Abstract
There is a continuous need for designing new and improved synthetic methods aiming at minimizing reaction steps while increasing molecular complexity. In this respect, catalytic, one-pot cascade methodologies constitute an ideal tool for the construction of complex molecules with high chemo-, regio-, and stereoselectivity. Herein, we describe two general and efficient cascade procedures for the synthesis of spiro-fused heterocylces. This transformation combines selective nucleophilic substitution (SN2′), palladium-catalyzed Heck and C–H activation reactions in a cascade manner. The use of allylic ammonium salts and specific Pd catalysts are key to the success of the transformations. The synthetic utility of these methodologies is showcased by the preparation of 48 spiro-fused dihydrobenzofuranes and indolines including a variety of fluorinated derivatives.
Subject terms: Catalytic mechanisms, Homogeneous catalysis, Synthetic chemistry methodology
Synthetic methods aiming at minimizing reaction steps while increasing molecular complexity are highly sought after by organic chemists. Here, the authors report two cascade procedures combining nucleophilic substitution, palladium-catalyzed Heck and C–H activation reactions for the synthesis of spiro-fused heterocycles.
Introduction
The development of novel chemical transformations increasing molecular complexity enables significant innovation potential in life and material sciences. In this respect, catalytic cascade or domino processes offer strong impetus for new methodology developments1–3. Compared to traditional consecutive procedures, they permit several practical advantages: In addition to improved step-economy, waste generation from multiple iterations of reaction, workup, and purification procedures are minimized. Consequently, diverse and complex organic molecules can be assembled not only in a faster, but also more sustainable way.
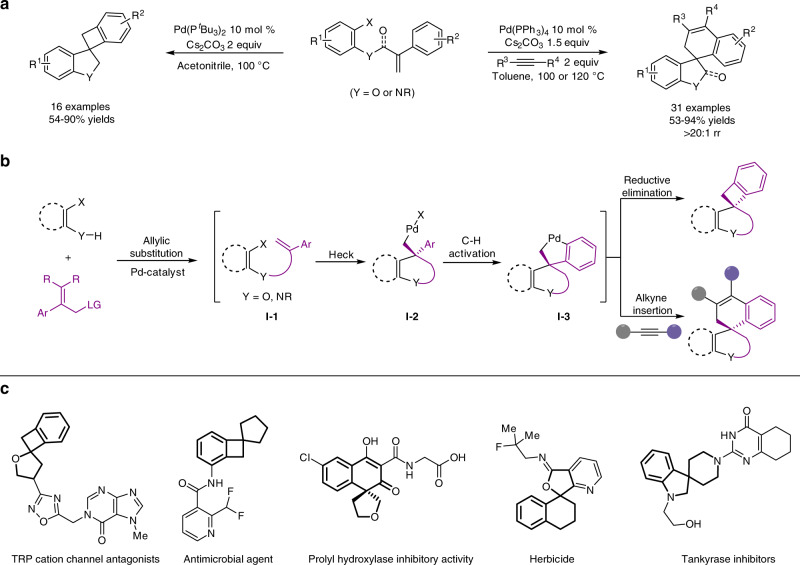
Since their discovery in the 1970s and 80s4–8, palladium-catalyzed C–C bond forming reactions have become the most popular homogeneous catalytic processes in organic chemistry and industrial fine chemical synthesis9–12. Their ability to form (stereo)selectively carbon-carbon bonds under mild conditions made them “a true power tool for organic synthesis”13. Specifically, the intramolecular Heck reaction provides an entry to useful palladium complexes with quaternary carbon centers as intermediates (Fig. 1b, I–2), which can be further valorized to a multitude of valuable building blocks14–18. Notably, the combination of this reaction with C–H activation processes has also been studied, giving access to structurally unique spiro compounds19–31, which possess interesting biological activities (Fig. 1c)32–38. However, the necessity of pre-synthesized starting materials in the existing methods limits the full exploitation of this elegant concept and is often tedious. In our quest for the development of new cascade methodologies, we had the idea to provide a more facile entry to this class of compounds by combining three (or four) palladium-catalyzed coupling processes, namely Tsuji–Trost and Heck reactions followed by selective C–H activation (and alkyne insertion) to assemble complex organic molecules from easily available substrates (Fig. 1).
Fig. 1. Concepts.
a Previous work: palladium-catalyzed intramolecular cyclization for the synthesis of spiroheterocycles. b This work: palladium-catalyzed allylic substitution/Heck/C–H activation(/alkyne) cascade processes. c Selected examples of related bio-active spiro compounds.
Herein, we describe our recent efforts to establish a palladium-catalyzed allylic substitution/Heck/C–H activation(/alkyne) cascade processes for the synthesis of spiro-fused heterocycles. Key challenges of such processes are obviously the compatibility of the well-matched reactant partners39, the required conditions of the individual reactions, the development of a general catalyst system able to promote all three (or four) transformations efficiently, and to achieve the needed high chemo-selectivity, regio-selectivity, and stereoselectivity throughout all elementary steps.
