Abstract
Spatial gradients of species richness can be shaped by the interplay between historical and ecological factors. They might interact in particularly complex ways in heterogeneous mountainous landscapes with strong climatic and geological contrasts. We mapped the distribution of 171 lizard species to investigate species richness patterns for all species (171), diurnal species (101), and nocturnal species (70) separately. We related species richness with the historical (past climate change, mountain uplifting) and ecological variables (climate, topography and vegetation). We found that assemblages in the Western Zagros Mountains, north eastern and north western parts of Central Iranian Plateau have the highest number of lizard species. Among the investigated variables, annual mean temperature explained the largest variance for all species (10%) and nocturnal species (31%). For diurnal species, temperature change velocity shows strongest explained variance in observed richness pattern (26%). Together, our results reveal that areas with annual temperature of 15–20 °C, which receive 400–600 mm precipitation and experienced moderate level of climate change since the Last Glacial Maximum (LGM) have highest number of species. Documented patterns of our study provide a baseline for understanding the potential effect of ongoing climate change on lizard diversity in Iran.
Subject terms: Biodiversity, Biogeography
Introduction
Exploring historical and current drivers of species richness can inform on how those gradients might be reshaped in the future1–3. Present environmental variables including climate, habitat heterogeneity, productivity represent important drivers of species distribution and diversity4–7. Diversity of present assemblages is not always in equilibrium with current climate. Thus, past climate changes may better reflect species richness2 or global endemism than current climate8,9. Beyond the Quaternary, mountain uplifting might also be associated to the formation of biodiversity gradients, shaping an association between habitat heterogeneity and species richness10,11. Together, it appears there is no single driver of species distribution across broad geographical range and species distributional patterns are shaped by the interplay between different historical and ecological factors2,3,11–13. The interplay between contemporary and historical variables in shaping biodiversity might vary between ecosystems types, biogeographic regions and taxonomic groups2,3,11,13.
With ca. 10,970 known species, reptiles are a highly diverse group of vertebrates14, but they are generally less studied compared to other vertebrate groups such as birds and mammals5,15,16. Reptiles are characterized by low dispersal ability and narrow ecological niches17. Thus, they are suitable biological models to assess the role of historical factors in shaping spatial distribution of biodiversity18,19. Global coarse scale distribution maps indicate that reptile richness is highest in pantropical including Central America, South America, south of Africa, Southeast Asia and Australia20. But some regions are less known than others, and lack high resolution distribution information. However, lizard richness is somehow different from reptile as their numbers are found to be maximum in both tropical and arid regions and reach a peak in Australia20. In particular, the highest proportions of threatened and Data Deficient21 reptiles are occurring in tropical areas20. Agriculture, biological resource use and urban development are the most important threats to reptile worldwide20.
Climate and topography are introduced as the most important contemporary determinants of reptile richness at global and regional scales22–25. In fact, reptile richness is highest in areas which characterized with high temperature and high topographic heterogeneity22–25. But the variables associated with the richness of reptile at regional scale in subtropical and desert areas were less investigated. While, reptiles have previously been the targets of richness mapping22,26–28, the influence of historical processes on their richness in southwest Asia was rarely quantified29–31. Among the historical factors, the influence of Quaternary climatic oscillations and mountains uplifting was observed on individual species, using genetic data and species distribution modelling32–37.
Iran is one of the biologically diverse countries in southwest Asia38, it is home to ca. 241 reptile species, of which ca. 71 are endemic to the country39. The country is a suitable place to test the effects of historical events on distribution of biodiversity. Iran’s current topography was shaped by uplifting of the different mountain ranges, which facilitated species diversification by providing new unoccupied niches34,35,37,40. The country also experienced several glacial periods41, which influenced reptile distribution36,42,43. Despite the fact that natural history studies have been initiated over 300 years ago in Iran38, the distribution and ecology of many species occupying that region remain poorly known38,44,45. Recent discoveries of novel lizards and snakes in Iran suggest that the biodiversity of Iran is still poorly explored and should receive further attention46,47. Distributions of some species like Heremites vittatus, Saara loricate and Phrynocephalus scutellatus were mapped using species distribution modeling45,48,49. Based on these studies, climate was the most important determinant of reptile distribution in Iran. Climate was also identified as a most influential variable in shaping reptile richness in the country30. However, there is a knowledge gap in the ecology and distribution of lizard species in Iran44,45,49. In this study, we mapped the species distribution of all lizards in Iran and quantified reptile richness. We associated species richness to ecological drivers of reptile distributions in Iran30, but considered in addition Quaternary climatic oscillations and mountains uplifting events. We had the following expectations:
The age of mountain uplifting have shaped topography as well as Quaternary glaciation should be associated to the present diversity of lizard species in Iran.
Among the ecological factors climatic variables are more influential in shaping lizard richness patterns.
Because nocturnal and diurnal species have different natural history, their richness is expected to be shaped by different historical and ecological variables.
Results
Species richness patterns
The 171 lizard species belonged to ten families and 47 genera. Family Gekkonidae and genus Eremias were most divers family and genus in Iran with 51 and 20 species respectively. Of 171 recognized lizard species in Iran 101 species (59.06%) were diurnal, 70 species nocturnal (40.93%) and 62 species endemic (36.25%).
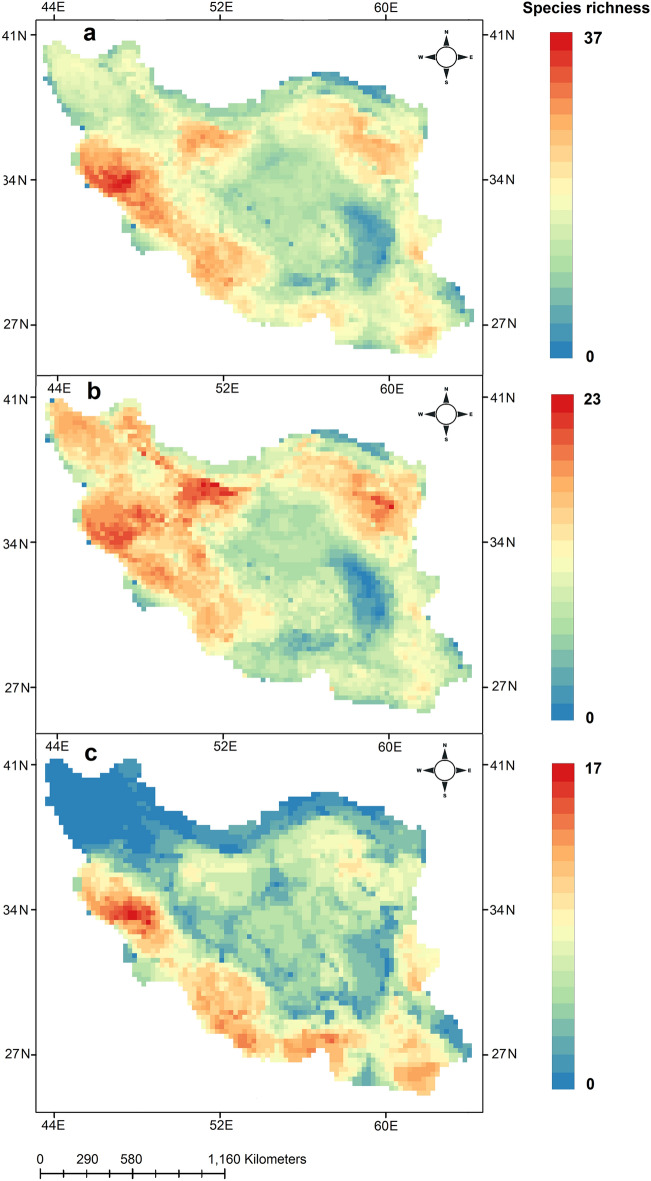
The mapping of the distribution of 171 lizard species showed that Western Zagros Mountains with 37 species, north western parts of Central Iranian Plateau with 30 species and north eastern parts with 28 species have the highest lizard richness in Iran (Fig. 1a). In contrast, northern parts of the country, Kopet-Dagh Mountains and Elburz Mountains with 2–14 species have relatively lower number of species. For diurnal species, Zagros Mountains with 22 species, north eastern and north western parts of Central Iranian Plateau with 23 species show the highest number of species (Fig. 1b). Nocturnal species hotspots occur in Western Zagros Mountains with 17 species, north of Persian Gulf and Oman Sea with 13 species, while we found that central parts of Iran with 1–6 species, Kopet-Dagh Mountains and Elburz Mountains with 0–4 species have much lower number of nocturnal species across the country (Fig. 1c). Results showed that hotspots of all lizards contain 37 species, diurnal lizard 23 and nocturnal lizards 17 species. Lut Desert contains lowest number of species for the three groups.
