Abstract
Objective:
To evaluate the result of an inpatient postpartum human papillomavirus (HPV) immunization pilot program in a diverse, low-income patient population from an urban, hospital-based obstetrics and gynecology clinic.
Methods:
In this cohort study, we present results from the first two years of the inpatient postpartum HPV immunization program, in which vaccine-eligible postpartum women were identified and immunized during their hospital stay. The program was implemented following educational outreach with prenatal and postpartum clinicians and nurses. Associations between receipt of HPV vaccine as an inpatient and characteristics of patients, and likelihood of and missed opportunities for receiving a subsequent dose of HPV vaccine as an outpatient were determined using logistic regression, time-to-event analyses, chi-squared tests and student’s t-tests.
Results:
From 04/11/2017 to 04/10/2019, 394 (59.2%) of 666 postpartum women were eligible for the inpatient postpartum HPV immunization program. The majority (265/394, 67.3%) received the IPP-HPV dose, 36/265 (13.6%) of whom completed the series with that dose. Among women due for additional doses after hospital discharge, those who received the inpatient dose were more likely to receive a subsequent outpatient dose (138/229) than were those who did not receive an inpatient dose (39/129; Hazard ratio: 2.51, 95% CI 1.76 to 3.58). On average, there were 30.7 fewer (95% CI 5.8–55.6, p<0.02) missed opportunities for subsequent outpatient doses for every 100 eligible visits among women who received the inpatient dose compared with women who did not. By the end of the study, the proportion of women who had completed the vaccine series was higher among women that received the inpatient dose (95/265, 35.8%) than in those who did not (12/129, 9.3%; OR 5.45, 95% CI 2.86–10.38).
Conclusion:
The inpatient postpartum HPV immunization program was associated with increased rates of immunization and addressed a previously missed opportunity. Inpatient immunization programs can serve as a critical way to address gaps in vaccine uptake.
Precis:
Programs to administer the human papillomavirus (HPV) vaccine to women as postpartum inpatients can be an effective way to increase uptake of HPV vaccine.
Introduction
Since 2006, HPV vaccine has been recommended for children starting as early as age 9 years, with routine immunization at 11 to 12 years old. Catch-up immunization is recommended for all adults through age 26 years and shared clinical decision-making is recommended for adults aged 27–45 years.(1, 2) The nine-valent HPV vaccine (9vHPV) prevents infection from high-risk HPV types that cause 85% of cervical cancer cases.(3) Despite established safety and efficacy of the HPV vaccine, as well as growing evidence of its clinical effectiveness,(1, 4–9) vaccine administration has lagged. Rates of series initiation and completion among adolescents aged 13–17 years remain low (68.1% and 51.1%, respectively).(10) Furthermore, the rate of vaccine series initiation among women ages 19–26 years who have never previously been vaccinated is even lower at 8.6%,(11) underscoring the importance of catch-up immunization even as efforts to improve adolescent rates are underway.
Administration of the HPV vaccine is not recommended during pregnancy. Consequently, pregnancy, associated with not completing the series, is a barrier to achieving higher rates of immunization.(12) The postpartum hospital stay is the first opportunity to vaccinate for HPV after delivery, and the vaccine is safe for breastfeeding infants.(13) Currently, the HPV vaccine is not routinely administered among postpartum women during their hospital admission, unlike other vaccines that have been more widely implemented into routine inpatient postpartum care: tetanus, diptheria, acellular pertussis (Tdap); influenza; and measles, mumps and rubella (MMR), resulting in increased immunization rates.(14–18)
Although HPV immunization prior to sexual debut is most effective, the vaccine can still prevent disease among women who have been sexually active. Results from a study of HPV-exposed and unexposed 15–25 years old women found vaccine effectiveness against precancerous lesions of moderate grade or worse (CIN2+) to be 30.4%, irrespective of prior HPV-type exposure.(19) The study also demonstrated a 24.7% reduction in the number of cervical excision procedures for the treatment of precancerous cervical lesions. In August 2019, the Centers for Disease Control and Prevention (CDC) expanded its HPV immunization recommendation, noting that some adults may benefit from receiving the vaccine through age 45 years.(1)
The postpartum hospital stay is, therefore, a critical immunization opportunity. We introduced an inpatient postpartum HPV immunization pilot program to improve HPV immunization rates and to decrease missed opportunities to initiate or complete the series. In this study, we evaluate the results of the inpatient postpartum HPV immunization program over the first two years of implementation, and one year of follow-up in a diverse, low-income patient population.
Methods
The inpatient postpartum HPV immunization pilot program was launched at Yale New Haven Hospital on April 11, 2017 as a quality improvement program providing HPV vaccine to postpartum women during their hospital admission. Data from the first two years of the program (04/11/2017 through 04/10/2019) were reviewed and a medical chart review was conducted on outpatient records through 10/10/2019, allowing at least 6 months from the date of delivery for follow-up of all patients. The Yale Institutional Review Board approved the review of program records and medical chart abstraction for this study.
As a pilot, the program was available to patients less than 27 years old, who received prenatal care at a single hospital-based ob-gyn clinic, and who had not completed the HPV vaccine series at the time of postpartum hospital admission. The recommendation for shared decision-making on HPV immunization with adults 27–45 years old occurred more than 2 years after initiation of the inpatient postpartum HPV immunization program and, given limited vaccine supply, program eligibility criteria was not changed. The ob-gyn clinic where program-eligible patients received prenatal care is located in an urban area and serves a low-income patient population, primarily of Black or Hispanic race and ethnicity. Most patients have public insurance and approximately one-quarter are uninsured. An interdisciplinary team of staff and clinicians provide general ob-gyn care at the clinic.
The program coordinator identified patients eligible for the inpatient postpartum HPV immunization program by reviewing the inpatient postpartum roster for practice site and patient age each day. The coordinator determined whether patients were adequately immunized by reviewing their immunization history in the electronic health record (EHR), and then informed the on-call clinician of all patients eligible for the program for that day. The on-call clinician would then place the order for 9vHPV. Additional routine clinical workflow for vaccines were then followed by the patient’s clinical team: discuss HPV vaccine with the patient, address questions, provide the vaccine information sheet from the CDC, administer the vaccine, document administration or declination of the vaccine, and update the patient EHR problem list if the patient would need subsequent doses following hospital discharge, as applicable.
