 |
1072.5 (594–1859) n = 348 |
0.001 | |
| Lactates, mmol/L | 1.30 (1.01–1.75) n = 651 |
1.51 (1.14–2.07) n = 66 |
1.28 (0.99–1.72) n = 585 |
0.005 | |
| D-dimer, μg/mL | 1.25 (0.62–2.64) n = 346 |
1.51 (1.13–3.25) n = 39 |
1.19 (0.59–2.55) n = 307 |
0.027 | |
| Prothrombin time, s | 13.9 (13.2–15.3) | 14.1 (12.8–15.5) | 13.9 (13.2–15.3) | 0.898 | |
| C-reactive protein, mg/L | 72.4 (31.2–134.6) | 136.7 (48.9–217.9) | 70.9 (29.5–129.8) | <0.0001 | |
| Procalcitonin, ng/mL | 0.52 (0.31–0.91) n = 382 |
0.68 (0.37–1.36) n = 49 |
0.52 (0.30–0.87) n = 333 |
0.215 | |
| Cytolysis score | 0.001 | ||||
| 0 | 405 (56.1%) | 29 (42.6%) | 376 (57.5%) | ||
| 1 | 149 (20.6%) | 15 (22.1%) | 134 (20.5%) | ||
| 2 | 107 (14.8%) | 10 (14.7%) | 97 (14.8%) | ||
| 3 | 61 (8.4%) | 14 (20.6%) | 47 (7.2%) | ||
| Inflammation score | <0.0001 | ||||
| 0 | 488 (67.6%) | 26 (38.8%) | 462 (70.5%) | ||
| 1 | 192 (26.6%) | 30 (44.8%) | 162 (24.7%) | ||
| 2 | 42 (5.8%) | 11 (16.4%) | 31 (4.7%) | ||
| Coagulation score | 0.144 | ||||
| 0 | 414 (66.6%) | 43 (64.2%) | 371 (66.8%) | ||
| 1 | 168 (27%) | 16 (23.9%) | 152 (27.4%) | ||
| 2 | 40 (6.4%) | 8 (11.9%) | 32 (5.8%) | ||
Cytolysis score included alanine aminotransferase, aspartate aminotransferase, lactate dehydrogenase. Inflammation score included ferritin and C-reactive protein. Coagulation score included D-dimer and prothrombin time. The three scores had a range of 0–2 or –3, with 0 corresponding to no abnormalities in inflammatory parameters levels and 2 or 3 corresponding to patients with markedly elevated values for all the considered laboratory parameters. See Methods and Supplementary Material sections for further details.
By χ-square or Fisher's exact test (categorical variables) or Wilcoxon rank-sum test (continuous variables).
Fig. 1.
Flow-chart of study cohort. ∗Who gave consent to be recorded in the COVID-BioB database and to use their data.
Biological immunosuppressive drugs were used in 129/731 patients (17.6%), among whom 14.0% (18/129) developed at least one secondary infection (median time from treatment initiation: 9 days (7–17)).
Data regarding steroid use was available for 483/731 patients (66.1%); among 108/483 patients (22.4%) who received steroids at any time during hospital stay, 11/108 (10.2%) developed at least one secondary infection compared to 28/375 (7.5%) who did not receive corticosteroid treatment (p 0.422).
Overall, 86/731 patients (11.8%) were admitted to the ICU (median days to ICU admission from hospital admission: 2 (0–6)). Patients admitted to the ICU in the first 48 hours (45/86, 52.3%) more frequently experienced a secondary infection compared to patients never admitted or admitted after 48 hours (Table 1). During 9720 PDFUs, 32/731 (4.4%) patients had 39 secondary infections outside the ICU for an incidence rate of 4.0 (2.9–5.5) per 1000 PDFUs, while during 1318 PDFUs 40/731 patients (5.5%) had 51 secondary infections during their ICU stay for an incidence rate of 38.7 (28.8–50.9) per 1000 PDFUs (p < 0.0001). The incidence rate of BSIs was 3.3 (2.3–4.6) per 1000 PDFUs in patients outside the ICU, compared to 31.9 (23.0–43.1) per 1000 PDFUs in patients admitted to the ICU (p < 0.0001), while for pLRTIs the incidence rate outside the ICU was 0.4 (0.1–1.1) compared to 15.2 (9.3–23.4) per 1000 PDFUs in patients inside the ICU (p < 0.0001).
