Graphical abstract
Keywords: Salinity, Plant growth promoting rhizobacteria, Remediation, Sustainable agriculture, Exopolysaccharides
Abstract
Background
The collective impact of climate change and soil salinity is continuously increasing the degraded lands across the globe, bringing agricultural productivity and food security under stress. The high concentration of salts in saline soils impose osmotic, ionic, oxidative and water stress in plants. Biological solutions can be the most reliable and sustainable approach to ensure food security and limit the use of agro-chemicals.
Aim of Review
Halo-tolerant plant growth promoting rhizobacteria (HT-PGPR) are emerging as efficient biological tools to mitigate the toxic effects of high salt concentrations and improve the growth of plants, simultaneously remediating the degraded saline soils. The review explains the role of HT-PGPR in mitigating the salinity stress in plants through diverse mechanisms and concurrently leading to improvement of soil quality.
Key Scientific Concepts of Review
HT-PGPR are involved in alleviating the salinity stress in plants through a number of mechanisms evoking multipronged physiological, biochemical and molecular responses. These include changes in expression of defense-related proteins, exopolysaccharides synthesis, activation of antioxidant machinery, accumulation of osmolytes, maintaining the Na+ kinetics and improving the levels of phytohormones and nutrient uptake in plants. The modification of signaling by HT-PGPR inoculation under stress conditions elicits induced systemic resistance in plants which further prepares them against salinity stress. The role of microbial-mechanisms in remediating the saline soil through structural and compositional improvements is also important. Development of novel bioinoculants for saline soils based on the concepts presented in the review can be a sustainable approach in improving productivity of affected agro-ecosystems and simultaneously remediating them.
Introduction
Soil salinity has emerged as one of the biggest constraints influencing crop productivity around the globe. In the last few decades, anthropogenic activities have exacerbated the rate of soil salinization [1], [2]. Saline soils are high in electrical conductivity (EC), low in water potential and excessive in amounts of ionic salts making survival of plant and other life forms difficult [3], [4]. It is estimated that salinity has affected over 800 million hectares of agricultural land around the globe [5]. There are reports claiming that each year around 1–2% of fertile soils are being degraded worldwide due to salinity [6]. It has also been forecasted that in the next 35 years, about 50% of the land on earth will suffer due to various degrees of salinity [7]. Additionally, data indicates that in past few decades the annual cost of salt-induced land degradation in irrigated areas was estimated to be US$ 27.3 billion [8].
The presence of an excess amount of salt in soil shows cumulative and far- reaching effects on crops [9]. Salt stress triggers ionic imbalance in plants, causes nutrient deficiency, perturbations in carbon (C) and nitrogen (N) assimilatory pathways, lowered rate of photosynthesis, generation of reactive oxygen species (ROS), osmotic and oxidative stress, thereby retarding growth and yield of crops [10], [11]. Salt stress also poses negative impacts on soil processes, pH, decomposition rate, nutrient composition, microbial biodiversity and water availability, leading to the prevalence of drought-like conditions [12], [13]. According to Attia et al. [14] in several agro-ecosystems, particularly in arid and semi-arid regions, drought and salinity occur simultaneously resulting in overlapping symptoms of both the stresses in the plants. Physical methods of treatment of saline soils, that include flushing, leaching, scraping and chemical amendments e.g. addition of gypsum and lime, are not sustainable [4]. Soil flushing and scraping has achieved only a little success and has limited practical significance as the salt accumulation tends to resurface due to lowering of water table and the problem intensifies with course of time. Soil leaching although comparatively successful but requires relatively low soil moisture and low ground water level [15] making it difficult for agro-ecosystems. Another drawback of this method is that important nutrients applied to the soil may also leach out lowering their availability in the plant root zones [16]. Among chemical methods, addition of gypsum is most commonly used. However, gypsum is a non-renewable mineral and also results in low solubility or availability of nutrients such as phosphorus (P), copper (Cu), iron (Fe) and zinc (Zn) to the plants [17], [18]. Apart from physical and chemical approaches, several other methods such as modification in breeding practices, shifting of crop calendars and introduction of genetically engineered salt-tolerant plant varieties have been evaluated for enhancement of crop productivity in salt-affected soils but have largely proved to be in-effective [19], [20]. These methods are time-consuming, costly and above all, cause genetic erosion of indigenous species [21], [22].
Application of halo-tolerant plant growth promoting rhizobacteria (HT-PGPR) has the potential of alleviating salt stress in plants through elicitation of several physiological and molecular mechanisms. This includes modification in root systems, inducing antioxidant machinery, production of exopolysaccharides (EPS) and siderophores, modulation of phytohormones, synthesis of osmolytes, uptake of minerals and control of phytopathogens [23], [24], [25]. Several species of halotolerant soil bacteria such as Arthrobacter, Azospirillum, Alcaligenes Bacillus, Burkholderia, Enterobactor, Flavobacterium, Pseudomonas and Rhizobium, have been reported to ameliorate salt stress in crops [26], [27]. Their use as bioinoculants is reported to increase soil organic matter, improve soil structure and water retention capacity. Apart from this, use of HT-PGPR in form of bioinoculants is an eco-friendly and sustainable method of improving productivity of saline agro-ecosystems [28]. In spite of overwhelming advantages the exact mechanisms of HT-PGPR in helping the plants are not precisely known. Hence it is important to find out the underlying molecular mechanisms of HT-PGPR involved in plant growth promotion. These findings can assist in ascertaining the role of HT-PGPR as efficient candidates for increasing production and remediation of saline soils.
HT-PGPR mediated salt tolerance in plants
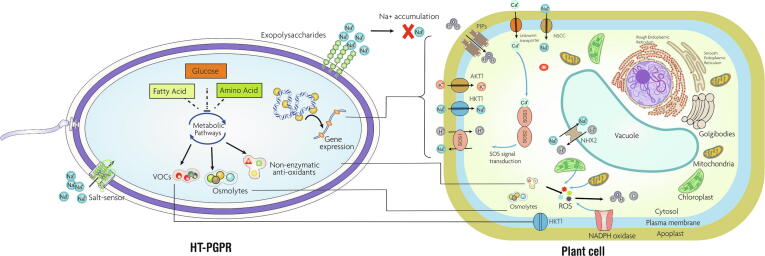
The intrinsic metabolic and genetic properties of the HT-PGPR have a direct role in lessening the harmful effects of salt stress in plants [6]. However, intriguing interactions happening in between HT-PGPR and plants under stress conditions are still a matter of further research. Probably, mitigation of salt stress by HT-PGPR may involve a three-tier entwined action cum association i.e. survival of bacteria itself under hyperosmotic environment, induction of salt tolerance events in plants and improvisation of soil quality through various mechanisms. Fig. 1 explains the mechanisms of salt-stress mitigation in plants inoculated with HT-PGPR. In last few years, research confirmed that the presence of HT-PGPR can modulate the expression of several genes responsible for the amelioration of salinity stress in plants (Table 1). The major aspect of salt stress-tolerance in plants mediated by HT-PGPR involves the generation of responsive machinery to pool out the toxicity and establishing an osmotic equilibrium state to avoid desiccation and flaccidity in plant’s cells. HT-PGPR constrict the uptake of Na+ by changing the composition of the cell wall/ cell membrane, which increases the electrogenic Na+/H+ ionic-porters along with increased expression of salt overly sensitive (SOS) genes and NHX transporters in plants [29], [44]. In a study it was shown that HT-PGPR Bacillus subtilis reduced the absorption of Na+ by Arabidopsis thaliana roots through down-regulation of high-affinity K+ transporter (HKT1) in salt-affected conditions [41]. Yasmin et al. [45] revealed that inoculation of salt-stressed soybean plants with Pseudomonas pseudoalcaligenes triggered synthesis of key defense enzymes that reduced the Na+ concentration in roots and shoots simultaneously balancing the cellular condition by increasing intracellular K+ levels. Similarly, Pseudomonas koreensis strain AK-1 reduced Na+ and elevated K+ levels in Glycine max L. Merrill [46]. Treatment of HT-PGPR, Burkholderia phytofirmans PsJN modified the expression patterns of ion-homeostasis related genes including Arabidopsis K + Transporter 1(AKT1), High-Affinity K + Transporter 1(HKT1), Sodium Hydrogen Exchanger 2 (NHX2), and SOS1 in salt-sensitive A. thaliana plants [29]. The upregulation of PtHKT1;5 and PtSOS1 and downregulation of PtHKT2;1 in B. subtilis GB03 inoculated plants reduced uptake of Na+ in halophyte grass Puccinellia tenuiflora [37]. The use of strain GB03 in improving drought tolerance in ryegrass [47] and salt tolerance in wheat [48], white clove [49] and Codonopsis pilosula [50] elucidates the involvement of some common genes and traits in mitigating both the intermingled abiotic stresses. Volatile organic compounds (VOCs) produced by HT-PGPR can trigger induction of HKT1 in shoots and reduction of HKT1 in roots, hence limiting Na + entry into roots and facilitating shoot-to-root Na + recirculation [51] Inoculation with HT-PGPR Dietzia natronolimnaea up-regulated HKT antiporters in salt-stressed wheat plants [33]. Similarly, upregulation of HKT family antiporters has also been reported in wheat inoculated with Serratia sp. Sl-12 [52], Arthrobacter nitroguajacolicus [39] and maize plants treated with Bacillus amyloliquefaciens SQR9 [36] under salinity stress. Detailing the response to salinity stress in wheat by the inoculation of Enterobacter cloacae SBP-8, Singh et al. [53] reported upregulation of defense related proteins by 2065%, photosynthesis related proteins by 792% and ion transport associated proteins by 765%. The upregulated ion-transporter proteins included malate transporter, and ROS-responsive calcium channel proteins to cope up the toxic ion influx and maintain ion homeostasis. The study also showed that bacterial inoculation upregulated expression of proteins involved in strengthening of cell wall and membrane integrity to prevent cellular damage and lateral diffusion of molecules in the endodermis. Similar mechanism of tolerance has also been reported in durum wheat when inoculated with HT-PGPR under drought and heat stress resulting in better adaption by the plants [54], [55]. This again suggests the correlation in between salinity and drought stresses along with common mechanisms at molecular level to combat them by the help of HT-PGPR.
Fig. 1.
Metabolic and genetic properties of HT-PGPR involved in salt tolerance in plants. Metabolic and genetic properties of the HT-PGPR have a direct role in the amelioration of salt-stress in plants. They can regulate the expression of ion transporters/channels such as high-affinity K+ transporter (HKT1), Arabidopsis K+ Transporter 1(AKT1), Sodium Hydrogen Exchanger 2 (NHX2), weakly voltage‐dependent nonselective cation channel (NSCC), and plasma membrane intrinsic proteins (PIPs) that collectively take part in ion homeostasis and osmatic balance in plants. All of these channels/transporters can mediate Na+ and K+ influx into plant cells and help a suitable K+: Na+ ratio in the cytoplasm which prevents cellular damage and nutrient deficiency. The presence of HT-PGPR can also modulate the Salt Overly Sensitive (SOS1) pathways. Compatible osmolytes produced by HT-PGPR can be uptaken by plant cells to reduce the osmotic potential and stabilize proteins and cellular structures from salt stress. Volatile organic compounds (VOCs) produced by HT-PGPR can also trigger the induction of HKT1 in shoots and reduction of HKT1 in roots that limit Na+ entry into roots and facilitating shoot-to-root Na+ recirculation. Apart from these, non-enzymatic anti-oxidants produced by HT-PGPR can control the formation of ROS in plant cells. Excretion of exopolysaccharides (EPS) facilitate binding of Na+ in roots cells and prevents their translocation to leaves thus acting as a physical barrier around the roots.
Table 1.