The HPV vaccine supply for the pilot program was provided through a drug-only grant from Merck & Co., Inc. (Merck) and was therefore available to patients at no cost. A new inpatient order for HPV vaccine was created in the EHR and the vaccine stock was managed by the hospital’s investigational pharmacy, given the non-formulary, grant-based vaccine supply. All patients eligible for the immunization program were scheduled to return to the ob-gyn clinic for outpatient postpartum care per standard practice. Subsequent doses of HPV vaccine were recommended and administered at this clinic as indicated. We used the 2-dose schedule (0, 6–12 months) for individuals who received their first dose < 15 years old and the 3-dose schedule (0, 1–2 months, 6 months) for all others per CDC guidelines (1).
Clinicians providing prenatal care were informed about the inpatient postpartum HPV immunization program prior to its initiation at monthly staff meetings and were encouraged to discuss IPP-HPV with eligible patients in the third trimester. In addition, patient education materials about the immunization progrma were developed and included HPV vaccine information adapted from the American College of Obstetricians and Gynecologists (ACOG).(20) Clinicians were encouraged to continue reviewing HPV immunization history as part of postpartum care to identify patients due for a subsequent dose of vaccine. Intermittent reminders were provided to outpatient clinicians to discuss HPV immunizations at both antenatal and postpartum visits.
Descriptive analyses were first conducted for the entire cohort of women who were eligible in the first 2 years of the immunization program. Next, analyses were conducted for the subgroup of women who were both eligible to receive one or more outpatient doses of the HPV vaccine after hospital discharge and had at least 12 months of follow-up time from date of delivery. Logistic regression analysis was used to determine if the likelihood of receiving the HPV vaccine, either the inpatient or subsequent outpatient doses, was associated with individual patient characteristics. Each patient characteristic was first assessed for association with receiving a dose of HPV vaccine (either inpatient or subsequent outpatient) individually using bivariate logistic regression models. Multivariable models were then built, which included all variables that were statistically significantly associated with receipt of the vaccine on bivariate analyses. A p-value of <0.05 was considered to be statistically significant. Variables assessed included age, patient self-reported race (American Indian or Alaska Native, Asian, Black or African American, Native Hawaiian, other Pacific Islander, White or Caucasian, other, unknown) and ethnicity (Hispanic or Latino, non-Hispanic, unknown) as categorized in the EHR, preferred language, marital status, insurance type, smoking status, parity, gravidity, mode of delivery, and previous HPV immunization history. Race and ethnicity were assessed in this study given well-documented racial and ethnic disparities in HPV immunization as well as cervical cancer incidence, morbidity and mortality.
Time-to-event analyses, using single-failure survival-time models with Gompertz distributions and non-parametric Kaplan Meier curves, were used to evaluate the association of inpatient immunization on the probability of receiving subsequent outpatient doses of HPV vaccine over time post-discharge among all women eligible for the inpatient postpartum HPV immunization program. For these survival models, the outcome of interest was the number of postpartum months until the next dose of HPV vaccine was received. Women were censored if they completed the vaccine series while hospitalized, aged out of eligibility for the HPV vaccine (27 years of age), or at the end of the study if they did not receive any additional doses of the vaccine after discharge.
Next, we examined if the proportion of women with at least one missed opportunity during a 12-month follow-up period differed between those that received and those that did not receive the inpatient dose using linear regression. A missed opportunity was defined as a vaccine-eligible visit at which the patient was due for HPV vaccine based on vaccine schedules described previously, but it was not administered.(21) A vaccine-eligible visit was defined as a visit to the ob-gyn clinic at which the patient was less than 27 years of age, was not pregnant, and had not completed the vaccine series. Vaccine administrations documented in the EHR that occurred outside the ob-gyn clinic were included in the vaccine-eligible visit count and in the administered count. The rate of missed opportunity was calculated as the ratio of missed opportunity to the total number of vaccine-eligible visits over a 12-month period. Poisson regression with robust variance estimators was used to estimate the rates of missed opportunity and test for differences in rates between the two groups. In these models, the number of missed opportunity was the dependent variable, receipt of the inpatient dose was a regressor, and the number of vaccine eligible visits were used as offsets (on a log-scale).
Statistical analyses were performed using STATA® software version 15.0 (College Station, TX).
Results
From April 11, 2017 to April 10, 2019, 666 women who were patients of the hospital-based ob-gyn clinic and under 27 years of age delivered at Yale New Haven Hospital. A total of 394/666 women (59.2%) were eligible to receive an inpatient dose of the HPV vaccine (Appendix 1, available online at http://links.lww.com/xxx). The remainder of women were noted to have completed their HPV vaccine series. Among women eligible for IPP-HPV, 265/394 received a dose during their hospital admission (67.3%). For 36/265 women, receipt of the inpatient dose led to vaccine series completion (13.6%), and the remaining 358 women were eligible to receive additional doses of HPV vaccine. Among these, 49.4% (n=177/358) went on to receive at least one subsequent dose. The proportion of women eligible for additional vaccine doses after hospital discharge and went on to receive a subsequent outpatient dose was significantly higher (30.1%, 95% CI 19.6%–40.4%) among those who had received the inpatient dose (138/229, 60.3%) than those who had not (39/129, 30.2%). The overall proportion of program-eligible women who completed the vaccine series at the end of the study was also higher among women that received the inpatient dose (95/265, 35.8%) compared with those who did not (12/129, 9.3%; OR 5.45, 95% CI 2.86–10.38).
The characteristics of the 394 women eligible for the immunization program are shown in Table 1. Of eligible women, 277/394 were Black or Hispanic (70.3%), 297/394 had public insurance and 82/394 had no insurance (75.4% and 20.8%, respectively), 104/394 identified Spanish as their preferred language (26.4%), and 316/394 had not received any prior doses of HPV vaccine (80.2%). The majority had previously been pregnant (250/394, 63.5%), although 189/394 had delivered for the first time (48%), and 334/394 delivered vaginally (84.8%).