Results
Reaction development
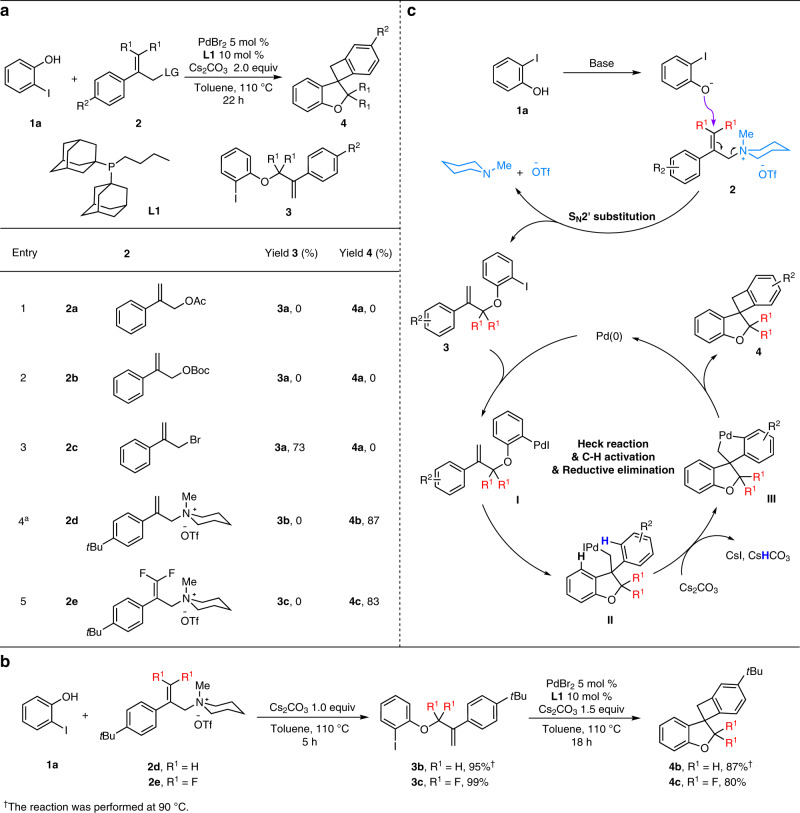
Recently, Lautens, Schoenebeck and co-workers reported the synthesis of spiro-fused heterocycles through an intramolecular Heck/C–H activation sequence using specific alkene-tethered aryl iodides (Fig. 1a)40–42. Regarding the starting materials, those substrates might be preferably prepared through an initial palladium-catalyzed Tsuji-Trost allylation of 2-halophenols, which would provide a more efficient and step-economic way43–45. Following this initial idea, we investigated the coupling of 2-iodophenol (1a) with 2-phenylallyl acetate (2a) in the presence of PdBr2/L1 (Fig. 2a) and various other palladium catalysts (for details see Supplementary Information, Supplementary Table 1). Unfortunately, in no case the desired spiro-fused product 4a was observed and no conversion took place. Similar results were obtained when the tert-butyl (2-phenylallyl) carbonate (2b) was used instead of 2a. When more reactive 2-phenylallyl bromide 2c was introduced, only the alkene-tethered aryl iodide 3a was isolated in 73% yield instead of product 4a. To improve the reactivity of the starting material further on, other allylic leaving groups were considered. In this respect, allylic ammonium salts, which have been largely neglected in intermolecular palladium-catalyzed allylic substitutions, attracted our attention39,46,47. This class of compounds are in general highly stable and can be conveniently prepared from a variety of amines. Surprisingly, testing 2d in the presence of the PdBr2/L1 catalyst, the desired cascade process took place and product 4b was obtained in 87% isolated yield! This means that each individual step proceeds with an efficiency of at least 95%. At this point, it should be mentioned that allylic ammonium salts are also known to undergo direct SN2-substitution or SN2’-substitution reactions under basic conditions48–50. To understand whether the first reaction step is really palladium-catalyzed, 2d was treated with 1a in the presence of 1 equiv. of base. Interestingly, the allyl aryl ether 3b was obtained in high yield (95%). Subsequent reaction in the presence of our regular palladium catalyst led to full consumption of 3b, providing the desired spiro-benzocyclobutane 4b in 87% yield (Fig. 2b). Obviously, applying substrate 2d does not allow to distinguish between SN2-mechanism and SN2’-mechanism in the first reaction step due to its symmetry. Based on the actual interest in fluorinated building blocks51–53, the gem-difluorinated allylic ammonium salt 2e39 was reacted with 2-iodophenol, which gave product 3c in 99% yield and excellent regioselectivity. Again, the following palladium-catalyzed steps took place smoothly and provided 4c in high yield (80%). Similarly, the direct conversion of 2e proceeded efficiently to give 4c in 83% isolated yield (Fig. 2a, entry 5). It should be noted that related fluorinated heterocycles in general cannot be easily prepared54 and that to the best of our knowledge no example of such spiro compounds has been reported yet. Apart from 2e, other related ammonium salts 2f–2i underwent similar coupling processes to provide the desired product 4c in slight lower yield (62–75%) (for details see Supplementary Information, Supplementary Table 2).
Fig. 2. Constructing molecular complexity: spiro-fused heterocycles from simple starting materials.
a Preliminary results show the importance of ammonium salts as the partners of the nucleophile. b Control experiments shown that the allylic aryl ether 3 was formed in situ as important intermediate during the cascade procedure. c A SN2’ substitution process was proposed for the first reaction step, followed by sequence palladium-catalyzed Heck reaction/C–H activation/Pd reductive elimination to provide the target product 4.
