Abstract
Immersive virtual reality(VR) consists of immersion in artificial environments through the use of real-time render technologies and the latest generation devices. The users feel just as immersed as they would feel in an everyday life situation, and this sense of presence seems to have therapeutic potentials. However, the VR mechanisms remain only partially known. This study is novel in that, for the first time in VR research, appropriate controls for VR contexts, immersive characteristics (ie, control VR), and multifaceted objective and subjective outcomes were included in a within-subject study design conducted on healthy participants. Participants received heat thermal stimulations to determine how VR can increase individual heat-pain tolerance limits (primary outcome) measured in degrees Celsius and seconds while recording concurrent autonomic responses. We also assessed changes in pain unpleasantness, mood, situational anxiety, and level of enjoyment (secondary outcomes). The VR induced a net gain in heat pain tolerance limits that was paralleled by an increase of the parasympathetic responses. Virtual Reality improved mood, situational anxiety, and pain unpleasantness when participants perceived the context as enjoyable, but these changes did not influence the increases in pain tolerance limits. Distraction increased pain tolerance limits but did not induce such mood and physiological changes. Immersive VR has been anecdotally applied to improve acute symptoms in contexts such as battlefield, emergency, and operating rooms. This study provides a mechanistic framework for VR as a low-risk, nonpharmacological intervention, which regulates autonomic, affective (mood and situational anxiety), and evaluative (subjective pain and enjoyment ratings) responses associated with acute pain.
Introduction
Immersive virtual reality (VR) affords users the sensation of being transported into interactive, three-dimensional worlds. Users can engage in a variety of activities in imaginary environments through 360° immersion in an alternate reality [13]. Several theories have been proposed on how exactly VR may alleviate symptoms of a variety of illnesses, such as post-traumatic stress disorders [34; 35; 44; 45] and pain [22; 29; 46; 54]. Exposure to the immersive VR contexts and distraction are leading hypotheses, attributing improvement to competing engagement of pathways for memory or emotions that detract from those devoted to stress and pain signaling and allow for improved stress and pain control [15; 32]. Researchers have yet to determine whether immersion in VR, per se, is enough to elicit symptom improvement or whether cognitive engagement, in which guided experiences would be paramount, is required. This distinction is clearly important for the applied, clinical use of VR [26].
VR stimulates the visual cortex while simultaneously engaging other senses. VR might be able to limit the user’s processing of pain signals [51]. The common nature of mobile high-performance computing has now reduced both the size and cost of VR devices, allowing for VR use in everyday settings like clinical and at home. As an alternative to opioids, VR has been proven to be effective in decreasing pain during severe burn wound bandage changes, IV line placements, and dental interventions [51]. In light of these findings, we designed a study to explore VR-induced increases in heat-pain tolerance limits and concurrent changes in the autonomic system and pain-related emotion responses.
Pain experiences are associated with autonomic body responses such as heart rate variability Standard Deviation from Normal to Normal (SDNN) [48; 53] and Galvanic Skin Responses (GSR) [5; 7; 12; 16; 47]. Significantly greater GSR during pain than non-pain conditions [5; 7; 47], and distinct heart rate responses between noxious heat and non-painful warm stimuli [28; 36] and non-painful cool and noxious cold stimuli [27] have been reported as critical measurements related to pain, relaxation and sympathetic/parasympathetic balance.
To determine the mechanism behind immersive VR-based pain tolerance limit gains and related physiological autonomic and psychological responses, we conducted a fully-powered, within-subjects design study in healthy participants. We used control conditions for the VR with non-immersive Ocean and Opera contexts (i.e. same video and audio contents delivered in a 2D manner). Moreover, we controlled for the attention/distraction demand by including a working memory condition (2-Back Task) [39]. We measured changes in affective and evaluative processes associated with experiencing pain such as mood, situational anxiety, pain unpleasantness, and level of enjoyment.
Our hypotheses were that being immersed in the VR with a context that is both relaxing and enjoyable would maximize the ability to tolerate acute experimental painful heat stimulations as compared to the control (non-immersive) VR and distraction controls. That gain in heat-pain tolerance limits would have been reflected in a re-set of the sympathetic and parasympathetic balance while moderating mood, situational anxiety and unpleasantness of pain.
Methods
Forty-nine subjects were enrolled (25 women and 24 men; age: 27.4 ±6.47 years; race: 20 Whites; 10 Afro-Americans or Black; 19 Asians; see Table 1). The criteria of inclusion were: 1. being between 18 and 55 years old; 2. being able to speak English, and 3. being right handed. Moreover, we excluded study participants based on the following criteria: left-handedness, impaired hearing, color blindness, any history of chronic pain, current ongoing pain, neurological, cardiovascular, pulmonary, kidney and liver diseases, psychiatric disorders, and use of pain and other over-the-counter medication. All participants gave written consent to participate in this study and were compensated $50 after completion of all study procedures. The local Internal Review Board of the University of Maryland, Baltimore approved the study. The study was conducted in accordance with the Declaration of Helsinki.
Table 1.
Demographic and vital information of the pain-free healthy participants (n=49)
| n/mean ± S.D. | ||
|---|---|---|
| Sex | ||
| Men | 24 | |
| Women | 25 | |
| Age (years) | 27.75 ± 6.7 | |
| Ethnicity and Race | ||
| Non-Hispanic White | 20 | |
| Non-Hispanic Afro-American or Black | 10 | |
| Asian | 19 | |
| Body Mass Index | 23.72 ± 3.45 | |
| Blood Pressure (mmHg) | ||
| Diastolic | 71.94 ± 8.41 | |
| Systolic | 117.98 ± 12.21 | |
| Heart Rate pre-experiment (beats/min) | 70.90 ± 11.44 | |
|
Temperature (°C) |
Duration (seconds) |
|
| Individual baseline pain sensitivity | ||
| Warmth detection | 35.74 ± 2.39 | 13.71 ± 8.71 |
| Painful threshold | 38.66 ± 2.34 | 24.42 ± 12.26 |
| Painful tolerance limit | 46.19 ± 2.93 | 15.96 ± 3.30 |
| Heat-pain assessment during VR Ocean | ||
| Warmth detection | 37.70 ± 2.75 | 20.91 ± 10.09 |
| Painful threshold | 40.50 ± 3.06 | 31.18 ± 12.21 |
| Painful tolerance limit | 47.09 ± 2.05 | 16.98 ± 2.31 |
| Heat-pain assessment during VR Opera | ||
| Warmth detection | 37.11 ± 2.62 | 18.75 ± 10.09 |
| Painful threshold | 39.69 ± 3.20 | 28.18 ± 11.72 |
| Painful tolerance limit | 46.46 ± 2.46 | 16.27 ± 2.76 |
| Heat-pain assessment during Control Ocean | ||
| Warmth detection | 37.78 ± 2.67 | 21.17 ± 9.80 |
| Painful threshold | 39.87 ±3.28 | 28.87 ± 12.04 |
| Painful tolerance limit | 46.33 ± 2.65 | 16.12 ± 2.98 |
| Heat-pain assessment during Control Opera | ||
| Warmth detection | 37.72 ± 2.63 | 20.99 ± 9.63 |
| Painful threshold limit | 40.09 ± 3.09 | 29.65 ± 11.34 |
| Painful tolerance | 46.23 ± 2.71 | 16.01 ± 3.05 |
| Heat-pain assessment during 2-Back Task | ||
| Warmth detection | 36.86 ± 2.89 | 17.81 ± 8.77 |
| Painful threshold | 40.11 ± 3.18 | 29.74 ± 11.65 |
| Painful tolerance limit | 46.54 ± 2.61 | 16.36 ± 2.93 |
Study procedures.
This study was designed to determine the influence of immersive VR contexts on heat-pain tolerance limits and related pain-induced autonomic and emotion responses. Changes in mood, situational anxiety, pain unpleasantness, and level of enjoyment were also assessed as secondary outcomes. The experiments were conducted in an insulated room with no external windows at the University of Maryland School of Nursing. Participants sat down comfortably in a zero gravity recliner chair.
The five experimental conditions were immersive and interactive VR Ocean, VR Opera, control (non-immersive) Ocean, control Opera, and attention/distraction task, respectively (see details below) (Fig. 1a). We modulated the context of the VR conditions by using two distinct VR contexts. Specifically, we used a commercially-available and ad-hoc immersive and interactive (i.e. head’s movements moved the contexts) VR context in which participants were immersed in an underwater scene featuring a myriad of jellyfish and rays of sunlight (VR Ocean) and were immersed onstage in a performance of La Clemenza di Tito, K. 621 opera composed by Wolfgang Amadeus Mozart (VR Opera) (Fig. 1b). We therefore measured the level of enjoyment of the two VR contexts (VR Ocean and VR Opera conditions).
Figure 1. Experimental paradigm and conditions.
