Abstract
Feeding difficulties are especially prevalent in preterm infants, although the mechanisms driving these difficulties are poorly understood due to a lack of data on healthy infants. One potential mechanism of dysphagia in adults is correlated with bolus volume. Yet, whether and how bolus volume impacts swallow safety in infant feeding is unknown. A further complication for safe infant swallowing is recurrent laryngeal nerve (RLN) injury due to patent ductus arteriosus surgery, which exacerbates the issues that preterm infants face and can increase the risk of dysphagia. Here, we used a validated animal model feeding freely to test the effect of preterm birth, postnatal maturation and RLN lesion and their interactions on swallow safety. We also tested whether bolus size differed with lesion or birth status, and the relationship between bolus size and swallow safety. We found very little effect of lesion on swallow safety, and preterm infants did not experience more penetration or aspiration than term infants. However, term infants swallowed larger boluses than preterm infants, even after correcting for body size. Bolus size was the primary predictor of penetration or aspiration, with larger boluses being more likely to result in greater degrees of dysphagia irrespective of age or lesion status. These results highlight that penetration and aspiration are likely normal occurrences in infant feeding. Further, when comorbidities, such as RLN lesion or preterm birth are present, limiting bolus size may be an effective means to reduce incidences of penetration and aspiration.
Keywords: Dysphagia, animal model, neonate, feeding, performance
Introduction
Multiple factors influence the pathophysiology of swallowing in infants. Many of these factors are patient characteristics, such as age or gestational age at birth (preterm or term), while others can be physical or behavioral [1–3]. However, these factors do not directly operate on the physiology of swallowing or necessarily result in penetration or aspiration. Swallows of known volumes are common practice for examining swallowing among the elderly and neurologically compromised [4–6]. These studies suggest that increases in bolus volume may be a mechanism that results in decreased swallow safety in adults [7–11], although other results suggest that bolus volume is not correlated with swallow safety [6,12]. Such studies are not feasible in infants due to their frailty and concerns over their exposure to videofluoroscopic radiation, and thus understanding how bolus volume impacts swallow safety in infant feeding has received little attention, despite the prevalence of dysphagia in this population.
Problems associated with feeding are especially prevalent in preterm infants, of which over 80% experience some feeding difficulties [13]. Compared to term infants, preterm infants often have reduced abilities to latch on to and acquire milk from the nipple [14,15], have decreased suck-swallow coordination [16], and have decreased pharyngeal motility and activity [17–20]. As such, preterm infants are considered to be a neurologically compromised population [21–24], with a higher risk for dysphagia than populations without neurological conditions [6,9]. The neurological issues that preterm infants face are often compounded by surgical procedures carried out to ensure their survival. One common injury that occurs during cardiovascular surgery to close a patent ductus arteriosus, is damage to the recurrent laryngeal nerve (RLN)[25,26].
RLN damage or lesion can result in unilateral vocal fold paralysis, stridor, as well as varying degrees of dysphagia [27,28]. In an animal model, RLN lesion has recently been shown to impact the neuromotor control of feeding [29], modify tongue kinematics [30], and reduce bolus size during swallowing [10], with variable changes to swallow safety [3,10]. The effect of RLN lesion on feeding performance can thus be pervasive, and the mechanism by which it reduces airway protection is distinct from other neurological insults such as lesion to the Superior Laryngeal Nerve (SLN) [31].
These results, however, apply to term infant pigs. We do not know if RLN lesion will impact preterm infant pigs in the same way. Furthermore, maturation associated with postnatal age can improve oral motor skills and aerodigestive coordination, especially in the context of RLN lesion [32,33], and has the potential to reduce incidences of dysphagia. Although preterm infants have been found to swallow smaller boluses than term infants in an animal model [34], we have little insight into the relationship between swallow safety and bolus size and how preterm birth, RLN lesion, and longitudinal maturation impact that relationship.
Delimiting the effects of, and the relationships among, bolus volume, gestational age at birth, neurological damage, and the effect of postnatal maturation is challenging in clinical settings, as these factors are often associated with other comorbidities [35]. It is also difficult to establish baseline levels of penetration and aspiration in non-compromised pediatric populations due to the desire to minimize radiation, which limits the extensive videofluoroscopy required to establish baseline levels of penetration and aspiration [36,37]. Thus while penetration and aspiration may occur in healthy individuals [38,39], good estimates of normal physiology are difficult to obtain.
Although preterm infants are at a greater risk of experiencing difficulties associated with feeding, we have little insight into whether those risks manifest into decreased swallow safety or relate to other aspects of feeding performance in comparison to term infants. Animal models present an opportunity to experimentally determine baseline performance in healthy animals while removing comorbidities [40]. They enable controlled experimental designs that can explicitly determine the individual effects of bolus volume, gestational age at birth, neurological damage, and postnatal maturation on swallow safety.
Here, we used a validated animal model for infant feeding to investigate how the combination of preterm birth and RLN lesion impact bolus size and swallow safety longitudinally from shortly after birth to the onset of weaning [40]. We measured bolus size and swallow safety using a repeated measures design, comparing performance of preterm and term infant pigs with and without RLN lesion at seven and 17 days of age (2–4 months and 6–9 months human age equivalent [41]). Our experimental design includes bolus size and swallow safety as dependent variables, with RLN lesion (control or lesion), birth age (term or preterm), and Age (seven or 17 days old, in a longitudinal design) as independent variables. This design allowed us to ask three specific questions that have not been addressed in clinical settings or in an animal model:
Does bolus size vary with birth age, post-natal age, or RLN lesion?
Does swallow safety vary with birth age, age, or RLN lesion?
Is there an interaction between bolus size and swallow safety?
We expect that the relationship among and between these variables will be complex and that bolus size will have a greater impact on swallow safety than RLN lesion, birth age, or postnatal age. Our results provide new insight into the impact of bolus volume on swallow safety and establishes baseline levels of performance for healthy infant pigs. These results have direct translational impacts on the design of studies of human infants with dysphagia and their clinical care.