Table 1.
Characteristics of Patients Eligible for IPP-HPV and Association with Receipt of Inpatient Vaccine Dose
| Characteristic | Overall, N= 394 | Given IPP Dose, N= 265 | Not Given IPP Dose, N=129 | OR | aOR | |||
|---|---|---|---|---|---|---|---|---|
| Age, years | 23 (21–25) | 23 (21–25) | 23 (21–25) | 0.98 (0.89–1.05)* | - | |||
| Race | ||||||||
| Hispanic | 171 (43.4) | 128 (48.3) | 43 (33.3) | 2.14 (1.07–4.30) | 1.31 (0.60–2.87) | |||
| Non-Hispanic Black | 106 (26.9) | 67 (25.3) | 39 (30.2) | 1.23 (0.60–2.55) | 1.17 (0.55–2.47) | |||
| Non-Hispanic White | 43 (10.9) | 25 (9.4) | 18 (14.0) | Referent | ||||
| Non-Hispanic Other□ | 52 (13.2) | 27 (10.2) | 25 (19.4) | 0.78 (0.34–1.76) | 0.91 (0.32–2.56) | |||
| Multirace | 18 (4.6) | 15 (5.7) | 3 (2.3) | 3.60 (0.91–14.31) | 2.78 (0.67–11.51) | |||
| Unknown | 4 (1.0) | 3 (1.1) | 1 (.8) | - | - | |||
| Language | ||||||||
| English | 242 (61.4) | 152 (57.4) | 90 (69.8) | Referent | ||||
| Spanish | 104 (26.4) | 87 (32.8) | 17 (13.2) | 3.03 (1.69–5.42) | 2.84 (1.41–5.67) | |||
| Other‡ | 48 (12.2) | 26 (9.8) | 22 (17.1) | 0.70 (0.37–1.31) | 1.14 (0.44–2.93) | |||
| Marital Status | ||||||||
| Single | 290 (73.6) | 202 (76.2) | 88 (68.2) | Referent | ||||
| Married | 92 (23.4) | 53 (20.0) | 39 (30.2) | 0.59 (0.37–0.96) | 0.59 (0.32–1.10) | |||
| Other | 8 (2.0) | 6 (2.3) | 2 (1.6) | 1.31 (0.26–6.60) | 0.93 (0.17–5.04) | |||
| Unknown | 4 (1.0) | 4 (1.5) | 0 (.0) | - | - | |||
| Insurance | ||||||||
| Private | 15 (3.8) | 8 (3.0) | 7 (5.4) | 0.6 (0.21–1.70) | - | |||
| Public | 297 (75.4) | 195 (73.6) | 102 (79.1) | Referent | - | |||
| Uninsured | 82 (20.8) | 62 (23.4) | 20 (15.5) | 1.62 (0.93–2.83) | - | |||
| Smoking History | ||||||||
| Never smoked | 297 (75.4) | 204 (77.0) | 93 (72.1) | Referent | - | |||
| Past or current smoker | 93 (23.6) | 59 (22.3) | 34 (26.4) | 0.79 (0.49–1.29) | - | |||
| Unknown | 4 (1.0) | 2 (.8) | 2 (1.6) | - | - | |||
| Prior HPV vaccine | ||||||||
| Unimmunized | 316 (80.2) | 207 (78.1) | 109 (84.5) | Referent | ||||
| 1 prior dose | 41 (10.4) | 27 (10.2) | 14 (10.9) | 1.02 (0.51–2.02) | 1.01 (0.49–2.09) | |||
| 2 prior doses | 37 (9.4) | 31 (11.7) | 6 (4.7) | 2.72 (1.10–6.72) | 3.07 (1.22–7.78) | |||
| Gravidity | ||||||||
| Primigravid | 144 (36.5) | 102 (38.5) | 42 (32.6) | 1.30 (0.50–1.20) | - | |||
| Parity | ||||||||
| Primiparous | 189 (48.0) | 133 (50.2) | 56 (43.4) | 1.31 (0.86–2.01) | - | |||
| Mode of delivery | ||||||||
| Vaginal | 334 (84.8) | 227 (85.7) | 107 (82.9) | ref | - | |||
| C-section | 60 (15.2) | 38 (14.3) | 22 (17.1) | 0.82 (0.46–1.44) | - | |||
Data are median(IQR), n (%), or value (95% CI) unless otherwise specified
OR, unadjusted odds ratio estimated using simple logistic regression where the outcome equals one if the individual received IPP HPV vaccine
aOR, adjusted odds ratio determined using multivariate logistic regression adjusting for race, language and marital status
Bold indicates significance (p<0.05)
Age considered a continuous variable, per year of age increase
Other Race: American Indian, Asian, and Native Hawaiian
Other language: Arabic, Turkish, Chinese, Farsi, French, Pashto, Serbian, Sign, Swahili, Tigrinya, Vietnamese and Hindi.
Bivariate and multivariate analyses of demographic and clinic characteristics associated with receipt of the inpatient dose are shown in Table 1. Hispanic women were significantly more likely to receive an inpatient dose of the HPV vaccine (OR 2.14, 95% CI 1.07–4.30), as were women identifying Spanish as their preferred language (OR 3.03, 95% CI 1.69–5.42). After adjusting for significant covariates, women with a preferred language of Spanish were still more likely to receive an inpatient dose (aOR 2.84, 95% CI 1.41–5.67). Women who were married were less likely to receive an inpatient dose (aOR 0.59, 95% CI 0.37–0.96), although after adjusting for significant covariates, this association was no longer significant (aOR 0.59, 95% CI 0.32–1.10). Women who had received 2 prior doses of the vaccine before their hospital admission were more likely to receive an inpatient dose (OR 2.72, 95% CI 1.10–6.72). This association was strengthened after adjusting for significant covariates (aOR 3.07, 95% CI 1.22–7.78). There was no significant difference in receipt of vaccine when comparing women with private or no insurance to those with public insurance.
