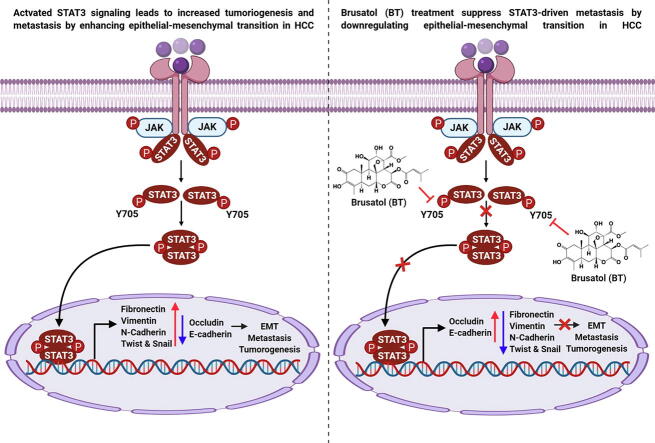
Graphical abstract
Keywords: EMT, Brusatol, HCC, Orthotopic mouse model, Metastasis
Highlights
-
•
Brusatol affects migration and invasion ability of HCC cells.
-
•
Brusatol affects EMT process through modulation of STAT3 activation pathway.
-
•
Brusatol mitigates tumorigenesis and metastasis in HCC preclinical model.
Abstract
Introduction
Epithelial-mesenchymal transition (EMT) is a process of transdifferentiation where epithelial cells attain mesenchymal phenotype to gain invasive properties and thus, can contribute to metastasis of tumor cells.
Objectives
The antimetastatic and antitumor efficacy of brusatol (BT) was investigated in a hepatocellular carcinoma (HCC) model.
Methods
We evaluated the action of BT on EMT process using various biological assays in HCC cell lines and its effect on tumorigenesis in an orthotopic mouse model.
Results
We found that BT treatment restored the expression of Occludin, E-cadherin (epithelial markers) while suppressing the levels of different mesenchymal markers in HCC cells and tumor tissues. Moreover, we observed a decline in the expression of transcription factors (Snail, Twist). Since the expression of these two factors can be regulated by STAT3 signaling, we deciphered the influence of BT on modulation of this pathway. BT suppressed the phosphorylation of STAT3Y705 and STAT3 depletion using siRNA resulted in the restoration of epithelial markers. Importantly, BT (1mg/kg) reduced the tumor burden in orthotopic mouse model with a concurrent decline in lung metastasis.
Conclusions
Overall, our results demonstrate that BT interferes with STAT3 induced metastasis by altering the expression of EMT-related proteins in HCC model.
Introduction
Metastasis is a process of dislodging of cancer cells from the primary tumor and their dissemination to the different organs through lymphatic system or circulation [1], [2], [3], [4], [5]. Metastasis contributes to about 90% of cancer-related deaths [6], [7]. The five-year survival rate of various early-stage cancers is above 50% and it falls to below 20% when cancer cells are metastasized to distant tissues [8], [9]. During metastasis, the immobile epithelial cancer cell undergoes trans differentiation to attain a mesenchymal phenotype that can permeate via extracellular matrix (ECM) through a process of epithelial-mesenchymal transition (EMT) [10], [11]. The mesenchymal phenotypes exhibit stem cell properties, enhanced production of ECM components along with increased cellular motility, and apoptotic resistance [12], [13], [14], [15].
EMT can be modulated by diverse transcription factors including zinc-finger E-box-binding (ZEB), Twist, Snail, and Slug [16], [17], [18]. STAT3 is frequently overactivated in different tumors including hepatocellular carcinoma (HCC) and can positively correlate with tumorigenicity, EMT, antiapoptosis, and metastasis [19], [20], [21], [22]. IL-6 activates STAT3 to promote EMT through the induction of Snail expression in cancers [8], [23]. Activated STAT3 can also induce the transcription of the Twist gene to promote oncogenic functions [24]. Therefore, it may be concluded that targeting STAT3 may be an appropriate strategy to counteract EMT and metastasis in advanced cancers.
Brusatol (BT) is a natural quassinoid that has been demonstrated as an inhibitor of nuclear factor erythroid 2-related factor-2 (Nrf-2) by several research groups [25]. BT can interfere with Nrf-2 signaling in cancer cells to enhance the chemotherapeutic potential of paclitaxel, cisplatin, 5-fluorouracil, gemcitabine, carboplatin, and etoposide [26], [27], [28] and also increase the radiosensitivity of lung cancer cells [29]. In addition, many studies have reported that various cellular targets can be affected by BT such as c-Myc, HIF-1α, JNK and p38 MAPK, and PI3K/Akt pathways [30]. On the contrary, Vartanian et al demonstrated that BT can interfere with global protein synthesis [31]. Moreover, Harder et al demonstrated that BT can localize in the endoplasmic reticulum of the cancer cells and terminate the cap-dependent and cap-independent protein translation which may affect various short-lived proteins including Nrf-2 [32]. The deficiency of succinate dehydrogenase subunit B (SDHB) is often observed in pheochromocytomas and paragangliomas (PCPGs) and they possess higher levels of ROS. Liu and colleagues reported that knockdown of SDHB (SDHBKD) in PCPGs resulted in increased cellular ROS levels and transcriptional activity of Nrf-2 [33]. Subsequently, the treatment of SDHBKD cells with BT disrupted Nrf-2 dependent transcriptional activity and induced oxidative DNA damage [33]. The same group also demonstrated that isocitrate dehydrogenase (IDH) 1-mutated glioma cells are dependent on Nrf-2 signaling cascade. The inhibition of Nrf-2 by BT increased oxidative damage to DNA with reduction in proliferation of IDH1-mutated cells [34]. They also reported that Nrf-2 can promote glutathione synthesis and thereby display protective function towards IDH1-mutated cells [35]. The abrogation of Nrf2/GSH pathway by BT resulted in potent anticancer effect on IDH1-mutated preclinical cancer models [35]. In addition, the effect of BT on glutathione metabolism, ROS production, and chemoresistance in breast cancer has been reported in the literature [33], [34], [35], [36], [37]. Besides, Yang et al described the effect of BT on various types of cancer cells, Nrf-2-guided gene transcription, and glutathione de novo synthesis [37]. In our previous report, we had reported that BT can effectively abrogate STAT3 phosphorylation in head and neck squamous cell carcinoma (HNSCC) cells, but did not analyze its actions on EMT process and tumor growth in preclinical settings [38]. Since, STAT3 is an inducer of EMT, we have assessed here whether the influence of BT on EMT may be mediated through the modulation of STAT3 in a HCC model.
Materials and methods
Reagents
Brusatol (BT) (CAS: 14907–98-3, purity ≥ 98% by HPLC analysis) was isolated from Bruceae
Fructus in our laboratory and its structural identity was confirmed by comparing its NMR and HRMS data with those published previously [27]. It was dissolved in dimethyl sulfoxide (DMSO) to prepare a stock (10 mM), stored at −80℃. Further, stock solution was diluted with culture medium as per experimental requirement. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), Sodium dodecyl sulfate (SDS), DMSO, and ribonuclease A were obtained from Sigma–Aldrich (St. Louis, MO, USA). Anti-Fibronectin, anti-Vimentin, anti-E-cadherin, anti-N-cadherin, anti-Occludin, and anti-Twist antibodies (diluent, 1: 5000) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-Snail, anti-p-STAT3 (Tyr 705), and anti-STAT3 antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA).
Cell culture
HCCLM3 cell line was obtained from from Prof Zhao-You Tang“s laboratory at The Liver Cancer Institute and Zhongshan Hospital, Fudan University, Shanghai China. This cell line has been completely characterized and published previously by us and others [39], [40]. They were maintained in DMEM containing 10% FBS and 1X penicillin/streptomycin.
Cell growth analysis
The growth behavior on BT treatment of cells was observed by xCELLigence RTCA DP instrument as done previously [41], [42], [43], [44]. HCCLM3 cells (5 103 cells/well) were seeded on E-plate. Then cells were treated by BT (0, 5, 10 nM) for 72 h, and analyzed every 15 min time intervals.
Western blot analysis
Western blotting was executed as elaborated before [45], [46], [47], [48].
Real-time polymerase chain reaction
Total RNA was extracted using Trizol and PCR was done as elaborated upon previously [49].