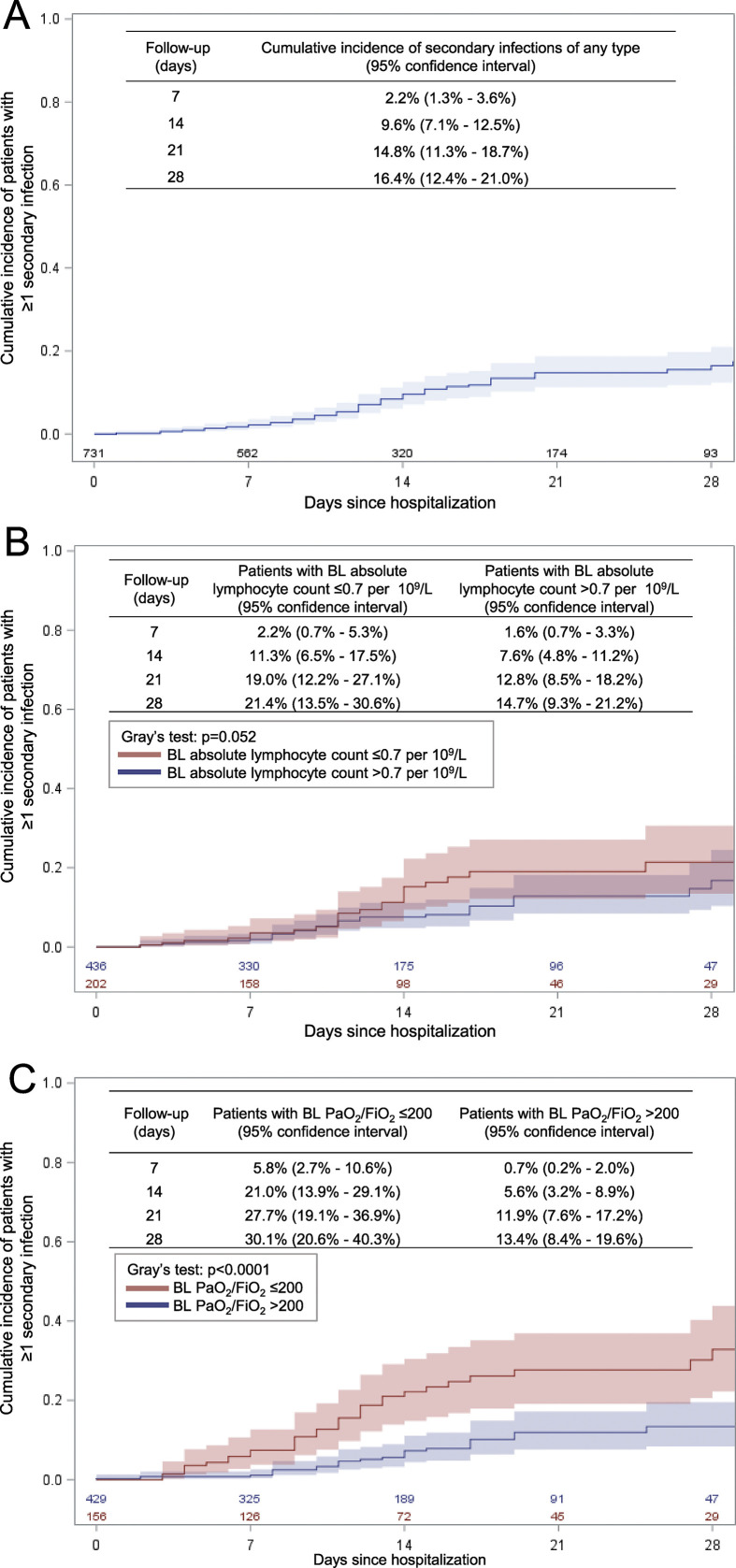
The cumulative incidence functions of secondary infection in the overall cohort and according to baseline PaO2/FiO2 ratio and absolute lymphocyte count are shown in Fig. 2 .