Methods
Animal Housing and Care
Infant pigs (Yorkshire/Landrace sows, Shoup Farms, Wooster, OH) were delivered via cesarean section at term (1 litter, 114 days gestation) or preterm (1 litter, 108 days gestation; human equivalent 30–32 weeks gestation [41]). Cesarean section for both litters ensures that performance differences between litters are due to the duration of gestation rather than the method of delivery. Detailed methods on C-section delivery are in Ballester et al., [33]. After delivery, newborns were fed colostrum within 2 hours of birth before transitioning to feeding on infant pig formula (Solustart Pig Milk Replacement, Land o’ Lakes, Arden Mills, MN) with a bottle and nipple over a period of 24 hours (NASCO Farm & Ranch, Fort Atkinson, WI). For the first week of life, infants were monitored for 24 hours a day, after which point care followed standard protocol for infant pigs [33,42,43].
Five days after birth, the right recurrent laryngeal nerve was lesioned on half the pigs following published protocols [3,30]. In short, in a sterile surgery, the nerve was identified in control and lesion individuals. In lesioned individuals, a 3 mm portion of the nerve was tied at both ends with sterile suture, capped using micro-hemoclips (Weck Ligation Solutions, NC) next to the suture, and then the 3 mm stretch of nerve between the pairs of suture and hemoclips was removed and the ends were displaced inside the neck of the individual. Persistence of lesion was confirmed postmortem by dissections of all lesioned individuals. In all pigs, radio-opaque markers were sewn into the tissues attached to the hyoid and thyroid for subsequent analyses of swallowing kinematics.
Data collection
All pigs had the same feeding experience prior to data collection. We collected feeding data at two ages: when the pigs were seven days old (2–3 months human equivalent), the first day piglets can maintain body temperature enough to be transported from the animal housing to the imaging facility, and 17 days old (6–9 months human equivalent, [41]), which is just prior to weaning [44]. Data were collected via videofluoroscopy (GE9400 C-Arm, 85 kV, 4MA), that digitally recorded images at 100 Hz using a high-speed camera (XC1M digital camera, XCitex, Cambridge, MA). To visualize milk during feeding, piglets were fed infant formula mixed with barium (E-Z Paque Barium Sulfate, EZ EM Inc., NY). We recorded at least 20 swallows per pig per feeding session, starting after the first 10 seconds of feeding, as the first few swallows occur at a faster rate than normal [45]. All animals fed willingly at this time, and usually for at least 50 swallows. Swallow volume was not controlled experimentally but was determined by the animal. Animal weight was recorded in the morning daily.
Data processing
Individual researchers were trained to identify swallows using single-blind procedures until intra and inter-rater reliability reached 95%. We identified a total of 947 swallows (N = 317 term (four control and four lesion individuals), N = 630 preterm (seven control and nine lesion individuals)), by determining at what frame the epiglottis began its posterior movement [33,34]. The Infant Mammalian Penetration-Aspiration Scale (IMPAS) was used to asses swallow safety [46]. This scale is generalizable to all infant mammals, and functions similarly to the Penetration-Aspiration Scale (PAS) in adult humans [47], whereby a 1 indicates a safe swallow, a 7 indicates silent aspiration (unlike in PAS, which is an 8 point scale), and values in between indicate varying degrees airway invasion and the response to penetration and aspiration [46].
Following published protocols, the frame prior to the initiation of the posterior movement of the epiglottis was isolated for analysis [10]. Swallows where the pig was not aligned parallel to the x-ray image were not included in analyses to control for the effect of parallax on apparent bolus area. The bolus in this frame was outlined using the free select tool in ImageJ [48] on a touch screen tablet with a stylus (Surface Pro 2, Microsoft Corporation, Redwood, WA). As milk in the pyriform recesses is quite variable within pigs and makes up a small amount of the total volume of the bolus [49], it was not outlined (Fig. S1). Prior to calculating bolus area in Image J [48], we scaled all images to mm2.
Statistical analyses
We performed analyses on raw bolus size, as well as bolus size scaled to pig mass (Area3/2/Mass) to control for differences in pig size between treatments and throughout growth during ontogeny. This approach differs from [10] in that it includes a correction for body mass on bolus size, in addition to raw measures of bolus size. To test question (1), differences in bolus area were tested using linear mixed models, with birth age (preterm or term), age (seven or 17), and lesion status (control or lesion) and their interactions as fixed effects, and individual pig as a random effect using lme4 in R (v. 3.5.0, www.r-project.org, [50]). P-values for main effects were obtained using the Anova() function in R by comparing the full model to simplified models in which the term was removed. In cases where interactions were significant, we performed planned contrasts analyses to test for differences between treatments.
To test for differences in question (2) between treatments in swallow safety, we performed logistic regression analyses, using birth age, age, and lesion status as main effects. We did not observe any swallows of IMPAS 4, 5, or 6, and grouped IMPAS scores of 1 (safe) and 2 (penetration but with clearance) for logistic regression analyses resulting in a total of three levels (IMPAS 1 and 2, IMPAS 3, and IMPAS 7). To test for the correlation between IMPAS and bolus size (question 3), we performed multinomial logistic regressions for each birth age – age group, with bolus area and lesion status as main effects. In logistic regression analyses, we calculated odds ratios, as well as p values using Wald chi-squared analyses. We performed separate analyses for raw bolus area and size-corrected bolus areas. To determine which variables were most likely to predict swallow safety, we performed a multinomial regression with the size-corrected bolus area, birth age, age, and lesion status as fixed effects. We then used Akaike’s information criterion (AIC) followed by model averaging to assess the importance of variables in determining penetration and aspiration [51]. These analyses differed from most previous work on RLN lesion in infant pigs in that they involve groupwise analyses, rather than analyses of the same individuals pre-and post-lesion, which were impossible to perform in this study, as term and preterm birth are mutually exclusive states.
