Abstract
Motor deficits in parkinsonism are caused by degeneration of dopaminergic nigral neurons. The success of disease-modifying therapies relies on early detection of the underlying pathological process, leading to early interventions in the disease phenotype. Healthy (n = 16), REM sleep behavior disorder (RBD) (n = 14), dementia with Lewy bodies (n = 10), and Parkinson’s disease (PD) (n = 20) participants underwent 18F-AV133 vesicular monoamine transporter type-2 (VMAT2) PET to determine the integrity of the nigrostriatal pathway. Clinical, neurophysiological and neuropsychological testing was conducted to assess parkinsonian symptoms. There was reduced VMAT2 levels in RBD participants in the caudate and putamen, indicating nigrostriatal degeneration. RBD patients also presented with hyposmia and anxiety, non-motor symptoms associated with parkinsonism. 18F-AV133 VMAT2 PET allows identification of underlying nigrostriatal degeneration in RBD patients. These findings align with observations of concurrent non-motor symptoms in PD and RBD participants of the Parkinson’s Progression Markers Initiative. Together, these findings suggest that RBD subjects have prodromal parkinsonism supporting the concept of conducting neuroprotective therapeutic trials in RBD-enriched cohorts. Ongoing longitudinal follow-up of these subjects will allow us to determine the time-window of clinical progression.
Subject terms: Neuroscience, Parkinson's disease
Introduction
Parkinson’s disease (PD) is associated with a period of latency in which dopaminergic neurodegeneration is occurring, yet no overt signs of parkinsonism are evident. Preservation of nigro-striatal neurons is paramount to delaying the progression of motor impairment. To date, there has been a failure of translation of neuroprotective drugs from preclinical research and there are no approved therapeutics that halt or modify the progression of the disease. Much of this failure can be attributed to an inability to identify at-risk patients in a preclinical/prodromal phase, where neuroprotective agents would be most efficacious. Currently, diagnosis of PD is based on clinical assessment and relies heavily upon the presentation of movement dysfunction. At this stage, it is estimated that there is already an irreversible loss of 50–70% of dopaminergic terminals in the putamen, based on post-mortem studies1 and in vivo neuroimaging of patients in the early stages of unilateral motor impairment2. This substantial neurodegeneration occurs before clinical diagnosis, significantly narrowing the optimal window for treatment. Hence, there is a critical need to develop preclinical/prodromal markers to identify individuals at-risk that can then be enrolled in early neuroprotective clinical trials.
PD is associated with preclinical non-motor symptoms, including REM sleep behavior disorder (RBD), hyposmia, and anxiety3. These symptoms are often reported concurrently and together increase the likelihood of identification of preclinical/prodromal PD4. RBD is a form of parasomnia in which people ‘act out their dreams’ during the normally paralytic stage of REM sleep. RBD is reported in 40–50% of PD patients5,6, and a number of long-term studies have reported that 65–90% of RBD patients will go on to develop a neurodegenerative disorder, predominantly PD, but also dementia with Lewy bodies (DLB) and multiple system atrophy7–9, collectively referred to as parkinsonism.
Between the extensive neurodegeneration that occurs before the onset of motor symptoms and the high likelihood of progression from RBD to a parkinsonism, neuroimaging assessment of the presynaptic nigro-striatal pathway in RBD patients may help the early identification of patients that are likely to progress to PD or another form of parkinsonism and provide a clinical marker for enrolment into preclinical trials.
A number of previous studies have demonstrated striatal dopaminergic neurodegeneration in RBD patients using presynaptic dopamine transporter (DAT) neuroimaging. An early study by Eisensehr et al. using [123I]IPT-single photon emission computerized tomography (SPECT) on 5 patients with chronic RBD and demonstrated a reduced DAT density in the putamen10. More recently, Kim et al. reported abnormal DAT imaging using [123I]FP-CIT in the putamen of three out of 14 RBD patients11, and Iranzo et al. demonstrated similar DAT findings in the striatum of 14 out of 43 RBD subjects12. These previous finding using DAT SPECT demonstrate a loss of integrity of dopamine circuits in the basal ganglia in RBD patients. Compared to DAT SPECT, VMAT2 PET imaging has improved image quality and quantification13, as such, we sought to investigate the dopamine system in RBD patients using the high affinity [18F]AV-133 PET tracer which selectively binds to VMAT2.
