Abstract
OBJECTIVES
The aim of this study was to test the hypothesis that transcatheter electrosurgery might allow intentional detachment of previously placed MitraClip(s) from the anterior leaflet to recreate a single mitral orifice for transcatheter mitral valve implantation (TMVI), leaving the retained MitraClip(s) securely fastened to the posterior leaflet and without interfering with the mitral bioprosthesis.
BACKGROUND
Patients with severe mitral regurgitation or stenosis despite edge-to-edge mitral repair with the MitraClip typically have few therapeutic options because the resultant double orifice precludes TMVI. Transcatheter electrosurgery may allow detachment of failed MitraClip(s) from the anterior leaflet to recreate a single orifice for TMVI.
METHODS
This was a single-center, 5-patient, consecutive, retrospective observational cohort. Patients underwent transcatheter electrosurgical laceration and stabilization of failed MitraClip(s) to recreate a single orifice, leaving the MitraClip(s) securely fastened to the posterior leaflet. Subsequently, patients underwent TMVI with an investigational device, the Tendyne mitral bioprosthesis, on a compassionate basis. Patients were followed up to 30 days.
RESULTS
MitraClip detachment from the anterior leaflet and Tendyne implantation were successful in all patients. All patients survived to discharge. All patients were discharged with grade 0 central mitral regurgitation. Two patients had moderate perivalvular mitral regurgitation that did not require reintervention. During the follow-up period of 30 days, there were no deaths, cases of valve dysfunction, or reintervention. There was no evidence of erosion or bioprosthetic valve dysfunction attributable to the retained MitraClip(s) still attached to the posterior leaflet.
CONCLUSIONS
Transcatheter electrosurgical detachment of failed MitraClips from the anterior leaflet followed by TMVI is technically feasible and safe at 30 days. Longer term study is needed to determine the clinical benefit of this approach and new algorithms for TMVI sizing following electrosurgical laceration and stabilization of a failed MitraClip to avoid perivalvular leak.
Keywords: MitraClip, mitral regurgitation, transcatheter electrosurgery, transcatheter mitral valve implantation
Residual mitral regurgitation (MR) following MitraClip (Abbott Vascular, Chicago, Illinois) implantation poses a clinical challenge and has limited transcatheter and surgical options. The reported mortality of patients undergoing surgery for residual or recurrent regurgitation after MitraClip remains prohibitively high, up to 47% at 1 year (1). The presence of an elevated mitral valve gradient often contraindicates additional MitraClip(s) (2–7) or other transcatheter annular reduction strategies (8,9). Conventional transcatheter mitral valve implantation (TMVI) cannot be performed, because the MitraClip creates a double-orifice mitral valve. There are currently no commercial catheter devices available to remove MitraClips to recreate a single orifice and allow TMVI.
LAMPOON-related techniques (10–12) have been successfully modified to facilitate laceration of a previously placed Alfieri stich prior to transseptal TMVI-in-ring (13). We hypothesized that transcatheter electrosurgery might similarly allow intentional detachment of previously placed MitraClip(s) from the anterior leaflet to recreate a single mitral orifice for TMVI, leaving the retained MitraClip(s) securely fastened to the posterior leaflet and without interfering with the mitral bioprosthesis. The U.S. Food and Drug Administration (FDA) granted a supplement to the Tendyne investigational device exemption protocol (G140240/S050) to facilitate a “clip management strategy” of electrosurgical laceration and stabilization of MitraClip (ELASTA-Clip), followed by TMVI with the investigational Tendyne bioprosthesis. We describe the procedural steps and clinical findings of the first 5 patients undergoing this technique at our institution.
METHODS
PATIENTS.
Patients were treated at a single center (Emory Structural Heart and Valve Center, Atlanta, Georgia). Each patient was evaluated by a multidisciplinary heart team composed of interventional cardiologists, cardiothoracic surgeons with expertise in mitral valve replacement, and multimodality imaging specialists. All patients underwent routine transthoracic echocardiography, transesophageal echocardiography, and time-resolved contrast-enhanced cardiac computed tomography (CT).
TENDYNE TMVI DEVICE.
