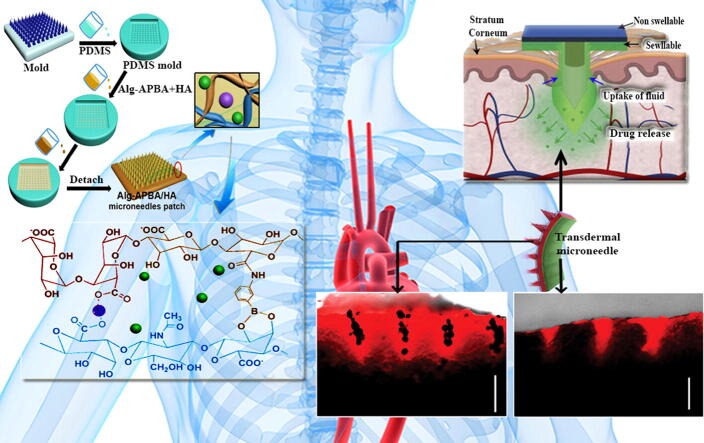
Graphical abstract
Keywords: Microneedle array, Drug delivery, Fabrication method, Hydrogel polymers, Dissolving polymers, Biodegradable polymers
Abstract
Background
Microscopic patches as quite promising platforms in transdermal drug delivery suffer from conventional injections. In other hand, a wide range of pharmacokinetics, ranging from fast oral administration to sustained drug delivery, can be implemented with the help of microneedle arrays (MNAs).
Aim of Review
Hence, in this paper, we overviewed different kinds of MNAs such as solid/coated, hollow, porous, hydrogel/swellable, and merged-tip geometry followed by introducing different types of material (silicon, glass, ceramics, dissolving and biodegradable polymers, and hydrogel) used for fabrication of MNAs. Afterwards, some conventional and brand-new simple and customizable MN mold fabrication techniques were surveyed. Polymeric MNAs have received a great deal of attention due to their potential biocompatibility and biodegradability in comparison to other materials. Therefore, we also covered different kinds of polymers such as hydrogel/swellable, dissolving and biodegradable analogues used for the development of MNAs as potential candidates in drug delivery systems (DDSs). Finally, we discussed different challenges and future perspectives in the aspect of MNAs-based drug delivery platforms.
Key Scientific Concepts of Review
This review may provide guidelines for the rational design of polymeric MNAs-based DDSs for promising programmable drug release and enhanced therapeutic effect.
Introduction
Microneedle arrays (MNAs) are known as a new platform for the treatment of skin disorders and drug delivery [1], [2]. This method surprisingly increases the concentration of collagen in the skin while at the same time regulating the collagen and elastin fibers in the damaged area [3], [4]. Nowadays, microneedling is one of the best collagen making methods that has produced amazing results with a very low cost [5]. Percutaneous collagen induction therapy (PCI), is a mechanical stimulation technique for repairing structural damage to the skin that results in repairing of damaged tissues [6]. The purpose of using MNAs is to improve the appearance and function of the skin [7], [8].
Introducing needles into the skin increases the release of growth factors from fibroblasts, blood platelets, and improves the function of transforming growth factor-α and -β [9]. This process results in increased production of collagen and elastin by fibroblasts. Keratinocytes are also transported to the epidermis where they proliferate, thereby increasing the thickness of the epidermis [10], [11]. In this method, a device called dermaroller creates pores and fine channels in the skin, forcing the skin to make high amounts of collagen, and if there is a defect such as scars or skin cracks, these problems would be potentially resolved [12], [13].
The wide surface of skin is easily accessible and therefore selected as an easy and non-invasive way of drug delivery [14]. Topical drug delivery from the surface of the skin has many benefits, including the possibility of using high concentrations of a drug on the skin, reducing systemic use of a drug and associated side effects, the possibility of prolonged presence of a drug on the surface and reducing frequency of drug use [15]. Despite these benefits, the skin has only been able to transmit less than a dozen available drugs systemically, because human skin is almost impermeable to drugs [16]. There are different types of MNAs that can be used for drug delivery through the skin [16]. Although there are differences in the flexibility of the tools and how to use them in drug delivery, they all follow a similar principle, transferring the drug through the skin tissue in a micro-controlled manner.
MNA technology
MNA technology is known as a safe drug delivery approach which can be used as an alternative platform to hypodermic needle technology. Indeed, micro-projections arrays from different materials are assembled in the form of MNAs having fine needles of different diameter and length with different types and materials. This minimally invasive and pain-free technology can serve as a potential DDS, which can be applied in programmable drug release [16].
A number of studies have shown that MNA can deliver a large number of drugs as well as provide greater safety for patients and operators [17], [18]. Determining a logical relationship between the use of MNAs and their design and fabrication parameters brings us to the most desirable results in the experiment. Three basic parameters in the design of the MNAs are: release strategy (type of MN), the constituent material, and the method of manufacture [19], [20]. There are different types of MNAs, namely, solid/coated, hollow, porous, hydrogel /swellable, and merged-tip geometry [21]. The properties of each MNA along with its advantageous and disadvantageous are tabulated in Table 1.
Table 1.
Different types of MNAs along with their advantages and disadvantages.
| Type of MNAs | Properties | Advantageous | Disadvantageous | Ref (s) |
|---|---|---|---|---|
| Solid/coated | Contain no drugs, or drug can be absorbed on its surface, synthesized by metals | Increase the permeability of drugs | Needs two step application, broken needles result in irritation, the high cost of fabrication method, non-biodegradability of metals | [43] |
| Hollow | Can be loaded with drugs, fabricated by self-assembly and molding of soft materials | Higher stiffness, hydrophilic behavior and resistance can be achieved by using proper materials | Needs precise and expensive manufacturing technology, strong leakage or uncontrolled drug release, blocking the narrow channels | [44] |
| Porous | Large variety of pore sizes can be achieved for drug loading, porosity and pore size can be controlled during synthesis | High drug loading capability, functionalization with different moieties, simple fabrication methods | Low strength and penetration ability, pore blockage, drug release | [45] |
| Hydrogel/ swellable | Minimally-invasive devices used for controlled drug release | Significant biocompatibility, degradability, safe, simple, cheap, controlled drug release | Low mechanical strength, not suitable for very wet wounds | [46] |
| Merged-tip MN | Uses elasto-capillarity-driven self-assembly for construction a micro merged-tip system | Can be tunable, simultaneous drug loading and volume control, no need for micromolding, controllable cavity volumes and fracture approaches, flexibility and fabrication simplicity | – | [47] |
Fabrication of MNAs
There are several materials which have been used in MNA fabrication (Table 2). Given the extensive amount of utilization and materials that may be applied, simple, cheap, and affordable methods in the construction of MNAs for pre-clinical and clinical purposes are required. There are a number of pivotal design criteria to take into consideration when designing and developing MNAs. Multiple factors such as needle height, tip radius, mechanical stiffness, and aspect ratio controls the insertion of MNAs into skin and following drug release. MNAs may be constructed for direct utilization or application as a sample for MN mold manufacturing. The latter strategy can be used in successive replica molding of MNAs from extensive agents as well as device manipulating in a clinical setting. Generally, some conventional fabrication techniques used in the production of MNAs along with their advantages and disadvantages are tabulated in Table 3.
Table 2.
Different types of material used for fabrication of MNAs along with their application.
| Material | Application | Ref (s) |
|---|---|---|
| Silicon | 1. Electroporation2. Transdermal drug delivery3. Deep brain drug infusion | [48], [49], [50] |
| Glass | 1. Recordings of force2. Microinjection3. Facial atrophic acne scar | [51], [52], [53] |
| Ceramics | 1. Transport interface2. Controlled release of a model vaccine3. Transdermal drug delivery | [54], [55] |
| Dissolving and biodegradable polymers | 1. Influenza vaccination2. Transdermal drug delivery3. Transdermal delivery of insulin4. Transdermal iron replenishment therapy5. Transdermal delivery of neurotoxin6. Patch and cream | [1], [56], [57], [58], [59], [60] |
| Swellable hydrogel polymers/ proteins | 1. Transdermal drug delivery2. Intradermal vaccination3. Regenerative internal/external surgical closure4. Glucose-responsive insulin delivery5. Sensing of specific circulating nucleic acids | [61], [62], [63], [64], [65] |
Table 3.