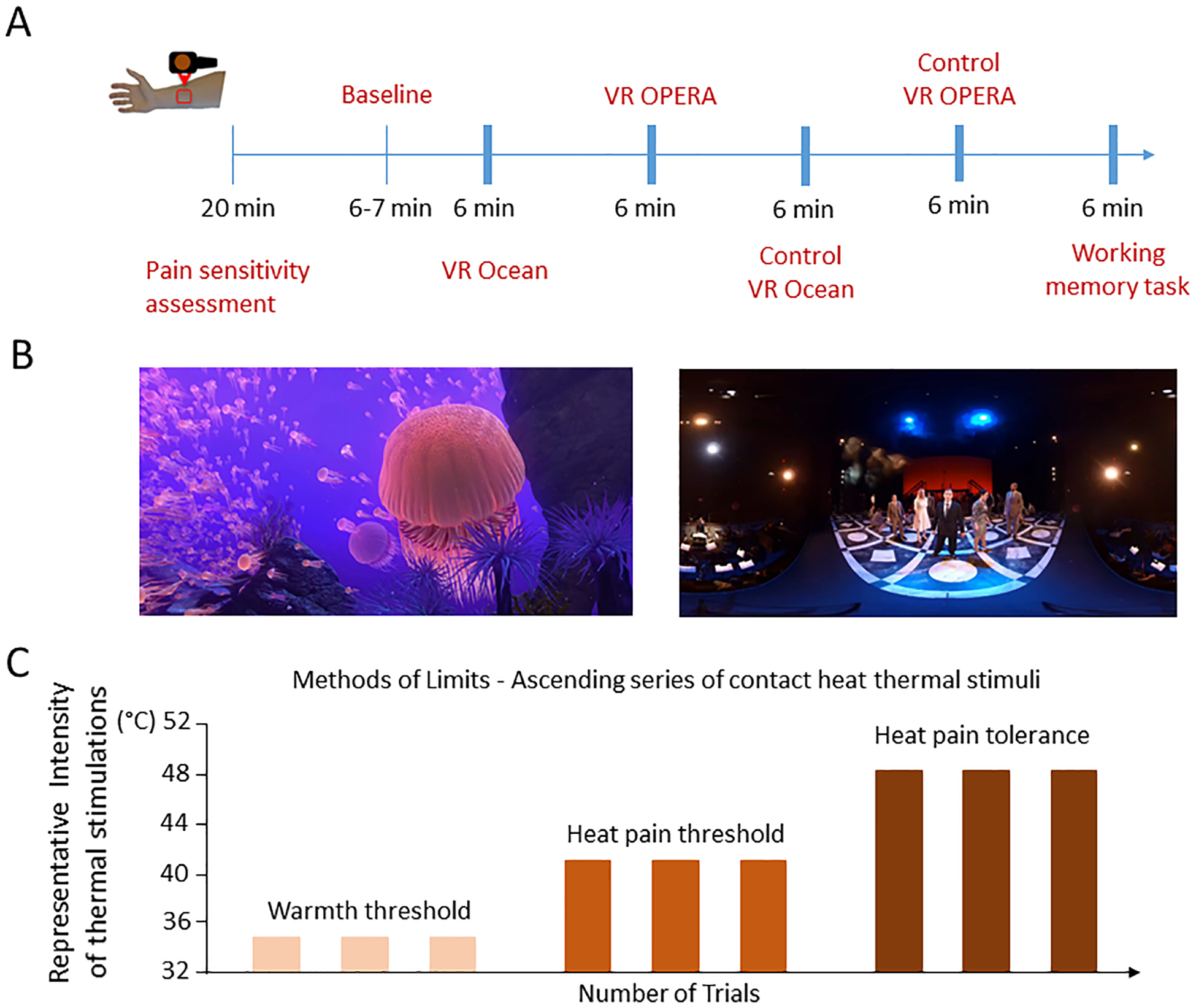
(a). Participants went through five conditions including VR Ocean, VR Opera, Control VR Ocean, Control VR Opera, and 2-Back Working Memory Task. First, participants underwent the pain sensitivity assessment followed by a Baseline familiarization phase before starting the VR/control interventions. The 6-min immersive VR and the control conditions were therefore delivered to assess changes in heat warmth, pain threshold and heat-pain tolerance limits. The condition to which the participants were first exposed was counterbalanced, and the order of conditions following was randomized after generating five sequences to control for time effects. Participants were able to stop the heat stimulation using a controller. Participants stopped the self-delivered heat stimulations and levels of degrees Celsius intensities and duration of the stimulations were recorded. At the end of the VR and control interventions, participants rated the overall perceived pain intensity, unpleasantness, mood, situational anxiety and level of enjoyment. Autonomic measurements were collected continuously. (b) Representative screen shot of VR Ocean condition (left) and screen shot of VR Opera condition (right). (c) We first assessed pain sensitivity. Afterwards a baseline familiarization phase was conducted followed by the assessment of warm detection, heat-pain threshold and heat-pain tolerance limit, respectively. Three series of stimuli were delivered for each modality under each VR and control conditions.
We included two ‘control’ conditions with non-immersive, non-interactive, 2D versions of the VR contexts to remove the VR-based ‘sense of presence’. Moreover, we added the distraction task to control for attentional demand. The condition to which the participants was first exposed was counterbalanced, and the order of conditions following was randomized after generating five sequences to control for time effects. During each VR/control condition, participants underwent heat stimulation and they were asked to stop the delivery of heat stimulation when they reached their perceptible warm, perceptible painful and maximum painful tolerable levels (see below). degrees Celsius and duration parameters were recorded during the VR/control interventions along with the autonomic measurements. At the end of each condition, participants rated their level of pain unpleasantness, mood, situational anxiety, and enjoyment using a specific Visual Analogue Scale (VAS), as reported below.
VR AND CONTROL CONDITION FEATURES.
IMMERSIVE VR OCEAN CONDITION.
The VR Ocean condition was implemented using the Blue Season 1 (Wevr, Venice, California, USA), an immersive VR series allowing participants to experience the wonders of the ocean through different habitats. Season 1 contains three episodes, out of which the Reef Migration episode (6 minutes 30 seconds) was used to create the mindful experience of being immersed in the depths of the ocean, surrounded by jellyfish, turtles, and other aquatic wildlife, while relaxing music is played in the background.
IMMERSIVE VIRTUAL REALITY OPERA.
This environment, captured, edited and rendered by research programmers at the Maryland Blended Reality Center, features a performance of the University of Maryland School of Music’s Maryland Opera Studio consisting of segments of a performance of La Clemenza di Tito, K. 621. Viewers wearing a head-mounted display can experience six minutes of 360-degree video and audio immersion, in which they are virtually placed directly onstage with the opera performers--an unusual and engaging way to view such a piece. This environment was produced as part of the MBRC’s Non-Opioid Pain Management Project, funded by Maryland’s MPowering the State initiative.
CONTROL (NON-IMMERSIVE) OCEAN.
Operationally, we created and defined the control non-immersive ocean as the condition with the auditory and visual features of the VR Ocean condition (i.e. the relaxing music and ocean-related calming, scenic images). This context was isolated from the Blue Season 1 (Wevr, Venice, California, USA), Reef Migration episode and delivered using a tablet (iPad Pro, 10.5 inch) and headphones (Audio-Technica ATH-M20x Professional Studio Monitor Headphones Deluxe Bundle). Thus, while the context was the same, it was non-immersive in that it lacked the ‘sense of presence’ of the VR Ocean condition and the experience of immersive features (e.g., scene changes with head movements). This environment was produced by the Maryland Blended Reality Center (MBRC).
CONTROL (NON-IMMERSIVE) OPERA.
Operationally, we created a non-immersive opera as the control condition that consisted of the auditory and visual features of the VR Opera condition (i.e. the opera music and theatre scene). Auditory and visual features were isolated from the performance at the University of Maryland School of Music’s Maryland Opera Studio of La Clemenza di Tito These isolated components were delivered using a tablet (iPad Pro, 10.5 inch) and headphones (Audio-Technica ATH-M20x Professional Studio Monitor Headphones Deluxe Bundle). Thus, while the context was the same, it was non-immersive in that it lacked the ‘sense of presence’ of the VR Opera condition and the experience of immersive features (e.g., scene changes with head movements). This environment was produced as part of MBRC.
A single workstation CPU with an NVIDIA K6000 GPU (Alienware 17 R4 - Alienware Miami, Florida, USA) drove an HTC Vive Pro headset (HTC Xindian, New Taipei, Taiwan). In this configuration, a single user could wear and experience the headset while the investigator watched the experience on a large LED display panel. The headset we used was the HTC Vive Pro (Vive, United Kingdom) (https://www.vive.com/uk/comparison/).
2-BACK WORKING MEMORY TASK.
The 2-back paradigm working memory task was chosen according to previous studies on distraction and pain [3]. A series of 90 capitalized letters were presented in the center of the screen one at a time in a pseudorandom sequence. Participants were asked to determine whether the letter was the same or different from the letter that was presented two positions prior. Each letter was presented as black Arial font with a white background for 500 ms, followed by a 1500ms blank screen. 30 out of 90 trials were target and 60 trials were non-target. Participants were asked to press thumb button for target and index button for non-target using the Celeritas® Fiber Optic Response System.