Results
Patient demographics and clinical data are summarized in Table 1. There was a higher proportion of men in all clinical groups compared with HC, and the RBD patients were younger than HC (63.5 [9.9] vs. 72.9 [5.1] years, P = 0.02). As expected, severe cognitive impairment was present in patients with DLB, as well as motor impairment in the DLB and PD groups compared to HC. There were no differences in dementia, cognitive, or motor scores between HC and RBD patients.
Table 1.
Patient demographic and clinical data.
| Mean (SD) | ||||
|---|---|---|---|---|
| HC | RBD | DLB | PD | |
| Sample size | 16 | 14 | 9 | 20 |
| Demographics | ||||
| Age, years | 72.9 (5.1) | 63.5 (9.9)* | 68.9 (7.9) | 67.0 (9.1) |
| Sex (M/F), no | 9/7 | 13/1 | 7/2 | 18/2 |
| Education, years | 14.75 (3.4) | 12.08 (4.0) | 12.67 (2.6) | 14.00 (4.3) |
| Symptom onset | – | 7.6 (4.7) | 3.03 (1.9) | 6.11 (4.4) |
| Disease duration | – | – | 0.87 (1.9) | 4.18 (4.0) |
| Neurophysiology | ||||
| UPDRSm scoreON | – | – | 11.7 (5.4)* | 13.9 (7.4)* |
| UPDRSm scoreOFF | 1.1 (1.1) | 1.5 (1.7) | – | 18.1 (9.2)* |
| UPDRSm subscale | ||||
| Bradykinesia | 0 | 0 | 0.9 (0.9)* | 2.1 (0.9)* |
| Rigitidity | 0 | 0 | 1.0 (0.5)* | 1.8 (0.6)* |
| H–Y scoreON | – | – | 1.93 (0.9)* | 1.60 (0.06)* |
| H–Y scoreOFF | 0.1 (0.3) | 0.2 (0.4) | – | 1.63 (0.6)* |
| Neuropsychology | ||||
| MMSE | 29.0 (1.0) | 29.3 (1.0) | 24.4 (2.9)* | 29.0 (1.2) |
| CDR | 0 | 0.1 (0.2) | 0.7 (0.4)* | 0.2 (0.5) |
| HADS | ||||
| Anxiety | 3.4 (2.6) | 6.9 (3.3)* | 7.8 (4.1)* | 5.1 (3.3) |
| Depression | 2.3 (2.9) | 4.1 (2.3) | 6.0 (3.5)* | 5.1 (2.4)* |
| ACE-R | 91.88 (4.9) | 92.07 (5.4) | – | – |
| Trail Making Test | ||||
| Trail A | 35.00 (12.8) | 32.29 (12.6) | – | – |
| Trail B | 79.44 (27.7) | 71.23 (29.0) | – | – |
| Grooved Pegboard | ||||
| Dominant | 80.6 (12.5) | 83.71 (13.7) | – | – |
| Non-dominant | 95.27 (19.7) | 98.93 (30.0) | – | – |
| Sniffin’ Sticks | 9.9 (1.5) | 8.2 (2.7)* | – | – |
| Mayo sleep Questionnaire | ||||
| Q1 response (Y/N) | 0/16 | 14/0 | – | – |
| General alertness rating | 9.33 (1.3) | 7.50 (1.3)* | – | – |
*Significantly different from HC (P < 0.05), bold values indicate significance
Patients with RBD and DLB had a higher anxiety score than HC (6.9 [3.3] and 7.8 [4.1] vs. 3.4 [2.6], P = 0.02, respectively). Further motor, cognitive, olfactory, and sleep examinations were conducted in the HC and RBD groups only. There was no difference between HC and RBD patients in cognition (ACE-R and Trail Making Test), or motor function (Grooved Pegboard), but RBD patients demonstrated impaired olfaction on the Sniffin’ Sticks (8.2 [2.7] vs. 9.9 [1.5], P = 0.035). Using an informant-based Mayo sleep questionnaire, none of the HC informants responded affirmatively to Q1 (n = 16) (Q1: have you ever seen the patient appear to “act out his/her dream” while sleeping?), and all of the RBD informants responded affirmatively (n = 14). Furthermore, RBD patients had decreased alertness, reflected by a decrease in the general alertness score (7.50 [1.3] vs. 9.33 [1.3], P = 0.0007, for RBD and HC, respectively) (Table 1).