Different methods for fabrication of MNAs along with advantages and disadvantages of each method.
| Methods | Description | Advantageous | Disadvantageous | Ref(s) |
|---|---|---|---|---|
| Micromilling | Uses cutting tools to fabricate microscale arrays | Cost effective start-up, high resolution, and versatility about characteristic geometries and material choices | Require burrs removal, damage to the micro-tools, poor surface quality | [66] |
| Direct laser micromachining | Molding of materials into desired morphology and dimension by laser | Simple, quick, precise method to handle, no contaminations, delicate designs on different metals, | High power consumption, burning or of the metal, side effects against human, not applicable for thick metals | [67] |
| Chemical wet etching | Chemical process for removing the surface layers | Simple equipment, high rate, high selectivity | Needs large amounts of chemicals | [68] |
| Electrical discharge machining | A device like MNA is fabricated by applying electrical discharges | Fabrication of complex shapes, high tolerance, Material hardness is not a concern, no distortion, well suited for delicate or fragile parts | Requires conductive materials, cost effective | [69] |
| Drawing lithography | A strategy (thermal, magnetorheological, UV, air blowing) for the construction of a MNAs directly from 2D planar polymers | Fabrication of ultrahigh-aspect ratio (UHAR), stepwise controlling, fabrication of hybrid electro-MN | Long drawing time, expensive, not applicable in producing complex shapes | [21], [70] |
| UV-lithography | A fabrication strategy based on the pattern parts of a thin film of an agent | Cost effective, production of smaller feature size | Increased cost for new technology, complexity, concern about the reliability | [71] |
| Deep reactive-ion etching | Plasma process for production of microstructure of silicon | Modifications can be made, creates a protective layer on the surface, creation of high sidewall angles | Requires suitable etch gasses, contamination of etch processes, requirement for dedicated machines | [72] |
| Projection-based direct light processing | A layer-by-layer strategy for fabrication of MNAs | Simple and rapid fabrication of 3D structures | Not high-quality MNAs, not convenient for large scale-up production | [73] |
| Fused filament fabrication (FFF) or fused deposition modeling (FDM) | Joint a filament of a material with the same material by heat or etc. | Uses low cost materials, fabricate more complex shaped MNAs | Resolution limitations, two-step process | [74] |
| Scanning-based SLA | Laser beam tracks and draws each layer into resin layer | Simple and rapid fabrication of 3D structures | Not high-quality MNAs, not convenient for large scale-up production | [75] |
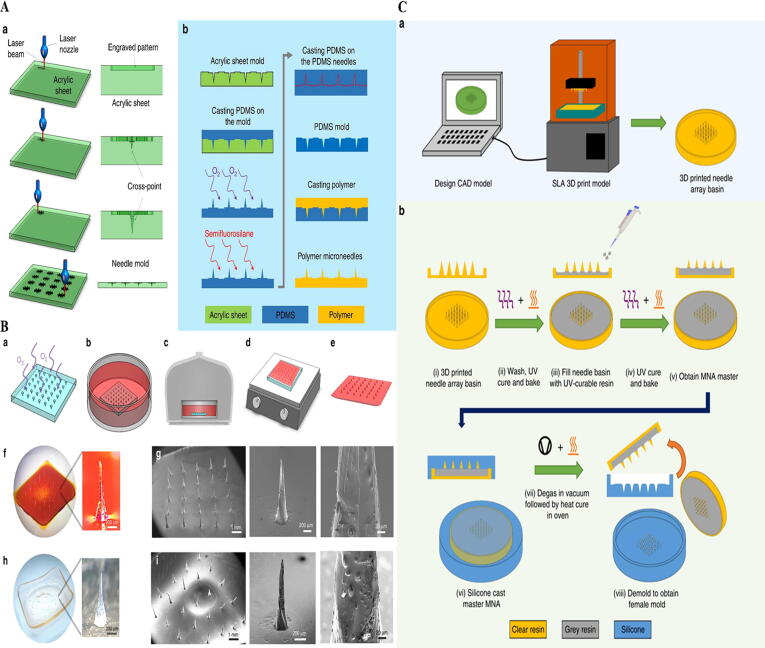
Reports to date employing these approaches, nevertheless, have only been able to produce MNAs which show several but not all of the geometric factors necessary for high-quality MNAs. Therefore, presentation of other customizable strategies for fabrication of MNAs in the research and clinical setting are required to overcome disadvantages of previous fabricated MNs. It may be proposed that using cross-over lines (COL) technique or optimizing the three-dimensional (3D) printers as a cost effective system to increase the tip radius and/or aspect ratios of fabricated MNs may open new avenues in biomedical science. For example, Nejad et al. [22] developed a cheap and cleanroom-free production of MNAs using molds patterned by COL technique by using laser ablation (Fig. 1A and B). Krieger et al. [21] carried out a parametric examination, revealing the printer’s abilities of printing needle geometry with optimized tip sharpness. To increase the resolution, they designed a two-step “Print & Fill” approach which permits the indirect production of custom-made MNAs for mold manufacturing utilizing stereolithography (SLA) 3D printing (Fig. 1C).
Fig. 1.
A: Fabrication of MNA mold. (a) CO2 laser cutter. (b) The acrylic mold was utilized to fabricate polydimethylsiloxane (PDMS) MNA mold [22]. B: Polymer casting and MNA fabrication approach. (a) Treating PDMS mold in oxygen. (b) Submerging the mold in polyvinyl alcohol (PVA) solution with phenol red dye. (c) Condensation of solution. (d) Dehydration of the mold. (e) Pilling off the MN patch. (f) Fabricated PVA MN patch (g) [22]. C: MNAs fabrication based on 3D printing. (a) MNA basin design by a Form 2 SLA printer. (b) MNA master mold construction approach (i) the 3D printed MNA basin; (ii) washing, UV curing and baking; (iii) filing with UV-curable resin; (iv) UV curing and baking; (v) MNA master; (vi) silicone casting; (vii) degassed process; (viii) demolding [21]. Reprined with permission from Refs [21], [22]. Copyright (2018) and (2019), Nature Publishing Group.
MNA in drug delivery
Today, stimuli-responsive platforms as smart DDS have numerous applications in the field of pharmaceutical science [23]. These systems intelligently release the drug as needed by changes in the amount of stimulus applied to it, such as temperature, pressure, pH, light, electric and magnetic fields. Of these smart DDSs, most research has been done on stimuli-responsive smart systems. Polymeric materials/smart hydrogels are often used in the preparation of such systems. Therefore, in practice, due to the effect of several stimuli, system responsiveness is impaired and the potential rate of drug release is not achieved. In addition, mathematical relationships governing their accountability are often complex and difficult. Indeed, a stimuli-responsive intelligent DDSs can be introduced in which the rate of drug release is only due to changing one stimulus like pH, while the mathematical relationships governing it are remarkably simple. The system, which consists of a multiple-layer composite plate structure, can be modeled in a quasi-steady state and the effect of the fundamental factors and characteristics of the system on its response can be investigated. Based on the results of system modeling, it can be found that the response of the system depends on the type of component in different layers (and their physical and chemical properties), the dimensions of the system, and other related factors. The results can be used to design and manufacture internal or external stimuli -responsive platforms as smart DDSs.
Transdermal delivery of drugs by using MNA
Although topical and hypodermic needles are used to potentially target drugs through the skin, the major concern is that this strategy is painful and the impacts are limited to the applied site. MNA may develop a promising approach to deliver drugs across the stratum corneum. Indeed, the capability of the polymer MNA for punching holes to transfer drugs to the skin seems to be a great idea. Treating the skin with MNAs may exhibit permeation of drugs in the skin and decrease the rate of disorders without stimulating side effects against major organs. Polymers that act as release controlling agents in DDSs can be divided into three main categories: hydrogel/swellable polymers, water-soluble polymers, and biodegradable polymers.
Hydrogels
Hydrogels are 3D polymeric networks with crosslinks that have the ability to absorb water or biological fluids even under pressure [24]. These compounds are chemically or physically cross-linked and can absorb a great amount of water [25]. The increasing attention to physical hydrogels is due to the relative ease of the process, the lack of lattice in their synthesis, and potential mechanical strength [25]. Also, natural hydrogels are very interesting because of the variety, abundance, cheapness, renewability, non-toxicity, as well as biodegradability and biocompatibility (Table 2) [26]. In the past few decades, hydrogels have been characterized by their unique properties in various industries such as pharmaceuticals, biomedical and bioengineering applications [27].