HEAT STIMULATIONS AND PAIN CALIBRATION.
Warm and painful thermal heat stimuli were delivered using the Medoc Pathway ATS system, with a 27-mm diameter ATS thermode (PATHWAY System, Medoc, Ramat Yishai, Israel). The thermode was placed on the participant’s non-dominant volar forearm. The Medoc PATHWAY maximum temperature was set at 52 degrees Celsius for safety to prevent any tissue damage. The duration of each pain stimulus depended on step within the paradigm (more than 1s and less than 22s). For the heat thermal stimulation, the rate of heat increase was set at 0.3°C/seconds and the decreasing rate at 1°C/seconds. To determine the maximum painful tolerance limit, the rate of increase was set at 1°C/seconds and the decreasing rate was set at 8°C/seconds.
We assessed the individual pain sensitivity. We used the methods of limits with ascending series of contact heat thermal stimuli (Fig. 1c) [11; 38], to identify heat-warmth detection, heat-pain threshold, and heat-pain tolerance limit, respectively in each participant. We operationally defined ‘warmth detection’ as the minimum temperature expressed in degrees Celsius that each participant was able to feel; ‘painful threshold’ as the temperature expressed in degrees Celsius that each participant was able to perceived as minimally painful; and ‘painful tolerance limit’ as the maximum temperature expressed in degrees Celsius that each participant could no longer endure the heat-pain stimuli [4; 55].
PAIN MEASUREMENTS AT BASELINE AND DURING VR (CONTROL) CONDITIONS.
Prior to actual VR procedure, participants had a baseline training session in which participants familiarize with the procedure they were going to do during the VR conditions. Each participant received three stimulations for the warmth, painful thresholds, and painful tolerance limit modalities. Our scope was to determine how VR would have induced increases in the individual warm, pain threshold and pain tolerance limit, respectively. Participants were asked to stop the delivery of heat stimulations when they reached their perceptible warm level, perceptible painful level and maximum tolerable painful limit, respectively (Fig. 1c). For each modality (i.e. warmth, pain threshold and pain tolerance limit), we measured intensities (expressed in degrees Celsius) and durations (expressed in seconds) as reported in Table 1.
SUBJECTIVE RATINGS.
Participants rated their pain intensity, pain unpleasantness, level of situational anxiety, mood, and level of enjoyment using a VAS ranging from 0 to 100. The following instructions were given: 1. Pain Intensity: “Please rate your overall pain intensity”; 2. Pain Unpleasantness: “Please rate your overall pain unpleasantness”; 3. A situational anxiety: “Please rate your situational anxiety during the session”; 4. Mood: “Please rate your mood level during the session”; and 5. Enjoyment: “Please rate how much you liked the session”. The anchors were set, respectively, with 0 being “no pain at all”, “not unpleasant at all”, “not anxious at all”, “extremely bad mood”, and “not enjoyable at all” and 100 being “maximum tolerable pain”, “very unpleasant”, “very anxious”, “extremely good mood”, and “very enjoyable”. VAS ratings were acquired using Eprime v2 (Psychology Software Tools, Sharpsburg, PA, USA) and participants operated a Celeritas Fiber Optic Response System (Sharpsburg, PA, USA), using their index and middle fingers to move a slider on the VAS scale. Eprime v2 (Psychology Software Tools Inc, Sharpsburg, USA) and Matlab were used to extract the data.
Participants were told that immersion in the VR may cause a sense of being in a closed environment and rarely, nausea and that if they had experienced any discomfort, the VR would have been removed quickly. No side effects were reported for any of the VR conditions during the experimental sessions and none of the study participants withdrew from the VR experience. When asked at the end of the experiment, participants referred to the VR as an experience that they would like to re-try.
AUTONOMIC MEASUREMENTS DATA ACQUISITION
Electrocardiogram (ECG) and GSR electrodes were placed on the participants to acquire their autonomic measurements. A three-lead ECG was used to record heart rate, with the positive and ground electrodes placed on the right and left shoulders, respectively, and the negative electrode placed on the left hip to form an Einthoven’s triangle. For the GSR, two electrodes were placed at the bottom of the left palm. Both the ECG and the GSR were collected using the BrainAmp ExG amplifier and BrainVision Recorder (Brain Products GmbH, Munich, Germany).
Given that our primary outcome was VR changes for pain tolerance limits, we analyzed the SDNN and GSR when participants reached their maximum pain tolerance limit and stopped the delivery of painful heat stimulation. Therefore, the ECG and GSR analyses were tailored to each participant.
SDNN data analysis
ECG was recorded and preprocessed using a Brain Vision Recorder/Analyzer (Brain Products GmbH, Munich, Germany). Continuous ECG data were high-pass filtered with the cut-off set as 0.04, since low frequency ECG is considered to be from 0.04 to 0.15 Hz. The raw ECG was visually inspected to remove abnormal heartbeats. R peaks were detected by Pan Tompkins algorithm [41] using the Matlab function “qrsdetecter-master” (https://github.com/danielwedekind/qrsdetector). RR-interval data were exported using the function “qrsdetecter-master” and imported to Kubios software to calculate the Heart Rate Variability (HRV) index for main analyses of SDNN [48; 53]. Decreasing HRV indices are associated with the activity of sympathetic system, while increasing of the HRV indices are associated with the activity of parasympathetic nervous system [53].
GSR data analysis
GSR data were analyzed using the LedaLab V3.4.6c software package for Matlab [2]. In preprocessing, the data were first down-sampled to 250 Hz [33] and smoothed with a 1000-samples moving average function [33]. Then the data were low-pass filtered with a cut-off frequency of 2 Hz [2]. Next, the preprocessed data were put through continuous deconvolution analysis (CDA) in which the data were decomposed into the tonic and the phasic components. The tonic component reflects baseline activity, while the phasic component reflects a direct response to a stimulus. For each trial, GSR from 1 second after heat onset to 4 second after heat offset to account for delayed autonomic responses. We calculated areas under the curve (AUC) for the GSR data. The average amplitude and AUC of GSR were calculated separately for each of the conditions and each of the three warmth and pain assessment (warmth detection vs. pain threshold vs. maximum pain tolerance limits). SDNN and GSR were calculated separately for each condition. Five participants were excluded from SDNN analysis and four participants were excluded from GSR analysis due to unanalyzable ECG and GSR data.
For ECG data, epochs were extracted continuously for three trials from the onset of the first painful stimulation to the end of the maximum duration of the increases in heat-pain tolerance limits of the last trial expressed in seconds (mean=62.21s, SD=6.71 s). For GSR data, epochs were extracted for each trial from 1 second after the onset of the painful stimulation to the 4 seconds after the maximum time duration to account for any response delays. Specifically, we averaged the three painful trials for each of five conditions and selected the maximum stimuli duration to segregate the epochs. Thus, the epoch lengths were the same across conditions but were tailored to each participant response to individual increases in heat-pain tolerance limits.
PSYCHOLOGICAL QUESTIONNAIRE.
We collected the Gaming Addiction Survey [14] in consideration that participants who used to play video games may have a greater appreciation of the VR tasks. Therefore, having this information may help to better interpret potential inter-individual differences towards VR contexts.
ANALYSES
Outcomes.
Primary outcomes were objective increases in heat-pain tolerance limits and SDNN/GSR associated changes. Secondary outcomes were subjective self-report VAS ratings for mood, anxiety, pain unpleasantness, and enjoyment levels.
Repeated measure ANCOVAs were performed with the five conditions (1. VR Ocean, 2. VR Opera, 3. Control Ocean, 4. Control Opera, and 5. 2-Back Working Memory task) as within-subjects factor and both increases in heat-pain tolerance limits (primary outcome) and associated secondary outcomes (mood, anxiety, pain unpleasantness, and enjoyment ratings) as dependent variables. Post-hoc analyses were applied using Bonferroni corrections for multiple comparisons. Greenhouse-Geisser corrections were applied when the sphericity assumption was violated. G*Power [8] and SPSS version 24 (SPSS Inc, Chicago, IL, USA) software were used to calculated the optimal sample size and to conducted the data-analysis. The significance level was set at p=0.05 for all the analyses.
Multilevel regression model.
