Abstract
A fast and accurate self-testing tool for COVID-19 diagnosis has become a prerequisite to comprehend the exact number of cases worldwide and to take medical and governmental actions accordingly. SARS-CoV-2 (formerly, 2019-nCoV) infection was first reported in Wuhan (China) in December 2019, and then it has rapidly spread around the world, causing ~14 million active cases with ~582,000 deaths as of July 2020. The diagnosis tools available so far have been based on a) viral gene detection, b) human antibody detection, and c) viral antigen detection, among which the viral gene detection by RT-PCR has been found as the most reliable technique. In this report, the current SARS-CoV-2 detection kits, exclusively the ones that were issued an “Emergency Use Authorization” from the U.S. Food and Drug Administration, were discussed. The key structural components of the virus were presented to provide the audience with an understanding of the scientific principles behind the testing tools. The methods that are still in the early research state were also reviewed in a subsection based on the reports available so far.
Keywords: COVID-19, SARS-CoV-2 diagnosis, SARS-CoV-2 detection, RT-PCR, Lateral flow assay, Loop-mediated isothermal amplification, Point of care devices
Highlights
-
•
The structural biomarkers of SARS-CoV-2 are described.
-
•
Viral gene, antigen, and antibody-based detection methods are discussed.
-
•
Emergency Use Authorization-issued commercial test kits are explained.
-
•
The methods at early research-stage are summarized.
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2, previously 2019-nCoV) is an enveloped, positive-sense single-stranded genomic RNA virus (+ssRNA), is the cause of the coronavirus disease 2019 (COVID-19). SARS-CoV-2, registered in the Wuhan City of China for the first time, is contagious in humans, and it has rapidly spread worldwide through close human interactions or the spilled respirational material (cough, sneeze) of the infected people. The Director-General of WHO declared the COVID-19 outbreak as “a pandemic” on March 12th, 2020, as a result of the increased infection rate out of China (WHO, 2020a). SARS-Cov-2 taxonomically belongs to coronaviruses family and Sarbecovirus subgenus that contain several other species causing mild to severe human diseases. SARS-CoV-2 is the seventh reported Coronavirus that has infected people after 229E, NL63, OC43, HKU1, MERS-CoV, and the previous SARS-CoV (Cui et al., 2019; Su et al., 2016; Zhu et al., 2020a). Khailany et al. (2020) reported a complete genome characterization study based on 95 SARS-CoV-2 sequences that were accessible on GenBank, National Microbiology Data Center (NMDC) and NGDC Genome Warehouse between December-2019 and early April-2020 in which NC_045512 genome sequence was used as the reference for the alignment. According to the report, the SARS-CoV-2 complete genome is around 30kb and two-third of 5’ contains orf1ab encoding orf1ab polyproteins, while the one-third of 3′ consists of genes encoding structural proteins, currently known as surface glycoprotein (S), an envelope protein (E), membrane protein (M), and nucleocapsid (N) proteins (Khailany et al., 2020). Additionally, the genomic comparison study revealed 116 mutations among which a) 8782C > T in ORF1ab gene, b) 28144T > C in ORF8 gene, and c) 29095C > T in the N gene are defined as the most common ones. The mutations might affect the gravity and extent of the SARS-CoV-2, as stated in the study.
The morphology and complete genome structure of the SARS-CoV-2 are illustrated in Fig. 1 based on the reports by Khailany et al. (2020), Walls et al. (2020) and Rosales-Mendoza et al. (2020). Significant sequence similarity between SARS-CoV-2 S and SARS-CoV S glycoproteins was shown by Walls et al. (2020), and the team also accomplished a comprehensive cryo-electron microscopy (cryo-EM) study disclosing the architecture of SARS-CoV-2 S glycoprotein subunits. The binding affinities of the S proteins of the two SARS viruses to the human angiotensin-converting enzyme 2 (ACE2), the host surface receptor used by the virus to enter the cells, were found in the low nanomolar range and comparable. The initial experiments performed by the SARS-CoV S-derived murine polyclonal antibodies demonstrated that the SARS-CoV-2 cell admission could be neutralized effectively (Walls et al., 2020), which was later approved by another publication of the same group (Pinto et al., 2020). A novel antibody, 47D11, against S protein of SARS-CoV-2 was also developed recently, where the humanized 47D11 antibodies effectively neutralized SARS-CoV and SARS-CoV-2 (C. C. Wang et al., 2020). Another team predicted by simulation that the SARS-CoV-2 S glycoprotein binds to the ACE2 receptor protein through Leu455, Phe486, Gln493, Asn501, and Tyr505 amino acid residues of which only a few were the same as SARS-CoV S protein (Liu et al., 2020). All these studies deliver an immense amount of information regarding the SARS-Cov-2 genome and structure, which we believe it has based the foundation for further diagnosis, treatment, and vaccine studies.
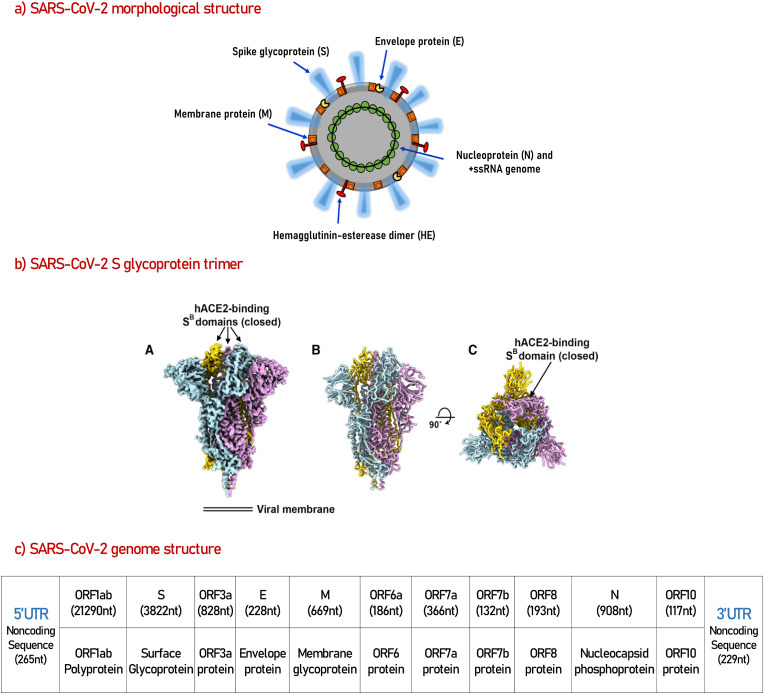
Fig. 1.
A visual artwork for whole SARS-CoV-2 virus (a), cryo-EM restructure of SARS-CoV-2 S trimer glycoprotein, which is responsible for host cell receptor binding (b), and the complete genome structure of SARS-CoV-2 virus (c).
a) The morphological structure of SARS-Cov-2 reproduced from ref (Rosales-Mendoza et al., 2020). “The envelope membrane is associated with the spike protein (S), which mediates binding to the host cell receptors and considered a critical target for the induction of antibodies capable of neutralizing the virus; hemagglutinin-esterase dimer (HE), which acts as a potent mediator of attachment and destruction of sialic acid receptors on the host cell surface; a membrane glycoprotein (M), which is important to generate the virus; and the envelope protein (E), which adheres to the M protein to form the viral envelope. The viral structure also comprises a nucleocapsid protein (N) that, along with the RNA genome, produces the nucleocapsid.” (Rosales-Mendoza et al., 2020).
b) The reconstruction of the closed SARS-CoV-2 S ectodomain trimer at 2.8Å resolution reproduced from ref (Walls et al., 2020). “(A) Closed SARS-CoV-2 S trimer unsharpened cryo-EM map. (B and C) Two orthogonal views from the side (B) and top (C) of the atomic model of the closed SARS- CoV-2” (Walls et al., 2020).
c) Complete genome structure of the SARS-CoV-2 virus, the data is redrawn based on the information and results of ref (Khailany et al., 2020).
Currently, many commercial SARS-CoV-2 detection kits, granted an Emergency Use Authorization (EUA) from FDA (U.SFDA, 2020a, USFDA, 2020k), can identify a) specific viral gene regions through nucleic acid amplification techniques (Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR) and isothermal nucleic acid amplification), b) the antibodies produced by the immune system in response to the viral infection (serology/Immunoglobulin M (IgM)/Immunoglobulin G (IgG) tests), and c) the antigen testing by lateral flow assays, all have to be entirely operated in designated laboratories by trained personnel, under specified experimental and Biological Safety Level (BSL) conditions.
As an exception, “home collection of specimens by COVID-19 RT-PCR Test” developed by LabCorp was issued a EUA from FDA in late April 2020 to be used by individuals to self-collect nasal samples at home (USFDA, 2020b, USFDA, 2020m). Presently, this sample collection kit comes for USD 119 each, and the company gives purchase priority for healthcare workers and first responders, consistent with CDC guidelines, due to the limited sample collection materials (LabCorp, 2020a). The kit is only for sample collection, and the collected sample has to be sent back to the authorized laboratory for nucleic acid testing, which remains to be the gold standard for COVID-19 detection, based on the clinically validated results.
On the other hand, the serology tests do not directly diagnose the presence of the virus, but the immune system molecules (immunoglobulins/antibodies), such as IgM and IgG that are produced by the body when encountered with the virus. IgG/IgM tests could play a central role in the fight against COVID-19 by accurately classifying the individuals who developed an immune response because of SARS-CoV-2 infection. Although the serology tests are suitable for indirect diagnosis, vast herd-immunity scanning, and mortality rate statistics, the antibody amounts produced on the first a few days of the infection may be insufficient for the detection. Furthermore, the lifetime of the produced antibodies, as well as the effectiveness, is still vague, restricting the engagement of the antibody-based rapid tests in the COVID-19 pandemic as a robust diagnosis and surveillance tool. A small number of serology tests have been EUA-authorized by FDA so far (U.SFDA, 2020a, USFDA, 2020k), but no country has performed an antibody testing on a national scale yet even though tremendous local efforts are on the way (ARUP Laboratories, 2020; Cohen, 2020b; Financial Times, 2020).
As of July 15th, 2020, the number of worldwide SARS-Cov-2 active cases is around 14 million, with 582,126 deaths and 7,881,023 recovered (Worldometer, 2020). Due to the limited test type and the number, only a small portion of the world population has been tested so far. As critically discussed by Morales-Narváez and Dincer, the development of high performance, fast, accurate, sensitive, and selective SARS-CoV-2 sensing tools has become pivotal for public health authorities (Morales-Narváez and Dincer, 2020). In this report, current SARS-CoV-2 diagnosis tests, mostly the ones with a EUA, have been methodically reviewed. Some non-commercial techniques that have been recently published in the literature are explicitly included in the report to provide the audience with the most recent research-based solutions and the initial outcomes.
2. Viral gene detection by RT-PCR method
RT-PCR is a version PCR method explicitly developed for (genomic) RNA detection. RT-PCR is sufficiently reliable and a fast technique, producing results in a few hours in a high throughput manner (Green et al., 2020). RT-PCR technique is based on two consecutive reactions: a) conversion of RNA into complementary DNA (cDNA) through reverse transcription enzyme and b) amplification of the cDNA sample by polymerase chain reaction using gene-specific primers and fluorescently labeled hydrolysis probes. The first step produces DNA templates to be used in the second step, where the copy number of the DNA is increased throughout repeated thermal cycles. Gene-specific primers guide the second reaction for the amplification of only the selected region on the genome while the probes produce fluorescent signals upon each successful amplification of the gene regions, allowing a quantifiable reaction system (Nolan et al., 2006). An illustration for RT-PCR based on well-known TaqMan hydrolysis probes (Holland et al., 1991), is presented in Fig. 2 a. The discovery of the RT-PCR method has paved the way for the detection of gene transcripts at trace levels, and the technique has been vastly utilized for contagious disease testing worldwide (Lee et al., 2001; Pabbaraju et al., 2009).