Among various applications, hydrogel-based DDSs have become an area of interest in the biomedical and biotechnological platforms. Hydrogels can protect the drug against aggressive microenvironmental factors [28]. Enzymes activity controllers, drug loading and pH-sensitive drug releasing, nanoreactors capable of precisely incorporating active groups in 3D space, intelligent microfluidics with responsive hydrogels, and energy conversion systems are promising applications of hydrogels in the medical and pharmaceutical fields [29], [30]. Indeed, hydrogels in the different forms can stimulate spatial and temporal drug delivery. Because of their tunable physical and chemical features and biodegradability, hydrogel acts as a promising platform on which a number of physicochemical reactions with the loaded drugs happen to control the drug release [31]. For example, Wang et al. [32] reported the development of dual-applicable transdermal drug delivery approach with adjustable drug release relied on thermo-responsive poloxamer hydrogel for treatment of atopic dermatitis (AD) disorder. Qu et al. [33] also developed the fabrication of a biocompatible conductive hydrogel derived from natural and synthetic polymers as electro-responsive drug delivery strategy for selective drug accumulation.
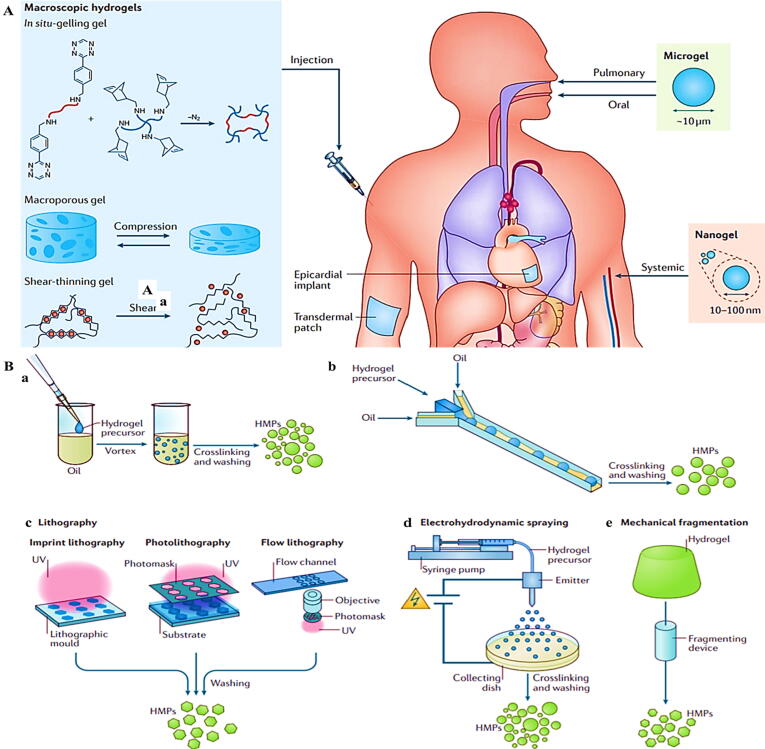
Macroscopic hydrogels such as in situ-gelling gel, microporous gel, and shear-thinning gel can be used for drug delivery through injection, epicardial implant, and transdermal patches. Microgels can be used as another drug delivery approaches through pulmonary and oral tracts. Finally, nanogels can be delivered into the body through systematic injection (Fig. 2A) [31]. Nanogels and microgels provide several advantages over their macroscopic compounds. First, the dimension is much smaller than the inner radius of classical needles. Their small size induces needle-injectable as well as supplying a large surface area for functionalization. Indeed, the small dimension results in simple bio-degradation and penetration through skin [31]. Therefore, much attention is focused on hydrogel microparticles in tissue engineering and DDSs. These particles can be fabricated by several methods such as batch emulsions [Fig. 2B (a)], microfluidic emulsions [Fig. 2B (b)], lithography [Fig. 2B (c)], electrohydrodynamic spraying [Fig. 2B (d)], and mechanical fragmentation [Fig. 2B (e)] [34].
Fig. 2.
A: Different kinds of hydrogels and delivery routes per each [31]. (B) Fabrication of hydrogel microparticles (HMPs) through (a) Batch emulsions. (b) Microfluidic emulsions. (c) Lithography. (d) Electrohydrodynamic spraying. (e) Mechanical fragmentation methods [34]. Reprined with permission from Refs [31], [34]. Copyright (2016) and (2019), Nature Publishing Group.
Water-soluble MNAs
Water-soluble MNAs based on polymers are used to provide short-term DDSs (from hours to days). The presence of hydrophilic functional groups such as hydroxylamine and carboxylic acid on the polymer chains dissolve these polymers in the aquatic environment of the living organism. Although all these polymers can be dissolved in water, they have different dissolution rates.
Drug release from MNAs fabricated by these polymers may be via different mechanisms. One of these mechanisms is the dissolution of a drug in the peripheral fluid. The level of contact that MNAs have with water - which is effective on its solubility - depends on the shape and size of the MNAs. In addition, drug content and solubility in the body affect the rate of drug release. Another factor is the penetration of a drug through the hydrated polymer to the surface and dissolution in the peripheral fluid.
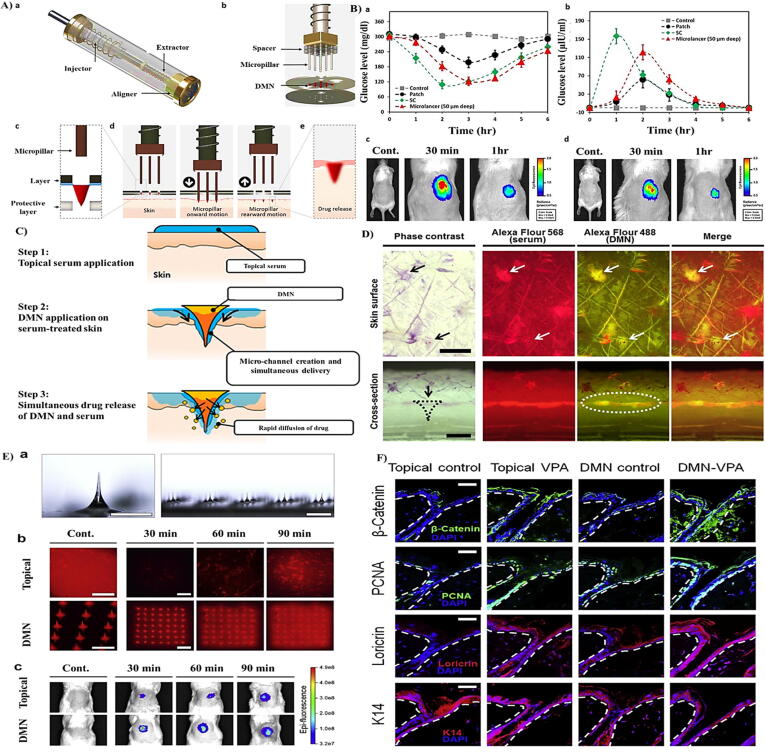
For example, Lahiji et al. [35] reported the fabrication of dissolving MNAs (DMNAs) that deliver loaded drugs in a minimally aggressive fashion. Indeed, DMNAs are currently placed onto patches that enhance their penetration into skin. Nevertheless, due to extensive variations in skin flexibility and surface curvature, the arrays prepared on the patch are normally not entirely slipped and substantial quantities of loaded drugs are not transferred. Therefore, they introduced the microlancer method, as a novel microarray-based approach by which patients can self-operate DMNAs with potential drug delivery ability (Fig. 3A). Also, the variation in the blood glucose level of diabetic mice and fluorescence intensity of insulin after the delivery of insulin by three different strategies of microlancer, patch and subcutaneous (SC) injection were studied (Fig. 3B). Kim et al. [36] also reported the developed transdermal delivery by simultaneous implementation of DMNAs on serum-treated skin. Indeed, they found that drugs in serum and encapsulated drugs in DMNAs are transferred into the skin through microchannels developed by implementation, hence promisingly enhancing the delivered level (Fig. 3C and D). Lahiji et al. [37] also revealed that transcutaneous implementation of valproic acid-encapsulated DMNA stimulates hair growth (Fig. 3E and F). Table 4 also summarizes the novel patches for skin disorder improvement by drug-loaded DMNAs.