The Tendyne TMVI device is an investigational transapical implant under investigation in the United States through an investigational device exemption license (NCT03433274). It is a porcine pericardial valve that consists of 2 self-expanding Nitinol frames. The outer frame is contoured to fit the native mitral annulus and does not rely on native mitral leaflets for anchoring. When appropriately sealed, the outer skirt should prevent perivalvular leak (PVL). The valve is anchored using a braided polyethylene tether attached to an epicardial pad placed via thoracotomy. Varying amounts of tension can be applied to the tether to optimize final valve positioning. The valve is deployed transapically, without the need for full cardiopulmonary bypass or rapid ventricular pacing. Pre-procedural cardiac CT is used to select valve size, left lateral thoracotomy site, and apical access site
PATIENT SELECTION.
All patients had symptoms attributed primarily to mitral valve regurgitation. All were deemed at prohibitive risk for operative mitral valve repair or replacement, ineligible for additional MitraClip implantation, and candidates for transcatheter mitral implantation, by a multidisciplinary heart team.
The main additional selection criteria for this study were as follows: 1) prior placement of at least 1 MitraClip; 2) recurrent or residual MR of greater than moderate severity; 3) New York Heart Association functional class III or IV attributable to MR; 4) ineligibility for placement of another MitraClip; 5) ineligibility for surgical mitral valve repair or replacement; and 6) eligibility for placement of a Tendyne prosthesis. Abbott Vascular reviewed all cases for anatomic feasibility, and manufacturer clinical field specialists were present at each case to provide clinical application support for the Tendyne implantation. Electrocardiographically gated multi-phase CT was used to measure mitral annular dimensions and to determine valve size and predict the risk for left ventricular outflow tract obstruction.
With vendor concurrence, and on a case-by-case basis, we petitioned the FDA for off-label use of the Tendyne bioprosthesis on a compassionate-use basis under 21 CFR §812.35(a). Each petition included review by an uninvolved physician and a description of patient protection measures. Patients were not excluded for severe left ventricular dysfunction, multivalve disease, right ventricular dysfunction, or pulmonary hypertension. All patients consented in writing. The Emory University Institutional Review Board approved the compassionate use and this communication.
ELECTROSURGICAL LACERATION.
The ELASTA-Clip procedure was designed by the authors. A large-bore inflatable-hub introducer sheath (DrySeal Flex, 26 F, W.L. Gore & Associates, Flagstaff, Arizona) is placed in the right femoral vein. This sheath design allows multiple parallel guiding sheaths through a single access site. The transseptal sheath is used to position two 0.018-inch guidewires (V-18 control wire, Boston Scientific, Marlborough, Massachusetts), which are used to exchange and reintroduce 2 transseptal deflectable guiding sheaths (Nagare, Terumo Interventional Systems, Somerset, New Jersey) into the left atrium through the single transseptal puncture.
With dual transseptal deflectable guiding sheaths in place, the anterior mitral leaflet is next straddled by an ELASTA-Clip laceration system. This is accomplished by crossing each orifice of the double-orifice mitral valve stepwise using balloon-wedge end-hole catheters to avoid chordal entrapment. These are each exchanged using V-18 guidewires for 6-F guiding catheters, IM in the lateral and JR4 in the medial orifice. A multiloop snare (Atrieve 18/30, Argon Medical, Frisco, Texas) is positioned through the JR4 in the left ventricle, and it is used to capture a 0.014-inch guidewire (Astato XS 20, Asahi-Intecc, Tokyo, Japan), pre-loaded with a locking hub-less insulating microcatheter, (Piggyback, Wire Convertor, Teleflex, Wayne, Pennsylvania) that is directed via the IM guiding catheter (Figure 1). In contrast to LAMPOON, the leaflet is not traversed by the 0.014-inch guide-wire. The ensnared guidewire is externalized, and a small segment is focally denuded and kinked to direct electrosurgical current along its inner curvature, as described previously, creating a “flying V” configu-ration (11,14). The “flying V” is reintroduced and positioned adjacent to the previously placed MitraClip(s) on the anterior mitral leaflet. This allows a focal electric contact of the lacerating surface against the anterior mitral leaflet tissue adjacent to the MitraClip(s).