Results
The impact of birth age, age, and RLN lesion on bolus size
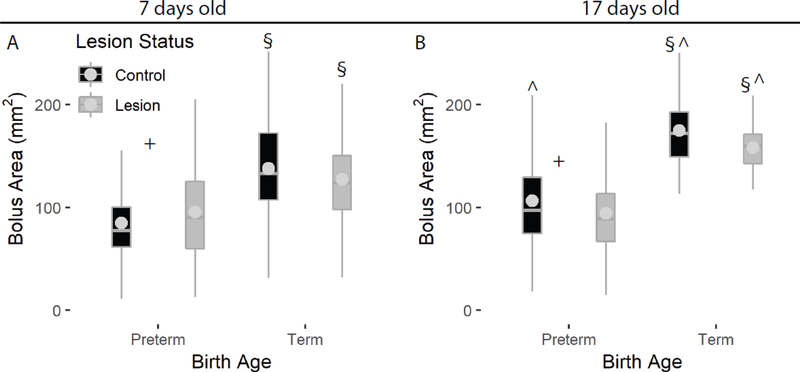
Age at birth (term/preterm) was a significant determinant of absolute bolus size, with term infants having larger boluses at seven days old (p < 0.001, Fig 1A). This difference was even more apparent by day 17, as term infants had nearly double the bolus size than preterm infants at this age (term mean = 167.35 cm2, preterm mean = 99.16 cm2, Table 1), regardless of lesion status (p<0.001, Fig 2B). In testing for interactions, RLN lesion did not impact bolus size in term infants (p > 0.05), but did have an effect on bolus size in preterm infants. Bolus size was larger directly after lesion at seven days (preterm lesion mean = 95.6 cm2, preterm control mean = 84.87, p = 0.03), and smaller at day 17 (preterm lesion mean = 94.6 cm2, preterm control mean = 106.5, p = 0.002). We found that with age, bolus size marginally increased in preterm non-lesion infants, and was much larger at day 17 for control term infants, as well as lesion term infants (Fig. 1, Table S1). In contrast, bolus size did not increase with age for preterm lesion infants (Fig. 1, Table S1).
Figure 1.
Term infants swallowed larger boluses than preterm infants at both ages, and although term infant bolus size increased substantially by day 17 (B), preterm bolus size did not. We found no effect of lesion status on bolus size in term infants. Effect of lesion indicated by ‘+’ within groups; effect of birth age indicated by ‘§’; effect of age indicated by ^ on the day 17 plot.
Table 1.
Post-hoc analyses for raw bolus size and corrected bolus size, highlighting the effect of lesion and age on bolus size.
| Effect tested | Contrast | Raw t-ratio(df) | Raw p | Std t-ratio (df) | Std p |
|---|---|---|---|---|---|
| Lesion | PT7C vs PT7L | 2.1 (794) | 0.03 | 1.9 (776) | 0.06 |
| Lesion | T7C vs T7L | −1.4 (794) | 0.17 | −1.6 (776) | 0.11 |
| Birth age | PT7C vs T7C | 8.0 (794) | <0.001 | 5.8 (776) | <0.001 |
| Birth age | PT7L vs T7L | 5.1 (794) | <0.001 | 2.6 (776) | 0.01 |
| Lesion | PT17C vs PT17L | −2.3 (794) | 0.02 | −3.6 (776) | <0.001 |
| Lesion | T17C vs T17L | −1.9 (794) | 0.06 | −2.2 (776) | 0.03 |
| Birth Age | PT17C vs T17C | 10.0 (794) | <0.001 | 7.7 (776) | <0.001 |
| Birth Age | PT17L vs T17L | 10.1 (794) | <0.001 | 8.3 (776) | <0.001 |
| Age | PT7C vs PT17C | 3.8 (794) | <0.001 | −0.8 (776) | 0.44 |
| Age | PT7L vs PT17L | −0.2 (794) | 0.83 | −7.2 (776) | <0.001 |
| Age | T7C vs T17C | 4.8 (794) | <0.001 | 1.1 (776) | 0.28 |
| Age | T7L vs T17L | 4.2 (794) | <0.001 | 0.5 (776) | 0.65 |
Bolded values indicate statistical significance.
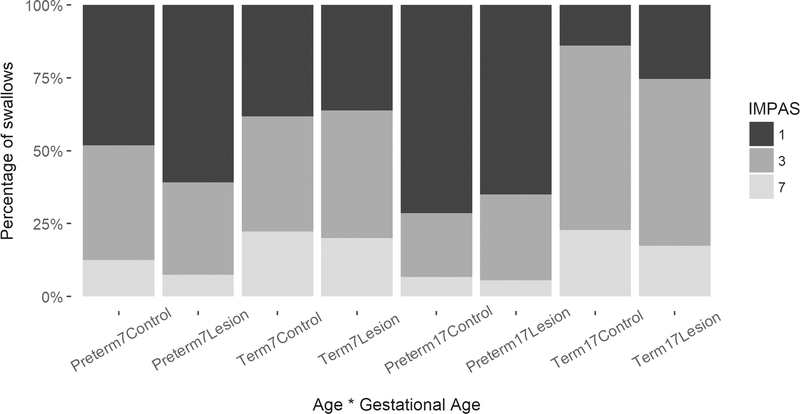
Figure 2.
Term infants exhibited more instances of penetration and aspiration than preterm infants, and there we no differences between ages or between lesion status.