Fig. 3.
A: (a) and (b) Microlancer. (c) The positioning of the hole. (d) Activation of the system. (e) Drug release and desolvation. B:(a) Plasma glucose level in diabetic mice over time. (b) Plasma insulin concentrations over time. (c) Insulin labeled FITC signal comparison of Microlancer and (d) patch [35]. C: Schematic illustration of simultaneous application of DMNA and serum. D: Distribution images of Alexa Fluor 568 dye (white arrow and dots) in serum and Alexa Fluor 488 dye (white arrow) loaded into DMNA patches (black arrow and dots) [36]. E: (a) Microscopic images of DMNAs. (b) Rhodamine delivery inside skin. (c) FITC delivery. F: Protein expression of β-catenin, PCNA, loricrin, and K14 [37]. Reprined with permission from Refs [35], [36], [37]. Copyright (2015), Nature Publishing Group; Copyright (2017), ACS; Copyright (2018), Elsevier.
Table 4.
Drug-loaded DMNAs and their potential use in treatment of skin disorders.
| Drug | Application | Ref(s) |
|---|---|---|
| Protein Delivery | Treatment of skin disorders | [56] |
| Human growth hormone | Wound healing | [76] |
| Soluble and particulate antigens | Vaccine/Treatment of skin disorders | [77] |
| Fibroblast growth factor | Local therapy of skin wounds | [78] |
| Green tea extracts | Antibacterial | [79] |
| Cosmeceutical relevant peptides | Treatment of skin disorders | [80] |
| Methylene blue | Photodynamic antimicrobial chemotherapy of infected wounds | [81] |
| Transdermal delivery of collagen I | Treatment of skin disorders | [82] |
| Cell delivery | Wound healing | [83] |
| Adenosine | Improve skin wrinkles, dermal density, elasticity and hydration | [84] |
| STAT3 siRNA | Melanoma | [85] |
| ROS‐responsive MN | Acne Vulgaris | [86] |
| Cosmeceuticals relevant nucleoside and peptides | Improvement of wrinkles | [12] |
| Bleomycin | Inhibiting hypertrophic scar | [87] |
| Triamcinolone | Treatment of keloids | [88] |
| Methotrexate | Treatment of psoriasis | [89] |
Biodegradable polymers
The term biodegradable polymer is used for those polymers that are initially insoluble in water, but after being exposed to environmental fluid by chemical reactions, they become soluble in water. There are several methods for solubilizing these polymers. If the polymer has side-chain bonding groups, these groups can be hydrated in the body and produce hydroxyl-carboxyl groups with other hydrolyzable groups, which are soluble polymers in water. Alternatively, the polymer is first fabricated by crosslinking agents, in which case the polymer is insoluble in water. After being exposed to the peripheral fluid, the hydrophilic lattice agent is dissolved. In this case, the polymer is soluble in water after removal of crosslinks. The third method is the use of water-insoluble polymers in which the hydrated functional groups are presented in the polymer body. As these groups are exposed to hydration in the chains, the main polymer chains become smaller chains that are prone to be dissolved in water. The main advantage of this group of polymers is the high molecular weight with good mechanical properties which can be used in the development of DDSs. These polymers eventually become smaller water-soluble components and are eliminated from the body.
Biodegradable polymers have attracted a great deal of attention in pharmaceutical companies due to their bioavailability and the ability to precisely control the degradation rate of DDSs [38]. These polymers are used in delivery of antibiotics, growth hormones and vaccines in the form of microcapsules, films, fibers and rods. SC as well as oral injection or implantation methods have been suggested for the administration of systems prepared with biodegradable polymers [1]. Shin et al. [39] applied biodegradable MNAs (BMNAs) to enhance the penetration of the steroid. They found that the penetration and efficacy of topical steroid was remarkably higher in the MNAs-treated skin. Lahiji et al. [40] also developed a scalp micro-pigmentation approach using BMNAs.
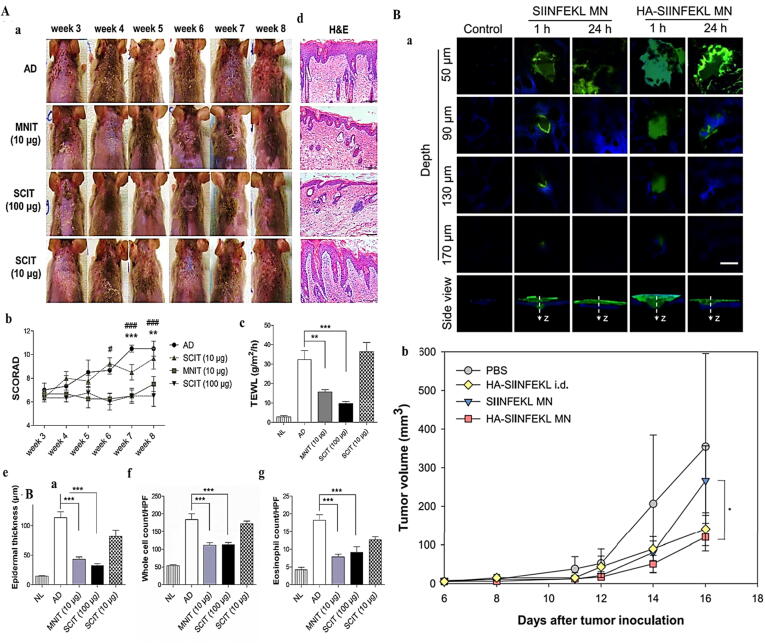
The use of MNAs has been used as a promising and effective transdermal drug delivery approach. Kim et al. [41] developed Dermatophagoides farinae extract (DfE)-loaded MNAs and examined their potential as a unique allergen-specific immunotherapy (SIT) strategy (Fig. 4A). Indeed, several approaches such as microneedle immunotherapy (MNIT) (10 μg) and SC immunotherapy were used in the treatment of AD. They found that MNIT provides more potential therapeutic outcomes than another strategy [41]. BMNAs loaded with peptides can be used to treat melanoma. For example, Kim et al. [42] revealed that hyaluronic acid/peptide-loaded BMNA can be used as a potential platform for melanoma immunotherapy (Fig. 4B).
Fig. 4.
A: (a) Variations in clinical morphology of skin. (b) Score of AD (SCORAD). (c) Trans-epidermal water loss (TEWL). (d) H&E-staining of skin. (e) Epidermal thickness. (f) Whole cell count. (g) Eosinophil count [41]. B: (a) Fluorescence microscopic images captured from skin 1 and 24 h after the administration of MNAs transferring FITC-labeled peptide. (b) Tumor growth [42]. Reprined with permission from Refs [41], [42]. Copyright (2018), Elsevier; Copyright (2019), ACS.
Challenges and future perspective
For the fabrication of stimuli-responsive MNAs, a variety of polymeric materials are utilized. However, due to conformational changes of biomaterials in response to environmental stimuli, the mechanism of drug release in polymeric MNAs have become more complex and only a few sets of a pre-clinical and in vivo model have been reported. These complexities and design of specific alignment of polymeric systems provide some challenges for semi-industrial scale production. In addition, low biocompatibility, low degradability and toxicity in some cases prevent the potential of polymeric systems being certificated by the Food and Drug Administration (FDA) and clinical approval. The use of polymeric MNAs in modern medicine and nanomedicine improves and facilitates some kinds of alternative therapies to improve the conventional treatments.
The most widely used approach in alternative medicines might be the improvement of MNAs-based drug delivery platforms. This type of MNAs with diverse properties may draw a step towards brighter futures to improve the quality of DDSs and underlying therapeutic approaches. One of the new approaches in the field of alternative medicine, especially in the drug release systems, is the use of stimuli-responsive materials. Indeed, these systems can play a pivotal role in efficacy of targeted drug delivery and potential extracellular delivery. Sources of stimulation can be internally related to the normal reaction of the tissue, such as hypoxic activity, pH, or enzymatic activities or external agents, such as magnetic field, temperature, and ultrasound (US).
