Abstract
HCC (hepatocellular carcinoma) is a major health threat for the Chinese population and has poor prognosis because of strong resistance to chemotherapy in patients. For instance, a considerable challenge for the treatment of HCC is sorafenib resistance. The aberrant glucose metabolism in cancer cells aerobic glycolysis is associated with resistance to chemotherapeutic agents. Drug-resistance cells and tumors were exposed to sorafenib to establish sorafenib-resistance cell lines and tumors. Western blotting and real-time PCR or IHC staining were used to analyze the level of CLCF1 in the sorafenib resistance cell lines or tumors. The aerobic glycolysis was analyzed by ECAR assay. The mechanism mediating the high expression of CLCF1 in sorafenib-resistant cells and its relationships with miR-130-5p was determined by bioinformatic analysis, dual luciferase reporter assays, real-time PCR, and western blotting. The in vivo effect was evaluated by xenografted with nude mice. The relation of CLCF1 and miR-30a-5p was determined in patients’ samples. In this study, we report the relationship between sorafenib resistance and increased glycolysis in HCC cells. We also show the vital role of CLCF1 in promoting glycolysis by activating PI3K/AKT signaling and its downstream genes, thus participating in glycolysis in sorafenib-resistant HCC cells. Furthermore, we also show that miR-30a-5p directly targets CLCF1 and that sorafenib-mediated suppression of miR-30a-5p results in the upregulation of CLCF1 in HCC cells resistant to sorafenib. We also found that when a cholesterol modified agomiR-30a-5p was delivered systemically to mice harboring sorafenib-resistant HCC tumors, tumor growth decreased significantly. There is an uncharacterized mechanism of biochemical resistance to hormone therapies orchestrated by the miR-30a-5p/CLCF1 axis to mediate sorafenib resistance and aerobic glycolysis in HCC. Therefore, this study indicates that targeting the miR-30a-5p/CLCF1 axis may hold promise for therapeutic intervention in HCC sorafenib resistance patients.
Subject terms: Cancer therapeutic resistance, Liver cancer
Introduction
On a global scale, HCC (hepatocellular carcinoma) ranks sixth among cancers and is the third leading cause of death due to cancer1. The incidence of this deadly disease is still on the rise, and curative treatments, including resection, ablation, and transplantation, are only feasible in patients diagnosed in the early stages1,2. The manifestation of HCC is more frequent in cirrhosis and liver dysfunction patients, which limits the interventions with systemic cytotoxic drugs3,4. HCC at the advanced stage is not amenable to surgery, and the only treatment strategy is chemotherapy5,6.
For HCC treatment, conventional chemotherapeutics such as doxorubicin and fluorouracil are not efficacious and exhibit potent side effects7,8. Significant progress in cancer treatment has been achieved with the advent of targeted drug therapy and small molecule kinase inhibitors9,10. The only clinical solution currently available for the treatment of HCC in patients who cannot undergo surgery is sorafenib. As an inhibitor of Raf kinase, sorafenib inhibits tumor growth by decreasing the activities of the B-RAF serine/threonine kinase and RAF-111,12; concurrently, sorafenib can also inhibit tumor growth by decreasing tumor angiogenesis via the tyrosine kinase activities of platelet-derived growth factor (PDGF), FMS-like tyrosine kinase 3 (FLT-3), Vascular Endothelial Growth Factor 2 (VEGF-2), and -3 receptors13,14. Nevertheless, HCC exhibits notable resistance to sorafenib, and only one-third of patients with HCC at an advanced stage respond to treatment11,15. Furthermore, the results of multiple random clinical cases were systematically analyzed and revealed an increase in the survival time by only 3 months, indicating the urgent need to discover more robust molecular targeted drugs against drug-resistant tumors and to further conduct in-depth evaluation of the HCC resistance mechanism and improve the sensitivity of HCC to sorafenib.
Cardiotrophin-like cytokine factor 1 (CLCF1, also referred to as BSF-3 or NNT-1) is a cytokine of the IL-6 family that is secreted in the form of a soluble CRLF1 (cytokine receptor cytokine-like factor 1) complex16,17. CLCF1 activates the tripartite CNTFR (ciliary neurotrophic factor receptor), which consists of LIFRβ, gp130, and CNTFRα18. The common signaling pathways shared by CLCF1 and CNTF indicate that the exhaustive evaluations conducted preclinically and clinically on CNTF may pave a way for the curative ability of CLCF119. In vitro studies and animal models of neurodegenerative diseases have demonstrated the significant neurotrophic activities of CNTF and have encouraged clinical trials in patients with amyotrophic lateral sclerosis, Huntington’s disease, and cases of retinal degeneration20. In addition, when administered systemically, CNTF leads to remarkable, unexpected weight loss, thus leading to preclinical assessments of CNTF in metabolic syndrome models21,22. When injections of CNTF were given daily, persistent anorexic effects were observed in leptin-deficient, diet-induced obese mice23. The conditions of hyperinsulinemia and hyperglycemia were reduced due to CNTF, indicating that type II diabetes could benefit and that leptin resistance could be overcome in obese individuals24,25. CNTF is believed to exert both systemic and central effects because CNTFR is expressed in the region of the brain directing energy balance, in skeletal muscle and in adipose tissues26. However, there is no report on the role of CLCF1 in sorafenib resistance in HCC. Hence, we studied the relationship between CLCF1 and sorafenib resistance in hepatocellular carcinoma.
MicroRNAs (miRNAs) are small noncoding RNAs that posttranscriptionally regulate eukaryotic gene expression. miRNAs have been shown to have crucial roles in the advancement and progression of cancers, and new anticancer therapies based on miRNA are actively being developed27,28. Additionally, miRNAs also act as important regulators in the Warburg effect (reprogramming of cellular energy metabolism) and in drug resistance in cancers29,30. Therefore, miRNA targeting may be a promising treatment strategy to interrupt aerobic glycolysis in cancer cells as well as in sorafenib-resistant HCC.
In the present study, we identified CLCF1 as a novel regulator in sustaining sorafenib resistance in HCC cells. We further found that CLCF1 acts as a key mediator to orchestrate sorafenib resistance and aerobic glycolysis in HCC cells. Mechanistically, upregulation of CLCF1 boosted PI3K/AKT signaling and thus activated its downstream glycolytic genes in sorafenib-resistant HCC cells. We further identified CLCF1 as a direct target of miR-30a-5p and revealed that sorafenib upregulated CLCF1 by downregulating miR-30a-5p. Functional studies showed that the miR-30a-5p/CLCF1 axis plays an important role in regulating both sorafenib resistance and aerobic glycolysis in sorafenib-resistant cells. Taken together, our findings reveal that the miR-30a-5p/CLCF1/PI3K/AKT regulatory cascade is part of an important mechanism of sorafenib resistance and aerobic glycolysis in HCC cells, suggesting that miR-30a-5p is a promising therapeutic target to reverse sorafenib resistance in HCC.
Materials and methods
Cell culture
The human HCC cell lines HepG2 and Huh7 were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA) and maintained in DMEM (Dulbecco’s modified Eagle’s medium) with streptomycin/penicillin (1%) and fetal bovine serum (FBS, 10%) in a humidified atmosphere of 5% CO2 at 37 °C. HepG2-S-R and Huh7-S-R, the sorafenib-resistant sublines, were derived from HepG2 and Huh7 cells as previously described31.
HCC specimens and Immunohistochemistry
HCC tumor specimens and paired normal adjacent tissues were obtained from Proteo Genex (CA, USA). EnVision™ Flex target retrieval solution was employed to deparaffinize and unmask epitopes in the samples. Endogenous peroxidase was blocked by adding 0.3% H2O2 or hydrogen peroxide for 5 min. This was followed by the addition of primary antibody at 4 °C overnight. Subsequently, the following treatments were done: 15 min with EnVision™ Flex linker rabbit, 30 min with EnVision™ Flex/HRP enzyme, and 10 min with 3′-3-diaminobenzidine tetrahydrochloride. Hematoxylin was added as a counterstain followed by dehydration and mounting on a Richard-Allan Scientific Cyto seal XYL (Thermo Scientific, USA).