The primary outcome in the multi-level model was the change in heat-pain tolerance limits. We aimed to assess how autonomic response SDNN, AUC, pain unpleasantness, mood, and situational anxiety influenced changes in pain tolerance limits. To avoid multi-collinearity in the regression model, enjoyment level was excluded due to its high correlation with mood (r = 0.774, p<0.001). The within-subjects experimental design resulted in a hierarchical nested data structure, with 44 observations for each variable (level 1: SDNN, AUC, pain unpleasantness, mood, and situational anxiety) nested within each condition (level 2: VR Ocean, VR opera, Control Ocean, Control Opera, and 2-Back Working Memory Task). Data from five study participants were not analyzable for SDNN due to incapability to collect autonomic measurements. Given the data structure, two separate multilevel regression models [1; 37] were conducted to examine 1. the effects of level 1 variables on changes in heat-pain tolerance limits and 2. the level 1 − level 2 cross-level interactions on changes in heat-pain tolerance limits. Specifically, when modeling the level 1 predictors’ influence on heat-pain tolerance limit changes (Model 1), we estimated both random slopes and random intercepts in the level 1 and level 2 variables, which allowed the level 1 predictor effects to vary between 5 conditions. The cross-level interaction (Model 2) was conducted to examine how the five conditions (level 2) moderated the relationship between SDNN, situational anxiety, mood, unpleasantness (level 1), and delta in changes for heat-pain tolerance limits (dependent variable). Following a previous study [9], the level 1 (AUC, pain unpleasantness, mood, and situational anxiety) − level 2 (5 conditions) cross-level interaction was only modeled with a random intercept component to retain the power for interaction analyses. In both multi-level models, the predictors were person-centered [6], and the sequence was treated as a covariate. The multi-level regression was performed using the “lmer” function in R studio i386.3.5.2 (R Studio, Inc., Boston, MA, USA).
Based on our previously published systematic review [25], we performed the power calculation on the primary outcome. We anticipated a moderate effect size (d=0.50) on the increased capability to tolerate pain resulting in a power calculation an N=44 that would have been required to achieve 0.99 power (1-β err prob). Five additional participants were enrolled in case of potential incomplete study session, missing data and dropouts leaving it a sample size of N=49.
Outliers for each outcome were identified by using the following Tukey formula: Upper=Q3+(2.2*(Q3−Q1); Lower=Q1−(2.2*(Q3−Q1)
Q1 and Q3 equal 25% and 75% percentiles respectively to define upper and lower boundaries. Results of regression and ANCOVA analyses did not change with the outlier removal and the full dataset was used for the analyses.
Results
VR effects at the level of individually-chosen heat temperatures
We found a significant main effect of the five conditions (1. immersive VR Ocean, 2. immersive VR Opera, 3. control (non-immersive) Ocean, 4. control (non-immersive) Opera, 5. 2-Back Memory Task) on heat-pain tolerance limit increases (F4,176=7.47, Greenhouse-Geisser corrected p<0.001; Fig. 2A). Bonferroni-corrected post-hoc comparisons indicated that immersion in the VR Ocean condition led to significantly greater increase in heat-pain tolerance limits (mean increase: 1.025±0.517 ℃, baseline temperature: 46.19±2.93 ℃; during VR Ocean: 47.09±2.05 ℃; scale from 32 to 52 ℃) than the VR Opera condition (p=0.001), control Ocean (p=0.001) and control Opera (p<0.001; Table 1). We also measured the duration expressed in seconds that the highest level of painful stimulations was tolerated. We found a main effect of conditions (F4,176=7.47, Greenhouse-Geisser corrected p<0.001; Fig. 2B). Bonferroni-corrected post-hoc comparisons indicated that immersion in the VR Ocean condition led to significantly greater increase in the duration (10.04±3.27%) of heat-pain tolerance limits than the VR Opera condition (4.47±2.67%; p=0.001), control Ocean (3±2.56%; p=0.001) and control Opera (1.53±1.95%;p<0.001). Not only did participants stop the heat stimulation at higher intensities but also tolerated the heat for longer time (expressed in seconds) as compared to the control interventions (see Table 1).
Figure 2. Heat-pain tolerance limit changes and heart rate variability SDNN among the five conditions.
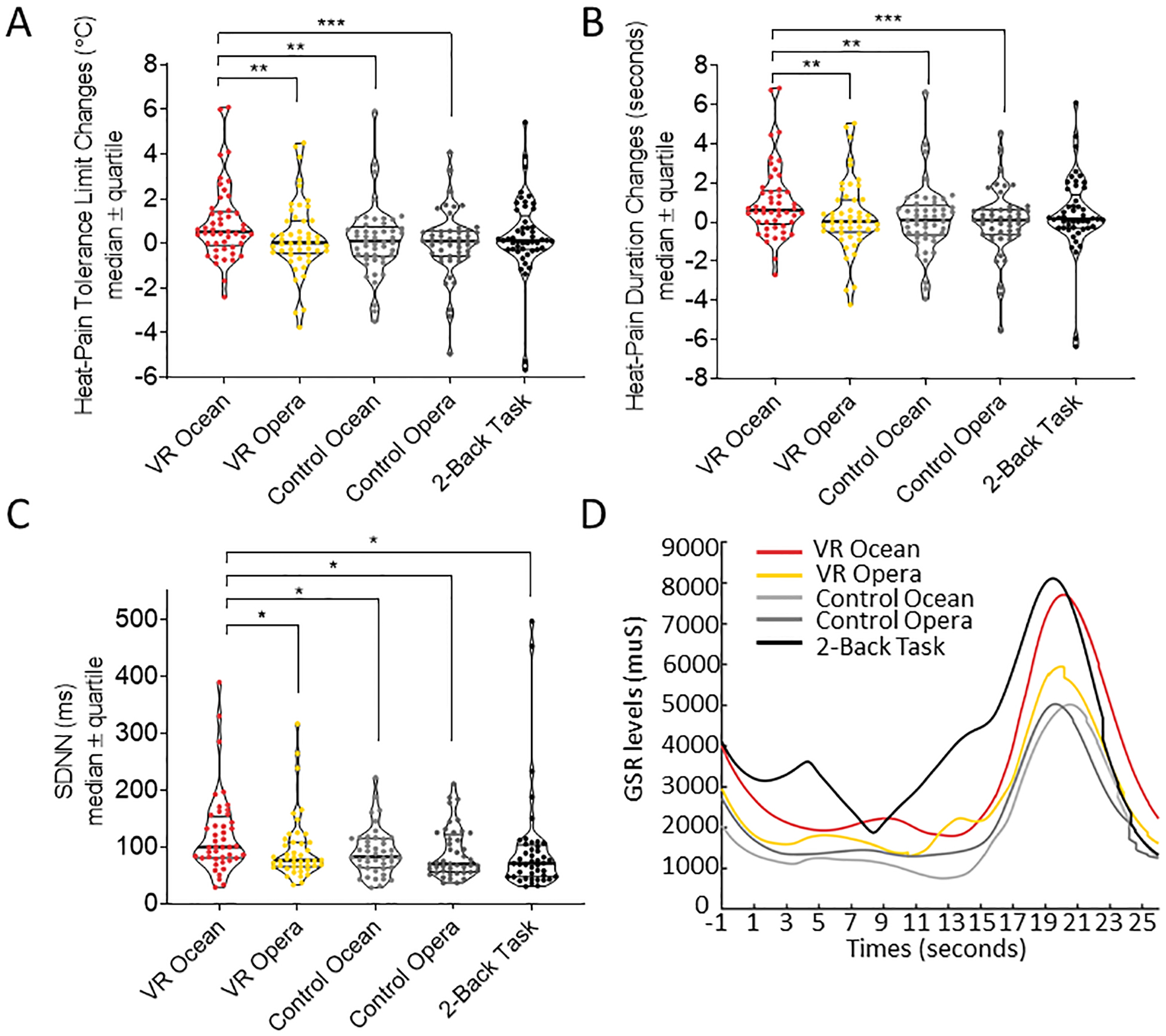
(a) The VR Ocean condition yielded higher limits for pain tolerance compared to VR Opera and control VR conditions, but it did not show significant differences from the 2-Back Working Memory Task condition. Heat-pain tolerance limit increases are expressed in degrees Celsius and as median ± quartile (b) The SDNN during VR Ocean condition was significantly higher than the other four conditions suggesting a greater level of parasympathetic system action. (c) Positive correlation between SDNN and temperature changes during VR Ocean condition. (d) GSR changes in the five experimental conditions. The area under curve (AUC) was calculated to represent the level of GSR response. The AUC for the VR Ocean condition was significantly larger than the AUC in Control Ocean condition (p=0.014).
These results indicated that VR Ocean condition significantly enhanced heat-pain tolerance limits compared to VR Opera (see below), which required a certain level of preference to be effective, and the two non-immersive control conditions. The gain in pain intensities that were tolerated during the VR interventions were not influenced by sex (all ps>0.252), race (p=0.689), and individual pain sensitivity levels.
Importantly, the immersive VR effect was maximized for heat-pain tolerance limits (maximum tolerated intensity of heat) and heat-pain threshold (minimum level of perceived pain to heat) but not warmth (minimum level of perceived warmth to heat stimulations). We found a significant main effect of the five conditions on pain threshold (F4,176=3.22, Greenhouse-Geisser corrected p=0.022). Pairwise comparisons applying Bonferroni correction indicated that being immersed in VR Ocean condition had greater increases in pain threshold compared to control VR Ocean (p=0.015) but did not differ from the other three conditions (VR Ocean vs. VR Opera: p=0.078; VR Ocean vs. control Opera: p=0.115; VR Ocean vs. 2-Back working memory: p=1.000). Controlling for sequence, the VR intervention did not change warmth threshold (F4,176=1.80, p=0.132).