For example, in the future by using polymers, the researchers can fabricate a hydrogel-based MNA that could control the programmable drug release. Researchers using US technology and the self-healing power of hydrogels would be able to provide MNAs to control the drug release at a predetermined time and tissue. This kind of hydrogel MNAs can control the drug release over a long period of time. US technology can temporarily disrupt the structure of the hydrogel and release a high dose of a drug.
The development of DMNAs and BMNAs will lead to the production of more efficient products in the future. This is possible by recognizing the capabilities and limitations of these polymers in various applications, especially DDSs. Polymeric MNAs-based drug delivery depends on the physical and chemical properties of the polymer. Indeed, particle size, polymer morphology, and selectivity of species can determine the output of the MNAs in development of DDSs. By modifying and controlling these factors, MNAs can be upgraded to a potential platform in advancement of DDSs. For example, in cases where rapid drug release and polymer degradation are considered after complete drug depletion, low-molecular-weight biodegradable polymers with hydrophilic groups may be required.
Also, the shape and morphology of MNA may play an important role in the drug release profile. For example, DMNAs or BMNAs with different shapes can be fabricated by using natural or synthetic-based material by anisotropic wet etching and a molding approach. The height, pitch, tip radius, penetration capability, and drug loading level of MNAs are very important factors in designing and development of MNAs.
Currently, MNAs are manufactured in clean and expensive factory rooms using plastic injection molds or lithography on stainless steel patterns. An important breakthrough in this field is the development of 3D printing capabilities for the use of materials such as fully functional electronic and biological polymers. The ability to print 3D arrays in biodegradable plastic can dramatically reduce the cost of MN patches and make them available anywhere. Also, there are some ways to incorporate proteins into the polymer matrix so that they can withstand the high heat present in the printing process.
Non-biodegradable polymeric particles in various aspects of medicine including targeted drug release, wound healing dressings, and antimicrobial medical coatings are used. However, chronic poisoning and inflammatory reactions are among the side effects of using these materials. These side effects have led to utilization of biocompatible materials with degradable capacity. Based on their low toxicity, dissolving and biodegradable polymeric material have been introduced as suitable alternative materials to non-biodegradable polymeric counterparts in nanomedicine due to the specific drug release pattern and increased biocompatibility. The gradually releasing nitric oxide can coagulate the blood vessels and increase blood flow to the injured area, which in turn improves the growth of healthy tissue by increasing the amount of oxygen-rich blood in the wound.
Nitric oxide not only increases blood flow but also shows some kind of antibacterial effects. But in severely damaged tissues, the body's natural mechanism for releasing this gas is kindly disrupted. For this purpose, sodium nitrite chemical with special activating gel can be delivered into the injury site by using MNAs. For example, the gel could consist of water and carboxylic acid (naturally occurring in fats and citrus fruits). When this acid comes in contact with sodium nitrite, the chemical reaction produces nitric oxide. The potential MNA platform can be designed to deliver a large amount of nitric oxide for up to several minutes continuously to initiate wound healing, then releases less gas within 24–48 h, and maybe several days.
Conclusion
The smart polymer-based MNAs, can be used in transdermal drug delivery to the selective site in the body, despite being thinner than conventional needles. 3D MNAs can be fabricated based on the technology of additive manufacturing or 3D printing as cost-effective and simple approaches. They are specifically designed for painless injections and can be used for a broad range of diagnostic and therapeutic agents from blood sampling for early detection of some disease to drug and vaccine injections. Doing more research based on polymeric MNAs at two pre-clinical and clinical levels would result in development of some therapeutic modalities.
CRediT authorship contribution statement
Fansu Meng: Conceptualization, Project administration, Resources, Writing - original draft. Anwarul Hasan: Conceptualization, Funding acquisition, Supervision, Writing - original draft, Writing - review & editing. Mohammad Mahdi Nejadi Babadaei: Conceptualization, Resources, Writing - original draft. Pegah Hashemi Kani: Conceptualization, Resources, Writing - original draft. Amir Jouta Talaei: Conceptualization, Resources, Writing - original draft. Majid Sharifi: Conceptualization, Resources, Writing - original draft. Tiange Cai: Conceptualization, Project administration, Resources. Mojtaba Falahati: Conceptualization, Supervision, Writing - original draft, Writing - review & editing. Yu Cai: Funding acquisition, Supervision, Writing - original draft, Writing - review & editing.
Acknowledgement
This research was made possible by the grants NPRP10-120-170-211 from Qatar National Research Fund (QNRF) under Qatar Foundation and GCC-2017-005 under the GCC collaborative research Program from Qatar University. This study was also supported by the Natural Science Foundation of Guangdong (2019A1515011286); and Open Project funded by Key laboratory of Carcinogenesis and Translational Research, Ministry of Education/Beijing (2019 Open Project-05). The statements made herein are the sole responsibility of the authors.
Biographies

Dr Anwarul Hasan, is an Assistant Professor in the Department of Mechanical and Industrial Engineering at Qatar University, Qatar. He joined the MIE department at QU in September 2015 prior to which he was an Assistant Professor of Biomedical Engineering and the Department of Mechanical Engineering at the American University of Beirut, Lebanon, and a visiting Assistant Professor at Harvard Medical School, USA. Earlier, Dr Hasan obtained his PhD from University of Alberta, Canada in 2010. He worked in industry in Canada during 2010-2011, and was a National Science and Engineering Research Council of Canada (NSERC) Research Fellow at Harvard and MIT during 2012-2013.

Dr. Mojtaba Falahati has completed his Bachelor Curriculum in the field of Biology majored in Zoology at Ferdowsi University in 2004 (Mashhad, Iran). Due to his high interests in Biophysical mechanisms related to diseases and pathological disorders, he performed his master thesis in Biophysics in the area of nerve membrane with focusing on medicinal polymers for the treatment of spinal cord injury at the Institute of Biochemistry and Biophysics (IBB) between 2005-2007 at the University of Tehran, Iran. Dr. Mojtaba Falahati received his PhD in Biophysics from the University of Tehran, Iran in 2011. During his PhD thesis he had a visit from Bremen University, Jacob University, Göttingen University, and Tübingen University in Germany. His main research area during the PhD was the immobilization of enzyme into the nanoporous materials and uncovering of the factors influencing the activity and stability of enzyme after interaction with nanoparticles. Since 2012, Dr. Falahati is an assistant professor at the Department of Nanotechnology, Faculty of Advanced Science and Technology, Tehran Medical Sciences, Islamic Azad University, Tehran, Iran. Dr. Falahati has recently received several national and international funding for the investigating the interaction of nanomaterials with proteins and cells, development of nanozymes, and nanomaterials-mediated drug delivery and tissue engineering.
Yu Cai is a Professor in the College of Pharmacy at Jinan University since 2003. He obtained a PhD in Traditional Chinese Medicine from the University of Guangzhou Traditional Chinese Medicine in 1999. His research interests include nanoparticles reverse multi-drug resistance, tumor microenvironment, active compound from herb in cancer treatment, etc. His research work is primarily supported by the National Natural Science Foundation of China and Guangdong Natural Science Foundation.
Footnotes
Peer review under responsibility of Cairo University.
Contributor Information
Anwarul Hasan, Email: ahasan@qu.edu.qa.
Mojtaba Falahati, Email: mojtaba.falahati@alumni.ut.ac.ir.
Yu Cai, Email: caiyu8@sohu.com.