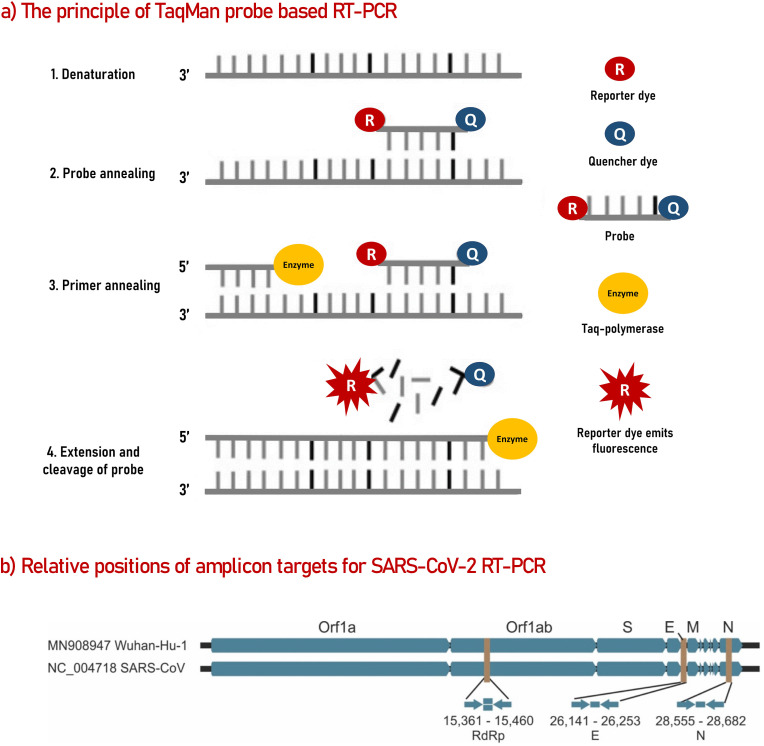
Fig. 2.
The principle of RT-PCR based on commercial TaqMan probes (a) and the relative amplicon positions for SARS-Cov-2 on GenBank data of the previous SARS-CoV and SARS-CoV-2 (b).
a) TagMan Probe-based RT-PCR steps. The illustration was redrawn based on Ref (Roy et al., 2019). The probe is modified with a fluorescent dye (reporter dye) at one end, and one quencher dye on the other end. The quencher blocks the fluorescent signal of the reporter dye due to proximity. The probe does not fluoresce in its native condition. When the polymerase enzyme starts the amplification and encounters with the labeled probe, the probe gets hydrolyzed, releasing its components away from each other, which constitutes a fluorescent signal. Each successful amplification produces fluorescent that is proportional to the amount of the target gene in the sample.
b) Relative positions of amplicon targets on SARS-CoV and Wuhan-CoV genome. S: Spike glycoprotein, E: Envelope protein, M: Membrane protein, N: nucleocapsid; ORF: open reading frame; RdRp: RNA-dependent RNA polymerase. Numbers below amplicon are genome positions according to SARS-CoV, NC_004718”. Reproduced from ref (Corman et al., 2020).
RT-PCR is currently the gold standard for SARS-Cov-2 detection due to its capacity to directly measure the viral genomic parts rather than the secondary biomarkers such as antigens or antibodies. Malaysian Institute for Medical Research (IMR) announced the SARS-CoV-2 specific RT-PCR primers and probes on January 11th, 2020 (General of Health Malaysia, 2020), the day when the scientists from China had released the entire genome sequence of the virus (Harun, 2020). Several other countries, such as England, Germany, South Korea, Turkey, Russia, the USA, and China, later declared their clinical-grade RT-PCR kits for SARS-CoV-2 detection. Some of the first SARS-CoV-2 Real-Time PCR Diagnostic kits delivered by the Central Disease Center (CDC) of the USA to national laboratories were found to cause inconclusive results and thereby limited the number of valid tests performed in February 2020 in the USA. The validated test kit from CDC, namely “CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel” was then approved by FDA under an Emergency Use Authorization (EUA) scheme in late February 2020 (USFDA, 2020c, USFDA, 2020n) to be used in qualified laboratories. Shortly, FDA has granted EUA for Abbott m2000 system (U.S. Food and Drug Administration, 2020d), Panther Fusion SARS-COV-2 Assay by Hologic (U.S. Food and Drug Administration, 2020e), COVID-19 RT-PCR test by LabCorp (USFDA, 2020b, USFDA, 2020m), and TaqPath COVID-19 Combo Kit by Thermo Fisher Scientific (U.S. Food and Drug Administration, 2020f) for authorized laboratory use. It should be noted that FDA gives EUAs for “unapproved medical products as well as the novel utilization of the existing, approved medical products” under section 564 of the Federal Food, Drug, and Cosmetic Act (Pilot, 2012), only for emergencies like public health threats, pandemics where no appropriate alternatives exist.
The list of EUA granted test kits, to be used only by authorized laboratories, is readily updated on FDA website (U.SFDA, 2020a, USFDA, 2020k). By June 6th, 2020, 72 commercial SARS-Cov-2 detection kits out of 88 EUA-issued were based on RT-PCR principle. On March 31st, 2020, FDA concluded that some other molecular detection-based tests developed by various laboratories could be authorized for use in the single laboratory that developed the test, and that is sanctioned under the “Clinical Laboratory Improvement Amendments (CLIA)” of 1988, 42 U.S.C. §263a to perform high complexity tests (U.SFDA, 2020a, USFDA, 2020k). FDA has issued EUA for another 23 kits under this category for singular/identified laboratory use as of early May 2020. RT-PCR kits have been used for samples from various infected parts of the body, including nasopharyngeal, oropharyngeal, or nasal swabs, upper and lower respiratory tract aspirates, bronchoalveolar lavage, and the sputum. The swaps taken from throat samples might cause misleading results since the virus starts moving towards the lower parts of the respiratory system following the first week of the infection. Therefore, the samples must be collected carefully from the deeper parts of the respiratory system wherever possible. A study conducted recently by Xu et al. (2020) reported that eight infected children out of ten continuously tested positive on rectal swab samples. However, their nasopharyngeal swab samples tested negative, indicating the potential of rectal samples for assessing the treatment efficacy as well as the quarantine period of the patients.
RT-PCR kits for SARS-CoV-2 detection mainly include the reverse transcription and amplification enzymes, two to three sets of primers and probes for amplification of the specific viral genome regions, and authorized reagents for negative, positive, and internal controls. The potential PCR amplicon regions for SARS-Cov-2 are illustrated in Fig. 2b (Corman et al., 2020). Control samples are processed in the same manner as the clinical patient samples and included in every plate in high throughput detection setups. For a valid test setup, all control samples must provide expected results as specified in the user guides of each kit. Some of the EUA-authorized RT-PCR kits have been explained here to highlight the significant differences in the principles. “NxTAG® CoV Extended Panel” by Luminex is a high throughput system that can process 96 samples in around 4 h (Rao et al., 2020). The platform is based on the amplification of viral ORF1ab, E gene, and N genes, and RNase P internal gene is used as an internal control. The synthetic double-stranded DNA gBlocks corresponding to SARS-CoV-2 gene fragments are used as positive control. “Real-time SARS-CoV-2 Assay” kit by Abbott relies on the amplification of RdRp and N genes from the samples collected only from respiratory system parts (Abbott, 2020a). Positive control of the kit contains non-infectious, recombinant SARS-CoV-2 RNA sequences that monitor the reagent or condition flops. The negative sample operates a buffer solution to check cross-contamination or non-specific byproducts. Finally, an internal/extraction control is included in the experimental setup to screen failures in the extraction protocol. However, persistent utilization of this automated high throughput system requires trained personnel as well as an installed Abbott m2000 System. Similarly, the “cobas® SARS-CoV-2” kit by Roche is high throughput RT-PCR detection platform that based on the presence of either cobas® 6800 or cobas® 8800 system in the laboratories (Roche, 2020). The main difference is that the cobas® SARS-CoV-2 kit targets ORF-1a and E-gene regions of the SARS-CoV-2 genome, and the platform also offers pan-sarbecovirus detection for the sarbecovirus subgenus family. It should be noted that FDA-EUA granted kits include at least three control samples, positive, negative, and internal controls, to provide consistent test results with maximum confidence level and minimum false positive/negatives as well as the reagent/experimental procedure failures.
A list of the target genes, corresponding primer, and probe sequences used in RT-PCR kits so far for SARS-CoV-2 detection is presented in Table 1 . WHO also provided a recipe document (Maschinen et al., 2020) for in house RT-PCR-based SARS-CoV-2 detection that summarizes the methods from well-known institutions worldwide, including China CDC (China), Institute Pasteur (France), USA CDC (USA), Charité (Germany), National Institute of Infectious Diseases (Japan), University of Hong Kong (Hong Kong) and National Institute of Health (Thailand). In a recent pre-print study conducted by Jung et al. (2020), the primers “2019-nCoV_N2 and N3” of the USA and the ORF1ab” of China were claimed to be the most sensitive primer-probe sets for detection of N and Orf1 genes, respectively. Another study conducted by Nalla et al. (2020) suggested that the E-gene primer/probe sets described by Corman et al. (2020) and N2 primer-probe set defined by the CDC (Jung et al., 2020) provided the most sensitive assays. The authors also stated that all the tested primer and probes sets were found to be highly specific for the SARS-CoV-2 genes, and no cross-reactivity with other respiratory viruses was observed. The SARS-COV-2 qRT-PCR primer-probe sets comparison study performed by Vogels et al. (2020) under identical PCR conditions (not optimized for each set) were in good agreement with the previous findings of the other groups except for the RdRp-SARSr set from Charité, Germany (Corman et al., 2020) that had low sensitivity under the specified experimental conditions.
Table 1.
A list of the target gene regions, primer and probe sequences used in different RT-PCR setups for SARS-CoV-2 detection.
| Reference | Genes | Forward Primer | Reverse Primer | Probe Sequencea |
|---|---|---|---|---|
| Corman et al. (2020) | RdRP | GTGARATGGTCATGTGTGGCGG | CARATGTTAAASACACTATTAGCATA | CAGGTGGAACCTCATCAGGAGATGC |
| E | ACAGGTACGTTAATAGTTAATAGCGT | ATATTGCAGCAGTACGCACACA | ACACTAGCCATCCTTACTGCGCTTCG | |
| N | CACATTGGCACCCGCAATC | GAGGAACGAGAAGAGGCTTG | ACTTCCTCAAGGAACAACATTGCCA | |
| Rao et al. (2020) | ORF1ab | CCCTGTGGGTTTTACACTTAA | ACGATTGTGCATCAGCTGA | CCGTCTGCG/ZEN/GTATGTGGAAAGGTTATGG |
| N | GGGGAACTTCTCCTGCTAGAAT | CAGACATTTTGCTCTCAAGCTG | TTGCTGCTGCTTGACAGATT | |
| U.S. Centers for Disease Control and Prevention (2020) | RdRP | AGATTTGGACCTGCGAGCG | GAGCGGCTGTCTCCACAAGT | TTCTGACCTGAAGGCTCTGCGCG |
| N1 | GACCCCAAAATCAGCGAAAT | TCTGGTTACTGCCAGTTGAATCTG | ACCCCGCAT/ZEN/TACGTTTGGTGGACC | |
| N2 | TTACAAACATTGGCGCAAA | GCGCGACATTCCGAAGAA | ACAATTTGCCCCCAGCGCTTCAG | |
| N3 | GGGAGCCTTGAATACACCAAAA | TGTAGCACGATTGCAGCATTG | AYCACATTGGCACCCGCAATCCTG | |
| Chu et al. (2020) | ORF1b | TGGGGYTTTACRGGTAACCT | AACRCGCTTAACAAAGCACTC | TAGTTGTGATGCWATCATGACTAG |
| N | TAATCAGACAAGGAACTGATTA | CGAAGGTGTGACTTCCATG | GCAAATTGTGCAATTTGCGG | |
| Nao et al. (2019) | N | AAATTTTGGGGACCAGGAAC | TGGCAGCTGTGTAGGTCAAC | ATGTCGCGCATTGGCATGGA |
| Institute of Pasteur (2020) | RdRP | ATGAGCTTAGTCCTGTTG | CTCCCTTTGTTGTGTTGT | AGATGTCTTGTGCTGCCGGTA |
| RdRP | GGTAACTGGTATGATTTCG | CTGGTCAAGGTTAATATAGG | TCATACAAACCACGCCAGG | |
| E | ACAGGTACGTTAATAGTTAATAGCGT | ATATTGCAGCAGTACGCACACA | ACACTAGCCATCCTTACTGCGCTTCG | |
| China CDC (2020) | ORF1ab | CCCTGTGGGTTTTACACTTAA ACG | ACGATTGTGCATCAGCTGACCG | CCGTCTGCGGTATGTGGAAAGGTTATGG |
| N | GGGGAACTTCTCCTGCTA GAA T | CAGACATTTTGCTCTCAA GCT G | TTGCTGCTGCTTGACAGATT | |
| Thailand Ministry of Public Health (2020) | N | CGTTTGGTGGACCCTCAGAT | CCCCACTGCGTTCTCCATT | CAACTGGCAGTAACCA |
All sequences are given in the direction of 5′ to 3’ (from left to right). Probe sequences are always labeled with a reporter dye at 5’ (usually FAM) and a quencher at the 3’ (usually BHQ).