After correcting bolus size for body mass, we found similar results for the effect of birth age on bolus size, with term infants having a larger corrected bolus area than preterm infants at both ages seven and 17 (Fig S2). RLN lesion resulted in decreased corrected bolus sizes for both preterm (p < 0.001) and term infants (p = 0.03) at day 17, but not at day 7 (Table S1). The correction of bolus area for body size resulted in no differences in bolus size between ages for preterm control infants or term infants, although we found that preterm lesioned infants had smaller size-corrected bolus areas at day 17 than they did at day 7 (Fig. S2, Table 2, Table S1). These results are consistent with previous research whereby bolus size was corrected for the length of the hard palate of each individual [34].
Table 2.
Results from multinomial logistic regression showing the contributions of birth age (T/PT), age (7 vs 17), and lesion status (C/L) to IMPAS. Terms were more likely to aspirate than preterms, and we found no effect of age or lesion on rates of aspiration.
| T/PT odds | Age odds | C/L odds | T/PT p | Age p | C/L p | |
|---|---|---|---|---|---|---|
| 3 | −3.42 | 0.05 | −0.29 | <0.001 | 0.92 | 0.46 |
| 7 | −4.64 | −0.65 | −1.03 | <0.001 | 0.29 | 0.09 |
Negative odds ratios indicate that the compared group is less likely to experience a three or seven than the reference group (control, term, seven).
The impact of birth age, age, and RLN lesion on IMPAS scores
We found that all pigs experienced safe swallows, swallows with penetration, and swallows with aspiration, regardless of birth age, age, or lesion status (Figure 2). We did not observe any IMPAS scores of 4, 5, or 6. Therefore, logistic regression analyses focus on differences between safe swallows (IMPAS 1 and 2), swallows with penetration (IMPAS 3), and swallows with aspiration (IMPAS 7). Logistic regression results indicate that the only significant effect on the probability to have penetration or aspiration was birth age, whereby term pigs were 3.42 times more likely to have an IMPAS score of 3 (penetration, p < 0.001) than preterm pigs and 4.64 times more likely to have an IMPAS score of 7 (aspiration, p < 0.001). Age and lesion status had no consistent effect on IMPAS (odds ratio < 0.4, p > 0.05, Table 2).
The relationship between bolus size and IMPAS
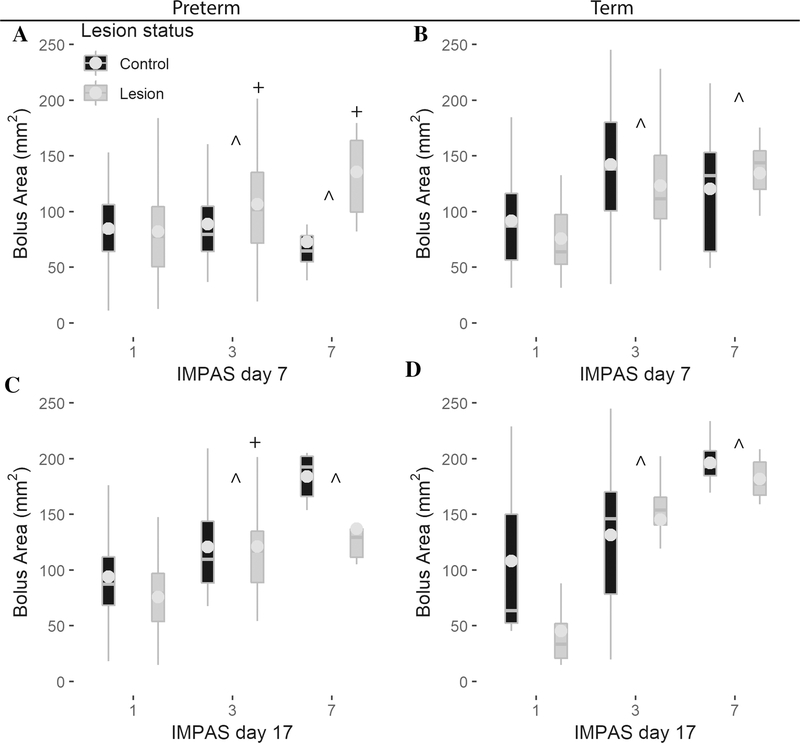
When testing for the relationship among IMPAS, bolus area, and lesion status, the primary predictor of penetration or aspiration was bolus area for preterm and term infants of both ages. We found an increase of bolus area by one cm2 increased the likelihood of penetration by 3.0 to 13.9 times, and increased the likelihood of aspiration (IMPAS 7) by up to 18.5 times (Table 3, all p < 0.001). In contrast, lesion only impacted IMPAS scores for preterm infants, whereby lesion slightly decreased the probability of penetration and aspiration at day 7, but increased the probability of penetration at day 17 (Fig. 3, Table 3).
Table 3.
Results from multinomial logistic regression of bolus area and lesion status within preterm and term infants at ages seven and 17.
| Bolus Area Odds | Lesion Odds | Bolus p | Lesion p | |||||
|---|---|---|---|---|---|---|---|---|
| 3 | 7 | 3 | 7 | 3 | 7 | 3 | 7 | |
| Preterm 7 | 3.0 | 3.8 | −1.6 | −2.4 | 0.001 | 0.007 | 0.03 | 0.03 |
| Term 7 | 13.9 | 11.69 | 2.5 | 0.8 | <0.001 | <0.001 | 0.37 | 0.79 |
| Preterm 17 | 7.9 | 13.1 | 2.5 | 2.5 | <0.001 | <0.001 | 0.007 | 0.14 |
| Term 17 | 6.3 | 18.5 | −1.9 | −0.8 | <0.001 | <0.001 | 0.17 | 0.65 |
Bolded values indicate odds ratios greater than one, or p values less than 0.05.
Negative odds ratios indicate that the compared group is less likely to experience a three or seven than the reference group (control).
Figure 3.
The probability of penetration and aspiration increases with bolus size for preterm infants at day 7 (A) and 17 (B) as well as for term infants at day 7 (C) and 17 (D). In contrast, lesion does not have an effect on swallow safety in term infants at either age, although preterm infants with lesion are less likely to aspirate at day 7 (A), whereas they are more likely to have penetration at day 17 (C).Lesion effect indicated by ‘+’, bolus size effect indicated by ′^′.