PGPR mediated expression of genes detected in plants responsible for amelioration of salinity stress.
| S. No | Plant species | Involved PGPR | Stress responsive gene | Function | Reference |
|---|---|---|---|---|---|
| 1. | Arabidopsis thaliana | Burkholderia phytofirmans PsJN | Transcription of RD29A, RD29B, APX2 and GLYI7 Downregulation of LOX2 Expression pattern of AKT1, NHX2, SOS1 and HKT1 |
Proline accumulation, abscisic acid signaling, ROS scavenging, detoxification, jasmonic acid biosynthesis pathway, ion homeostasis | [29] |
| 2. | Glycine max | Pseudomonas simiae strain AU | Upregulation of POX, CAT, VSP and NR Downregulation of HKTI, LOX, PPO and P5CS |
Production of antioxidant enzymes such as peroxidase, catalase and also vegetative storage protein, nitrite reductase | [30] |
| 3. | Abelmoschus esculentus | Enterobacter sp. UPMR18 | Upregulation of CAT, APX, GR and DHAR | Reactive oxygen species (ROS) pathway, production of antioxidant enzymes such as peroxidase, catalase | [31] |
| 4. | A. thaliana | Pseudomonas putida PS01 | Upregulation of LOX2 Downregulation of APX2 and GLY17 No changes in expression of RD29A and RD29B |
Jasmonic acid synthesis pathway, ROS scavenging activity, detoxification, abscisic acid (ABA) signaling |
[32] |
| 5. | Wheat | Bacillus safensis W10 | Upregulation of expansins, endotransglucosylase/hydrolase, sulphur rich thionin, S adenosylmethinoine secarboxylase precursor and metallothionines Downregulation of Flavonone hydroxylase, oxalate oxidase, protein phosphatase |
Alleviation of salt stress | [21] |
| 6. | Wheat | Dietzia natronolimnaea STR1 | Upregulation of TaABARE and TaOPR1 Enhanced expression of TaST, APX, MnSOD, CAT, POD, GPX, GR Tissue-specific responses of TaNHX1, TaHAK, TaHKT1, ABARE and TaOPR1 |
Abscisic acid signaling, reactive oxygen species scavenging, antioxidant enzyme activity, increased expression of ion transporters, maintenance of high K+/Na + ratio | [33] |
| 7. | A. thaliana |
Enterobacter spp. EJ01 |
Increased expression of DREB2b, RD29A, RD29B and RAB18 Expression of P5CS1, P5CS2, MPK3 and MPK6 |
Proline biosynthesis, regulation of defense pathways and salt responses | [34] |
| 8. | Solanum lycopersicum | Bacillus megaterium | Expression of MT2 and GR1 | Metallothionein Glutathione reductase enzyme synthesis | [35] |
| 9.. | G. max | Bacillus firmus SW5 | Upregulation of GmVSP, GmPHD2, GmbZIP62, GmWRKY54, GmOLPb and CHS Expression of APX, CAT, Fe-SOD and POD |
Antioxidant enzyme production, tolerance to salinity, flavonoid biosynthetic pathway |
[24] |
| 10. | Zea mays L. | Bacillus amyloliquefaciens SQR9 | Upregulation of RBCS, RBCL, H+‐PPase, HKT1, NHX1, NHX2 and NHX3 Downregulation of NCED |
Photosynthesis, Na + export and sequestration | [36] |
| 11. | Puccinellia tenuiflora | Bacillus subtilis (GB03) | Upregulation of PtHKT1;5 and PtSOS1 Downregulation of PtHKT2;1 |
Modulation of Na + homeostasis | [37] |
| 12. | S. lycopersicum | P. putida UW4 | Expression of Toc GTPase | Regulation of chloroplast import apparatus components | [38] |
| 13. | Wheat | Arthrobacter nitroguajacolicus | Upregulation of AA0618700, AA0359620, APX, GPX Expression of AA0410390, AA1982260, AA0412840 and AA1872340 |
Plant cell wall biosynthesis, phenylpropanoid biosynthetic pathway, glutathione-ascorbate cycle, modulation of celular osmotic balance, ROS scavenging activity |
[39] |
| 14. | Oryza sativa | B. amyloliquefaciens SN13 | Upregulation of NADP-Me2, EREBP, SOSI, BADH and SERK1 Repression of GIG and SAPK4 NADP-Me2, EREBP, SOSI, BADH and SERK1 Accumulation of MAPK5 |
Na+/H+ antiporter system, plant growth and development, abiotic stress response, oxidative decarboxylation of L-malate, Ion homeostasis | [40] |
| 15. | A. thaliana | B. subtilis GB03 | Expression of HKT1 | Na + import in roots | [41] |
| 16. | A. thaliana | Paenibacillus yonginensis DCY84T | Expression of AtRSA1, AtVQ9 and AtWRKY8, AtERD15, AtRAB18, and AtLT178 | Detoxification of reactive oxygen species, Na+ homeostasis, responses to abiotic stress | [42] |
| 17. | O. sativa | Root-associated plant growth-promoting rhizobacteria (PGPR) |
Expression of RAB18 | Intracellular protein transport | [43] |
Apart from the direct exclusion of the salts from cells/roots/shoots, HT-PGPR also help the plant in accumulating low molecular weight osmolytes (soluble sugars, amino acids, quaternary amines, polyols and tetrahydropyrimidines) for maintaining ionic equilibrium in the cytoplasm under salinity stress [56]. It has been found that instead of synthesizing de novo, plants prefer uptake of osmolytes liberated by HT-PGPR when exposed to high salt conditions [57], [28]. Osmotic accumulation is found to be negatively correlated with cellular osmotic potential. Due to increased concentration of osmolytes in cells under osmotic and water stresses the osmotic potential becomes negative and causes endosmosis of water which thereby maintains the turgor pressure and integrity of cells [58]. After the stress is over, osmolytes help in repairing the endodermis and cortical cell layers of plants and act as source of nitrogen and energy [59], [60]. Bacillus fortis SSB21 was reported to increase biosynthesis of proline and upregulation of stress- related genes CAPIP2, CaKR1, CaOSM1, and CAChi2 in capsicum under saline conditions [5]. Inoculation of HT-PGPR strains, Acinetobacter sp. (Br3), Pseudomonas putida (Br18) and Curtobacterium sp. (Br20) in Sulla carnosa, enhanced chlorophyll content, photosynthetic activity, total soluble sugars and antioxidant activities under saline stress [61]. With varying salt concentrations, transcription of genes responsible for osmoprotectants synthesis may express differentially in some HT-PGPR. For example, in B. amyloliquefaciens FZB42 trehalose and proline synthesis were found to be differentially expressed at 0 mM and 100 mM NaCl concentrations facilitating stress adaption in A. thaliana [62]. Among the class of transcription factors (TFs), WRKY induces several adaption responses in plants against abiotic stresses [63], [64]. Safdarian et al. [39] reported the role of WRKY TFs in mitigating salinity and drought stress through modulation of osmotic balance, scavenging of ROS and triggering of stress related genes. The study also proved that inoculation of wheat with A. nitroguajacolicus had higher WRKY28 gene expression and promoted growth of the plants under salinity stress.
Along with ionic imbalance, long term exposure of salinity generates water deficit conditions which affect plant growth. The growth of plants is majorly affected in the osmotic phase due to decrease in the soil water potential and the mitigation requires changes at molecular, cellular and physiological levels. HT-PGPR are reported to increase the expression of genes for aquaporins (AQPs) thereby, channeling the uptake of water by plants in saline and associated drought stress [65]. HT-PGPR such as Azospirillum brasilense, Pantoea agglomerans, and Bacillus megaterium were found to induce the expression of PIP2, ZmPIP1-1, and HvPIP2-1 genes involved in AQPs synthesis. The up-regulation of these genes stimulates water uptake and maintains desired water potential [66], [67]. Uptake of water by Chenopodium quinoa was improved when inoculated with HT-PGPR Enterobacter sp. MN17 and Bacillus sp. MN54 [68]. An increase in water availability was reported in mung bean when inoculated with Bacillus drentensis P16 and E. cloacae P6 under saline conditions [69]. Apart from channeling the uptake of water, PIP1 subgroup of aquaporin TaAQP8 has been reported to confer salinity mitigation in transgenic tobacco through K+ /Na+ ion homeostasis, retaining calcium content, reducing H2O2 accumulation, membrane damage and stimulation of antioxidant systems [70].
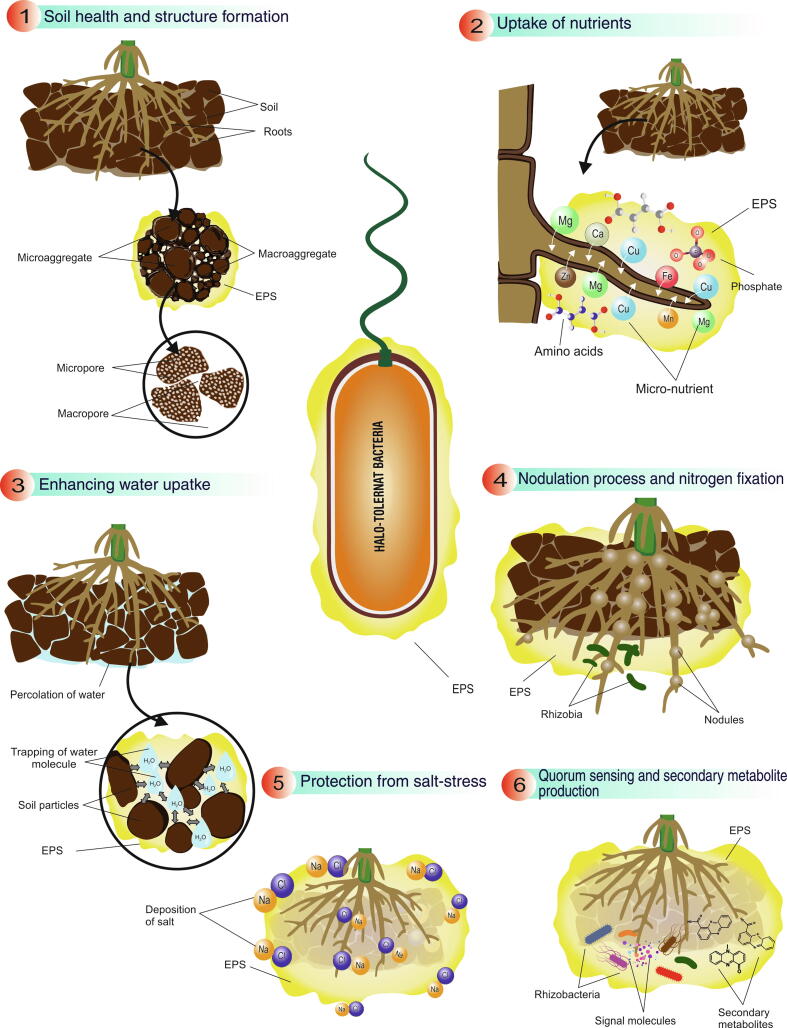
Production of natural polysaccharides or EPS during unfavorable conditions is a well-known characteristic of HT-PGPR. EPS produced by HT-PGPR help in the formation of a watery-nutrient rich layer around the root surface, known as rhizosheath [71]. There is evidence that rhizosheath serves as physical barrier against deposition of ionic salts and also acts as an active site of nutrient cycling, cation uptake, symbiotic association, nodulation and maintaining osmotic equilibrium in root region of plants exposed to high salt concentrations [72], [73], [74]. EPS are found to be useful in soil aggregate formation, humification, increase in water retention, quorum sensing, nodulation and establishing microbial diversity in saline soils (Fig. 2). Isfahani et al. [75] elucidated the combined action of silicon nanoparticles, bacterial EPS and bacterial inoculum on growth of Solanum lycopersicum L. grown under various salinity levels (0.3, 2, 4, 6, and 8 dS m−1). The combination was found to increase the root length/ shoot length, fresh weight and dry weight of the plant (upto 8 dS m−1 salinity level) in comparison to control. HT-PGPR namely Bacillus aryabhattai, Achromobacter denitrificans and Ochrobactrum intermedium have been reported to have significant role in increasing productivity of rice under salinity stress (200 mM NaCl) by production of high amounts of EPS along with efficient nitrogen fixation and phosphate solubilization, thus facilitating nutrients to the plant under adverse conditions [76]. EPS produced by Pseudomonas fluorescens D11, DR7, Enterobacter hormaechei DR16 enhanced the colonization of bacteria in root hairs and promoted the growth of foxtail millet under drought stress. The authors also reported increased soil/root tissue ratio due to higher adhesion of soils caused by gel-like nature of EPS [77]. Endophytic and rhizospheric isolates Halomonas, Bacillus, Virgibacillus and Zhihengiluella from the halophytic species Salicornia rubra, Sarcocornia utahensis, and Allenrolfea occidentalis growing in Central Utah (salinity level 5–27%), when inoculated in alfalfa grown under saline stress, stimulated growth and stress resilience [78]. The possible mechanism suggested by the authors included binding of sodium ions by bacteria along with production of volatile compounds, biofilm formation involving EPS through modification of signaling and expression of stress resistant genes.
Fig. 2.
Benefits of biofilm formation by EPS producing PGPR in improving the nutrient status and structure of saline soil suitable for plant and microbial associations.