FIGURE 1. Key Steps in the Electrosurgical Laceration and Stabilization of MitraClip Procedure.
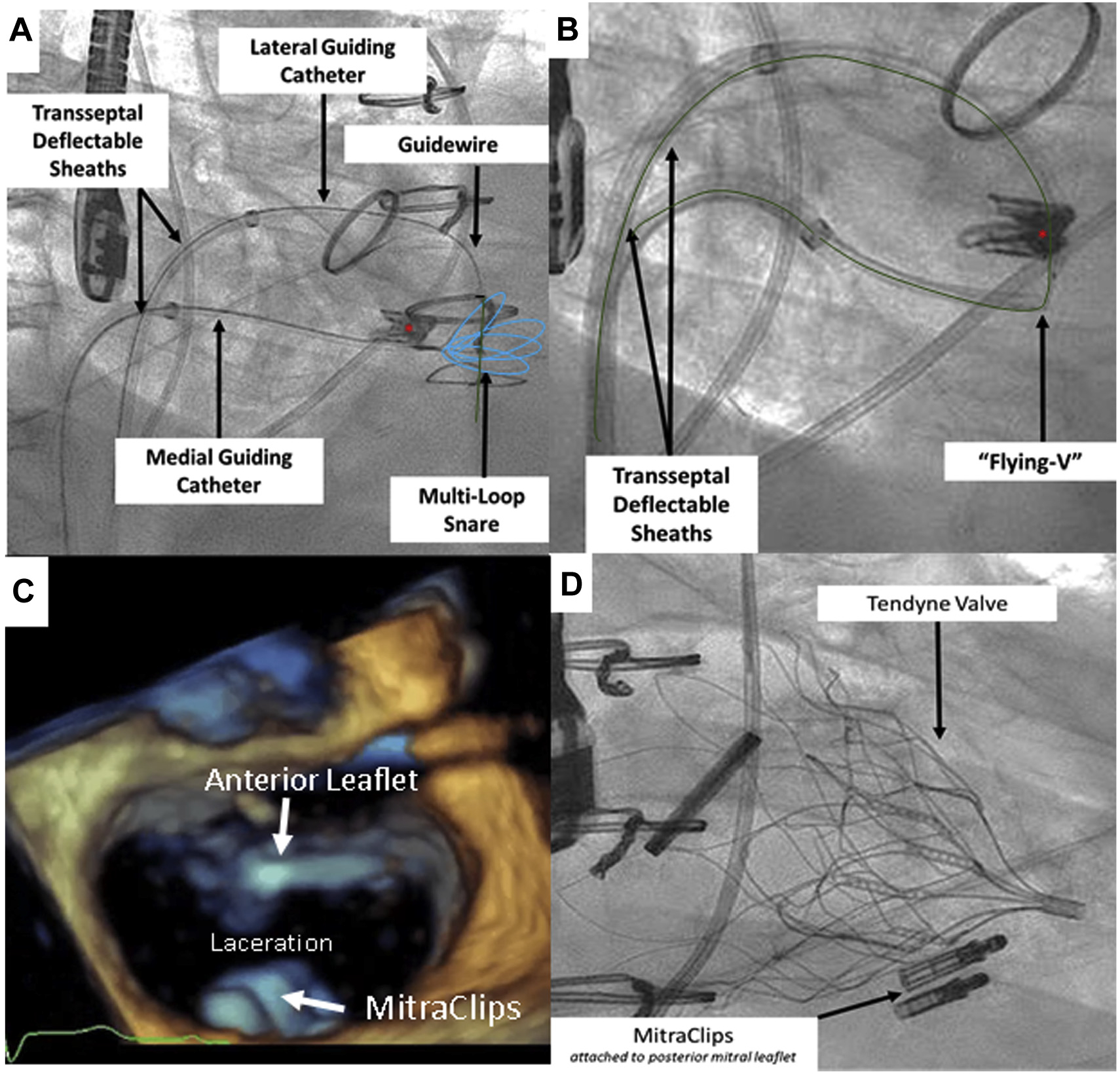
(A) After cannulation of the “double-orifice” mitral valve, an 0.014-inch guidewire (green) is advanced into a prepositioned snare (blue). Asterisk denotes MitraClip. (B) The “flying V” is created and positioned on the anterior mitral leaflet. (C) Following laceration, the singleorifice mitral valve is created. Note that the MitraClips remain attached to the posterior mitral leaflet. (D) The Tendyne valve is deployed while MitraClips remain attached to the posterior mitral leaflet.