When examining the overall relationship among IMPAS, corrected bolus area, lesion status, age, and birth age, we found that three models had a delta AIC of less than three, indicating a strongly supportive model (Table S2). All three models found that corrected bolus area and birth age were predictive of IMPAS, with one model finding an impact of age, and another finding an impact of lesion status (Table 4). The best fitting model found that lesion status and age had no impact on IMPAS scores, and that only corrected bolus area and birth age were significantly predictive of IMPAS score.
Table 4.
Model selection table of the top three models.
| Age | Std Area | L.C | PT_T | Delta AIC | |
|---|---|---|---|---|---|
| Model 1 | + | + | 0 | ||
| Model 2 | + | + | + | 1.15 | |
| Model 3 | + | + | + | 2.88 |
+: indicates a variable that was significant for a given model.
Discussion
We found that the primary predictor of penetration and aspiration was birth age, whereby term infant pigs actually experienced penetration and aspiration more frequently than preterm infant pigs. We found no changes in the frequency of penetration or aspiration with post-natal age (7 or 17 days) or with RLN lesion. Although it may at first be surprising that term infant pigs experienced fewer safe swallows than preterm infant pigs, this is probably due to the fact that they swallowed larger boluses, and that a larger bolus was the most important predictor of unsafe swallows. This suggests that unsafe swallows are more likely to occur due to the physical properties of the bolus, and the sensory response to that bolus, rather than a result of decreased neuromotor control induced by either preterm birth, neurological maturation during development, or RLN lesion.
The impact of preterm birth on the maturation of bolus size and swallow safety
Preterm birth had a strong effect on bolus size, and was especially apparent when examining it longitudinally, as even control (non-lesioned) preterm pigs showed very little change in bolus size. Lesion may also compound the ability to swallow larger boluses longitudinally in preterm infant pigs, as they did not increase their bolus size at all as they grew. These results concur with previous work on infant pigs without RLN lesion, which found that preterm pigs did not show changes in bolus size or shape as they grew, in contrast to term pigs which had larger, more elongate boluses at older ages [33]. These results are important clinically, as a larger bolus has been suggested to indicate improvements in swallow physiology with maturity in human infants [52]. The lack of change in bolus size in preterm pigs could relate to their reduced abilities to acquire milk [32,53], but could also relate to decreased esophageal motility, inhibiting swallows of larger boluses [18–20]. Alternatively, small boluses in preterm infant pigs could arise from immature neural control of feeding physiology [16], and they could be swallowing small boluses to reduce incidences of penetration and aspiration.
Our finding that RLN lesion has no impact on the likelihood of aspiration is surprising given the clinical and experimental documentation of increased aspiration rates in human infants with RLN lesion [3,28]. Two possible complimentary hypotheses might account for this discrepancy. On the one hand, as noted previously, videofluroscopic swallow studies on intact human infants are rare and limited in scope. Thus, it is possible that aspiration rates in intact human infants have been underreported and aspiration rates in humans with RLN lesion overreported. Additionally, a key difference between this study and previous work on RLN lesion in infant pigs lies in the design of the experiments. In previous work, each animal was compared to itself prior to and following RLN lesion, whereas in this study we compared one group of pigs with lesion, and one without. Previous work noted the high amount of interindividual variation following RLN lesion, suggesting that in this study, the effect size resulting from RLN lesion is likely small relative to the amount of interindividual variation. It is notable that this is not the case for bolus size as a parameter, as its effect is still clear even when comparing two groups.
The importance of bolus size for swallow safety
Bolus volume is widely appreciated to play a role in determining swallow safety in human and animal models [7–11], although some research has found that smaller boluses do not relate to the risk of aspiration or PAS score in adults [6,12]. Our results agree with the general body of knowledge, in that a larger bolus decreases swallow safety, regardless of birth age, age, or lesion status. Previous work on RLN lesion in term pigs has found that lesion impacts tongue kinematics [30], neuromotor control of feeding [29], and bolus size [10] but that the impact of lesion on swallow safety was more variable. This could be because lesioned animals had a decreased bolus size, thereby reducing the risk of penetration or aspiration. Similarly, lesion to the superior laryngeal nerve (SLN), the nerve responsible for sensation of the bolus in the valleculae, results in increased bolus volume [49] and increased penetration and aspiration [46,49]. These results are important from a clinical perspective, as they suggest that this specific neurological compromise itself is not responsible for increased rates of penetration and aspiration, but rather that the neurological compromise results in changes to the oropharyngeal kinematics impacting bolus size, which is what drives swallow safety.
As term pigs swallowed much larger boluses than preterm pigs at both ages, our finding that term pigs actually experience greater rates of penetration and aspiration than preterm pigs is not surprising. Despite the increased rates of aspiration, all term pigs were healthy and showed no comorbidities. Indeed, all aspirations would have been judged as silent traces, and were only identified after feeding analysis of the videos. Many studies on the rates of penetration and aspiration in healthy patients have found that silent aspiration is a regular occurrence in adults and infants [8,38,39]. This suggests that penetration and aspiration of trace amounts of liquid are likely a normal part of infant feeding, and that the potential comorbidities associated with penetration and aspiration (such as failure to thrive, decreased suction generation, decreased neuromotor coordination, or reduced pharyngeal pressures during swallowing) should be accounted for in making decisions about the care of infants with dysphagia [16,35,52,54]. The fact that bolus size is strongly related to the likelihood of aspiration, yet still increases in term, but not preterm infant pigss, suggests that increasing bolus size, and thus efficiency of food acquisition, may be more important in normal infant development than avoiding aspiration. Additionally, the frailty of preterm infants may result in aspiration providing a larger health risk to preterm infants than it does to term infants.