Fig. 2.
Cumulative incidence of secondary infections of any type (panel A), according to baseline (BL) absolute lymphocyte count (panel B) and to BL PaO2/FiO2 (panel C).
While baseline C-reactive protein (CRP) values were significantly different at baseline, procalcitonin was not significantly higher in patients who experienced a secondary infection (Table 2). Levels of CRP at the time of the first secondary infection were significantly lower when compared to values at the time of the first microbiological specimen requested in patients with negative cultures (85 mg/L (36–135) versus 118 mg/L (52–181), p 0.040), while procalcitonin was similar between the two groups (0.88 ng/mL (0.46–1.94), n = 62, versus 0.69 ng/mL (0.33–1.94), n = 119; p 0.157).
Among patients with secondary BSIs (Supplementary Material Table S1), median time to the first secondary BSI after hospital admission was 13 days (8–16). Incidence rate was 6.7 (5.3–8.4) per 1000 PDFUs. The majority of BSIs were due to Gram-positive pathogens (76/106 isolates, 71.7%), specifically coagulase-negative staphylococci (53/76, 69.7%), while among Gram-negatives (23/106, 21.7%) Acinetobacter baumanii (7/23, 30.4%) and Escherichia coli (5/23, 21.7%) predominated.
Among patients with secondary pLRTIs (Supplementary Material Table S2), median time to the first secondary pLRTI after hospital admission was 16 days (10–29). Incidence rate was 2.1 (1.4–3.2) per 1000 PDFUs. pLRTIs were caused mainly by Gram-negative pathogens (14/26, 53.8%), principally Pseudomonas aeruginosa (6/14, 42.9%). Microbial aetiology and multidrug-resistant organisms (MDROs) are detailed in Supplementary Material Table S3.
Eleven patients were diagnosed with putative aspergillosis; 10/11 were diagnosed in the ICU, only one patient received biological immunosuppressive drugs.
Overall, 194/731 patients (26.5%) died: 30/68 (44.1%) with secondary infections and 164/663 (24.7%) without further infectious events (p 0.001). Median time to death after the first secondary infection was 9 days (4–19).
At multivariable analysis (Table 3 ), the factors associated with the development of secondary infections were baseline lymphocyte count, baseline PaO2/FiO2 ratio, and ICU admission in the first 48 hours after hospital admission. These findings were confirmed in additional models including a higher number of variables and observations (Supplementary Material Table S4), and after excluding patients who died in the first 5 days, to minimize the risk of confounding due to immortal time bias (Supplementary Material Tables S5 and S6).
Table 3.
Multivariable analysis on the risk of secondary infections of any type in patients hospitalized with coronavirus disease 19 (COVID-19)
| Baseline characteristics (n = 512) |
Subdistribution hazard ratio | 95% confidence interval | |
|---|---|---|---|
| Age, >65 versus ≤65 years | 0.57 | 0.30 | 1.10 |
| Comorbidities, yes versus no | 0.77 | 0.40 | 1.46 |
| Cytolysis score, per 1 point higher | 1.06 | 0.82 | 1.37 |
| Inflammation score, per 1 point higher | 1.14 | 0.76 | 1.70 |
| Lymphocyte count, ≤0.7 versus >0.7 per 109/L | 1.93 | 1.11 | 3.35 |
| PaO2/FiO2, per 100 points lower | 1.56 | 1.21 | 2.04 |
| ICU admission <48 hours from hospital admission, yes versus no | 2.51 | 1.04 | 6.05 |
| Use of biological immunosuppressive drugs, yes versus no | 1.74 | 0.88 | 3.43 |
Cytolysis score included alanine aminotransferase, aspartate aminotransferase, lactate dehydrogenase. Inflammation score included ferritin and C-reactive protein. See Methods and Supplementary Material sections for further details.