To obtain optimal results, an extensive evaluation of the reaction conditions of the model systems was performed (for details see Supplementary Information, Supplementary Tables 1–9) and revealed three significant points: (1) The catalyst system is crucial in this cascade process and only in the presence of sterically hindered and electron-rich diadamantyl phosphines such as L1, the desired product was obtained in high yield. (2) Cs2CO3 and toluene were independently identified as the most effective base and solvent, which nearly doubled the product yield compared to other common bases and solvents. (3) In addition, the concentrations of substrates are decisive. Using an equimolar amount of both substrates led to the best result while an excess of either ammonium salt or aryl halide considerably decreased the yield of 4. Based on all these observations, a plausible mechanism for the formation of benzocyclobutane derivative 4 is proposed in Fig. 2c: Initially, 2-iodophenol 1 and ammonium salt 2 underwent a base mediated SN2’ allylic substitution in a highly regioselective manner. To further confirm the SN2’ route, deuterium substituted ammonium salt was tested, details see Supplementary Information, Supplementary Fig. 1. Next, intramolecular palladium-catalyzed Heck reaction of the in situ generated compound 3 followed by site-selective C–H activation forms the spiropalladacycle III. Final reductive elimination regenerates the palladium species and produces the desired product40. Noteworthily, this novel cascade reaction is a rare example of a domino process involving SN2´ substitution with subsequent metal-catalyzed transformations55,56.
Scope for the formation of spiro-fused benzocyclobutene derivatives
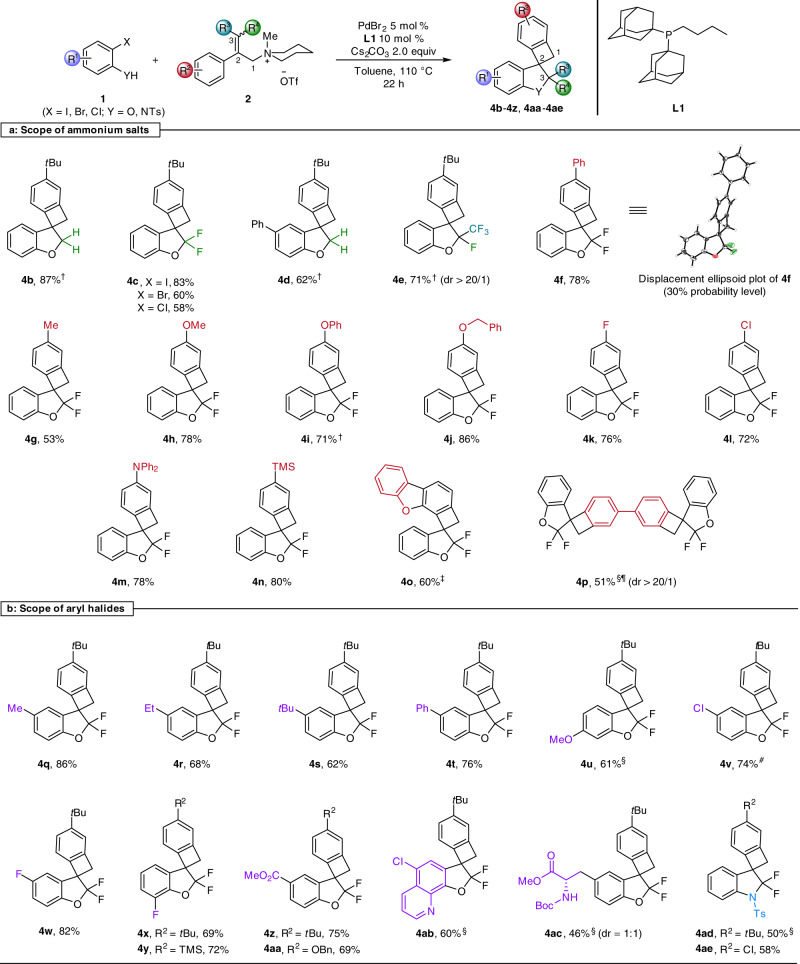
With the optimized reaction conditions in hand, the general feasibility of this approach was examined. As shown in Fig. 3, allylic ammonium salts with different substituents in 3-position including H, F, CF3, directly afforded 4b–4e in all cases in good to high isolated yields. For disubstituted substrate 2j with –F and –CF3 substituents in 3-position, high diastereoselectivity for two adjacent quaternary carbon centers was obtained (4e, 71%). Next, the reaction of gem-difluorinated allylic ammonium salts 2k–2u with aryl halides 1a–1o (for details see Supplementary Information, Supplementary Fig. 2) was investigated. Most of the ammonium salts were conveniently obtained from commercially available phenylboronic acid and vinyl bromides via Suzuki reaction, base-mediated amination, and final N-methylation39. With regard to the cascade reaction, both electron-donating groups including alkyl, aryl, alkoxy and aryloxy and electron-withdrawing groups including fluoro and chloro were perfectly compatible with the conditions, and the corresponding products 4f–4l were obtained in 53 − 86% yield. The molecular structure of these highly strained 5,4-spirocycles was unambiguously confirmed by X-ray crystal structure analysis of 4f. Both diphenylamino-substituted and trimethylsilyl-substituted spiro compounds 4m and 4n were successfully formed in high yield. Furthermore, dibenzofuran-derived ammonium salt underwent the cascade process, leading to the construction of the heterocycle-embedded tetracyclic framework 4o in 60% yield. Gratifyingly, the more complex derivative 4p containing two spiro-fused benzocyclobutanes was smoothly generated in 51% yield via a consecutive two-fold cascade process using the corresponding bis-ammonium salt as the reagent.