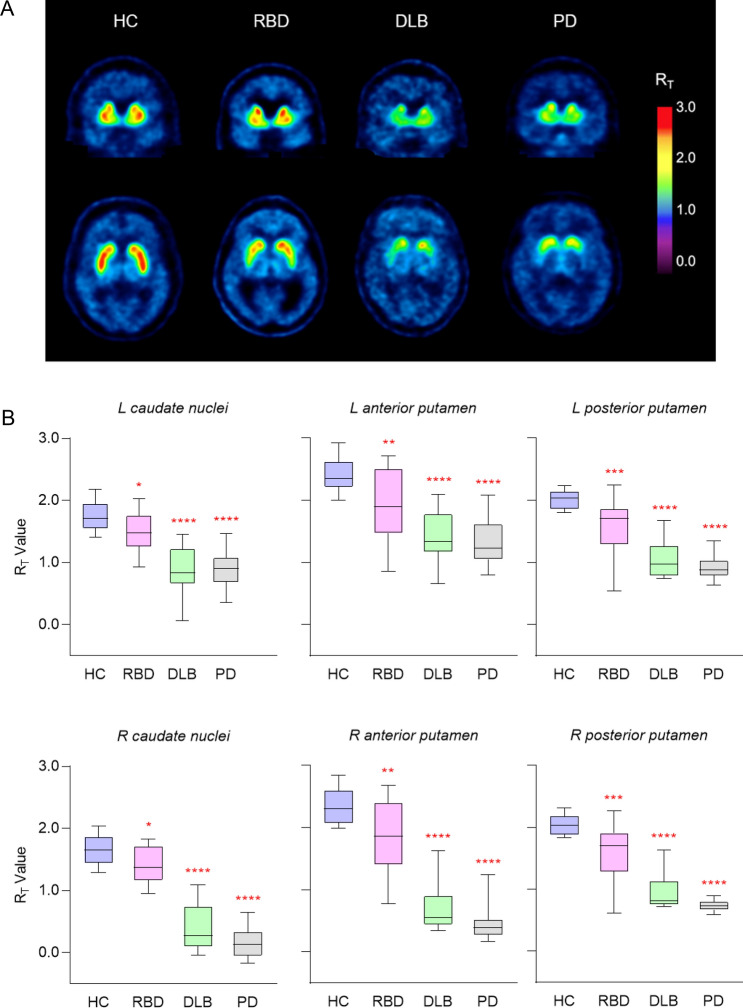
Visual inspection showed lower [18F]AV-133 binding in RBD, DLB, and PD patients (Fig. 1A). There was a significantly lower RT in all groups compared to HC in the left caudate nuclei, right caudate nuclei, left anterior putamen, right anterior putamen, left posterior putamen, and right posterior putamen (Fig. 1B). There was no significant difference in the anterior midbrain between HC and RBD, however, there was a significant reduction of midbrain RT in the DLB and PD patients. PD patients had significantly lower [18F]AV-133 binding compared to RBD patients in all brain regions. (Table 2). There were three RBD participants who had [18F]AV-133 binding within the normal range (HC mean ± SD).
Figure 1.
RBD patients have reduced [18F]AV-133 binding in the caudate nuclei and putamen. (A) Representative [18F]AV-133 tissue ratio (RT) positron emission tomography images. (B) [18F]AV-133 positron emission tomography RT values from volumes of interest analysis. Box lines represent median and interquartile range, whiskers represent minimum and maximum values. *Significantly different from HC. *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.0001.
Table 2.