Figure 1.
Richness map of all (a) diurnal (b) and nocturnal (c) lizards in Iran. All species richness range from 0 to 37, diurnal from 0 to 23 and nocturnal from 0 to 17. Maps were generated using QGIS 3.4.1 (https://www.qgis.org).
Drivers of lizard richness in Iran
We found that all historical and ecological variables are significantly correlated with lizard richness (Table 1). We determined which variable explained highest proportion of variance among ecological and historical variable. Results showed that annual mean temperature explained largest variance for all species (10%) and nocturnal species (31%). For diurnal species, velocity of temperature shows strongest effect in explaining variance in observed richness pattern (26%). The distribution of lizard richness has a positive relationship with annual mean temperature and the number of species increases with higher mean temperatures for the three groups (all species, diurnal and nocturnal). Topographic heterogeneity is positively correlated with lizard species richness but NDVI was negatively correlated with richness for the three groups. Richness is negatively correlated with precipitation for the three groups. Temperature change velocity is positively associated with higher number of species for all lizards, diurnal and nocturnal lizards and maximum number of species occur in areas with moderate level of temperature change velocity.
Table 1.
Results of generalized linear model with quasi-Poisson distribution.
| Group | Predictor | Slope (linear) | z value | AIC | D2 | Predictor | Slope (quadratic) | z value | AIC | D2 |
|---|---|---|---|---|---|---|---|---|---|---|
| All species | ||||||||||
| Intercept | 2.21E+00 | 75.74*** | Intercept | 2.796446 | 585.197*** | |||||
| Temperature | 1.34E−03 | 27.795*** | 18,740 | 0.0494 | Temperature | − 8.5712 | − 18.493*** | 16,830 | 0.1055 | |
| Temperature velocity | 1.24E−03 | 8.614*** | 18,760 | 0.0457 | Precipitation | − 2.14503 | − 7.236*** | 18,370 | 0.0253 | |
| Precipitation | − 1.59E−03 | − 14.073*** | 18,910 | 0.0224 | Temperature velocity | − 1.7505 | − 3.887*** | 18,630 | 0.0654 | |
| Topo-heterogeneity | 5.71E−03 | 1.961* | 18,940 | 0.0177 | NDVI | − 0.98842 | − 3.883*** | 18,700 | 0.0559 | |
| Precipitation velocity | 4.67E−04 | 5.635*** | 19,050 | 0.0014 | Precipitation velocity | 0.174253 | 0.581 | 18,830 | 0.0355 | |
| NDVI | − 1.22E+00 | − 21.612*** | 19,060 | 0.0002 | Topo-heterogeneity | 0.958962 | 3.633*** | 18,880 | 0.0274 | |
| Geo | 5.40E−10 | 1.931 | 19,060 | 0 | Geo | 2.833519 | 9.111*** | 19,060 | 0 | |
| Full model | – | – | 17,680 | 0.213 | Full model | – | – | 16,830 | 0.3437 | |
| Diurnal | ||||||||||
| Intercept | 2.36E+00 | 71.152*** | Intercept | 2.409271 | 444.02*** | |||||
| Temperature velocity | 2.01E−03 | 16.463*** | 18,060 | 0.1243 | Temperature velocity | − 9.40342 | − 16.718*** | 17,220 | 0.2685 | |
| Temperature | 1.11E−03 | 21.284*** | 18,080 | 0.1079 | NDVI | − 0.30654 | − 0.612 | 17,750 | 0.1715 | |
| Precipitation velocity | 1.36E−03 | 14.359*** | 18,300 | 0.0704 | Temperature | − 3.00657 | − 8.113*** | 18,020 | 0.122 | |
| Precipitation | − 1.37E−03 | − 7.857*** | 18,400 | 0.0526 | Precipitation velocity | − 0.21334 | − 0.573 | 18,220 | 0.0846 | |
| NDVI | − 8.31E−01 | − 13.794*** | 18,460 | 0.041 | Precipitation | − 1.23191 | − 4.006*** | 18,320 | 0.0656 | |
| Topo-heterogeneity | 3.58E−02 | 10.995*** | 18,630 | 0.0092 | Topo-heterogeneity | − 0.40011 | − 1.257 | 18,630 | 0.009 | |
| Geo | 1.04E−09 | 3.25** | 18,680 | 0 | Geo | 3.325387 | 8.851*** | 18,680 | 0.0009 | |
| Full model | – | – | 17,240 | 0.2658 | Full model | 16,600 | 0.5221 | |||
| Nocturnal | ||||||||||
| Intercept | − 9.98E−02 | − 1.919*** | Intercept | 1.42242 | 137.94*** | |||||
| Temperature | 8.63E−03 | 45.972*** | 16,750 | 0.292 | Temperature | − 7.34695 | − 11.508*** | 16,530 | 0.3135 | |
| NDVI | − 3.13E+00 | − 25.14*** | 18,830 | 0.0845 | Precipitation velocity | − 16.5552 | − 20.062*** | 17,910 | 0.1759 | |
| Topo-heterogeneity | 7.43E−02 | 15.469*** | 19,440 | 0.0245 | NDVI | − 11.7424 | − 10.3*** | 18,680 | 0.1 | |
| Precipitation velocity | − 1.99E−03 | − 12.669*** | 19,550 | 0.013 | Topo-heterogeneity | − 0.04915 | − 0.104 | 19,370 | 0.0314 | |
| Geo | 3.38E−09 | 7.673*** | 19,640 | 0.004 | Temperature velocity | − 2.05889 | − 4.303*** | 19,610 | 0.0072 | |
| Precipitation | − 2.06E−03 | − 24.405*** | 19,670 | 0.0011 | Geo | 0.45501 | 0.803 | 19,620 | 0.0067 | |
| Temperature velocity | 1.57E−03 | 5.727*** | 19,670 | 0.0016 | Precipitation | − 5.05909 | − 5.223*** | 19,660 | 0.0023 | |
| Full model | – | – | 14,930 | 0.4726 | Full model | – | – | 13,590 | 0.6062 | |
The table shows linear and quadratic estimates, associated z‐ values with p‐values (asterisks), the Akaike information criterion values (AIC) and explained deviance (D2) of variables. The AIC values and the explained deviance were estimated for each predictor separately and in combination for full model. The most important variables for all lizards, diurnal lizards and nocturnal lizards were indicated in bold. Significance codes: 0 ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05.