In addition, receipt of other postpartum vaccines was analyzed for association with receipt of the inpatient dose. Among women eligible for the immunization program, 52/394 (13.2%) were also due for a Tdap vaccine, of whom 15/52 (28.8%) received it during their postpartum hospitalization. Similarly, 67/394 (17.0%) women were also due for an influenza vaccine, of whom 21/67 (31.3%) received it during their postpartum hospitalization. Receipt of Tdap or influenza vaccine was not significantly correlated with receipt of the inpatient dose (OR 2.11; 95% CI 0.60–7.38 and OR 1.33; 95% CI 0.47–3.77, respectively).
Patients eligible for the inpatient postpartum HPV immunization program who were not series complete at the time of hospital discharge (n = 358) were included in the time-to-event analysis to evaluate the association of the program with the probability of receiving subsequent outpatient doses over time post-discharge. Women who had received an inpatient dose had a higher probability of receiving subsequent outpatient doses of HPV vaccine compared with those who did not receive the inpatient dose (HR 2.51, 95% confidence interval (CI) 1.76 to 3.58). Kaplan-Meier curves for this analysis are shown in Figure 1.
Figure 1:
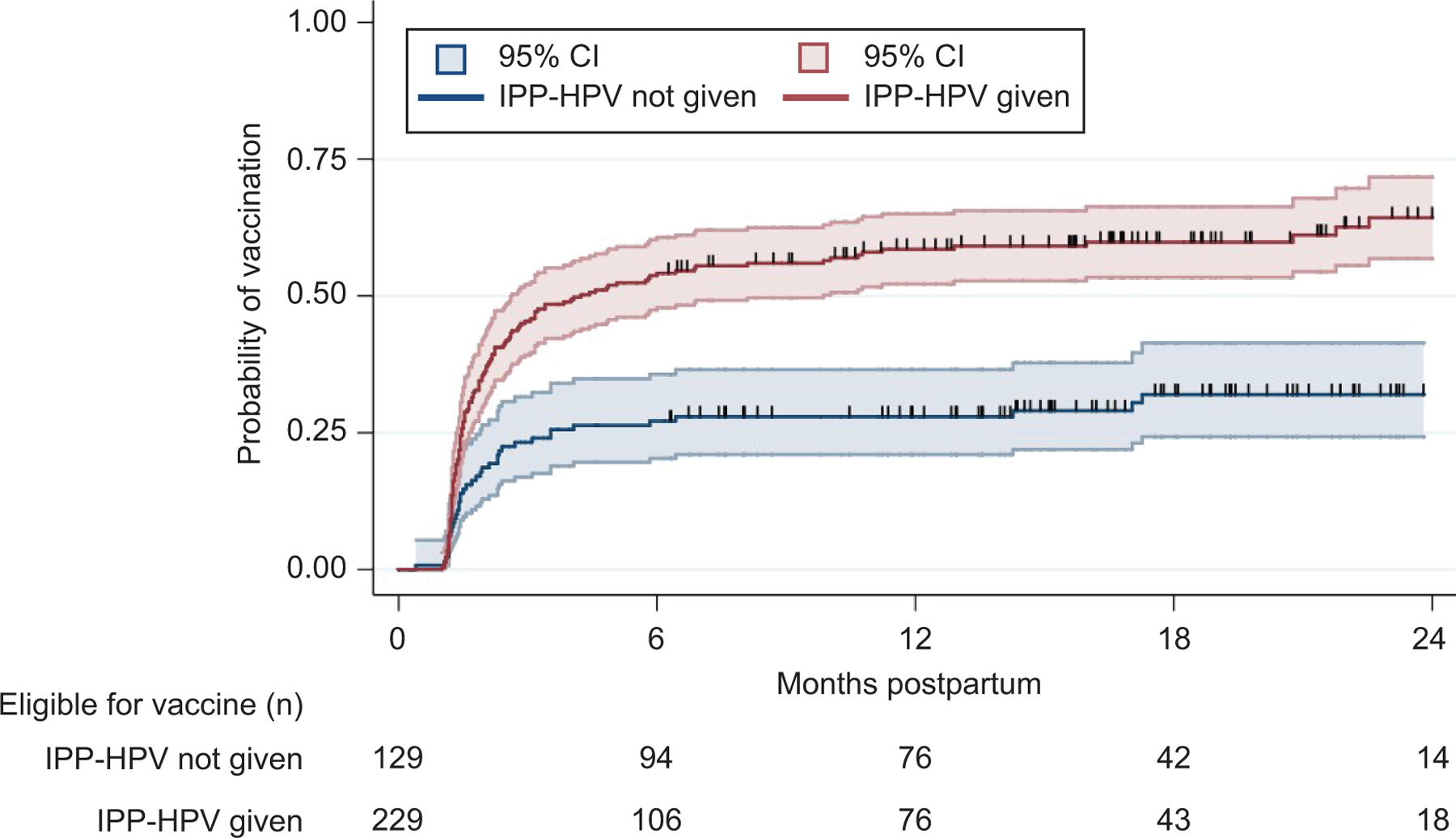
Time-to-event analyses on the probability of receiving subsequent doses of outpatient human papillomavirus (HPV) vaccine over time posthospital discharge among all women eligible for the inpatient postpartum HPV immunization pilot program (IPP-HPV) during the study period, based on receipt of IPP-HPV dose.
Of the 358 women who had not completed their vaccine series at hospital discharge, 295 women had 12 months of follow-up time after delivery (Table 2). This group was similar to the overall group eligible for the inpatient postpartum HPV immunization program described above and in Table 1. The odds of receiving an outpatient dose of HPV vaccine during the first 12 postpartum months was higher among Hispanic women (OR 2.60, 95% CI 1.15–5.90) and among women who preferred speaking Spanish (OR 2.11, 95% CI 1.22–3.64). However, these associations did not remain significant after adjusting for covariates (aOR 1.83, 95% CI 0.63–5.34; aOR 0.98, 95% CI 0.47–2.08). Overall, 189/295 (64.1%) of women in this group had received the inpatient dose, and these women were significantly more likely to receive a subsequent outpatient dose (OR 3.76, 95% CI 2.26–6.29), even after adjusting for significant covariates (aOR 3.14, 95% CI 1.71–5.77). For every additional outpatient follow-up visit attended, the odds of getting the outpatient dose increased almost 3-fold (aOR 2.88, 95% CI 2.08–3.99).