Regional RT values for [18F]AV-133 PET.
| Mean (SD) | ||||||||
|---|---|---|---|---|---|---|---|---|
| HC | RBD | P (vs. HC) | P (vs. PD) | DLB | P (vs. HC) | PD | P (vs. HC) | |
| L caudate nuclei | 1.76 (0.2) | 1.47 (0.3) | 0.01 | < 0.0001 | 0.87 (0.4) | < 0.0001 | 0.88 (0.3) | < 0.0001 |
| R caudate nuclei | 1.66 (0.2) | 1.39 (0.3) | 0.01 | < 0.0001 | 0.39 (0.4) | < 0.0001 | 0.16 (0.2) | < 0.0001 |
| L anterior putamen | 2.40 (0.3) | 1.90 (0.6) | 0.004 | 0.0011 | 1.39 (0.4) | < 0.0001 | 1.33 (0.4) | < 0.0001 |
| R anterior putamen | 2.34 (0.3) | 1.82 (0.6) | 0.005 | < 0.0001 | 0.71 (0.4) | < 0.0001 | 0.45 (0.3) | < 0.0001 |
| L posterior putamen | 2.02 (0.1) | 1.56 (0.5) | 0.0006 | < 0.0001 | 1.04 (0.3) | < 0.0001 | 0.93 (0.2) | < 0.0001 |
| R posterior putamen | 2.04 (0.2) | 1.56 (0.5) | 0.0006 | < 0.0001 | 0.95 (0.3) | < 0.0001 | 0.73 (0.1) | < 0.0001 |
| Anterior midbrain | 0.74 (0.1) | 0.61 (0.1) | 0.12 | < 0.0001 | 0.34 (0.3) | < 0.0001 | 0.33 (0.2) | < 0.0001 |
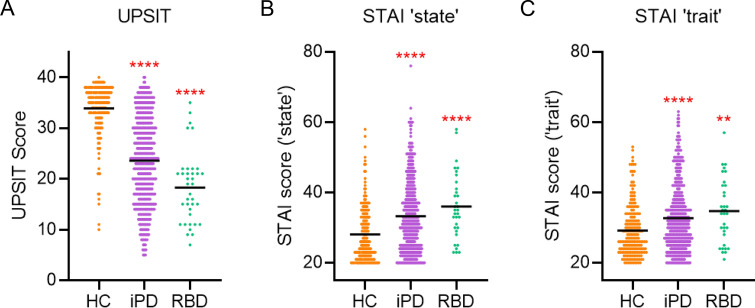
The presence of both hyposmia and anxiety in the RBD group from this study is aligned with observations from the large PPMI dataset. The PPMI data, including participants with iPD (n = 492, 55.1% female) and RBD (n = 37, 14.9% female) were compared with HCs (n = 197, 56.0% female). The RBD group was significantly older (70.2 ± 6.4 years) than the iPD and the HC groups (61.7 ± 9.9 years, P < 0.0001; 60.8 ± 11.2 years, P < 0.0001; respectively). There was hyposmia and increased anxiety in the iPD (UPSIT: 23.57 [8.5], P < 0.0001; STAIstate: 33.20 [10.1], P < 0.0001; STAItrait: 32.66 [9.5], P < 0.0001) and prodromal RBD groups (UPSIT: 18.30 [7.0], P < 0.0001; STAIstate: 36.00 [9.3], P < 0.0001; STAItriat: 34.67 [9.1], P = 0.001) compared to HC (UPSIT: 33.87 [5.1], STAIstate: 28.09 [7.9]; STAItrait: 29.15 [7.5]) (Fig. 2).
Figure 2.
RBD participants from the PPMI have concurrent hyposmia and increased anxiety. (A) Olfactory data of HC (n = 197), iPD (n = 492), and RBD (n = 37). (B) ‘State’ anxiety of HC (n = 200), iPD (493), and RBD (n = 33). (C) ‘trait’ anxiety data of HC (n = 200), iPD (n = 493), and RBD (n = 33). *Significantly different from HC. **P < 0.01, ****P < 0.0001.