Discussion
We mapped the richness of all recognized lizard species in Iran, using distribution data of 171 lizard species and documented pronounced gradients of species richness, which were different between nocturnal and diurnal species. The Western Zagros Mountains, north eastern and north western parts of Central Iranian Plateau have the highest number of species, with a peak specifically in the Zagros Mountains.
Climatic variables were identified as the important drivers of reptile distribution around the globe5,22. Our results supported previous effects of climate and showed that temperature was the best explaining variable for richness of all and nocturnal species, while temperature change velocity explained highest fraction of variation in diurnal species richness. Hosseinzadeh, et al.30 also showed that temperature is the most important predictor of reptile distribution in Iran. A positive association was frequently reported for reptile richness and temperature around the world50. Body temperature, which is the most important ecophysiological variable affecting the performance of reptiles, is regulated by ambient energy input51. Annual mean temperature is an indicator of ambient energy input52 and it is often used as a measure of environmental energy53. Thus, areas with higher temperature and consequently higher ambient energy can support more species52,53. This is why a positive association was found between lizard richness and temperature in all three groups. In agreement with our expectations, nocturnal and diurnal lizard richness was shaped by different contemporary and historical variables. Moreover, we found that areas which receive less precipitation have more lizard species, while in other group of reptiles, such as turtles, richness may show the opposite response and increase with precipitation54,55. Nocturnal lizards are richest in the tropics and deserts, and their richness decreases with latitude56. Nocturnal lizards, especially those of the families Gekkonidae, Sphaerodactylidae and Phyllodactylidae are more divers in the south of Iran than the rest of the country38,39. Annual mean temperature is increased toward southern Iran, that could be linked to the increased diversity of nocturnal lizards. In warm environments, the hot days increases the cost of diurnal activity, whereas nocturnal activity provides a shelter from these extreme conditions, for e.g. feeding and reproduction.
Quaternary climatic change was associated with distribution biodiversity in high latitude57. Our results show that past climate change also played important role in shaping biodiversity distribution in lower latitude as well. During the ice ages, mountains of Iran were covered by snow and ice line and snow line was much lower than what we see today41. So reptiles of Iran have undergone several cycles of range expansion–contraction due to the climate fluctuations which in turn shaped their current distribution36,42,43. We found that temperature change velocity was the most important variable in explaining variation in diurnal species richness and the second most important predictor of all lizard richness. Past climate change was not important in shaping nocturnal species richness because their richness reach a peak at south of Iran38,39 which was not influenced by past climatic changes41.
Our results agree with previous studies, which have shown that past climate is a major determinant of reptile richness13,31. For example, Araújo, et al.13 showed that past climate played an important role in shaping large-scale species richness patterns of reptiles and amphibians across Europe. In another study Ficetola et al. 31 quantified the importance of past climate on reptiles of the Western Palearctic. In both studies, reptile richness and endemism were highest in areas with high climate stability, low climate change velocity. Our findings showed that lizard richness was positively associated with temperature change velocity, areas with moderate climate change velocity were correlated with maximum number of species. Our finding is in line with previous studies which highlighted the role of Quaternary climatic oscillations on Iran’s biodiversity using genetic data and species distribution modeling34,42,43. Zagros Mountains which contain highest number of species was a refugium for several taxonomic groups during past climatic oscillations including reptiles34,42,43,58, amphibians59,60 birds61,62, and mammals63–65.
As pointed by Wines and Graham66, there might be strong correlation between species richness and some environmental variables but environmental variables (in our case temperature) cannot change the number of species in particular region66. Species richness pattern linked to processes like speciation, dispersal and extinction66,67. Zagros uplifting caused speciation by splitting populations of species and by providing unoccupied niches for species of the genus Eremias, Mesalina, Timon and Sarra32,35,38,68. Climatic changes also strongly influenced lizard distribution pattern and played important role in their isolations and dispersal in Zagros region34,69. There are some sky island species which remained in climatic refugia in Zagros Mountains like Iranolacerta brandtii and Iranolacerta zagrosica during past climatic oscillations34. In addition, Zagros acted as dispersal barrier and known as dispersal corridor for different species of lizards38,69,70. According to our knowledge, there is not any specific driver of extinction of lizard in the area. Thus, among the three main processes which are linked to species richness in each region, speciation and dispersal due to Zagros uplifting and past climatic fluctuations, are the main drivers of lizard richness patterns (high richness in Zagros region).
In this study, Zagros Mountains were identified as hotspot of lizard richness in Iran. This region was also identified as hotspot of biodiversity for mammals and plants in the country7,71. Our analyses indicate that the Zagros Mountains are a region of moderate climate stability and high topographic heterogeneity, providing a high diversity of climatic niches. Furthermore, the Zagros Mountains are located in the Irano-Anatolian biodiversity hotspot72, showing that there are local hotspots of biodiversity nested within regional biodiversity hotspots7. Zagros Mountains were also identified as an endemism area for reptiles of the Western Palearctic31. Beside reptiles, there are some high prioritized species for conservation such as the Luristan newt (Neurergus kaiseri), which was ranked as 45th among the world’s 100 top priority amphibian species73. This combination of high diversity and endemism makes Zagros Mountains a most valuable target for conservation of biodiversity in Iran.
Conclusion
Altogether, our results suggest that lizard richness can be explained by current and past climate in Iran. There are studies which quantified the role of ecological factors on reptile richness in southwest Asia (for instance29–31), while historical drivers of reptile richness remain poorly understood31. For better understanding the drivers of current species distribution, we need to look at past and investigate the role of historical factors on species distribution. This study took in to account both historical and ecological factors effects on the distribution patterns of 171 lizard species in Iran. Our findings support the fact that there is no single driver for biodiversity distribution and there is always a set of ecological and historical factors shaping species richness3,11,12,74. Since lizard richness is strongly associated with climate we speculate that lizard diversity and distribution will be affected by future climatic changes. Documented patterns of our study provide a baseline for understanding the potential effect of ongoing climate change on lizards in Iran.