VR-induced SDNN changes
At the level of the physiological autonomic measurements, we tested the hypothesis that VR-induced increases in heat-pain tolerance limits would be associated with an increase of SDNN that was calculated for each condition. Five participants were excluded from the SDNN analyses due to unanalyzable autonomic measurement data. Repeated measures ANCOVA revealed a significant main effect of the five conditions on SDNN (F4,156=4.34, Greenhouse-Geisser corrected p=0.013). Bonferroni-corrected post-hoc comparisons indicated that the immersive VR Ocean condition yielded significantly higher SDNN compared to immersive VR Opera (p=0.017), non-immersive control Ocean (p=0.022), non-immersive control Opera (p=0.023) and 2-Back Memory Task (p=0.013). These results provided evidence that the immersive VR Ocean intervention induced a larger activation of the parasympathetic nervous system compared to the other four conditions (Fig. 2C). Importantly, the immersive VR Ocean condition was characterized by a higher level of SDNN, which was associated with greater gain in the painful intensities that were tolerated (r=0.529, p<0.001).
Data from 2 participants were identified as outliers. After removing the two outliers the results did not change. The main effect of the five conditions on SDNN remained significant (F4,148=8.70, p<0.001, Greenhouse-Geisser corrected). Post-hoc analysis applying Bonferroni correction indicated that the SDNN for VR Ocean condition (mean=118.14, sem=7.85) were significantly greater than VR Opera (mean=88.74, sem=7.84, p=0.014), Control Ocean (mean=89.68, sem=7.29, p=0.009), Control Opera (mean=88.09, sem=7.66, p=0.023), and working memory task (mean=77.18, sem=6.86, p<0.001).
We further compared the SDNN responses to high painful versus warm heat thermal stimuli. We found that during the delivery of warm heat stimuli, SDNN values (mean=131.562, sem=16.192) were greater than SDNN ones observed during the delivery of high painful heat stimuli (mean=98.701, sem=4.34, F1,142.20=3.59, one-tailed correction p=0.030). This finding suggests higher vagal activation during the delivery of warm than high painful stimuli.
VR-induced GSR changes
In terms of GSR responses, we observed an increase in the GSR related-AUC (main effect of conditions: F4,140=6.95, Greenhouse-Geisser corrected p=0.001) with the Immersive VR Ocean intervention having significantly larger AUC compared to the control (non-immersive) VR Ocean conditions (p=0.014, Fig. 2d).
These SDNN/GSR results may reflect the differential body’s response to the heat pain. In fact, higher intensities of heat pain (expressed in degrees Celsius and seconds for intensity and duration, respectively) were paralleled by the increases in SDNN/GRS responses recorded while participants were able to tolerate during the immersive VR conditions (VR Ocean and VR Opera).
Behavioral results: self-reported pain unpleasantness, mood, anxiety and enjoyment ratings
At the level of affective and cognitive measurements, we found distinct actions of the immersive VR interventions based on the context.
Pain unpleasantness.
We observed significant main effects of the five conditions for VAS pain unpleasantness ratings (F4,176=10.34, p<0.001). Specifically, participants reported significantly lower pain unpleasant ratings for the immersive VR Ocean than immersive VR Opera (p=0.004), control (non-immersive) Ocean (p=0.011), control Opera (p<0.001), and 2-Back Memory Task (p<0.001) (Fig. 3a), therefore VR Ocean reduced pain unpleasantness compared to the other four conditions.
Figure 3. The differences in pain unpleasantness, mood, level of enjoyment, and situational anxiety among the five conditions.
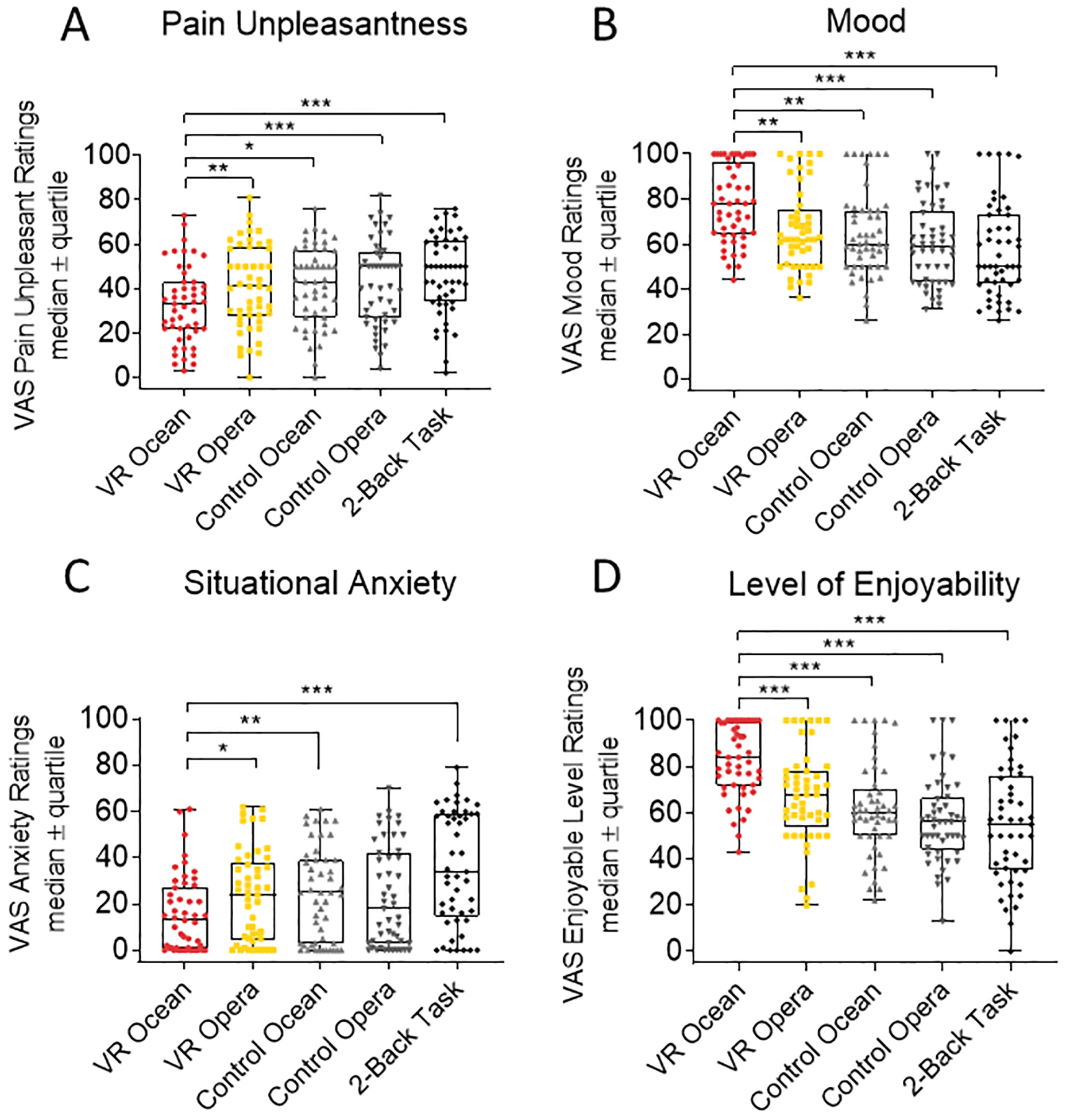
(a) Pain unpleasantness ratings for VR Ocean were significantly lower than in the other four conditions. (b) Mood ratings for VR Ocean was significantly higher than the remaining four conditions. (c) Situational anxiety level for the VR Ocean condition was significantly lower than VR Opera, Control Ocean and 2-Back Working Memory Task conditions. (d) Level of enjoyment for the VR Ocean condition was significantly higher than the other four conditions. Participants rated each outcome at the end of the experimental session. Data are expressed as median ± quartiles.
Mood ratings.
We observed a significant main effect of conditions on mood ratings (F4,176=13.41, Greenhouse-Geisser corrected p<0.001). In line with our hypothesis, the immersive VR Ocean condition enhanced mood as compared to the immersive VR Opera (p=0.004), control Ocean (p=0.003), control Opera (p<0.001) and 2-Back Memory Task (p<0.001) (Fig. 3B).
Situational anxiety.
A significant main effect of conditions as also found for situational anxiety ratings F4,176=17.14, Greenhouse-Geisser corrected p<0.001). The immersive VR Ocean condition reduced situational anxiety (Fig. 3C) in comparison with the VR Opera (p=0.027), control Ocean (p=0.003), 2-Back Memory Task (p<0.001) and marginally less situational anxiety than control Opera (p=0.087).
Level of enjoyment.
The level of enjoyment experienced by the participants varied across conditions (F4,176=25.30, Greenhouse-Geisser corrected p<0.001). In fact, participants reported that they enjoyed the immersive VR Ocean condition Fig. 3D) more than Immersive VR Opera (p<0.001), control VR Ocean (p<0.001), control Opera (p<0.001) and 2-Back Memory Task (p<0.001).