Fig. 3. Palladium-catalyzed cascade reaction of ammonium salts with aryl halides.
Standard reaction conditions: 1 (0.2 mmol), 2 (0.2 mmol), Cs2CO3 (0.4 mmol), PdBr2 (0.01 mmol), L1 (0.02 mmol), toluene (2.5 mL), the reaction mixture was performed at 110 °C under argon atmosphere for 22 h, isolated yield, the diastereoselectivity of 4e, 4p, and 4ac was determined by crude 19F NMR analyses. †The reaction mixture was performed at 90 °C. ‡1 mol% PdBr2 and 2 mol% L1 were used. §10 mol% PdBr2 and 20 mol% L1 were used. ¶1 (0.2 mmol), 2 (0.1 mmol) were used. #2 mol% PdBr2 and 4 mol% L1 were used.
Next, we explored the scope of our methodology with respect to the aryl halide coupling partner. In addition to 2-iodophenols 1a-1l, 2-bromophenol 1m, 2-chlorophenol 1n, and 2-iodoaniline 1o were also investigated. The latter case highlights the possibility to construct 3-spiro-indolines, specifically 2-fluorinated indolines (4ad and 4ae), which are of interest as natural products and pharmaceutical molecules57,58. As depicted in Fig. 3, several different aryl halides gave the expected tetracyclic products under the standard conditions. Interestingly, considering the three-step cascade, these transformations proceeded in good to excellent yields with either electron-rich or electron-deficient substituents. Notably, substrates containing heteroarenes, such as the quinoline derivative 1k, provided the N,O-fused heterocycle 4ab in 60% yield. Moreover, the L-tyrosine derived product 4ac was obtained by a concise cascade transformation (dr = 1:1).
Three-component spirocyclization reaction
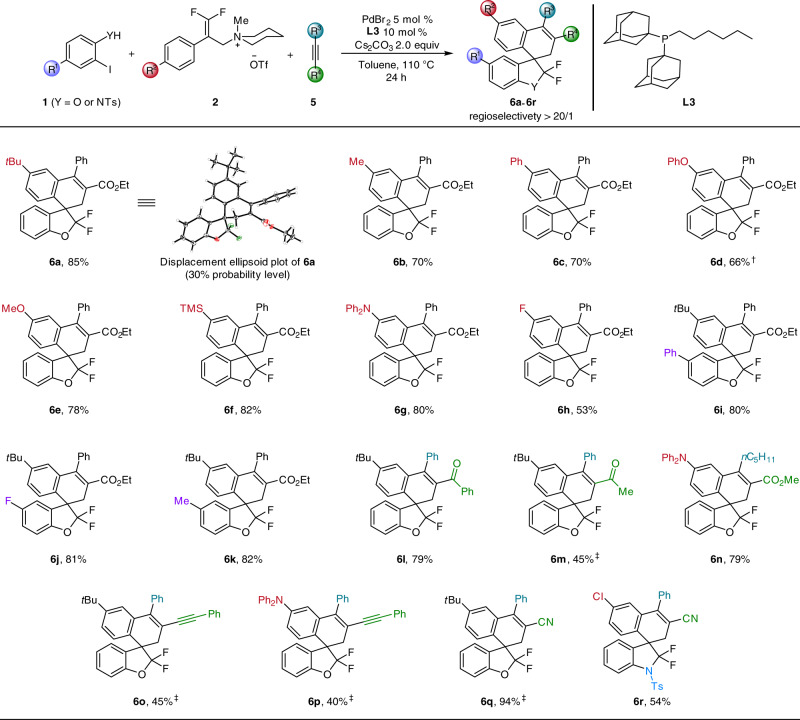
Considering the versatility of the in-situ-generated palladacycles III16,26,27, subsequent functionalization including carbene and alkyne insertion should allow for the efficient construction of other classes of novel spiro compounds29,30,41,42. To demonstrate this synthetic potential, we performed the reaction of 1a and 2e with two equiv. of an additional unsymmetrical alkyne 5a (ethyl 3-phenyl-propynoate). Indeed, the envisioned cascade process combining SN2’ substitution, palladium-catalyzed Heck/C–H activation and final alkyne insertion provided in a straightforward manner only one regioisomer of the respective 6,5-spirocycles 6 (regioselectivity: >20:1). Under standard conditions, the desired product 6a was obtained in 73% yield; however, in this case the highly reactive palladacycle III also underwent minor reductive elimination and the 5,4-spirocycle 4c was detected in 14% yield. Pleasantly, increasing substrate concentration in the presence of the extended ligand L3 provided exclusively 6a in high yield (85% isolated yield; for a brief evaluation of reaction conditions see Supplementary Information, Supplementary Table 10).