The commercial kits based on the RT-PCR method are only operated in laboratory conditions equipped with specialized tools and instruments because of the sophisticated nature of the RT-PCR reactions as well as safety reasons. Therefore, it may be challenging to use the RT-PCR kits for rapid surveillance or screening of the nations for the current pandemic. COVID-19 RT-PCR Test kit by Laboratory Corporation of America (LabCorp) was granted an FDA-EUA in mid-March 2020 for the qualitative detection of nucleic acid from SARS-CoV-2 in upper and lower respiratory specimens. Recently, in April 2020, LabCorp requested an amendment for its COVID-19 RT-PCR Test kit to be used with a “home specimen collection method” and received a swift authorization from FDA (U.S. Food and Drug Administration, 2020g). Nasal swab specimens now can be self-collected by individuals at home using the “Pixel by LabCorp COVID-19 home collection kit” when found suitable by healthcare personnel following a COVID-19 questionnaire. The COVID-19 RT-PCR Test kit targets three different nucleocapsid proteins from the viral genome (N1, N2 and N3), and it includes a) RNase P (RP) reagents as the internal control, b) an in vitro transcribed SARS-CoV-2 RNA as the positive control, c) a clinically negative patient sample as the negative extraction control and d) nuclease-free, molecular-grade water as the no-template or negative control. On the other hand, the Pixel by LabCorp COVID-19 Test Home Collection Kit contains a shipping box, pre-labeled return FedEx envelope, nasal specimen collection swab, saline tube, insulated specimen pouch, gel pack (for sample cooling), specimen biohazard bag and the user guideline. Appropriately collected home nasal swab sample is placed in the provided saline tube, packaged in the specimen pouch, placed in between the cooling peds, and sent back to the laboratory in the pre-labeled FedEx return envelopes. The test results are provided online and repeated as seen necessary. Still, the home kit is available at limited numbers and only for a limited number of locations, in its current state. In this way, the patients are not required to visit hospitals for regular checks, the frontline healthcare personnel are genuinely protected, and the overloads in the hospitals are reduced, allowing the patients to be tested and screened at their homes. The test has a LOD of 6.2 genomic copy/ul sample and slightly cross-reacts with the SARS-Cov-2 N3 gene, according to the cross-reactivity tests, conducted using in silico analysis, purified nucleic acid extracts or whole organisms (U.S. Food and Drug Administration, 2020h). The interpretation of the results is the same for RT-PCR kits, differing by the type of targeted viral gene. The RT-PCR results interpretation table for COVID-19 RT-PCR Single Plex Test kit by LabCorp (2020b) is represented in Table 2 , as an example. As can be seen from the table, the test becomes “invalid” when all the genes tested negative. The test result becomes “SARS-CoV-2 positive” when all the viral N genes are tested positive. If only one target viral gene tests positive, the result is considered “intermediate,” and the sample requires another testing. Finally, the test result becomes “SARS-CoV-2 negative” when all the viral N genes are tested negative.
Table 2.
LabCorp COVID-19 RT-PCR test interpretation-SINGLEPLEX. Reproduced from ref (LabCorp, 2020b).
| SARS-CoV-2 N1 (FAM) | SARS-CoV-2 N2 (FAM) | SARS-CoV-2 N3 (FAM) | RNAse P (FAM) | Interpretation | Report | Actions (Clinical Site Samples) | Actions (Pixel Home Collection Kit samples) |
|---|---|---|---|---|---|---|---|
| + | + | + | +/− | SARS-CoV-2 detected | DETECTED | Report results to sender and appropriate public health authorities | Report results to PWN Health, who will call the patient. Report the result to the Pixel portal. Report the result to the appropriate public health authorities |
| If only one target is positive | +/− | +/− | SARS-CoV-2 Indeterminate |
INDETERMINATE | The sample is repeated once. If results remain the same, it is reported to the sender as indeterminate and recommend recollection if the patient is still clinically indicated | The sample is repeated once. If results remain the same, it is reported to the sender as indeterminate to PWN Health, who will call the patient. Report the result to the Pixel Portal | |
| - | - | - | + | SARS-CoV-2 Not Detected | NOT DETECTED | Report results to sender | Report results to PWN Health and the Pixel Portal |
| - | - | - | - | Invalid Result | INVALID | The sample is repeated once. If a second failure occurs, it is reported to the sender as invalid and recommend recollection if the patient is still clinically indicated | The sample is repeated once. If a second failure occurs, it is reported to PWN Health. Pixel's customer service will contact the patient to discuss options. Report the result to the Pixel Portal |
Although the RT-PCR remains to be the most reliable method for SARS-CoV-2 detection so far, only a limited number of tests could be performed each day, which prevents the authorities from obtaining consistent data on the prevalence of the virus in the entire world population. The sensitivity of this method usually depends on the RNA amount in each sample. The patients can be categorized as SARS-CoV-2 positive (active case) or SARS-CoV-2 negative based on the outcome of the RT-PCR results. However, this technique does not give information for the patients who have already recovered from the SARS-CoV-2, since the viral load is cleared out of the body after recovery. Equally, the patients who are in the very first days of the viral infection may not respond “positive” with this test due to the inadequate amount of the virus on the swabs. Therefore, swab samples from different parts of the body may be required for a confirmation test. The development of faster detection kits that do not rely on trained personnel, advanced reactions, or equipped laboratories is of great importance. Antigen tests that target the viral biomarkers such as spike, envelope, or nucleocapsid proteins could be useful to support the current RT-PCR-based systems and accelerate the detection speed worldwide. On the other hand, antibody or serology tests are equally important to see how many people have already had the virus and developed protective antibodies, which may be later used as a databank to survey potential plasma donors for the disease treatment.
3. Antibody detection methods
An antibody is a protein produced by the immune system in response to an antigen. Each antibody has sites that can bind only one specific type of antigen to remove it from the body. This specificity is determined by Complementary Determining Regions (CDRs), localized on N-terminus of the antibody. Antibodies are also called immunoglobulins (Ig). Five classes of antibodies exist; IgM, IgD, IgG, IgA, and IgE, which are distinguished by C-terminus regions. IgM is the first antibody produced during an infection, while the IgG is the most common and abundant one in serum. Antibodies are secreted in mucosa and blood; here, they neutralize pathogens by binding to and inactivating antigens. Therefore, antibody neutralization prevents the virus from infecting the cells (Jacofsky et al., 2020). An antibody test can measure the presence and concentration of IgG and IgM levels in the blood/serum/plasma samples to determine if the body is fighting with a pathogen, for example, a contagious virus. A “recombinant” antigen that mimics the virus can be produced in the laboratory, and antibodies with specific target binding affinity can be produced by using these artificial pathogens (Loeffelholz and Tang, 2020). The most common antibody tests are based on lateral flow type assays (LFA) and enzyme-linked immunosorbent type assays (ELISA).
In an LFA, the presence of the molecule(s) of interest is determined by antibodies immobilized onto a membrane. The device is composed of a cassette, enclosing a strip of the polymer membrane, usually containing two lines: a control line and a test line. In an antibody LFA test, the molecules of interest are the specific antibodies present in the patient's blood sample. The sample is deposited onto the sample pad through a port and moves through the strip by capillary action. When it encounters the first line, antibodies labeled with gold nanoparticles bind to the target molecule in the sample. Then as the sample continues to move, the gold-labeled antibodies are bound by the capture antibodies in the lines. The gold-labeled antibodies that are in excess then move further along the strip and are captured at the control line. Even in the absence of the target molecule in the sample solution, the gold-labeled antibodies must be captured at the control line, making the control line for the validity of the test (Soh et al., 2020). Depending on whether the test is for IgG- or IgM-class antibodies, or both, the display window should show either one, two, or three stripes. If the test is only for one class of antibody and is negative, it should display one stripe, only at the control line. If the test is positive, it should display two stripes, both at the control line and the test line. If the test is for both antibodies and negative, it should, again, display one stripe at the control line. If it is positive for both, three stripes will be displayed: one at the control line, one at the IgG test line, and one at the IgM test line. Additionally, in an IgG/IgM test, only one antibody may be present, which could be indirect indicators of the course of the infection or direct immune response.
In ELISA tests, the recombinant viral antigen is coated onto the surfaces of plastic wells as the target molecules. Once the wells are prepared, the sample, i.e., the patient's serum, is added to the well. If antibodies (IgG or IgM) against the target antigen are present in the sample, a binding event occurs. The excess sample is washed out several times to ensure all unbound substrate is removed. Then, a second solution containing labeled, secondary anti-human antibodies are added and allowed to bind; if the antibody of interest is absent in the sample, no binding occurs. The excess is again removed by washing, and the binding of target antibodies is confirmed by an enzyme-dependent (usually horseradish peroxidase) color changing reaction. A spectrometer reads the color change, and the concentration of the antibody of interest can be determined. In chemiluminescent immunoassays (CLIA, also called modified-ELISA), the binding of the secondary antibody is confirmed by a separate chemiluminescent substrate (Lin et al., 2020).
As of June 2020, there were only 16 serology tests issued EUA by the FDA, some of which are detailed in Table 3 . All these tests require laboratories that are authorized to perform moderate to high complexity tests or high complexity tests, as specified by the CLIA of 1988. There are, however, six more tests, developed by countries outside the USA, in China and South Korea, two of which, the SARS-CoV-2 Rapid Test by ScanWell Health/INNOVITA and the (COVID-19) IgM/IgG Rapid Test by Aytu BioScience, Inc./Orient Gene Biotech., are waiting for authorization by the FDA. Additionally, several other tests with CE/IVD approval are available for purchase by research labs and, in some cases, healthcare professionals. The CE/IVD approval is a legal requirement for all on-market medical products to aid in patient care and decision making. The authorization is valid within the European Union (EU), European Free Trade Area (EFTA), Switzerland, and Turkey (Global Product Certification, 2020). These companies include Ray Biotech, Eagle Biosciences, Biomedomics, Sanuo Biotech, Shenzhen Yhlo Biotech Company, GenBody, Snibe Co., Liming Bio, BioEasy, Sensing self, Sugentech, Euroimmun AG, and Livzon Diagnostics (Johns Hopkins Bloomberg School of Public Health, 2020). There are several more US-based tests approved under the FDA Policy for Diagnostic Tests for Coronavirus Disease-2019, wherein Section IV-A specifies that laboratories authorized under the CLIA of 1988 will be granted EUAs in the absence of significant problems. Section IV-D specifies that FDA plans to not object to the development and distribution of serological tests by commercial manufacturers, as long as proper disclaimers are given and the application of said tests are only in research laboratories or with healthcare professionals at point-of-care. The suggested disclaimers include: the test is not FDA-approved, should not be the only basis for a diagnosis, a positive result may be due to a past coronavirus infection, and that a negative result does not guarantee that the patient is not infected. If the patient has been in contact with the virus, they should undergo molecular testing (USFDA, 2020b, USFDA, 2020m).
Table 3.