References
- 1.Hong X., Wei L., Wu F., Wu Z., Chen L., Liu Z. Dissolving and biodegradable microneedle technologies for transdermal sustained delivery of drug and vaccine. Drug Des, Develop Therapy. 2013;7:945. doi: 10.2147/DDDT.S44401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saleh F.Y., Abdel-Azim E.S., Ragaie M.H., Guendy M.G. Topical tranexamic acid with microneedling versus microneedling alone in treatment of melasma: clinical, histopathologic, and immunohistochemical study. J Egypt Women’s Dermatol Soc. 2019;16(2):89. [Google Scholar]
- 3.Hou A., Cohen B., Haimovic A., Elbuluk N. Microneedling: a comprehensive review. Dermatol Surg. 2017;43(3):321–339. doi: 10.1097/DSS.0000000000000924. [DOI] [PubMed] [Google Scholar]
- 4.Schoenberg E., O’Connor M., Wang J.V., Yang S., Saedi N. Microneedling and PRP for acne scars: a new tool in our arsenal. J Cosmet Dermatol. 2019 doi: 10.1111/jocd.12988. [DOI] [PubMed] [Google Scholar]
- 5.Singh A., Yadav S. Microneedling: advances and widening horizons. Indian Dermatol Online J. 2016;7(4):244. doi: 10.4103/2229-5178.185468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lima E., Lima M., Martins S. Percutaneous collagen induction with microneedles. Chem Phys Proc. 2018:175–183. [Google Scholar]
- 7.Ramaut L., Hoeksema H., Pirayesh A., Stillaert F., Monstrey S. Microneedling: where do we stand now? A systematic review of the literature. J Plastic, Reconstr Aesthetic Surg. 2018;71(1):1–14. doi: 10.1016/j.bjps.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Waghule T., Singhvi G., Dubey S.K., Pandey M.M., Gupta G., Singh M. Microneedles: a smart approach and increasing potential for transdermal drug delivery system. Biomed Pharmacother. 2019;109:1249–1258. doi: 10.1016/j.biopha.2018.10.078. [DOI] [PubMed] [Google Scholar]
- 9.McCrudden M.T., McAlister E., Courtenay A.J., González-Vázquez P., Raj Singh T.R., Donnelly R.F. Microneedle applications in improving skin appearance. Exp Dermatol. 2015;24(8):561–566. doi: 10.1111/exd.12723. [DOI] [PubMed] [Google Scholar]
- 10.Zeitter S., Sikora Z., Jahn S., Stahl F., Strauß S., Lazaridis A. Microneedling: matching the results of medical needling and repetitive treatments to maximize potential for skin regeneration. Burns. 2014;40(5):966–973. doi: 10.1016/j.burns.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Badran K.W., Nabili V. Lasers, microneedling, and platelet-rich plasma for skin rejuvenation and repair. Facial Plastic Surg Clin. 2018;26(4):455–468. doi: 10.1016/j.fsc.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Lee A.-R.-C. Microneedle-mediated delivery of cosmeceutically relevant nucleoside and peptides in human skin: challenges and strategies for dermal delivery. J Pharm Invest. 2019:1–15. [Google Scholar]
- 13.Duncan D.I. Microneedling with biologicals: advantages and limitations. Facial Plastic Surg Clin. 2018;26(4):447–454. doi: 10.1016/j.fsc.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Naspolini A.P., Boza J.C., da Silva V.D., Cestari T.F. Efficacy of microneedling versus fractional non-ablative laser to treat Striae Alba: a randomized study. Am J Clin Dermatol. 2019;20(2):277–287. doi: 10.1007/s40257-018-0415-0. [DOI] [PubMed] [Google Scholar]
- 15.Rzhevskiy A.S., Singh T.R.R., Donnelly R.F., Anissimov Y.G. Microneedles as the technique of drug delivery enhancement in diverse organs and tissues. J Control Release. 2018;270:184–202. doi: 10.1016/j.jconrel.2017.11.048. [DOI] [PubMed] [Google Scholar]
- 16.Dharadhar S., Majumdar A., Dhoble S., Patravale V. Microneedles for transdermal drug delivery: a systematic review. Drug Dev Ind Pharm. 2019;45(2):188–201. doi: 10.1080/03639045.2018.1539497. [DOI] [PubMed] [Google Scholar]
- 17.Luzuriaga M.A., Berry D.R., Reagan J.C., Smaldone R.A., Gassensmith J.J. Biodegradable 3D printed polymer microneedles for transdermal drug delivery. Lab Chip. 2018;18(8):1223–1230. doi: 10.1039/c8lc00098k. [DOI] [PubMed] [Google Scholar]
- 18.Luo Z., Sun W., Fang J., Lee K., Li S., Gu Z. Biodegradable gelatin methacryloyl microneedles for transdermal drug delivery. Adv Healthcare Mater. 2019;8(3):1801054. doi: 10.1002/adhm.201801054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhatnagar S., Kumari P., Pattarabhiran S.P., Venuganti V.V.K. Zein microneedles for localized delivery of chemotherapeutic agents to treat breast cancer: drug loading, release behavior, and skin permeation studies. AAPS PharmSciTech. 2018;19(4):1818–1826. doi: 10.1208/s12249-018-1004-5. [DOI] [PubMed] [Google Scholar]
- 20.Stewart S.A., Domínguez-Robles J., Donnelly R.F., Larrañeta E. Implantable polymeric drug delivery devices: classification, manufacture, materials, and clinical applications. Polymers. 2018;10(12):1379. doi: 10.3390/polym10121379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krieger K.J., Bertollo N., Dangol M., Sheridan J.T., Lowery M.M., O’Cearbhaill E.D. Simple and customizable method for fabrication of high-aspect ratio microneedle molds using low-cost 3D printing. Microsyst Nanoeng. 2019;5(1):1–14. doi: 10.1038/s41378-019-0088-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nejad H.R., Sadeqi A., Kiaee G., Sonkusale S. Low-cost and cleanroom-free fabrication of microneedles. Microsyst Nanoeng. 2018;4:17073. [Google Scholar]
- 23.Serrano-Castañeda P., Escobar-Chavez J.J., Rodríguez-Cruz I.M., Melgoza L.M., Martinez-Hernandez J. Microneedles as enhancer of drug absorption through the skin and applications in medicine and cosmetology. J Pharm Pharm Sci. 2018;21:73–93. doi: 10.18433/jpps29610. [DOI] [PubMed] [Google Scholar]
- 24.Dimatteo R., Darling N.J., Segura T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv Drug Deliv Rev. 2018;127:167–184. doi: 10.1016/j.addr.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y., Sui Y., Liu C., Liu C., Wu M., Li B. A physically crosslinked polydopamine/nanocellulose hydrogel as potential versatile vehicles for drug delivery and wound healing. Carbohydr Polym. 2018;188:27–36. doi: 10.1016/j.carbpol.2018.01.093. [DOI] [PubMed] [Google Scholar]
- 26.Wang L., Chen S., Zhu Y., Zhang M., Tang S., Li J. Triple-modal imaging-guided chemo-photothermal synergistic therapy for breast cancer with magnetically targeted phase-shifted nanoparticles. ACS Appl Mater Interfaces. 2018;10(49):42102–42114. doi: 10.1021/acsami.8b16323. [DOI] [PubMed] [Google Scholar]
- 27.Mantha S., Pillai S., Khayambashi P., Upadhyay A., Zhang Y., Tao O. Smart hydrogels in tissue engineering and regenerative medicine. Materials. 2019;12(20):3323. doi: 10.3390/ma12203323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu X., Hosta-Rigau L., Chandrawati R., Cui J. Multi-stimuli-responsive polymer particles, films, and hydrogels for drug delivery. Chem. 2018;4(9):2084–2107. [Google Scholar]
- 29.Fu J. Hydrogel properties and applications. J Mater Chem B. 2019;7(10):1523–1525. doi: 10.1039/c9tb90023c. [DOI] [PubMed] [Google Scholar]
- 30.Yu Y., Feng R., Yu S., Li J., Wang Y., Song Y. Nanostructured lipid carrier-based pH and temperature dual-responsive hydrogel composed of carboxymethyl chitosan and poloxamer for drug delivery. Int J Biol Macromol. 2018;114:462–469. doi: 10.1016/j.ijbiomac.2018.03.117. [DOI] [PubMed] [Google Scholar]