ICU, intensive care unit.
Discussion
We described the incidence and characteristics of secondary infections in a large cohort of patients hospitalized with COVID-19. Among 731 patients, 68 (9.3%) had at least one secondary infection, with an overall 28-day cumulative incidence of 16.4% (95%CI 12.4–21.0%). Our results are coherent with reports from available large cohorts, where the proportion of patients with secondary infections ranged from 5% to 30% [[8], [9], [10],[17], [18], [19], [20]].
In our cohort, the incidence rate of BSIs appears significantly higher compared to those in previous reports concerning nosocomial and ICU-related BSIs in European countries [21,22] (ranges 0.5–1.3 and 0.7–6.6 per 1000 PDFUs, respectively). Similarly, the incidence rate of pLRTIs among patients with COVID-19 admitted to the ICU appears to be higher than in historical European cohorts [22] (7.3 per 1000 PDFUs for ICU-acquired pneumonia and 9.5 per 1000 PDFUs for intubation-associated pneumonia).
BSIs constituted the majority of secondary infections, with 58/731 patients (7.9%) experiencing at least one event. We documented a high rate of BSIs due to coagulase-negative staphylococci and a noteworthy proportion of patients with multiple isolates. This finding may reflect a high burden of catheter-associated infections. However, data regarding the presence of intravascular catheters were not available. Several factors may have contributed to a higher incidence of BSIs due to coagulase-negative staphylococci. First, being in the epicentre of the COVID-19 pandemic in our country, and given the unprecedented strain on our healthcare system, critically ill patients with multiple devices were managed outside the ICU, possibly leading to increased rates of BSIs due to common skin colonizers. Second, the pandemic setting may have reduced the adherence to strict aseptic procedures, especially in critically ill patients managed outside the ICU or in overcrowded or makeshift ICUs. Furthermore, the adequate use of personal protective equipment may be challenging and may have potentially led to reduced compliance with aseptic techniques in managing intravascular devices.
pLRTIs were documented in 22/731 patients (3.0%), and Gram-negative pathogens predominated, with the majority of cases due to non-fermenting bacteria. Eleven patients were considered to have putative invasive aspergillosis. Other authors reported invasive fungal infections in patients with COVID-19 admitted to the ICU [23,24]. The diagnosis of invasive aspergillosis in patients with COVID-19 without other known underlying risk factors may be challenging [25]. Nevertheless, in our experience, 9/11 patients had a positive culture or galactomannan assay from a bronchoscopy-guided BAL, strengthening the results of our study.
The burden of antimicrobial resistance was substantial. Indeed, when compared with a historical cohort of BSIs from our institution (year 2017, unpublished data), patients hospitalized with COVID-19 had a significantly higher incidence rate of BSIs due to Gram-negative MDROs (11.8 (6.3–20.1) per 1000 PDFUs versus 4.9 (4.2–5.7) per 1000 PDFUs, p 0.006), and specifically due to Acinetobacter baumannii (6.3 (2.5–13.1) per 1000 PDFUs versus 0.4 (0.2–0.7) per 1000 PDFUs, p < 0.001), while only vancomycin-resistant Enterococcus faecium was more frequently implicated among Gram-positive bacteria (3.6 (1.0–9.3) per 1000 PDFUs versus 0.5 (0.3–0.8) per 1000 PDFUs, p 0.004). Given the possible misuse of empirical broad-spectrum antibiotics in patients with severe COVID-19, the focus should be on prevention and careful use of antimicrobials to reduce the development of resistance [20,26].