The generality of this second three-component cascade procedure is shown by variation of five aryl iodides, nine ammonium salts and six alkynes (Fig. 4). In all cases, the domino reaction proceeded smoothly with valuable substituents and functional groups, including alkoxy, aryloxy, halide, silyl, and amino, giving the corresponding products 6a–6h in good to high yields with excellent regioselectivities. The molecular structure of 6a was confirmed by X-ray crystallography. Substituents on the phenyl ring of aryl iodide displayed only a minor influence on the reactivity and provided 6i–6k in high yields. Notably, various unsymmetrical alkynes with different substituents on the triple bond afforded 6l–6q with excellent degrees of both chemoselectivities and regioselectivities. For example, internal alkynes bearing −COPh, −COCH3, and −CO2Me substituents gave the corresponding products 6l–6n in 79%, 45 and 79% isolated yield, respectively. It is worthy to note that 3-phenyl-2-propynenitrile and 1,3-diynes were compatible in this transformation, affording 6o–6q in 40–94% isolated yield. Finally, the construction of 3-spiro-indoline 6r was also achieved in 54% yield.
Fig. 4. Palladium-catalyzed three-component cascade spriocyclizations.
Standard reaction conditions: 1 (0.2 mmol), 2 (0.2 mmol), 5 (0.4 mmol), Cs2CO3 (0.4 mmol), PdBr2 (0.01 mmol), L3 (0.02 mmol), toluene (1.0 mL), the reaction mixture was stirred at 110 °C under argon atmosphere for 24 h, isolated yield, the regioselectivity of 6 was determined by crude 19F NMR analyses. †2 mol% PdBr2 and 4 mol% L3 were used. ‡10 mol% PdBr2 and 20 mol% L3 were used.
Discussion
In summary, we have developed two efficient cascade processes involving allylic substitution (via SN2’-mechanism), palladium-catalyzed Heck, remote C–H activation, and reductive elimination or alkyne insertion for the straightforward synthesis of 5,4-spiroheterocycles and 6,5-spiroheterocycles in good to high yields with excellent selectivities. Crucial for the success of these transformations is the use of specifically activated allylic substrates (ammonium salts) in combination with special PdBr2/AlkylPAd2 catalytic systems. Under optimal conditions diverse (fluorinated) spiro-dihydrobenzofurans and spiro-indolines are achieved in an unprecedented fast and step-economic way, without need for purification of intermediates. We believe these methodologies demonstrate the potential of catalytic cascade processes for a straightforward increase of molecular complexity of simple and easily available aryl halides.
Methods
General procedure for the preparation of spiro-fused benzocyclobutanes 4
To a 25 ml oven-dried pressure tube equipped with a magnetic stir bar were added 2-halophenol or aniline 1 (0.2 mmol), ammonium salt 2 (0.2 mmol), Cs2CO3 (130 mg, 0.4 mmol), PdBr2 (2.7 mg, 0.01 mmol), L1 (7.2 mg, 0.02 mmol), and then degassed toluene (2.5 mL) was introduced under argon atmosphere. The sealed pressure tube was heated and stirred at 110 °C for 22 h. The reaction mixture was allowed cooling to room temperature, diluted with ethyl acetate (10 ml), and filtered through a short pad of celite eluting with ethyl acetate (3 × 10 ml). After evaporation, the residue was purified by chromatography on basic aluminum oxide (It is worthy to note that the 2-fluorinated product can only be separated without decomposition using basic aluminum oxide) to afford the desired product 4.
General procedure for the preparation of spiro-fused dihydronaphthalenes 6
To a 25 ml oven-dried pressure tube equipped with a magnetic stir bar were added 2-halophenol or aniline 1 (0.2 mmol), ammonium salt 2 (0.2 mmol), alkyne 5 (0.4 mmol), Cs2CO3 (130 mg, 0.4 mmol), PdBr2 (2.7 mg, 0.01 mmol), L3 (7.7 mg, 0.02 mmol), and then degassed toluene (1 ml) was introduced under argon atmosphere. The sealed pressure tube was heated and stirred at 110 °C for 24 h. The reaction mixture was allowed cooling to room temperature, diluted with ethyl acetate (10 ml), and filtered through a short pad of celite eluting with ethyl acetate (3 × 10 ml). After evaporation, the residue was purified by chromatography on basic aluminum oxide (It is worthy to note that the 2-fluorinated product can only be separated without decomposition using basic aluminum oxide) to afford the desired product 6.
Supplementary information
Acknowledgements
We are grateful for financial support from the State of Mecklenburg-Western Pomerania and the Federal State of Germany (BMBF). We also thank the analytic department (LIKAT) for their kind support.
Author contributions
M.B. and F.Y. conceived and designed the experiments. F.Y. and Y.G. performed the experiments and analyzed the data. A.S. performed the X-ray analysis. H.N. participated in the discussions and supported the project. M.B. and F.Y. prepared the manuscript with feedback from all authors.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The authors declare that all the data supporting this study, including the experimental details, data analysis, and spectra for all unknow compounds, see Supplementary Files. All data underlying the findings of this work are available from the corresponding author upon reasonable request. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 2006609 (4f) and 2006610 (6a). These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Fei Ye, Yao Ge.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-020-19110-3.