- 31.Li J., Mooney D.J. Designing hydrogels for controlled drug delivery. Nat Rev Mater. 2016;1(12):16071. doi: 10.1038/natrevmats.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang W., Wat E., Hui P.C., Chan B., Ng F.S., Kan C.-W. Dual-functional transdermal drug delivery system with controllable drug loading based on thermosensitive poloxamer hydrogel for atopic dermatitis treatment. Sci Rep. 2016;6:24112. doi: 10.1038/srep24112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qu J., Liang Y., Shi M., Guo B., Gao Y., Yin Z. Biocompatible conductive hydrogels based on dextran and aniline trimer as electro-responsive drug delivery system for localized drug release. Int J Biol Macromol. 2019;140:255–264. doi: 10.1016/j.ijbiomac.2019.08.120. [DOI] [PubMed] [Google Scholar]
- 34.Daly A.C., Riley L., Segura T., Burdick J.A. Hydrogel microparticles for biomedical applications. Nat Rev Mater. 2019 doi: 10.1038/s41578-019-0148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lahiji S.F., Dangol M., Jung H. A patchless dissolving microneedle delivery system enabling rapid and efficient transdermal drug delivery. Sci Rep. 2015;5:7914. doi: 10.1038/srep07914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim S., Dangol M., Kang G., Lahiji S.F., Yang H., Jang M. Enhanced transdermal delivery by combined application of dissolving microneedle patch on serum-treated skin. Mol Pharm. 2017;14(6):2024–2031. doi: 10.1021/acs.molpharmaceut.7b00111. [DOI] [PubMed] [Google Scholar]
- 37.Lahiji S.F., Seo S.H., Kim S., Dangol M., Shim J., Li C.G. Transcutaneous implantation of valproic acid-encapsulated dissolving microneedles induces hair regrowth. Biomaterials. 2018;167:69–79. doi: 10.1016/j.biomaterials.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 38.Park J.-H., Allen M.G., Prausnitz M.R. Biodegradable polymer microneedles: fabrication, mechanics and transdermal drug delivery. J Control Release. 2005;104(1):51–66. doi: 10.1016/j.jconrel.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 39.Shin J.U., Kim J.D., Kim H.K., Kang H.K., Joo C., Lee J.H. The use of biodegradable microneedle patches to increase penetration of topical steroid for prurigo nodularis. Eur J Dermatol. 2018;28(1):71–77. doi: 10.1684/ejd.2017.3164. [DOI] [PubMed] [Google Scholar]
- 40.Lahiji S.F., Um D.J., Kim Y., Jang J., Yang H., Jung H. Scalp micro-pigmentation via transcutaneous implantation of flexible tissue interlocking biodegradable microneedles. Pharmaceutics. 2019;11(11):549. doi: 10.3390/pharmaceutics11110549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim J.H., Shin J.U., Kim S.H., Noh J.Y., Kim H.R., Lee J. Successful transdermal allergen delivery and allergen-specific immunotherapy using biodegradable microneedle patches. Biomaterials. 2018;150:38–48. doi: 10.1016/j.biomaterials.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 42.Kim H., Seong K.-Y., Lee J.H., Park W., Yang S.Y., Hahn S.K. Biodegradable microneedle patch delivering antigenic peptide-hyaluronate conjugate for cancer immunotherapy. ACS Biomater Sci Eng. 2019 doi: 10.1021/acsbiomaterials.9b00961. [DOI] [PubMed] [Google Scholar]
- 43.van der Maaden K., Sekerdag E., Jiskoot W., Bouwstra J. Impact-insertion applicator improves reliability of skin penetration by solid microneedle arrays. AAPS J. 2014;16(4):681–684. doi: 10.1208/s12248-014-9606-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Niu L., Chu L.Y., Burton S.A., Hansen K.J., Panyam J. Intradermal delivery of vaccine nanoparticles using hollow microneedle array generates enhanced and balanced immune response. J Control Release. 2019;294:268–278. doi: 10.1016/j.jconrel.2018.12.026. [DOI] [PubMed] [Google Scholar]
- 45.Li J., Zhou Y., Yang J., Ye R., Gao J., Ren L. Fabrication of gradient porous microneedle array by modified hot embossing for transdermal drug delivery. Mater Sci Eng, C. 2019;96:576–582. doi: 10.1016/j.msec.2018.11.074. [DOI] [PubMed] [Google Scholar]
- 46.Economidou S.N., Lamprou D.A., Douroumis D. 3D printing applications for transdermal drug delivery. Int J Pharm. 2018;544(2):415–424. doi: 10.1016/j.ijpharm.2018.01.031. [DOI] [PubMed] [Google Scholar]
- 47.Lim J., Tahk D., Yu J., Min D.-H., Jeon N.L. Design rules for a tunable merged-tip microneedle. Microsyst Nanoeng. 2018;4(1):29. doi: 10.1038/s41378-018-0028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilke N., Hibert C., O’Brien J., Morrissey A. Silicon microneedle electrode array with temperature monitoring for electroporation. Sens Actuators, A. 2005;123:319–325. [Google Scholar]
- 49.Narayanan S.P., Raghavan S. Solid silicon microneedles for drug delivery applications. Int J Adv Manuf Technol. 2017;93(1–4):407–422. [Google Scholar]
- 50.Das A., Singha C., Bhattacharyya A. Development of silicon microneedle arrays with spontaneously generated micro-cavity ring for transdermal drug delivery. Microelectron Eng. 2019;210:14–18. [Google Scholar]
- 51.Chaen S., Oiwa K., Shimmen T., Iwamoto H., Sugi H. Simultaneous recordings of force and sliding movement between a myosin-coated glass microneedle and actin cables in vitro. Proc Natl Acad Sci. 1989;86(5):1510–1514. doi: 10.1073/pnas.86.5.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee S., Jeong W., Beebe D.J. Microfluidic valve with cored glass microneedle for microinjection. Lab Chip. 2003;3(3):164–167. doi: 10.1039/b305692a. [DOI] [PubMed] [Google Scholar]
- 53.Chae W.S., Seong J.Y., Jung H.N., Kong S.H., Kim M.H., Suh H.S. Comparative study on efficacy and safety of 1550 nm Er: Glass fractional laser and fractional radiofrequency microneedle device for facial atrophic acne scar. J Cosmet Dermatol. 2015;14(2):100–106. doi: 10.1111/jocd.12139. [DOI] [PubMed] [Google Scholar]
- 54.Verhoeven M., Bystrova S., Winnubst L., Qureshi H., De Gruijl T.D., Scheper R.J. Applying ceramic nanoporous microneedle arrays as a transport interface in egg plants and an ex-vivo human skin model. Microelectron Eng. 2012;98:659–662. [Google Scholar]
- 55.Ita K. Ceramic microneedles and hollow microneedles for transdermal drug delivery: two decades of research. J Drug Delivery Sci Technol. 2018;44:314–322. [Google Scholar]
- 56.Sullivan S.P., Murthy N., Prausnitz M.R. Minimally invasive protein delivery with rapidly dissolving polymer microneedles. Adv Mater (Deerfield Beach Fla.) 2008;20(5):933–938. doi: 10.1002/adma.200701205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ling M.H., Chen M.C. Dissolving polymer microneedle patches for rapid and efficient transdermal delivery of insulin to diabetic rats. Acta Biomater. 2013;9(11):8952–8961. doi: 10.1016/j.actbio.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 58.Maurya A., Nanjappa S.H., Honnavar S., Salwa M., Murthy S.N. Rapidly dissolving microneedle patches for transdermal iron replenishment therapy. J Pharm Sci. 2018;107(6):1642–1647. doi: 10.1016/j.xphs.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 59.Yao W., Tao C., Zou J., Zheng H., Zhu J., Zhu Z. Flexible two-layer dissolving and safing microneedle transdermal of neurotoxin: a biocomfortable attempt to treat Rheumatoid Arthritis. Int J Pharm. 2019;563:91–100. doi: 10.1016/j.ijpharm.2019.03.033. [DOI] [PubMed] [Google Scholar]
- 60.Kang G., Kim S., Yang H., Jang M., Chiang L., Baek J.H. Combinatorial application of dissolving microneedle patch and cream for improvement of skin wrinkles, dermal density, elasticity, and hydration. J Cosmet Dermatol. 2019;18(4):1083–1091. doi: 10.1111/jocd.12807. [DOI] [PubMed] [Google Scholar]
- 61.Yin Z., Kuang D., Wang S., Zheng Z., Yadavalli V.K., Lu S. Swellable silk fibroin microneedles for transdermal drug delivery. Int J Biol Macromol. 2018;106:48–56. doi: 10.1016/j.ijbiomac.2017.07.178. [DOI] [PubMed] [Google Scholar]