Limitations and future directions
One limitation of this study is that we used a two-dimensional measure of bolus size, rather than directly measuring total bolus volume. However, bolus size and volume are tightly correlated in infant pigs [49], and thus our results are likely to approximate volume fairly accurately. Additionally, our results delineate the impact of birth age, age, and RLN lesion on swallow safety, but do not elucidate the mechanisms driving those differences. Preterm infant pigs qualitatively consume food over a longer period of time, and this may compensate for their smaller bolus sizes to ensure that they intake adequate nutrition for growth. Future studies should explore differences in the oropharyngeal kinematics and kinetics between these groups in order to develop an explanation for why preterm infants swallow smaller boluses and do not increase bolus size with age. Although this study identifies bolus size as a predictor of aspiration, it does not identify a mechanism of airway protection failure. Future studies should examine how known airway protective mechanisms, such as laryngeal vestibule closure timing might correlate with bolus size to identify mechanisms of airway protection failure [55].Finally, although this study uses a validated animal model for infant feeding function [40], how bolus size interacts with birth age, age, and RLN lesion in human infants is not known.
Conclusions
We found two major impacts on feeding performance through ontogeny: that preterm birth disrupts the maturation of bolus size and airway protection, and that bolus size is the most important predictor of penetration and aspiration in infant pigs. In contrast, we found that RLN lesion does not appear to act directly to increase aspiration rates in term or preterm infant pigs, despite the fact that it has a pervasive impact on other aspects of the feeding cycle [10,29,30]. Thus, for preterm infants with feeding difficulties, care should focus not just on reducing rates of aspiration. Rather, focusing on improving the ability of preterm infants to acquire and process milk, and coordinate between different behaviors during the feeding cycle to enable improved feeding function might have more impact on reducing dysphagia [16,52,56]. Additionally, for infants that show comorbidities associated with high incidences of aspiration, limiting bolus volume is likely to be an effective way to improve health, either through the use of slow-flow nipples, or increasing sensitivity to bolus volume to trigger the swallow reflex at lower volumes [49,57,58].
Supplementary Material
Acknowledgements
We would like to thank C. Tennant and E. Catchpole for their assistance with data collection and animal care, Claire Lewis, Katlyn McGrattan and Kayla Hernandez for assistance with animal care, and the Biomechanics journal club at NEOMED for their helpful comments on an earlier version of this manuscript.
Funding: This project was funded by NIH R01 HD088561 to R.Z.G.
Footnotes
Competing interests: The authors declare no competing interests
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Gewolb IH, Vice FL. Maturational changes in the rhythms, patterning, and coordination of respiration and swallow during feeding in preterm and term infants. Dev Med Child Neurol [Internet]. 2006;48:589–94. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Citation&list_uids=16780629 [DOI] [PubMed] [Google Scholar]
- 2.Amaizu N, Shulman RJ, Schanler RJ, Lau C. Maturation of oral feeding skills in preterm infants. Acta Paediatr. 2008;97:61–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gould FDH, Lammers AR, Ohlemacher J, Ballester A, Fraley L, Gross A, et al. The Physiologic Impact of Unilateral Recurrent Laryngeal Nerve (RLN) Lesion on Infant Oropharyngeal and Esophageal Performance. Dysphagia. 2015/08/20. 2015;30:714–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Humbert IA, Lokhande A, Christopherson H, German R, Stone A. Adaptation of swallowing hyo-laryngeal kinematics is distinct in oral vs. pharyngeal sensory processing. J Appl Physiol [Internet]. 2012;112:1698–705. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Citation&list_uids=22403349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyoka Y, Ashida I, Kawakami S, Tamaki Y, Miyaoka S. Activity patterns of the suprahyoid muscles during swallowing of different fluid volumes. J Oral Rehabil. 2010;37:575–82. [DOI] [PubMed] [Google Scholar]
- 6.Park JW, Sim GJ, Yang DC, Lee KH, Chang JH, Nam KY, et al. Increased bolus volume effect on delayed pharyngeal swallowing response in post-stroke oropharyngeal dysphagia: A pilot study. Ann Rehabil Med. 2016;40:1018–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hiss SG, Treole K, Stuart A. Effects of age, gender, bolus volume, and trial on swallowing apnea duration and swallow/respiratory phase relationships of normal adults. Dysphagia. 2001;16:128–35. [DOI] [PubMed] [Google Scholar]
- 8.Butler SG, Stuart A, Leng X, Rees C, Williamson J, Kritchevsky SB. Factors influencing aspiration during swallowing in healthy older adults. Laryngoscope. 2010;120:2147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belo LR, Gomes NAC, Coriolano MDGWDS, De Souza ES, Moura DAA, Asano AG, et al. The relationship between limit of dysphagia and average volume per swallow in patients with Parkinson’s disease. Dysphagia. 2014;29:419–24. [DOI] [PubMed] [Google Scholar]
- 10.Gould FDH, Yglesias B, Ohlemacher J, German RZ. Pre-pharyngeal Swallow Effects of Recurrent Laryngeal Nerve Lesion on Bolus Shape and Airway Protection in an Infant Pig Model. Dysphagia [Internet]. 2017;32:362–73. Available from: https://www.ncbi.nlm.nih.gov/pubmed/27873091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butler SG, Stuart A, Markley L, Feng X, Kritchevsky SB. Aspiration as a Function of Age, Sex, Liquid Type, Bolus Volume, and Bolus Delivery Across the Healthy Adult Life Span. Ann Otol Rhinol Laryngol 2018;127:21–32. [DOI] [PubMed] [Google Scholar]