Methods
Study area
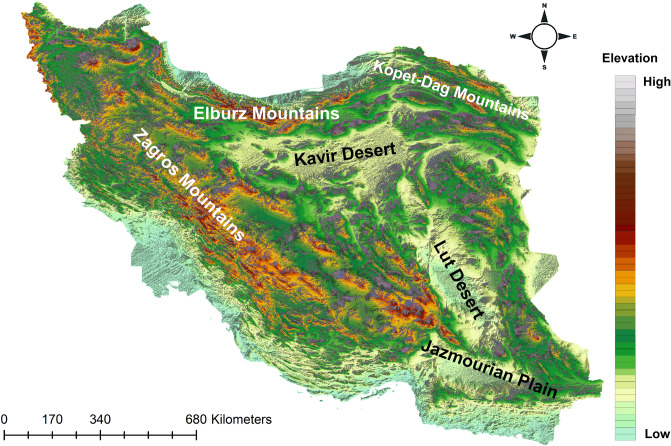
Iran covers 164.8 million hectares, located in the Palearctic region at the crossroads of three biogeographic realms; Afrotropic, Palearctic and Indomalaya. Iran hosts over ca. 8000 plant species75,76 and more than 1214 vertebrate species of which many are endemic to the country38,39,71,77. The elevation ranges from − 26 to 5770 m and a large fraction of the country has an elevation above 1200 m. Iran’s current topography (Fig. 2) was shaped mainly via tectonic activities of Arabia–Eurasia continent collision78. This collision generated in around early Miocene, approximately 19 Mya79,80 and the last mass tectonic event in this zone occurred in late Miocene and the beginning of the Pliocene, 5 Mya, when progressive anticlockwise rotation of the Arabian Peninsula associated with the formation of the Red Sea and Gulf of Aden81. The collision of Arabia – Eurasia continents resulted in multi stage uplift of the Zagros Mountains, as well as the uplift of the whole plateau. The Elburz Mountains in northern Iran are stretching from west to east at the southern coast of the Caspian Sea and form the northern border of the Iranian Plateau. They are more than 1000 km long and the width varies from 30 to 130 km in different parts. The northern slopes of Elburz Mountains are covered by Hyrcanian forests. Kopet Dagh Mountains is a large mountain range (about 650 kms along) which is located in the northeast of Iran between Iran and Turkmenistan, stretching from near the Caspian Sea to the Harirud River. Zagros Mountains form the western and south-western borders of the Iranian Plateau, covering 1500 km from Lake Van in Turkish Kurdistan to south-eastern Iran. Iran has two well-known deserts, the Kavir Desert and the Lut Desert which are among the hottest areas in the world, located in Iran's central plateau. The Quaternary has been a period of global climate oscillation and several Quaternary climatic changes occurred in Iran, also on the dry Iranian highlands82. The most recent glaciation, termed the Riss-Würm, reached its maximum about 18,000–21,000 years ago, and subsequently replaced by Holocene Climatic Optimum (HGO), 9000–5000 years ago83,84. During this period, in northern and western Iran climate changed between dry and cold climatic conditions during the glaciation and moist and warm conditions during the interglacials82–84.
Figure 2.
A topographic overview of Iran with its major geomorphological features. Map was generated using QGIS 3.4.1 (https://www.qgis.org).
Distribution points
Distribution records of all 171 recognised lizard species of Iran (see Appendix S1 for annotated checklist of lizards of Iran) were collected from multiple sources; (1) through opportunistic observations and long term own and other colleagues fieldworks from 2009 to 2020 (Using random field surveys different habitat types within the county were investigated (See Appendix S2 Figs. S1–S36 for examples habitats surveyed during fieldworks and observed lizard species in each habitat)), (2) published papers and books (see Appendix S3) and (3) from the Global Biodiversity Information Facility (GBIF: https://www.gbif.org/). Observed lizards were identified following Anderson38, Rastegar-Pouyani et al.39 and Narabadi et al.85. In total, we collected 8620 distribution points from these sources. Since our distribution records come from multiple sources, we carefully checked distribution data, removing duplicate records and distribution records lacking geographic coordinates. Reliability of all 171 lizard species’ distribution records was examined by mapping each species records separately in DIVA-GIS 7.586. We also thinned distribution records of each species to 1 km reduce clustering. Finally, we used 6245 species presence records.
Environmental variables
We selected seven variables (Table 2) related to climate: topography, the normalized difference vegetation index (NDVI), climate change velocity and geology which we expected to be important in shaping distribution of reptiles22–24,30,32–35,38,45,48,49,87. To quantify climate effect on reptile distribution, we used temperature and annual precipitation13. Current climate data was downloaded from WorldClim (www.worldclim.org). We quantified topographic heterogeneity by measuring the standard deviation of elevation values in area grid cells of 1 km from a 90 m resolution88. Elevation layer obtained from the Shuttle Radar Topography Mission (SRTM) elevation model89. The climate change velocity was calculated following Sandel et al.9. We used temperate and precipitation data of current climatic conditions (1970–2000) and Last Glacial Maximum (LGM; 21,000 years before present) to calculate climate change velocity as the rate of climate change in time divided by the local climate change in space. Climate change velocity is a measurement for long-time climate variability9,90 and it shows the direction and rate at which organisms must have moved to maintain a given climate under climate change9,91. In previous research, climate change velocity was identified to be strongly associated with species richness and endemism at regional and global scales9,90,92. LGM data were obtained from CCSM4 and MIROC 3.2 (averaged values) downloaded from WorldClim (www.worldclim.org). Temperature and precipitation change velocity were calculated in R 3.393 environment using the packages raster94, gdistance95 matrixStats96 and SDMTools97. To explore the possible role of mountains uplifting on lizard richness we assembled information on uplift age of each major mountain range based on the available literatures78,98–102, then combined this information in a raster layer containing uplift age of the various mountain ranges in Iran. The layer with uplifting age of mountain ranges was created using QGIS 3.4.1103.To avoid collinearity among variables a variance inflation factor (VIF104) was calculated for the variables using the ‘vifstep’ function in the ‘usdm’ package105 in R 3.393 environment and results showed that collinearity among variables is low (VIF values: annual mean temperature = 1.356, annual mean precipitation = 3.86, topographic heterogeneity = 1.614, NDVI = 3.041, temperature change velocity = 1.185, precipitation change velocity = 1.153, mountains uplifting = 1.056).
Table 2.
List of ecological and historical predictors used to explore drivers of lizard richness.
| Units | Variable (abbreviation) and references |
|---|---|
| Degrees celsius | Annual mean temperature (temperature)106 |
| Millimeters | Annual precipitation (precipitation)106 |
| Meters | Topographic heterogeneity (topo-heterogeneity)89 |
| – | Normalized Difference Vegetation Index (NDVI)107 |
| Degrees celsius | Temperature change velocity (temperature velocity)108 |
| Millimeters | Precipitation change velocity (precipitation velocity)108 |
| Million years | Mountain uplifting age (Geo) 78,98–102 |
Species richness mapping
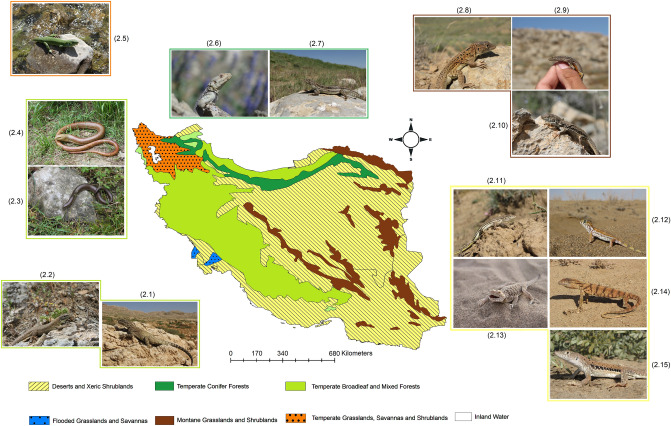
We followed the method applied by Pellissier et al.11 and Albouy et al.109 to map species ranges and then multiply them to create lizard richness in Iran. This method uses species occurrence records, border of biomes (Fig. 3) of the study area and a climatic layer (here we used annual mean temperature an important factor in shaping reptile distribution) to create species range maps. We separated Iran into six biomes based on the World Wildlife Fund (WWF) Terrestrial Ecoregions including the following; Temperate Broadleaf and Mixed Forests, Temperate Grasslands, Savannahs and Shrublands, Temperate Conifer Forests, Montane grasslands and shrublands, and Deserts and Xeric Shrublands110. This approach goes through several steps to map species distribution; first, we created a convex hull polygon around all the observations within one bioregion. If there is only one observation in a bioregion, it will create a buffer around it of 50 km. Then to remove potential outliers, we quantified the 2.5th and 97.5th temperature values from the occurrence, where a species is found. We removed areas that are outside those temperature conditions. Together, we created range maps of all 171 lizard species, and we stacked the species distribution maps into species richness using raster package94 in R environment93 to create three richness maps; all lizards, diurnal lizards and nocturnal lizards.