Table 2.
Characteristics of Patients with 12-months Follow-up Due for Additional Doses of HPV Vaccine After Hospital Discharge and Association with Receipt of Subsequent Outpatient Dose, N=295
| Characteristic | Overall, N= 295 | Received Outpatient Dose, N = 143 | Did Not Receive Outpatient Dose, N=152 | OR | aOR | |||
|---|---|---|---|---|---|---|---|---|
| Age, median (IQR), y | 23 (21–25) | 23 (21–25) | 23 (21–25) | 0.95 (0.87–1.04)* | - | |||
| Race, No. (%) | ||||||||
| Hispanic | 124 (42.0) | 73 (51.0) | 51 (33.6) | 2.60 (1.15–5.90) | 1.83 (0.63–5.34) | |||
| Non-Hispanic Black | 79 (26.8) | 30 (21.0) | 49 (32.2) | 1.11 (0.47–2.64) | 1.16 (0.41–3.33) | |||
| Non-Hispanic White | 31 (10.5) | 11 (7.7) | 20 (13.2) | Referent | ||||
| Non-Hispanic Other□ | 41 (13.9) | 18 (12.6) | 23 (15.1) | 1.42 (0.54–3.71) | 2.01 (0.51–7.92) | |||
| Multi-race | 16 (5.4) | 8 (5.6) | 8 (5.3) | 1.82 (0.53–6.19) | 1.33 (0.33–5.48) | |||
| Unknown | 4 (1.4) | 3 (2.1) | 1 (.7) | - | - | |||
| Language, No. (%) | ||||||||
| English | 180 (61.0) | 80 (55.9) | 100 (65.8) | Referent | ||||
| Spanish | 78 (26.4) | 49 (34.3) | 29 (19.1) | 2.11 (1.22–3.64) | 0.98 (0.47–2.08) | |||
| Other‡ | 37 (12.5) | 14 (9.8) | 23 (15.1) | 0.76 (0.37–1.57) | 0.44 (0.14–1.41) | |||
| Marital Status, No. (%) | ||||||||
| Single | 216 (73.2) | 104 (72.7) | 112 (73.7) | Referent | - | |||
| Married | 68 (23.1) | 31 (21.7) | 37 (24.3) | 0.90 (0.52–1.56) | - | |||
| Other | 7 (2.4) | 7 (4.9) | 0 (.0) | - | - | |||
| Unknown | 4 (1.4) | 1 (.7) | 3 (2.0) | - | - | |||
| Insurance, No. (%) | ||||||||
| Private | 11 (3.7) | 6 (4.2) | 5 (3.3) | 1.45 (0.42–4.89) | - | |||
| Public | 223 (75.6) | 101 (70.6) | 122 (80.3) | Referent | - | |||
| Uninsured | 61 (20.7) | 36 (25.2) | 25 (16.4) | 1.74 (0.98–3.09) | - | |||
| Smoking History, No. (%) | ||||||||
| Never smoked | 225 (76.3) | 116 (81.1) | 109 (71.7) | Referent | - | |||
| Past or current smoker | 66 (22.4) | 26 (18.2) | 40 (26.3) | 0.61 (0.35–1.07) | - | |||
| Unknown | 4 (1.4) | 1 (.7) | 3 (2.0) | - | - | |||
| Gravidity, No. (%) | ||||||||
| Primagravid | 108 (36.6) | 60 (42.0) | 48 (31.6) | 1.57 (0.97–2.52) | - | |||
| Parity, No. (%) | ||||||||
| Primiparous | 141 (47.8) | 73 (51.0) | 68 (44.7) | 1.29 (0.81–2.04) | - | |||
| Mode of delivery, No. (%) | ||||||||
| Vaginal | 255 (86.4) | 122 (85.3) | 133 (87.5) | Referent | - | |||
| C-section | 40 (13.6) | 21 (14.7) | 19 (12.5) | 1.20 (0.62–2.35) | - | |||
| IPP-HPV Vaccine, No. (%) | ||||||||
| IPP Dose Not Given | 106 (35.9) | 30 (21.0) | 76 (50.0) | Referent | ||||
| IPP Dose Given | 189 (64.1) | 113 (79.0) | 76 (50.0) | 3.76 (2.26–6.29) | 3.14 (1.71–5.77) | |||
| Postpartum Visits Attended | 3.16 (2.30–4.35) | 2.88 (2.08–3.99) | ||||||
| Median number (IQR) | 1(1–2) | 2 (1–2) | 1 (0–1) | |||||
Data are median(IQR), n (%), or value (95% CI) unless otherwise specified
OR, unadjusted odds ratio estimated using simple logistic regression where the outcome equals one if the individual received subsequent outpatient doses of HPV vaccine
aOR, adjusted odds ratio determined using multivariate logistic regression adjusting for race, language and marital status
Bold indicates significance (p<0.05)
Age considered a continuous variable, per year of age increase
Other Race: American Indian, Asian, and Native Hawaiian
Other language: Arabic, Turkish, Chinese, Farsi, French, Pashto, Serbian, Sign, Swahili, Tigrinya, Vietnamese and Hindi.
Among women who remained vaccine eligible after discharge, 222/358 (62.0%) had a vaccine eligible clinical encounter in the 12-month follow-up period. Of these, the number of women who had at least one missed opportunity was significantly lower (23.4% lower, 95%CI: 9.4%–23.3%) in the subgroup that received the inpatient dose (75/154, 48.7%) than in those who did not (49/68; 72.1%). On average, there were 30.7 fewer (95%CI: 5.8–55.6, p<0.02) missed opportunities for every 100 eligible visits among women who received an inpatient dose compared with those that did not (83.2 vs 52.5 missed opportunities per 100 visits, respectively).
