Abstract
A 4-year-old boy presented with a 6-day history of severe non-limbic–sparing conjunctivitis. Atypical Stevens–Johnson syndrome with a possible cause of Mycoplasma pneumoniae was suspected as the precipitant of the clinical symptoms. The patient recovered with amniotic membrane transplantation and intravenous immunoglobulin therapy despite an initial delay in diagnosis.
INTRODUCTION
Mycoplasma pneumoniae is considered the most common infectious cause of Stevens–Johnson syndrome (SJS), particularly in children.1 It can cause an atypical presentation limited to ocular, oral, and genitourinary mucous membranes that spares the skin. It has been suggested that M. pneumoniae-associated mucositis be considered its own distinct clinical entity.2 Up to 25% of people with M. pneumoniae infections experience extrapulmonary symptoms before, during, or after onset of pulmonary signs or in the complete absence of respiratory symptoms.3
There have been reports of M. pneumoniae-associated SJS in patients who all exhibited preceding pulmonary symptoms such as cough, congestion, and pharyngitis.4 We report a case of severe ocular involvement in atypical SJS with a possible cause of M. pneumoniae in a previously healthy boy lacking respiratory symptoms.
CASE REPORT
A 4-year-old boy with no medical history experienced 3 days of self-limiting night fevers, without cough, pharyngitis, or respiratory symptoms (Table 1). Four days after his fevers resolved without medication, he presented to the emergency department with purulent bilateral conjunctivitis and one oral tongue ulcer. He was discharged with a prescription of cefdinir and ofloxacin drops. His condition worsened despite antibiotics for 5 days, with more oral ulcers and periorbital edema. He subsequently presented to the emergency department a second time and was discharged with Magic Mouthwash (CutisPharma, Inc., Wilmington, MA) for suspected Coxsackievirus. Two days later, he was admitted to the pediatric intensive care unit for 11 days with odynophagia and inability to open his eyes, and was given empiric intravenous acyclovir.
TABLE 1.
Disease Time Course and Events Before and After Hospital Admission
| Time Course (Days) | Events |
|---|---|
| Pre-hospital | |
| 1 | Night fevers; no medicationsa |
| 3 | Fevers resolve; no medications |
| 7 | Presented with bilateral purulent conjunctivitis and oral tongue ulcer; diagnosis of bacterial conjunctivitis; discharged with ofloxacin and cefdinir eye drops |
| 12 | Presented with more oral ulcers and worsening conjunctivitis; diagnosis of Cocksackievirus; ofloxacin and cefdinir discontinued and Magic Mouthwashb started |
| 14 | Presented with odynophagia and severe conjunctivitis; diagnosis of herpes-simplex virus gingivostomatitis with conjunctivitis or Stevens–Johnson syndrome; Magic Mouthwash discontinued and empiric intravenous acyclovir started |
| Hospital | |
| 1 | Presented with bilateral non-limbic–sparing conjunctivitis with symblepharon; started artificial tears, Lacri-Lube, prednisolone acetate, and tobramycin-dexamethasone drops |
| 3 | Underwent lip and oral mucosa biopsy, dilated funduscopic examination under anesthesia, symblerpharon lysis, and amniotic membrane transplantation; no herpetic lesions or corneal epithelial defects were found; started artificial tears, Lacri-Lube, 0.05% cyclosporine, 0.5% moxifloxacin, and 0.1% dexamethasone |
| 4 | Acyclovir discontinued, intravenous immunoglobulin started |
| 6 | Intravenous immunoglobulin discontinued |
| 11 | Patient discharged |
| 6-week follow-up | Bilateral white and quiet eyes |
No ibuprofen, acetaminophen, or other medication was taken for fevers as per the patient’s mother.
Magic Mouthwash formulation contained nystatin, diphenhydramine, Mylanta, viscous lidocaine 2%, and distilled water.
Magic Mouthwash is manufactured by CutisPharma, Inc., Wilmington, MA; Lacri-Lube is manufactured by Allergan, Dublin, Ireland; and Mylanta is manufactured by McNeil Consumer Pharmeuticals Co., Lancaster, PA.
Laboratory results showed a high white blood cell count, C-reactive protein level, and erythrocyte sedimentation rate. Throat herpes-simplex virus cultures and direct fluorescent antibody test, throat varicella-zoster virus direct fluorescent antibody, throat enterovirus culture, and blood and urine cultures were negative. M. pneumoniae IgM titer was positive on serology and immunofluorescent antibody test and IgG was positive on serology. Polymerase chain reaction test for M. pneumoniae IgM and paired sera for IgM and IgG were not performed. Lip and buccal mucosa biopsies stained negative for organisms on periodic acid-Schiff stain and showed necrotic tissue with acute and chronic inflammation and no viable epidermis, consistent with SJS.