Immunocytochemistry (ICC) for Vimentin and Occludin localization
Immunocytochemistry was carried out as per the prior reported protocol [50].
Invasion assay
Roche xCELLigence Real-Time Cell Analyzer instrument was used to calculate invasion as reported formerly [51], [52], [53].
Boyden chamber assay
In vitro invasion assay was executed using micro chemotaxis Boyden chamber as described earlier [54]. Matrigel-coated 8-μm polycarbonate membrane was prepared on trans-well chamber. HCCLM3 cells (2 × 104 cells/well) were seeded on top chamber with BT (10 nM) in medium then incubated at 37 °C in 5% CO2 conditions.
siRNA transfections
siRNA transfection was carried out as described earlier [55]. To determine whether BT interferes with EMT through modulating STAT3 signaling, HCCLM3 cells were transfected with STAT3 siRNA (Santa Cruz Biotechnology [sc-29493]) and scrambled control with transfection reagent (Intron Biotechnology, Seoul, Korea).
Acute toxicity studies
The study was conducted as per the protocol approved by the SingHealth Institutional Animal Use and Care Committee (protocol number: 2013/SHS/870). Thereafter the experiments were performed using eight-week-old NCr nude female mice following treatment with intraperitoneal injections of 5 and 15 mg/kg of BT, and vehicle (0.1% DMSO) as described previously [56].
Preclinical experiments
In vivo experiments were performed as per the protocol approved by the SingHealth Institutional Animal Use and Care Committee (protocol number: 2013/SHS/870). NCr nude mice were injected subcutaneously with 100 μl of HCCLM3-Luc cells (5 x106) in the right flank region. After tumor reaching the size of 1 cm3, it was removed and cut into small pieces of 2 mm3 and placed into the liver of NCr nude mice orthotopically. Tumor development was measured weekly twice by quantifying the bioluminescence signals after intraperitoneal injection of BT (1 mg/kg) twice a week, for four weeks.
Statistical analysis
The significance of differences between groups was evaluated by Student’s t-test and one-way analysis of variance, (ANOVA) test. p < 0.05 was considered as statistically significant. * p < 0.05; ** p < 0.01 and *** p < 0.001. All results are presented as the mean ± S.D. of three independent experiments.
Results
BT moderately affects proliferation of HCC cells
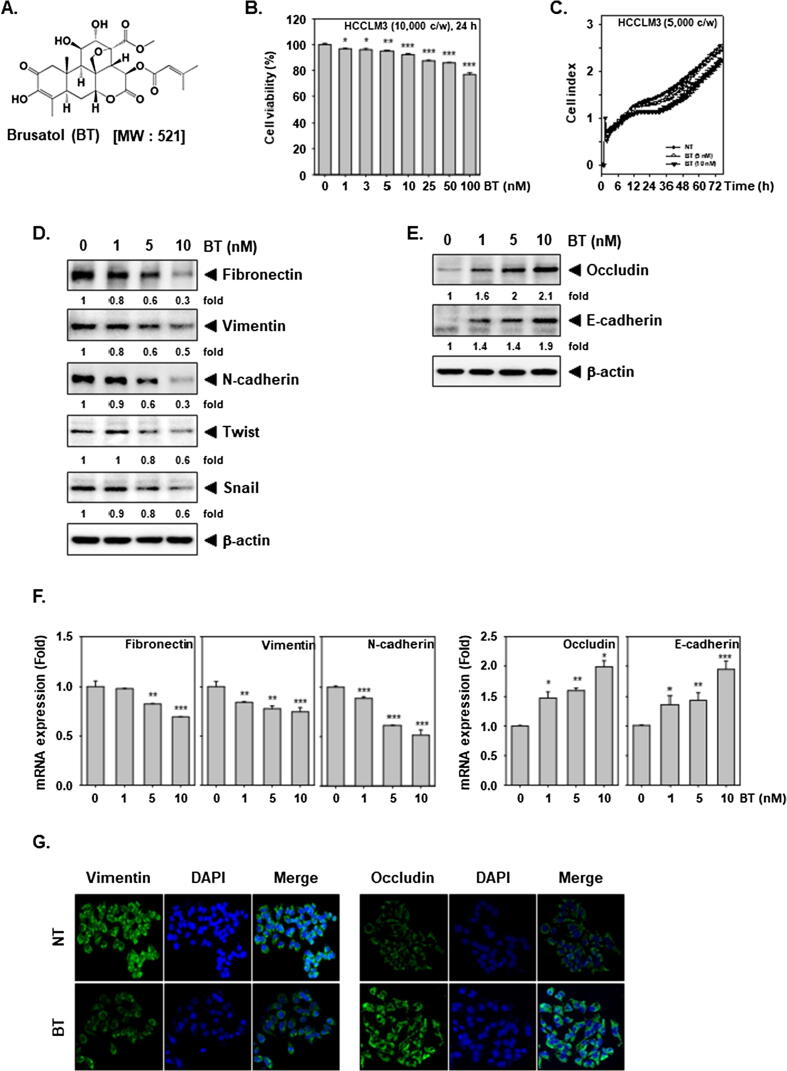
Firstly, the action of BT (structure shown in Fig. 1A) on viability/proliferation of HCC cells was elucidated. BT modestly decreased the cell viability of HCCLM3 cells (Fig. 1B), and the differences in the proliferation were observed at 5 and 10 nM doses (Fig. 1C).
Fig. 1.
BT changes the levels of EMT markers. (A) The structure of BT. (B) HCCLM3 cells were exposed to BT (0, 1, 3, 5, 10, 25, 50, 100 nM) for 24 h and viability was calculated by MTT method. (C) HCCLM3 cells were exposed to BT and proliferation assay was performed using RTCA for 72 h. (D-E) HCCLM3 cells were exposed to BT for 24 h and Western blotting was executed. (F) Total RNA was measured via real-time PCR for levels of different genes. * p < 0.05; ** p < 0.01 and *** p < 0.001 as measured by (G) HCCLM3 cells were exposed to 10 nM of BT for 24 h, and then distribution of Vimentin and Occludin was studied by immunocytochemistry.
BT alters the transcription and protein expression of EMT-related proteins
We then evaluated the effects of BT on EMT markers. It reduced the protein expression of Fibronectin, Vimentin, N-cadherin, Twist, and Snail (Fig. 1D) and increased expression of Occludin, and E-cadherin (Fig. 1E). In addition, we also noted that mRNA levels of Fibronectin, Vimentin, N-cadherin were attenuated (Fig. 1F left) whereas Occludin and E-cadherin mRNAs were elevated upon BT exposure (Fig. 1F right). We analyzed the expression of Vimentin and Occludin in control and BT-treated cells using immunofluorescence. BT impeded the level of Vimentin but triggered that of Occludin (Fig. 1G) and thus can influence the EMT process.
BT suppresses migration as well as invasion in HCC cells
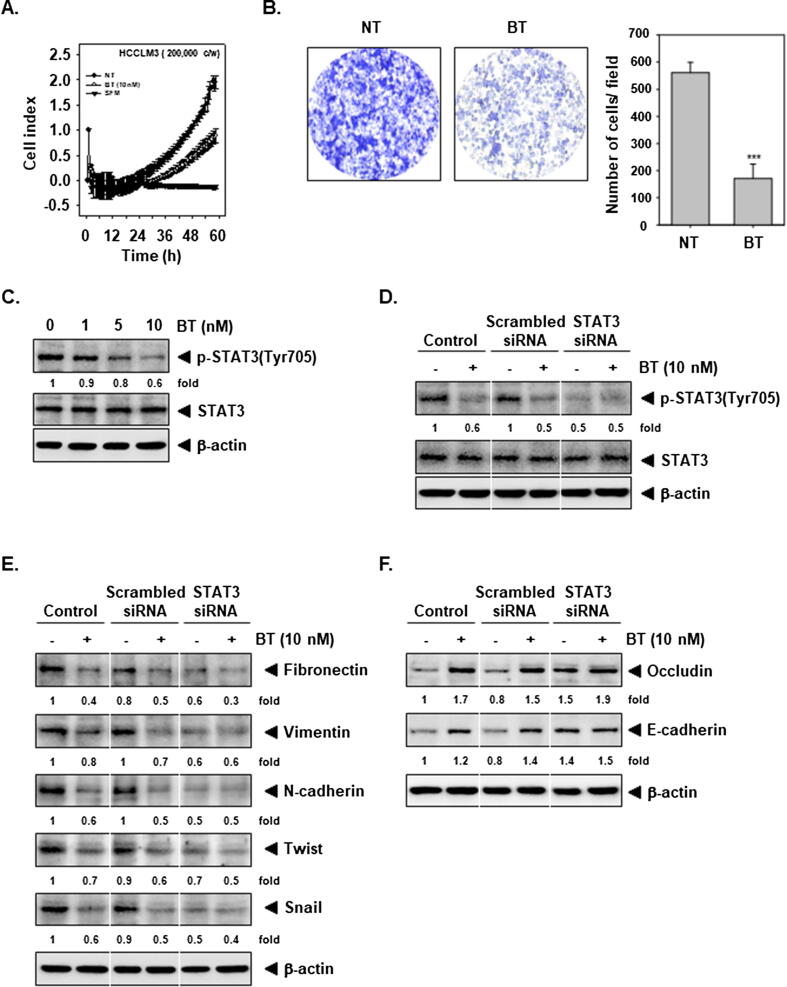
Next, whether BT regulates HCCLM3 cell migration was explored using xCELLigence RTCA DP and Boyden chamber assay. Interestingly, it was also noted that BT substantially counteracted the invasiveness of HCCLM3 cells (Fig. 2A). In addition, HCCLM3 cells appeared to be able to migrate efficiently as noted in Boyden chamber assay but BT inhibited the cell migration (Fig. 2B). These data suggested that BT can reduce cancer cell motility in vitro.