- 12.Omari TI, Dejaeger E, Tack J. Effect of bolus volume and viscosity on pharyngeal automated impedance manometry variables derived for broad dysphagia patients. Dysphagia. 2013;28:146–52. [DOI] [PubMed] [Google Scholar]
- 13.Bryant-Waugh R, Markham L, Kreipe RE, Walsh BT. Feeding and eating disorders in childhood. Int J Eat Disord. 2010;43:98–111. [DOI] [PubMed] [Google Scholar]
- 14.Rommel N, van Wijk M, Boets B, Hebbard G, Haslam R, Davidson G, et al. Development of pharyngo-esophageal physiology during swallowing in the preterm infant. Neurogastroenterol Motil [Internet]. 2011;23:e401–8. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Citation&list_uids=21827583 [DOI] [PubMed] [Google Scholar]
- 15.Lau C Development of suck and swallow mechanisms in infants. Ann Nutr Metab. 2015;66:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayerl CJ, Gould FDH, Bond LE, Stricklen BM, Buddington RK, German RZ. Preterm birth disrupts the development of feeding and breathing coordination. J Appl Physiol. 2019;126:1681–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rasch S, Sangild PT, Gregersen H, Schmidt M, Omari T, Lau C. The preterm piglet - a model in the study of oesophageal development in preterm neonates. Acta Paediatr [Internet]. 2010;99:201–8. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Citation&list_uids=19878132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Staiano A, Boccia G, Salvia G, Zappulli D, Clouse RE. Development of esophageal peristalsis in preterm and term neonates. Gastroenterology. 2007;132:1718–25. [DOI] [PubMed] [Google Scholar]
- 19.Prabhakar V, Hasenstab KA, Osborn E, Wei L, Jadcherla SR. Pharyngeal contractile and regulatory characteristics are distinct during nutritive oral stimulus in preterm-born infants: Implications for clinical and research applications. Neurogastroenterol Motil 2019;31:E13650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jadcherla SR, Shubert TR, Gulati IK, Jensen PS, Wei L, Shaker R. Upper and lower esophageal sphincter kinetics are modified during maturation: effect of pharyngeal stimulus in premature infants. Pediatr Res [Internet]. 2015;77:99–106. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Citation&list_uids=25279989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flamand VH, Nadeau L, Schneider C. Brain motor excitability and visuomotor coordination in 8-year-old children born very preterm. Clin Neurophysiol 2012;123:1191–9. [DOI] [PubMed] [Google Scholar]
- 22.Pitcher JB, Schneider LA, Burns NR, Drysdale JL, Higgins RD, Ridding MC, et al. Reduced corticomotor excitability and motor skills development in children born preterm. J Physiol. 2012;590:5827–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pitcher JB, Riley AM, Doeltgen SH, Kurylowicz L, Rothwell JC, McAllister SM, et al. Physiological evidence consistent with reduced neuroplasticity in human adolescents born preterm. J Neurosci. 2012;32:1640–16416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oudgenoeg-Paz O, Mulder H, Jongmans MJ, van der Ham IJM, Van der Stigchel S. The link between motor and cognitive development in children born preterm and/or with low birth weight: A review of current evidence. Neurosci Biobehav Rev [Internet]. Elsevier; 2017;80:382–93. Available from: 10.1016/j.neubiorev.2017.06.009 [DOI] [PubMed] [Google Scholar]
- 25.Benjamin JR, Smith PBS, Cotten CM, Jaggers J, Goldstein RF, Malcom WF. Long-term morbidities associated with vocal cord paralysis after surgical closure of a patent ductus arteriosus in extremely low birth weight infants. J Perinatol. 2010;30:408–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pereira KD, Firpo C, Gasparin M, Teixeira AR, Dornelles S, Bacaltchuk T, et al. Evaluation of swallowing in infants with congenital heart defect. Int Arch Otorhinolaryngol. 2015;19:55–60. doi: 10.1055/s-0034-1384687. Epub 2014 Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pereira KD, Webb BD, Blakely ML, Cox CS, Lally KP. Sequelae of recurrent laryngeal nerve injury after patent ductus arteriosus ligation. Int J Pediatr Otorhinolaryngol. 2006;70:1609–12. [DOI] [PubMed] [Google Scholar]
- 28.Nichols BG, Jabbour J, Hehir DA, Ghanayem NS, Beste D, Martin T, et al. Recovery of vocal fold immobility following isolated patent ductus arteriosus ligation. Int J Pediatr Otorhinolaryngol [Internet]. 2014;78:1316–9. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Citation&list_uids=24882459 [DOI] [PubMed] [Google Scholar]
- 29.DeLozier KR, Gould FDH, Ohlemacher J, Thexton AJ, German RZ. The impact of recurrent laryngeal nerve lesion on oropharyngeal muscle activity and sensorimotor integration in an infant pig model. J Appl Physiol. 2018/04/13. 2018;125:159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gould FDH, Ohlemacher J, Lammers AR, Gross A, Ballester A, Fraley L, et al. Central nervous system integration of sensorimotor signals in oral and pharyngeal structures: oropharyngeal kinematics response to recurrent laryngeal nerve lesion. J Appl Physiol [Internet]. 2016;120:495–502. Available from: http://jap.physiology.org/lookup/doi/10.1152/japplphysiol.00946.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gould FDH, Lammers AR, Mayerl CJ, German RZ. Specific vagus nerve lesion have distinctive physiologic mechanisms of dysphagia. Fontiers Neurol. 2019;10:1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lau C, Alagugurusamy R, Schanler RJ, Smith EO, Shulman RJ. Characterization of the developmental stages of sucking in preterm infants during bottle feeding. Acta Paediatr [Internet]. 2000;89:846–52. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Citation&list_uids=10943969 [PubMed] [Google Scholar]