The kits cleared a EUA from FDA for SARS-CoV-2 antibody detection. The commercial kit list was retrieved from the FDA website in early May 2020 (U.SFDA, 2020a, USFDA, 2020l). Instruction for Use (IFU) and Fact Sheet for Healthcare Providers (HCP) documents were also retrieved from the same FDA website.
| Entity/Company | Targeted Molecules and Kit Name | Details |
|---|---|---|
| Abbott Laboratories Inc. | IgG only SARS-CoV-2 IgG assay |
The test can be used only in authorized laboratories on Abbott's Architect i1000SR and i2000SR instruments. This platform can run 100–200 tests/hour, being faster than RT-PCR. It is reported that the kit has a sensitivity of 100% and specificity of 99.5% (IFU). The company plans to develop an IgM test too, which would be the 4th product of the company in the market for the COVID-19 diagnosis (Cairns, 2020). |
| Autobio Diagnostics Co. Ltd. | IgM and IgG Anti-SARS-CoV-2 Rapid Test |
This is a rapid LFA test based on a one-step capture protocol. The samples can be taken from serum or plasma. The membrane pads are decorated with anti-Spike, anti-IgM, and IgG antibodies lines, which serve as a control, IgG detection, and IgM detection lines, respectively. The spike protein-coated gold nanoparticles are employed to capture the antibodies and form a reddish color on the pads for fast, visual detection. The protocol implies that the tests are more accurate when used after around 15 days following the infection (IFU). Such a test can be used by individuals since they do not require trained personnel or sophisticated equipment. |
| DiaSorin Inc. | IgG only LIAISON SARS-CoV-2 S1/S2 IgG |
This kit is a two-step automated immunoassay for the qualitative detection of anti-SARS-CoV-2 IgG antibodies in serum/plasma samples. The assay is based on chemiluminescent microparticle immunoassay technology (CMIA) in which the particles are functionalized with the SARS-CoV-2 antigens. Further addition of the Anti-human IgG acridinium-labeled conjugate into the incubation medium creates a chemiluminescent signal that is measured as relative light units. The positive percent agreement (PPA) was reported as 100% for 88 clinically validated positive samples that are measured after at least 14 days following the infection, whereas this value was ≤25% when tested in the first week (n = 12) (IFU). This test can only be performed at laboratories; however, the sensitivity of the test is high enough to make a decision without further confirmation. |
| Ortho-Clinical Diagnostics, Inc. | IgG only VITROS Immunodiagnostic Products Anti-SARS-CoV-2 IgG Reagent |
This two-step kit is performed with Anti-SARS-CoV-2 IgG Reagent Pack, the Anti-SARS-CoV-2 IgG Calibrator on the ECi/ECiQ/3600 Immunodiagnostic Systems and the 5600/XT 7600 Integrated Systems, all available from Ortho-Clinical Diagnostics, Inc. In the first step, anti-SARS-CoV-2 antibodies bind to the wells that are decorated with the virus spike proteins. The wash step removes the unbound molecules from the sample environment, which is followed by the addition of the horseradish-peroxidase-linked anti IgG antibodies. If there is no spike protein-IgG complex in the sample, the second antibody will be cleared off the well with the second washing step. If positive, the addition of a final enzymatic substrate results in a color change that is measured and correlated with the virus presence. The test showed 100% negative agreement (n = 407) and 87.5% (n = 48) positive agreement (IFU). The positive agreement value of the test is relatively low that probably requires a second confirmatory test. |
| Mount Sinai Laboratory | IgG only COVID-19 ELISA IgG Antibody Test |
The ELISA-based test kit is only authorized for use at Mount Sinai Laboratory (New York, USA), certified CLIA (1988, 42 U.S.C. §263a), to perform high complexity tests (HCP). The ELISA kits cannot be performed out of laboratory; however, they are both reliable and high throughput and thus a very efficient alternative to the RT-PCR method. |
| Ortho Clinical Diagnostics, Inc. | Total Antibody VITROS Immunodiagnostic Products Anti-SARS-CoV-2 Total Reagent Pack |
The SARS-CoV-2 Total Antibody kit by Ortho Clinical, Inc. is quite similar to the one other VITROS kit, listed earlier in this table except for the second step, which involves the use of horseradish peroxidase (HRP)-labeled recombinant SARS-CoV-2 antigen instead of horseradish-peroxidase-linked anti IgG antibodies. The clinical agreement of the kit was reported as 100% negative agreement (n = 400) and 83.3% positive agreement, which is less than the IgG antibody kit agreement values, provided by the same company (IFU). |
| Chembio Diagnostic System, Inc. | IgM and IgG DPP COVID-19 IgM/IgG System |
The DPP® COVID-19 IgM/IgG System is a single-use rapid test (a version of LFA performed on a device for readout) for qualitative IgG and IgM detection in blood, serum, or plasma samples. The platform includes the DPP COVID-19 IgM/IgG Test Devices and the DPP Micro Reader/DPP Micro Reader 2 for use with the kit. For this dual antibody detection platform, the positive agreement was reported as 100% (n = 13) for both IgG and IgM (for the samples tested after the first two weeks of the infection). The negative agreements were reported as 100% for IgM and 95.9% for IgG, 95.9% for IgG, and IgM dual test line (n = 49) (IFU). |
| Cellex Inc. | IgM and IgG qSARS-CoV-2 IgG/IgM Rapid Test | This test is an LFA that can detect antibodies produced against the new Coronavirus. The cassette includes colloidal gold conjugated- SARS-CoV-2 antigens and rabbit IgG-gold conjugates to be spiked with the actual sample. One of the test lines contains an anti-human IgG antibody for IgG testing; the other one contains anti-human IgM antibodies for IgM detection. The control line contains anti-rabbit IgG antibodies produced in goat and work regardless of the presence of anti-SARS-CoV-2 IgG/IgM antibodies in the sample. The overall clinical agreement values were reported as 93.8% for the positive percent (n = 128), and 96% for the negative percent (n = 250) (IFU). The clinical agreement values are higher than the previous kits. |
| The Other Serology Kits | |||
|---|---|---|---|
| Supplier | Target Molecules and Kit Name | Sensitivity/Specificity | Country/Use/Reference |
| Aytu BioScience, Inc./Orient Gene Biotech. | IgM and IgG (COVID-19) IgM/IgG Rapid Test |
The sensitivity of lgM only test is 89.2% (n = 112). The specificity is 100% (n = 14). The sensitivity of lgG & IgM test is 91.9% (n = 112), and the specificity is 100% (n = 14) |
China For only professional use (Aytu BioScience Inc, 2020) |
| ScanWell Health/INNOVITA | IgM and IgG SARS-CoV-2 Rapid Test |
87.3% sensitivity and 100% specificity in clinical trials | China For use at-home with smartphone readout (Murphy, 2020) |
| SD Biosensor, Inc. | IgM and IgG Standard Q COVID-19 IgM/IgG Duo Rapid Test |
The positive percent value for IgM/IgG was reported as 98.1% (n = 52) for the samples measured following around two weeks of the infection. This percent was increased up10 100% following almost three weeks of the infection. The negative percent value was reported as 96.1& (n = 205). | South Korea/Point-of-care (SDBiosensor Inc., 2020) |
The benefit of an LFA over the ELISA test is that the device can be used at home without training, such as at-home pregnancy tests. ELISA is a diagnostic technique that can be conducted only in laboratories with authorization to perform moderate or high complexity tests, as the protocol involves multistep and long turnaround time and requires skilled personnel and specific instrumentation. These limitations increase the need for point-of-care rapid testing. Therefore Sars-Cov-2 studies have focused on improving the sensitivity and specificity of LFAs. To increase the accuracy, sensitivity, and detection throughput, new approaches are in development, such as signal amplification and multiplexed detection. Especially in resource-poor areas, because of the lack of early diagnosis, many cases remain undifferentiated, and this leads to an uncontrollable spread of the virus. Multiplexed LFAs have become necessary to test multiple biomarkers simultaneously by reducing the reagents, turnaround time, and labor costs (Soh et al., 2020). However, both methods suffer from setbacks.
The biggest issue concerning serological tests is cross-reactivity. Cross-reactivity occurs when antibodies bind with an antigen different from the target antigen, often due to the similarities between the molecules. For example, cross-reactivity was observed by Okba et al. for SARS-CoV-2 spike (S) protein with S proteins of SARS-CoV and MERS-CoV (Okba et al., 2020). However, no cross-reactivity was observed for the S1 subunit of the spike protein for MERS-CoV. The S2 is more highly conserved among coronaviruses, which could make the S1 subunit a better candidate for serological tests. The biggest issue with this cross-reactivity is that it can lead to false positives: a patient previously infected with SARS-CoV may test positive for SARS-CoV-2, although uninfected. Additionally, because antibodies are developed through the course of an infection, a negative result on an antibody test may not confirm that the patient is uninfected. A majority of patients develop an antibody after around 14 days in response to SARS-CoV-2 infection, while a recent study shows that both IgG and IgM antibodies were detected five days later (Cevik et al., 2020; Loeffelholz and Tang, 2020). This general late response may, therefore, lead to false negatives: where the patient is infected but has not yet produced antibodies at detectable levels. It is, therefore, essential to establish the sensitivity and specificity values of tests to comprehend the possibilities of false positives and false negatives. Sensitivity is the percentage of true positives among all positive results, and the percentage of true negatives among all negative results (Li et al., 2020). On the other hand, due to the late detection of antibodies, serological studies may be retroactively beneficial in vaccine studies, epidemiologic studies, and late complications, where RT-PCR testing may yield false negatives due to shedding or low viral loads in the tested samples (Woo et al., 2020). Dr. Edward Wright, senior lecturer in microbiology at the University of Sussex, says that an antibody test cannot tell us who is infected as the antibodies are generated up to two weeks later when the virus should have been cleared from the system. However, it can tell us who developed an immunity to the virus and what proportion of the population has been infected (Kent, 2020).
4. Antigen detection methods
An antigen is a particle/fragment/molecule that can trigger the immune system and induce antibody production to kill the pathogens, thereby protecting the body. The antigen tests, unlike PCR-based methods, detect viral components (i.e., S glycoprotein, M protein, or released N protein) or the virus directly without thermal amplification steps (Green et al., 2020). Like the PCR-based methods, antigen tests only reveal the active viral infection, not the recovery situation. Because antigens precede antibodies and are target-specific, they could be more reliable than antibody tests. Antigen tests can be operated on LFA strips for rapid detection purposes or in ELISA format for better sensitivity, and high throughput uses (the simultaneous measurement of 96 samples). Diao et al. developed a fluorescent immunochromatographic LFA assay for detecting the nucleocapsid (N) protein of SARS-CoV-2 (Diao et al., 2020). The assay utilizes anti-N mouse antibodies and goat anti-rabbit IgG antibodies to create the test and control lines, respectively. It uses anti-N rabbit IgG marked with carboxylate-modified polystyrene Europium (III) chelate microparticles as signal particles, unlike conventional gold nanoparticles. Nasopharyngeal swabs and urine were used as the samples. When compared with nucleic acid testing, the assay was shown to have a sensitivity of 68% and a specificity of 100%. However, such studies are at early research state that it could take years to put into the market even if they present excellent sensitivity and selectivity.
Although ELISA and LFA techniques are well-established, and antigen tests are urgently and immensely needed, there is only one EUA-issued antigen test in the market so far for SARS-COV-2 detection that is “Sofia 2 SARS Antigen Test Kit” developed by QUIDEL (U.S. Food and Drug Administration, 2020i). Sofia 2 rapid antigen test kit is based on a sandwich-type immunofluorescence strip technology that is used with an instrument to detect N protein of both SARS-CoV and SARS-CoV-2, meaning that the kit cannot differentiate between these two closely related virus species. Furthermore, the kit utilizes nasal and nasopharyngeal swab samples, and it is only intended to be operated by medical professionals. The clinical sensitivity of the kit was found as 80%, whereas the specificity was 100% out of 47 positive and 96 negative clinical samples (U.S. Food and Drug Administration, 2020j). After the failures of several antibody kits (Cohen, 2020a; Royal Statistical Society Covid-19 Task Force, 2020), WHO and FDA warned scientific and medical communities on the use of the rapid commercial tests for SARS-CoV-2 detection (U.S. Food and Drug Administration, 2020c). The original quote from WHO was as follows: “with the limited data now available, WHO does not currently recommend the use of antigen-detecting rapid diagnostic tests for patient care, although research into their performance and potential diagnostic utility is highly encouraged” (WHO, 2020b). Two companies are trying to develop reliable antigen tests. In Table 4 , we briefly covered these future technologies based on the information available from online sources so far. It is for sure that the sensitive and selective rapid antibody/antigen tests will dramatically change the spread of the virus worldwide because they basically can be operated by anyone.
Table 4.