Fig. 2.
BT reduces invasion and blocks the STAT3 pathway. (A) HCCLM3 invasive activity in Matrigel-coated plate was determined. (B) HCCLM3 cells were exposed to 10 nM of BT for 8 h and invasion assay was done. (C) HCCLM3 cells were exposed to BT for 4 h and Western blot was executed. (D) HCCLM3 cells were transiently transfected with scrambled or STAT3 siRNA and then exposed to10 nM of BT for 4 h and blotting was carried out. (E-F) Transfection was done with 50 nM STAT3 siRNA or scrambled siRNA for 24 h as narrated above in D. The cells were processed as narrated in C and blotting was conducted.
BT inhibits constitutively active STAT3 in HCC cells
Our previous report suggests that BT can modulate STAT3 signaling in HNSCC cells and since this transcription factor can regulate the EMT process. Therefore, the action of BT on phosphorylation of STAT3Y705 in HCCLM3 cells was deciphered. It was noted that BT concentration-dependently inhibited constitutive STAT3 activation (Fig. 2C), thus suggesting that BT may affect EMT through targeting STAT3 pathway.
BT regulates EMT through affecting STAT3 signaling
To decipher the possible role of STAT3 in modulating EMT, we carried out the transient transfection using STAT3 siRNA. Fig. 2D indicates that STAT3-siRNA transfection successfully depleted STAT3 from the cells. In parallel, knockdown of STAT3 using siRNA can substantially reverse the alteration of EMT markers expression by BT (Fig. 2E and 2F).
BT did not exhibit toxicity in preclinical studies
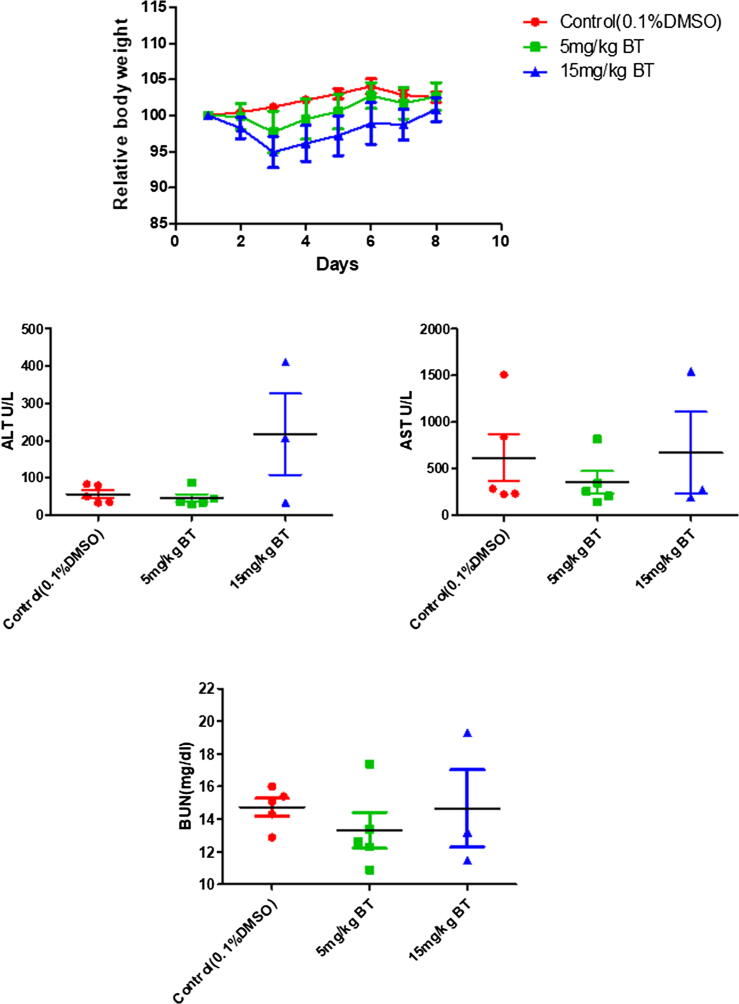
Initially, we conducted acute toxicity studies to identify if any adverse effects can be noted in mice treated with BT. We found that no mortality in mice was observed and BT-treated animals did not show major alterations in feed and water consumption, and body weight. We also noticed that there were no variations in the biochemical parameters of serum in BT-treated groups such as blood urea nitrogen (BUN), alanine aminotransferase (ALT), and aspartate aminotransferase (AST). Overall, these results suggest that BT treatment did not impart notable toxicity in tested mice (Fig. 3).
Fig. 3.
The consequence of intraperitoneal administration of BT on body weight change and various biochemical parameters was measured. The nude mice n = 5 per group were exposed to one single dose of BT (5 or 15 mg/kg) and 0.1% DMSO control.
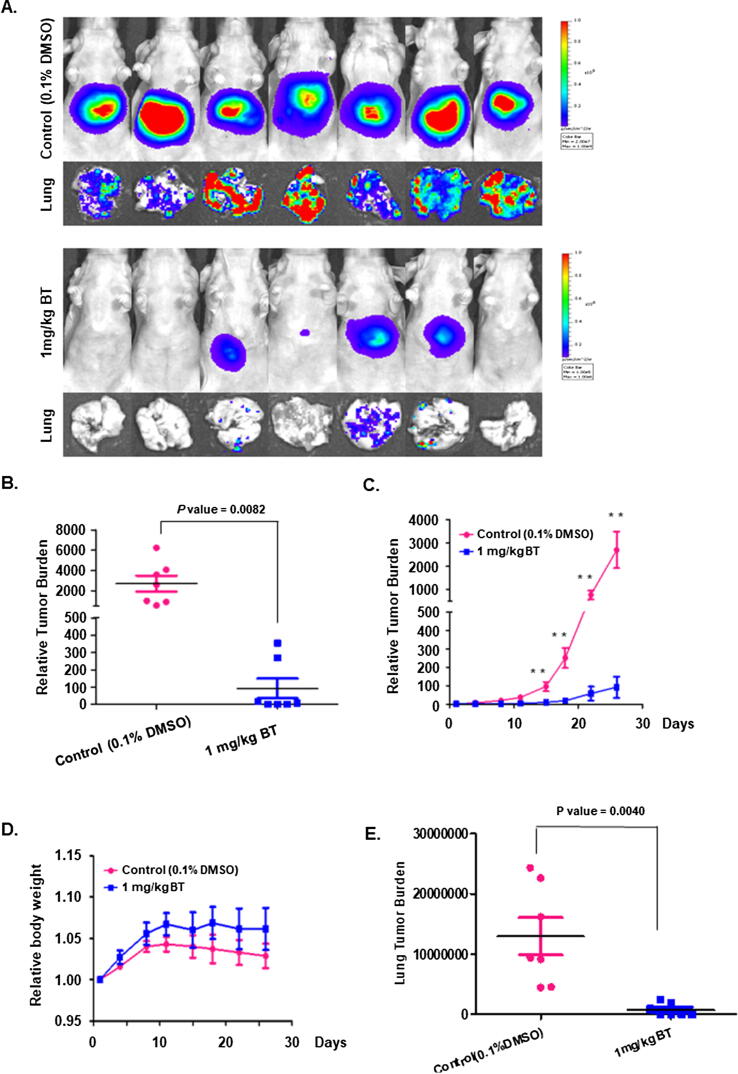
BT attenuates tumorigenesis and metastasis in vivo
We next established an orthotopic HCC mouse model as described in methods and examined the antitumor activity of BT. The intraperitoneal injection of BT (1 mg/kg, twice a week for four weeks) dramatically reduced tumor burden (Fig. 4A-C). The tumor burden was quantified by measuring photon counts before the first administration of BT and at the last dose as described in our previous studies [57]. We also observed a slight increase in body weight of mice in the brusatol treated group compared to vehicle control mice, however no significant difference observed. The mice in both the groups were found to be healthy (Fig. 4D). Interestingly, a significant decrease in metastasis to lungs upon BT exposure as compared to the vehicle-treated group (0.1% DMSO) was also noted (Fig. 4E).