RNA oligonucleotides, plasmid constructs, and antibodies
The lentiviral vector pLVX-3×Flag-Puro was used to clone the cDNA of human CLCF1 to obtain the pLVX-CLCF1-3×Flag-Puro plasmid. The pmirGLO dual-luciferase reporter from Promega was used to clone the human CLCF1 3′-UTR to obtain the wild-type pmirGLO-CLCF1-3′-UTR reporter. In the mutant reporter pmirGLO-CLCF1-3′-UTR-mut, mutations of seven nucleotides in the CLCF1 3′-UTR that correspond to the miR-30a-5p seed sequences were performed. DNA sequencing was performed to confirm all constructs. The agomiR-30a-5p, miR-30a-5p mimics, CLCF1 siRNA and their respective control RNAs and anti-miR-30a-5p reagents were procured (RiboBio, China).
Transfection of cells and viral transduction
Cell transfection was performed with Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Specifically, for each transfection, RNA oligonucleotide (50 pmol) or plasmid DNA (2 μg) was used in a 6-well plate. The pLVX-CLCF1-3×Flag-Puro lentivirus or control vector was packaged into a pseudovirus with the corresponding packaging plasmids as per standard protocol. Centrifugation of the supernatant was performed at 5 × 104 g for 1.5 h at 4 °C, and lentivirus at high titer (>1 × 108 transduction units/mL) was prepared. Lentiviruses expressing CLCF1 shRNA or miR-30a-5p at high titer were procured from GeneChem (Shanghai, China). Lentiviruses were incubated with target cells in media supplemented with polybrene (8 μg/mL) for 24 hours. After transduction, puromycin (1 μg/mL) was added for the selection.
Luciferase reporter assay
Luciferase reporter assay was performed as previously described29, luciferase reporter assays were carried out. Briefly, cotransfection of HepG2 cells with Renilla luciferase reporter vectors, siRNAs or protein expression vectors, or/and miRNA mimics was performed in 24-well plates. The Promega (USA) supplied Dual-Luciferase Reporter Assay System was used to assess luciferase activity, and the internal control was the firefly luciferase plasmid pGL3, with luciferase activity normalized to the corresponding controls.
Cell viability
Viability assays were conducted as previously described32,33. Briefly, viable cells in equal number were seeded in DMEM with sorafenib or regorafenib at varying concentrations in 96-well plates. Cell viability was determined by MTT assay after 3 days of incubation.
Apoptosis assay
Apoptosis was assessed using the Fluorometric TUNEL System from Promega, as per the manufacturer’s instructions. Briefly, the indicated cells were treated and stained using the apoptosis detection kit Annexin V-FITC/PI from KeyGen (China) according to the manufacturer’s instructions. Finally, apoptosis was detected by flow cytometry. Analysis of more than 1 × 106 cells was performed per measurement.
Metabolic parameters of glucose utilization and production of lactate
The assays were performed using a glucose assay kit from Sigma and a lactate assay kit from BioVision as per the provided protocols. The cell number was used to normalize lactate production and glucose consumption rates.
Determination of cellular ATP
The level of ATP in cells was measured using a luminometer and a CellTiter-Glo 2.0 Assay kit from Promega as per instructions. The total protein in the corresponding cell lysate was used to normalize the relative intracellular ATP level (measured as luminescence).
Measurement of ECAR (extracellular acidification rate)
The mitochondrial functions and capacity for glycolysis were assessed by Seahorse Bioscience XF96 Extracellular Flux Analyzer, as instructed by the Seahorse XF Cell Mito Stress Test Kit or Glycolysis Stress Test Kit from Seahorse Bioscience (MA, USA). Cells (initial density of 4 × 104 cells/well) were plated in Cell Culture Microplates XF96 (Seahorse Bioscience) the day before measurement. Next, the addition of oligomycin (1 μM), 2-deoxy-glucose (100 mM) and glucose (10 mM) was performed to determine the ECAR value. Cell numbers were used to normalize the final ECAR values.
Real-time PCR
After extracting total RNA with TRIzol reagent obtained from Invitrogen (USA), reverse transcription (RT) was performed with PrimeScript RT reagent from TaKaRa (Dalian, China) to obtain cDNA. Quantitative real-time PCR was performed on an ABI 7900HT Real-Time PCR System from Applied Biosystems (USA) to evaluate the expression of specific genes and β-actin. Each reaction was run independently in triplicate. The 2−ΔΔCT method was used to calculate the mRNA levels. The primers are list in Table 1.
Table 1.
Primers for real-time PCR.
| Gene | Forward | Reverse |
|---|---|---|
| CLCF1 | 5′-CTTAGCTGGGACCTACCTGAA-3′ | 5′-CCACACTTCCAAGTTGACCGT-3′ |
| CCL2 | 5′-AGGTCCCTGTCATGCTTCTG-3′ | 5′-TCTGGACCCATTCCTTCTTG-3′ |
| IL-6 | 5′-TAGTCCTTCCTACCCCAATTTCC-3′ | 5′-TTGGTCCTTAGCCACTCCTTC-3′ |
| CCL7 | 5′-CTGCTCTCCAGCGCTCTCA-3′ | 5′-GTAAGAAAAGCAGCAGGCGG-3′ |
| TGFB1 | 5′-TATCGACATGGAGCTGGTGA-3′ | 5′-CAGCTTGGACAGGATCTGGC-3′ |
| IL-10 | 5′-TGCAGGACTTTAAGGGTTAC-3′ | 5′-GGCCTTGTAGACACCTTGGTC-3′ |
| CNTF | 5′-GGAAGATTCGTTCAGACCTGAC-3′ | 5′-CCCATCAGCCTCATTTTCAGGG-3′ |
| IL-1α | 5′-ATCAGTACCTCACGGCTGCT-3′ | 5′-TGGGTATCTCAGGCATCTCC-3′ |
| NGF | 5′-ACAGGAGCAAGCCCTCCT-3′ | 5′-GCCTGCTTGCCGTCCAT-3′ |
| CD40 | 5′-TCGGCTTCTTCTCCAATCAGT-3′ | 5′-TGAAATTTGGTGTCTACTGT-3′ |
| CXCL14 | 5′-CCAAGATTCGCTATAGCGAC-3′ | 5′-CCTGCGCTTCTCGTTCCAGG-3′ |
| LIF | 5′-CTCTTCCCATCACCCCTGTA-3′ | 5′-TGGTCTTCTCTGTCCCGTTG-3′ |
| OSM | 5′-CCAGACTTCCTCCTTTCCGTG-3′ | 5′-ACACCCTGCCGCTGTTACAG-3′ |
| IL-19 | 5′-GGTCTGGTTGGATCCCAATG-3′ | 5′-CCCATCCTTGATCAGCTTCCT-3′ |
| IFNAR1 | 5′-ATTTACACCATTTCGCAAAGCTC-3′ | 5′-TCCAAAGCCCACATAACACTA-3′ |
| BAFF | 5′-TGCCTTGGAGGAGAAAGAGA-3′ | 5′-GGCAGTGTTTTGGGCATATT-3′ |
| IFNAR2 | 5′-TGAGCAGGATGCGTTCAC-3′ | 5′-TCTCTAGGCTCGCAGACACC-3′ |
| ALK-7 | 5′-ATAGCGGACTTAGGGTTG-3′ | 5′-CTGGTTTGGGATACTTGG-3′ |
| CCL1 | 5′-GCTCCAATGAGGGCTTAATC-3′ | 5′-ATTTTTCAGTGCCTCTGAACC-3′ |
| β-actin | 5′-TGTGTGACGACGAAGTCGCC-3′ | 5′-TGGTCGTGAAGGTGTAACCA-3′ |
| miR-30a-5p | 5′-AACGAGACGACGACAGAC-3′ | 5′-TGTAAACATCCTCGACTGGAAG-3′ |
| U6 | 5′-GTGCTCGCTTCGGCAGCA-3′ | 5′-CAAAATATGGAACGCTTC-3′ |
| GLUT3 | 5′-TTCTGGTCGGAATGCTCTTC-3′ | 5′-AATGTCCTCGAAAGTCCTGC-3′ |
| HK2 | 5′-CTTGCGAATATGGTTGCCTCA-3′ | 5′-CCTCCCAATGCCTGATCTGAT-3′ |
| PDK1 | 5′-TTACGGATTGCCCATCTCACG-3′ | 5′-CCCGGTCACTCATCTTCACAGT-3′ |
Western blotting
Western blotting was performed as previously study34,35. Briefly, cells were lysed using RIPA (Abmole, USA) buffer. The supernatant was collected after lysate centrifugation at 12,000 rpm for 30 min at 4 °C. The protein concentration was measured using the BCA (bicinchoninic acid) method, and the proteins were electrophoresed on a 10% SDS-PAGE gel. The separated proteins were transferred onto a PVDF membrane using a Trans-Blot Transfer Slot (both from Bio-Rad), and the membrane was blocked using milk (fat-free; 5%) for 120 min at RT (room temperature). Then, the primary antibody was added and incubated overnight at 4 °C, followed by the addition of a secondary antibody and another incubation for 60 min at RT. The immunoblot was developed with an ECL reagent. β-Actin was used as the internal reference. The primary antibodies are list as follows: CLCF1 (HPA042444, Sigma), p-AKT (#9611, Cell Signaling Technology), AKT (#9272, Cell Signaling Technology), p-mTOR (#5536, Cell Signaling Technology), mTOR (#2972, Cell Signaling Technology), β-actin (A5441, Sigma), Ki-67 (#9449, Cell Signaling Technology), GLUT3 (ab41525, Abcam), HK2 (ab209147, Abcam), PDK1 (#5662, Cell Signaling Technology).