The antigen tests under development for SARS-CoV-2 detection.
| Companies | Target | Method and Possible Outcome | Ref. |
|---|---|---|---|
| QUIDELa | N protein | Sofia 2 SARS Antigen Test Kit is based on a sandwich-type immunofluorescence strip technology that is used with an instrument to detect N protein of both SARS-CoV and SARS-CoV-2. The clinical sensitivity of the kit was found as 80%, whereas the specificity was 100% out of 47 positive and 96 negative clinical samples. | (U.S. Food and Drug Administration, 2020i) |
| Avacta and Cytiva (formerly GE Healthcare Life Sciences) | SARS-CoV-2 viral antigens, S glycoproteins | The developer of Affimer® biotherapeutics and reagents, Avacta Group plc (AIM: AVCT), announced in late April (2020) a collaborative project to develop rapid diagnostic assays for SARS-CoV-2 antigen detection. Affimer binders® have been produced against S proteins of the SARS-COV-2. The developed affinity reagents are planned to be characterized by Avacta, which then be transferred to Cytiva for employment in strip assays, like those handy pregnancy tests. The affinity agents are reported to be highly specific to the new virus, not to the previous versions (SAR or MERS). The technology will be available as mass testing units as well as strip tests. | Avacta Life Sciences Limited (2020) |
| OraSure Inc. | A panel of SARS-CoV-2 viral antigens-not specifically disclosed | OraSure Inc. was awarded around USD 700,000 from Biomedical Advanced Research and Development Authority (BARDA) to develop a rapid pan-home test for SARS-CoV-2 detection that could be issued a EUA from FDA as early as September. The test will not require any trained personnel, complex solutions, or instruments and will be available self-testing at home. The platform, based on OraSure's OraQuick® technology, will allow testing in less than half an hour by using oral samples such as saliva. The company had successfully developed an oral-sample-HIV home test previously, which was used by millions around the globe. | OraSure (2020) |
Sofia 2 SARS Antigen Test Kit by QUIDEL was received and EUA from FDA in mid-May 2020.
5. Other methods
The prompt testing of the SARS-CoV-2 is a subject of the emergency as the virus is continuing to spread. It would be a tremendous help to fight against the virus if a precise and rapid test was developed since the methods based on rapid antibody and antigens tests are still in question regarding their accuracy and selectivity worldwide (Maxmen, 2020). The researchers all around the globe have been thriving to develop a test that could deliver fast and accurate results, without compromising the sensitivity and selectivity. In this subsection, some of the novel research-stage methods proposed for SARS-CoV-2 detection in the literature were elaborated. The content is also summarized in Table 5 for a quick review of the procedures and the outcomes. So far, many of the published methods have relied on field-effect transistors (FET) and surface plasmon resonance (SPR) principles that are known to deliver fast and sensitive signals. SPR principle is based on the collective oscillation of electrons on a given noble metal surface, and it is commonly used to monitor molecular interactions in proximity between protein-protein, protein-DNA, protein-RNA, antigen-antibody, enzyme-substrate samples in real-time, with high sensitivity (Kurt et al., 2019). The SPR method proposed by Djaileb et al. was based on the gold chips modified by the viral nucleocapsid proteins, and it detected the antibodies in diluted human serum samples in 15 min with nanomolar sensitivity (Djaileb et al., 2020), but no clinical testing was performed. The system was also utilized to fast screen some potential antibody candidates that showed the best binding affinity against the immobilized nucleocapsid proteins. Another SPR study, based on plasmonic photothermal effect (PPT) and localized surface plasmon resonance (LSPR), was published by Qui et al. in which the gold nanoparticles were decorated with oligonucleotides that were complementary to the selected viral gene regions (Qiu et al., 2020). The dual-function system successfully detected the RdRp gene, E gene, ORF1a gene regions with picomolar sensitivity. SPR systems are now widely available in laboratories, and especially the ones with automated handling systems can analyze several samples without human interference. The system works in real-time and gives reliable results in minutes on the antigen or antibody-modified surfaces in minutes. Therefore, SPR can be considered an alternative testing station to laboratory-based antigen and antibody kits for faster diagnosis of the clinical samples.
Table 5.
Early research stage SARS-COV-2 detection strategies in the literature.
| Target | Method | Result | Ref |
|---|---|---|---|
| A new framework to predict COVID-19 using onboard smartphone sensors | Measurement of well-known disease symptoms (fever, fatigue, headache, nausea, dry cough, lung CT imaging features, and shortness of breath) through the sensors already embedded in smartphones (cameras, inertial sensors, microphone, and temperature sensor) | The proposed framework is expected to read the smartphone sensors' signal measurements to predict the grade of severity of pneumonia. Although the sensitivity is still a question, such systems can be useful to monitor and act on mass populations worldwide online. | Maghdid et al. (2020b) |
| An electrical probe to detect COVID-19 spike protein S1 | Receptor Binding Domain-modified graphene field-effect transistor was used to identify spike proteins | The sensor can capture S1 protein at a limit of detection down to 0.2 pM, in a real-time and label-free manner. The platform could also be used to screen high-affinity antibodies. Portable electrochemical measurement systems are widely available at low-cost, and thus FET principle may be quickly transformed into a biomedical instrument for on-site diagnosis, especially in rural areas. | Zhang et al. (2020) |
| An artificial intelligence tool (based on deep learning and transfer learning algorithms) to predict COVID-10 cases | Collection of X-rays and CT scan images from multiple sources and processing by simple convolution neural network (CNN) and modified the pre-trained Alex Net model. | The constructed models provide accuracy up to 98% via a pre-trained network and 94.1% accuracy by using the modified CNN. Such algorithms can be quite fast and effective for health and regulatory institutions to monitor the pandemic in real-time and obtain fast-track data of individuals who are at risk. | Maghdid et al. (2020a) |
| A field-effect transistor device for SARS-CoV-2 spike protein detection in clinical samples | The graphene sheets coated by SARS-CoV-2 spike protein-specific antibodies were used as the transducer for sensing the signal production | The sensor detected SARS-CoV-2 in culture medium (limit of detection [LOD]: 1.6 × 101 pfu/mL) and clinical samples (LOD: 2.42 × 102 copies/mL) | Seo et al. (2020) |
| RT-LAMP-coupled with Nanoparticles are suggested for COVID-19 diagnosing | The assay is based on two sets of LAMP primers against ORF1ab and N genes of the virus. The assay results were interpreted through the nanoparticles | The sensitivity was reported as 12 copies/reaction, and no cross-reactivity was generated from non-SARS-CoV-2 templates. The analytical sensitivity was 100% (33/33) in the clinically validated oropharynx swab samples, and the specificity was also 100% (96/96) when analyzed with samples from non-COVID-patients. RT-LAMP has rapidly become an efficient tool for viral gene detection because of faster reaction times and increased sensitivity. These systems can be an alternative to the existent RT-PCR method because RT-LAMP can also be incorporated with LFA technology for individual implementation (please see the previous sections). | (Zhu et al., 2020b) |
| A microfluidic ELISA technology for rapid (15–20 min) detection of viral IgG and viral S antigen | The portable, microfluidic-based ELISA array has 12 channels. The signal intensities of the microfluidic ELISA were measured with the chemiluminescent imaging method, using a CMOS camera. Multiple exposures with adjustable exposure time were applied to enhance the dynamic ranges of the ELISA | A candidate IgG with a high binding affinity towards the SARS-CoV-2 S1 protein was identified. The microfluidic ELISA platform was used for the detection of anti-S1 monoclonal antibodies. No clinical testing was performed. The current rapid antibody tests are mainly LFA-based, and many of them still rely on instruments for increased sensitivity. Although ELISA is also instrument-dependent, it can give high throughput results with better sensitivity. | Tan et al. (2020) |
| The selected gene sequences (RdRp, E gene, ORF1ab) from SARS-CoV- through nucleic acid hybridization | A dual-functional plasmonic biosensor combining the plasmonic photothermal (PPT) effect and localized surface plasmon resonance (LSPR) was proposed for COVID-19 diagnosis. The two-dimensional gold nano islands-modified with complementary DNA receptors was used to detect the selected viral sequences |
A reasonably low detection limit of 0.22 pM was reported. SPR instruments are quite sensitive in general and easy to operate in the laboratories. They only require chip or surface modification by antigens or antibodies. They, therefore, can be a good alternative to current diagnostic tools that are limited by RT-PCR instruments. | Qiu et al. (2020) |
| Reverse transcription loop-mediated isothermal amplification (RT-LAMP) methodology was reported for simultaneous SARS-CoV-2 RNA region detection (ORF1a and N regions) | ORF1a and N gene regions from SARS-CoV-2 RNA were targeted with the RT-LAMP system using China-CDC approved primers | 45 SARS-CoV-2 positive and 25 negative samples were employed in the study. The dual gene RT-LAMP assay was found to be 95% accurate in detecting positive cases and showed no cross-reactivity or false-positive results in non-COVID- 19 samples | Butt et al. (2020) |
| A portable Surface Plasmon Resonance (SPR)-based platform for SARS-CoV-2 specific antibody detection | The SPR chip surface was modified with the viral N proteins to be able to detect the antibodies against the nucleocapsid proteins of the virus in diluted human serum samples. An SPR sensor coated with a peptide monolayer and functionalized with SARS-CoV-2 nucleocapsid recombinant protein detected anti-SARS-CoV-2 antibodies in the nanomolar range. This bioassay was performed on a portable. |
SPR instrument used with undiluted human serum samples provided binding affinities in the nanomolar range. The results were collected within 15 min of sample/sensor contact. | Djaileb et al. (2020) |
| CRISPR, Cas12-based lateral flow assay for detection of SARS-CoV-2 from RNA extracts. | The platform performs RT-LAMP with RNA samples extracted from nasopharyngeal or oropharyngeal swabs in universal transport media (UTM). Then, selected SARS-CoV-2 sequences are detected by Cas 12 enzyme, and the cleavage of a reporter dye confirms the presence of the virus | The method was validated by employing contrived reference samples and 40 clinical samples from infected US patients. The results said to be comparable with the US CDC SARS-CoV-2 real-time RT-PCR assay. | Broughton et al. (2020) |
| SARS-CoV-2 spike protein detection in saliva | A built in- house electrochemical measurement platform (eCovSens) was used for the detection. The fluorine-doped tin oxide and the gold nanoparticle (signal amplifier)-based electrode was decorated with anti-spike monoclonal antibodies to monitor the change in the conductivity upon target binding | The limit of detection was reported as small as 90 fM for the spiked saliva samples. | Mahari et al. (2020) |
| One-pot detection of SARS-CoV-2 and | The assay platform, AIOD-CRISPR, was based on dual CRISPR-Cas12a components and the LAMP principles. The assays were conducted in one pot at one step and one temperature. | The optimized system was able to detect 1.2 copies of DNA targets, and 4.6 copies of RNA targets in 40 min without the need for a preamplification step. | Ding et al. (2020) |
| SARS- CoV-2 genome sequences | Nanopore target sequencing (NTS) platform was developed to detect novel coronavirus as well as previous respiratory viruses in a simultaneous manner in 6–10 h. | The NTS platform was compared with the approved qPCR for 61 samples from suspected clinical samples. The results demonstrated that the NTS could detect more positive cases. Besides, the mutated sequences can be found along with the other respiratory viruses present in the sample. The sequencing-based virus diagnosis strategies are quite rare but highly needed since the revealed genomic and proteomic elements of the virus form the basis for the current and future diagnosis tools. | (M. M. Wang et al., 2020) |
| SARS-CoV-2 nucleic acids: N1, N2, and N3 gene regions | The diagnosis system was based on the combined use of a portable mini PCR machine and a 96-well plate reader. EvaGreen dye was employed as an intercalator for fluorescent detection of the targeted regions. | The method detected ~625 to 2 × 105 DNA copies through direct PCR amplification, and the signal measurements were performed on a plate reader, which was proposed as a portable alternative for viral gene sequencing. | Gonzalez-Gonzalez et al. (2020) |
Graphene has been a vital component of FET-based label-free sensors for the last decade (Ohno et al., 2010; Yüce and Kurt, 2017) based on the key properties, such as optical transparency, electrical conductivity, robust pi-interactions with macromolecules and tuneability, rivaling the similar materials in the field. In FET-based biosensors, biological molecules change the charge distribution of the surface, or they induce a surface potential by binding to the surface, which is then measured as a conductance value with high sensitivity (Chen et al., 2019). In the study conducted by Seo et al., the graphene sheets coated by SARS-CoV-2 spike protein-specific antibodies were used as the active surface for monitoring the signal production out of viral protein binding (Seo et al., 2020). The platform achieved a limit of the detection value of 242 copies/mL of in clinical samples, which is like the ones obtained in standard RT-PCR setups (around five genomic copies/ul). Another electrical probe was reported by Zhang et al., where the FET surface was modified with the receptor binding domain to be able to detect viral S glycoprotein (Zhang et al., 2020). The platform showed an excellent sensitivity down to the picomolar level in real-time and without specific labels. Graphene-based commercial electrodes have become common in many laboratories specialized in electrochemical techniques. Because the suggested sensing strategies are based on simple surface modification of the graphene electrodes, many of the viral antigens are antibodies have become commercially available, portable electrochemical detection systems are available, such research-wise tools can be quickly converted into biomedical instruments for field use after clinical validations.