HT-PGPR are also known for triggering antioxidant defense machinery in plants which are involved in synthesis of anti-oxidant enzymes such as superoxide dismutase (SOD), peroxidase, catalase (CAT), nitrate reductase (NR), glutathione reductase (GR), polyphenol oxidase (PO), guaiacol peroxidase (GP), monohydrate dehydrogenase (MDHAR) and dehydroascorbate reductase (DHAR) against oxidative stress caused by ROS during salt stress [79], [80]. SOD has been reported as key antioxidant enzyme and first line of defense to mitigate abiotic stresses for scavenging H2O2, reducing Haber–Weiss reaction and hydroxyl ion formation [81]. Enzymes CAT, APX (ascorbate peroxidase) and POD (peroxidase) act as the second line of defense found to mitigate the products of SOD [82]. Inoculation of plants with anti-oxidant enzymes producing HT-PGPR exhibited higher expression of APX, CAT, SOD genes under salt stress which attenuated the risks of cellular damage due to ROS in plants [24]. Inoculation of HT-PGPR (B. megaterium UPMR2 and Enterobacter sp. UPMR18) enhanced the levels of SOD, APX, and CAT and triggered the regulation of genes involved in ROS pathway (CAT, APX, GR, and DHAR) in okra plants under salinity stress [31]. Priming of Panax ginseng with salt-tolerant Paenibacillus yonginensis DCY84T facilitated the expression of various antioxidant genes such as PgAPX and PgCAT, increasing the plant tolerance against salinity stress [83]. Along with enzymatic ROS scavenging, HT-PGPR also produce non-enzymatic antioxidants such as ascorbate (AsA), carotenoids, tocopherols, glutathione (GSH) and phenolics which act as defense molecules for the plant under saline conditions [84]. HT-endophyte Pseudomonas stutzeri ISE12 isolated from halophyte Salicornia europaea upon inoculation to Brassica napus L. triggered antioxidant system and rearrangement of plant cell wall, consecutively inducing the tolerance in plants [85]. Inoculation of cowpea with HT-PGPR Bradyrhizobium and Pseudomonas graminis showed the accumulation of AsA and GSH and helped plants survival under salt stress [86].
Solubilization of inaccessible form of soil minerals into bioavailable form is a noted activity of HT-PGPR. HT-PGPR employ a vast array of mechanisms including production of low molecular weight organic acids, chelating agents and ion exchange for solubilization of P, Zn and potassium (K) in saline soil [87], [88]. Minimizing the rhizospheric competition in nutrient scant stressed soils, some microbes through penetration or nodulation establish themselves as endophytes and in turn benefit the plant by assimilating nutrients and increasing survival/ adaption rate [89]. P is an important macronutrient required in large quantities by the plant i.e. ~ 0.2%- 0.8% [90]. But the enhanced level of salt in soil messes up with the availability of P to plants. In alkaline and calcareous soils, lime reacts with P forming strong calcium phosphate compounds, thereby exacerbating the chelation by plants [91]. Thus, along with phosphatic fertilizers application of phosphate solubilizing microorganisms (PSMs) are gaining attention. Bacterial isolates from saline mangrove swamps exhibited the property to solubilize rock phosphate with maximum chelation of 97% by Oceanobacillus picturae [87]. HT-PGPR such as Vibrio, Arthrobacter, Bacillus, Azospirillum, O. picturae, and Phyllobacterium are reported to increase the P uptake by plants in saline soils [92], [6]. Apart from the macro-nutrients, availability of Fe is another challenge, limiting the growth and productivity of crops. Chelation of Fe is mainly governed by the pH of the soil system. Under saline conditions, higher content of bicarbonates inactivates the soluble Fe and thus, the uptake (of Fe by plants) is reduced. Saline soils tend to have pH more than 6.5 which causes oxidation of soluble ferrous to insoluble ferric oxide [93]. Siderophore production by HT-PGPR fulfill the demand of Fe in crops grown under salinity stress [94], [95]. Mukherjee et al. [96] reported the role of siderophore production by Halomonas sp. in chelating Fe and growth promotion of rice under salinity stress. Goswami et al. [97] noted that Bacillus licheniformis strain A2 isolated from rhizosphere of salt tolerant desert plant Suaeda fruticosa solubilized phosphate and produced siderophore contributing to increment in plant biomass and height when grown under salinity stress. Another issue faced by the plants under salt stress is the deficiency of N due to negative influence on population and distribution of microbes. Process of dinitrogen fixation can be hampered in presence of salinity unless and until proper strains are not selected and inoculated [98]. A novel study determining the diversity of diazotrophic endophytes in salt tolerant plant S. europaea L (growing at 55 and 112 dS/ m salinity) highlighted the presence of wide range of efficient nitrogen fixing genera including Curtobacterium, Microbacterium, Rhodococcus, Mycobacterium, Cellulomonas, Sanguibacter, Clavibacter, Cryocola/Labedella, Frigoribacterium, Agreia, Herbiconiux, and Plantibacter [99]. The study also reported that nitrogen fixing Actinobacteria have higher salinity tolerance abilityb than Proteobacteria which are often reported at lower salinity. Similarly, Jha et al. [100] reported novel diazotrophic spectra of HT-PGPR including Brachybacterium saurashtrense sp. nov., Zhihengliuella sp., Brevibacterium casei, Haererehalobacter sp., Halomonas sp., Vibrio sp., Cronobacter sakazakii, Pseudomonas spp., Rhizobium radiobacter, and Mesorhizobium sp. from the rhizosphere of extreme halophyte Salicornia brachiate. The isolates were found to be potent nitrogen fixers, efficient IAA (indole acetic acid) producers, phosphate solubilizers along with positive for 1-aminoacyclopropane 1-carboxylate (ACC) deaminase activity (ACCD). Gordonia sp., a HT- PGPR was reported positive for nifH gene and siderophore production, stimulating the growth of pearl millets under saline conditions [101]. The lesser explored extreme habitats should thus be explored to discover the potential strains of stress tolerant PGPR so as to utilize them in strained agro-ecosystems.
Modulation of phytohormones and accumulation of nutrients against the hyperosmotic and hypo-nutritive conditions is an important role of HT-PGPR to confer symbiotic association and promote growth and productivity of plants. Studies confirmed that under salt stress, expression of genes for auxin like compounds increase in HT-PGPR and compensate plant’s requirement of growth hormone [102], [89]. Apart from the improvement in growth, there is also evidence that phytohormones produced by HT-PGPR showed role in the exclusion of ionic salts. Rojas-Tapias et al. [103] reported improved K+ uptake, Na+ ion exclusion and plant growth promotion in maize upon inoculation with auxin producing Azotobacter C5 and C9 strains under salt stress. Priming the seeds of Triticum durum var. waha with potent IAA producing HT-PGPR A. brasilense NH along with the extract of Marine Alga Ulva lactuca served as a novel bioformulation and enhanced the growth parameters of plants under saline conditions [104]. Synthesis of IAA is transcriptionally related to soaring level of ethylene in plants via the expression of ACC synthase gene (producing ethylene) [105]. Ethylene increases salt tolerance in plants but at the expenditure of growth and productivity [106]. The first small peak of ethylene under abiotic stresses takes up ACC and initiates expression of genes responsible for defense mechanism, while second larger peak is majorly deleterious to plants causing leaf chlorosis abscission, and senescence [107]. Mitigating this second ethylene peak, HT-PGPR synthesize ACC deaminase (ACCD) enzyme for growth and development of stressed plants [108]. ACCD encoding gene acdS are found in halotolerant microbes functioning to convert ACC (precursor of ethylene) to ammonia, α-ketoglutaraldehyde and utilizing for energy and N source [109]. Concluding from the direct correlation between IAA and ethylene it can be suggested that HT-PGPR with combined property of IAA production and ACCD should be used for stress amelioration [110], [111].
Under salt stress PGPR also modulate ‘emergency hormone’ abscisic acid (ABA) biosynthesis and ABA-mediated signaling pathways for growth elevation of plants. Relatively, inoculation of wheat plants with halotolerant strain D. natronolimnaea STR1 under salinity stress caused the up-regulation of TaABARE (ABA-responsive gene) and TaOPR1 (12-oxophytodienoate reductase 1) stimulating TaMYB and TaWRKY following the expression of salt-stress induced gene TaST [33]. P. fluorescens inoculation to rice plants under drought stress was found to be enhancing the intrinsic tolerance level of plant through elevated expression of ABA synthetic genes, particularly at later reproductive stage [112]. The authors reported increased expression of nced1, an ABA biosynthesis gene in tomato plants, upon inoculation with bacteria. The increased ABA content helped in stomatal closure to prevent loss of water from plants in a water-deficit condition. Gibberellic acid (GA) is another class of phytohormones inducing the physiological responses, germination and plant growth promotion. Under salt stress the mitigation strategy by GA involves antioxidant activity, inhibiting lipid peroxidation, chlorophyll synthesis, elevated root-shoot length, photosynthetic activity and plant biomass [113]. Kang et al. [114] reported that inoculation of salt and drought stressed soybean plants with P. putida H2-3, elevated the production of gibberellins and helped in regulating the hormonal and stress physiology.
Cytokinin phytohormonal signaling events also impart osmotic balance and help plant acclimatize to salinity [115]. These purine derivatives are directly involved in root callus differentiation, root hair and embryo vasculature formation, increase in leaf area, chlorophyll synthesis, and nutrient signaling [116]. Although cytokinins are less studied in comparison to auxins but their role in salt stress tolerance is equally significant. Sandhya et al. [117] found that a quarter of pseudomonads that were isolated from plants growing in arid and semi-arid areas showed increased cytokinin production under osmotic stress conditions. Elucidating the role of cytokinins under saline stress, a hypothesis illustrates that may be limiting the root proliferation leads to reduced uptake of salts by plants, but the larger picture of cytokinin driven tolerance mechanism is still unknown [118].
Apart from governing the molecular and physical interactions in plants, PGPR also elicit induced systemic resistance/ tolerance (ISR/ IST) through signaling [119]. ISR is primarily governed by ethylene and jasmonic acid (JA) signaling and the complex transcriptome analysis reveals involvement of MYB72, β-glucosidase U42 (BGLU42) and MYC2, in rhizobacteria mediated ISR [120]. PGPR produce plethora of signaling molecules including volatile organic compounds (VOCs), quorum sensing molecules such as N-acyl homoserine lactone (AHL) and cyclodipeptides (CDPs) which are effective in induction of systemic resistance in plants [121], [122], [123]. The exogenous application of N-3-oxo-hexanoyl-homoserine lactone (3OC6-HSL) (molecule of AHL family) enhanced the salt tolerance in Arabidopsis and wheat plants. 3OC6-HSL was found to be increasing the root and shoot length, plant biomass, proline and chlorophyll content and reduced MDA (malondialdehyde) and Na+ levels. The molecular mechanism revealed that 3OC6-HSL was up-regulating expression of salinity-tolerance genes including COR15a, RD22, ADH and P5CS1 (ABA-dependent osmotic stress regulating genes), ERD1 (ABA-independent pant signaling gene), and SOS1, SOS2 and SOS3 (salinity responsive genes) in both the plants [124]. Correlatively, red rice plants inoculated with Gluconacetobacter diazotrophicus under drought stress showed synthesis of AHL molecules by LuxI proteins (autoinducer proteins) which enhanced tolerance in plants through IST [125]. Also two stress marker genes, PR-1 (for salicylic acid SA pathway) and PR-10 (for JA/ ethylene pathway) were induced transcriptionally to activate ISR. Therefore, G. diazotrophicus strain Pal 5 was suggested as future bioinoculant to reduce the damages in plants due to abiotic stresses and AHL an elicitor of plant defense systems [126].