- 62.Courtenay A.J., Rodgers A.M., McCrudden M.T.C., McCarthy H.O., Donnelly R.F. Novel hydrogel-forming microneedle array for intradermal vaccination in mice using ovalbumin as a model protein antigen. Mol Pharm. 2019;16(1):118–127. doi: 10.1021/acs.molpharmaceut.8b00895. [DOI] [PubMed] [Google Scholar]
- 63.Jeon E.Y., Lee J., Kim B.J., Joo K.I., Kim K.H., Lim G. Bio-inspired swellable hydrogel-forming double-layered adhesive microneedle protein patch for regenerative internal/external surgical closure. Biomaterials. 2019;222 doi: 10.1016/j.biomaterials.2019.119439. [DOI] [PubMed] [Google Scholar]
- 64.Chen S., Matsumoto H., Moro-oka Y., Tanaka M., Miyahara Y., Suganami T. Smart Microneedle fabricated with silk fibroin combined semi-interpenetrating network hydrogel for glucose-responsive insulin delivery. ACS Biomater Sci Eng. 2019;5(11):5781–5789. doi: 10.1021/acsbiomaterials.9b00532. [DOI] [PubMed] [Google Scholar]
- 65.Al Sulaiman D., Chang J.Y.H., Bennett N.R., Topouzi H., Higgins C.A., Irvine D.J. Hydrogel-coated microneedle arrays for minimally invasive sampling and sensing of specific circulating nucleic acids from skin interstitial fluid. ACS Nano. 2019;13(8):9620–9628. doi: 10.1021/acsnano.9b04783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Friedrich C.R., Vasile M.J. The micromilling process for high aspect ratio microstructures. Microsyst Technol. 1996;2(3):144–148. [Google Scholar]
- 67.Lim D., Kamotani Y., Cho B., Mazumder J., Takayama S. Fabrication of microfluidic mixers and artificial vasculatures using a high-brightness diode-pumped Nd:YAG laser direct write method. Lab Chip. 2003;3(4):318–323. doi: 10.1039/b308452c. [DOI] [PubMed] [Google Scholar]
- 68.Jung P.G., Lee T.W., Oh D.J., Hwang S.J., Jung I.D., Lee S.M. Nickel microneedles fabricated by sequential copper and nickel electroless plating and copper chemical wet etching. Sens Mater. 2008;20(1):45–53. [Google Scholar]
- 69.Tathireddy P., Rakwal D., Bamberg E., Solzbacher F. TRANSDUCERS 2009–2009 International Solid-State Sensors, Actuators and Microsystems Conference. IEEE; 2009. Fabrication of 3-dimensional silicon microelectrode arrays using micro electro discharge machining for neural applications; pp. 1206–1209. [Google Scholar]
- 70.Lee K., Jung H. Drawing lithography for microneedles: a review of fundamentals and biomedical applications. Biomaterials. 2012;33(30):7309–7326. doi: 10.1016/j.biomaterials.2012.06.065. [DOI] [PubMed] [Google Scholar]
- 71.Tomono T. Puncture performance of sharpen microneedles by using inclined contact UV lithography. Microsyst Technol. 2018;24(9):3589–3599. [Google Scholar]
- 72.Li Y., Zhang H., Yang R., Tazrin F., Zhu C., Kaddoura M. In-plane silicon microneedles with open capillary microfluidic networks by deep reactive ion etching and sacrificial layer based sharpening. Sens Actuators, A. 2019;292:149–157. [Google Scholar]
- 73.Lim S.H., Ng J.Y., Kang L. Three-dimensional printing of a microneedle array on personalized curved surfaces for dual-pronged treatment of trigger finger. Biofabrication. 2017;9(1) doi: 10.1088/1758-5090/9/1/015010. [DOI] [PubMed] [Google Scholar]
- 74.Chen Z., Lin Y., Lee W., Ren L., Liu B., Liang L. Additive manufacturing of honeybee-inspired microneedle for easy skin insertion and difficult removal. ACS Appl Mater Interfaces. 2018;10(35):29338–29346. doi: 10.1021/acsami.8b09563. [DOI] [PubMed] [Google Scholar]
- 75.Farias C., Lyman R., Hemingway C., Chau H., Mahacek A., Bouzos E. Three-dimensional (3D) printed microneedles for microencapsulated cell extrusion. Bioengineering. 2018;5(3):59. doi: 10.3390/bioengineering5030059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee J.W., Choi S.O., Felner E.I., Prausnitz M.R. Dissolving microneedle patch for transdermal delivery of human growth hormone. Small. 2011;7(4):531–539. doi: 10.1002/smll.201001091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matsuo K., Yokota Y., Zhai Y., Quan Y.-S., Kamiyama F., Mukai Y. A low-invasive and effective transcutaneous immunization system using a novel dissolving microneedle array for soluble and particulate antigens. J Control Release. 2012;161(1):10–17. doi: 10.1016/j.jconrel.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 78.Takada K., Ito Y., Matsumoto K., Sato Y., Nishio M., Tadano Y. Usefulness of basic fibroblast growth factor (bFGF) loaded dissolving microneedles for local therapy of skin wounds. J Biomater Nanobiotechnol. 2013;4(03):256. [Google Scholar]
- 79.Park S.Y., Lee H.U., Lee Y.-C., Kim G.H., Park E.C., Han S.H. Wound healing potential of antibacterial microneedles loaded with green tea extracts. Mater Sci Eng, C. 2014;42:757–762. doi: 10.1016/j.msec.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 80.Mohammed Y.H., Yamada M., Lin L.L., Grice J.E., Roberts M.S., Raphael A.P. Microneedle enhanced delivery of cosmeceutically relevant peptides in human skin. PLoS ONE. 2014;9(7) doi: 10.1371/journal.pone.0101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Caffarel-Salvador E., Kearney M.-C., Mairs R., Gallo L., Stewart S., Brady A. Methylene blue-loaded dissolving microneedles: potential use in photodynamic antimicrobial chemotherapy of infected wounds. Pharmaceutics. 2015;7(4):397–412. doi: 10.3390/pharmaceutics7040397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun W., Inayathullah M., Manoukian M.A., Malkovskiy A.V., Manickam S., Marinkovich M.P. Transdermal delivery of functional collagen via polyvinylpyrrolidone microneedles. Ann Biomed Eng. 2015;43(12):2978–2990. doi: 10.1007/s10439-015-1353-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gualeni B., Coulman S., Shah D., Eng P., Ashraf H., Vescovo P. Minimally invasive and targeted therapeutic cell delivery to the skin using microneedle devices. Br J Dermatol. 2018;178(3):731–739. doi: 10.1111/bjd.15923. [DOI] [PubMed] [Google Scholar]
- 84.Kang G., Tu T., Kim S., Yang H., Jang M., Jo D. Adenosine-loaded dissolving microneedle patches to improve skin wrinkles, dermal density, elasticity and hydration. Int J Cosmet Sci. 2018;40(2):199–206. doi: 10.1111/ics.12453. [DOI] [PubMed] [Google Scholar]
- 85.Pan J., Ruan W., Qin M., Long Y., Wan T., Yu K. Intradermal delivery of STAT3 siRNA to treat melanoma via dissolving microneedles. Sci Rep. 2018;8(1):1117. doi: 10.1038/s41598-018-19463-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang Y., Feng P., Yu J., Yang J., Zhao J., Wang J. ROS-responsive microneedle patch for acne vulgaris treatment. Adv Therap. 2018;1(3) [Google Scholar]
- 87.Xie Y., Wang H., Mao J., Li Y., Hussain M., Zhu J. Enhanced in vitro efficacy for inhibiting hypertrophic scar by bleomycin-loaded dissolving hyaluronic acid microneedles. J Mater Chem B. 2019;7(42):6604–6611. doi: 10.1039/c9tb01449g. [DOI] [PubMed] [Google Scholar]
- 88.Tan C.W., Tan W.D., Srivastava R., Yow A.P., Wong D.W., Tey H.L. Dissolving triamcinolone-embedded microneedles for the treatment of keloids: a single-blinded intra-individual controlled clinical trial. Dermatol Ther. 2019;9(3):601–611. doi: 10.1007/s13555-019-00316-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Du H., Liu P., Zhu J., Lan J., Li Y., Zhang L., Zhu J., Tao J. Hyaluronic acid dissolving microneedle patch loaded with methotrexate for improved treatment of psoriasis. ACS Appl Mater Interfaces. 2019 doi: 10.1021/acsami.9b15668. [DOI] [PubMed] [Google Scholar]