Figure 3.
Iran’s terrestrial biomes110, together with representative lizards species occur in Temperate Broadleaf and Mixed Forests: 2.1 Paralaudakia microlepis, 2.2 Timon kurdistanicus, 2.3 Anguis colchica, 2.4 Pseudopus apodus; Temperate Grasslands, Savannahs, and Shrublands: 2.5 Lacerta media; Temperate Conifer Forests: 2.6 Paralaudakia caucasia, 2.7 Eremias papenfussi; Montane grasslands and shrublands: 2.8 Eremias kopetdaghica, 2.9 Darevskia kopetdaghica, 2.10 Eremias isfahanica; Deserts and Xeric Shrublands: 2.11 Eremias fasciata, 2.12 Teratoscincus scincus, 2.13 Phrynocephalus mystaceus, 2.14 Varanus griseus 2.15 Eremias persica. Map was generated using QGIS 3.4.1 (https://www.qgis.org).
Statistical analyses
We fitted a generalized linear model (GLM) with quasi-Poisson distribution in order to explore the relationship between reptile richness (number of species in each raster pixel) and the different historical and ecological factors. We estimated the Akaike information criterion values (AIC) and computed the explained deviance for each predictor separately and in combination for full model using the ‘ecospat.adj.D2.glm’ function in the R‐package ‘ecospat’111. Nocturnal and diurnal species have different natural and evolutionary history and their distribution patterns are different in Iran38,44. For example, most of the nocturnal species are occur in south of Iran which characterized with high temperature and more stable climate since LGM68. Thus, nocturnal and diurnal lizard distribution is most likely affected by different ecological and historical variables. So, we did the analysis for all lizard, diurnal and nocturnal separately to test whether there are different drivers for diurnal and nocturnal species. Analyses were carried out in R 3.393.
Supplementary information
Acknowledgements
We would like to thank Ali Khani, Farhad Ataei, Mehdi Yousefi and Mohammad Ebrahim Kafash for their help during fieldwork. We thank Fabian Fopp for his help in producing the richness maps. This research was supported by the Iranian National Science Foundation (project number: 96000492), Ministry of Science Research and Technology of Iran.
Author contributions
A.K. and L.P conceived and designed the research; A.K. and M.Y. collected the data; A.K. analyzed the data with the help of M.G.; A.K wrote the original draft; all authors contributed to the writing and reviewing the manuscript.
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials, or the references cited here within.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-74867-3.
References
- 1.Thuiller W, et al. Predicting global change impacts on plant species' distributions: future challenges. Perspect. Plant Ecol. Evol. Syst. 2008;9:137–152. doi: 10.1016/j.ppees.2007.09.004. [DOI] [Google Scholar]
- 2.Pellissier L, et al. Quaternary coral reef refugia preserved fish diversity. Science. 2014;344:1016–1019. doi: 10.1126/science.1249853. [DOI] [PubMed] [Google Scholar]
- 3.Antonelli A, et al. Geological and climatic influences on mountain biodiversity. Nat. Geosci. 2018;11:718–725. doi: 10.1038/s41561-018-0236-z. [DOI] [Google Scholar]
- 4.Kerr JT, Packer L. Habitat heterogeneity as a determinant of mammal species richness in high-energy regions. Nature. 1997;385:252–254. doi: 10.1038/385252a0. [DOI] [Google Scholar]
- 5.McCain CM. Global analysis of reptile elevational diversity. Glob. Ecol. Biogeogr. 2010;19:541–553. [Google Scholar]
- 6.Hortal J, et al. Species richness can decrease with altitude but not with habitat diversity. PNAS. 2013;110:E2149–E2150. doi: 10.1073/pnas.1301663110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noroozi J, et al. Hotspots within a global biodiversity hotspot - areas of endemism are associated with high mountain ranges. Sci. Rep. 2018;8:10345. doi: 10.1038/s41598-018-28504-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jansson R. Global patterns in endemism explained by past climatic change. Proc. R. Soc. B. 2003;270:583–590. doi: 10.1098/rspb.2002.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandel B, et al. The influence of late quaternary climate-change velocity on species endemism. Science. 2011;334:660–664. doi: 10.1126/science.1210173. [DOI] [PubMed] [Google Scholar]
- 10.Craw D, et al. Rapid biological speciation driven by tectonic evolution in New Zealand. Nat. Geosci. 2016;9:140. doi: 10.1038/ngeo2618. [DOI] [Google Scholar]
- 11.Pellissier L, Heine C, Rosauer DF, Albouy C. Are global hotspots of endemic richness shaped by plate tectonics? Biol. J. Linn. Soc. 2017;123:247–261. doi: 10.1093/biolinnean/blx125. [DOI] [Google Scholar]
- 12.Graham CH, Smith TB, Languy M. Current and historical factors influencing patterns of species richness and turnover of birds in the Gulf of Guinea highlands. J. Biogeogr. 2005;32:1371–1384. doi: 10.1111/j.1365-2699.2005.01284.x. [DOI] [Google Scholar]
- 13.Araújo MB, et al. Quaternary climate changes explain diversity among reptiles and amphibians. Ecography. 2008;31:8–15. doi: 10.1111/j.2007.0906-7590.05318.x. [DOI] [Google Scholar]
- 14.Uetz, P. Freed, P. & Hošek J. The Reptile Database. https://www.reptile-database.org (accessed Aug 6, 2019] (2019).
- 15.Rodriguez MA, Belmontes JA, Hawkins BA. Energy, water and large-scale patterns of reptile and amphibian species richness in Europe. Acta Oecol. 2005;28:65–70. doi: 10.1016/j.actao.2005.02.006. [DOI] [Google Scholar]
- 16.Guedes TB, et al. Patterns, biases and prospects in the distribution and diversity of Neotropical snakes. Glob. Ecol. Biogeogr. 2017;27:14–21. doi: 10.1111/geb.12679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pough H, et al. Herpetology. Upper Saddle River: Prentice Hall; 2001. [Google Scholar]
- 18.Doan TM. A south-to-north biogeographic hypothesis for Andean speciation: evidence from the lizard genus Proctoporus (Reptilia, Gymnophthalmidae) J. Biogeogr. 2003;30:361–374. doi: 10.1046/j.1365-2699.2003.00833.x. [DOI] [Google Scholar]
- 19.Agarwal I, Bauer AM, Jackman TR, Karanth KP. Insights into Himalayan biogeography from geckos: a molecular phylogeny of Cyrtodactylus (Squamata: Gekkonidae) Mol. Phylogenet. Evol. 2014;80:145–155. doi: 10.1016/j.ympev.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 20.Böhm M, et al. The conservation status of the world’s reptiles. Biol. Conserv. 2013;157:372–385. doi: 10.1016/j.biocon.2012.07.015. [DOI] [Google Scholar]
- 21.IUCN. The IUCN Red List of Threatened Species. Version 2019.3. https://www.iucnredlist.org. (2019).