Discussion
In this study, we present an evaluation of a pilot quality improvement program to immunize postpartum women with HPV vaccine prior to hospital discharge. The goals of this program were to increase rates of HPV immunization in a young adult population at high risk for HPV-associated disease, to create a new opportunity for immunization and thereby decrease missed opportunities, and to serve as a demonstration project for consideration of future expansion and scaling. Through the inpatient postpartum HPV immunization program, 265 women received HPV vaccine prior to hospital discharge that would not otherwise have received a dose, for an uptake rate of 67.3%. Those who received the inpatient dose were three times more likely to get subsequent outpatient doses and the time to their next dose was sooner. Postpartum women who received the inpatient dose had fewer missed opportunities for subsequent immunization in the outpatient clinic.
Previous studies have examined various strategies for improving HPV vaccine uptake among adolescents – improving the quality of clinicians’ recommendations; increasing education for parents, adolescents and clinicians; improving clinical workflows with best practice alerts or standing orders.(22–26) A few studies have also demonstrated the effectiveness of using similar strategies among young adults.(26–29) However, identifying new opportunities for HPV immunization is also critical and can potentially have a systems-level impact. Alternate arenas for immunization that remain underused include schools, pharmacies, and non-traditional clinical opportunities such as immunizing parents at pediatricians’ offices or patients admitted to the hospital.
Although it is most effective to immunize children and adolescents prior to initiation of sexual activity, individuals who have been sexually active can still benefit from catch-up immunization with HPV vaccine. This is particularly important with 9vHPV, as most women have only been exposed to 1–2 HPV types and remain susceptible to several types that the current vaccine protects against (30). One study has shown inpatient postpartum HPV immunization to be feasible and acceptable under a research protocol.(31) However, a research consent was required of women to receive the vaccine and eligibility was limited to women delivering at ≥ 32 weeks gestation who had received no prior doses. A program for women delivering at The University of Texas Medical Branch (UTMB) is the only other program we are aware of that routinely provides HPV vaccine to postpartum women less than 27 years of age prior to hospital discharge.(32) The proportion of eligible women who received the inpatient dose in our program (67.3%) was similar although slightly lower than that (75.4%) reported by investigators at UTMB.
The UTMB program differs in several ways from our program. The UTMB cohort was more likely to have never received prior HPV vaccine (74.2% versus 46.9%) and was less likely to be vaccine series complete at the time of program eligibility screening (15.5% versus 40.8%) compared with our cohort. This could indicate that among our study cohort, more women had been counseled on HPV vaccine previously and had already made a decision or formed opinions about the vaccine. Additionally, the UTMB program uses resource-intense patient navigators and text messaging-based patient reminders in its outpatient follow-up process. We used existing workflows for delivering subsequent outpatient doses of HPV vaccine. Previous initiatives have been implemented in our ob-gyn clinic to improve HPV immunization rates and decrease missed opportunities.(29) We, therefore, introduced minimal additional processes in implementing the inpatient postpartum HPV immunization program which included guidance and intermittent reminders to outpatient clinicians both to discuss the program as part of prenatal care and to review HPV immunization history during postpartum visits to facilitate completion of the HPV vaccine series.
The program evaluated in this study was successful in immunizing women at higher risk for developing cervical cancer and other HPV-associated disease. Incidence of cervical cancer is higher among lower income women and also among Black and Hispanic women in the U.S. compared with white and non-Hispanic women.(33–35) In our study cohort, the majority of women eligible for the inpatient postpartum HPV immunization program, regardless of race or ethnicity, received the inpatient dose of the HPV vaccine. In addition, among women with a preferred language of Spanish, the odds of receiving the inpatient dose was nearly 3 times higher compared with women with a preferred language of English. This may represent a group of women that perhaps did not have many prior opportunities for immunization due to a lack of access to the vaccine by geography or resources.
The American Cancer Society and the President’s Cancer Panel in 2014 and in 2018 have endorsed using every opportunity for immunizing against HPV infection.(36–38) There are also intangible benefits to such a strategy such as promoting a continued emphasis on the woman’s future health and well-being beyond pregnancy – setting the stage for overall preventive health, including vaccines, for an entire family. Even for women who did not receive the inpatient dose of HPV vaccine, there could be some benefit due to the positive messaging about the vaccine that affects the likelihood of receiving it at a subsequent outpatient visit.
The success of the inpatient postpartum HPV immunization program may reach beyond the benefits to those women who have been immunized. The HPV vaccine supply for this program currently comes from a grant and is therefore resource-limited. The Medicaid billing structure in Connecticut uses the diagnosis-related group (DRG) codes for inpatient hospital admissions. With this structure, individual medications or vaccines are not reimbursed and therefore adding items to the inpatient formulary must be carefully considered. The high cost of the vaccine makes it challenging to include the HPV vaccine on the inpatient formulary. Additionally, the role of a program coordinator can also be a barrier to sustainability. The success of this pilot program, however, has laid the groundwork for a significant investment by the hospital system to explore, develop, and implement an adapted version of the the inpatient postpartum HPV immunization program that will be both sustainable and accessible to all vaccine-eligible women delivering in the Yale New Haven Health System, the largest healthcare system in Connecticut. The adapted program is expected to leverage the electronic health record and health informatics in the creation of a “virtual outpatient immunization clinic” to scale up the immunization program, eliminate the need for a program coordinator, and allow for the vaccine to be charged for and covered as an outpatient insurance benefit thereby eliminating reliance on an external supply of vaccine.