Fig. 4.
Effect of BT on tumor development. (A) Bioluminescence images of tumors in mice. HCCLM3-Luc cells-induced tumors are orthotopically placed followed by treatment with 0.1% DMSO (n = 7) or BT (n = 7) (administered 1 mg/kg intraperitoneally, twice a week, for four weeks). Lung tissues were also analyzed for metastasis using bioluminescence imaging (B) The scattered plot indicates the tumor burden was quantified by measuring photon counts before the first administration of BT and at the last dose (**p < 0.01). (C) Tumor burden was recorded in vehicle-treated or BT-treated tumor-bearing mice throughout the study duration. (D) The graph represents the body weight of experimental animals throughout the study duration. (E) The quantitative estimation of lung tumor burden after BT treatment.
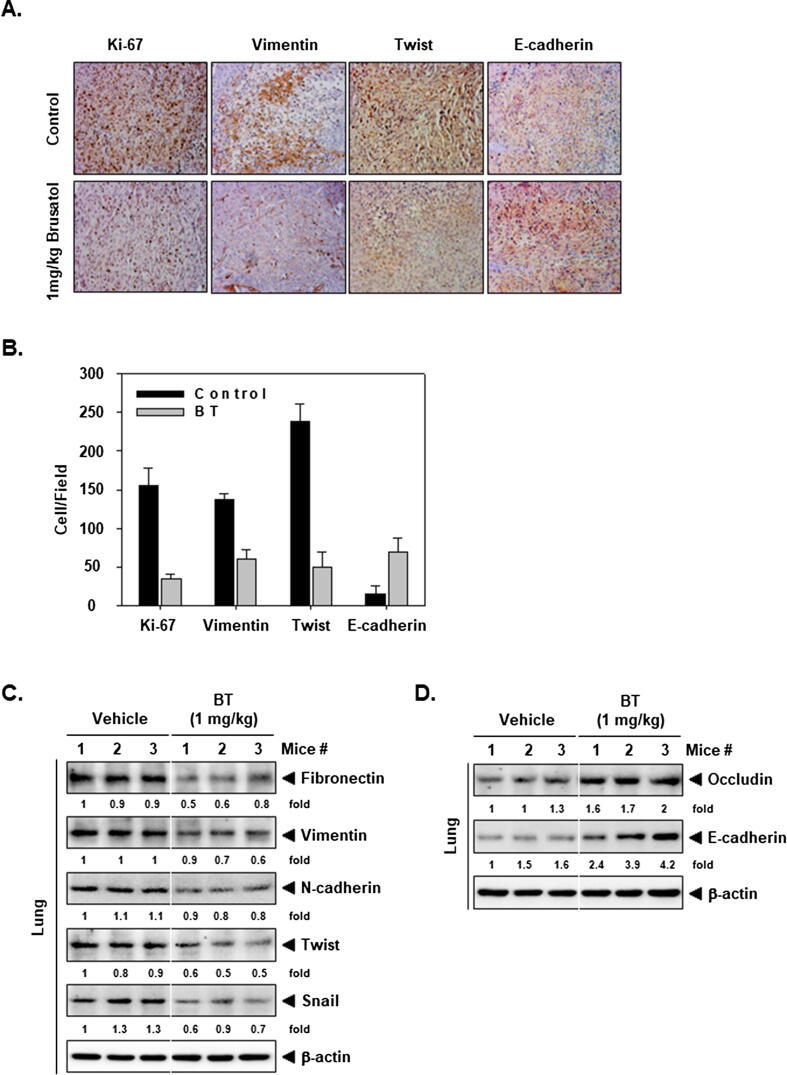
BT alters the levels of various proteins in tumor tissues
We determined the levels of EMT and proliferation markers in the tumor tissues. The intensity of Ki-67, Vimentin, and Twist was markedly reduced by BT treatment, whereas E-cadherin was markedly elevated in the tissues (Fig. 5A and 4B). Besides, the level of mesenchymal markers was downregulated and epithelial markers upregulated in BT-treated group. These observations are consistent with our in vitro findings (Fig. 5C and 5D).
Fig. 5.
The action of BT on EMT in tissues. (A) Analysis of EMT-related proteins by IHC. Magnification 200x. (B) Quantification of IHC. (C-D) The levels of various proteins was checked in tumor tissues.
Discussion
EMT can control embryonic development, wound healing, tissue remodeling, repair, and malignant transformation. Improper activation of EMT in cancer cells can contribute to their metastasis [58]. We report here that BT can significantly alter EMT through affecting STAT3 activation. An initial evaluation revealed that BT can suppress cell proliferation only at lower doses. Western blotting, Real-Time PCR, and ICC analysis suggested that BT attenuated the levels of mesenchymal markers with a subsequent increase in epithelial markers (Fig. 6). An elevated N-cadherin expression can be positively linked with metastasis in HCC and colon cancer tissues with poor survival rates [59], [60]. Fibronectin and integrin levels are often augmented in tumors and can increase regulate abnormal proliferation [61]. In addition, Fibronectin may also promote EMT in breast cancer cells [62].
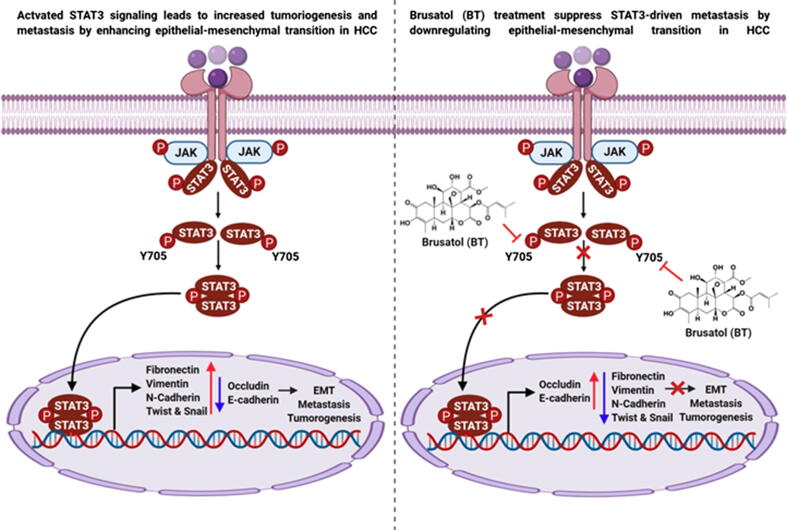
Fig. 6.
A graphic demonstrating the action of BT in regulating EMT process in HCC model.
Vimentin is ubiquitously expressed in non-diseased mesenchymal cells and overexpressed in a broad range of epithelial cancers, which can be positively correlated with elevated tumor proliferation, metastasis, and reduced survival [63], [64]. A decrease in E-cadherin can lead to the promotion of invasiveness, and resistance to standard chemotherapeutics in colorectal cancer cells [65], and knockdown of Occludin can contribute to the progression of breast cancer [66]. Next, we were interested to investigate the cause behind the altered expression of EMT-related proteins. Therefore, we deciphered the levels of major transcription factors that can affect EMT such as Snail and Twist. Interestingly, expression of both these proteins was downmodulated thereby indicating that EMT-related proteins may be suppressed by BT at the transcription level. For instance, Snail can repress the levels of E-cadherin and Occludin, and induce that of fibronectin, and MMP-9 [67]. The expression of N-cadherin is dependent on the integrin-mediated nuclear translocation of Twist1 [68]. Besides, Twist can regulate the levels of E-cadherin and may contribute to altered levels of various mesenchymal proteins [69].