- 33.Ballester A, Gould FDH, Bond L, Stricklen B, Ohlemacher J, Gross A, et al. Maturation of the coordination between respiration and deglutition with and without recurrent laryngeal nerve lesion in an animal model Dysphagia [Internet]. Springer US; 2018;33:627–35. Available from: 10.1007/s00455-018-9881-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mayerl CJ, Myrla AM, Bond LE, Stricklen BM, German RZ, Gould FDH. Premature birth impacts bolus size and shape through nursing in infant pigs. Pediatr Res. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jadcherla S Dysphagia in the high-risk infant: Potential factors and mechanisms. Am J Clin Nutr. 2016;103:622S–628S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henderson M, Miles A, Holgate V, Peryman S, Allen J. Application and Verification of Quantitative Objective Videofluoroscopic Swallowing Measures in a Pediatric Population with Dysphagia. J Pediatr [Internet]. Elsevier Inc.; 2016;178:200–205.e1. Available from: 10.1016/j.jpeds.2016.07.050 [DOI] [PubMed] [Google Scholar]
- 37.Ongkasuwan J, Chiou EH. Pediatric Dysphagia: Challenges and Controversies [Internet]. Ongkasuwan J, Chiou EH, editors. Cham, Switzerland: Springer; 2018. Available from: https://link.springer.com/content/pdf/10.1007%2F978-3-319-97025-7.pdf [Google Scholar]
- 38.Gleeson K, Eggli DF, Maxwell SL. Quantitative aspiration during sleep in normal subjects. Chest [Internet]. The American College of Chest Physicians; 1997;111:1266–72. Available from: 10.1378/chest.111.5.1266 [DOI] [PubMed] [Google Scholar]
- 39.Catchpole E, Bond L, German R, Mayerl C, Stricklen B, Gould FDH. Reduced Coordination of Hyolaryngeal Elevation and Bolus Movement in a Pig Model of Preterm Infant Swallowing. Dysphagia [Internet]. Springer US; 2019; Available from: 10.1007/s00455-019-10033-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.German RZ, Crompton AW, Gould FDH, Thexton AJ. Animal models for dysphagia studies: what have we learnt so far. Dysphagia [Internet]. 2017;32:73–7. Available from: https://www.ncbi.nlm.nih.gov/pubmed/28132098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eiby YA, Wright LL, Kalanjati VP, Miller SM, Bjorkman ST, Keates HL, et al. A pig model of the preterm neonate: anthropometric and physiological characteristics. PLoS One 2013/07/23. 2013;8:e68763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.German RZ, Crompton AW, Thexton AJ. The coordination and interaction between respiration and deglutition in young pigs. J Comp Physiol. 1998;182:539–47. [DOI] [PubMed] [Google Scholar]
- 43.German RZ, Crompton AW, Thexton AJ. Integration of the reflex pharyngeal swallow into rhythmic oral activity in a neurologically intact pig model. J Neurophysiol. 2009/06/12. 2009;102:1017–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thexton AJ, Crompton AW, German RZ. Transition from suckling to drinking at weaning: a kinematic and electromyographic study in miniature pigs. J Exp Zool. 1998;280:327–43. [DOI] [PubMed] [Google Scholar]
- 45.Gierbolini-Norat EM, Holman SD, Ding P, Bakshi S, German RZ. Variation in the Timing and Frequency of Sucking and Swallowing over an Entire Feeding Session in the Infant Pig Sus scrofa. Dysphagia [Internet]. Springer US; 2014;29:1–8. Available from: 10.1007/s00455-014-9532-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holman SD, Campbell-Malone R, Ding P, Gierbolini-Norat EM, Griffioen AM, Inokuchi H, et al. Development, reliability, and validation of an infant mammalian penetration-aspiration scale. Dysphagia. 2013;28:178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11:93–8. [DOI] [PubMed] [Google Scholar]
- 48.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods [Internet]. 2012;9:671–5. Available from: http://www.nature.com/doifinder/10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ding P, Fung GS, Lin M, Holman SD, German RZ. The effect of bilateral superior laryngeal nerve lesion on swallowing: a novel method to quantitate aspirated volume and pharyngeal threshold in videofluoroscopy. Dysphagia [Internet]. 2015;30:47–56. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Citation&list_uids=25270532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw [Internet]. 2015;67:1–48. Available from: http://arxiv.org/abs/1406.5823 [Google Scholar]
- 51.Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach (2nd ed) [Internet]. Ecol. Modell. 2002. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0304380003004526 [Google Scholar]
- 52.Lau C, Smith EO, Schanler RJ. Coordination of suck-swallow and swallow respiration in preterm infants. Acta Paediatr [Internet]. 2003;92:721–7. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Citation&list_uids=12856985 [PubMed] [Google Scholar]
- 53.Gewolb IH, Vice FL, Schweitzer-Kenney EL, Taciak VL, Bosma JF. Developmental patterns of rhythmic suck and swallow in preterm infants. Dev Med Child Neurol. 2001;43:22–7. [DOI] [PubMed] [Google Scholar]
- 54.Prasse JE, Kikano GE. An overview of pediatric dysphagia. Clin Pediatr (Phila). 2009;48:247–51. [DOI] [PubMed] [Google Scholar]
- 55.Gross A, Ohlemacher J, German RZ, Gould FDH. LVC Timing in Infant Pig Swallowing and the Effect of Safe Swallowing. Dysphagia. 2017/08/07. 2018;33:51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gewolb IH, Vice FL. Maturational changes in the rhythms, patterning, and coordination of respiration and swallow during feeding in preterm and term infants. Dev Med Child Neurol [Internet]. 2006;48:589–94. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Citation&list_uids=16780629 [DOI] [PubMed] [Google Scholar]
- 57.Steele CM, Miller AJ. Sensory input pathways and mechanisms in swallowing: a review. Dysphagia [Internet]. 2010;25:323–33. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Citation&list_uids=20814803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McGrattan KE, McFarland DHJC, Hill E, White DR, Martin-Harris B. Effect of single-use, laser-cut, slow-flow nipples on respiration and milk ingestion in preterm infants. Am J Speech-Language Pathol. 2017;26:832–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.