Our study and the inpatient postpartum HPV immunization program had limitations. As a single-site study, the findings may have limited generalizability. The program was only available to patients from the hospital-based clinic. Therefore, we are unable to determine the effect of such a program in patients from other practice settings delivering in the same hospital. In addition, the inpatient postpartum HPV immunization program was possible due to a grant for vaccine supply. Thus, the implementation of similar programs in other settings may be challenging if the HPV vaccine is not on hospital formulary, however this is a hospital and state-specific factor. Determination of a patient’s HPV immunization history was limited by data available in the EHR, outside medical records, and by patient self-report, similar to the outpatient setting. Without an immunization registry that extends beyond age 2, accurate determination of vaccine history remains challenging. We used EHR data to determine the administration of subsequent outpatient HPV vaccine doses. However, if vaccine administration occurred outside of the health system’s EHR, we would not have been able to capture this information. Despite an overall successful program, 50% of eligible women needing subsequent outpatient doses did not receive any additional vaccine. This highlights the potential need for additional program components to further improve HPV vaccine series completion.
In this study, we demonstrated how substantial improvements in HPV vaccine uptake can be achieved with an inpatient postpartum HPV immunization program, thereby creating a new opportunity for catch-up immunization. Given the inability to receive the HPV vaccine in pregnancy, immunizing women in the postpartum period is critical. Using the hospital admission allows for efficient and timely care that is patient-centered as it facilitates faster completion of the HPV vaccine series with a reduced number of visits. Those women that participated in the program had fewer missed opportunities for subsequent outpatient immunization and received their next dose due sooner than women who did not receive the inpatient dose. Further work is needed to understand what additional programmatic components are needed to achieve higher rates of immunization with subsequent outpatient doses of HPV vaccine in the postpartum period. Inpatient postpartum HPV immunization programs should be more routinely considered by hospitals and health systems as part of a larger effort to improve immunization rates in the community.
Supplementary Material
Acknowledgements:
The authors thank the following individuals for their contributions and support in implementing the inpatient postpartum HPV immunization clinical program: Christian Pettker, MD; Liz O’Mara, RN; Meaghan Scalley, APRN; Sarah Kelly, PharmD; Jan Kozakiewez, PharmD; Jessica Illuzzi, MD; Nicole Spaulding, RN; Doreen Picagli, APRN; Susan Griggs, RN.
The study described in this manuscript has previously been presented at the following meetings: Society for Academic Specialists in General Obstetrics and Gynecology (SASGOG) Annual Meeting, May 2, 2019, Nashville, TN; and ASCCP Annual Meeting, April 2, 2020.
This work was supported, in part, from grants by the National Institutes of Health grant numbers R01AI123204 (Niccolai, Sheth, Shapiro, Oliveira), K07CA230234 (Sheth), KL2TR001862 (Sheth, Shapiro, Oliveira), UL1TR000142 (Shapiro) from the National Center for Advancing Translational Science (NCATS) at the National Institutes of Health and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIH.
Footnotes
Financial Disclosure
Ashlynn Torres disclosed receiving a Merck “drug only” award. Eugene D. Shapiro disclosed receiving funds for Expert testimony for several law firms and of University of Pittsburgh honorarium for giving Grand Rounds (neither of which included topics covered by this article). He also received royalties for writing for UptoDate about Lyme disease. Linda Niccolai has served as Scientific Advisor to Merck. The other authors did not report any potential conflicts of interest.
The 9vHPV (Gardasil 9) vaccine utilized by the program evaluated in this study was provided through the Merck Investigator Studies Program as a “drug only” award.
References
- 1.Meites E, Szilagyi PG, Chesson HW, Unger ER, Romero JR, Markowitz LE. Human papillomavirus Vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 2019. August 16;68:698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Advisory Committee on Immunization Practices. ACIP Shared Clinical Decision-Making Recommendations. 2020; https://www.cdc.gov/vaccines/acip/acip-scdm-faqs.html. Accessed June 22, 2020.
- 3.Saraiya M, Unger ER, Thompson TD, Lynch CF, Hernandez BY, Lyu CW, et al. US assessment of hpv types in cancers: implications for current and 9-valent hpv vaccines. J Natl Cancer Inst 2015;107(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serrano B, Alemany L, Tous S, Bruni L, Clifford GM, Weiss T, et al. Potential impact of a nine-valent vaccine in human papillomavirus related cervical disease. Infect Agent Cancer 2012;7(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McClung NM, Lewis RM, Gargano JW, Querec T, Unger ER, Markowitz LE. Declines in vaccine-type human papillomavirus prevalence in females across racial/ethnic groups: data drom a national survey. J of Adolesc Health 2019;65:715–722. [DOI] [PubMed] [Google Scholar]
- 6.Cleveland AA, Gargano JW, Park IU, Griffin MR, Niccolai LM, Powell M, et al. Cervical adenocarcinoma in situ: human papillomavirus types and incidence trends in five states, 2008–2015. Int J Cancer 2020;146:810–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niccolai LM, Meek JI, Brackney M, Hadler JL, Sosa LE, Weinberger DM. Declines in human papillomavirus (hpv)-associated high-grade cervical lesions after introduction of hpv vaccines in Connecticut, United States, 2008–2015. Clin Infect Dis 2017;65:884–9. [DOI] [PubMed] [Google Scholar]
- 8.Oakley F, Desouki MM, Pemmaraju M, Gargano JM, Markowitz LE, Steinau M, et al. Trends in high-grade cervical cancer precursors in the human papillomavirus vaccine era. Am J Prev Med 2018;55:19–25. [DOI] [PubMed] [Google Scholar]
- 9.Shing JZ, Hull PC, Zhu Y, Gargano JW, Markowitz LE, Cleveland AA, et al. Trends in anogenital wart incidence among Tennessee Medicaid enrollees, 2006–2014: The impact of human papillomavirus vaccination. Papillomavirus Res 2019;7:141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker TY, Elam-Evans LD, Yankey D, Markowitz LE, Williams CL, Fredua B, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 Years - United States, 2018. MMWR Morb Mortal Wkly Rep 2019;68:718–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hung MC WW, Lu PJ, Kim DK, Grohskopf LA, Pilishvili T, Skoff TH, et al. Vaccination coverage among adults in the United States, National Health Interview Survey, 2016. Available at: https://www.cdc.gov/vaccines/imz-managers/coverage/adultvaxview/pubs-resources/NHIS-2016.html#hpv. Retrieved November 8, 2019.