Anterior leaflet laceration around the MitraClip does not retain a normal coapting surface. This theoretically increases the risk for hemodynamic compromise due to MR. To mitigate this risk, we attempt to minimize the time between laceration and TMVI as follows. Before laceration, the apex is punctured with an 18-gauge needle, and a transapical wire-and-balloon combination is positioned in a pulmonary vein to facilitate rapid progression to deployment of the TMVI. In addition, an intra-aortic balloon is electively placed at the start of each procedure, and counterpulsation is initiated before laceration to help hemodynamic stability.
The flying V is positioned immediately adjacent to the MitraClip(s) (anterior and basal) under fluoroscopic and echocardiographic guidance (Figure 2). Laceration of the anterior leaflet is achieved in 2 simultaneous steps. Blood is displaced by flushing the guiding catheters with 5% dextrose in water to eliminate alternative current paths except where the flying V contacts the leaflet. Continuous-duty “cutting” electrosurgery is applied at 70 W during traction of the free ends of the lacerating guidewire toward the fulcrum established in the left atrium by the paired deflectable guiding sheaths. Laceration is expected to require 15 to 30 s during light traction and frees the MitraClip(s) from the anterior mitral leaflet while the clip remains attached to the posterior mitral leaflet. Following laceration, the transseptal lacerating catheters and guidewires are removed from the body. Tendyne implantation is performed in the standard fashion. The retained MitraClip(s) are retained posteriorly against the left ventricular myocardium by the TMVI device (Video 1).
FIGURE 2. The ELASTA-Clip Lacerating System.
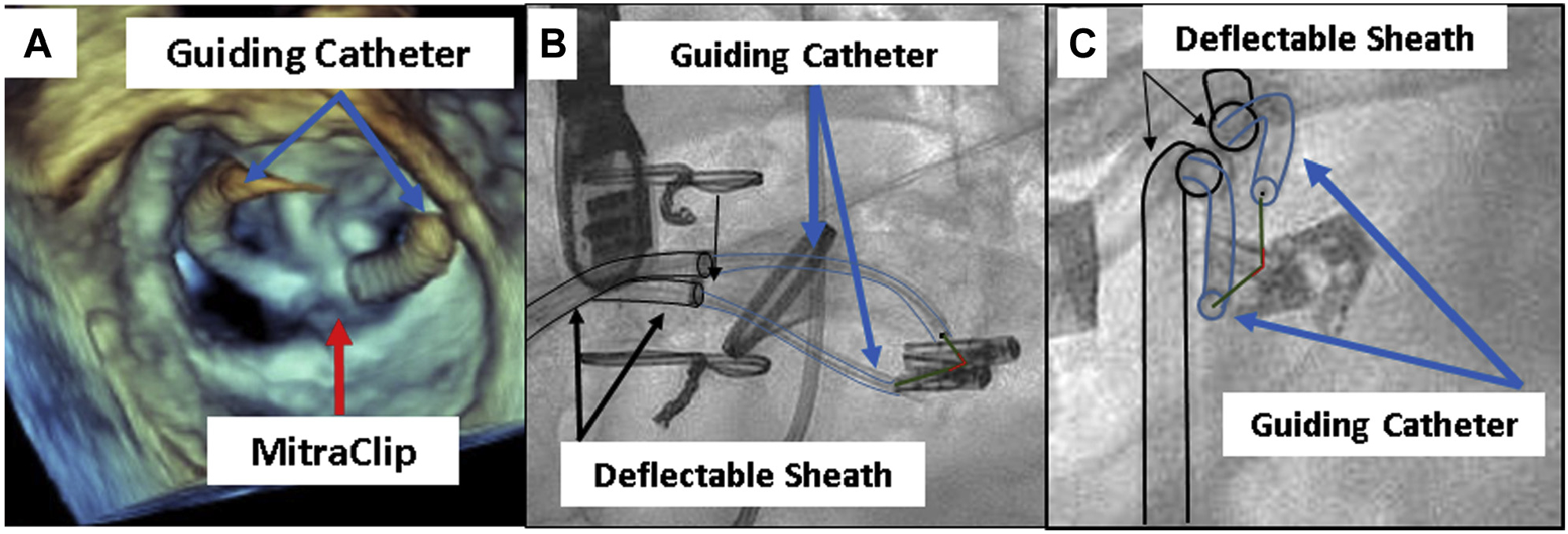
(A) Three-dimensional transesophageal echocardiogram of the electrosurgical laceration and stabilization of MitraClip (ELASTA-Clip) lacerating system in the surgeon’s view. (B,C) Orthogonal projections of the ELASTA-Clip lacerating system. Note in (C) (left anterior oblique caudal projection) that the lacerating system is on the anterior mitral leaflet.