HT-PGPR as soil remediators
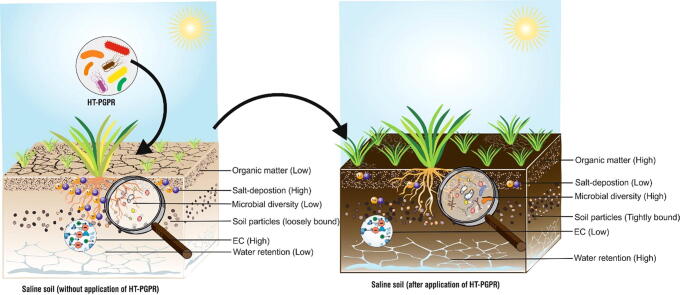
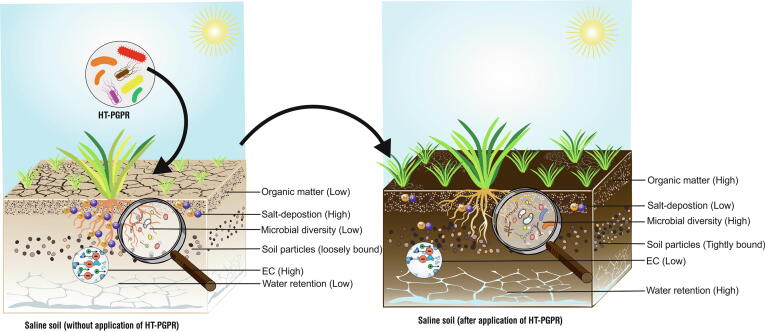
The presence of HT-PGPR in saline soil greatly influences soil quality and fertility parameters. Studies have confirmed that HT-PGPR improve nutrient status, soil structure, organic matter, pH, EC, and deposition of ionic salts in soil [127]. HT-PGPR mitigate ionic toxicity through cation bridging, hydrogen bonding, and anion adsorption [128]. There are reports where application of HT-PGPR has improved salt index of saline soil [129]. Mitigating the nutrient status, HT-PGPR improve N, C, P, Fe and Zn content of saline soils, thereby reviving the lost vegetative index and accelerating the agricultural sustainability. Under saline conditions the N content and population of nitrogen fixers are found to be decreasing. Thus, acting as an efficient reclamation strategy, the symbiotic and asymbiotic biological nitrogen fixation by salt tolerant microbes enhances the N content as well as improves fertility of soil [88], [130], [131]. The enrichment of saline soil using nitrogen fixing PGPR Pseudomonas aeruginosa, along with N compost stimulated the level of nitrogen as compared with un-inoculated control [132]. Revival of arid and saline soil by utlizing salt-tolerant rhizobia can help in improving the fertility and productivity of these stressed agro-ecosystems [133]. Hassan et al. [134] utilized root powder of a halophyte Cenchrus ciliaris as carrier to develop inoculant from HT-PGPR Bacillus cereus, Pseudomonas moraviensis, and Stenotrophomonas maltophilia. The developed bioinoculant when applied in field improved growth of wheat and simultaneously resulted in better texture, EC, pH and organic matter of saline-sodic soil. Along with N, HT-PGPR can stimulate the P, Zn and Fe content of saline soils. P. moraviensis reclaimed saline sodic soil by improving P, nitrate (NO3–), N and K content by almost 18–35% [135]. Increase in P content of saline soil was observed by inoculation with phosphate solubilizing B. licheniformis MH48 strain. Reduction in soil pH, EC and enhanced availability of macro-nutrients (NPK), micronutrients (Fe, Zn, Mn and Cu) and organic matter was reported when saline soil was inoculated with HT-PGPR and phosphogypsum [136].
Besides nutrition, aggregation is also an important soil quality which promotes water percolation, root penetration, aeration and micropore formation [137]. HT-PGPR improve soil structure and aggregation by production of EPS (in stress conditions) resulting in formation of microaggregates (<250 µm) and macroaggregates (greater than250 µm) thus entrapping nutrients and water molecules. Formation of coherent EPS-soil complex in protected environment against odds of salinity helps in protection of both the plants and HT-PGPR population in the rhizosphere [138], [139]. Qurashi and Sabri [140]. concluded that significant increase in soil aggregation was found due to EPS producing PGPR Planococcus rifietoensis (RT4) and Halomonas variabilis (HT1). Acting as protective sponge, EPS in the biofilm complex also increase the water holding capacity of soil and improves the water activity in plants under stress conditions of salinity and drought [74]. Improvement in water holding capacity and aggregation of soil upon application of EPS extracted from P. putida GAP-P45 under salt and drought stress was reported [128]. Similarly, Boukhelata et al. [141] testified that EPS produced by Alcaligenes latus absorbed 1000 times more water than its own dry weight, while water absorbing capacity of EPS from P. aeruginosa strain CMG1421 was 400 times [142] and that from Rhizobium sp. and Paenibacillus sp., 100 times more than the dry weight [143]. EPS is an excellent soil conditioner and can even be utilized as an additive for development of bioformulations [144].
Increase in salinity by more than 5% significantly lowers the population of bacteria and actinobacteria [145]. The exogenous application of HT-PGPR while improving the soil structure also participates in assimilating the organic matter and increasing the microbial interactions. The establishment of biofilm in soil aggregates or on root surface is characterized by high concentration of root exudates, signaling molecules, organic matter and water content. This complex acts as a dragging force in selecting and establishing microbial diversity. The primary content of biofilm (EPS) regulates the organic matter by serving as C source and coagulating soil particles thereby ensuring the formation of humic substances which are stable organic carbon forms [146]. Improvement of C cycling in saline soil is reported when inoculated with PGPR [147]. Another mechanism of action reported by Lipińska et al. [148] highlights that bacterial inoculation increases the dehydrogenase activity which is suggested to be directly correlated with soil microbial biomass. Islam et al. [149] described increase in microbial biomass carbon and dehydrogenase activity in saline soil upon inoculation with HT-PGPR B. cereus Pb25. Research thus clearly shows the role and possible utilization of HT-PGPR in improving the quality of soils impacted with abiotic stresses such as salinity (Fig. 3).
Fig. 3.
Application of HT-PGPR showing rejuvenation of saline soil by different mechanisms for better productivity.
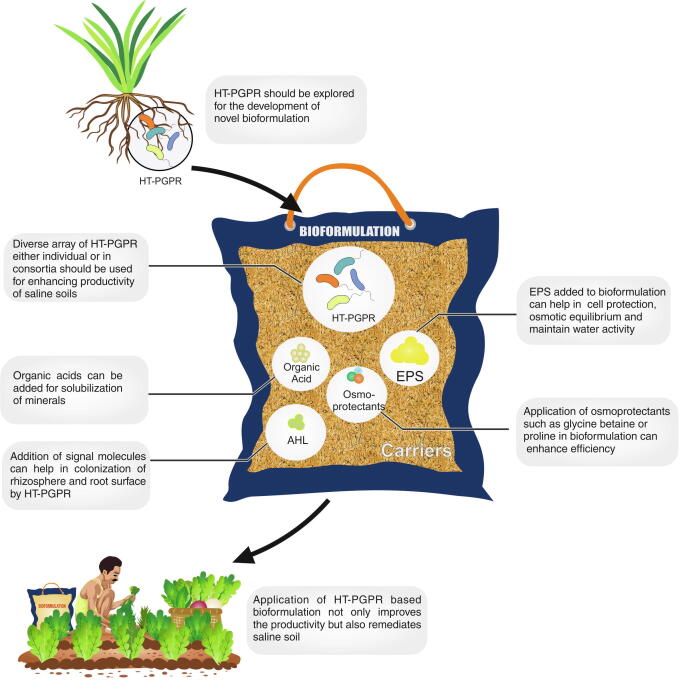
Future prospects
Rapid increase in salinization of soil has not only affected agricultural production but also posed a threat to food security. Although still in its infancy, recent research shows that application of HT-PGPR has proven effective and a sustainable solution for the reclamation of saline soils. With the advancement of methodologies and techniques wide array of metabolites and genes (by PGPR) have been identified in response to salinity stress. However, further insights are required into the metabolome of the HT-PGPR during their interaction with plants both under physiologically stressed and non-stressed conditions [150]. ‘Interactomics’ is the emerging mapping technique involving bioinformatics tools to analyze the relationships between biomolecules such as proteins/ enzymes from both plants and bacterial cells so as to determine the pathways involved in communication between both the partners under salt stress [151]. The decoding of the signaling and interactions between microbe-to-plant or plant-to-microbe can be used to modify the phytomicrobiome for the benefit of stressed plants. The connection between plant stress responses, signaling molecules and assemblages of microbiome can be further explored for development of stress ‘smart agriculture’ [152]. Limitations of bioformulations such as shelf-life and inability to perform in conditions prevalent under abiotic stresses have to be overcome. Inducing the stress ameliorating factors by adding additives or metabolites to attract the microbes can be explored to improve the quality of bioformulations for stressed agro-ecosystems. AHL can be used as one such elicitor for enhancing the plant–microbe communication and ensuring physiological, biochemical and molecular changes to prevent salt-injury in crops [153]. Osmoprotectants or cell-protectants can also be added in the novel formulations, along with HT-PGPR, to help them overcome the initial stress and get acclimatized to the conditions. Exogenous application of glycine betaine and proline to culture medium resulted in enhanced salinity tolerance of fluorescent pseudomonads [154]. Hence this can also be utilized for development of bioinoculants for saline soils. Novel bioformulations can thus be developed by utilizing diverse HT-PGPR or their metabolites for improving productivity and quality of saline soils (Fig. 4). Amending gene elicitors inducing salt-stress responses and aiding in formation of biofilms can not only help in preparing the plant and microbe against stress like salinity, but also protect the introduced microbe from initial shock. Biofilm formation by HT-PGPR has already been reported in protection and growth promotion of plants in saline conditions [155]. Improvement of productivity and quality of saline soils utilizing HT-PGPR can play important role in improving the microbial diversity, soil organic matter, water activity, EC, pH and nutrient availability. Bioinoculants based on HT-PGPR can thus be of multiple utility. These novel formulations can not only protect and improve the crops’ yields but also play crucial role in remediating stressed agro-ecosystems.
Fig. 4.
HT-PGPR and their metabolites for development and application of novel bioformulation for saline soil.
Conclusion
Switching from unsustainable traditional methods, microbiological approaches are emerging as the most significant biological tools to remediate and increase the productivity of saline soils. HT-PGPR have an array of mechanisms in their armor to manage and overcome the harmful impacts of soil-salinity. The application of these under-used but potential microorganisms to improve the productivity and instigate remediation of saline soils is required to be explored. Further research is needed to develop novel bioinoculants to tackle the menace of soil-salinity. To be a replicable and reliable technology, more scientific inputs will be required so as to understand the intricacies of the plant–microbe-microbe interactions under the complex stresses elicited due to soil salinity. This novel microbial technology needs to be explored further for improving crop-productivity and control the pandemic of soil salinity.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
NKA conceptualized the idea. All authors equally contributed in writing of the manuscript. NKA supervised the whole study. NKA and JM prepared the illustrations. TF thanks UGC-MANF for fellowship, SV received UGC-NFOBC fellowship, RV and CB thank CSIR-UGC for the fellowship.
Biographies

Naveen Kumar Arora, Fellow of International Society of Environmental Botanists (FISEB), Professor and Head, Department of Environmental Science, Babasaheb Bhimrao Ambedkar University, Lucknow, India is a renowned researcher in the field of Environmental Microbiology and Biotechnology. His specific area of research is rhizosphere biology and plant growth-promoting rhizobacteria (PGPR). He has more than 70 research articles published in premium international journals and is an editor of 25 books published by Springer Nature. He is also Editor-in-Chief of the journal “Environmental Sustainability” published by Springer Nature. He has been awarded by national and international bodies. His current research interest mainly focusses on salt-tolerant rhizobacteria and characterization of associated metabolites and development of novel bioformulation for remediation of saline soils.

Tahmish Fatima is a PhD candidate in Environmental Microbiology under the supervision of Prof. Naveen Kumar Arora, at Babasaheb Bhimrao Ambedkar University, Lucknow, India. She has a Bachelor degree in Science (2012) and Master degree in Environmental Microbiology (2014). Currently, her line of research is focused on isolation, characterization and in vitro and in vivo application of salt tolerant plant growth promoting rhizobacteria on growth enhancement of plants and reclamation of soil.

Jitendra Mishra holds a Ph.D. in Environmental Microbiology. He completed his master’s in Environmental Microbiology in 2009 from Babasaheb Bhimrao Ambedkar University, Lucknow, and subsequently gained experience in research and teaching alike. He has worked as an Assistant Professor (Guest) at the Department of Environmental Microbiology, Babasaheb Bhimrao Ambedkar University, Lucknow. He is serving as an Assistant Editor for the journal “Environmental Sustainability” published by Springer-Nature. He has published his research findings in the form of book, book chapters, research articles, and reviews in several journals of repute. Currently, he is a Research Associate at the DST-Centre for Policy Research, BBAU, Lucknow.

Isha Mishra is currently a PhD fellow in Environmental Microbiology under Prof Naveen Kumar Arora at Babasaheb Bhimrao Ambedkar University, Lucknow, India. Completing her Bachelor degree in Science (2012) and Master degree in Environmental Microbiology (2014). Currently, her research interest is about the characterization and role of PGPR-extracted biosurfactants in growth promotion and disease protection in plants.