References
- 1.Wasilke J, Obrey SJ, Baker RT, Bazan GC. Concurrent tandem catalysis. Chem. Rev. 2005;105:1001–1020. doi: 10.1021/cr020018n. [DOI] [PubMed] [Google Scholar]
- 2.Fogg DE, Dos Santos EN. Tandem catalysis: a taxonomy and illustrative review. Coord. Chem. Rev. 2004;248:2365–2379. doi: 10.1016/j.ccr.2004.05.012. [DOI] [Google Scholar]
- 3.Parsons PJ, Penkett CS, Shell AJ. Tandem reactions in organic synthesis: novel strategies for natural product elaboration and the development of new synthetic methodology. Chem. Rev. 1996;96:195–206. doi: 10.1021/cr950023+. [DOI] [PubMed] [Google Scholar]
- 4.Heck RF. Palladium-catalyzed reactions of organic halides with olefins. Acc. Chem. Res. 1979;12:146–151. doi: 10.1021/ar50136a006. [DOI] [Google Scholar]
- 5.Negishi E. Palladium- or nickel-catalyzed cross coupling. A new selective method for carbon-carbon bond formation. Acc. Chem. Res. 1982;15:340–348. doi: 10.1021/ar00083a001. [DOI] [Google Scholar]
- 6.Stille JK. The palladium-catalyzed cross-coupling reactions of organotin reagents with organic electrophiles. Angew. Chem. Int. Ed. 1986;25:508–524. doi: 10.1002/anie.198605081. [DOI] [Google Scholar]
- 7.Miyaura N, Suzuki A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995;95:2457–2483. doi: 10.1021/cr00039a007. [DOI] [Google Scholar]
- 8.Trost BM. New rules of selectivity: allylic alkylations catalyzed by palladium. Acc. Chem. Res. 1995;13:385–393. doi: 10.1021/ar50155a001. [DOI] [Google Scholar]
- 9.Wu XF, Neumann H, Beller M. Palladium-catalyzed carbonylative coupling reactions between Ar–X and carbon nucleophiles. Chem. Soc. Rev. 2011;40:4986–5009. doi: 10.1039/c1cs15109f. [DOI] [PubMed] [Google Scholar]
- 10.Biffis A, Centomo P, Del Zotto A, Zecca M. Pd metal catalysts for cross-couplings and related reactions in the 21st century: a critical review. Chem. Rev. 2018;118:2249–2295. doi: 10.1021/acs.chemrev.7b00443. [DOI] [PubMed] [Google Scholar]
- 11.Brennführer A, Neumann H, Beller M. Palladium-catalyzed carbonylation reactions of aryl halides and related compounds. Angew. Chem. Int. Ed. 2009;48:4114–4133. doi: 10.1002/anie.200900013. [DOI] [PubMed] [Google Scholar]
- 12.Johansson Seechurn CC, Kitching MO, Colacot TJ, Snieckus V. Palladium-catalyzed cross-coupling: a historical contextual perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed. 2012;51:5062–5085. doi: 10.1002/anie.201107017. [DOI] [PubMed] [Google Scholar]
- 13.Nicolaou KC, Sorensen EJ. Classics in Total Synthesis. Weinheim: VCH; 1996. p. 566. [Google Scholar]
- 14.Jones DJ, Lautens M, Mcglacken GP. The emergence of Pd-mediated reversible oxidative addition in cross coupling, carbohalogenation and carbonylation reactions. Nat. Catal. 2019;2:843–851. doi: 10.1038/s41929-019-0361-0. [DOI] [Google Scholar]
- 15.Nicolaou KC, Bulger PG, Sarlah D. Palladium-catalyzed cross-coupling reactions in total synthesis. Angew. Chem. Int. Ed. 2005;44:4442–4489. doi: 10.1002/anie.200500368. [DOI] [PubMed] [Google Scholar]
- 16.Ping Y, Li Y, Zhu J, Kong W. Construction of quaternary stereocenters by palladium-catalyzed carbopalladation-initiated cascade reactions. Angew. Chem. Int. Ed. 2019;58:1562–1573. doi: 10.1002/anie.201806088. [DOI] [PubMed] [Google Scholar]
- 17.Shibasaki M, Vogl EM, Ohshima T. Asymmetric heck reaction. Adv. Synth. Catal. 2004;346:1533–1552. doi: 10.1002/adsc.200404203. [DOI] [Google Scholar]
- 18.Yang X, Kalita SJ, Maheshuni S, Huang Y-Y. Recent advances on transition-metal-catalyzed asymmetric tandem reactions with organoboron reagents. Coord. Chem. Rev. 2019;392:35–48. doi: 10.1016/j.ccr.2019.04.009. [DOI] [Google Scholar]
- 19.Grigg R, Meerholtz PFC, Sridharan V. Palladium catalysed synthesis of spiroindolines. Tetrahedron. 1994;50:359–370. doi: 10.1016/S0040-4020(01)80760-2. [DOI] [Google Scholar]