To assess the role of individual preferences on the effect of VR Opera, we split participants into those who liked opera and those who disliked it. VR Opera likers versus dislikers did not show differential increases in heat-pain tolerance limits (p>0.673). However, in those who liked opera a negative correlation between VAS pain unpleasantness and VAS enjoyment ratings (Pearson correlation r=−0.43, p=0.034, Fig. 4A), VAS pain unpleasantness and VAS situational anxiety ratings (Pearson correlation r=−0.52, p=0.009, Fig. 4B) was observed indicating that the level of enjoyment was negatively correlated with pain unpleasantness and anxiety. Moreover, there was a significant positive correlation between VAS mood and VAS enjoyment ratings (Pearson correlation r=0.898, p<0.001, Fig. 4C): the more participants liked the VR Opera the higher the mood improvement. These effects were not present in those who did not like the Opera context (enjoyment and unpleasantness ratings: Pearson correlation r=−0.273, p=0.187; enjoyment and anxiety ratings: r=−0.318, p=0.122; enjoyment and mood ratings: r=−0.384, p=0.058, trend).
Figure 4. Median splitting of study participants into opera likers and dislikers.
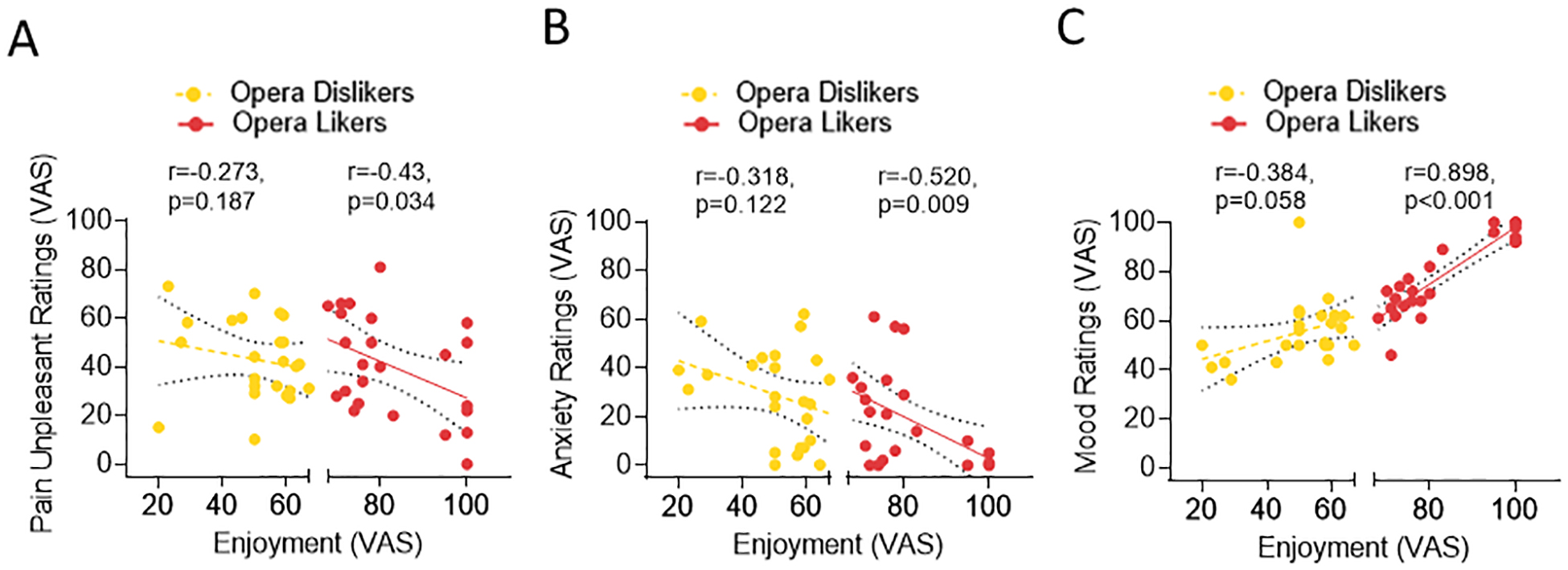
(a) Distribution of likers (in red) and dislikers (in yellow) based in the median of the enjoyment ratings (Fig. 4a). (b) Those who liked opera did have a negative correlation between VAS pain unpleasantness and VAS enjoyment ratings (Pearson correlation r=−0.43, p=0.034, Fig. 4b) and VAS pain unpleasantness and VAS situational anxiety ratings (Pearson correlation r=−0.52, p=0.009, Fig. 4c). There was a significant positive correlation between VAS mood and VAS enjoyment ratings (Pearson correlation r=0.898, p<0.001, Fig. 4d). These effects were not present in those participants who did not enjoy opera (enjoyment and unpleasantness ratings: Pearson correlation r=−0.273, p=0.187; enjoyment and anxiety ratings: r=−0.318, p=0.122; enjoyment and mood ratings: r=−0.384, p=0.058).
Finally, we explored the relationships among the increases in temperature that was tolerated, VR-induced body SDNN/AUC autonomic changes and self-reported changes in pain unpleasantness, mood, anxiety. The cross-level interaction regression model results indicated that there was a significant interaction between SDNN and VR interventions in predicting heat-pain tolerance limit delta (Table S1). Namely, for the VR Ocean condition, the SDNN of heart rate variability which reflected likely the participant’s relaxation level, predicted the increase in individual heat-pain tolerance limits (see statistical values in Table S1).
Participants who like video games may have a higher interest in VR interventions. The Gaming Addiction Survey total score was not associated with the VR-induced pain-heat tolerance limit increases (all p>0.157). One participant scored high (i.e., real life of the participant, such as school or work performance, daily living and social relationships, were disrupted by the video games); removing this participant from the planned analyses, did not change the findings with a significant main effect of the 5 conditions on pain-heat tolerance limit gains (F4,172=5.33, Greenhouse-Geisser corrected p<0.001), therefore we kept this participant in the overall analyses.
Discussion
In sum, we determined that immersive VR increases heat-pain tolerance limits, while improving mood and decreasing situational anxiety. We also found that contexts and preferences are important in the VR effects. As compared to the Opera, attention and non-immersive conditions, VR Ocean that was perceived as the most enjoyable condition, induced not only an increase in the level of pain that participants can tolerate (higher intensity heat stimulations expressed in Celsius degrees and longer duration expressed in seconds) via a reset of parasympathetic and sympathetic activity, but also led to an improvement of mood, situational anxiety and pain unpleasantness.
Despite showing a higher sympathetic activity along with the gain in heat-pain tolerance limits, the VR Ocean condition was paralleled by an increase of SDNN that might reflect a relaxation. There was a significant lower pain unpleasantness and anxiety and higher mood ratings when participants were immersed in the VR Ocean condition. Finally, the level of enjoyment of the VR context (i.e. opera likers) was associated with mood improvements and reduction of situational anxiety and pain unpleasantness ratings suggesting that the extent, to which the immersive VR context is appreciated, influences affective and evaluative processes associated with VR interventions.
An important component of VR is the subjective experience of being virtually present, even when one is physically elsewhere. This notion of ‘presence’ has long been considered central to evaluating both the effectiveness and quality of virtual environments. Slater (2009) developed the idea of place illusion, which refers to the aspects of presence constrained by sensorimotor contingencies of the specific virtual reality system [49]. Sensorimotor contingencies are actions used in the process of perceiving the virtual world, such as moving the head and eyes to change gaze direction or seeing around occluding objects to gain an understanding of the space [39]. Slater (2009) concluded that establishing presence, or ‘being there,’ is not feasible for lower-order immersive systems such as desktops [49]. In contrast, the sensorimotor contingencies of walking and looking around facilitated by head-mounted displays contribute to their higher-order immersion establishing ‘presence’. This study is novel in that, for the first time in VR research, appropriate control groups for VR context, VR immersion, and distraction were included in a within-subjects study. In fact, many VR studies lack either the appropriate controls (i.e. non-immersive VR) and/or analyses of the VR contexts (see for a systematic review [25]). Therefore, we included two ‘control’ conditions with non-immersive, 2D versions of the VR Ocean and VR Opera (control Ocean and control Opera, respectively). These 2D control conditions accounted for the effect of immersion and interaction on the outcomes by removing the ‘sense of presence’ and they delivered the same sounds/music and contexts as in the fully immersive versions via headphones and the same visual context via a tablet. Moreover, we added the distraction task to control for attentional demand [39] and found that being immersed in a relaxant context of VR induced changes that are not explainable with a mere attention/distraction demand. We modulated the context of the VR conditions by using two distinct music-based contexts (i.e., relaxing music in VR Ocean versus VR Opera music). This allowed us to understand that the amount to which people relax is of paramount relevance in driving heat-pain tolerance limit increases. However, enjoying the VR context (i.e. opera likers) is still relevant for regulation of behaviors such as pain unpleasantness, mood and situational anxiety.