Video 1:
This video details key steps in the Elasta-Clip procedure.
FOLLOW-UP.
Clinical and echocardiographic follow-up was performed at 30 days. Clinical, procedural, echocardiographic, and angiographic details were abstracted from the medical records of each patient’s index procedure and 30-day follow-up visit. Device and procedural criteria were defined using the Mitral Valve Academic Research Consortium (15) criteria and Bleeding Academic Research Consortium criteria (16).
DATA ANALYSIS.
Data are expressed as median (interquartile range [IQR]).
RESULTS
PATIENTS.
The patients were predominantly women (3 of 5), were severely symptomatic (100% in New York Heart Association functional class ≥III), had a median age of 79 years (IQR: 69.5 to 88 years), and had few comorbidities (Table 1). The median ejection fraction was 55% (IQR: 45% to 55%) in the setting of severe MR. The median baseline mitral gradient prior to TMVI was 4.5 ± 1.0 mm Hg (Table 1). The median time from initial MitraClip placement to ELASTA-Clip was 2 years (IQR: 1.3 to 3.5 years). Table 1 shows the indications for MitraClip implantation. MitraClips all had been placed in a central position, and there were no single-leaflet device attachments prior to electrosurgical laceration. Baseline computed tomography parameters are detailed in Table 2.
TABLE 1.
Baseline Patient Characteristics (N = 5)
| Age (yrs) | 79 (69.5–88) |
| Female | 3 (60) |
| BSA (m2) | 1.8 (1.6–2.1) |
| Comorbidities | |
| Severe COPD | 0 |
| Diabetes | 1 (20) |
| Coronary artery disease | 1 (20) |
| Prior coronary artery bypass grafting | 0 |
| Prior aortic valve replacement | 2 (40) |
| NYHA functional class III or IV | 5 (100) |
| ≥2 cardiac surgical procedures | 2 (40) |
| STS Predicted Risk of Mortality | 7.0 (4.0–8.4) |
| MitraClip setting | |
| Primary MR | 2 |
| Secondary MR | 1 |
| Mixed MR | 1 |
| Unknown | 1 |
| Time from MitraClip placement (yrs) | 2 (1.3–3.5) |
| Baseline hemoglobin (mg/dl) | 12.8 (10.95–13.6) |
| Glomerular filtration rate (ml/min/1.73 m2) | 54 (31–67.5) |
| Medications | |
| ACE inhibitor, ARB, or vasodilator | 3 (60) |
| Beta-receptor antagonist | 3 (60) |
| Aspirin or antiplatelet agent | 4 (80) |
| Transthoracic echocardiographic parameters | |
| Left ventricular ejection fraction % | 55 (45–55) |
| Baseline mean mitral valve gradient (mm Hg) | 4.5 (4.0–5.0) |
| Grade III or IV MR severity | 5 (100) |
| Right ventricular dysfunction | |
| Normal | 3 (60) |
| Mild | 1 (20) |
| Moderate | 1 (20) |
| Severe | 0 |
| Severe tricuspid regurgitation | 1 (20) |
Values are median (interquartile range), n (%), or n.
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; BSA = body surface area; COPD = chronic obstructive pulmonary disease; MR = mitral regurgitation; NYHA = New York Heart Association; STS = Society of Thoracic Surgeons.