Xenograft assays
The Ethics Committee of University of Pittsburgh approved all experiments with animals in compliance with the Care and Use of Laboratory Animals Guide. To carry out assays on tumor xenografts, suspensions of cells (5 × 105) were made in Matrigel from BD Biosciences and subcutaneously injected into athymic nude BALB/c mice (female; n = 5; 6–8 weeks of age). For the sorafenib assay, mice were randomly divided into two groups and no blinding was done, and the sorafenib treatment group was administered sorafenib (25 mg/kg) through oral gavage five times per week until week 7. Sorafenib was dissolved in ethanol Cremephor EL/95% (50:50) to prepare a stock solution (4×) and was diluted in sterile water to obtain the final concentration prior to use. For treatment using miR-30a-5p, agomiR (chemically modified) along with the neutral, lipid-based delivery reagent “In Vivo RNA-LANCEr II” (BIOO Scientific) was formulated and injected (1.5 mg/kg mice body weight) into mouse tail veins for five cycles of one injection per week for seven weeks. All mice were euthanized at the end of the seventh week, and surgical resection of tumors was performed for further analyses. Once per week for seven weeks, tumor volumes were determined and assessed for tumor width (W) and length (L) and volume was calculated using the formula LW2/2.
In situ hybridization
In situ hybridization kits were purchased from Boster Sci corp, Wuhan, China to conduct In situ hybridization by excellent probes (locked nucleic acid (LNA)-modified antisense oligonucleotides). 5′-CTTCCAGTCGAGGATGTTTACA-3′ was the nucleotides sequence of the LNA modified probe (miR-30a-5p). In short, the tissues sections (deparaffinized) were incubated with 25 μg/mL proteinase-K (at 37 °C for 8 min). The Scrambled LNA™ and miR-30a-5p (labeled with DIG) probes were treated at 30 nM concentration with Exiqon hybridization buffer for 60 min (57 °C). The probes were visualized at 30 °C by using anti-DIG-alkaline phosphatase for 60 min, treated with the fresh substrate (NBT-BCIP) comprising of levamisole with 0.2 nM concentration at 30 °C.
Statistical analyses
The mean ± SD (standard deviation) was used to present all results. The variations among the control and treated groups were assessed using Student’s t test. The cell viability and tumor growth data were analyzed using one-way ANOVA. The P-values (***: P < 0.001, **: P < 0.01; *: P < 0.05) denoted different levels of statistical significance. The correlation between control and treated groups as well as regression were calculated by Pearson’s correlation analyses.
Results
Upregulation of CLCF1 in sorafenib resistance HCC cells
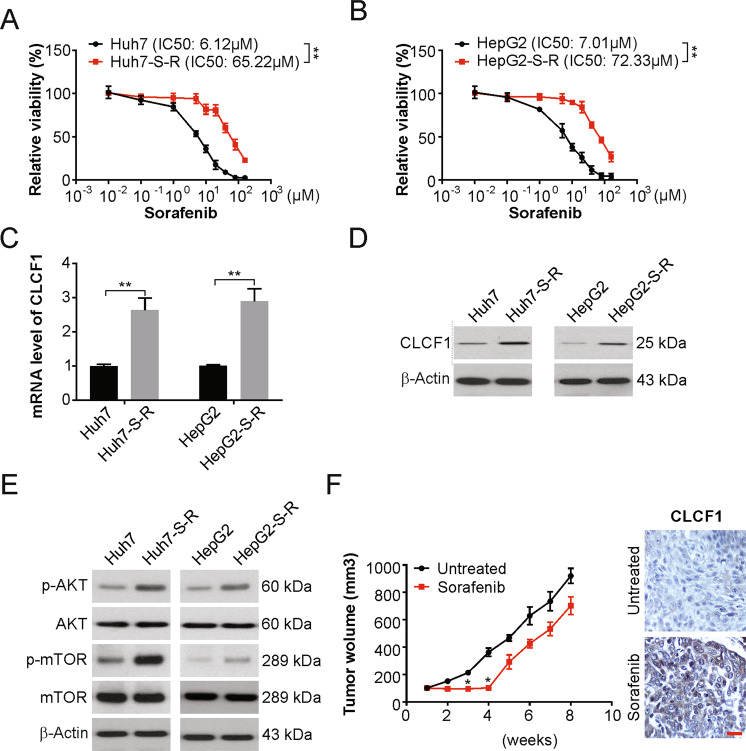
To investigate the mechanisms of sorafenib resistance in HCC, we generated Huh7-S-R and HepG2-S-R sorafenib-resistant human HCC cell lines by exposing Huh7 and HepG2 cells, respectively, to sorafenib at increasing concentrations. The Huh7-S-R and HepG2-S-R cells were resistant to sorafenib cytotoxicity even at higher concentrations, as per the cell viability assay (Fig. 1a, b). Interestingly, both cells were also regorafenib resistant (Fig. S1A, S1B). Proinflammatory cytokines in the tumor microenvironment can promote tumor growth, yet their value in sorafenib resistance remains underexploited. We next analyzed a series of proinflammatory cytokines in sorafenib resistance cells compared with the parental cells, and found CLCF1 upregulated in the resistance cells by real-time PCR (Figs. 1c and S1C). Our findings also showed a remarkable enhancement of CLCF1 levels in cells resistant to sorafenib using western blotting (Fig. 1d). We also observed that PI3K/AKT signaling and its downstream effector mTOR were activated noticeably in Huh7-S-R and HepG2-S-R cells (Fig. 1e). Hence, CLCF1 is upregulated, and PI3K/AKT signaling is activated in HCC cells resistance to sorafenib.
Fig. 1. Increased CLCF1 expression in sorafenib-resistant HCC cells.
a Huh7 and Huh7-S-R cells were treated with increasing dose of sorafenib for 3 days. Relative cell viability was analyzed by MTT assay. The significance of the difference in IC50 values was analyzed by extra sum-of-squares F test. b HepG2 and HepG2-S-R cells were treated with increasing dose of sorafenib for 3 days. Relative cell viability was analyzed by MTT assay. The significance of the difference in IC50 values was analyzed by extra sum-of-squares F test. c mRNA level of CLCF1 in Huh7-S-R, HepG2 and their parental cells was analyzed by real-time PCR. d Protein level of CLCF1 in Huh7-S-R, HepG2 and their parental cells was analyzed by Western blotting. e Indicated protein level in Huh7-S-R, HepG2 and their parental cells was analyzed by Western blotting. f HepG2 tumors resistant to sorafenib were generated in nude mice. Left, schematics of the experimental design. Post 14 days of HepG2 cells implantation subcutaneously, treatment of mice was done with a control vehicle (non-therapeutic) or with sorafenib five times per week across week seven; bottom left, tumor growth assessed through the time course. Right, HepG2 tumor sections from sorafenib or vehicle-treated mice were immunohistochemically stained for CLCF1. Scale bar: 25 µm. All data are mean ± SD of three separate experiments. ** P < 0.01.