Sushma Verma is a PhD student in Environmental Microbiology at Babasaheb Bhimrao Ambedkar University, Lucknow, India. She did her bachelor in Science (2013) from Lucknow University and completed masters in Environmental Microbiology from Babasaheb Bhimrao Ambedkar University in the year 2015. She joined the research group of Prof. Naveen Kumar Arora in 2015. Her research work primarily focuses on the exploration and characterization of plant microbiota subjected to abiotic stresses. She has publications (original articles, book chapters) in reputed journals.

Renu Verma is a PhD scholar in Environmental Microbiology at the Babasaheb Bhimrao Ambedkar University, Lucknow and jointly associated with ICAR- Indian Institute of pulses research for her research. She did BSc in Biology (2004) and MSc in Microbiology (2009) from Kanpur University. She is currently working under the supervision of Prof. Naveen Kumar Arora. Her research work focuses on diversity of rhizobia associated with chickpea germplasm in relation to nodulation and nitrogen fixation.

Maya Verma is presently a Microbiologist at Uttar Pradesh Pollution Control Board (UPPCB), Lucknow, India. She received her bachelor degree in Science and Masters in Environmental Microbiology. She has done her PhD in Environmental Microbiology at Babasaheb Bhimrao Ambedkar University, Lucknow, India, under the supervision of Prof. Naveen Kumar Arora. Her research topic was to determine the rhizobial diversity in wild medicinal legumes and their role in supporting better growth of plants. Currently, her research is focused on river quality monitoring in the area of Uttar Pradesh, India.

Ankita Bhattacharya is a PhD student in the Department of Environmental Science at Babasaheb Bhimrao Ambedkar University, Lucknow, India. She received her BSc in Biotechnology (2016) from AKS University Satna, India and MSc in Environmental Science (2018) from Banaras Hindu University, Varanasi, India. She joined her research under Prof. Naveen Kumar Arora in the year 2018. Her area of research is plant-microbe interactions in agriculture particularly focusing on endophytic salt-tolerant bacteria.

Priyanka Verma is a PhD student in Environmental Science at Babasaheb Bhimrao Ambedkar University, Lucknow. She received BSc in Science (2015) from M. B. P. Govt. P.G. College and MSc in Environmental Science (2018) from Babasaheb Bhimrao Ambedkar University. She is currently pursuing PhD under Prof. Naveen Kumar Arora. Her research focuses on role of plant growth promoting rhizobacteria in biocontrol against phytopathogens along with the metabolites and mechanism involved in action.

Priya Mishra earned her Master’s degree in Environmental Microbiology from Babasaheb Bhimrao Ambedkar University, Lucknow in 2014. Currently, she is pursuing a PhD in the Department of Environmental Science, Babasaheb Bhimrao Ambedkar University, Lucknow under the close guidance of Prof. Naveen Kumar Arora, and trying to explore the potential of fluorescent pseudomonads in biofortification of wheat under salt-stress conditions.

Chanda Bharti is a PhD scholar in Department of Environmental Science at Babasaheb Bhimrao Ambedkar University, Lucknow. She completed her graduation in 2015 from Kamla Nehru Institute of Science and Technology, Sultanpur and Masters in Environmental Science and Technology from Institute of Environment and Sustainable Development, Banaras Hindu University, Varanasi, India (2017). She is now pursuing her PhD under the supervision of Prof. Naveen Kumar Arora. Her research work mainly focuses on plant-microbe interactions involving salt tolerant endophytic microbes.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Lambers H. Introduction: Dryland salinity: A key environmental issue in southern Australia. Plant Soil. 2003;257:5–7. doi: 10.1023/B:PLSO.0000003909.80658.d8. [DOI] [Google Scholar]
- 2.Bargaz A., Lyamlouli K., Chtouki M., Zeroual Y., Dhiba D. Soil microbial resources for improving fertilizers efficiency in an integrated plant nutrient management system. Front. Microbiol. 2018 doi: 10.3389/fmicb.2018.01606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mishra J, Fatima T, Arora NK. Plant Microbiome: Stress Response. In: Egamberdieva D, Ahmad P, eds. Role of secondary metabolites from plant growth-promoting rhizobacteria in combating salinity stress. Singapore: Springer; 2018. p. 127-163. doi:10.1007/978-981-10-5514-0_6.
- 4.Egamberdieva D., Wirth S., Bellingrath-Kimura S.D., Mishra J., Arora N.K. Salt-tolerant plant growth promoting rhizobacteria for enhancing crop productivity of saline soils. Front. Microbiol. 2019;10:2791. doi: 10.3389/fmicb.2019.02791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yasin N.A., Akram W., Khan W.U., Ahmad S.R., Ahmad A., Ali A. Halotolerant plant-growth promoting rhizobacteria modulate gene expression and osmolyte production to improve salinity tolerance and growth in Capsicum annum L. Environ. Sci. Pollut. Res. 2018;25(23):23236–23250. doi: 10.1007/s11356-018-2381-8. [DOI] [PubMed] [Google Scholar]
- 6.Etesami H., Beattie G.A. Mining halophytes for plant growth-promoting halotolerant bacteria to enhance the salinity tolerance of non-halophytic crops. Front. Microbiol. 2018;9:148. doi: 10.3389/fmicb.2018.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thiem D., Gołębiewski M., Hulisz P., Piernik A., Hrynkiewicz K. How does salinity shape bacterial and fungal microbiomes of Alnusglutinosa roots? Front. Microbiol. 2018;9:651. doi: 10.3389/fmicb.2018.00651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qadir M., Quillérou E., Nangia V., Murtaza G., Singh M., Thomas R. Economics of salt-induced land degradation and restoration. Nat. Resour. Forum. 2014;38:282–295. doi: 10.1111/1477-8947.12054. [DOI] [Google Scholar]
- 9.Artiola J., Walworth J., Musil S., Crimmins M. Soil and land pollution. Environ. Pollut. Sci. 2019:219–235. doi: 10.1016/B978-0-12-814719-1.00014-8. [DOI] [Google Scholar]
- 10.Hashem A., Abd_Allah E., Alqarawi A., Al-Huqail A., Shah M. Induction of osmoregulation and modulation of salt stress in Acacia gerrardii Benth. by arbuscular mycorrhizal fungi and Bacillus subtilis (BERA 71) Biomed. Res. Int. 2016:6294098. doi: 10.1155/2016/6294098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan J., Peng F., Xue X., You Q., Zhang W., Wang T. The growth promotion of two salt-tolerant plant groups with PGPR inoculation: a meta-analysis. Sustainability. 2019;11(2):378. doi: 10.3390/su11020378. [DOI] [Google Scholar]
- 12.Mishra J., Arora N.K. Secondary metabolites of fluorescent pseudomonads in biocontrol of phytopathogens for sustainable agriculture. Appl. Soil Ecol. 2018;125:35–45. doi: 10.1016/j.apsoil.2017.12.004. [DOI] [Google Scholar]
- 13.Bulgari R., Franzoni G., Ferrante A. Biostimulants application in horticultural crops under abiotic stress conditions. Agronomy. 2019;9(6):306. doi: 10.3390/agronomy9060306. [DOI] [Google Scholar]
- 14.Attia H., Alamer K.H., Ouhibi C., Oueslati S., Lachaal M. Interaction between salt stress and drought stress on some physiological parameters in two pea cultivars. Int. J. Botany. 2020;16:1–8. doi: 10.3923/ijb.2020.1.8. [DOI] [Google Scholar]
- 15.Osman KT. Saline and sodic soils. In: Osman KT, ed. Management of soil problems. Cham: Springer; 2018. p. 255-298. doi:10.1007/978-3-319-75527-4_1.
- 16.Cuevas J., Daliakopoulos I.N., del Moral F., Hueso J.J., Tsanis I.K. A review of soil-improving cropping systems for soil salinization. Agronomy. 2019;9(6):295. doi: 10.3390/agronomy9060295. [DOI] [Google Scholar]
- 17.Elrashidi M.A., West L.T., Seybold C.A., Benham E.C., Schoeneberger P.J., Ferguson R. Effects of gypsum addition on solubility of nutrients in soil amended with peat. Soil Sci. 2010;175:162–172. doi: 10.1097/SS.0b013e3181dd51d0. [DOI] [Google Scholar]
- 18.Freedman B. Environmental Science: A Canadian perspective, Dalhousie University Libraries Digital Editions, (2018) Pressbooks.
- 19.Ceccarelli S. Efficiency of plant breeding. Crop Sci. 2015;55(1):87. doi: 10.2135/cropsci2014.02.0158. [DOI] [Google Scholar]
- 20.Arora N.K. Impact of climate change on agriculture production and its sustainable solutions. Environ. Sustain. 2019;2:95–96. doi: 10.1007/s42398-019-00078-w. [DOI] [Google Scholar]
- 21.Chakraborty U., Chakraborty B.N., Dey P.L., Chakraborty A.P. Bacillus safensis from wheat rhizosphere promotes growth and ameliorates salinity stress in wheat. Indian J. Biotech. Pharm. 2018;17:466–479. http://nopr.niscair.res.in/handle/123456789/45279 [Google Scholar]
- 22.Anderson J.A., Ellsworth P.C., Faria J.C., Head G.P., Owen M.D.K., Pilcher C.D. Genetically engineered crops: Importance of diversified integrated pest management for agricultural sustainability. Front. Bioeng. Biotechnol. 2019;7 doi: 10.3389/fbioe.2019.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shahzad R., Khan A.L., Bilal S., Waqas M., Kang S.M., Lee I.J. Inoculation of abscisic acid-producing endophytic bacteria enhances salinity stress tolerance in Oryza sativa. Environ. Exp. Bot. 2017;136:68–77. doi: 10.1016/j.envexpbot.2017.01.010. [DOI] [Google Scholar]
- 24.El-Esawi M.A., Alaraidh I.A., Alsahli A.A., Alamri S.A., Ali H.M., Alayafi A.A. Bacillus firmus (SW5) augments salt tolerance in soybean (Glycine max L.) by modulating root system architecture, antioxidant defense systems and stress-responsive genes expression. Plant Physiol. Biochem. 2018;132:375–384. doi: 10.1016/j.plaphy.2018.09.026. [DOI] [PubMed] [Google Scholar]
- 25.Arora N.K., Fatima T., Mishra I., Verma M., Mishra J., Mishra V. Environmental sustainability: challenges and viable solutions. Environ. Sustain. 2018;1(4):309–340. doi: 10.1007/s42398-018-00038-w. [DOI] [Google Scholar]
- 26.Egamberdiyeva D. Plant-growth-promoting rhizobacteria isolated from a Calcisol in a semi-arid region of Uzbekistan: biochemical characterization and effectiveness. J. Plant Nutr. Soil Sci. 2005;168(1):94–99. doi: 10.1002/jpln.200321283. [DOI] [Google Scholar]
- 27.Saghafi D., Ghorbanpour M., Ajirloo H.S., Lajayer B.A. Enhancement of growth and salt tolerance in Brassica napus L. seedlings by halotolerant Rhizobium strains containing ACC-deaminase activity. Plant Physiol. Rep. 2019;24(2):225–235. doi: 10.1007/s40502-019-00444-0. [DOI] [Google Scholar]
- 28.Fatima T, Arora NK. Plant growth-promoting rhizospheric microbes for remediation of saline soils. In: Arora NK, Narendra K, eds. Phyto and Rhizo Remediation. Singapore: Springer; 2019. p.121-146. doi:10.1007/978-981-32-9664-0_5.