At multivariable analysis, severe hypoxaemia, severe lymphopenia, and need for ICU in the first 48 hours after hospital admission were shown to be predictive factors for secondary infections. Patients with acute respiratory distress syndrome have diffuse alveolar damage with inflammatory infiltrates, potentially predisposing to superinfection [27]. The need for ICU and invasive procedures likely contributes to the risk of secondary infections in patients with COVID-19, regardless of clinical severity. Lymphopenia is common in patients with severe COVID-19 [17] and a known predictive factor for mortality [28]. Whether the occurrence of secondary infections may also be related to the higher mortality seen in patients with lymphopenia needs to be explored.
Our study has several limitations. First, being a single-centre experience from a tertiary-care hospital in the epicentre of the COVID-19 pandemic, our results may not be generalizable. Second, data regarding empirical antimicrobial use were not available, limiting the assessment of its impact on the development of secondary infections. Moreover, no data regarding the presence of intravascular catheters were available, preventing us from evaluating the proportion of catheter-related infections. Third, the distinction between infection and contamination/colonization may not always be possible, thus leading to an overestimation of secondary infections, while the inclusion of microbiologically documented infections and the adoption of a 7-day cut-off from the first positive blood culture to define a single episode of secondary BSI may lead to an underestimation of the true burden of secondary infections. Nevertheless, the exclusion of potential contaminants from blood cultures and the inclusion of patients with positive cultures obtained only from invasive respiratory procedures strengthen our results. Finally, clinical and laboratory data used in the multivariable models were not available for all patients in our cohort, potentially leading to a loss of precision of the reported estimates. Nevertheless, the supplementary model including more than 85% of our cohort confirmed the results obtained in the main analysis. We recognize that the low number of events was associated with a different degree of class imbalance in some predictive variables, and might have influenced our findings. However, based on several sensitivity analyses, we are confident that the suggested factors are those likely associated with the risk of secondary infections.
In conclusion, we have described the incidence and predictive factors of secondary bloodstream and lower respiratory tract infections in patients with COVID-19, showing a high burden of infections due to Gram-positive pathogens and multidrug-resistant Gram-negative bacteria, along with a non-negligible proportion of patients with putative invasive aspergillosis. Patients with respiratory failure, severe lymphopenia, and early need for ICU treatment were shown to be at higher risk of secondary infections.
Author contributions
Conceptualization: MR, AM, PS, AC. Methodology: LG. Software: LG, AP. Formal analysis: LG, AP. Investigation: MR, CO, VS, AM, CM, GM, GDL, PS. Resources: LG, AP, GL, LD, MC, PRQ, FC, MT, AL, AZ. Data curation: MR, CO, VS, AP, AM, CM. Writing—Original draft: MR, AM, LG. Writing—Review & Editing: MR, LG, AP, CO, VS, AM, CM, GM, GDL, GL, LD, MC, PRQ, FC, MT, AL, AZ, PS, AC. Visualization: MR, LG, AP. Supervision: GL, LD, MC, PRQ, FC, MT, AL, AZ, PS, AC. Project administration: GL, LD, MC, PRQ, FC, MT, AL, AZ, PS, AC. All authors approved the final version.
Transparency declaration
AC has received consultancy payments and speaking fees from Bristol-Myers Squibb, Gilead, ViiV Healthcare, Merck Sharp & Dohme and Janssen-Cilag. AL has received consultancy payments and speaking fees from Bristol-Myers Squibb, Gilead Sciences, ViiV Healthcare, Merck Sharp & Dohme, AbbVie and Janssen-Cilag. All other authors declare that they have no conflicts of interest. There was no funding source for this study.
Acknowledgments
We thank all the doctors, nurses and site personnel. We thank Dr Mila Ann Kalapurackal for language revision.
Editor: E.J. Kuipers
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2020.10.021.