STAT3 is a major transcription factor that promotes malignant progression, antiapoptosis, angiogenesis, and metastasis [70], [71], [72], [73], [74], [75], [76]. Activation of STAT3 can be achieved by forming a positive feedback loop and crosstalk with other oncogenic mediators in the tumor microenvironment [77], [78], [79], [80], [81], [82]. Moreover, HCC patients with increased phosphorylated STAT3 in tumor tissues showed poor prognosis after transarterial chemoembolization and post-liver resection [83]. In addition, hyperactivated STAT3 signaling contributed to EMT in the same study [84]. IL-6, that can stimulate STAT3 activation [85], [86], can promote metastasis by promoting EMT through JAK-STAT3-Snail axis in HNSCC [8]. In addition, TGFβ-induced EMT is also dependent on JAK-STAT3 cascade in lung cancer [87]. In our previous investigation, we identified that STAT3 signaling can be downmodulated by BT in HNSCC cells [38]. Here we found that BT suppressed the phosphorylation of STAT3Y705 in HCC cells that can contribute to its effect on various hallmarks of cancer, specifically EMT. The knockdown of STAT3 using STAT3 targeted siRNA caused a substantial decline of epithelial and increase in mesenchymal markers thus indicating that the STAT3 can modulate EMT. In parallel, we also observed a reduction in the levels of Snail and Twist. This effect could be due to the regulation of Snail and Twist expression by STAT3 [8], [23], [24].
It has also been previously documented that STAT3 can affect EMT by modulating Snail gene expression in pancreatic cancer [88]. Similarly, bergamottin, a furanocoumarin present in grapefruits, attenuated STAT3 signaling and mitigated metastasis through inhibition of EMT [49]. In addition, we have previously demonstrated that several STAT3 signaling inhibitors can suppress metastatic ability of cancer cells [89], [90], [91], [92], [93]. Furthermore, we evaluated the action of BT on the invasive and migratory potential and the results demonstrated a significant decrease in cellular motility. The alterations of EMT-related proteins by BT may mediate its repressive actions on the invasive ability of HCC cells.
Since BT did not display any major toxic effects (up to 15 mg/kg), we next investigated its antitumor actions in HCC model. BT imparted significant antitumor potential in orthotopic model at a very low dose of 1 mg/kg. Lu and colleagues also reported the non-toxic nature of BT (2 mg/kg) in nude mice when intraperitoneally administered for 28 consecutive days [94]. Importantly, lung metastasis was also significantly inhibited with an alteration in the expression profile of Ki-67, Vimentin, Twist, and E-cadherin. The modulation in the expression of these proteins is consistent with our in vitro experimental findings.
Conclusion
EMT has been linked with metastasis of cancer cells and commonly observed in advanced tumors. Blocking of STAT3 activation by BT may interfere with mesenchymal phenotype and can downmodulate metastasis potential. Our results demonstrate that BT can attenuate STAT3-driven metastasis by altering the levels of EMT-related proteins in HCC preclinical settings.
Compliance with ethics requirements
All Institutional and National Guidelines for the care and use of animals (fisheries) were followed.
Declaration of Competing Interest
The authors have declared no conflict of interest.
Acknowledgments
Acknowledgements
K.S.R. thanks the University Grants Commission, New Delhi for providing the Basic Science Research faculty fellowship. K.S.R., and C.D.M., thanks DST-Promotion of University Research and Scientific (PURSE), Institution of Excellence, University of Mysore for arranging laboratory facility. H.K.M is supported by grant from the National Medical Research Council of Singapore (NRNMRPR18101). The authors also thank the International Scientific Partnership Program ISPP at King Saud University for funding this research work through ISPP# 0091.
Authors Contributions
Experiments performed by: J.H.L., C.D.M., A.D., Y.Y.J.
Study design, data interpretation: S.R., S.B., A.C., T.A.A., S.A.A., M.G., Z.X.L., K.S.R.
Writing of the manuscript: J.H.L., C.D.M., G.S., K.M.H., K.S.A.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (NRF-2018R1D1A1B07042969).
Footnotes
Peer review under responsibility of Cairo University.
Contributor Information
Gautam Sethi, Email: phcgs@nus.edu.sg.
Kam Man Hui, Email: cmrhkm@nccs.com.sg.
Kwang Seok Ahn, Email: ksahn@khu.ac.kr.
References
- 1.Chaffer C.L., Weinberg R.A. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 2.Mittal V. Epithelial mesenchymal transition in tumor metastasis. Annual Rev Pathol. 2018;13:395–412. doi: 10.1146/annurev-pathol-020117-043854. [DOI] [PubMed] [Google Scholar]
- 3.Chua A.W., Hay H.S., Rajendran P., Shanmugam M.K., Li F., Bist P. Butein downregulates chemokine receptor CXCR4 expression and function through suppression of NF-κB activation in breast and pancreatic tumor cells. Biochem Pharmacol. 2010;80(10):1553–1562. doi: 10.1016/j.bcp.2010.07.045. [DOI] [PubMed] [Google Scholar]
- 4.Syn N., Wang L., Sethi G., Thiery J.P., Goh B.C. Exosome-mediated metastasis: from epithelial-mesenchymal transition to escape from immunosurveillance. Trends Pharmacol Sci. 2016;37(7):606–617. doi: 10.1016/j.tips.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Shanmugam M.K., Manu K.A., Ong T.H., Ramachandran L., Surana R., Bist P. Inhibition of CXCR4/CXCL12 signaling axis by ursolic acid leads to suppression of metastasis in transgenic adenocarcinoma of mouse prostate model. Int J Cancer. 2011;129(7):1552–1563. doi: 10.1002/ijc.26120. [DOI] [PubMed] [Google Scholar]
- 6.Seyfried T.N., Huysentruyt L.C. On the origin of cancer metastasis. Crit Rev Oncog. 2013;18(1–2):43–73. doi: 10.1615/critrevoncog.v18.i1-2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta G.P., Massagué J. Cancer metastasis: building a framework. Cell. 2006;127(4):679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Yadav A., Kumar B., Datta J., Teknos T.N., Kumar P. IL-6 promotes head and neck tumor metastasis by inducing epithelial-mesenchymal transition via the JAK-STAT3-SNAIL signaling pathway. Molecul Cancer Res MCR. 2011;9(12):1658–1667. doi: 10.1158/1541-7786.MCR-11-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore W., Talati R., Bhattacharji P., Bilfinger T. Five-year survival after cryoablation of stage i non-small cell lung cancer in medically inoperable patients. J Vasc Interv Radiol. 2015;26(3):312–319. doi: 10.1016/j.jvir.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Diepenbruck M., Christofori G. Epithelial-mesenchymal transition (EMT) and metastasis: yes, no, maybe? Curr Opin Cell Biol. 2016;43:7–13. doi: 10.1016/j.ceb.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y., Weinberg R.A. Epithelial-to-mesenchymal transition in cancer: complexity and opportunities. Front Medicine. 2018;12(4):361–373. doi: 10.1007/s11684-018-0656-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalluri R., Weinberg R.A. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng J.-T., Wang L., Wang H., Tang F.-R., Cai W.-Q., Sethi G. Insights into biological Role of LncRNAs in epithelial-mesenchymal transition. Cells. 2019;8(10):1178. doi: 10.3390/cells8101178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baek S.H., Ko J.H., Lee J.H., Kim C., Lee H., Nam D. Ginkgolic acid inhibits invasion and migration and TGF-β-induced EMT of lung cancer cells through PI3K/Akt/mTOR inactivation. J Cell Physiol. 2017;232(2):346–354. doi: 10.1002/jcp.25426. [DOI] [PubMed] [Google Scholar]