- 29.Pinedo I., Ledger T., Greve M., Poupin M.J. Burkholderia phytofirmans PsJN induces long-term metabolic and transcriptional changes involved in Arabidopsis thaliana salt tolerance. Front. Plant Sci. 2015;6:466. doi: 10.3389/fpls.2015.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vaishnav A., Kumari S., Jain S., Varma A., Tuteja N., Choudhary D.K. PGPR-mediated expression of salt tolerance gene in soybean through volatiles under sodium nitroprusside. J. Basic Microbiol. 2016;56:1274–1288. doi: 10.1002/jobm.201600188. [DOI] [PubMed] [Google Scholar]
- 31.Habib S.H., Kausar H., Saud H.M. Plant growth-promoting rhizobacteria enhance salinity stress tolerance in Okra through ROS-scavenging enzymes. Biomed. Res. Int. 2016:6284547. doi: 10.1155/2016/6284547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chu T.N., Tran B., Van Bui L., Hoang M. Plant growth-promoting rhizobacterium Pseudomonas PS01 induces salt tolerance in Arabidopsis thaliana. BMC Res. Notes. 2019;12:11. doi: 10.1186/s13104-019-4046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bharti N., Pandey S.S., Barnawal D., Patel V.K., Kalra A. Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Sci. Rep. 2016;6:34768. doi: 10.1038/srep34768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim K., Jang Y.J., Lee S.M., Oh B.T., Chae J.C., Lee K.J. Alleviation of salt stress by Enterobacter sp. EJ01 in tomato and Arabidopsis is accompanied by up-regulation of conserved salinity responsive factors in plants. Mol. Cell. 2014;37:109–117. doi: 10.14348/molcells.2014.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zameer M., Zahid H., Tabassum B., Ali Q., Nasir I.A., Saleem M. PGPR potentially improve growth of tomato plants in salt-stressed environment. Turk. J. Agric. Food Sci. Technol. 2016;4:455–463. doi: 10.24925/turjaf.v4i6.455-463.614. [DOI] [Google Scholar]
- 36.Chen L., Liu Y., Wu G., Njeri K.V., Shen Q., Zhang N. Induced maize salt tolerance by rhizosphere inoculation of Bacillus amyloliquefaciens SQR9. Physiol. Plant. 2016;158(1):34–44. doi: 10.1111/ppl.12441. [DOI] [PubMed] [Google Scholar]
- 37.Niu S.Q., Li H.R., Paré P.W., Aziz M., Wang S.M., Shi H. Induced growth promotion and higher salt tolerance in the halophyte grass Puccinellia tenuiflora by beneficial rhizobacteria. Plant Soil. 2016;407(1–2):217–230. doi: 10.1007/s11104-015-2767-z. [DOI] [Google Scholar]
- 38.Yan J., Smith M.D., Glick B.R., Liang Y. Effects of ACC deaminase containing rhizobacteria on plant growth and expression of Toc GTPases in tomato (Solanum lycopersicum) under salt stress. Botany. 2014;92:775–781. doi: 10.1139/cjb-2014-0038. [DOI] [Google Scholar]
- 39.Safdarian M., Askari H., Shariati J.V., Nematzadeh G. Transcriptional responses of wheat roots inoculated with Arthrobacter nitroguajacolicus to salt stress. Sci. Rep. 2019;9:1792. doi: 10.1038/s41598-018-38398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nautiyal C.S., Srivastava S., Chauhan P.S., Seem K., Mishra A., Sopory S.K. Plant growth-promoting bacteria Bacillus amyloliquefaciens NBRISN13 modulates gene expression profile of leaf and rhizosphere community in rice during salt stress. Plant Physiol. Biochem. 2013;66:1–9. doi: 10.1016/j.plaphy.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 41.Zhang H., Kim M.S., Sun Y., Dowd S.E., Shi H., Paré P.W. Soil bacteria confer plant salt tolerance by tissue-specific regulation of the sodium transporter HKT1, Mol. Plant Microbe Interact. 21(6) (2008) 737–744. doi:10.1094/ MPMI -21-6-07. [DOI] [PubMed]
- 42.Sukweenadhi J., Kim Y.J., Choi E., Koh S., Lee S., Kim Y. Paenibacillus yonginensis DCY84(T) induces changes in Arabidopsis thaliana gene expression against aluminum, drought, and salt stress. Microbiol. Res. 2015;172:7–15. doi: 10.1016/j.micres.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 43.Jha Y., Sablok G., Subbarao N., Sudhakar R., Fazil M.H.U.T., Subramanian R.B. Bacterial-induced expression of RAB18 protein in Orzya sativa salinity stress and insights into molecular interaction with GTP ligand. J. Mol. Recognit. 2014;27:521–527. doi: 10.1002/jmr.2371. [DOI] [PubMed] [Google Scholar]
- 44.Etesami H., Maheshwari D.K. Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: action mechanisms and future prospects. Ecotox. Environ. Safe. 2018;156:225–246. doi: 10.1016/j.ecoenv.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 45.Yasmin H., Naeem S., Bakhtawar M., Jabeen Z., Nosheen A., Naz R. Halotolerant rhizobacteria Pseudomonas pseudoalcaligenes and Bacillus subtilis mediate systemic tolerance in hydroponically grown soybean (Glycine max L.) against salinity stress. PLoS ONE. 2020;15(4) doi: 10.1371/journal.pone.0231348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kasotia A., Varma A., Choudhary D.K. Pseudomonas-mediated mitigation of salt stress and growth promotion in Glycine max. Agric. Res. 2015;4(1):31–41. doi: 10.1007/s40003-014-0139-1. [DOI] [Google Scholar]
- 47.Su A.Y., Niu S.Q., Liu Y.Z., He A.L., Zhao Q., Paré P.W. Synergistic effects of Bacillus amyloliquefaciens (GB03) and water retaining agent on drought tolerance of perennial ryegrass. Int. J. Mol. Sci. 2017;18(12):2651. doi: 10.3390/ijms18122651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang J.L., Aziz M., Qiao Y., Han Q.Q., Li J., Wang Y.Q. Soil microbe Bacillus subtilis (GB03) induces biomass accumulation and salt tolerance with lower sodium accumulation in wheat. Crop Pasture Sci. 2014;65(5):423–427. doi: 10.1071/CP13456. [DOI] [Google Scholar]
- 49.Han Q.Q., Lü X.P., Bai J.P., Qiao Y., Paré P.W., Wang S.M. Beneficial soil bacterium Bacillus subtilis (GB03) augments salt tolerance of white clover. Front. Plant Sci. 2014:525. doi: 10.3389/fpls.2014.00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han Q.Q., Wu Y.N., Gao H.J., Xu R., Paré P.W., Shi H. Improved salt tolerance of medicinal plant Codonopsis pilosula by Bacillus amyloliquefaciens GB03. Acta Physiol. Plant. 2017;39(1):35. doi: 10.1007/s11738-016-2325-1. [DOI] [Google Scholar]
- 51.Qin Y., Druzhinina I.S., Pan X., Yuan Z. Microbially mediated plant salt tolerance and microbiome-based solutions for saline agriculture. Biotechnol. Adv. 2016;34(7):1245–1259. doi: 10.1016/j.biotechadv.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 52.Singh R.P., Jha P.N. Alleviation of salinity-induced damage on wheat plant by an ACC deaminase-producing halophilic bacterium Serratia sp. SL-12 isolated from a salt lake. Symbiosis 69(2) 2016:101–111. doi: 10.1007/s13199-016-0387-x. [DOI] [Google Scholar]
- 53.Singh R.P., Runthala A., Khan S., Jha P.N. Quantitative proteomics analysis reveals the tolerance of wheat to salt stress in response to Enterobacter cloacae SBP-8. PLoS ONE. 2017;12(9) doi: 10.1371/journal.pone.0183513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu Y., Spollen W.G., Sharp R.E. Root growth maintenance at low water potentials: increased activity of xyloglucan endotransglyosylase and its possible regulation by abscisic acid. Plant Physiol. 1994;106:607–615. doi: 10.1104/pp.106.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tenhaken R. Cell wall remodeling under abiotic stress. Front. Plant Sci. 2015;771 doi: 10.3389/fpls.2014.00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Acosta-Motos J.R., Penella C., Hernández J.A., Díaz-Vivancos P., Sánchez-Blanco M.J., Navarro J.M. Towards a sustainable agriculture: Strategies involving phyto protectants against salt stress. Agronomy. 2020;10(2):194. doi: 10.3390/agronomy10020194. [DOI] [Google Scholar]
- 57.Zhu M., Zhou M., Shabala L., Shabala S. Linking osmotic adjustment and stomatal characteristics with salinity stress tolerance in contrasting barley accessions. Funct. Plant Biol. 2015;42(3):252–263. doi: 10.1071/FP14209. [DOI] [PubMed] [Google Scholar]
- 58.Sharma A., Shahzad B., Kumar V., Kohli S.K., Sidhu G.P.S., Bali A.S. Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules. 2019;9(7):285. doi: 10.3390/biom9070285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hayat S., Hayat Q., Alyemeni M.N., Wani A.S., Pichtel J., Ahmad A. Role of proline under changing environments: a review. Plant Signal. Behav. 2012;11:1456–1466. doi: 10.4161/psb.21949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Calvo-Polanco M., Sánchez-Castro I., Cantos M., García J.L., Azcón R., Ruiz-Lozano J.M. Effects of different arbuscular mycorrhizal fungal backgrounds and soils on olive plants growth and water relation properties under well-watered and drought conditions. Plant Cell Environ. 2016;39(11):2498–2514. doi: 10.1111/pce.12807. [DOI] [PubMed] [Google Scholar]
- 61.Hmaeid N., Wali M., Mahmoud O.M.B., Pueyo J.J., Ghnaya T., Abdelly C. Efficient rhizobacteria promote growth and alleviate NaCl-induced stress in the plant species Sulla carnosa. Appl. Soil Ecol. 2019;133:104–113. doi: 10.1016/j.apsoil.2018.09.011. [DOI] [Google Scholar]
- 62.Liu S., Hao H., Lu X., Zhao X., Wang Y., Zhang Y. Transcriptome profiling of genes involved in induced systemic salt tolerance conferred by Bacillus amyloliquefaciens FZB42 in Arabidopsis thaliana. Sci. Rep. 2017;7(1):10795. doi: 10.1038/s41598-017-11308-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scarpeci T.E., Zanor M.I., Mueller-Roeber B., Valle E.M. Overexpression of AtWRKY30 enhances abiotic stress tolerance during early growth stages in Arabidopsis thaliana. Plant Mol. Biol. 2013;83:265–277. doi: 10.1007/s11103-013-0090. [DOI] [PubMed] [Google Scholar]
- 64.Yan H., Jia H., Chen X., Hao L., An H., Guo X. The cotton WRKY transcription factor GhWRKY17 functions in drought and salt stress in transgenic Nicotiana benthamiana through ABA signaling and the modulation of reactive oxygen species production. Plant Cell Physiol. 2014;55:2060–2076. doi: 10.1093/pcp/pcu133. [DOI] [PubMed] [Google Scholar]
- 65.Moshelion M., Halperin O., Wallach R., Oren R.A.M., Way D.A. Role of aquaporins in determining transpiration and photosynthesis in water stressed plants: Crop water use efficiency, growth and yield. Plant Cell Environ. 2015;38(9):1785–1793. doi: 10.1111/pce.12410. [DOI] [PubMed] [Google Scholar]
- 66.Marulanda A., Azcón R., Chaumont F., Ruiz-Lozano J.M., Aroca R. Regulation of plasma membrane aquaporins by inoculation with a Bacillus megaterium strain in maize (Zea mays L.) plants under unstressed and salt-stressed conditions. Planta. 2010;232(2):533–543. doi: 10.1007/s00425-010-1196-8. [DOI] [PubMed] [Google Scholar]
- 67.Gond S.K., Bergen M.S., Torres M.S., White J.F., Jr. Endophytic Bacillus spp. produce antifungal lipopeptides and induce host defence gene expression in maize. Microbiol. Res. 2015;172:79–87. doi: 10.1016/j.micres.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 68.Yang A., Akhtar S.S., Iqbal S., Amjad M., Naveed M., Zahir Z.A. Enhancing salt tolerance in quinoa by halotolerant bacterial inoculation. Funct. Plant Biol. 2016;43(7):632–642. doi: 10.1071/FP15265. [DOI] [PubMed] [Google Scholar]
- 69.Mahmood S., Daur I., Al-Solaimani S.G., Ahmad S., Madkour M.H., Yasir M. Plant growth promoting rhizobacteria and silicon synergistically enhance salinity tolerance of mung bean. Front. Plant Sci. 2016;7:876. doi: 10.3389/fpls.2016.00876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hu W., Yuan Q., Wang Y., Cai R., Deng X., Wang J. Overexpression of a wheat aquaporin gene, TaAQP8, enhances salt stress tolerance in transgenic tobacco. Plant Cell Physiol. 2012;53:2127–2141. doi: 10.1093/pcp/pcs154. [DOI] [PubMed] [Google Scholar]
- 71.Naseem H., Ahsan M., Shahid M.A., Khan N. Exopolysaccharides producing rhizobacteria and their role in plant growth and drought tolerance. J. Basic Microbiol. 2018;58(12):1009–1022. doi: 10.1002/jobm.201800309. [DOI] [PubMed] [Google Scholar]