- 22.Powney GD, Grenyer R, Orme CD, Owens IP, Meiri S. Hot, dry and different: Australian lizard richness is unlike that of mammals, amphibians and birds. Glob. Ecol. Biogeogr. 2010;19:386–396. doi: 10.1111/j.1466-8238.2009.00521.x. [DOI] [Google Scholar]
- 23.Qian H. Environment–richness relationships for mammals, birds, reptiles, and amphibians at global and regional scales. Ecol. Res. 2010;25:629–637. doi: 10.1007/s11284-010-0695-1. [DOI] [Google Scholar]
- 24.Coops NC, Rickbeil GJM, Bolton DK, Andrew ME, Brouwers NC. (2018), Disentangling vegetation and climate as drivers of Australian vertebrate richness. Ecography. 2018;41:1147–1160. doi: 10.1111/ecog.02813. [DOI] [Google Scholar]
- 25.Skeels A, Esquerré D, Cardillo M. Alternative pathways to diversity across ecologically distinct lizard radiations. Glob. Ecol. Biogeogr. 2020;29:454–469. doi: 10.1111/geb.13044. [DOI] [Google Scholar]
- 26.Soares C, Brito JC. Environmental correlates for species richness among amphibians and reptiles in a climate transition area. Biodivers. Conserv. 2007;16:1087. doi: 10.1007/s10531-006-9070-5. [DOI] [Google Scholar]
- 27.Tallowin O, Allison A, Algar AC, Kraus F, Meiri S. Papua New Guinea terrestrial-vertebrate richness: elevation matters most for all except reptiles. J. Biogeogr. 2017;44:1734–1744. doi: 10.1111/jbi.12949. [DOI] [Google Scholar]
- 28.Kissling DW, Blach-Overgaard A, Zwaan RE, Wagner P. Historical colonization and dispersal limitation supplement climate and topography in shaping species richness of African lizards (Reptilia: Agaminae) Sci. Rep. 2016;6:34014. doi: 10.1038/srep34014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ficetola GF, Bonardi A, Sindaco R, Padoa-Schioppa E. Estimating patterns of reptile biodiversity in remote regions. J. Biogeogr. 2013;40:1202–1211. doi: 10.1111/jbi.12060. [DOI] [Google Scholar]
- 30.Hosseinzadeh M, Aliabadian M, Rastegar-Pouyani E, Rastegar-Pouyani N. The roles of environmental factors on reptile richness in Iran. Amphib. Reptil. 2014;35:215–225. doi: 10.1163/15685381-00002946. [DOI] [Google Scholar]
- 31.Ficetola GF, Falaschi M, Bonardi A, Padoa-Schioppa E, Sindaco R. Biogeographical structure and endemism pattern in reptiles of the Western Palearctic. Prog. Phys. Geogr. 2018;42:220–236. doi: 10.1177/0309133318765084. [DOI] [Google Scholar]
- 32.Rastegar-Pouyani E, Rastegar-Pouyani N, Kazemi-Noureini S, Joger U, Wink M. Molecular phylogeny of the Eremias persica complex of the Iranian plateau (Reptilia: Lacertidae), based on mtDNA sequences. Zool. J. Linn. Soc. 2010;158:641–660. doi: 10.1111/j.1096-3642.2009.00553.x. [DOI] [Google Scholar]
- 33.Rastegar-Pouyani E, Kazemi-Noureini S, Rastegar-Pouyani N, Joger U, Wink M. Molecular phylogeny and intraspecific differentiation of the Eremias velox complex of the Iranian Plateau and Central Asia (Sauria, Lacertidae) J. Zool. Syst. Evol. 2012;50:220–229. doi: 10.1111/j.1439-0469.2012.00662.x. [DOI] [Google Scholar]
- 34.Ahmadzadeh F, et al. Inferring the effects of past climate fluctuations on the distribution pattern of Iranolacerta (Reptilia, Lacertidae): Evidence from mitochondrial DNA and species distribution models. Zool. Anz. 2013;252:141–148. doi: 10.1016/j.jcz.2012.05.002. [DOI] [Google Scholar]
- 35.Ahmadzadeh F, Carretero MA, Harris DJ, Perera A, Böhme W. A molecular phylogeny of the eastern group of ocellated lizard genus Timon (Sauria: Lacertidae) based on mitochondrial and nuclear DNA sequences. Amphib. Reptil. 2012;33:1–10. doi: 10.1163/156853811X619718. [DOI] [Google Scholar]
- 36.Macey JR. Testing hypotheses for vicariant separation in the agamid lizard Laudakia caucasia from mountain ranges of the Northern Iranian plateau. Mol. Phylogenet. Evol. 2000;14:479–483. doi: 10.1006/mpev.1999.0722. [DOI] [PubMed] [Google Scholar]
- 37.Rajabizadeh M, et al. Alpine-Himalayan orogeny drove correlated morphological, molecular, and ecological diversification in the Persian dwarf snake (Squamata: Serpentes: Eirenis persicus) Zool. J. Linn. Soc. 2016;176:878–913. doi: 10.1111/zoj.12342. [DOI] [Google Scholar]
- 38.Anderson SC. The Lizards of Iran. Oxford: Society for the Study of Amphibians and Reptiles; 1999. [Google Scholar]
- 39.Eskandarzadeh N, et al. Annotated checklist of the endemic Tetrapoda species of Iran. Zoosystema. 2018;40:507–537. doi: 10.5252/zoosystema2018v40a24. [DOI] [Google Scholar]
- 40.Saberi-Pirooz R, et al. Dispersal beyond geographic barriers: a contribution to the phylogeny and demographic history of Pristurus rupestris Blanford, 1874 (Squamata: Sphaerodactylidae) from southern Iran. Zoology. 2019;134:8–15. doi: 10.1016/j.zool.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 41.Ahmadi H, Feiznia S. Quaternary Formations (Aeoretical and Applied Principles in Natural Resources) Tehran: University of Tehran Press; 2006. [Google Scholar]
- 42.Stümpel N, Rajabizadeh M, Avcı A, Wüster W, Joger U. Phylogeny and diversification of mountain vipers (Montivipera, Nilson etal. 2013) triggered by multiple Plio-Pleistocene refugia and high-mountain topography in the Near and Middle East. Mol. Phylogenet. Evol. 2016;101:336–351. doi: 10.1016/j.ympev.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 43.Yousefi M, et al. Upward altitudinal shifts in habitat suitability of mountain vipers since the Last Glacial Maximum. PLoS ONE. 2015;10:e0138087. doi: 10.1371/journal.pone.0138087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rastegar-Pouyani N, Rastegar-Pouyani E, Jawaheri M. Field Guide to the Reptiles of Iran. Kermanshah: Razi University Press; 2007. [Google Scholar]
- 45.Kafash A, Kaboli M, Köhler G, Yousefi M, Asadi A. Ensemble distribution modeling of the Mesopotamian spiny-tailed lizard, Saara loricate (Blanford, 1874), in Iran: an insight into the impact of climate change. Turk. J. Zool. 2016;40:262–271. doi: 10.3906/zoo-1504-10. [DOI] [Google Scholar]
- 46.Faizi H, et al. A new species of Eumeces Wiegmann 1834 (Sauria: Scincidae) from Iran. Zootaxa. 2017;4320:289–304. doi: 10.11646/zootaxa.4320.2.5. [DOI] [Google Scholar]
- 47.Torki F. Description of a new species of Lytorhynchus (Squamata: Colubridae) from Iran. Zool. Middle East. 2017;63:109–116. doi: 10.1080/09397140.2017.1299319. [DOI] [Google Scholar]
- 48.Fattahi R, et al. Modelling the potential distribution of the Bridled Skink, Trachylepis vittata (Olivier, 1804), in the Middle East. Zool. Middle East. 2014;60:208–216. doi: 10.1080/09397140.2014.944428. [DOI] [Google Scholar]
- 49.Kafash A, et al. Phrynocephalus scutellatus (Olivier, 1807) in Iranian Plateau: The degree of niche overlap depends on the phylogenetic distance. Zool. Middle East. 2018;64:47–54. doi: 10.1080/09397140.2017.1401309. [DOI] [Google Scholar]
- 50.Rodríguez MÁ, Belmontes JA, Hawkins BA. Energy, water and large-scale patterns of reptile and amphibian species richness in Europe. Acta Oecol. 2005;28:65–70. doi: 10.1016/j.actao.2005.02.006. [DOI] [Google Scholar]
- 51.Angilletta MJ, Niewiarowski PH, Navas CA. The evolution of thermal physiology in ectotherms. J. Therm. Biol. 2002;27:249–268. doi: 10.1016/S0306-4565(01)00094-8. [DOI] [Google Scholar]