Loop-mediated isothermal amplification (LAMP), on the other hand, is another tool being developed for rapid SARS-CoV-2 detection. LAMP is a cost-effective alternative to standard PCR since it does not require expensive thermocycler instruments that operate alternating temperatures for the amplification (“Loop-mediated isothermal amplification of DNA,” n.d.). The method can synthesize up to 109 copies of the target gene in less than an hour. All the amplification reaction is processed in one single tube at around 60–65 °C by using two or three different gene-specific primer sets and strand-displacement polymerase enzymes (Green et al., 2020). Magnesium pyrophosphate byproduct is formed during the amplification process, which can be measured by the naked eye or simple analytical devices, such as turbidimetry or photometry, for increased sensitivity. Dye molecules could also be employed in the reaction for selective quantitative detection of the target genes as well as the data correlation purposes. Compared to PCR, a finely-tuned LAMP test could be faster and more sensitive to rapid testing (Nguyen et al., 2020). Reverse Transcription LAMP (RT-LAMP) applies an additional transcription step for the detection of RNA targets. So far, only one RT-LAMP-based kit, “SherlockTM CRISPR SARS-CoV-2 kit developed by Sherlock Biosciences, Inc., has received and EUA from FDA (U.S. Food & Drug Administration, 2020k). The Sherlock kit is designed to target ORF1ab, N, and RP genes, the third being the internal extraction control gene.
Likewise, Butt et al. reported an RT-LAMP assay for the simultaneous detection of ORF1a and N genes (Butt et al., 2020). The test showed 95% accuracy in clinically validated positive samples (n = 45), whereas no cross-reactivity was observed with any of the clinically validated negative samples (n = 25). Similarly, a nanoparticle-based RT-LAMP assay was reported to be 100% accurate in clinically validated oropharynx swab samples (n = 33), and the specificity was again 100% with the negative clinical samples (n = 96) tested (Zhu et al., 2020b). The analytical tools that rely on the LAMP principle for SARS-CoV-2 detection, however, may give “false negative” results for the patients who passed the infection and improved already (similar to RT-PCR) (Green et al., 2020). Hence, the researchers are now fine-tuning the current models to improve the test sensitivity as well as the specificity, mainly by decreasing the required viral load for more reliable testing.
Based on a previously established “ID NOW rapid detection” technology, Abbott Inc. has developed an “Abbott ID NOW COVID-19” test kit for SARS-COV-2 detection using LAMP principles and cleared a EUA from FDA in late March 2020 (Abbott, 2020b; U.SFDA, 2020a, USFDA, 2020k). The new Abbott ID NOW COVID-19 test is operated on a small instrument that could perform around 50,000 tests per day, giving results only in five minutes per sample. This technology has been the fastest rapid diagnostic tool so far that issued and EUA from the FDA and sold out in thousands in the USA. Combined with the RealTime SARS-CoV-2 platform, Abbott plans to deliver around 5 million tests in late April 2020.
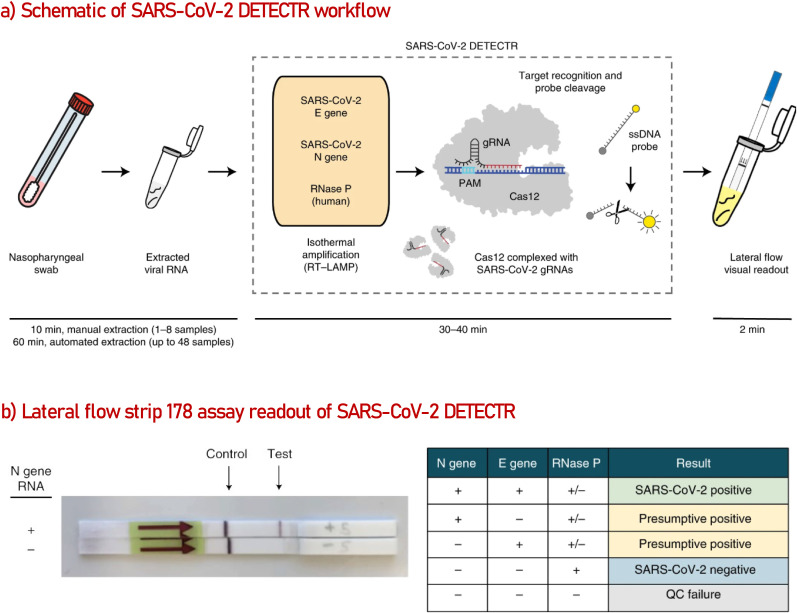
A sophisticated method, combining RT-LAMP, Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) (Jia et al., 2020; Mustafa and Makhawi, 2020) and LFA principles in one assay (Posthuma-Trumpie et al., 2009) was reported by a group of scientists from Mammoth Biosciences Inc., University of California, Abbott Viral Diagnostics and Discovery Inc. and Center California Department of Public Health (Broughton et al., 2020). As represented in Fig. 3 , the method named “SARS-CoV-2 DETECTR” processes the extracted RNA samples (10 min) first with RT-PCR to increase the copy numbers of the selected E, N, and RNAse P genes in the sample solution (62 °C for 20). Second, the CRISPR-Cas 12 enzyme takes the role and detect the genomic copies from the specified sequences, producing a fluorescent signal because of the reporter dye cleavage (37 °C for 10 min). The optimized assay was tested on 11 respiratory swab samples from patients with COVID-19 infection and 12 swab samples from patients with influenza. SARS-CoV-2 DETECTR was found 90% sensitive and 100% specific for SARS-COV-2 detection, which was reported to be relative to CDC qRT-PCR standard assay values. It was also indicated that the primer-probe set designed for the N2 gene was able to distinguish SARS-CoV-2 from SARS-CoV and MERS-CoV at a single nucleotide level. The group is focusing on validating the assay with clinical samples to be able to receive a EUA from the FDA (Dutton, 2020).
Fig. 3.
(a) Illustration of the method workflow. Standard RNA extraction solution or original sample matrix can be used as an input to detect E, N, and RNase P genes. The assay is monitored by a fluorescent reader or lateral flow strip. (b) Lateral flow readout of SARS-CoV-2 positive sample. Detection of at least two genes is necessary for positive sample confirmation. Reproduced from ref (Broughton et al., 2020).
6. Conclusion
The COVID-19 pandemic has proven the worth of rapid diagnostics. As comprehensively reviewed in this paper (based on the information available so far), the detection of the viral genomic regions has been almost solely dependent on the RT-PCR method, or molecular tests in general. Rapid antibody (serology) tests are certainly needed for vast immune screening, but they do not confirm the presence of the virus. The antigen tests are currently under development, and it would take some time to develop a very accurate one since the virus is mutating without control. Despite the unprecedented collective effort put by the researchers into developing sensitive, selective and rapid test kits, still, only a small portion of the world population has been tested by now, and the testing priority has been understandably given to those who show the severe clinical symptoms of the disease as well as the frontline healthcare providers. As a result, the total lockdown, mask use, and social distancing have become the most effective practice to prevent the spread of the disease and overload in pandemic clinics. The global fight against COVID-19 could be a long one until we develop effective and clinically validated treatments above and beyond the vaccines. Until then, analytical experts and antigen/antibody manufacturers must focus on the development of self-testing home kits to be able to see the real extent and progress of the disease worldwide and act accordingly.
Author contributions
Elif Filiztekin and Korin Gasia Özkaya equally contributed to this work and listed alphabetically.
CRediT authorship contribution statement
Meral Yüce: Writing - original draft, Designed the paper, wrote and revised all sections. Elif Filiztekin: contributed to a part of the antibody detection methods section, equally contributed to this work and listed alphabetically. Korin Gasia Özkaya: contributed to a part of the antibody detection methods section, equally contributed to this work and listed alphabetically.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors would like to thank Sabanci University and Sabanci University SUNUM Nanotechnology Research and Application Center for the swift and efficient actions taken during the pandemic, which made this work possible.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bios.2020.112752.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Abbott . molecular.abbott; 2020. Abbott RealTime SARS-CoV-2 Assay (EUA) | Abbott Molecular.https://www.molecular.abbott/us/en/products/infectious-disease/RealTime-SARS-CoV-2-Assay [WWW Document] accessed 4.25.20. [Google Scholar]
- Abbott . abbott.com; 2020. Detect COVID-19 in as Little as 5 Minutes | Abbott Newsroom.https://www.abbott.com/corpnewsroom/product-and-innovation/detect-covid-19-in-as-little-as-5-minutes.html [WWW Document] accessed 4.29.20. [Google Scholar]
- A, RUP Laboratories . aruplab.com; 2020. ARUP Laboratories Announces Nationwide Rollout of COVID-19 Antibody Testing | ARUP Laboratories.https://www.aruplab.com/news/4-16-2020/antibody-testing-rollout [WWW Document] accessed 4.26.20. [Google Scholar]
- Avacta Life Sciences Limited . avacta.com; 2020. Avacta/Cytiva Partnership Ahead of Schedule.https://avacta.com/avacta-cytiva-partnership-ahead-of-schedule/ [WWW Document] accessed 4.29.20. [Google Scholar]
- Aytu BioScience Inc . accesswire.com; 2020. Aytu BioScience Secures Exclusive U.S. Distribution Agreement for Coronavirus 2019 (COVID-19) Point-of-Care Rapid Test.https://www.accesswire.com/579898/Aytu-BioScience-Secures-Exclusive-US-DistributionAgreement-for-Coronavirus-2019-COVID-19-Point-of-Care-Rapid-Test [WWW Document] [Google Scholar]
- Broughton J.P., Deng X., Yu G., Fasching C.L., Singh J., Streithorst J.A., Granados A., Sotomayor-Gonzalez A., Zorn K., Gopez A., Hsu E., Gu W., Miller S., Pan C., Guevara H., Wadford D.A., Chen J.S., Chiu C.Y., Servellita V., Singh J., Miao X., Streithorst J.A., Granados A., Sotomayor-Gonzalez A., Zorn K., Gopez A., Hsu E., Gu W., Miller S., Pan C., Guevara H., Wadford D.A., Chen J.S., Chiu C.Y. 2020. Rapid Detection of 2019 Novel Coronavirus SARS-CoV-2 using a CRISPR-based DETECTR Lateral Flow Assay. medRxiv 2020.3.6.20032334. [DOI] [Google Scholar]
- Butt A.M., Siddique S., An X., Tong Y. 2020. Development of a dual-gene loop-mediated isothermal amplification (LAMP) detection assay for SARS-CoV-2: a preliminary study. medRxiv 2020.04.08.20056986. [DOI] [Google Scholar]
- Cairns E. evaluate.com; 2020. New Covid-19 test and a decent first quarter buoy Abbott | Evaluate.https://www.evaluate.com/vantage/articles/news/corporate-strategy/new-covid-19-test-and-decent-first-quarter-buoy-abbott [WWW Document] accessed 4.30.20. [Google Scholar]
- Cevik M., Bamford C., Ho A. COVID-19 pandemic – a focused review for clinicians. Clin. Microbiol. Infect. 2020 doi: 10.1016/j.cmi.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Liu Y., Fang X., Li Z., Pu H., Chang J., Chen J., Mao S. Ultratrace antibiotic sensing using aptamer/graphene-based field-effect transistors. Biosens. Bioelectron. 2019;126:664–671. doi: 10.1016/j.bios.2018.11.034. [DOI] [PubMed] [Google Scholar]
- China C.D.C. 2020. 病毒病控制所 [WWW Document]. ivdc.chinacdc.cn.http://ivdc.chinacdc.cn/kyjz/202001/t20200121_211337.html accessed 4.28.20. [Google Scholar]