Ophthalmology consultation on the first hospital day was significant for bilateral symblepharon and severe non-limbic–sparing conjunctivitis. At this time, culture and direct fluorescent antibody results were not available, and the diagnosis considered was herpes-simplex virus gingivostomatitis with conjunctivitis. For this reason, conservative management was initiated with artificial tears, Lacri-Lube (Allergan, Dublin, Ireland), prednisolone acetate, and tobramycin-dexamethasone drops, with eyelid hygiene for symblepharon.
Symptoms did not improve. Dilated funduscopic examination and amniotic membrane transplantation were performed on the third hospital day. No intraocular disease, herpetic lesions, or ulcers were found. Fluorescein stain demonstrated bilateral inferior linear corneal epithelial defects, likely due to exposure. The fornices were swept with muscle hooks and symblepharon and eyelid adhesions were lysed. Cryopreserved amniotic membranes (Ambio 5; Okto Ophtho, Costa Mesa, CA) were grafted within all fornices using two 6–0 polyglactin 910 mattress sutures on the medial and lateral sides of each fornix (Figure 1B). Symblepharon rings (FCI Ophthalmics, Pembroke, MA) were then placed to maintain the fornix.
Figure 1.
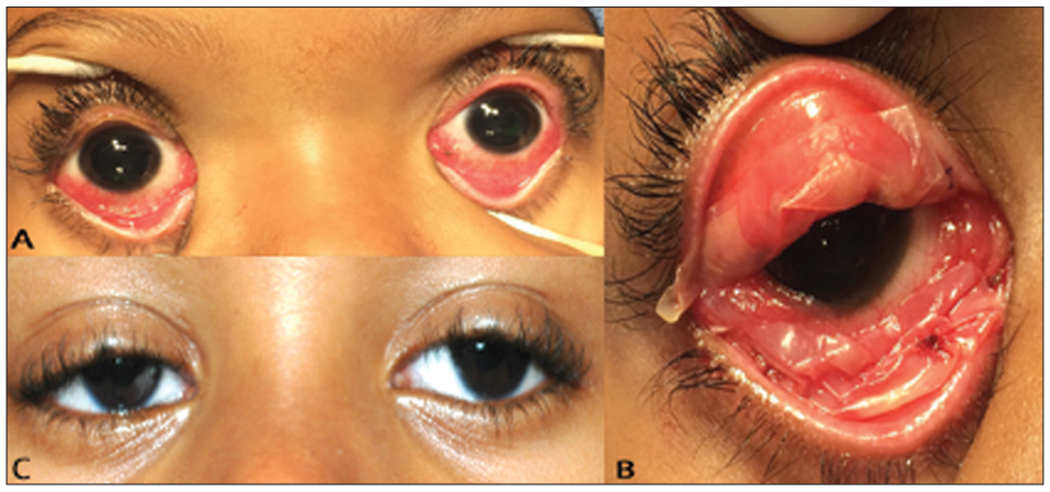
(A) Clinical appearance of a patient with atypical Stevens–Johnson syndrome during the acute phase, after symblepharon lysis and immediately before bilateral amniotic membrane transplantation on day 3 of the hospital course. Symblepharons are not pictured. (B) The right superior and inferior fornices immediately after bilateral amniotic membrane transplantation. (C) The patient 6 weeks after amniotic membrane transplantation with bilateral white and quiet eyes. Symblepharon rings are still in place.
Postoperative management included a 3-day course of 1 g/kg/d intravenous immunoglobulin, as well as an ophthalmic regimen including artificial tears, Lacri-Lube, 0.05% cyclosporine, 0.5% moxifloxacin, and 0.1% dexamethasone. The patient recovered fully with intravenous immunoglobulin and amniotic membrane transplantation. Additional antibiotic therapy was not given because the patient’s symptoms improved with intravenous immunoglobulin and no pulmonary manifestations were present.
DISCUSSION
This case demonstrates the ongoing challenges in the early diagnosis of atypical SJS. Delays in diagnosis and ophthalmologic management can potentially result in end-stage corneal blindness if not caught during the acute phase.5 Pediatric ophthalmologists should be aware that atypical SJS with a possible cause of M. pneumoniae could occur in a child without any pulmonary manifestations.
Typically, in children younger than 5 years, M. pneumoniae infections manifest as coryza and wheezing, and uncommonly progress to pneumonia.6 Initial pharyngitis, cough, sore throat, hoarseness, and fever also tend to occur. Because children may carry M. pneumoniae without respiratory symptoms before manifestation of a more severe condition, the timely correct diagnosis of SJS is particularly important. Initially, this patient was suspected of having either a bacterial or viral infection and treated accordingly. With no skin rash present, SJS was not considered at the onset. Neither antibiotics nor Magic Mouthwash improved the patient’s conjunctivitis or oral lesions, delaying SJS diagnosis for approximately 9 days after initial ocular symptoms.