We then evaluated whether a similar effect of CLCF1 is observed in HCC tumors resistant to sorafenib. For this, subcutaneous injection of HepG2 cells into female nude mice was administered, and mice were treated with low-dose sorafenib one-week post-implantation (Fig. 1f). Indeed, tumor growth was significantly inhibited after sorafenib treatment for the first four weeks but was ineffective later (Fig. 1f). At the end of treatment, tumors of similar sizes were observed in the treatment and control groups (Fig. 1f). Thus, sorafenib resistance was developed in due course in xenografted tumors in the sorafenib treatment group. After sacrificing all mice and harvesting xenografted tumors at week seven for further analyses, IHC staining showed significantly increased CLCF1 levels in HepG2 tumors in the sorafenib-treated group, in contrast to the controls (Fig. 1f). Therefore, there is a likely association between CLCF1 and sorafenib resistance in HCC.
Maintenance of resistance to sorafenib in HCC cells requires CLCF1
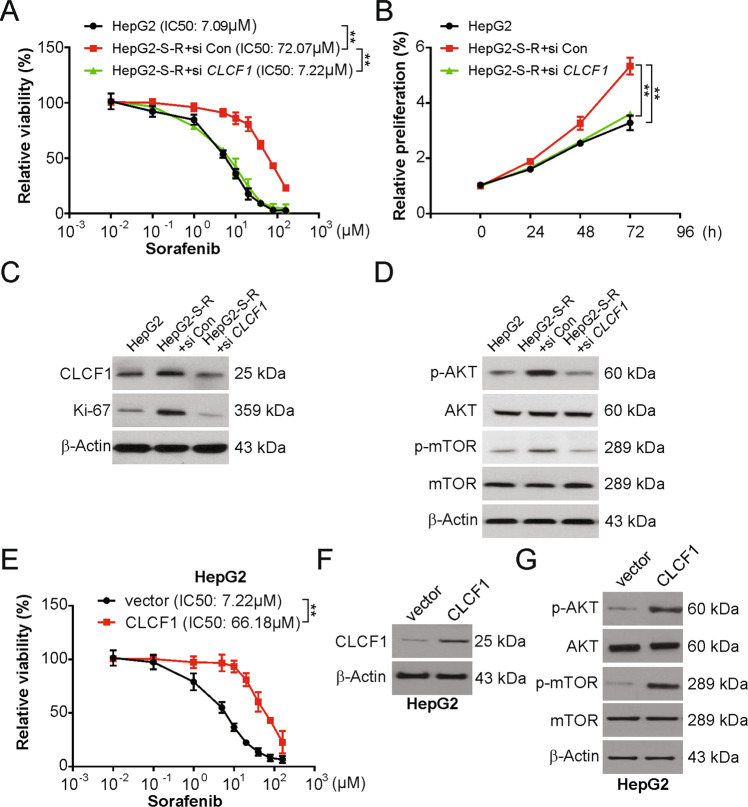
Next, we evaluated whether CLCF1 is required for resistance to sorafenib in HCC cells and discovered that knockdown of CLCF1 led to re-sensitization of HepG2-S-R cells to sorafenib (Fig. 2a) along with potentially increased apoptosis (Fig. S2A). Moreover, knockdown of CLCF1 led to significantly reduced HepG2-S-R cell proliferation (Fig. 2b), as confirmed by western blotting indicating a decline in Ki-67, a cell proliferation marker (Fig. 2c), as well as p-AKT and p-mTOR in cells transfected with CLCF1 siRNA (Fig. 2d). The similar results were obtained in Huh7 and Huh7-S-R cells (Fig. S2B–S2F). Conversely, an effective impairment of the inhibitory effect of sorafenib and better survival of cells was found after ectopic expression of CLCF1 in the parental HepG2 and Huh7 cells (Figs. 2e, f and S2J). Likewise, overexpression of CLCF1 enhanced p-mTOR and p-AKT levels in HepG2 and Huh7 cells (Figs. 2g and S2K). Therefore, our results indicate that CLCF1 is important for sorafenib resistance in HCC cells.
Fig. 2. HCC cells require CLCF1 to sustain sorafenib resistance.
a HepG2 cells transfected with si CLCF1 were treated with increasing dose of sorafenib for 72 h. Relative cell viability was analyzed by MTT. The significance of the difference in IC50 values was analyzed by extra sum-of-squares F test. b Cell proliferation was analyzed in the HepG2 cells with si CLCF1 transfection. c HepG2 cells were transfected with si CLCF1. Indicated protein level was analyzed by Western blotting. d HepG2 cells were transfected with si CLCF1. Indicated protein level was analyzed by Western blotting. e HepG2 cells transfected with CLCF1 overexpression plasmid were treated with increasing dose of sorafenib for 72 hours. Relative cell viability was analyzed by MTT. The significance of the difference in IC50 values was analyzed by extra sum-of-squares F test. f CLCF1 transfection efficiency was analyzed by Western blotting. g HepG2 cells were transfected with CLCF1 overexpression plasmid. Indicated protein level was analyzed by Western blotting. All data are mean ± SD of three separate experiments. ** P < 0.01.
Enhanced glycolysis in sorafenib-resistant HCC cells requires CLCF1
Several cancers have been associated with aerobic glycolysis and chemo-resistance36. We next assessed whether metabolism in HCC cells is altered by sorafenib resistance and observed a significant increase in lactate and ATP production, glucose consumption (Fig. S3A–S3C), and ECAR (Fig. S3D) in HepG2-S-R cells in comparison to the parental HepG2 cells. Therefore, sorafenib resistance leads to an increase in glycolysis in HCC cells. The above observations of CLCF1 upregulation in HCC cells resistant to sorafenib, along with the participation of PI3K/AKT signaling in the regulation of aerobic glycolysis in HCC cells, prompted us to speculate that CLCF1 contributes to the enhanced rate of glycolysis in HCC cells resistant to sorafenib. To test this, we first treated HepG2-S-R cells with an inhibitor of AKT, called MK-2206, and found a strong decrease in lactate production, glucose consumption, and cellular ATP levels (Fig. S3E–S3G). Previous studies have shown that PI3K/AKT signaling mediates the activation of several glycolytic genes, such as GLUT3 (glucose transporter type 3), HK2 (hexokinase 2), and PDK1 (pyruvate dehydrogenase kinase 1). Accordingly, upregulation of each of these genes was observed in HepG2-S-R cells (Fig. S3H), whereas a significant decline in their levels was observed after treatment with MK-2206 (Fig. S3H). We also found that the xenografted HepG2-S-R tumor growth in nude mice was remarkably inhibited by systemic delivery of MK-2206 (Fig. S3I), thus supporting the important role of AKT/PI3K signaling in glucose metabolism regulation in HCC cells resistant to sorafenib.
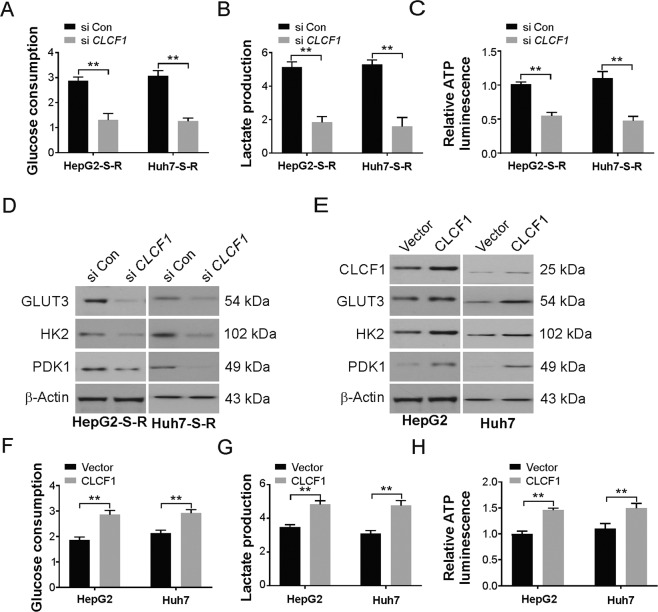
Next, the role of CLCF1 in the increased rate of glycolysis in HCC cells resistant to sorafenib was assessed. A significant decrease in lactate production, glucose consumption, and intracellular ATP levels was observed in HepG2-S-R and Huh7 cells with CLCF1 knockdown (Fig. 3a–c). In addition, HK2, GLUT3, and PDK1 protein levels were decreased in HepG2-S-R cells transfected with CLCF1 siRNA (Fig. 3d). In an alternate assay, our findings indicated that overexpression of ectopic Flag-CLCF1 enhanced the levels of HK2, GLUT3, and PDK1 (Fig. 3e), as well as glycolysis was remarkably promoted due to the expression of ectopic Flag-CLCF1 in HepG2 and Huh7 parental cells (Fig. 3f–h). Thus, aerobic glycolysis is promoted in HCC cells resistant to sorafenib by CLCF1. As the functions of aerobic glycolysis in cancer cell proliferation and survival are well known, our above results indicate that CLCF1 may enhance glycolysis to confer sorafenib resistance in HCC cells.