- Chu D.K.W., Pan Y., Cheng S.M.S., Hui K.P.Y., Krishnan P., Liu Y., Ng D.Y.M., Wan C.K.C., Yang P., Wang Q., Peiris M., Poon L.L.M. Molecular diagnosis of a novel Coronavirus (2019-nCoV) Causing an outbreak of Pneumonia. Clin. Chem. 2020;66:549–555. doi: 10.1093/clinchem/hvaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E. cnn.com; 2020. Coronavirus antibody tests have “really terrible” accuracy, researcher says - CNN.https://edition.cnn.com/2020/04/28/health/coronavirus-antibody-tests-terrible/index.html [WWW Document] accessed 4.29.20. [Google Scholar]
- Cohen J. Unprecedented nationwide blood studies seek to track U.S. coronavirus spread. Science (80-. ) 2020 doi: 10.1126/science.abc1319. [DOI] [Google Scholar]
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K.W., Bleicker T., Brünink S., Schneider J., Schmidt M.L., Mulders D.G.J.C., Haagmans B.L., Van Der Veer B., Van Den Brink S., Wijsman L., Goderski G., Romette J.L., Ellis J., Zambon M., Peiris M., Goossens H., Reusken C., Koopmans M.P.G., Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J., Li F., Shi Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao B., Wen K., Chen Jian, Liu Y., Yuan Z., Han C., Chen Jiahui, Pan Y., Chen L., Dan Y., Wang J., Chen Y., Deng G., Zhou H., Wu Y. 2020. Diagnosis of Acute Respiratory Syndrome Coronavirus 2 Infection by Detection of Nucleocapsid Protein. medRxiv 2020.3.7.20032524. [DOI] [Google Scholar]
- Ding X., Yin K., Li Z., Liu C. 2020. All-in-One Dual CRISPR-Cas12a (AIOD-CRISPR) Assay: a Case for Rapid, Ultrasensitive and Visual Detection of Novel Coronavirus SARS-CoV-2 and HIV virus. bioRxiv 2020.3.19.998724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djaileb A., Charron B., Jodaylami M.H., Thibault V., Coutu J., Forest S., Live L.S., Boudreau D., Pelletier J.N., Masson J. 2020. A Rapid and quantitative Serum Test for SARS-CoV-2 Antibodies with Portable Surface Plasmon Resonance Sensing. chemrxiv.org 0–13. [DOI] [Google Scholar]
- Dutton G. biospace.com; 2020. Mammoth's CRISPR Assay for Asymptomatic COVID-19 Carriers Gains Peer-Reviewed Validation | BioSpace.https://www.biospace.com/article/mammoth-s-crispr-assay-for-asymptomatic-covid-19-carriers-gains-peer-reviewed-validation-/ [WWW Document] accessed 4.28.20. [Google Scholar]
- Financial Times . ft.com; 2020. Germany to run Europe's first large-scale antibody test programme | Financial Times.https://www.ft.com/content/fe211ec7-0ed4-4d36-9d83-14b639efb3ad [WWW Document] accessed 4.26.20. [Google Scholar]
- General of Health Malaysia . kpkesihatan.com; 2020. Laboratory Readiness for Detecting the 2019 novel coronavirus (2019-nCoV) infection in Malaysia – From the Desk of the Director-General of Health Malaysia.https://kpkesihatan.com/2020/02/13/laboratory-readiness-for-detecting-the-2019-novel-coronavirus-2019-ncov-infection-in-malaysia/ [WWW Document] accessed 4.25.20. [Google Scholar]
- Global Product Certification . gpc.center; 2020. CE IVD.https://www.gpc.center/ce_ivd_/585 [WWW Document] [Google Scholar]
- Gonzalez-Gonzalez E., Santiago G.T., Lara-Mayorga I.M., Martinez-Chapa S.O., Alvarez M.M. 2020. Portable and accurate diagnostics for COVID-19: Combined use of the miniPCR thermocycler and a well-plate reader for SARS-Co2 virus detection. medRxiv 2020.04.03.20052860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K., Winter A., Dickinson R., Graziadio S., Wolff R., Mallett S., Allen A.J., Park E.B. 2020. What tests could potentially be used for the screening , diagnosis and monitoring of COVID-19 and what are their advantages and disadvantages ?https://www.cebm.net/covid-19/what-tests-could-potentially-be-used-for-the-screening-diagnosis-and-monitoring-of-covid-19-and-what-are-their-advantages-and-disadvantages/ [WWW Document] accessed 4.29.20. [Google Scholar]
- Harun H.N. New StraitsTimes; 2020. IMR Created Reagent to Handle 2019-nCoV Outbreak Ahead of the WHO. [Google Scholar]
- Holland P.M., Abramson R.D., Watson R., Gelfand D.H. Detection of specific polymerase chain reaction product by utilizing the 5′ → 3′ exonuclease activity of Thermus aquaticus DNA polymerase. Proc. Natl. Acad. Sci. U.S.A. 1991 doi: 10.1073/pnas.88.16.7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Pasteur . 2020. Protocol : Real-Time RT-PCR Assays for the Detection of SARS-CoV-2 1–3. [Google Scholar]
- Jacofsky D., Jacofsky E.M., Ph D., Officer C.E., Jacofsky M., Ph D. Understanding antibody testing for COVID-19. J. Arthroplasty. 2020 doi: 10.1016/j.arth.2020.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia F., Li X., Zhang C., Tang X. The expanded development and application of CRISPR system for sensitive nucleotide detection. Protein Cell. 2020;9 doi: 10.1007/s13238-020-00708-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns Hopkins Bloomberg School of Public Health . Cent. Heal. Secur; 2020. Serology-based Tests for COVID-19 [WWW Document] [Google Scholar]
- Jung Y.J., Park G.-S., Moon J.H., Ku K., Beak S.-H., Kim Seil, Park E.C., Park D., Lee J.-H., Byeon C.W., Lee J.J., Maeng J., Kim S.J., Kim Seung Il, Kim B.-T., Lee M.J., Kim H.G. 2020. Comparative Analysis of Primer-probe Sets for the Laboratory Confirmation of SARS-CoV-2. bioRxiv 2020.2.25.964775. [DOI] [PubMed] [Google Scholar]
- Kent C. 2020. Different Paths to the Same Destination : Screening for Covid-19. [WWW Document] [Google Scholar]
- Khailany R.A., Safdar M., Ozaslan M. Genomic characterization of a novel SARS-CoV-2. Gene Rep. 2020;19 doi: 10.1016/j.genrep.2020.100682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt H., Eyüpoǧlu A.E., Sütlü T., Budak H., Yüce M. Plasmonic Selection of ssDNA Aptamers against fibroblast growth factor Receptor. ACS Comb. Sci. 2019;21:578–587. doi: 10.1021/acscombsci.9b00059. [DOI] [PubMed] [Google Scholar]
- LabCorp . pixel.labcorp.com; 2020. COVID-19 Test (At-Home Kit) | Pixel by LabCorp.https://www.pixel.labcorp.com/at-home-test-kits/covid-19-test [WWW Document] accessed 4.26.20. [Google Scholar]
- LabCorp . fda.gov; 2020. LabCorp COVID-19 RT-PCR test EUA Summary America)https://www.fda.gov/media/136151/download [WWW Document] accessed 4.25.20. [Google Scholar]
- Lee M.S., Chang P.C., Shien J.H., Cheng M.C., Shieh H.K. Identification and subtyping of avian influenza viruses by reverse transcription-PCR. J. Virol. Methods. 2001 doi: 10.1016/S0166-0934(01)00301-9. [DOI] [PubMed] [Google Scholar]
- Li Z., Yi Y., Luo X., Xiong N., Liu Y., Li S., Sun R., Wang Y., Hu B., Chen W., Zhang Y., Wang J., Huang B., Lin Y., Yang J., Cai W., Wang X., Cheng J., Chen Z., Sun K., Pan W., Zhan Z., Chen L., Ye F. Development and clinical application of A Rapid IgM‐IgG combined antibody Test for SARS‐CoV‐2 infection Diagnosis. J. Med. Virol. 2020 doi: 10.1002/jmv.25727. 0–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D., Liu L., Zhang M., Hu Y., Yang Q., Guo J., Dai Y., Xu Y., Cai Y., Chen X., Huang K., Zhang Z. 2020. Evaluations of serological test in the diagnosis of 2019 novel coronavirus (SARS-CoV-2) infections during the COVID-19 outbreak. medRxiv 2020.3.27.20045153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Xiao X., Wei X., Li J., Yang J., Tan H., Zhu J., Zhang Q., Wu J., Liu L. Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS‐CoV‐2. J. Med. Virol. 2020;92:595–601. doi: 10.1002/jmv.25726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeffelholz M.J., Tang Y.-W. Laboratory diagnosis of emerging human coronavirus infections – the state of the art. Emerg. Microb. Infect. 2020;9:747–756. doi: 10.1080/22221751.2020.1745095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NA [WWW Document], n.d. URL https://www.ncbi.nlm.nih.gov/pmc/articles/PMC102748/(accessed 4.28.20).
- Maghdid H.S., Asaad A.T., Ghafoor K.Z., Sadiq A.S., Khan M.K. 2020. Diagnosing COVID-19 Pneumonia from X-Ray and CT Images Using Deep Learning and Transfer Learning Algorithms. [Google Scholar]
- Maghdid H.S., Ghafoor K.Z., Sadiq A.S., Curran K., Rabie K. A Novel AI-enabled Framework to Diagnose Coronavirus COVID 19 using Smartphone Embedded Sensors. Des. Stud. 2020;1–5 [Google Scholar]
- Mahari S., Roberts A., Shahdeo D., Gandhi S. 2020. eCovSens-Ultrasensitive Novel In-House Built Printed Circuit Board Based Electrochemical Device for Rapid Detection of nCovid-19. bioRxiv 2020.4.24.059204. [DOI] [Google Scholar]
- Maschinen B., Investition A., Beschaffungen G., Ersatzbeschaffungen B., Mittelherkunft S. 2020. WHO Summary in House Assays for RASR-CoV-2 Detection by RT-PCR [WWW Document] [Google Scholar]
- Maxmen A. The researchers taking a gamble with antibody tests for coronavirus. Nature. 2020 doi: 10.1038/d41586-020-01163-5. [DOI] [PubMed] [Google Scholar]
- Morales-Narváez E., Dincer C. The impact of biosensing in a pandemic outbreak: COVID-19. Biosens. Bioelectron. 2020;163 doi: 10.1016/j.bios.2020.112274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. pharmacytimes.com/; 2020. First Clinical-Grade Rapid At-Home Test to Aid in COVID-19 Crisis.https://www.pharmacytimes.com/news/first-clinical-grade-rapid-at-home-test-to-aid-in-covid-19-crisis [WWW Document] accessed 5.1.20. [Google Scholar]
- Mustafa M.I., Makhawi A.M. 2020. SHERLOCK and DETECTR : CRISPR-Cas Systems as Potential Rapid Diagnostic Tools for Emerging Infectious Diseases and Cancer-Associated Mutations. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Nalla A.K., Casto A.M., Huang M.-L.W., Perchetti G.A., Sampoleo R., Shrestha L., Wei Y., Zhu H., Jerome K.R., Greninger A.L. Comparative performance of SARS-CoV-2 Detection assays using Seven Different primer/probe Sets and one assay Kit. J. Clin. Microbiol. 2020 doi: 10.1128/JCM.00557-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nao N., Shirato K., Katano H., Matsuyama S., Takeda M. Department of Virology III, National Institute of Infectious Diseases; 2019. Detection of Second Case of 2019-nCoV Infection in Japan (Corrected Version), Laboratory of Acute Viral Respiratory Infections and Cytokines. 4-7-1 Gakuen, Musashimurayama, 208-0011 Tokyo, Japan: 2Department of Pathology, National Institute of Infectious Disease. doi:.1037//0033-2909.I26.1.78. [Google Scholar]
- Nguyen T., Duong Bang D., Wolff A. 2019 novel Coronavirus Disease (COVID-19): Paving the Road for Rapid Detection and point-of-care Diagnostics. Micromachines. 2020;11:306. doi: 10.3390/mi11030306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan T., Hands R.E., Bustin S.A. Quantification of mRNA using real-time RT-PCR. Nat. Protoc. 2006;1:1559–1582. doi: 10.1038/nprot.2006.236. [DOI] [PubMed] [Google Scholar]
- Ohno Y., Maehashi K., Matsumoto K. Label-free biosensors based on aptamer-Modified graphene field-effect transistors. J. Am. Chem. Soc. 2010;132:18012–18013. doi: 10.1021/ja108127r. [DOI] [PubMed] [Google Scholar]