SJS may be drug-induced, idiopathic, or secondary to infection. In this case, ophthalmology, dermatology, and infectious disease teams postulated a possible cause of M. pneumoniae given single positive IgM and IgG titers. It is possible that SJS had a different cause, because additional confirmatory polymerase chain reaction and paired serology tests for IgM and IgG were not performed. IgM may persist for weeks to months after M. pneumoniae infection, making the timing of infection difficult to deduce from a single IgM titer. The ofloxacin and cefdinir drops the patient took are another potential cause. However, he presented with simultaneous conjunctivitis and oral ulcers before initiating antibiotics and had not taken any prior medications. Previous research also suggests that ocular involvement is more common in M. pneumoniae-associated SJS than drug-induced SJS.7,8
Mild forms of SJS can improve with conservative treatment and not all patients develop serious ocular sequelae. However, amniotic membrane transplantation has been recommended in patients with more severe conjunctival inflammation at higher risk for long-term complications.9 In such patients, it is most ideal to initiate amniotic membrane transplantation in the hyperacute (< 72 hours) or the acute (72 hours to 4 weeks) phase.10 Amniotic membrane promotes epithelialization, possessing anti-inflammatory and anti-fibrotic effects.11 In this case, amniotic membrane transplantation was prudently initiated during the acute phase and proved effective. This confirms reports that amniotic membrane transplantation is a successful treatment for ocular involvement in severe SJS when applied early.12 Two weeks after amniotic membrane transplantation, the patient’s cornea and conjunctiva completely healed with no irritation present and remained so at 6 weeks (Figure 1C).
This case demonstrates that SJS limited to ocular and oral mucous membranes may still be missed clinically at the onset. In children with a mucositis-type presentation, pediatric ophthalmologists should consider atypical SJS with a possible cause of M. pneumoniae from the start, even in the absence of respiratory symptoms. Although amniotic membrane transplantation is now regarded as an effective ocular treatment in severe SJS, further investigations are needed to elucidate how specific delays in amniotic membrane transplantation initiation, as in this case, affect visual outcomes. Knowing the exact time periods within the acute phase of SJS during which amniotic membrane transplantation initiation will still have positive effects would improve ocular management guidelines.
Acknowledgments
The authors thank the Department of Medical Education at the Icahn School of Medicine at Mount Sinai for support in the copyediting of this case report.
Footnotes
The authors have no financial or proprietary interest in the materials presented herein.
REFERENCES
- 1.Vanfleteren I, Van Gysel D, De Brandt C. Stevens-Johnson syndrome: a diagnostic challenge in the absence of skin lesions. Pediatr Dermatol. 2003;20:52–56. [DOI] [PubMed] [Google Scholar]
- 2.Schalock PC, Dinulos JG. Mycoplasma pneumoniae-induced Stevens-Johnson syndrome without skin lesions: fact or fiction? J Am Acad Dermatol. 2005;52:312–315. [DOI] [PubMed] [Google Scholar]
- 3.Kashyap S, Sarkar M. Mycoplasma pneumonia: clinical features and management. Lung India. 2010;27:75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vujic I, Shroff A, Posch C, et al. Mycoplasma pneumoniae-associated mucositis: case report and systematic review of literature. J Eur Acad Dermatol Venereol. 2015;29:595–598. [DOI] [PubMed] [Google Scholar]
- 5.Iyer G, Srinivasan B, Agarwal S, Kamala Muradharan S, Arumugam S. Comprehensive approach to ocular consequences of Stevens Johnson syndrome: the aftermath of a systemic condition. Graefes Arch Clin Exp Ophthalmol. 2014;252:457–467. [DOI] [PubMed] [Google Scholar]
- 6.Cassell GH, Clyde WA Jr, Davis JK. Mycoplasma respiratory infections In: Razin S, Barile MD, eds. The Mycoplasma, vol. 4 New York: Academic Press; 1985:65–106. [Google Scholar]
- 7.Moreau J, Watson R, Hartman M, Linde-Zwirble WT, Ferris LK. Epidemiology of ophthalmologic disease associated with erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis in hospitalized children in the United States. Pediatr Dermatol. 2014;31:163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kunimi Y, Hirata Y, Aihara M, Yamane Y, Ikezawa Z. Statistical analysis of Stevens-Johnson syndrome caused by Mycoplasma pneumoniae infection in Japan. Allergol Int. 2011;60:525–532. [DOI] [PubMed] [Google Scholar]
- 9.Gregory DG. The ophthalmologic management of acute Stevens-Johnson syndrome. Ocul Surf. 2008;6:87–95. [DOI] [PubMed] [Google Scholar]
- 10.Ciralsky J, Sippel K. Prompt versus delayed amniotic membrane application in a patient with acute Stevens-Johnson syndrome. Clin Ophthalmol. 2013;7:1031–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honavar SG, Bansal AK, Sangwan VS, Rao GN. Amniotic membrane transplantation for ocular surface reconstruction in Stevens-Johnson syndrome. Ophthalmology. 2000;107:975–979. [DOI] [PubMed] [Google Scholar]
- 12.Gregory DG. Treatment of acute Stevens-Johnson syndrome and toxic epidermal necrolysis using amniotic membrane: a review of 10 consecutive cases. Ophthalmology. 2011;118:908–914. [DOI] [PubMed] [Google Scholar]