- 15.Dai X., Ahn K.S., Wang L.Z., Kim C., Deivasigamni A., Arfuso F. Ascochlorin enhances the sensitivity of doxorubicin leading to the reversal of epithelial-to-mesenchymal transition in hepatocellular carcinoma. Mol Cancer Ther. 2016;15(12):2966–2976. doi: 10.1158/1535-7163.MCT-16-0391. [DOI] [PubMed] [Google Scholar]
- 16.Itoh Y., Saitoh M., Miyazawa K. Smad3-STAT3 crosstalk in pathophysiological contexts. Acta Biochim Biophy Sin. 2018;50(1):82–90. doi: 10.1093/abbs/gmx118. [DOI] [PubMed] [Google Scholar]
- 17.Loh C.-Y., Chai J.Y., Tang T.F., Wong W.F., Sethi G., Shanmugam M.K. The E-cadherin and N-cadherin switch in epithelial-to-mesenchymal transition: signaling, therapeutic implications, and challenges. Cells. 2019;8(10):1118. doi: 10.3390/cells8101118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J.H., Chinnathambi A., Alharbi S.A., Shair O.H.M., Sethi G., Ahn K.S. Farnesol abrogates epithelial to mesenchymal transition process through regulating Akt/mTOR pathway. Pharmacol Res. 2019;150 doi: 10.1016/j.phrs.2019.104504. [DOI] [PubMed] [Google Scholar]
- 19.Lee J.H., Kim C., Lee J., Um J.Y., Sethi G., Ahn K.S. Arctiin is a pharmacological inhibitor of STAT3 phosphorylation at tyrosine 705 residue and potentiates bortezomib-induced apoptotic and anti-angiogenic effects in human multiple myeloma cells. Phytomed Int J Phytotherapy Phytopharmacol. 2019;55:282–292. doi: 10.1016/j.phymed.2018.06.038. [DOI] [PubMed] [Google Scholar]
- 20.Lee M., Hirpara J.L., Eu J.Q., Sethi G., Wang L., Goh B.C. Targeting STAT3 and oxidative phosphorylation in oncogene-addicted tumors. Redox Biol. 2019;25 doi: 10.1016/j.redox.2018.101073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baburajeev C.P., Mohan C.D., Patil G.S., Rangappa S., Pandey V., Sebastian A. Nano-cuprous oxide catalyzed one-pot synthesis of a carbazole-based STAT3 inhibitor: a facile approach via intramolecular C-N bond formation reactions. RSC Adv. 2016;6(43):36775–36785. [Google Scholar]
- 22.Mohan C.D., Bharathkumar H., Bulusu K.C., Pandey V., Rangappa S., Fuchs J.E. Development of a novel azaspirane that targets the Janus kinase-signal transducer and activator of transcription (STAT) pathway in hepatocellular carcinoma in vitro and in vivo. J Biol Chem. 2014;289(49):34296–34307. doi: 10.1074/jbc.M114.601104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamouille S., Xu J., Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng G.Z., Zhang W.Z., Sun M., Wang Q., Coppola D., Mansour M. Twist is transcriptionally induced by activation of STAT3 and mediates STAT3 oncogenic function. J Biol Chem. 2008;283(21):14665–14673. doi: 10.1074/jbc.M707429200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olayanju A., Copple I.M., Bryan H.K., Edge G.T., Sison R.L., Wong M.W. Brusatol provokes a rapid and transient inhibition of Nrf2 signaling and sensitizes mammalian cells to chemical toxicity-implications for therapeutic targeting of Nrf2. Free Radic Biol Med. 2015;78:202–212. doi: 10.1016/j.freeradbiomed.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen H.M., Lai Z.Q., Liao H.J., Xie J.H., Xian Y.F., Chen Y.L. Synergistic antitumor effect of brusatol combined with cisplatin on colorectal cancer cells. Int J Mol Med. 2018;41(3):1447–1454. doi: 10.3892/ijmm.2018.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ren D., Villeneuve N.F., Jiang T., Wu T., Lau A., Toppin H.A. Brusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanism. PNAS. 2011;108(4):1433–1438. doi: 10.1073/pnas.1014275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiang Y, Ye W, Huang C, Yu D, Chen H, Deng T, et al. Brusatol enhances the chemotherapy efficacy of gemcitabine in pancreatic cancer via the Nrf2 signalling pathway. Oxidative medicine and cellular longevity. 2018; 2018. [DOI] [PMC free article] [PubMed]
- 29.Sun X., Wang Q., Wang Y., Du L., Xu C., Liu Q. Brusatol enhances the radiosensitivity of A549 cells by promoting ros production and enhancing DNA damage. Int J Molecul Sci. 2016;17(7) doi: 10.3390/ijms17070997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ye R., Dai N., He Q., Guo P., Xiang Y., Zhang Q. Comprehensive anti-tumor effect of Brusatol through inhibition of cell viability and promotion of apoptosis caused by autophagy via the PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Biomed Pharmacother. 2018;105:962–973. doi: 10.1016/j.biopha.2018.06.065. [DOI] [PubMed] [Google Scholar]
- 31.Vartanian S., Ma T.P., Lee J., Haverty P.M., Kirkpatrick D.S. Application of Mass Spectrometry Profiling to Establish Brusatol as an Inhibitor of Global Protein Synthesis. 2016;15(4):1220–1231. doi: 10.1074/mcp.M115.055509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harder B., Tian W., La Clair J.J., Tan A.-C., Ooi A., Chapman E. Brusatol overcomes chemoresistance through inhibition of protein translation. Mol Carcinog. 2017;56(5):1493–1500. doi: 10.1002/mc.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y., Pang Y., Caisova V., Ding J., Yu D., Zhou Y. Targeting NRF2-Governed Glutathione Synthesis for SDHB-Mutated Pheochromocytoma and Paraganglioma. Cancers (Basel). 2020;12(2):280. doi: 10.3390/cancers12020280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y., Lu Y., Celiku O., Li A., Wu Q., Zhou Y. Targeting IDH1-Mutated Malignancies with NRF2 Blockade. J Natl Cancer Inst. 2019;111(10):1033–1041. doi: 10.1093/jnci/djy230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang X., Fu X., Liu Y., Yu D. Blockade of Glutathione Metabolism in IDH1-Mutated. Glioma. 2020;19(1):221–230. doi: 10.1158/1535-7163.MCT-19-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu T., Harder B.G., Wong P.K., Lang J.E., Zhang D.D. Oxidative stress, mammospheres and Nrf2–new implication for breast cancer therapy? Mol Carcinog. 2015;54(11):1494–1502. doi: 10.1002/mc.22202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cai S.J., Liu Y., Han S., Yang C. Brusatol, an NRF2 inhibitor for future cancer therapeutic. Cell Biosci. 2019;9:45. doi: 10.1186/s13578-019-0309-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee J.H., Rangappa S., Mohan C.D., Basappa Sethi G, Lin Z.-X. Brusatol, a Nrf2 inhibitor targets STAT3 signaling cascade in head and neck squamous cell carcinoma. Biomolecules. 2019;9(10):550. doi: 10.3390/biom9100550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang J., Qin L.X., Li Y., Ye S.L., Liu Y.K., Gao D.M. Molecular cytogenetic characteristics of the human hepatocellular carcinoma cell line HCCLM3 with high metastatic potential: comparative genomic hybridization and multiplex fluorescence in situ hybridization. Cancer Genet Cytogenet. 2005;158(2):180–183. doi: 10.1016/j.cancergencyto.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 40.Thura M., Al-Aidaroos A.Q., Gupta A., Chee C.E., Lee S.C., Hui K.M. PRL3-zumab as an immunotherapy to inhibit tumors expressing PRL3 oncoprotein. Nat Commun. 2019;10(1):2484. doi: 10.1038/s41467-019-10127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee J.H., Mohan C.D., Basappa S., Rangappa S., Chinnathambi A., Alahmadi T.A. The IκB kinase inhibitor ACHP targets the STAT3 signaling pathway in human non-small cell lung carcinoma cells. Biomolecules. 2019;9(12):875. doi: 10.3390/biom9120875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee J.H., Kim C., Ko J.-H., Jung Y.Y., Jung S.H., Kim E. Casticin inhibits growth and enhances ionizing radiation–induced apoptosis through the suppression of STAT3 signaling cascade. J Cell Biochem. 2019;120(6):9787–9798. doi: 10.1002/jcb.28259. [DOI] [PubMed] [Google Scholar]