Contributor Information
COVID-BioB study group:
Andrea Andolina, Martina Baiardo Redaelli, Giorgia Bigai, Alba Bigoloni, Giorgia Borio, Simona Bossolasco, Elena Bruzzesi, Maria Grazia Calabrò, Stefania Calvisi, Corrado Campochiaro, Diana Canetti, Valentina Canti, Jacopo Castellani, Barbara Castiglioni, Giulio Cavalli, Ludovica Cavallo, Massimo Cernuschi, Matteo Chiurlo, Marta Cilla, Elena Cinel, Paola Cinque, Caterina Conte, Valentina Da Prat, Anna Danise, Rebecca De Lorenzo, Antonio Dell’Acqua, Raffaele Dell’Acqua, Emanuel Della Torre, Liviana Della Torre, Gaetano Di Terlizzi, Iulia Dumea, Federica Farolfi, Marica Ferrante, Claudia Frangi, Luca Fumagalli, Gabriele Gallina, Bruno Germinario, Nicola Gianotti, Monica Guffanti, Hamid Hasson, Francesca Lalla, Marco Lanzillotta, Raffaele Li Voti, Emanuela Messina, Chiara Molinari, Elena Moizo, Marco Montagna, Giulia Morsica, Silvia Nozza, Maria Pascali, Alessandro Patrizi, Marina Pieri, Antonella Poloniato, Dario Prestifilippo, Giuseppe Ramirez, Martina Ranzenigo, Jacopo Sapienza, Federico Seghi, Giuseppe Tambussi, Chiara Tassan Din, Stefano Turi, Caterina Uberti-Foppa, and Concetta Vinci
Appendix.
Members of the COVID-BioB study group: Andrea Andolina, Martina Baiardo Redaelli, Giorgia Bigai, Alba Bigoloni, Giorgia Borio, Simona Bossolasco, Elena Bruzzesi, Maria Grazia Calabrò, Stefania Calvisi, Corrado Campochiaro, Diana Canetti, Valentina Canti, Jacopo Castellani, Barbara Castiglioni, Giulio Cavalli, Ludovica Cavallo, Massimo Cernuschi, Matteo Chiurlo, Marta Cilla, Elena Cinel, Paola Cinque, Caterina Conte, Valentina Da Prat, Anna Danise, Rebecca De Lorenzo, Antonio Dell’Acqua, Raffaele Dell’Acqua, Emanuel Della Torre, Liviana Della Torre, Gaetano Di Terlizzi, Iulia Dumea, Federica Farolfi, Marica Ferrante, Claudia Frangi, Luca Fumagalli, Gabriele Gallina, Bruno Germinario, Nicola Gianotti, Monica Guffanti, Hamid Hasson, Francesca Lalla, Marco Lanzillotta, Raffaele Li Voti, Emanuela Messina, Chiara Molinari, Elena Moizo, Marco Montagna, Giulia Morsica, Silvia Nozza, Maria Pascali, Alessandro Patrizi, Marina Pieri, Antonella Poloniato, Dario Prestifilippo, Giuseppe Ramirez, Martina Ranzenigo, Jacopo Sapienza, Federico Seghi, Giuseppe Tambussi, Chiara Tassan Din, Stefano Turi, Caterina Uberti-Foppa, Concetta Vinci.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Coronavirus Disease (COVID-19) situation reports. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports
- 2.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020 doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X., Yu Y., Xu J., Shu H., Xia J’an, Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jamilloux Y., Henry T., Belot A., Viel S., Fauter M., El Jammal T. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun Rev. 2020:102567. doi: 10.1016/j.autrev.2020.102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu Y., Xu D., Fu S., Zhang J., Yang X., Xu L. Patients with COVID-19 in 19 ICUs in Wuhan, China: a cross-sectional study. Crit Care. 2020;24:219. doi: 10.1186/s13054-020-02939-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dudoignon E., Caméléna F., Deniau B., Habay A., Coutrot M., Ressaire Q. Bacterial pneumonia in COVID-19 critically ill patients: a case series. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes S., Troise O., Donaldson H., Mughal N., Moore L.S. Bacterial and fungal coinfection among hospitalised patients with COVID-19: a retrospective cohort study in a UK secondary care setting. Clin Microbiol Infect. 2020;26:1395–1399. doi: 10.1016/j.cmi.