Discussion
This study demonstrates the utility of 18F-AV133 VMAT2 PET to indicate the loss of integrity of the nigrostriatal pathway in the striatum of RBD patients in the absence of a classical Parkinson’s motor phenotype. All of the RBD participants held a clinical diagnosis of RBD plus had a positive response to the core Q1 on the Mayo Sleep Questionnaire, which has a sensitivity of 100% and a specificity of 95% in detecting RBD14. Furthermore, these patients have anxiety and olfactory deficits, which are recognized symptoms associated with PD3. It has been previously shown that there is more severe degeneration in the posterior putamen, followed by the anterior putamen and the caudate nucleus in PD15, and this pattern is observed in the RBD patients reported here. Lower VMAT2 levels in the striatum is concordant with findings from a smaller previous study using [11C]dihydrotetrabenazine16, and these findings recapitulate reduced presynaptic dopamine transporter (DAT) levels using single-photon emission computed tomography in RBD populations17. The advantage of assessing VMAT2 over DAT is that while both reflect nigrostriatal degeneration, VMAT2 is less susceptible than DAT to pharmacological challenges and compensatory changes associated with the loss of dopaminergic neurons18.
This study demonstrates the concurrence of three prevalent non-motor symptoms with clear reduced VMAT2 levels in disease-associated brain regions, suggesting an increased likelihood that these patients have preclinical/prodromal parkinsonism. To confirm the concurrence of non-motor symptoms was not an artifact of this study, we analysed olfactory and anxiety scores in the large PPMI dataset and confirmed the presence of anxiety and hyposmia in both iPD and prodromal RBD groups.
Attempts at developing disease-modifying therapies have ended in failure, attributable to the substantial loss of axons/synapses at the time of clinical diagnosis. Testing protective treatments where advanced neuronal loss has already occurred dooms these approaches to failure. Ideal subjects are those where nigrostriatal degeneration has begun but not progressed to the point of irreversible damage and profound motor impairments (prodromal PD). RBD patients represent ideal candidates for recruitment into clinical trials for neuroprotective/disease-modifying drugs due to early nigrostriatal neurodegeneration and their increased likelihood of developing clinical PD. Our results suggest that these RBD subjects have prodromal parkinsonism and support the concept that trials of neuroprotective treatments need to be tested in RBD-enriched cohorts.
The main limitations of the present study are the relatively small number of participants and the lack of polysomnography to confirm the extent of RBD. It is also important to note that [18F]AV-133 VMAT2 PET may not be reflective of the true extent of nigro-striatal degeneration, as mechanisms such as sprouting of terminals may be compensating for the progressive neuronal/terminal loss19,20. 18F-AV133 VMAT2 PET allows identification of underlying nigrostriatal degeneration in RBD patients. Ongoing longitudinal follow-up of these subjects will allow to determine the time-window of clinical progression.
Methods
Participants
Fourteen patients with clinically diagnosed RBD, along with 10 DLB, 20 PD, and 16 healthy controls (HC) were included in the study. Written informed consent was obtained from all participants, and from a legal guardian of the participants with DLB and PD. The RBD group were referred to the study with a clinical RBD diagnosis, which was the only inclusion criteria for RBD subject recruitment. The participants’ bed partners completed the Informant Mayo Sleep Questionnaire (IMSQ), of which the core question (Q1) on the IMSQ has a sensitivity of 100% and a specificity of 95% in detecting RBD14. Data from the DLB and PD patients has been previously published15. Approval for the study was obtained from the Austin Health Research Ethics Committee. With regards to dopaminergic medications, 15 patients with PD were receiving carbidopa-levodopa; two selegiline; two, levodopa and benserazide and one, pramipexole. Three DLB patients were receiving carbidopa-levodopa and two, levodopa and benserazide.
Clinical data
The neurological evaluation of participants included duration of disease and/or symptoms, the Hoehn–Yahr score (H–Y), the motor subscale (Section III) of the Unified Parkinson’s Disease Rating Scale (UPDRSm) both on and off medication (medication stopped 24 h prior to examination, and resumed just before the PET scan). The neuropsychological evaluation included the Mini-Mental State Examination (MMSE), Clinical Dementia Rating scale (CDR), and Hospital Anxiety and Depression Scale (HADS) for all groups. HC and RBD participants underwent Trail Making Test, Grooved Pegboard assessment, Addenbrook’s Cognitive Assessment (ACE-R), Sniffin’ Sticks (MediSense, Netherlands) and participants bed partners completed the Informant Mayo Sleep Questionnaire.