- 72.Bhaskar P.V., Bhosle N.B. Bacterial extracellular polymeric substance (EPS): A carrier of heavy metals in the marine food-chain. Environ. Int. 2006;32(2):191–198. doi: 10.1016/j.envint.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 73.Ercole C., Cacchio P., Botta A.L., Centi V., Lepidi A. Bacterially induced mineralization of calcium carbonate: the role of exopolysaccharides and capsular polysaccharides. Microsc. Microanal. 2007;13(1):42–50. doi: 10.1017/S1431927607070122. [DOI] [PubMed] [Google Scholar]
- 74.Costa O.Y., Raaijmakers J.M., Kuramae E.E. Microbial extracellular polymeric substances: Ecological function and impact on soil aggregation. Front. Microbiol. 2018;9:1636. doi: 10.3389/fmicb.2018.01636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Isfahani F.M., Tahmourespour A., Hoodaji M., Ataabadi M., Mohammadi A. Influence of Exopolysaccharide-Producing Bacteria and SiO 2 Nanoparticles on Proline Content and Antioxidant Enzyme Activities of Tomato Seedlings (Solanum lycopersicum L.) under. Salinity Stress, Pol. J. Environ. Stud. 28. 2019 doi: 10.15244/pjoes/81206. [DOI] [Google Scholar]
- 76.Sultana S., Paul S.C., Parveen S., Alam S., Rahman N., Jannat B. Isolation and identification of salt-tolerant plant-growth-promoting rhizobacteria and their application for rice cultivation under salt stress. Can. J. Microbiol. 2020;66 doi: 10.1139/cjm-2019-0323. [DOI] [PubMed] [Google Scholar]
- 77.Niu X., Song L., Xiao Y., Ge W. Drought-tolerant plant growth-promoting rhizobacteria associated with foxtail millet in a semi-arid agroecosystem and their potential in alleviating drought stress. Front. Microbiol. 2018;8:2580. doi: 10.3389/fmicb.2017.02580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kearl J., McNary C., Lowman J.S., Mei C., Aanderud Z.T., Smith S.T. Salt-tolerant halophyte rhizosphere bacteria stimulate growth of alfalfa in salty soil. Front. Microbiol. 2019;10:1849. doi: 10.3389/fmicb.2019.01849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chawla S., Jain S., Jain V. Salinity induced oxidative stress and antioxidant system in salt-tolerant and salt-sensitive cultivars of rice (Oryza sativa L.) J. Plant Biochem. Biotechnol. 2013;22:27–34. doi: 10.1007/s00709-011-0365-3. [DOI] [Google Scholar]
- 80.Islam F., Ali B., Wang J., Farooq M.A., Gill R.A., Ali S. Combined herbicide and saline stress differentially modulates hormonal regulation and antioxidant defense system in Oryza sativa cultivars. Plant Physiol. Biochem. 2016;107:82–95. doi: 10.1007/s13205-017-1074-1. [DOI] [PubMed] [Google Scholar]
- 81.Abd_Allah E.F., Alqarawi A.A., Hashem A., Radhakrishnan R., Al-Huqail A.A., Al-Otibi F.O.N. Endophytic bacterium Bacillus subtilis (BERA 71) improves salt tolerance in chickpea plants by regulating the plant defense mechanisms. J. Plant Interact. 2018;13:37–44. doi: 10.1080/17429145.2017.1414321. [DOI] [Google Scholar]
- 82.Jincy M., Djanaguiraman M., Jeyakumar P., Subramanian K., Jayasankar S., Paliyath G. Inhibition of phospholipase D enzyme activity through hexanal leads to delayed mango (Mangifera indica L.) fruit ripening through changes in oxidants and antioxidant enzymes activity. Sci. Hortic. 218. 2017:316–325. doi: 10.1016/j.scienta.2017.02.026. [DOI] [Google Scholar]
- 83.Sukweenadhi I., Balusamy S.R., Kim Y.J., Lee C.H., Kim Y.J., Koh S.C. A growth promoting bacteria, Paenibacillus yonginensis DCY84T enhanced salt stress tolerance by activating defense-related systems in Panax ginseng. Front. Plant Sci. 2018;9:813. doi: 10.3389/fpls.2018.00813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sharma P., Jha A.B., Dubey R.S., Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012;184:57–67. doi: 10.1155/2012/217037. [DOI] [Google Scholar]
- 85.Szymańska S., Dąbrowska G.B., Tyburski J., Niedojadło K., Piernik A., Hrynkiewicz K. Boosting the Brassica napus L. tolerance to salinity by the halotolerant strain Pseudomonas stutzeri ISE12. Environ. Exp. Bot. 2019;163:55–68. doi: 10.1016/j.envexpbot.2019.04.007. [DOI] [Google Scholar]
- 86.Santos A.D.A., Silveira J.A.G.D., Bonifacio A., Rodrigues A.C., Figueiredo M.D., Santos V.B. Antioxidant response of cowpea co-inoculated with plant growth-promoting bacteria under salt stress. Braz. J. Microbiol. 2018;49(3):513–521. doi: 10.1016/j.bjm.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.El-Tarabily K.A., Youssef T. Enhancement of morphological, anatomical and physiological characteristics of seedlings of the mangrove Avicennia marina inoculated with a native phosphate-solubilizing isolate of Oceanobacillu spicturae under greenhouse conditions. Plant Soil. 2010;332(1–2):147–162. doi: 10.1007/s11104-010-0280-y. [DOI] [Google Scholar]
- 88.Rashid M.I., Mujawar L.H., Shahzad T., Almeelbi T., Ismail I.M., Oves M. Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol. Res. 2016;183:26–41. doi: 10.1016/j.micres.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 89.Khan M.A., Asaf S., Khan A.L., Ullah I., Ali S., Kang S.M. Alleviation of salt stress response in soybean plants with the endophytic bacterial isolate Curtobacterium sp. SAK1. Ann. Microbiol. 2019;69(8):797–808. doi: 10.1007/s13213-019-01470-x. [DOI] [Google Scholar]
- 90.Mills HA, Jones Jr JB, Plant analysis handbook II. Athens. GA: MicroMacro Publishing, (1996), 185-414.
- 91.Mahmood I.A., Arshad A., Aslam M., Shahzad A., Sultan T., Hussain F. Phosphorus availability in different salt-affected soils as influenced by crop residue incorporation. Int. J. Agric. Biol. 2013;15(3) [Google Scholar]
- 92.Bashan Y., Moreno M., Troyo E. Growth promotion of the seawater-irrigated oilseed halophyte Salicornia bigelovii inoculated with mangrove rhizosphere bacteria and halotolerant Azospirillum spp. Biol. Fertil. Soils. 2000;32(4):265–272. doi: 10.1007/s003740000246. [DOI] [Google Scholar]
- 93.Dahiya S.S., Singh M. Effect of salinity, alkalinity and iron sources on availability of iron. Plant Soil. 1979;51(1):13–18. doi: 10.1007/BF02205922. [DOI] [Google Scholar]
- 94.Morrissey I., Guerinot M.L. Iron uptake and transport in plants: the good, the bad, and the ionome. Chem. Rev. 2009;109(10):4553–4567. doi: 10.1021/cr900112r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Morgan J.B., Connolly E.L. Plant-soil interactions: nutrient uptake. Nature Education Knowledge. 2013;4(8):2. [Google Scholar]
- 96.Mukherjee P., Mitra A., Roy M. Halomonas rhizobacteria of Avicennia marina of Indian Sundarbans promote rice growth under saline and heavy metal stresses through exopolysaccharide production. Front. Microbiol. 2019;10:1207. doi: 10.3389/fmicb.2019.01207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Goswami D., Dhandhukia P., Patel P., Thakker J.N. Screening of PGPR from saline desert of Kutch: growth promotion in Arachis hypogea by Bacillus licheniformis A2. Microbiol. Res. 2014;169(1):66–75. doi: 10.1016/j.micres.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 98.Saleem M.T., Ahmed N. Proceedings of the First National Congress on “Soil Science”. 1988. Soil fertility research and fertilizer use in Pakistan; pp. 93–97. [Google Scholar]
- 99.Hrynkiewicz K., Patz S., Ruppel S. Salicornia europaea L. as an underutilized saline-tolerant plant inhabited by endophytic diazotrophs. J. Adv. Res. 2019:49–56. doi: 10.1016/j.jare.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jha B., Gontia I., Hartmann A. The roots of the halophyte Salicornia brachiata are a source of new halotolerant diazotrophic bacteria with plant growth-promoting potential. Plant Soil. 2012;356(1–2):265–277. doi: 10.1007/s11104-011-0877-9. [DOI] [Google Scholar]
- 101.Kayasth I., Kumar V., Gera R. Gordonia sp.: a salt tolerant bacterial inoculant for growth promotion of pearl millet under saline soil conditions, 3. Biotech. 2014:553–557. doi: 10.1007/s13205-013-0178-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sorty A.M., Meena K.K., Choudhary K., Bitla U.M., Minhas P.S., Krishnani K.K. Effect of plant growth promoting bacteria associated with halophytic weed (Psoralea corylifolia L) on germination and seedling growth of wheat under saline conditions. Appl. Biochem. 2016;180(5):872–882. doi: 10.1007/s12010-016-2139-z. [DOI] [PubMed] [Google Scholar]
- 103.Rojas-Tapias D., Moreno-Galván A., Pardo-Díaz S., Obando M., Rivera D., Bonilla R. Effect of inoculation with plant growth-promoting bacteria (PGPB) on amelioration of saline stress in maize (Zea mays) Appl. Soil. Ecol. 2012;61:264–272. doi: 10.1016/j.apsoil.2012.01.006. [DOI] [Google Scholar]
- 104.Nabti E., Sahnoune M., Ghoul M., Fischer D., Hofmann A., Rothballer M. Restoration of growth of durum wheat (Triticum durum var. waha) under saline conditions due to inoculation with the rhizosphere bacterium Azospirillum brasilense NH and extracts of the marine alga Ulva lactuca. J. Plant Growth Regul. 2010;29(1):6–22. doi: 10.1007/s00344-009-9107-6. [DOI] [Google Scholar]
- 105.Glick B.R. Plant growth-promoting bacteria: mechanisms and applications. Scientifica. 2012 doi: 10.6064/2012/963401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nabti E., Schmid M., Hartmann A. Application of halotolerant bacteria to restore plant growth under salt stress. In: Maheshwari DK, Saraf M, Halophiles, editors. Biodiversity and Sustainable Exploitation. Springer; Cham: 2015. pp. 235–259. [DOI] [Google Scholar]
- 107.Glick B.R., Cheng Z., Czarny J., Duan J. Promotion of plant growth by ACC deaminase-producing soil bacteria. Eur. J. Plant Pathol. 2007;119:329–339. doi: 10.1007/s10658-007-9162-4. [DOI] [Google Scholar]
- 108.Ojuederie O.B., Olanrewaju O.S., Babalola O.O. Plant growth promoting rhizobacterial mitigation of drought stress in crop plants: Implications for sustainable agriculture. Agronomy. 2019;9(11):712. doi: 10.3390/agronomy9110712. [DOI] [Google Scholar]
- 109.Glick B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014;169(1):30–39. doi: 10.1016/j.micres.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 110.Sziderics A.H., Rasche F., Trognitz F., Sessitsch A., Wilhelm E. Bacterial endophytes contribute to abiotic stress adaptation in pepper plants (Capsicum annuum L.) Can. J. Microbiol. 2007;53(11):1195–1202. doi: 10.1139/W07-082. [DOI] [PubMed] [Google Scholar]
- 111.Ilangumaran G., Smith D.L. Plant growth promoting rhizobacteria in amelioration of salinity stress: a systems biology perspective. Front. Plant Sci. 2017;8:1768. doi: 10.3389/fpls.2017.01768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Saakre M., Baburao T.M., Salim A.P., Ffancies R.M., Achuthan V.P., Thomas G. Identification and characterization of genes responsible for drought tolerance in rice mediated by Pseudomonas fluorescens. Rice Sci. 2017;24(5):291–298. doi: 10.1016/j.rsci.2017.04.005. [DOI] [Google Scholar]
- 113.Maggio A., Barbieri G., Raimondi G., De Pascale S. Contrasting effects of GA 3 treatments on tomato plants exposed to increasing salinity. J. Plant Growth Regul. 2010;29(1):63–72. doi: 10.1007/s00344-009-9114-7. [DOI] [Google Scholar]
- 114.Kang S.M., Radhakrishnan R., Khan A.L., Kim M.J., Park J.M., Kim B.R. Gibberellin secreting rhizobacterium, Pseudomonas putida H-2-3 modulates the hormonal and stress physiology of soybean to improve the plant growth under saline and drought conditions. Plant Physiol. Biochem. 2014;84:115–124. doi: 10.1016/j.plaphy.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 115.Waśkiewicz A, Gładysz O, Goliński P. Plant Hormones under Challenging Environmental Factors. In: Ahammed GJ, Yu JQ, eds. Participation of phytohormones in adaptation to salt stress. Dordrecht: Springer; 2016. p. 75-115. doi: 10.1007/978-94-017-7758-2_4.