At the level of autonomic responses related to the VR (and control) interventions, we found that GSR was significantly higher in VR Ocean condition compared to control ocean reflecting the body response when higher levels of pain were tolerated. Importantly, we also found an increase of the parasympathetic activity as expressed by the SDNN, concurrently with the GSR greater changes suggestive of a status of relaxation (during the painful heat stimulation).
This is in line the previous results, where a significantly greater GSR during pain than non-pain conditions [5; 7; 47] was observed. Heart rate responses between noxious heat and non-painful warm stimuli [28; 36], non-painful cool stimuli, and noxious cold stimuli vary [27]. Despite high painful stimulations cause an activation of sympathetic activity, the VR Ocean intervention might have favored a state of relaxation with an activation of the parasympathetic system (SDNN changes). By leveraging these findings, we speculate that the VR interventions that are experienced as relaxant might result in a re-balance of the autonomic nervous system whereby a GSR activation related to the pain being tolerated, might be counterbalanced by the parasympathetic activation that reflects a status of relaxation despite the self-delivered high painful heat stimulation. This information might hint to a potential mechanism for VR induced increase of the capability to tolerate pain. Upon replication of these findings in chronic pain patients, such a mechanism could be potentially used as a foundation for future therapeutic applications. These findings might outline that a VR context that induces relaxation (i.e. Ocean VR) via modulation of the autonomic responses can achieve a gain in acute clinical pain tolerability.
Our findings also suggest that VR interventions affect multiple sensorial pathways which are connected to mood, situational anxiety and a sense of pleasure. Therefore, VR works as a multifaceted intervention, which could play a role in the experience of presence, and into the therapeutic benefits of that experience.
Despite the novelty, creativity and strengths of this study, there are limitations. First, to fully understand the top-down brain mechanistic bases of VR, we would need to include brain imaging. Yet, our approach represents a comprehensive, multifaceted evaluation of the VR mechanisms of action at the level of gains in the capability to tolerate pain, its related-autonomic responses as well as mood, situational anxiety, pain unpleasantness, and condition-related enjoyment. Our study might lead to a new framework and appreciation of VR mechanisms with respect to behavioral and physiological mechanisms. Second, we used two distinct VR contexts: the immersive VR Opera and VR Ocean contexts. To understand how preferences and eventually preference-related mechanisms influence VR effects, there is a need for expanded libraries of VR contexts that can be tailored to individuals’ preferences. Third, we conducted this study in healthy participants, which might limit the ability to translate these findings to clinical acute pain and other symptoms (i.e. mood, situational anxiety, and discomfort). For example, it has been showed that VR in hospitalized patients reduced acute post-operative pain up to 3 days [52]. Forth, we explored the VR effects in one single session. The long-term effects and the cumulative session-by-session effects (i.e. additive) of immersive VR should be further explored to understand the therapeutic actions of VR in mollifying chronic symptoms. Finally, while we controlled for baseline GSR recordings [30], we did not include a baseline recording for SDNN preventing us from drawing definitive conclusions about the VR effects on the vagal responses. Additionally, there was a lack of a sham condition (i.e. wearing a non-immersive, non-interactive VR headset with the same contexts) to account for placebo effects. We controlled for the sense of presence (i.e. immersive VR) with identical contexts delivered as 2D video with audio versus the same contexts experienced in the immersive VR. A condition with VR headset without immersive visual and auditory parts can potentially be added in future research to control for ritual effects. Importantly, these results might indicate that immersive VR provides a unique resource that allows individuals to transcend acute pain and related symptoms in clinical encounters when it is unfeasible to relocate patients to relaxing environments (i.e. a theater, the ocean).
Overall, our findings point to immersive VR as a highly promising non-pharmacological intervention that contributes to increased individual heat-pain tolerance limits through mechanisms that include autonomic system and emotion regulation, and ‘being immersed’ in a largely appreciated context [10; 15; 17–26; 31; 32; 43; 50]. Integrating VR interventions with established pain therapeutics may represent an innovative approach to optimizing symptoms management and improving clinical outcomes, and it clearly warrants further investigation. Given the current epidemic of opioid over-prescription, overuse, and abuse [40; 42], finding non-invasive, non-pharmacological interventions that can effectively improve individual heat-pain tolerance and reduce the use of pain medication is of the utmost importance. As discussed here, VR might offer a fruitful area for future translational and large-scale clinical trials within this scope of research.
Supplementary Material
Acknowledgments.
This research was supported by the MPowering the State grant (LC, SM, AV) and NCCIH (LC, R01AT010333-01A1). The funding agencies did not have a role in interpreting these results.
We thank Steve Butkus and Scott Murray for their valuable feedback during the preparation of the setting and equipment. Nathaniel Haycock for his comments on the first draft of this manuscript and John A. Melnicki for commenting on the latest version of this manuscript.
Footnotes
Competing interests.
LC reports grants from NIDCR and NCCIH, grants from PCORI, grants from MPowering the State, grants from UM Grants, personal fees from Elsevier during the conduct of the study. NR, YW, TA, BBC, GCC, CK, AV, SM have no competing interests to declare.
References
- [1].Affleck G, Zautra A, Tennen H, Armeli S. Multilevel daily process designs for consulting and clinical psychology: A preface for the perplexed. Journal of consulting and clinical psychology 1999;67(5):746. [DOI] [PubMed] [Google Scholar]
- [2].Benedek M, Kaernbach C. Decomposition of skin conductance data by means of nonnegative deconvolution. Psychophysiology 2010;47(4):647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Buhle J, Wager TD. Performance-dependent inhibition of pain by an executive working memory task. Pain 2010;149(1):19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Defrin R, Ohry A, Blumen N, Urca G. Sensory determinants of thermal pain. Brain 2002;125(Pt 3):501–510. [DOI] [PubMed] [Google Scholar]
- [5].Dube AA, Duquette M, Roy M, Lepore F, Duncan G, Rainville P. Brain activity associated with the electrodermal reactivity to acute heat pain. Neuroimage 2009;45(1):169–180. [DOI] [PubMed] [Google Scholar]
- [6].Enders CK, Tofighi D. Centering predictor variables in cross-sectional multilevel models: a new look at an old issue. Psychological methods 2007;12(2):121. [DOI] [PubMed] [Google Scholar]
- [7].Eriksson M, Storm H, Fremming A, Schollin J. Skin conductance compared to a combined behavioural and physiological pain measure in newborn infants. Acta Paediatr 2008;97(1):27–30. [DOI] [PubMed] [Google Scholar]
- [8].Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior research methods 2007;39(2):175–191. [DOI] [PubMed] [Google Scholar]
- [9].Finan PH, Zautra AJ, Davis MC, Lemery-Chalfant K, Covault J, Tennen H. COMT moderates the relation of daily maladaptive coping and pain in fibromyalgia. PAIN® 2011;152(2):300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Flores A, Linehan MM, Todd SR, Hoffman HG. The Use of Virtual Reality to Facilitate Mindfulness Skills Training in Dialectical Behavioral Therapy for Spinal Cord Injury: A Case Study. Front Psychol 2018;9:531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fruhstorfer H, Lindblom U, Schmidt WC. Method for quantitative estimation of thermal thresholds in patients. J Neurol Neurosurg Psychiatry 1976;39(11):1071–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fujita T, Fujii Y, Okada SF, Miyauchi A, Takagi Y. Fall of skin impedance and bone and joint pain. JBone MinerMetab 2001;19(3):175–179. [DOI] [PubMed] [Google Scholar]
- [13].Garrett B, Taverner T, Masinde W, Gromala D, Shaw C, Negraeff M. A rapid evidence assessment of immersive virtual reality as an adjunct therapy in acute pain management in clinical practice. Clin J Pain 2014;30(12):1089–1098. [DOI] [PubMed] [Google Scholar]
- [14].Gentile D Pathological video-game use among youth ages 8 to 18: a national study. Psychol Sci 2009;20(5):594–602. [DOI] [PubMed] [Google Scholar]