Fig. 3. Enhanced glycolysis requires CLCF1 in HCC cells resistant to sorafenib.
a Glucose consumption in HepG2-S-R and Huh7-S-R cells transfected with siRNA against CLCF1. b Lactate production in HepG2-S-R and Huh7-S-R cells transfected with siRNA against CLCF1. c Relative ATP luminescence in HepG2-S-R and Huh7-S-R cells transfected with siRNA against CLCF1. d Western blotting of indicated protein level in HepG2-S-R and Huh7-S-R cells transfected with siRNA against CLCF1. e Western blotting of indicated protein in HepG2 and Huh7 cells were transfected with CLCF1 overexpression plasmid. f Glucose consumption in HepG2 and Huh7 cells transfected with CLCF1 overexpression plasmid. g Lactate production in HepG2 and Huh7 cells transfected with CLCF1 overexpression plasmid. h Relative ATP luminescence in HepG2 and Huh7 cells transfected with CLCF1 overexpression plasmid. All data are mean ± SD of three separate experiments. ** P < 0.01.
miR-30a-5p targets CLCF1, and sorafenib upregulates CLCF1 through miR-30a-5p downregulation
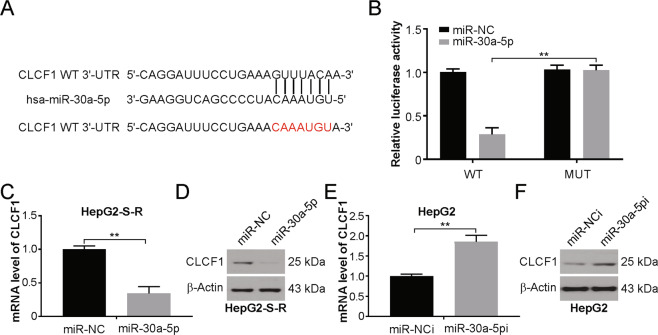
It is well known that miRNAs play a role in the progression of cancer and in the development of drug resistance37. Hence, we investigated if CLCF1 expression is controlled by any miRNA(s) in HepG2-S-R cells. We used bioinformatic tools to search for miRNAs targeting the human CLCF1 3′-UTR and discovered potential miR-30a-5p binding sites (Fig. 4a). Curiously, a marked downregulation of miR-30a-5p was found by real-time PCR assay in HepG2-S-R cells in comparison to the parental HepG2 cells (Fig. S4A). We also found significantly reduced miR-30a-5p expression in sorafenib-treated HepG2 tumors (Fig. S4B). Likewise, a time-dependent decrease in miR-30a-5p was observed in cultured HepG2 cells treated with sorafenib (Fig. S4C), along with increased CLCF1 levels (Fig. S4D). These data thus indicate that miR-30a-5p downregulation may play a part in the upregulation of CLCF1 induced by sorafenib in HCC cells.
Fig. 4. CLCF1 is targeted directly by miR-30a-5p.
a Prediction of CLCF1 as a target of miR-30a-5p. miR-30a-5p binding site, as predicted in CLCF1 3′-UTR. b Assay using dual-luciferase reporters. Normalization of activity of renilla luciferase was done with firefly luciferase activity. c, d CLCF1 expression in miR-30a-5p mimic-transfected HepG2-S-R cells was assessed through real-time PCR or Western blotting. e, f CLCF1 expression in miR-30a-5p inhibitor-transfected HepG2 cells transfected with anti-miR-30a-5p was analyzed by real-time PCR and Western blotting. All data are mean ± SD of three separate experiments. ** P < 0.01.
To experimentally confirm the above observation, luciferase reporters containing the wild-type CLCF1 3′-UTR or the mutant miR-30a-5p seed with site mutations were constructed (Fig. 4a), and HepG2 cells were cotransfected with reporters and miR-30a-5p mimics or negative control RNA. We observed noticeable inhibition of the wild-type vector, but not the mutant construct, by miR-30a-5p (Fig. 4b). Furthermore, a decrease in CLCF1 expression in HepG2-S-R and Huh7-S-R cells due to overexpression of miR-30a-5p was confirmed by western blotting and real-time PCR (Figs. 4c, d and S4E, S4F), whereas anti-miR-30a-5p-mediated inhibition of miR-30a-5p led to enhanced expression of CLCF1 in HepG2 and Huh7 cells at the transcript and protein levels (Figs. 4e, f and S4G, S4H). Taken together, the results demonstrate that CLCF1 is a direct target of miR-30a-5p, and a role of the miR-30a-5p/CLCF1 regulatory axis is indicated in HCC cells resistant to sorafenib.
Sorafenib resistance and tumorigenesis is regulated by the miR-30a-5p/CLCF1 axis
Our next obvious step was to determine whether this miR-30a-5p/CLCF1 axis plays a role in regulating resistance to sorafenib in HCC cells. Indeed, HepG2-S-R cells were re-sensitized to sorafenib (Fig. 5a) upon increased expression of miR-30a-5p, indicating the suppressive action of miR-30a-5p on sorafenib resistance in HCC cells. Furthermore, apoptosis was significantly enhanced in HepG2-S-R cells overexpressing miR-30a-5p (Fig. S5A), in addition to a remarkable reduction in the proliferation of HepG2-S-R cells (Fig. S5B). To investigate whether miR-30a-5p exerts the abovementioned effects on sorafenib resistance by targeting CLCF1, ectopic expression of CLCF1 was achieved through p3xFlag-CLCF1, which did not encode the CLCF1 3′-UTR, making this construct resistant to miR-30a-5p. There was an effective recovery of sorafenib resistance, cell proliferation and survival in HepG2-S-R cells overexpressing miR-30a-5p (Figs. 5a, S5A, and S5B). We also observed AKT/PI3K signaling inhibition in sorafenib-resistant HCC cells by miR-30a-5p targeting CLCF1 (Fig. 5b). Thus, sorafenib resistance and cell growth are suppressed by miR-30a-5p by targeting CLCF1.
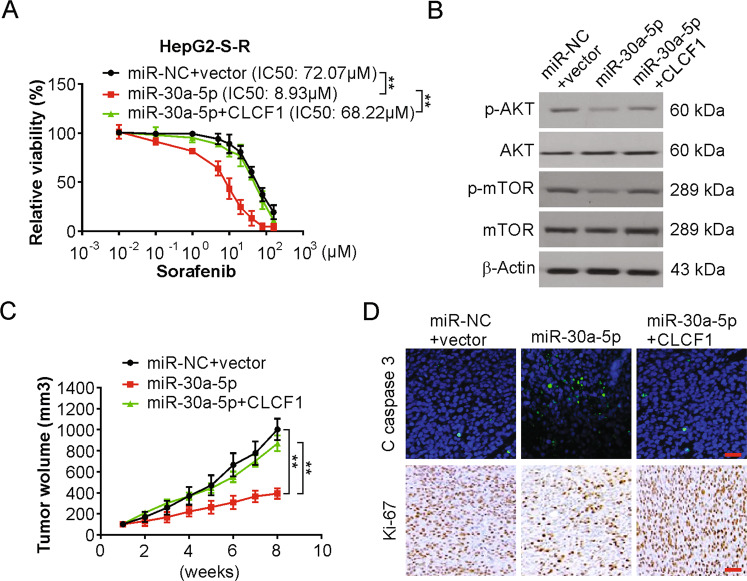
Fig. 5. The miR-30a-5p/CLCF1 axis regulates tumorigenesis and sorafenib resistance in HCC cells resistant to sorafenib.
a HepG2-S-R cells transfected with miR-30a-5p with or without CLCF1 cotransfection were treated with increasing dose sorafenib for 72 h. Relative cell viability was analyzed by MTT. The significance of the difference in IC50 values was analyzed by extra sum-of-squares F test. b Western blotting of indicated proteins in HepG2-S-R cells transfected with miR-30a-5p with or without CLCF1 cotransfection. c The growth of HepG2-S-R tumors in mice is controlled by the miR-30a-5p/CLCF1 axis. d Paraffin-embedded sections of HepG2-S-R tumor tissues from mice treated as in (a) were analyzed by IHC staining for Ki-67, or IF staining for cleaved caspase 3. Scale bars: 25 µm.