- 20.Piou T, Neuville L, Zhu J. Spirocyclization by palladium-catalyzed domino heck-direct C-H arylation reactions: synthesis of spirodihydroquinolin-2-ones. Org. Lett. 2012;14:3760–3763. doi: 10.1021/ol301616w. [DOI] [PubMed] [Google Scholar]
- 21.Liu JG, Chen WW, Gu CX, Xu B, Xu MH. Access to spiroindolines and spirodihydrobenzofurans via Pd-catalyzed domino Heck spiroyclization through C-H activation and carbene insertion. Org. Lett. 2018;20:2728–2732. doi: 10.1021/acs.orglett.8b00935. [DOI] [PubMed] [Google Scholar]
- 22.Piou T, Neuville L, Zhu J. Activation of a C(sp3)-H bond by a transient sigma-alkylpalladium(II) complex: synthesis of spirooxindoles through a palladium-catalyzed domino carbopalladation/C(sp3)-C(sp3) bond-forming process. Angew. Chem. Int. Ed. 2012;51:11561–11565. doi: 10.1002/anie.201206267. [DOI] [PubMed] [Google Scholar]
- 23.Ruck RT, et al. Palladium-catalyzed tandem Heck reaction/C-H functionalization-preparation of spiro-indane-oxindoles. Angew. Chem. Int. Ed. 2008;47:4711–4714. doi: 10.1002/anie.200800549. [DOI] [PubMed] [Google Scholar]
- 24.Satyanarayana, G., Maichle-Mossmer, C. & Maier, M. E. Formation of pentacyclic structures by a domino sequence on cyclic enamides. Chem. Commun. 1571–1573 (2009). [DOI] [PubMed]
- 25.Shao C, Wu Z, Ji X, Zhou B, Zhang Y. An approach to spirooxindoles via palladium-catalyzed remote C-H activation and dual alkylation with CH2Br2. Chem. Commun. 2017;53:10429–10432. doi: 10.1039/C7CC06196J. [DOI] [PubMed] [Google Scholar]
- 26.Franzoni I, Yoon H, Garcia-Lopez JA, Poblador-Bahamonde AI, Lautens M. Exploring the mechanism of the Pd-catalyzed spirocyclization reaction: a combined DFT and experimental study. Chem. Sci. 2018;9:1496–1509. doi: 10.1039/C7SC04709F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pérez-Gómez M, et al. Synthesis and reactivity of model intermediates proposed for the Pd-catalyzed remote C–H functionalization of N-(2-haloaryl)acrylamides. Organometallics. 2017;36:4465–4476. doi: 10.1021/acs.organomet.7b00702. [DOI] [Google Scholar]
- 28.Sickert M, Weinstabl H, Peters B, Hou X, Lautens M. Intermolecular domino reaction of two aryl iodides involving two C-H functionalizations. Angew. Chem. Int. Ed. 2014;53:5147–5151. doi: 10.1002/anie.201400807. [DOI] [PubMed] [Google Scholar]
- 29.Pérez-Gómez M, García-López JA. Trapping sigma-alkyl-palladium(II) intermediates with arynes encompassing intramolecular C-H activation: spirobiaryls through Pd-catalyzed cascade reactions. Angew. Chem. Int. Ed. 2016;55:14389–14393. doi: 10.1002/anie.201607976. [DOI] [PubMed] [Google Scholar]
- 30.Pérez-Gómez M, Hernandez-Ponte S, Bautista D, García-López JA. Synthesis of spiro-oxoindoles through Pd-catalyzed remote C-H alkylation using alpha-diazocarbonyl compounds. Chem. Commun. 2017;53:2842–2845. doi: 10.1039/C7CC00065K. [DOI] [PubMed] [Google Scholar]
- 31.Ye J, Lautens M. Palladium-catalysed norbornene-mediated C−H functionalization of arenes. Nat. Chem. 2015;7:863–870. doi: 10.1038/nchem.2372. [DOI] [PubMed] [Google Scholar]
- 32.Carreira EM, Fessard TC. Four-membered ring-containing spirocycles: synthetic strategies and opportunities. Chem. Rev. 2014;114:8257–8322. doi: 10.1021/cr500127b. [DOI] [PubMed] [Google Scholar]
- 33.Trost, B. & Brennan, M. Asymmetric syntheses of oxindole and indole spirocyclic alkaloid natural products. Synthesis2009, 3003–3025 (2009).
- 34.Terrett, J. A. et al. Oxadiazole transient receptor potential channel inhibitors. US20190284179A1 (2019).
- 35.Bubost, C. et al. Benzocyclobutane carboxamides. WO2015063086A1 (2015).
- 36.Allen, J. et al. Naphthalenone compounds exhibiting prolyl hydroxylase inhibitory activity, composition, and uses thereof. WO2008076427A2 (2008).
- 37.Waespe, H.-R., Van Lommen, G. R. E. & Sipido, V. K. Isonicotinic acid derivatives and related spiro compounds with herbicidal action. WO1992009577A1 (1992).