- 12.Perry R, Rankin K, Yu MC, Harwood B. Factors associated with human papillomavirus vaccination completion on a catch-up schedule. Obstet Gynecol 2014;124:76–81. [DOI] [PubMed] [Google Scholar]
- 13.Markowitz LE, Dunne EF, Saraiya M, Chesson HW, Curtis CR, Gee J, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommendations and reports 2014;63:1–30. [PubMed] [Google Scholar]
- 14.Beigi RH, Fortner KB, Munoz FM, Roberts J, Gordon JL, Han HH, et al. Maternal immunization: opportunities for scientific advancement. Clin Infect Dis 2014;59:S408–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Committee opinion no. 608: influenza vaccination during pregnancy. Obstet Gynecol 2014. September;124(3):648–51. [DOI] [PubMed] [Google Scholar]
- 16.Committee Opinion No. 718: update on immunization and pregnancy: tetanus, diphtheria, and pertussis vaccination. Obstet Gynecol 2017;130:e153–e7. [DOI] [PubMed] [Google Scholar]
- 17.Ding HBC, Ball SW, Fink R, Parker Fiebelkor A,Bridges CB, Kahn KE, et al. Pregnant women and flu vaccination, internet panel survey, United States, November 2016. Available at: https://www.cdc.gov/flu/fluvaxview/pregnant-women-nov2016.htm#place. Retrieved September 4, 2017.
- 18.Healy CM, Rench MA, Castagnini LA, Baker CJ. Pertussis immunization in a high-risk postpartum population. Vaccine 2009;27(:5599–602. [DOI] [PubMed] [Google Scholar]
- 19.Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009;374:301–14. [DOI] [PubMed] [Google Scholar]
- 20.American College of Obstetricians and Gynecologists. Human Papillomavirus (HPV) Vaccination Frequently Asked Questions: Women’s Health. Available at: https://www.acog.org/patient-resources/faqs/womens-health/hpv-vaccination. Retrieved March 25, 2020.
- 21.Oliveira CR, Rock RM, Shapiro ED, Xu X, Lundsberg L, Zhang LB, et al. Missed opportunities for HPV immunization among young adult women. Am J Obstet Gynecol 2018;218:326.e1–326.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niccolai LM, Hansen CE. Practice- and community-based interventions to increase human papillomavirus vaccine coverage: a systematic review. JAMA Pediatr 2015;169:686–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilkey MB, Parks MJ, Margolis MA, McRee AL, Terk JV. Implementing evidence-based strategies to improve hpv vaccine delivery. Pediatr 2019;144: e20182500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brewer NT, Hall ME, Malo TL, Gilkey MB, Quinn B, Lathren C. Announcements versus conversations to improve hpv vaccination coverage: a randomized trial. Pediatr 2017;139:e20161764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dempsey AF, Pyrznawoski J, Lockhart S, Barnard J, Campagna EJ, Garrett K, et al. Effect of a health care professional communication training intervention on adolescent human papillomavirus vaccination: a cluster randomized clinical trial. JAMA Pediatr 2018;172:e180016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruffin MTt Plegue MA, Rockwell PG Young AP, Patel DA Yeazel MW. Impact of an electronic health record (ehr) reminder on human papillomavirus (hpv) vaccine initiation and timely completion. J Am Board of Fam Med 2015;28:324–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smulian EA, Mitchell KR, Stokley S. Interventions to increase hpv vaccination coverage: a systematic review. Hum Vaccin Immunother 2016;12:1566–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soon R, Sung S, Cruz MRD, Chen JJ, Hiraoka M. Improving human papillomavirus (hpv) vaccination in the postpartum setting. J Community Health 2017;42:66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deshmukh UOC, Griggs S, Coleman E, Avni-Singer L, Pathy S, Shapiro E, Sheth SS. Impact of a clinical interventions bundle on uptake of hpv vaccine at an ob/gyn clinic. Vaccine 2018;36:3599–3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu G, Markowitz LE, Hariri S, Panicker G, Unger ER. Seroprevalence of 9 human papillomavirus types in the United States, 2005–2006. J Infect Dis. 2016;213:191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wright JD, Govindappagari S, Pawar N, Cleary K, Burke WM, Devine PC, et al. Acceptance and compliance with postpartum human papillomavirus vaccination. Obstet Gynecol 2012;120:771–82. [DOI] [PubMed] [Google Scholar]
- 32.Berenson AB, Rahman M, Hirth JM, Rupp RE, Sarpong KO. A human papillomavirus vaccination program for low-income postpartum women. Am J Obstet Gynecol 2016;215:318e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boscoe FP, Johnson CJ, Sherman RL, Stinchcomb DG, Lin G, Henry KA. The relationship between area poverty rate and site-specific cancer incidence in the United States. Cancer 2014;120:2191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh GK, Williams SD, Siahpush M, Mulhollen A. Socioeconomic, rural-urban, and racial inequalities in us cancer mortality: part I—all cancers and lung cancer and part II—colorectal, prostate, breast, and cervical cancers. J Cancer Epidemiol 2011;2011:107497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Viens LJ, Henley SJ, Watson M, Markowitz LE, Thomas CC, Thompson TD, et al. Human papillomavirus-associated cancers - United States, 2008–2012. MMWR Morb Mortal Wkly Rep 2016;65:661–666. [DOI] [PubMed] [Google Scholar]
- 36.Accelerating HPV Vaccine Uptake: Urgency for Action to Prevent Cancer A Report to the President of the United States from the President’s Cancer Panel. Bethesda, MD: National Cancer Institute; 2014. [Google Scholar]
- 37.HPV Vaccination for Cancer Prevention: Progress, Opportunities, and a Renewed Call to Action A Report to the President of the United States from the Chair of the President’s Cancer Panel. Bethesda, MD: National Cancer Institute; 2018. [Google Scholar]
- 38.American Cancer Society. Steps for increasing HPV vaccination in practice: An action guide to implement evidence-based strategies for clinicians. Available at: https://www.cancer.org/content/dam/cancer-org/online-documents/en/pdf/flyers/steps-for-increasing-hpv-vaccination-in-practice.pdf. Retrieved March 9, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