Imaging procedures
18F-(+)Fluropropyldihydrotetrabenazine (18F-AV-133) was synthesized using previously described methods21. Each participant underwent a 20-min static acquisition (4 × 5 min frames) at 120–140 min after injection of ~ 250 MBq of [18F]AV-133. The sorted sinograms were reconstructed using a 3D RAMLA algorithm. As described earlier15, volumes of interest (VOIs) were placed over the standard space MRI over the caudate nucleus, anterior and posterior putamen, midbrain and primary visual cortex by an operator blind to clinical diagnosis. Volumes of interest were then transferred onto the individual [18F]AV-133 PET images.
Image analysis
Tissue ratios (RT) of the caudate, anterior and posterior putamen and the anterior midbrain were generated using the primary visual cortex, a region relatively devoid of monoaminergic terminals, as reference region22.
Parkinson’s Progressive Marker Initiative (PPMI) analysis
The PPMI is an observational multicentre study including clinical assessments of olfaction using the University of Pennsylvania Smell Identification Test (UPSIT), and anxiety using the State-Trait Anxiety Inventory (STAI). For the purposes of the data presented in this paper, only a subset of the entire PPMI cohort were analysed including HC, diagnosed iPD, and participants recruited with prodromal RBD. Participating PPMI sites received approval from an ethical standards committee and written informed consent was obtained from all participants.
Statistical analysis
Statistical comparison of normal data between HC and the three disease groups was performed using a Dunnet test. Non-normal data were analyzed using the Kruskal–Wallis one-way ANOVA. Statistical comparison between HC and RBD groups only was conducted using an unpaired Student’s T test. PPMI data were analysed using a Kruskal–Wallis one-way ANOVA, significance refers to a difference to the HC group. Data were corrected for age-differences. Statistical analysis was performed using GraphPad Prism version 8.0.0 for Windows and significance for each analysis was defined as P < 0.05. Data are presented as mean [SD].
Ethical approval
All methods were carried out in accordance with relevant guidelines and regulations laid out by the Declaration of Helsinki, under ethics approval from the Austin Health Research Ethics Committee.
Acknowledgements
We acknowledge participants and organisers of the PPMI. PPMI is funded by The Michael J. Fox Foundation for Parkinson’s Research and funding partners including Abbott, Biogen Idec, F. Hoffman-La Roche Ltd., GE Healthcare, Genentech, Pfizer Inc., Meso Scale Diagnostics and Life Molecular Imaging (formerly Piramal). J.Q.T. is supported, in part, by P50 NS053488 University of Pennsylvania.
Author contributions
L.B. performed the statistical analysis, wrote the first draft of the manuscript and prepared the figures. V.V., V.D, and C.R. were responsible for the conception, organization and execution of the research project. D.F., A.B., and K.B. helped with the design and execution of statistical analysis. All authors contributed to review and critique of the manuscript.
Data availability
All data generated or analysed during this study are included in this published article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Scherman D, et al. Striatal dopamine deficiency in Parkinson's disease: role of aging. Ann. Neurol. 1989;26:551–557. doi: 10.1002/ana.410260409. [DOI] [PubMed] [Google Scholar]
- 2.Lee CS, et al. In vivo positron emission tomographic evidence for compensatory changes in presynaptic dopaminergic nerve terminals in Parkinson's disease. Ann. Neurol. 2000;47:493–503. doi: 10.1002/1531-8249(200004)47:4<493::AID-ANA13>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 3.Siderowf A, et al. Impaired olfaction and other prodromal features in the Parkinson At-Risk Syndrome Study. Mov. Disord. 2012;27:406–412. doi: 10.1002/mds.24892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berg D, et al. MDS research criteria for prodromal Parkinson's disease. Mov. Disord. 2015;30:1600–1611. doi: 10.1002/mds.26431. [DOI] [PubMed] [Google Scholar]