- 43.Gilandoust M., Harsha K.B., Mohan C.D., Raquib A.R., Rangappa S., Pandey V. Synthesis, characterization and cytotoxicity studies of 1,2,3-triazoles and 1,2,4-triazolo [1,5-a] pyrimidines in human breast cancer cells. Bioorg Med Chem Lett. 2018;28(13):2314–2319. doi: 10.1016/j.bmcl.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 44.Keerthy H.K., Mohan C.D., Sivaraman Siveen K., Fuchs J.E., Rangappa S., Sundaram M.S. Novel synthetic biscoumarins target tumor necrosis factor-α in hepatocellular carcinoma in vitro and in vivo. J Biol Chem. 2014;289(46):31879–31890. doi: 10.1074/jbc.M114.593855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sethi G., Chatterjee S., Rajendran P., Li F., Shanmugam M.K., Wong K.F. Inhibition of STAT3 dimerization and acetylation by garcinol suppresses the growth of human hepatocellular carcinoma in vitro and in vivo. Molecular Cancer. 2014;13:66. doi: 10.1186/1476-4598-13-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Subramaniam A., Shanmugam M.K., Ong T.H., Li F., Perumal E., Chen L. Emodin inhibits growth and induces apoptosis in an orthotopic hepatocellular carcinoma model by blocking activation of STAT3. Br J Pharmacol. 2013;170(4):807–821. doi: 10.1111/bph.12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sulaiman N.B., Mohan C.D., Basappa S., Pandey V., Rangappa S., Bharathkumar H. An azaspirane derivative suppresses growth and induces apoptosis of ER-positive and ER-negative breast cancer cells through the modulation of JAK2/STAT3 signaling pathway. Int J Oncol. 2016;49(3):1221–1229. doi: 10.3892/ijo.2016.3615. [DOI] [PubMed] [Google Scholar]
- 48.Li F., Rajendran P., Sethi G. Thymoquinone inhibits proliferation, induces apoptosis and chemosensitizes human multiple myeloma cells through suppression of signal transducer and activator of transcription 3 activation pathway. Br J Pharmacol. 2010;161(3):541–554. doi: 10.1111/j.1476-5381.2010.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim S.M., Lee J.H., Sethi G., Kim C., Baek S.H., Nam D. Bergamottin, a natural furanocoumarin obtained from grapefruit juice induces chemosensitization and apoptosis through the inhibition of STAT3 signaling pathway in tumor cells. Cancer Lett. 2014;354(1):153–163. doi: 10.1016/j.canlet.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 50.Pandey V., Wang B., Mohan C.D., Raquib A.R., Rangappa S., Srinivasa V. Discovery of a small-molecule inhibitor of specific serine residue BAD phosphorylation. PNAS. 2018;115(44):E10505–E10514. doi: 10.1073/pnas.1804897115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baburajeev C.P., Dhananjaya Mohan C., Ananda H., Rangappa S., Fuchs J.E., Jagadish S. Development of Novel Triazolo-Thiadiazoles from Heterogeneous “Green” Catalysis as Protein Tyrosine Phosphatase 1B Inhibitors. Sci Rep. 2015;5(1):14195. doi: 10.1038/srep14195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mohan C.D., Srinivasa V., Rangappa S., Mervin L., Mohan S., Paricharak S. Trisubstituted-imidazoles induce apoptosis in human breast cancer cells by targeting the oncogenic PI3K/Akt/mTOR signaling pathway. PLoS ONE. 2016;11(4) doi: 10.1371/journal.pone.0153155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee J.H., Kim C., Sethi G., Ahn K.S. Brassinin inhibits STAT3 signaling pathway through modulation of PIAS-3 and SOCS-3 expression and sensitizes human lung cancer xenograft in nude mice to paclitaxel. Oncotarget. 2015;6(8):6386–6405. doi: 10.18632/oncotarget.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heo J.Y., Kim H.J., Kim S.M., Park K.R., Park S.Y., Kim S.W. Embelin suppresses STAT3 signaling, proliferation, and survival of multiple myeloma via the protein tyrosine phosphatase PTEN. Cancer Lett. 2011;308(1):71–80. doi: 10.1016/j.canlet.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 55.Mohan C.D., Anilkumar N.C., Rangappa S., Shanmugam M.K., Mishra S., Chinnathambi A. Novel 1,3,4-oxadiazole induces anticancer activity by targeting NF-κB in hepatocellular carcinoma cells. Front Oncol. 2018;8:42. doi: 10.3389/fonc.2018.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dai X., Wang L., Deivasigamni A., Looi C.Y., Karthikeyan C., Trivedi P. A novel benzimidazole derivative, MBIC inhibits tumor growth and promotes apoptosis via activation of ROS-dependent JNK signaling pathway in hepatocellular carcinoma. Oncotarget. 2017;8(8):12831–12842. doi: 10.18632/oncotarget.14606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dai X., Ahn K.S., Kim C., Siveen K.S., Ong T.H., Shanmugam M.K. Ascochlorin, an isoprenoid antibiotic inhibits growth and invasion of hepatocellular carcinoma by targeting STAT3 signaling cascade through the induction of PIAS3. Mol Oncol. 2015;9(4):818–833. doi: 10.1016/j.molonc.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wendt M.K., Balanis N., Carlin C.R., Schiemann W.P. STAT3 and epithelial-mesenchymal transitions in carcinomas. JAKSTAT. 2014;3(1) doi: 10.4161/jkst.28975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhuo H., Jiang K., Dong L., Zhu Y., Lü L., Lü Y. Overexpression of N-cadherin is correlated with metastasis and worse survival in colorectal cancer patients. Chin Sci Bull. 2013;58(28):3529–3534. [Google Scholar]
- 60.Sebastian A., Pandey V., Mohan C.D., Chia Y.T., Rangappa S., Mathai J. Novel adamantanyl-based thiadiazolyl pyrazoles targeting EGFR in triple-negative breast cancer. ACS Omega. 2016;1(6):1412–1424. doi: 10.1021/acsomega.6b00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang J.P., Hielscher A. Fibronectin: how its aberrant expression in tumors may improve therapeutic targeting. J Cancer. 2017;8(4):674–682. doi: 10.7150/jca.16901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li C.L., Yang D., Cao X., Wang F., Hong D.Y., Wang J. Fibronectin induces epithelial-mesenchymal transition in human breast cancer MCF-7 cells via activation of calpain. Oncology Lett. 2017;13(5):3889–3895. doi: 10.3892/ol.2017.5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wei J., Xu G., Wu M., Zhang Y., Li Q., Liu P. Overexpression of vimentin contributes to prostate cancer invasion and metastasis via src regulation. Anticancer Res. 2008;28(1a):327–334. [PubMed] [Google Scholar]
- 64.Satelli A., Li S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cellular Mol Life Sci CMLS. 2011;68(18):3033–3046. doi: 10.1007/s00018-011-0735-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen X., Wang Y., Xia H., Wang Q., Jiang X., Lin Z. Loss of E-cadherin promotes the growth, invasion and drug resistance of colorectal cancer cells and is associated with liver metastasis. Mol Biol Rep. 2012;39(6):6707–6714. doi: 10.1007/s11033-012-1494-2. [DOI] [PubMed] [Google Scholar]
- 66.Martin T.A., Mansel R.E., Jiang W.G. Loss of occludin leads to the progression of human breast cancer. Int J Mol Med. 2010;26(5):723–734. doi: 10.3892/ijmm_00000519. [DOI] [PubMed] [Google Scholar]
- 67.Wu Y., Zhou B.P. Snail: more than EMT. Cell Adhesion Migration. 2010;4(2):199–203. doi: 10.4161/cam.4.2.10943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alexander N.R., Tran N.L., Rekapally H., Summers C.E., Glackin C., Heimark R.L. N-cadherin gene expression in prostate carcinoma is modulated by integrin-dependent nuclear translocation of twist1. Cancer Res. 2006;66(7):3365. doi: 10.1158/0008-5472.CAN-05-3401. [DOI] [PubMed] [Google Scholar]