- [15].Gold JI, Belmont KA, Thomas DA. The neurobiology of virtual reality pain attenuation. Cyberpsychol Behav 2007;10(4):536–544. [DOI] [PubMed] [Google Scholar]
- [16].Harrison D, Boyce S, Loughnan P, Dargaville P, Storm H, Johnston L. Skin conductance as a measure of pain and stress in hospitalised infants. Early HumDev 2006;82(9):603–608. [DOI] [PubMed] [Google Scholar]
- [17].Hoffman H, Vu D. Virtual reality: teaching tool of the twenty-first century? Acad Med 1997;72(12):1076–1081. [DOI] [PubMed] [Google Scholar]
- [18].Hoffman HG, Doctor JN, Patterson DR, Carrougher GJ, Furness TA 3rd. Virtual reality as an adjunctive pain control during burn wound care in adolescent patients. Pain 2000;85(1–2):305–309. [DOI] [PubMed] [Google Scholar]
- [19].Hoffman HG, Patterson DR, Carrougher GJ. Use of virtual reality for adjunctive treatment of adult burn pain during physical therapy: a controlled study. Clin J Pain 2000;16(3):244–250. [DOI] [PubMed] [Google Scholar]
- [20].Hoffman HG, Patterson DR, Carrougher GJ, Sharar SR. Effectiveness of virtual reality-based pain control with multiple treatments. Clin J Pain 2001;17(3):229–235. [DOI] [PubMed] [Google Scholar]
- [21].Hoffman HG, Patterson DR, Seibel E, Soltani M, Jewett-Leahy L, Sharar SR. Virtual reality pain control during burn wound debridement in the hydrotank. Clin J Pain 2008;24(4):299–304. [DOI] [PubMed] [Google Scholar]
- [22].Hoffman HG, Richards TL, Coda B, Bills AR, Blough D, Richards AL, Sharar SR. Modulation of thermal pain-related brain activity with virtual reality: evidence from fMRI. Neuroreport 2004;15(8):1245–1248. [DOI] [PubMed] [Google Scholar]
- [23].Hoffman HG, Richards TL, Van Oostrom T, Coda BA, Jensen MP, Blough DK, Sharar SR. The analgesic effects of opioids and immersive virtual reality distraction: evidence from subjective and functional brain imaging assessments. Anesth Analg 2007;105(6):1776–1783, table of contents. [DOI] [PubMed] [Google Scholar]
- [24].Hoffman HG, Sharar SR, Coda B, Everett JJ, Ciol M, Richards T, Patterson DR. Manipulating presence influences the magnitude of virtual reality analgesia. Pain 2004;111(1–2):162–168. [DOI] [PubMed] [Google Scholar]
- [25].Honzel E, Murthi S, Brawn-Cinani B, Colloca G, Kier C, Varshney A, Colloca L. Virtual reality, music, and pain: developing the premise for an interdisciplinary approach to pain management. Pain 2019;160(9):1909–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Keefe FJ, Huling DA, Coggins MJ, Keefe DF, Zachary Rosenthal M, Herr NR, Hoffman HG. Virtual reality for persistent pain: a new direction for behavioral pain management. Pain 2012;153(11):2163–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kregel KC, Seals DR, Callister R. Sympathetic nervous system activity during skin cooling in humans: relationship to stimulus intensity and pain sensation. J Physiol 1992;454:359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lavigne GJ, Zucconi M, Castronovo V, Manzini C, Veglia F, Smirne S, Ferini-Strambi L. Heart rate changes during sleep in response to experimental thermal (nociceptive) stimulations in healthy subjects. Clin Neurophysiol 2001;112(3):532–535. [DOI] [PubMed] [Google Scholar]
- [29].Li A, Montano Z, Chen VJ, Gold JI. Virtual reality and pain management: current trends and future directions. Pain management 2011;1(2):147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Loggia ML, Juneau M, Bushnell MC. Autonomic responses to heat pain: Heart rate, skin conductance, and their relation to verbal ratings and stimulus intensity. Pain 2011;152(3):592–598. [DOI] [PubMed] [Google Scholar]
- [31].Loreto-Quijada D, Gutierrez-Maldonado J, Gutierrez-Martinez O, Nieto R. Testing a virtual reality intervention for pain control. Eur J Pain 2013;17(9):1403–1410. [DOI] [PubMed] [Google Scholar]
- [32].Loreto-Quijada D, Gutierrez-Maldonado J, Nieto R, Gutierrez-Martinez O, Ferrer-Garcia M, Saldana C, Fuste-Escolano A, Liutsko L. Differential effects of two virtual reality interventions: distraction versus pain control. Cyberpsychol Behav Soc Netw 2014;17(6):353–358. [DOI] [PubMed] [Google Scholar]
- [33].Mischkowski D, Palacios-Barrios EE, Banker L, Dildine TC, Atlas LY. Pain or nociception? Subjective experience mediates the effects of acute noxious heat on autonomic responses. Pain 2018;159(4):699–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Miyahira SD, Folen RA, Hoffman HG, Garcia-Palacios A, Schaper KM. Effectiveness of brief VR treatment for PTSD in war-fighters: a case study. Stud Health Technol Inform 2010;154:214–219. [PubMed] [Google Scholar]
- [35].Miyahira SD, Folen RA, Hoffman HG, Garcia-Palacios A, Spira JL, Kawasaki M. The effectiveness of VR exposure therapy for PTSD in returning warfighters. Stud Health Technol Inform 2012;181:128–132. [PubMed] [Google Scholar]
- [36].Moltner A, Holzl R, Strian F. Heart rate changes as an autonomic component of the pain response. Pain 1990;43(1):81–89. [DOI] [PubMed] [Google Scholar]
- [37].Nezlek JB. An introduction to multilevel modeling for social and personality psychology. Social and Personality Psychology Compass 2008;2(2):842–860. [Google Scholar]
- [38].Nielsen CS, Price DD, Vassend O, Stubhaug A, Harris JR. Characterizing individual differences in heat-pain sensitivity. Pain 2005;119(1–3):65–74. [DOI] [PubMed] [Google Scholar]
- [39].O’Regan JK, Noe A. A sensorimotor account of vision and visual consciousness. Behav Brain Sci 2001;24(5):939–973; discussion 973–1031. [DOI] [PubMed] [Google Scholar]
- [40].Ostling PS, Davidson KS, Anyama BO, Helander EM, Wyche MQ, Kaye AD. America’s Opioid Epidemic: a Comprehensive Review and Look into the Rising Crisis. Curr Pain Headache Rep 2018;22(5):32. [DOI] [PubMed] [Google Scholar]
- [41].Pan J, Tompkins WJ. A real-time QRS detection algorithm. IEEE Trans Biomed Eng 1985;32(3):230–236. [DOI] [PubMed] [Google Scholar]
- [42].Pattullo GG, Colloca L. The opioid epidemic: could enhancing placebo effects be part of the solution? Br J Anaesth 2019;122(6):e209–e210. [DOI] [PubMed] [Google Scholar]
- [43].Pourmand A, Davis S, Marchak A, Whiteside T, Sikka N. Virtual Reality as a Clinical Tool for Pain Management. Curr Pain Headache Rep 2018;22(8):53. [DOI] [PubMed] [Google Scholar]
- [44].Rizzo AA, Difede J, Rothbaum BO, Johnston S, McLay RN, Reger G, Gahm G, Parsons T, Graap K, Pair J. VR PTSD exposure therapy results with active duty OIF/OEF combatants. Stud Health Technol Inform 2009;142:277–282. [PubMed] [Google Scholar]
- [45].Rizzo AA, Graap K, Perlman K, McLay RN, Rothbaum BO, Reger G, Parsons T, Difede J, Pair J. Virtual Iraq: initial results from a VR exposure therapy application for combat-related PTSD. Stud Health Technol Inform 2008;132:420–425. [PubMed] [Google Scholar]
- [46].Scapin S, Echevarria-Guanilo ME, Boeira Fuculo Junior PR, Goncalves N, Rocha PK, Coimbra R. Virtual Reality in the treatment of burn patients: A systematic review. Burns 2018. [DOI] [PubMed] [Google Scholar]
- [47].Schestatsky P, Valls-Sole J, Costa J, Leon L, Veciana M, Chaves ML. Skin autonomic reactivity to thermoalgesic stimuli. Clin Auton Res 2007;17(6):349–355. [DOI] [PubMed] [Google Scholar]
- [48].Shaffer F, Ginsberg JP. An Overview of Heart Rate Variability Metrics and Norms. Front Public Health 2017;5:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Slater M Place illusion and plausibility can lead to realistic behaviour in immersive virtual environments. Philos Trans R Soc Lond B Biol Sci 2009;364(1535):3549–3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Small C, Stone R, Pilsbury J, Bowden M, Bion J. Virtual restorative environment therapy as an adjunct to pain control during burn dressing changes: study protocol for a randomised controlled trial. Trials 2015;16:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Spiegel B Virtual Reality for Management of Pain in Hospitalized Patients: A Randomized Comparative Effectiveness Trial (unpublished). [DOI] [PMC free article] [PubMed]
- [52].Spiegel B, Fuller G, Lopez M, Dupuy T, Noah B, Howard A, Albert M, Tashjian V, Lam R, Ahn J, Dailey F, Rosen BT, Vrahas M, Little M, Garlich J, Dzubur E, IsHak W, Danovitch I. Virtual reality for management of pain in hospitalized patients: A randomized comparative effectiveness trial. PLoS One 2019;14(8):e0219115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Sztajzel J Heart rate variability: a noninvasive electrocardiographic method to measure the autonomic nervous system. Swiss Med Wkly 2004;134(35–36):514–522. [DOI] [PubMed] [Google Scholar]
- [54].Wiederhold BK, Soomro A, Riva G, Wiederhold MD. Future directions: advances and implications of virtual environments designed for pain management. Cyberpsychol Behav Soc Netw 2014;17(6):414–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Yarnitsky D, Ochoa JL. Studies of heat pain sensation in man: perception thresholds, rate of stimulus rise and reaction time. Pain 1990;40(1):85–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