TABLE 2.
Case Planning
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| Reason for surgical ineligibility | Frailty | Frailty | Pulmonary fibrosis | Third redo sternotomy | 86 years of age |
| Septal-lateral diameter (mm) | 33.4 | 36.9 | 29.3 | 27.9 | 31.9 |
| Intercommissural diameter (mm) | 41.5 | 47.7 | 42.3 | 43.2 | 45.4 |
| Perimeter (mm) | 126.4 | 141.3 | 122.9 | 116.7 | 128.9 |
| Area (cm2) | 11.5 | 13 | 10.5 | 9.7 | 12.1 |
| Implanted valve size | 35M LP | 37L SP | 33M LP | 33M LP | 37M LP |
| Septal-lateral percentage oversizing | 3.3 | −1.1 | 10.8 | 12.1 | 14.4 |
| Intercommissural percentage oversizing | 16.9 | 10.1 | 10.0 | 8.1 | 9.0 |
| Perimeter percentage oversizing | 13.9 | 10.4 | 17.1 | 13.3 | 16.4 |
| Predicted neo-LVOT (mm2) | 571 | 294 | 306 | 296 | 341 |
| End-systolic aortomitral angle (deg) | 32.2 | NA | NA | 52.1 | 54.2 |
| End-diastolic aortomitral angle (deg) | 33.7 | NA | NA | 53 | 49.3 |
Values were derived using contrast-enhanced gated cardiac computed tomography for the 5 patients undergoing electrosurgical laceration and stabilization of MitraClip.
LVOT = left ventricular outflow tract; NA = not applicable.
PROCEDURAL AND 30-DAY OUTCOMES.
Procedural characteristics are shown in Table 3. Electrosurgical laceration followed by TMVI with Tendyne bioprosthesis was technically successful in all patients. The median interval between laceration and TMVI was 20 min (IQR: 15 to 24 min). At the conclusion of the procedure, all 5 patients (100%) had grade 0 central MR. Two patients had mild to moderate perivalvular MR that was not believed to warrant intervention. There were no embolizations of the prior MitraClips.
TABLE 3.
Procedural and In-Hospital Outcomes (N = 5)
| Successful electrosurgical laceration | 5 (100) |
| Number of clips lacerated (per patient) | 2 |
| Successful deployment and correct positioning of Tendyne valve | 5 (100) |
| Need for valve retrieval | 0 |
| Procedure time (min) | 205 (198.5–237.5) |
| Fluoroscopy duration (min) | 59.5 (54.6–66.4) |
| Planned intra-aortic balloon pump | 5 (100) |
| Hospital length of stay (days) | 13 (11–37) |
| Procedural complications | |
| Death | 0 |
| Cardiac arrest | 0 |
| Tamponade | 0 |
| Cardiac perforation | 0 |
| LVOT obstruction | 0 |
| Required conversion to open surgery | 0 |
| Significant hemodynamic compromise | 1 (20) |
| Laceration of nontarget structure | 0 |
| Valve embolization | 0 |
| Stroke | 0 |
| Major vascular complication | 0 |
| Life-threatening bleed | 2 (40) |
| Hemothorax | 2 (40) |
| Acute renal failure | 1 (20) |
| Perivalvular leak greater than mild | 2 (40) |
| Discharge echocardiographic outcomes | |
| Left ventricular ejection fraction (%) | 55 (50.0–57.5) |
| Mean mitral gradient (mm Hg) | 5.0 (4.3–5.5) |
| Central MR grade 0 | 5 (100) |
| Perivalvular leak greater than mild | 2 (40) |
| Survival | |
| Survived immediate procedure | 5 (100) |
| Survived to discharge | 5 (100) |
All patients survived the immediate procedure without left ventricular outflow tract obstruction, cardiopulmonary arrest, or need to escalate mechanical circulatory support. There was no laceration or injury of a nontarget structure. All MitraClip(s) remained attached to the posterior leaflet and were immobilized posteriorly between the Tendyne TMVI device and the posterior wall of the left ventricle. The Tendyne valve was successfully deployed in all cases, without need to recapture the valve. There was no evidence that the displaced MitraClips interacted with or interfered with the Tendyne valve leaflets. The median procedure time was 205 min (IQR: 198.5 to 237.5) min. One patient developed hemodynamic instability that was attributed to embolization of air to the right coronary artery. This resolved with conservative management. Two patients experienced clinically significant bleeding at the apical access site requiring return to the operating room for reexplo-ration and evacuation of a hemothorax. One patient returned to the operating room on the same day as the ELASTA-Clip procedure, and the second patient underwent surgery on post-TMVI day 8. In both cases, bleeding was attributed to “oozing,” despite what had been 7considered satisfactory hemostatic closure of apical access in patients also treated with antithrombin agents afterward. Both of these patients survived to discharge.