- 69.Yang J., Mani S.A., Donaher J.L., Ramaswamy S., Itzykson R.A., Come C. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117(7):927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 70.Lee J.H., Kim C., Baek S.H., Ko J.H., Lee S.G., Yang W.M. Capsazepine inhibits JAK/STAT3 signaling, tumor growth, and cell survival in prostate cancer. Oncotarget. 2017;8(11):17700–17711. doi: 10.18632/oncotarget.10775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee J.H., Kim C., Lee S.-G., Sethi G., Ahn K.S. Ophiopogonin D, a steroidal glycoside abrogates STAT3 signaling cascade and exhibits anti-cancer activity by causing GSH/GSSG imbalance in lung Carcinoma. Cancers (Basel) 2018;10(11):427. doi: 10.3390/cancers10110427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arora L., Kumar A.P., Arfuso F., Chng W.J., Sethi G. The role of signal transducer and activator of transcription 3 (STAT3) and its targeted inhibition in hematological malignancies. Cancers (Basel) 2018;10(9):327. doi: 10.3390/cancers10090327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chai E.Z., Siveen K.S., Shanmugam M.K., Arfuso F., Sethi G. Analysis of the intricate relationship between chronic inflammation and cancer. Biochem J. 2015;468(1):1–15. doi: 10.1042/BJ20141337. [DOI] [PubMed] [Google Scholar]
- 74.Loh C.Y., Arya A., Naema A.F., Wong W.F., Sethi G., Looi C.Y. Signal transducer and activator of transcription (STATs) proteins in cancer and inflammation: functions and therapeutic implication. Front Oncol. 2019;9:48. doi: 10.3389/fonc.2019.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Siveen K.S., Sikka S., Surana R., Dai X., Zhang J., Kumar A.P. Targeting the STAT3 signaling pathway in cancer: role of synthetic and natural inhibitors. BBA. 2014;1845(2):136–154. doi: 10.1016/j.bbcan.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 76.Wong A.L.A., Hirpara J.L., Pervaiz S., Eu J.Q., Sethi G., Goh B.C. Do STAT3 inhibitors have potential in the future for cancer therapy? Expert Opin Invest Drugs. 2017;26(8):883–887. doi: 10.1080/13543784.2017.1351941. [DOI] [PubMed] [Google Scholar]
- 77.Baek S.H., Ko J.H., Lee H., Jung J., Kong M., Lee J.W. Resveratrol inhibits STAT3 signaling pathway through the induction of SOCS-1: Role in apoptosis induction and radiosensitization in head and neck tumor cells. Phytomed Int J Phytotherapy Phytopharmacol. 2016;23(5):566–577. doi: 10.1016/j.phymed.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 78.Baek SH, Lee JH, Kim C, Ko JH, Ryu SH, Lee SG, et al. Ginkgolic Acid C 17:1, Derived from Ginkgo biloba Leaves, Suppresses Constitutive and Inducible STAT3 Activation through Induction of PTEN and SHP-1 Tyrosine Phosphatase. Molecules (Basel, Switzerland). 2017;22(2). [DOI] [PMC free article] [PubMed]
- 79.Chai E.Z., Shanmugam M.K., Arfuso F., Dharmarajan A., Wang C., Kumar A.P. Targeting transcription factor STAT3 for cancer prevention and therapy. Pharmacol Ther. 2016;162:86–97. doi: 10.1016/j.pharmthera.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 80.Zhang J., Ahn K.S., Kim C., Shanmugam M.K., Siveen K.S., Arfuso F. Nimbolide-Induced Oxidative Stress Abrogates STAT3 Signaling Cascade and Inhibits Tumor Growth in Transgenic Adenocarcinoma of Mouse Prostate Model. Antioxid Redox Signal. 2016;24(11):575–589. doi: 10.1089/ars.2015.6418. [DOI] [PubMed] [Google Scholar]
- 81.Gyamfi J., Lee Y.-H., Eom M., Choi J. Interleukin-6/STAT3 signalling regulates adipocyte induced epithelial-mesenchymal transition in breast cancer cells. Sci Rep. 2018;8(1):8859. doi: 10.1038/s41598-018-27184-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee J.H., Kim C., Kim S.H., Sethi G., Ahn K.S. Farnesol inhibits tumor growth and enhances the anticancer effects of bortezomib in multiple myeloma xenograft mouse model through the modulation of STAT3 signaling pathway. Cancer Lett. 2015;360(2):280–293. doi: 10.1016/j.canlet.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 83.Zheng X., Xu M., Yao B., Wang C., Jia Y., Liu Q. IL-6/STAT3 axis initiated CAFs via up-regulating TIMP-1 which was attenuated by acetylation of STAT3 induced by PCAF in HCC microenvironment. Cell Signal. 2016;28(9):1314–1324. doi: 10.1016/j.cellsig.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 84.Gai X., Zhou P., Xu M., Liu Z., Zheng X., Liu Q. Hyperactivation of IL-6/STAT3 pathway leaded to the poor prognosis of post-TACE HCCs by HIF-1α/SNAI1 axis-induced epithelial to mesenchymal transition. J Cancer. 2020;11(3):570–582. doi: 10.7150/jca.35631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim C., Cho S.K., Kapoor S., Kumar A., Vali S., Abbasi T. β-Caryophyllene oxide inhibits constitutive and inducible STAT3 signaling pathway through induction of the SHP-1 protein tyrosine phosphatase. Mol Carcinog. 2014;53(10):793–806. doi: 10.1002/mc.22035. [DOI] [PubMed] [Google Scholar]
- 86.Lee J.H., Chiang S.Y., Nam D., Chung W.S., Lee J., Na Y.S. Capillarisin inhibits constitutive and inducible STAT3 activation through induction of SHP-1 and SHP-2 tyrosine phosphatases. Cancer Lett. 2014;345(1):140–148. doi: 10.1016/j.canlet.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 87.Liu R.Y., Zeng Y., Lei Z., Wang L., Yang H., Liu Z. JAK/STAT3 signaling is required for TGF-β-induced epithelial-mesenchymal transition in lung cancer cells. Int J Oncol. 2014;44(5):1643–1651. doi: 10.3892/ijo.2014.2310. [DOI] [PubMed] [Google Scholar]
- 88.Huang C., Yang G., Jiang T., Zhu G., Li H., Qiu Z. The effects and mechanisms of blockage of STAT3 signaling pathway on IL-6 inducing EMT in human pancreatic cancer cells in vitro. Neoplasma. 2011;58(5):396–405. doi: 10.4149/neo_2011_05_396. [DOI] [PubMed] [Google Scholar]
- 89.Jung Y.Y., Lee J.H., Nam D., Narula A.S., Namjoshi O.A., Blough B.E. Anti-myeloma effects of icariin are mediated through the attenuation of JAK/STAT3-dependent signaling cascade. Front Pharmacol. 2018;9:531. doi: 10.3389/fphar.2018.00531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim C., Lee S.G., Yang W.M., Arfuso F., Um J.Y., Kumar A.P. Formononetin-induced oxidative stress abrogates the activation of STAT3/5 signaling axis and suppresses the tumor growth in multiple myeloma preclinical model. Cancer Lett. 2018;431:123–141. doi: 10.1016/j.canlet.2018.05.038. [DOI] [PubMed] [Google Scholar]
- 91.Shanmugam M.K., Rajendran P., Li F., Kim C., Sikka S., Siveen K.S. Abrogation of STAT3 signaling cascade by zerumbone inhibits proliferation and induces apoptosis in renal cell carcinoma xenograft mouse model. Mol Carcinog. 2015;54(10):971–985. doi: 10.1002/mc.22166. [DOI] [PubMed] [Google Scholar]
- 92.Rajendran P., Li F., Shanmugam M.K., Vali S., Abbasi T., Kapoor S. Honokiol inhibits signal transducer and activator of transcription-3 signaling, proliferation, and survival of hepatocellular carcinoma cells via the protein tyrosine phosphatase SHP-1. J Cell Physiol. 2012;227(5):2184–2195. doi: 10.1002/jcp.22954. [DOI] [PubMed] [Google Scholar]
- 93.Tan S.M., Li F., Rajendran P., Kumar A.P., Hui K.M., Sethi G. Identification of beta-escin as a novel inhibitor of signal transducer and activator of transcription 3/Janus-activated kinase 2 signaling pathway that suppresses proliferation and induces apoptosis in human hepatocellular carcinoma cells. J Pharmacol Experimen Therapeutics. 2010;334(1):285–293. doi: 10.1124/jpet.110.165498. [DOI] [PubMed] [Google Scholar]
- 94.Lu Z., Lai Z.-Q., Leung A.W.N., Leung P.S., Li Z.-S., Lin Z.-X. Exploring brusatol as a new anti-pancreatic cancer adjuvant: biological evaluation and mechanistic studies. Oncotarget. 2017;8(49):84974–84985. doi: 10.18632/oncotarget.17761. [DOI] [PMC free article] [PubMed] [Google Scholar]