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zangrillo A., Beretta L., Scandroglio A.M., Monti G., Fominskiy E., Colombo S. Characteristics, treatment, outcomes and cause of death of invasively ventilated patients with COVID-19 ARDS in Milan, Italy. Crit Care Resusc. 2020;22:200–211. doi: 10.1016/S1441-2772(23)00387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pagnesi M., Baldetti L., Beneduce A., Calvo F., Gramegna M., Pazzanese V. Pulmonary hypertension and right ventricular involvement in hospitalised patients with COVID-19. Heart. 2020;106:1324–1331. doi: 10.1136/heartjnl-2020-317355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciceri F., Castagna A., Rovere-Querini P., De Cobelli F., Ruggeri A., Galli L. Early predictors of clinical outcomes of COVID-19 outbreak in Milan, Italy. Clin Immunol. 2020;217:108509. doi: 10.1016/j.clim.2020.108509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blot S.I., Taccone F.S., Van den Abeele A.-M., Bulpa P., Meersseman W., Brusselaers N. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am J Respir Crit Care Med. 2012;186:56–64. doi: 10.1164/rccm.201111-1978OC. [DOI] [PubMed] [Google Scholar]
- 15.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Statist Assoc. 1999;94:496–509. doi: 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 16.Kim H.T. Cumulative incidence in competing risks data and competing risks regression analysis. Clin Cancer Res. 2007;13:559–565. doi: 10.1158/1078-0432.ccr-06-1210. [DOI] [PubMed] [Google Scholar]
- 17.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao J., Tu W.-J., Cheng W., Yu L., Liu Y.-K., Hu X. Clinical features and short-term outcomes of 102 patients with corona virus disease 2019 in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goyal P., Choi J.J., Pinheiro L.C., Schenck E.J., Chen R., Jabri A. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020 doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clancy C.J., Nguyen M.H. COVID-19, superinfections and antimicrobial development: what can we expect? Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goto M., Al-Hasan M.N. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect. 2013;19:501–509. doi: 10.1111/1469-0691.12195. [DOI] [PubMed] [Google Scholar]
- 22.European Centre for Disease Prevention and Control . ECDC; Stockholm: 2018. Incidence and attributable mortality of healthcare-associated infections in intensive care units in Europe, 2008–2012.https://www.ecdc.europa.eu/sites/default/files/documents/surveillance-report-HAI-Net-ICU-mortality-2008-2012.pdf [Google Scholar]
- 23.van Arkel A.L.E., Rijpstra T.A., Belderbos H.N.A., van Wijngaarden P., Verweij P.E., Bentvelsen R.G. COVID-19 associated pulmonary aspergillosis. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202004-1038LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alanio A., Dellière S., Fodil S., Bretagne S., Mégarbane B. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30237-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verweij P.E., Gangneux J.-P., Bassetti M., Brüggemann R.J.M., Cornely O.A., Koehler P. Diagnosing COVID-19-associated pulmonary aspergillosis. Lancet Microbe. 2020;1:e53–e55. doi: 10.1016/S2666-5247(20)30027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huttner B.D., Catho G., Pano-Pardo J.R., Pulcini C., Schouten J. COVID-19: don’t neglect antimicrobial stewardship principles! Clin Microbiol Infect. 2020;26:808–810. doi: 10.1016/j.cmi.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan L., Zhang H.-T., Goncalves J., Xiao Y., Wang M., Guo Y. An interpretable mortality prediction model for COVID-19 patients. Nat Mach Intell. 2020;2:283–288. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.