- 5.Zhang X, Sun X, Wang J, Tang L, Xie A. Prevalence of rapid eye movement sleep behavior disorder (RBD) in Parkinson's disease: a meta and meta-regression analysis. Neurol. Sci. 2017;38:163–170. doi: 10.1007/s10072-016-2744-1. [DOI] [PubMed] [Google Scholar]
- 6.Sixel-Doring F, Trautmann E, Mollenhauer B, Trenkwalder C. Associated factors for REM sleep behavior disorder in Parkinson disease. Neurology. 2011;77:1048–1054. doi: 10.1212/WNL.0b013e31822e560e. [DOI] [PubMed] [Google Scholar]
- 7.Postuma RB, et al. Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology. 2009;72:1296–1300. doi: 10.1212/01.wnl.0000340980.19702.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schenck CH, Boeve BF, Mahowald MW. Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: a 16-year update on a previously reported series. Sleep Med. 2013;14:744–748. doi: 10.1016/j.sleep.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Iranzo A, et al. Neurodegenerative disorder risk in idiopathic REM sleep behavior disorder: study in 174 patients. PLoS ONE. 2014;9:e89741. doi: 10.1371/journal.pone.0089741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisensehr I, et al. Reduced striatal dopamine transporters in idiopathic rapid eye movement sleep behaviour disorder. Comparison with Parkinson's disease and controls. Brain. 2000;123(6):1155–1160. doi: 10.1093/brain/123.6.1155. [DOI] [PubMed] [Google Scholar]
- 11.Kim YK, et al. The implication of nigrostriatal dopaminergic degeneration in the pathogenesis of REM sleep behavior disorder. Eur. J. Neurol. 2010;17:487–492. doi: 10.1111/j.1468-1331.2009.02854.x. [DOI] [PubMed] [Google Scholar]
- 12.Iranzo A, et al. Decreased striatal dopamine transporter uptake and substantia nigra hyperechogenicity as risk markers of synucleinopathy in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study [corrected] Lancet Neurol. 2010;9:1070–1077. doi: 10.1016/S1474-4422(10)70216-7. [DOI] [PubMed] [Google Scholar]
- 13.Xu SS, et al. Diagnostic accuracy of imaging brain vesicular monoamine transporter type 2 (VMAT2) in clinically uncertain parkinsonian syndrome (CUPS): a 3-year follow-up study in community patients. BMJ Open. 2018;8:e025533. doi: 10.1136/bmjopen-2018-025533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boeve BF, et al. Validation of the Mayo Sleep Questionnaire to screen for REM sleep behavior disorder in a community-based sample. J. Clin. Sleep Med. 2013;9:475–480. doi: 10.5664/jcsm.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villemagne VL, et al. In vivo assessment of vesicular monoamine transporter type 2 in dementia with Lewy bodies and Alzheimer disease. Arch. Neurol. 2011;68:905–912. doi: 10.1001/archneurol.2011.142. [DOI] [PubMed] [Google Scholar]
- 16.Albin RL, et al. Decreased striatal dopaminergic innervation in REM sleep behavior disorder. Neurology. 2000;55:1410–1412. doi: 10.1212/wnl.55.9.1410. [DOI] [PubMed] [Google Scholar]
- 17.Barber TR, Klein JC, Mackay CE, Hu MTM. Neuroimaging in pre-motor Parkinson's disease. NeuroImage Clin. 2017;15:215–227. doi: 10.1016/j.nicl.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin I, Dawson VL, Dawson TM. The impact of genetic research on our understanding of Parkinson's disease. Prog. Brain Res. 2010;183:21–41. doi: 10.1016/S0079-6123(10)83002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashimoto M, Masliah E. Cycles of aberrant synaptic sprouting and neurodegeneration in Alzheimer's and dementia with Lewy bodies. Neurochem. Res. 2003;28:1743–1756. doi: 10.1023/a:1026073324672. [DOI] [PubMed] [Google Scholar]
- 20.Finkelstein DI, et al. Axonal sprouting following lesions of the rat substantia nigra. Neuroscience. 2000;97:99–112. doi: 10.1016/s0306-4522(00)00009-9. [DOI] [PubMed] [Google Scholar]
- 21.Okamura N, et al. In vivo measurement of vesicular monoamine transporter type 2 density in Parkinson disease with (18)F-AV-133. J. Nucl. Med. 2010;51:223–228. doi: 10.2967/jnumed.109.070094. [DOI] [PubMed] [Google Scholar]
- 22.Bohnen NI, et al. Positron emission tomography of monoaminergic vesicular binding in aging and Parkinson disease. J. Cereb. Blood Flow Metab. 2006;26:1198–1212. doi: 10.1038/sj.jcbfm.9600276. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.