Next, the activity of the miR-30a-5p/CLCF1 axis in sorafenib-resistant HCC cell tumorigenesis was tested. HepG2-S-R cells were infected with pseudoviruses expressing miR-30a-5p and CLCF1, and nude mice were implanted with these infected cells. To maintain sorafenib resistance in HepG2-S-R tumors, the mice bearing tumors were treated systematically with sorafenib. Overexpression of miR-30a-5p greatly inhibited the growth of HepG2-S-R tumors, which was effectively rescued by coexpression of the Flag-CLCF1 protein (Figs. 5c and S5C), supporting the functional importance of the miR-30a-5p/CLCF1 axis in the regulation of tumorigenesis in sorafenib-resistant HCC cells. In addition, the AKT/mTOR signaling pathway was inhibited in miR-30a-5p transfected tumors, which was attenuated by CLCF1 overexpression (Fig. S5D). Furthermore, miR-30a-5p overexpression resulted in a decrease in the level of Ki-67 protein, but an increase in the cell apoptosis marker cleaved caspase-3 protein was observed by IHC staining in HepG2-S-R tumors and in comparison to the controls (Fig. 5d). Additionally, restoring CLCF1 expression substantially negated the action of miR-30a-5p on the expression of both cleaved caspase-3 and Ki-67 (Fig. 5d). These results further support the role of the miR-30a-5p/CLCF1 axis in regulating tumorigenesis and sorafenib resistance in sorafenib-resistant HCC cells.
Aerobic glycolysis in HCC cells resistant to sorafenib is regulated by the miR-30a-5p/CLCF1 axis
After observing the requirement of CLCF1 for aerobic glycolysis in HCC cells resistant to sorafenib, we next tested the role of the miR-30a-5p/CLCF1 axis in regulating glucose metabolism in these cells. As expected, overexpression of miR-30a-5p led to a remarkable reduction in lactate production, glucose consumption, ECAR, and intracellular ATP levels in HepG2-S-R cells (Fig. 6a–d), as well as suppressed the glycolytic genes HK2, GLUT3, and PDK1 (Figs. 6e, 6f and S6A, S6B). In an alternate experiment, an increase in glucose utilization, intracellular ATP levels and lactate production in HepG2 cells following anti-miR-30a-5p treatment mediated inhibition of miR-30a-5p (Fig. S6C–S6E). Thus, miR-30a-5p functions as an aerobic glycolysis repressor in sorafenib-resistant HCC cells.
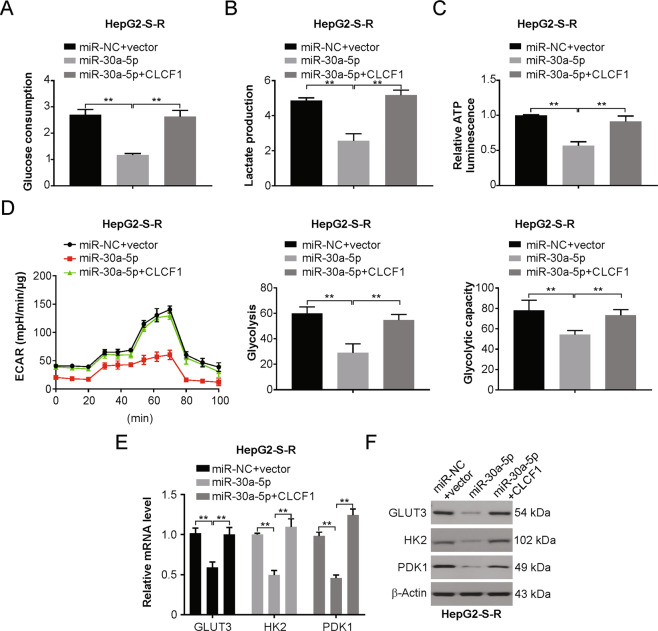
Fig. 6. The miR-30a-5p/CLCF1 axis is important in regulating metabolism of glucose in HCC cells resistant to sorafenib.
a Glucose consumption in HepG2-S-R cells transfected with miR-30a-5p with or without CLCF1 cotransfection. b Lactate production in HepG2-S-R cells transfected with miR-30a-5p with or without CLCF1 cotransfection. c Relative ATP luminescence in HepG2-S-R cells transfected with miR-30a-5p with or without CLCF1 cotransfection. d Left, real-time measurement of extracellular acidification rate (ECAR) in HepG2-S-R cells transfected with miR-30a-5p with or without CLCF1 cotransfection. The value of glycolysis (Middle) and glycolytic capacity (Right) in the indicated cells. e Relative mRNA level of GLUT3, HK2, and PDK1 in HepG2-S-R cells transfected with miR-30a-5p with or without CLCF1 cotransfection. f Relative protein level of GLUT3, HK2, and PDK1 in HepG2-S-R cells transfected with miR-30a-5p with or without CLCF1 cotransfection. All data are mean ± SD of three separate experiments. ** P < 0.01.
We next investigated whether miR-30a-5p targets CLCF1 to inhibit aerobic glycolysis. We observed that Flag-CLCF1 protein coexpression remarkably abrogated the inhibitory action of miR-30a-5p on glucose metabolism in HepG2-S-R cells (Fig. 6a–d), which followed the restoration of GLUT3, HK2 and PDK1 levels (Figs. 6e, 6f and S6A, S6B). Therefore, the miR-30a-5p/CLCF1 axis plays an important role in the regulation of aerobic glycolysis in HCC cells and tumors resistant to sorafenib.
We next investigated whether the restoration of miR-30a-5p expression may exert a therapeutic effect on HCC cells resistant to sorafenib. To assess this, we performed a treatment study by injecting 2′-O-methyl-modified miR-30a-5p (agomiR-30a-5p) conjugated with cholesterol into the tail veins of nude mice and enveloped them in delivery vehicles made of natural lipids two weeks after HepG2-S-R cell implantation. The injections were given for five cycles (n = 5), one every week. Control groups received agomiR-NC treatments. We found that HepG2-S-R tumor growth was effectively suppressed compared with agomiR-NC when agomiR-30a-5p was systemically delivered (Fig. S6F). Hence, our findings demonstrated the therapeutic efficacy of agomiR-30a-5p against sorafenib-resistant HCC.
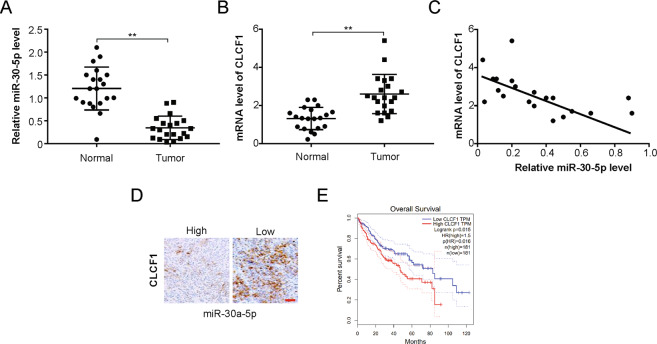
Inverse relationship between levels of miR-30a-5p and CLCF1 in human HCC specimens
Next, the relevance of the above findings was clinically assessed in HCC patients. Paired specimens from HCC patients are difficult to obtain before initiating sorafenib treatment and post-development of sorafenib resistance due to ethical issues; alternatively, miR-30a-5p and CLCF1 transcript levels in 20 primary HCC and paired adjacent normal tissue samples were evaluated by real-time PCR (Table 2). A significant decrease in the expression of miR-30a-5p was observed in HCC compared to normal tissues (Fig. 7a), while there was a remarkable increase in CLCF1 levels (Fig. 7b). Importantly, an inverse relationship was observed between CLCF1 mRNA levels and miR-30a-5p in tumors (Fig. 7c). Additionally, IHC staining of tumor sections showed that HCC with a low miR-30a-5p level produced stronger CLCF1 staining (Fig. 7d), while tumors with a high miR-30a-5p level had weaker CLCF1 signal (Fig. 7d). In addition, the data from the TCGA dataset indicated that HCC patients with high CLCF1 had poor survival (Fig. 7e).