We believe that the patients included in this initial compassionate series were more frail and ill than others enrolled in reported feasibility studies of the Tendyne valve (17). Despite technically successful procedures in all, the overall post-TMVI course was complex and protracted. The first patient experienced post-operative tachyarrhythmia causing cardiogenic shock, requiring inotropic support days post-procedure. The patient ultimately underwent atrioventricular node ablation and biventricular pacing. The fourth patient, who underwent the procedure after a 3-week admission for decompensated heart failure, experienced post-operative volume overload, renal failure, and surgical evacuation of a hemothorax.
At the time of discharge, 2 patients had PVLs that were graded mild to moderate in severity and not believed to be clinically significant. The median ejection fraction was 55% (IQR: 50% to 58%), and the median mitral gradient at discharge was 5.0 mm Hg (IQR: 4.3 to 5.5 mm Hg). Of the 5 patients undergoing ELASTA-Clip, 4 were discharged home and 1 was discharged to a subacute rehabilitation facility.
THIRTY-DAY OUTCOMES.
All patients we alive at 30-day follow-up. Two had moderate perivalvular MR at 30-day follow-up not requiring intervention. One reported recurrent “dizziness” and underwent magnetic resonance imaging that showed punctate acute cerebral infarctions that were likely embolic in origin but did not correspond anatomically with the symptoms according to consulting neurologists. It remains unclear whether this event was related to the ELASTA-Clip or TMVI procedures (Central Illustration).
CENTRAL ILLUSTRATION.
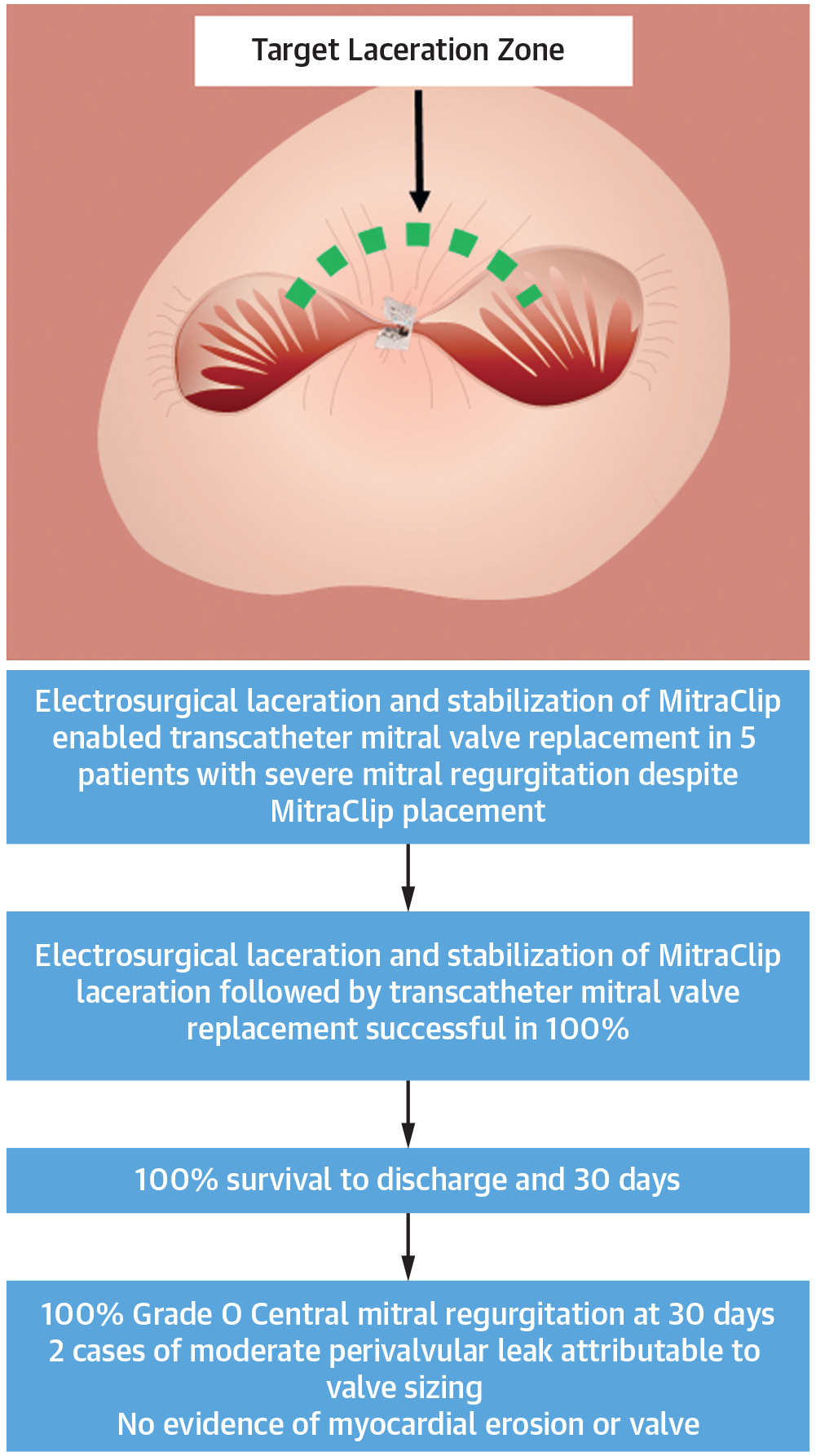
The Electrosurgical Laceration and Stabilization of MitraClip Procedure Successfully and Reproducibly Allows Transcatheter Mitral Valve Implantation in Patients With Previous MitraClip(s)