- 38.Shirai F, et al. Discovery of novel spiroindoline derivatives as selective tankyrase inhibitors. J. Med. Chem. 2019;62:3407–3427. doi: 10.1021/acs.jmedchem.8b01888. [DOI] [PubMed] [Google Scholar]
- 39.Tang L, Liu ZY, She W, Feng C. Selective single C-F bond arylation of trifluoromethylalkene derivatives. Chem. Sci. 2019;10:8701–8705. doi: 10.1039/C9SC01966A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ye J, et al. Remote C-H alkylation and C-C bond cleavage enabled by an in situ generated palladacycle. Nat. Chem. 2017;9:361–368. doi: 10.1038/nchem.2631. [DOI] [PubMed] [Google Scholar]
- 41.Yoon H, Lossouarn A, Landau F, Lautens M. Pd-Catalyzed spirocyclization via C-H activation and benzyne insertion. Org. Lett. 2016;18:6324–6327. doi: 10.1021/acs.orglett.6b03213. [DOI] [PubMed] [Google Scholar]
- 42.Yoon H, Rolz M, Landau F, Lautens M. Palladium-catalyzed spirocyclization through C-H activation and regioselective alkyne insertion. Angew. Chem. Int. Ed. 2017;56:10920–10923. doi: 10.1002/anie.201706325. [DOI] [PubMed] [Google Scholar]
- 43.Trost BM, Crawley ML. Asymmetric transition-metal-catalyzed allylic alkylations: applications in total synthesis. Chem. Rev. 2003;103:2921–2943. doi: 10.1021/cr020027w. [DOI] [PubMed] [Google Scholar]
- 44.Butt NA, Zhang W. Transition metal-catalyzed allylic substitution reactions with unactivated allylic substrates. Chem. Soc. Rev. 2015;44:7929–7967. doi: 10.1039/C5CS00144G. [DOI] [PubMed] [Google Scholar]
- 45.Trost BM. Metal catalyzed allylic alkylation: its development in the trost laboratories. Tetrahedron. 2015;71:5708–5733. doi: 10.1016/j.tet.2015.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soheili A, Tambar UK. Tandem catalytic allylic amination and [2,3]-Stevens rearrangement of tertiary amines. J. Am. Chem. Soc. 2011;133:12956–12959. doi: 10.1021/ja204717b. [DOI] [PubMed] [Google Scholar]
- 47.West TH, Daniels DS, Slawin AM, Smith AD. An isothiourea-catalyzed asymmetric [2,3]-rearrangement of allylic ammonium ylides. J. Am. Chem. Soc. 2014;136:4476–4479. doi: 10.1021/ja500758n. [DOI] [PubMed] [Google Scholar]
- 48.Arfaoui A, Saâdi F, Nefzi A, Amri H. Easy conversion of dimethylα-(bromomethyl)fumarate into functionalized allyl ethers mediated by DABCO. Synth. Commun. 2015;45:2627–2635. doi: 10.1080/00397911.2015.1093144. [DOI] [Google Scholar]
- 49.Baidya M, Remennikov GY, Mayer P, Mayr H. SN2’ versus SN2 reactivity: control of regioselectivity in conversions of Baylis-Hillman adducts. Chem. Eur. J. 2010;16:1365–1371. doi: 10.1002/chem.200902487. [DOI] [PubMed] [Google Scholar]
- 50.Kulchat S, Lehn JM. Dynamic covalent chemistry of nucleophilic substitution component exchange of quaternary ammonium salts. Chem. Asian J. 2015;10:2484–2496. doi: 10.1002/asia.201500604. [DOI] [PubMed] [Google Scholar]
- 51.Hu XG, Hunter L. Stereoselectively fluorinated N-heterocycles: a brief survey. Beilstein J. Org. Chem. 2013;9:2696–2708. doi: 10.3762/bjoc.9.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang J, et al. Fluorine in pharmaceutical industry: fluorine-containing drugs introduced to the market in the last decade (2001–2011) Chem. Rev. 2014;114:2432–2506. doi: 10.1021/cr4002879. [DOI] [PubMed] [Google Scholar]
- 53.Guo P, Zhang R, Wang X, Wang Z, Ding K. Synthesis of chiral tertiary alpha,alpha-difluoromethyl carbinols by Cu-catalyzed asymmetric propargylation. Chem. Eur. J. 2019;25:16425–16434. doi: 10.1002/chem.201904543. [DOI] [PubMed] [Google Scholar]
- 54.Fujita T, Sugiyama K, Sanada S, Ichitsuka T, Ichikawa J. Platform for ring-fluorinated benzoheterole derivatives: palladium-catalyzed regioselective 1,1-difluoroallylation and heck cyclization. Org. Lett. 2016;18:248–251. doi: 10.1021/acs.orglett.5b03390. [DOI] [PubMed] [Google Scholar]
- 55.Sharma UK, Sharma N, Vachhani DD, Van Der Eycken EV. Metal-mediated post-Ugi transformations for the construction of diverse heterocyclic scaffolds. Chem. Soc. Rev. 2015;44:1836–1860. doi: 10.1039/C4CS00253A. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Z, et al. Tandem SN2’ nucleophilic substitution/oxidative radical cyclization of aryl substituted allylic alcohols with 1,3-dicarbonyl compounds. Org. Biomol. Chem. 2017;15:3239–3247. doi: 10.1039/C7OB00620A. [DOI] [PubMed] [Google Scholar]
- 57.Zheng C, You SL. Exploring the chemistry of spiroindolenines by mechanistically-driven reaction development: asymmetric pictet-spengler-type reactions and beyond. Acc. Chem. Res. 2020;53:974–987. doi: 10.1021/acs.accounts.0c00074. [DOI] [PubMed] [Google Scholar]
- 58.Cheng D, Ishihara Y, Tan B, Barbas CF. Organocatalytic asymmetric assembly reactions: synthesis of spirooxindoles via organocascade strategies. ACS Catal. 2014;4:743–762. doi: 10.1021/cs401172r. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all the data supporting this study, including the experimental details, data analysis, and spectra for all unknow compounds, see Supplementary Files. All data underlying the findings of this work are available from the corresponding author upon reasonable request. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 2006609 (4f) and 2006610 (6a). These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.