- 52.Currie DJ. Energy and large-scale patterns of animal and plant-species richness. Am Nat. 1991;137:27–49. doi: 10.1086/285144. [DOI] [Google Scholar]
- 53.Whittaker RJ, Nogues-Bravo D, Araujo MB. Geographical gradients of species richness: a test of the water-energy conjecture of Hawkins et al. (2003) using European data for five taxa. Glob. Ecol. Biogeogr. 2007;16:76–89. doi: 10.1111/j.1466-8238.2006.00268.x. [DOI] [Google Scholar]
- 54.Iverson JB. Species richness maps of the freshwater and terrestrial turtles of the world. Smithsonian Herpet. Inform. Serv. 1992;88:1–18. [Google Scholar]
- 55.Schall JJ, Pianka ER. Geographical trends in number of species. Science. 1978;201:679–686. doi: 10.1126/science.201.4357.679. [DOI] [PubMed] [Google Scholar]
- 56.Vidan E, et al. The Eurasian hot nightlife: environmental forces associated with nocturnality in lizards. Glob. Ecol. Biogeogr. 2017;26:1316–1325. doi: 10.1111/geb.12643. [DOI] [Google Scholar]
- 57.Hewitt GM. Genetic consequences of climatic oscillations in the Quaternary. Philos. Trans. R. Soc. Lond. B. 2004;359:183–195. doi: 10.1098/rstb.2003.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rajabizadeh M, et al. Geographic variation, distribution and habitat of Natrix tessellata in Iran. Mertensiella. 2011;18:414–429. [Google Scholar]
- 59.Veith M, Schmidtler JF, Kosuch J, Baran I, Seitz A. Palaeoclimatic changes explain Anatolian mountain frog evolution: a test for alternating vicariance and dispersal events. Mol. Ecol. 2003;12:185–199. doi: 10.1046/j.1365-294X.2003.01714.x. [DOI] [PubMed] [Google Scholar]
- 60.Farasat H, Akmali V, Sharifi M. Population Genetic Structure of the Endangered Kaiser’s Mountain Newt, Neurergus kaiseri (Amphibia: Salamandridae) PLoS ONE. 2016;11:e0149596. doi: 10.1371/journal.pone.0149596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perktaş U, Barrowclough GF, Groth JG. Phylogeography and species limits in the green woodpecker complex (Aves: Picidae): multiple Pleistocene refugia and range expansion across Europe and the Near East. Biol. J. Linn. Soc. 2011;104:710–723. doi: 10.1111/j.1095-8312.2011.01750.x. [DOI] [Google Scholar]
- 62.Perktas U, Quintero E. A wide geographical survey of mitochondrial DNA variation in the great spotted woodpecker complex, Dendrocopos major (Aves: Picidae) Biol. J. Linn. Soc. 2013;108:173–188. doi: 10.1111/j.1095-8312.2012.02003.x. [DOI] [Google Scholar]
- 63.Haddadian-Shad H, Darvish J, Rastegar-Pouyani E, Mahmoudi A. Subspecies differentiation of the house mouse Mus musculus Linnaeus, 1758 in the center and east of the Iranian plateau and Afghanistan. Mammalia. 2016;81:1–22. [Google Scholar]
- 64.Dianat M, Darvish J, Cornette R, Aliabadian M, Nicolas V. Evolutionary history of the Persian Jird, Meriones persicus, based on genetics, species distribution modelling and morphometric data. J. Zool. Syst. Evol. 2016;55:29–45. doi: 10.1111/jzs.12145. [DOI] [Google Scholar]
- 65.Ashrafzadeha MR, Rezaei HR, Khalilipourc O, Kuszad S. Genetic relationships of wild boars highlight the importance of Southern Iran in forming a comprehensive picture of the species’ phylogeography. Mamm. Biol. 2018;92:21–29. doi: 10.1016/j.mambio.2018.04.001. [DOI] [Google Scholar]
- 66.Wiens JJ, Graham CH. Niche conservatism: integrating evolution, ecology, and conservation biology. Annu. Rev. Ecol. Evol. Syst. 2005;36:519–539. doi: 10.1146/annurev.ecolsys.36.102803.095431. [DOI] [Google Scholar]
- 67.Wiens JJ, Donoghue MJ. Historical biogeography, ecology, and species richness. Trends Ecol. Evol. 2004;19:639–644. doi: 10.1016/j.tree.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 68.Ahmadzadeh F, et al. The evolutionary history of two lizards (Squamata: Lacertidae) is linked to the geological development of Iran. Zool. Anz. 2017;270:49–56. doi: 10.1016/j.jcz.2017.09.003. [DOI] [Google Scholar]
- 69.Nilson G, Rastegar-Pouyani N, Rastegar-Pouyani E, Andrén C. Lacertas of South and Central Zagros Mountains, Iran, with descriptions of two new taxa. Russ J. Herpetol. 2003;10:11–24. [Google Scholar]
- 70.Šmíd J, Frynta D. Genetic variability of Mesalina watsonana (Reptilia: Lacertidae) on the Iranian plateau and its phylogenetic and biogeographic affinities as inferred from mtDNA sequences. Acta. Herpetol. 2012;7:139–153. [Google Scholar]
- 71.Yusefi GH, Faizolahi K, Darvish J, Safi K, Brito JC. The species diversity, distribution, and conservation status of the terrestrial mammals of Iran. J. Mammal. 2019;100:55–71. doi: 10.1093/jmammal/gyz002. [DOI] [Google Scholar]
- 72.Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- 73.Isaac NJB, Redding DW, Meredith HM, Safi K. Phylogenetically-Informed Priorities for Amphibian Conservation. PLoS ONE. 2012;7:e43912. doi: 10.1371/journal.pone.0043912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hagen O, et al. Mountain building, climate cooling and the richness of cold-adapted plants in the Northern Hemisphere. J. Biogeogr. 2019;46:1792–1807. doi: 10.1111/jbi.13653. [DOI] [Google Scholar]
- 75.Noroozi J, Moser D, Essl F. Diversity, distribution, ecology and description rates of alpine endemic plant species from Iranian mountains. Alp. Bot. 2016;126:1–9. doi: 10.1007/s00035-015-0160-4. [DOI] [Google Scholar]
- 76.Noroozi J, Akhani H, Breckle SW. Biodiversity and phytogeography of the alpine flora of Iran. Biodivers. Conserv. 2008;17:493–521. doi: 10.1007/s10531-007-9246-7. [DOI] [Google Scholar]
- 77.Ahmadzadeh F, et al. Cryptic speciation patterns in Iranian rock lizards uncovered by Integrative Taxonomy. PLoS ONE. 2013;8:e80563. doi: 10.1371/journal.pone.0080563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Darvishzadeh A. Geology of Iran. Tehran: Amirkabir Publication; 2003. [Google Scholar]
- 79.Rögl F. Mediterranean and Paratethys. Facts and hypotheses of an Oligocene to Miocene paleogeography (short overview) Geol. Carpath. 1999;50:339–349. [Google Scholar]
- 80.Okay AI, Zattin M, Cavazza W. Apatite fission-track data for the Miocene Arabia-Eurasia collision. Geology. 2010;38:35–38. doi: 10.1130/G30234.1. [DOI] [Google Scholar]
- 81.Girdler RW. The evolution of the Gulf of Aden and Red Sea in space and time. Deep-Sea Res. 1984;316:747–762. doi: 10.1016/0198-0149(84)90039-6. [DOI] [Google Scholar]
- 82.Kehl M. Quaternary climate change in Iran—the state of knowledge. Erdkunde. 2009;63:1–17. doi: 10.3112/erdkunde.2009.01.01. [DOI] [Google Scholar]
- 83.Ehlers J, Gibbard PL. Quaternary Glaciations Extent and Chronology: Part I: Europe. Amsterdam: Elsevier; 2004. [Google Scholar]
- 84.Kaufman DS, et al. Holocene thermal maximum in the western Arctic (0–180 W) Quat. Sci. Rev. 2004;23:529–560. doi: 10.1016/j.quascirev.2003.09.007. [DOI] [Google Scholar]
- 85.Nasrabadi R, Rastegar-Pouyani N, Rastegar-Pouyani E, Gharzi A. A revised key to the lizards of Iran (Reptilia: Squamata: Lacertilia) Zootaxa. 2017;4227:431–443. doi: 10.11646/zootaxa.4227.3.9. [DOI] [PubMed] [Google Scholar]
- 86.Hijmans, R.J., Guarino, L. & Mathur, P. “DIVA-GIS.” https://www.diva-gis.org/documentation (2012).