Table 2.
Patient characteristics and univariate P value.
| Characteristics | N (%) | P |
|---|---|---|
| Gender | 0.41 | |
| Male | 12 (60) | |
| Female | 8 (40) | |
| Age (years) | 0.03 | |
| <60 | 18 (90) | |
| ≥60 | 2 (10) | |
| Size of tumor (diameter/cm) | 0.24 | |
| <5 | 16 (80) | |
| ≥5 | 4 (20) | |
| TNM classification | 0.33 | |
| I–II | 13 (65) | |
| III–IV | 7 (35) | |
| Child–Pugh classification | 0.63 | |
| A | 11 (55) | |
| B | 9 (45) |
Fig. 7. Clinical relevance of miR-30a-5p/CLCF1 expression in human HCC.
a Comparison of mRNA level of miR-30a-5p in primary human HCC tumors and normal paired adjacent tissues. b Comparison of mRNA level of CLCF1 in primary human HCC tumors and normal paired adjacent tissues. c A negative correlation was seen by Pearson’s correlation analyses in above comparison in human HCC tumor samples. r = −0.78, p < 0.001. d Human HCC tumor sections were IHC stained and representative images of CLCF1 were acquired with low or high miR-30a-5p expression. Scale bars, 25 μm. e TCAG dataset shows that High CLCF1 expression predicate a poor survival in HCC. All data are mean ± SD of three separate experiments. ** P < 0.01.
Discussion
The first and the primary targeted therapy for advanced HCC is sorafenib38,39. However, patients that acquire sorafenib resistance are deprived of this drug’s benefits15,40. Sorafenib is the most approved and widely used systemic drug and occupies a unique position in the international effort against HCC41. Nevertheless, there are frequent cases of drug resistance in HCC patients post sorafenib treatment and can be categorized into primary or intrinsic resistance and secondary or acquired resistance. However, the mechanisms of sorafenib resistance remain unclear, and it becomes imperative to assess the inherent mechanism of the acquired resistance to sorafenib. Here, we found that sorafenib potentiates glucose metabolism in HCC cells because enhanced glycolysis was observed in HCC cells as a result of sorafenib resistance. Furthermore, an important regulatory pathway of the miR-30a-5p/CLCF1 axis was found to modulate sorafenib resistance and aerobic glycolysis in HCC cells.
A ubiquitous increase in CLCF1 has been observed in several cancers, indicating an oncogenic function of CLCF142. However, the exact activity of CLCF1 in sorafenib resistance in HCC remains unexplored. As per the data of this study, resistance to sorafenib increased CLCF1 expression, which is crucial for sustaining sorafenib resistance in HCC cells. The development of resistance to sorafenib in HCC may be facilitated by PI3K/AKT signaling, which is a yet unexplored mechanism. In this study, we also showed that upregulation of CLCF1 led to PI3K/AKT signaling activation in sorafenib-resistant HCC cells. Hence, the findings of this study combined with those of previous studies firmly support the regulatory cascade of CLCF1/PI3K/AKT underlying sorafenib resistance development in HCC.
A major reason for relapse and mortality in HCC is resistance to sorafenib43. The regulatory activity of miRNAs in HCC growth and progression is well known44. miRNAs may be regulated during carcinogenesis and can function as tumor suppressors or oncogenes depending on the characteristics of their target genes45. Aberrant expression of miRNAs is a universal feature of solid tumors and HCC46. In this study, we observed a direct binding of miR-30a-5p to the 3′-UTR of CLCF1 mRNA to repress its protein expression.
The cytokine CLCF1 has been suggested to be involved in the pathogenesis of multiple myelomas, but its role in HCC has not yet been reported47. The signaling of CLCF1 and IL-6 occurs via multimeric complexes, including the β-subunit/gp130 receptor, and consequently activates a signaling cascade moderated by the JAK-STAT, PI3K/AKT, and MAPK pathways42. Specific cellular responses by these cytokines are managed by the different α-subunit receptor expression patterns, IL-6R for IL-6, and CNTFR for CLCF1, which can independently be switched on by each cytokine16,42. In addition, a previous study showed that CLCF1-CNTFR axis inhibition may be a potential approach for the treatment of cancer19.
As an aberrant trait of cancer cell metabolism, aerobic glycolysis has been associated with resistance to chemotherapies in several human cancers, although the mechanism of how resistance to sorafenib is attributed to cancer cell metabolism is still unclear48. Cancer cell survival and proliferation requires aerobic glycolysis, which also favors cancer cell metastasis and invasion. Interestingly, significantly enhanced glycolysis was found in HCC cells and tumors resistant to sorafenib, indicating that glucose metabolism is associated with sorafenib resistance in HCC cells. We further observed enhanced glycolysis by CLCF1 via PI3K/AKT signaling activation and the glycolytic genes GLUT3, HK2, and PDK1 functioning downstream in sorafenib-resistant HCC cells. Concurrent with the observations of this study, previous reports also found that HCC cells become sensitized to sorafenib after targeting PDK1, thus supporting the requirement of aerobic glycolysis for the maintenance of sorafenib resistance in HCC49. In an earlier study, sorafenib was shown to inhibit oxygen consumption through inhibition of complex I of mitochondria, ultimately affecting glycolysis in HCC cells50. We also demonstrated that the CLCF1/PI3K/AKT regulatory cascade governs aerobic glycolysis and sorafenib resistance in HCC cells. Additionally, in accordance with the critical role of miRNAs in cancer cells, we found that miR-30a-5p regulates both glycolysis and sorafenib resistance in HCC cells and tumors by targeting CLCF1 directly. In particular, miR-30a-5p, the cognate miRNA from the opposite arm of pre-miR-30a-5p, was downregulated by several chemotherapy drugs and acts to improve chemosensitivity in several kinds of tumors. miR-30a-5p further attenuates metabolism in cancer cells by downregulating HIF-1α and GLUT1, in accordance with our findings that miR-30a-5p is a key regulator of resistance to anticancer therapeutics and aerobic glycolysis in HCC cells.
Conclusions
In summary, this is the first study to report that the miR-30a-5p/CLCF1/PI3K/AKT regulatory cascade is a pathway linking sorafenib resistance to metabolism in HCC cells. More importantly, agomiR-30a-5p has a therapeutic effect on sorafenib-resistant HCC, further corroborating the potential of miRNAs as targets for the treatment of sorafenib-resistant HCC.
Supplementary information
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81900371), Natural Science Foundation of Hunan Province (2019JJ50899), China Scholarship Council (201506370112) to Z.Z.
Author contributions
Z.Z., Z.S., and J.S.T. developed the hypothesis and designed the experiments. Z.Z., X.T., J.L., and H.Y. performed the statistical analyses. Z.Z., X.T., Z.S., and J.S.T. conducted experiments and wrote the main manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Edited by I. Amelio
This article has been retracted. Please see the retraction notice for more detail: 10.1038/s41419-025-07492-5
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
3/7/2025
This article has been retracted. Please see the Retraction Notice for more detail: 10.1038/s41419-025-07492-5
Contributor Information
Zhongzhou Si, Email: zhongzsi@csu.edu.cn.
Jing-Shan Tong, Email: tongjingshan@gmail.com.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41419-020-03123-3).