- Okba N., Muller M., Li W., Wang C., GeurtsvanKessel C., Corman V., Lamers M., Sikkema R., de Bruin E., Chandler F., Yazdanpanah Y., Le Hingrat Q., Descamps D., Houhou-Fidouh N., Reusken C., Bosch B.-J., Drosten C., Koopmans M., Haagmans B. SARS-CoV-2 specific antibody responses in COVID-19 patients. Emerg. Infect. Dis. 2020. 2020 doi: 10.1101/2020.03.18.20038059. 03.18.20038059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OraSure I. orasure.com; 2020. OraSure Technologies - In Development.https://www.orasure.com/innovation/innovation-in-development.asp [WWW Document] accessed 4.29.20. [Google Scholar]
- Pabbaraju K., Wong S., Wong A.A., Appleyard G.D., Chui L., Pang X.L., Yanow S.K., Fonseca K., Lee B.E., Fox J.D., Preiksaitis J.K. Design and validation of real-time reverse transcription-PCR assays for detection of pandemic (H1N1) 2009 virus. J. Clin. Microbiol. 2009 doi: 10.1128/JCM.01103-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilot L.R. Pharmacy Law Desk Reference. 2012. Federal food, drug, and cosmetic act; pp. 25–39. [DOI] [Google Scholar]
- Pinto D., Park Y.-J., Beltramello M., Walls A.C., Tortorici M.A., Bianchi S., Jaconi S., Culap K., Zatta F., De Marco A., Peter A., Guarino B., Spreafico R., Cameroni E., Case J.B., Chen R.E., Havenar-Daughton C., Snell G., Telenti A., Virgin H.W., Lanzavecchia A., Diamond M.S., Fink K., Veesler D., Corti D. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020:1–10. doi: 10.1038/s41586-020-2349-y. [DOI] [PubMed] [Google Scholar]
- Posthuma-Trumpie G.A., Korf J., van Amerongen A. Lateral flow (immuno)assay: its strengths, weaknesses, opportunities and threats. A literature survey. Anal. Bioanal. Chem. 2009;393:569–582. doi: 10.1007/s00216-008-2287-2. [DOI] [PubMed] [Google Scholar]
- Qiu G., Gai Z., Tao Y., Schmitt J., Kullak-Ublick G.A., Wang J. Dual-functional plasmonic photothermal biosensors for highly accurate Severe acute Respiratory Syndrome Coronavirus 2 Detection. ACS Nano. 2020 doi: 10.1021/acsnano.0c02439. [DOI] [PubMed] [Google Scholar]
- Rao A., Goldstein D.Y., Wolk D.M., Wolf L.A. 2020. White-paper: Development and Evaluation of Two SARS-CoV-2 RT-PCR Laboratory Developed Tests on the ARIES ® Automated, Sample-To-Answer, Real-Time PCR System [WWW Document]. luminexcorp.com. [Google Scholar]
- Roche . diagnostics.roche.com; 2020. cobas® SARS-CoV-2 Test.https://diagnostics.roche.com/global/en/products/params/cobas-sars-cov-2-test.html [WWW Document] accessed 4.25.20. [Google Scholar]
- Rosales-Mendoza S., Márquez-Escobar V.A., González-Ortega O., Nieto-Gómez R., Arévalo-Villalobos J.I. What Does Plant-based vaccine technology offer to the fight against COVID-19? Vaccines. 2020;8:183. doi: 10.3390/vaccines8020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy J., Jain N., Singh G., Das B., Mallick B. AGO-driven Non-Coding RNAs. Elsevier; 2019. Small RNA proteome as disease biomarker: an incognito treasure of clinical utility; pp. 101–136. [DOI] [Google Scholar]
- Royal Statistical Society Covid-19 Task Force . Reiczegel; 2020. Statement on Covid-19 Antibody Testing, issued on 14th April 2020. [WWW Document]. rss.org.uk. [DOI] [Google Scholar]
- SDBiosensor Inc . sdbiosensor.com; 2020. Products - STANDARD Q COVID-19 IgM/IgG Duo.http://sdbiosensor.com/xe/product/7662 [WWW Document] accessed 5.1.20. [Google Scholar]
- Seo G., Lee G., Kim M.J., Baek S.-H., Choi M., Ku K.B., Lee C.-S., Jun S., Park D., Kim H.G., Kim S.-J., Lee J.-O., Kim B.T., Park E.C., Kim S. Il. Rapid Detection of COVID-19 Causative virus (SARS-CoV-2) in human nasopharyngeal Swab Specimens using field-effect transistor-based biosensor. ACS Nano. 2020 doi: 10.1021/acsnano.0c02823. [DOI] [PubMed] [Google Scholar]
- Soh J.H., Chan H.M., Ying J.Y. Strategies for developing sensitive and specific nanoparticle-based lateral flow assays as point-of-care diagnostic device. Nano Today. 2020;30 doi: 10.1016/j.nantod.2019.100831. [DOI] [Google Scholar]
- Su S., Wong G., Shi W., Liu J., Lai A.C.K., Zhou J., Liu W., Bi Y., Gao G.F. Epidemiology, genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X., Lin C., Zhang J., Oo M.K.K., Fan X. 2020. Rapid and quantitative detection of COVID-19 markers in micro-liter sized samples. bioRxiv 2020.4.20.052233. [DOI] [Google Scholar]
- Thailand Ministry of Public Health . who.int; 2020. Diagnostic detection of Novel coronavirus 2019 by Real time RTPCR.https://www.who.int/docs/default-source/coronaviruse/conventional-rt-pcr-followed-by-sequencing-for-detection-of-ncov-rirl-nat-inst-health-t.pdf?sfvrsn=42271c6d_4 [WWW Document] accessed 4.25.20. [Google Scholar]
- U.S. Centers for Disease Control and Prevention . 2020. 2019-Novel Coronavirus (2019-nCoV) Real-Time rRT-PCR Panel Primers and Probes 2–3. [Google Scholar]
- U.S. Food & Drug Administration . fda.gov; 2020. Emergency Use Authorizations | FDA.https://www.fda.gov/medical-devices/emergency-situations-medical-devices/emergency-use-authorizations#covid19ivd [WWW Document] accessed 4.25.20. [Google Scholar]
- U.S. Food & Drug Administration . fda.gov; 2020. EUA-LabCorp COVID-19 RT-PCR- Letter of Authorization.https://www.fda.gov/media/136148/download [WWW Document] [Google Scholar]
- U.S. Food & Drug Administration . fda.gov; 2020. EUA-CDC Real-Time RT-PCR Diagnostic Panel-Letter of Authorization.https://www.fda.gov/media/134922/download [WWW Document] [Google Scholar]
- U.S. Food & Drug Administration . fda.gov; 2020. EUA-Abbott-nCoV-LOA-Letter of Authorization.https://www.fda.gov/media/136255/download [WWW Document] [Google Scholar]
- U.S. Food & Drug Administration . 2020. EUA-Hologic- Panther Fusion SARS-CoV-2-Letter of Authorization.https://www.fda.gov/media/136153/download [WWW Document] [Google Scholar]
- U.S. Food & Drug Administration . fda.gov; 2020. EUA-Thermo Fisher-TaqPath COVID-19 Combo Kit-Letter of Authorization.https://www.fda.gov/media/136113/download [WWW Document] [Google Scholar]
- U.S. Food & Drug Administration . 2020. EUA-LabCorp COVID-19 RT-PCR- Letter of Authorization.https://www.fda.gov/media/136148/download [WWW Document]. [Google Scholar]
- U.S. Food & Drug Administration . 2020. LabCorp COVID-19 RT-PCR test EUA Summary.https://www.fda.gov/media/136151/download [WWW Document] [Google Scholar]
- U.S. Food & Drug Administration . fda.gov; 2020. EUA: Sofia 2 SARS Antigen FIA by Quidel Corporation.https://www.fda.gov/media/137886/download [WWW Document] [Google Scholar]
- U.S. Food & Drug Administration . da.gov; 2020. Sofia 2 SARS Antigen FIA Kit Instructions for Use.https://www.fda.gov/media/137885/download [WWW Document] [Google Scholar]
- U.S. Food & Drug Administration . fda.gov; 2020. EUA: Sherlock CRISPR SARS-CoV-2 Kit by Sherlock BioSciences, Inc.https://www.fda.gov/media/136598/download [WWW Document] [Google Scholar]
- U.S. Food and Drug Administration . fda.gov; 2020. Coronavirus (COVID-19) Update: Serological Tests.https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-serological-tests [WWW Document] accessed 4.25.20. [Google Scholar]
- U.S. Food and Drug Administration . fda.gov; 2020. Policy for Coronavirus Disease-2019 Tests During the Public Health Emergency - Serology Template for Manufacturers.https://www.fda.gov/regulatory-information/search-fda-guidance-documents/policy-coronavirus-disease-2019-tests-during-public-health-emergency-revised [WWW Document] [Google Scholar]
- U.S. Food and Drug Administration . fda.gov; 2020. Coronavirus (COVID-19) Update: daily Roundup May 5, 2020 | FDA.https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-daily-roundup-may-5-2020 [WWW Document] accessed 5.6.20. [Google Scholar]
- Vogels C.B.F., Brito A.F., Wyllie A.L., Fauver J.R., Ott I.M., Kalinich C.C., Petrone M.E., Casanovas-Massana A., Muenker M.C., Moore A.J., Klein J., Lu P., Lu-Culligan A., Jiang X., Kim D.J., Kudo E., Mao T., Moriyama M., Oh J.E., Park A., Silva J., Song E., Takehashi T., Taura M., Tokuyama M., Venkataraman A., Weizman O.-E., Wong P., Yang Y., Cheemarla N.R., White E., Lapidus S., Earnest R., Geng B., Vijayakumar P., Odio C., Fournier J., Bermejo S., Farhadian S., Cruz C. Dela, Iwasaki A., Ko A.I., Landry M.-L., Foxman E.F., Grubaugh N.D. 2020. Analytical sensitivity and efficiency comparisons of SARS-COV-2 qRT-PCR primer-probe sets. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and Antigenicity of the SARS-CoV-2 Spike glycoprotein. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Li W., Drabek D., Okba N.M.A., van Haperen R., Osterhaus A.D.M.E., van Kuppeveld F.J.M., Haagmans B.L., Grosveld F., Bosch B.-J. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat. Commun. 2020;11:2251. doi: 10.1038/s41467-020-16256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Fu A., Hu B., Tong Y., Liu R., Gu J., Liu J., Jiang W., Shen G., Zhao W., Men D., Yu L., Deng Z., Li Y., Liu T. 2020. Nanopore target sequencing for accurate and comprehensive detection of SARS-CoV-2 and other respiratory viruses. medRxiv 2020.3.4.20029538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . who.int; 2020. WHO announces COVID-19 outbreak a pandemic.https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 [WWW Document] accessed 4.26.20. [Google Scholar]
- WHO . who.int; 2020. Advice on the use of point-of-care immunodiagnostic tests for COVID-19.https://www.who.int/news-room/commentaries/detail/advice-on-the-use-of-point-of-care-immunodiagnostic-tests-for-covid-19 [WWW Document] accessed 4.29.20. [Google Scholar]
- Woo C.H., Jang S., Shin G., Jung G.Y., Lee J.W. 2020. Sensitive One-step Isothermal Detection of Pathogen-derived RNAs. medRxiv 2020.03.05.20031971. [DOI] [Google Scholar]
- Worldometer Coronavirus update (live): Cases and deaths from COVID-19 virus pandemic. Worldometers. 2020 [Google Scholar]
- Xu Y., Li X., Zhu B., Liang H., Fang C., Gong Y., Guo Q., Sun X., Zhao D., Shen J., Zhang H., Liu H., Xia H., Tang J., Zhang K., Gong S. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat. Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yüce M., Kurt H. How to make nanobiosensors: surface modification and characterisation of nanomaterials for biosensing applications. RSC Adv. 2017;7:49386–49403. doi: 10.1039/C7RA10479K. [DOI] [Google Scholar]
- Zhang X., Qi Q., Jing Q., Ao S., Zhang Zhihong, Ding M., Wu M., Liu K., Wang W., Ling Y., Zhang Zhengjun, Fu W. 2020. Electrical Probing of COVID-19 Spike Protein Receptor Binding Domain via a Graphene Field-Effect Transistor; pp. 1–20. [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel Coronavirus from patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Wang X., Han L., Chen T., Wang L., Li H., Li S., He L., Fu X., Chen S., Mei X., Chen H., Wang Y. 2020. Reverse transcription loop-mediated isothermal amplification combined with nanoparticles-based biosensor for diagnosis of COVID-19. medRxiv 2020.3.17.20037796. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.