- 87.Kafash A, Ashrafi S, Ohler A, Schmidt BR. Environmental predictors for the distribution of the Caspian Green Lizard, Lacerta strigata Eichwald, 1831 along elevational gradients of the Elburz Mountains in northern Iran. Turk. J. Zool. 2019;43:106–113. doi: 10.3906/zoo-1808-15. [DOI] [Google Scholar]
- 88.Descombes P, Leprieur F, Albouy C, Heine C, Pellissier L. Spatial imprints of plate tectonics on extant richness of terrestrial vertebrates. J. Biogeogr. 2017;44:1185–1197. doi: 10.1111/jbi.12959. [DOI] [Google Scholar]
- 89.Jarvis, A., Reuter, H. I., Nelson, A. & Guevara, E. Hole-Filled SRTM for the Globe Version 4. Available from the CGIAR-CSI SRTM 90m Database. https://srtm.csi.cgiar.org (2008).
- 90.Loarie SR, Duffy PB, Hamilton H, Asner GP, Field CB, Ackerly DD. The velocity of climate change. Nature. 2009;462:1052–1055. doi: 10.1038/nature08649. [DOI] [PubMed] [Google Scholar]
- 91.Grünig M, Beerli N, Ballesteros-Mejia L, Kitching IJ, Beck J. How climatic variability is linked to the spatial distribution of range sizes: Seasonality versus climate change velocity in sphingid moths. J. Biogeogr. 2017;44:2441–2450. doi: 10.1111/jbi.13051. [DOI] [Google Scholar]
- 92.Soultan A, Wikelski M, Safi K. Classifying biogeographic realms of the endemic fauna in the Afro-Arabian region. Ecol Evol. 2020;10:8669–8680. doi: 10.1002/ece3.6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. [Google Scholar]
- 94.Hijmans, R.J. raster: Geographic Data Analysis and Modeling. R package version 3.3-7 (2020).
- 95.van Etten J. R package gdistance: Distances and routes on geographical grids. J. Stat. Softw. 2017;76:1–21. [Google Scholar]
- 96.Bengtsson, H. matrixStats: Functions That Apply to Rows and Columns of Matrices (and to Vectors). R package version 0.56.0. (2020).
- 97.VanDerWal, J. et al. SDMTools: Species Distribution Modelling Tools: Tools for Processing Data Associated with Species Distribution Modelling Exercises. R package ver. 1.1‐221.1. (2019).
- 98.Alavi M. Tectonics of the Zagros orogenic belt of Iran: New data and interpretations. Tectonophysics. 1994;229:211–238. doi: 10.1016/0040-1951(94)90030-2. [DOI] [Google Scholar]
- 99.Agard P, Omrani J, Jolivet L, Mouthereau F. Convergence history across Zagros (Iran): constraints from collisional an earlier deformation. Int. J. Earth Sci. 2005;94:401–419. doi: 10.1007/s00531-005-0481-4. [DOI] [Google Scholar]
- 100.Monthereau F. Timing of uplift in the Zagros belt/Iranian plateau and accommodation of late Cenozoic Arabia-Eurasia convergence. Geol. Mag. 2011;148:726–738. doi: 10.1017/S0016756811000306. [DOI] [Google Scholar]
- 101.Rezaeian M, Carter A, Hovius N, Allen MB. Cenozoic exhumation history of the Alborz Mountains, Iran: new constraints from low-temperature chronometry. Tectonics. 2012;31:TC004. doi: 10.1029/2011TC002974. [DOI] [Google Scholar]
- 102.Madanipour S, Ehlers TA, Yassaghi A, Enkelmann E. Accelerated middle Miocene exhumation of the Talesh Mountains constrained by U-Th/He thermochronometry: evidence for the Arabia-Eurasia collision in the NW Iranian Plateau. Tectonics. 2017;36:1538–1561. doi: 10.1002/2016TC004291. [DOI] [Google Scholar]
- 103.QGIS Development Team. QGIS Geographic Information System (version 3.4.1). Software (2018).
- 104.Quinn GP, Keough MJ. Experimental Designs and Data Analysis for Biologists. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- 105.Naimi, B. Uncertainty Analysis for Species Distribution Models. R package version 1.1-15 (2015).
- 106.Fick SE, Hijmans RJ. Worldclim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017;37:4302–4315. doi: 10.1002/joc.5086. [DOI] [Google Scholar]
- 107.Broxton PD, Zeng X, Scheftic W, Troch PA. A MODIS-based global 1-km maximum green vegetation fraction dataset. J. Appl. Meteorol. Clim. 2014;53:1996–2004. doi: 10.1175/JAMC-D-13-0356.1. [DOI] [Google Scholar]
- 108.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005;25:1965–1978. doi: 10.1002/joc.1276. [DOI] [Google Scholar]
- 109.Albouy C, et al. The marine fish food web is globally connected. Nat. Ecol. Evol. 2019;3:1153–1161. doi: 10.1038/s41559-019-0950-y. [DOI] [PubMed] [Google Scholar]
- 110.Olson D, et al. Terrestrial ecoregions of the world: a new map of life on Earth: a new global map of terrestrial ecoregions provides an innovative tool for conserving biodiversity. Bioscience. 2001;51:933–938. doi: 10.1641/0006-3568(2001)051[0933:TEOTWA]2.0.CO;2. [DOI] [Google Scholar]
- 111.Di Cola V, et al. ecospat: an R package to support spatial analyses and modeling of species niches and distributions. Ecography. 2017;40:774–787. doi: 10.1111/ecog.02671. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials, or the references cited here within.