- 116.Goswami D., Thakker J.N., Dhandhukia P.C. Portraying mechanics of plant growth promoting rhizobacteria (PGPR): A review. Cogent Food Agric. 2016;2(1):1127500. doi: 10.1080/23311932.2015.1127500. [DOI] [Google Scholar]
- 117.Sandhya V.S.K.Z., Ali S.Z., Grover M., Reddy G., Venkateswarlu B. Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress. Plant Growth Regul. 2010;62(1):21–30. doi: 10.1007/s10725-010-9479-4. [DOI] [Google Scholar]
- 118.Kudoyarova G., Arkhipova T.N., Korshunova T., Bakaeva M., Loginov O., Dodd I.C. Phytohormone mediation of interactions between plants and non-symbiotic growth promoting bacteria under edaphic stresses. Front. Plant Sci. 2019;10:1368. doi: 10.3389/fpls.2019.01368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang J., Kloepper J.W., Ryu C.M. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 2009;14(1):1–4. doi: 10.1016/j.tplants.2008.10.004-8739-5_4. [DOI] [PubMed] [Google Scholar]
- 120.Sharma S., Chen C., Navathe S., Chand R., Pandey S.P. A halotolerant growth promoting rhizobacteria triggers induced systemic resistance in plants and defends against fungal infection. Sci. Rep. 2019;9(1):1–17. doi: 10.1038/s41598-019-40930-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schikora A., Schenk S.T., Hartmann A. Beneficial effects of bacteria-plant communication based on quorum sensing molecules of the N-acyl homoserine lactone group. Plant Mol. Biol. 2016;90(6):605–612. doi: 10.1007/s11103-016-0457-8. [DOI] [PubMed] [Google Scholar]
- 122.Rosier A., Medeiros F.H., Bais H.P. Defining plant growth promoting rhizobacteria molecular and biochemical networks in beneficial plant-microbe interactions. Plant Soil. 2018;428(1–2):35–55. doi: 10.1007/s11104-018-3679-5. [DOI] [Google Scholar]
- 123.Hartmann A., Fischer D., Kinzel L., Chowdhury S.P., Hofmann A., Baldani J.I. Assessment of the structural and functional diversities of plant microbiota: achievements and challenges – a review. J. Adv. Res. 2019;19:3–13. doi: 10.1016/j.jare.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhao Q., Xiang-Yun Y., Yao L., Fang L., Xiang–Yu C., Zhen-Hua Jia J., Shui-Shan S. N-3-oxo-hexanoyl-homoserine lactone, a bacterial quorum sensing signal, enhances salt tolerance in Arabidopsis and wheat. Bot. Stud. 2020;61(8) doi: 10.1186/s40529-020-00283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Filgueiras L., Silva R., Almeida I., Vidal M., Baldani J.I., Meneses C.H.S.G. Gluconacetobacter diazotrophicus mitigates drought stress in Oryza sativa L. Plant Soil. 2019:1–17. doi: 10.1007/s11104-019-04163-1. [DOI] [Google Scholar]
- 126.Silva R., Filgueiras L., Santos B., Coelho M., Silva M., Estrada-Bonilla G. Gluconacetobacter diazotrophicus Changes the Molecular Mechanisms of Root Development in Oryza sativa L. Growing Under Water Stress, Int. J. Mol. Sci. 2020;21(1):333. doi: 10.3390/ijms21010333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Arora S, Vanza M. Microbial approach for bioremediation of saline and sodic soils. In: Arora S, Singh AK, Singh YP, eds. Bioremediation of Salt Affected Soils: An Indian Perspective. Cham: Springer; 2017. p. 87-100. doi:10.1007/978-3-319-48257-6.
- 128.Sandhya V.D., Ali S. The production of exopolysaccharide by Pseudomonas putida GAP-P45 under various abiotic stress conditions and its role in soil aggregation. Microbiology. 2015;84:512–519. doi: 10.1134/S0026261715040153. [DOI] [Google Scholar]
- 129.Arora S., Singh Y., Vanza M., Sahni D. Bio-remediation of saline and sodic soils through halophilic bacteria to enhance agricultural production. J. Soil Water Conserv. 2016;15:302–305. doi: 10.5958/2455-7145.2016.00027.8. [DOI] [Google Scholar]
- 130.Mishra I, Arora NK. Rhizoremediation: a sustainable approach to improve the quality and productivity of polluted soils. In: Arora NK, Narendra K, eds. Phyto and Rhizo Remediation. Singapore: Springer; 2019. p. 33-66. doi:10.1007/978-981-32-9664-0_2.
- 131.Verma M, Mishra J, Arora NK. Environmental Biotechnology: For Sustainable Future. In: Sobti R, Arora NK, et al, eds. Plant growth-promoting rhizobacteria: diversity and applications. Singapore: Springer; 2019. p. 129-173. doi:10.1007/978-981-10-7284-0_6.
- 132.Arif M.S., Shahzad S.M., Riaz M., Yasmeen T., Shahzad T., Akhtar M.J. Nitrogen-enriched compost application combined with plant growth-promoting rhizobacteria (PGPR) improves seed quality and nutrient use efficiency of sunflower. J. Plant Nutri. Soil Sci. 2017;180(4):464–473. doi: 10.1007/978-3-319-58841-4_21. [DOI] [Google Scholar]
- 133.Zahran H.H. Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol. Mol. Biol. Rev. 1999;63(4):968–989. doi: 10.1128/MMBR.63.4.968-989.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hassan T.U., Bano A., Naz I. Halophyte root powder: an alternative biofertilizer and carrier for saline land. J. Soil Sci. Plant Nutri. 2018;64(5):653–661. doi: 10.1080/00380768.2018.1509676. [DOI] [Google Scholar]
- 135.Hassan T.U., Bano A. Construction of IAA-deficient mutants of Pseudomonas moraviensis and their comparative effects with wild type strains as bio-inoculant on wheat in saline sodic soil. Geomicrobiology. 2019;36(4):376–384. doi: 10.1080/01490451.2018.1562498. [DOI] [Google Scholar]
- 136.Al-Enazy A., Al-Barakah F., Al-Oud S., Usman A. Effect of phosphogypsum application and bacteria co-inoculation on biochemical properties and nutrient availability to maize plants in a saline soil. Arch. Agron. Soil Sci. 2018;64:1394–1406. doi: 10.1080/03650340.2018.1437909. [DOI] [Google Scholar]
- 137.Rillig M.C., Muller L.A., Lehmann A. Soil aggregates as massively concurrent evolutionary incubators. ISME J. 2017;11(9):1943–1948. doi: 10.1038/ismej.2017.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Siebers N., Kruse J. Short-term impacts of forest clear-cut on soil structure and consequences for organic matter composition and nutrient speciation: A case study. PLoS ONE. 2019;14(8) doi: 10.1371/journal.pone.0220476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Arora N.K., Fatima T., Mishra I., Verma S. Microbe-based inoculants: role in next green revolution. In: Shukla V., Kumar N., editors. Environmental Concerns and Sustainable Development. Springer; Singapore: 2020. pp. 191–246. [DOI] [Google Scholar]
- 140.Qurashi A.W., Sabri A.N. Bacterial exopolysaccharide and biofilm formation stimulate chickpea growth and soil aggregation under salt stress. Braz. J. Microbiol. 2012;43:1183–1191. doi: 10.1590/S1517-838220120003000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Boukhelata N., Taguett F., Kaci Y. Characterization of an extracellular polysaccharide produced by a Saharan bacterium Paenibacillus tarimensis REG 0201M. Ann. Microbiol. 2019;69(2):93–106. doi: 10.1007/s13213-018-1406-3. [DOI] [Google Scholar]
- 142.Jamil N., Ahmed N. Production of biopolymers by Pseudomonas aeruginosa isolated from marine source. Braz. Arch. Biol. 2008;51(3):457–464. doi: 10.1590/S1516-89132008000300004. [DOI] [Google Scholar]
- 143.Kaci Y., Heyraud A., Barakat M., Heulin T. Isolation and identification of an EPS-producing Rhizobium strain from arid soil (Algeria): characterization of its EPS and the effect of inoculation on wheat rhizosphere soil structure. Res. Microbiol. 2005;156(4):522–531. doi: 10.1016/j.resmic.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 144.Arora N.K., Mishra J. Prospecting the roles of metabolites and additives in future bioformulations for sustainable agriculture. Appl. Soil Ecol. 2016;107:405–407. doi: 10.1016/j.apsoil.2016.05.020. [DOI] [Google Scholar]
- 145.Omar S.A., Abdel-Sater M.A., Khallil A.M., Abdalla M.H. Growth and enzyme activities of fungi and bacteria in soil salinized with sodium chloride. Folia Microbiol. 1994;39(1):23–28. doi: 10.1007/BF02814524. [DOI] [Google Scholar]
- 146.Burns R.G., DeForest J.L., Marxsen J., Sinsabaugh R.L., Stromberger M.E., Wallenstein M.D. Soil enzymes in a changing environment: current knowledge and future directions. Soil Biol. Biochem. 2013;58:216–234. doi: 10.1016/j.soilbio.2012.11.009. [DOI] [Google Scholar]
- 147.Canfora L., Bacci G., Pinzari F., Lo Papa G., Dazzi C., Benedetti A. Salinity and bacterial diversity: to what extent does the concentration of salt affect the bacterial community in a saline soil?, PLoS. ONE 9(9) 2014 doi: 10.1371/journal.pone.0106662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lipińska A., Wyszkowska J., Kucharski J. Diversity of organotrophic bacteria, activity of dehydrogenases and urease as well as seed germination and root growth Lepidium sativum, Sorghum saccharatum and Sinapis alba under the influence of polycyclic aromatic hydrocarbons. Environ. Sci. Pollut. Res. Int. 2015;22(23):18519–18530. doi: 10.1007/s11356-015-5329-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Islam S., Akanda A.M., Prova A., Islam M.T., Hossain M.M. Isolation and identification of plant growth promoting rhizobacteria from cucumber rhizosphere and their effect on plant growth promotion and disease suppression. Front. Microbiol. 2015;6:1360. doi: 10.3389/fmicb.2015.01360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Meena K.K., Sorty A.M., Bitla U.M., Choudhary K., Gupta P., Pareek A. Abiotic stress responses and microbe-mediated mitigation in plants: the omics strategies. Front. Plant Sci. 2017;8:172. doi: 10.3389/fpls.2017.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yadav BS, Mani A. Microbial Genomics in Sustainable Agroecosystems In: Tripathi V, Kumar P, et al, eds. Functional genomics and systems biology approach for understanding agroecosystems. Springer: Singapore; 2019. p. 67-86. doi:10.1007/978-981-13-8739-5_4.
- 152.Rodriguez I.A., Rothballer M., Chowdhury S.P., Nussbaumer T., Gutjahr C., Falter-Braun P. Systems biology of plant-microbiome interactions. Mol. Plant. 2019;12:804–821. doi: 10.1016/j.molp.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 153.Zhou N., Zhao S., Tian C. Effect of halotolerant rhizobacteria isolated from halophytes on the growth of sugar beet (Beta vulgaris L.) under salt stress. FEMS Microbiol. Lett. 2017;364:11. doi: 10.1093/femsle/fnx091. [DOI] [PubMed] [Google Scholar]
- 154.Arif F., Ghoul M. Halotolerance of indigenous fluorescent pseudomonads in the presence of natural osmoprotectants. Annu. Res. Rev. Biol. 2018:1–11. doi: 10.9734/ARRB/2018/39690. [DOI] [Google Scholar]
- 155.Ansari F.A., Ahmad I. Plant growth promoting attributes and alleviation of salinity stress to wheat by biofilm forming Brevibacterium sp. FAB3 isolated from rhizospheric soil, Saudi. J. Biol. Sci. 2018 doi: 10.1016/j.sjbs.2018.08.003. [DOI] [Google Scholar]