References
- 1.Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin.69, 7–34 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Yang, J. D. et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol.16, 589–604 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anstee, Q. M., Reeves, H. L., Kotsiliti, E., Govaere, O. & Heikenwalder, M. From NASH to HCC: current concepts and future challenges. Nat. Rev. Gastroenterol. Hepatol.16, 411–428 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Llovet, J. M., Montal, R., Sia, D. & Finn, R. S. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat. Rev. Clin. Oncol.15, 599–616 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daher, S., Massarwa, M., Benson, A. A. & Khoury, T. Current and future treatment of hepatocellular carcinoma: an updated comprehensive review. J. Clin. Transl. Hepatol.6, 69–78. (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruix, J., da Fonseca, L. G. & Reig, M. Insights into the success and failure of systemic therapy for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol.16, 617–630 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Kumari, R., Sahu, M. K., Tripathy, A., Uthansingh, K. & Behera, M. Hepatocellular carcinoma treatment: hurdles, advances and prospects. Hepat. Oncol.5, HEP08 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forner, A., Gilabert, M., Bruix, J. & Raoul, J. L. Treatment of intermediate-stage hepatocellular carcinoma. Nat. Rev. Clin. Oncol.11, 525–535 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Dubbelboer, I. R. et al. Treatment of intermediate stage hepatocellular carcinoma: a review of intrahepatic doxorubicin drug-delivery systems. Ther. Deliv.5, 447–466 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Finn, R. S. et al. Therapies for advanced stage hepatocellular carcinoma with macrovascular invasion or metastatic disease: a systematic review and meta-analysis. Hepatology67, 422–435 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Zhu, Y. J., Zheng, B., Wang, H. Y. & Chen, L. New knowledge of the mechanisms of sorafenib resistance in liver cancer. Acta Pharm. Sin.38, 614–622 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao, Q. et al. Increased expression of SLC46A3 to oppose the progression of hepatocellular carcinoma and its effect on sorafenib therapy. Biomed. Pharmacother.114, 108864 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Ziogas, I. A. & Tsoulfas, G. Evolving role of Sorafenib in the management of hepatocellular carcinoma. World J. Clin. Oncol.8, 203–213 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng, G. L., Zeng, S. & Shen, H. Chemotherapy and target therapy for hepatocellular carcinoma: new advances and challenges. World J. Hepatol.7, 787–798 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mendez-Blanco, C., Fondevila, F., Garcia-Palomo, A., Gonzalez-Gallego, J. & Mauriz, J. L. Sorafenib resistance in hepatocarcinoma: role of hypoxia-inducible factors. Exp. Mol. Med50, 134 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pasquin, S. et al. Cardiotrophin-like cytokine factor 1 exhibits a myeloid-biased hematopoietic-stimulating function. Front Immunol.10, 2133 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nahle, S. et al. Cardiotrophin-like cytokine (CLCF1) modulates mesenchymal stem cell osteoblastic differentiation. J. Biol. Chem.294, 11952–11959 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savin, V. J. et al. Renal and hematological effects of CLCF-1, a B-cell-stimulating cytokine of the IL-6 family. J. Immunol. Res2015, 714964 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim, J. W. et al. Antitumor activity of an engineered decoy receptor targeting CLCF1-CNTFR signaling in lung adenocarcinoma. Nat. Med.25, 1783–1795 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emerich, D. F. & Thanos, C. G. Intracompartmental delivery of CNTF as therapy for Huntington’s disease and retinitis pigmentosa. Curr. Gene Ther.6, 147–159 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Pasquin, S., Sharma, M. & Gauchat, J. F. Ciliary neurotrophic factor (CNTF): new facets of an old molecule for treating neurodegenerative and metabolic syndrome pathologies. Cytokine Growth Factor Rev.26, 507–515 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Cui, M. X. et al. Therapeutic effects of a recombinant mutant of the human ciliary neurotrophic factor in a mouse model of metabolic syndrome. Pharmazie65, 279–283 (2010). [PubMed] [Google Scholar]
- 23.Bongioanni, P., Reali, C. & Sogos, V. Ciliary neurotrophic factor (CNTF) for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst. Rev.3, CD004302 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma, M. et al. Involvement of ciliary neurotrophic factor in early diabetic retinal neuropathy in streptozotocin-induced diabetic rats. Eye (Lond.)32, 1463–1471 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo, X. & Liu, X. Nogo receptor knockdown and ciliary neurotrophic factor attenuate diabetic retinopathy in streptozotocin-induced diabetic rats. Mol. Med Rep.16, 2030–2036 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Askvig, J. M., Leiphon, L. J. & Watt, J. A. Neuronal activity and axonal sprouting differentially regulate CNTF and CNTF receptor complex in the rat supraoptic nucleus. Exp. Neurol.233, 243–252 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Treiber, T., Treiber, N. & Meister, G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat. Rev. Mol. Cell Biol.20, 5–20 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Alberti, C. & Cochella, L. A framework for understanding the roles of miRNAs in animal development. Development144, 2548–2559 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Zhang, J. et al. MicroRNA-1231 exerts a tumor suppressor role through regulating the EGFR/PI3K/AKT axis in glioma. J. Neurooncol.139, 547–562 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu, B. et al. MicroRNA-31-5p enhances the Warburg effect via targeting FIH. FASEB J.33, 545–556 (2019). [DOI] [PubMed] [Google Scholar]
- 31.Makol, A. et al. Vimentin as a potential therapeutic target in sorafenib resistant HepG2, a HCC model cell line. Clin. Mol. Hepatol.26, 45–53 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang, Z. et al. GNA13 promotes tumor growth and angiogenesis by upregulating CXC chemokines via the NF-kappaB signaling pathway in colorectal cancer cells. Cancer Med.7, 5611–5620 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Tan, X., Zhang, Z., Yao, H. & Shen, L. Tim-4 promotes the growth of colorectal cancer by activating angiogenesis and recruiting tumor-associated macrophages via the PI3K/AKT/mTOR signaling pathway. Cancer Lett.436, 119–128 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Tong, J. S. et al. Icaritin causes sustained ERK1/2 activation and induces apoptosis in human endometrial cancer cells. PLoS ONE6, e16781 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tong, J. S. et al. ER-alpha36, a novel variant of ER-alpha, mediates estrogen-stimulated proliferation of endometrial carcinoma cells via the PKCdelta/ERK pathway. PLoS ONE5, e15408 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ganapathy-Kanniappan, S. Molecular intricacies of aerobic glycolysis in cancer: current insights into the classic metabolic phenotype. Crit. Rev. Biochem Mol. Biol.53, 667–682 (2018). [DOI] [PubMed] [Google Scholar]
- 37.An, X., Sarmiento, C., Tan, T. & Zhu, H. Regulation of multidrug resistance by microRNAs in anti-cancer therapy. Acta Pharm. Sin. B7, 38–51 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rimassa, L., Pressiani, T. & Merle, P. Systemic treatment options in hepatocellular carcinoma. Liver Cancer8, 427–446 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raoul, J. L., Frenel, J. S., Raimbourg, J. & Gilabert, M. Current options and future possibilities for the systemic treatment of hepatocellular carcinoma. Hepat. Oncol.6, HEP11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ray, E. M. & Sanoff, H. K. Optimal therapy for patients with hepatocellular carcinoma and resistance or intolerance to sorafenib: challenges and solutions. J. Hepatocell. Carcinoma4, 131–138 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bangaru, S., Marrero, J. A. & Singal, A. G. Review article: new therapeutic interventions for advanced hepatocellular carcinoma. Aliment Pharm. Ther.51, 78–89 (2020). [DOI] [PubMed] [Google Scholar]
- 42.Vicent, S. et al. Cross-species functional analysis of cancer-associated fibroblasts identifies a critical role for CLCF1 and IL-6 in non-small cell lung cancer in vivo. Cancer Res.72, 5744–5756 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jindal, A., Thadi, A. & Shailubhai, K. Hepatocellular carcinoma: etiology and current and future drugs. J. Clin. Exp. Hepatol.9, 221–232 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fornari, F. et al. MicroRNAs in Animal Models of HCC. Cancers (Basel)11, 1906 (2019). [DOI] [PMC free article] [PubMed]
- 45.Tan, W. et al. MicroRNAs and cancer: key paradigms in molecular therapy. Oncol. Lett.15, 2735–2742 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu, X. et al. The role of MicroRNAs in hepatocellular carcinoma. J. Cancer9, 3557–3569 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sims, N. A. Cardiotrophin-like cytokine factor 1 (CLCF1) and neuropoietin (NP) signalling and their roles in development, adulthood, cancer and degenerative disorders. Cytokine Growth Factor Rev.26, 517–522 (2015). [DOI] [PubMed] [Google Scholar]
- 48.Lunt, S. Y. & Vander Heiden, M. G. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev. Cell Dev. Biol.27, 441–464 (2011). [DOI] [PubMed] [Google Scholar]
- 49.Wu, C. X. et al. Blocking CDK1/PDK1/beta-Catenin signaling by CDK1 inhibitor RO3306 increased the efficacy of sorafenib treatment by targeting cancer stem cells in a preclinical model of hepatocellular carcinoma. Theranostics8, 3737–3750 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tesori, V. et al. The multikinase inhibitor Sorafenib enhances glycolysis and synergizes with glycolysis blockade for cancer cell killing. Sci. Rep.5, 9149 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.