At follow-up, 4 of 5 patients were ambulatory and living independently. One other patient remained in a skilled rehabilitation facility. That patient fell while on therapeutic anticoagulation, developed a hemothorax, and underwent a therapeutic thoracentesis. There was no evidence of valve thrombosis, dysfunction, hemolysis, erosion, or embolization of the posteriorly displaced MitraClip(s) or need for reintervention (Table 4).
TABLE 4.
Thirty-Day Clinical Follow-Up (N = 5)
| Clinical outcomes | |
| Any mortality | 0 |
| Cardiovascular mortality | 0 |
| Stroke or TIA | 1 (20) |
| Reintervention for MV dysfunction | 0 |
| BARC 2, 3, or 5 bleeding | 1 (20) |
| Bioprosthetic valve dysfunction | |
| Embolization | 0 |
| Thrombosis | 0 |
| Erosion, migration | 0 |
| Echocardiographic outcomes | (n = 5)* |
| Left ventricular ejection fraction (%) | 55 |
| Mean mitral gradient (mm Hg) | 5.3 (4.5–6.5) |
| Central MR grade 0 | 5 |
| PVL (moderate) | 2 |
Values are n, n (%), or median (range).
One patient’s 30-day echocardiographic study was completed to discharge.
BARC = Bleeding Academic Research Consortium; MR = mitral regurgitation; MV = mitral valve; PVL = paravalvular leak; TIA = transient ischemic attack.
DISCUSSION
In the present study, we investigated the feasibility of electrosurgical detachment of MitraClips from the anterior mitral leaflet to recreate a single mitral orifice for TMVI with the Tendyne bioprosthesis. The key findings from this study are as follows: 1) electrosurgical MitraClip detachment from the anterior leaflet followed by TMVI is technically straightforward and feasible, with 100% technical success, 100% procedural survival, 100% survival to discharge, and 100% survival to 30 days; 2) TMVI was associated with significant morbidity in this cohort ineligible for conventional mitral surgery; 3) at 30 days after ELASTA-Clip, the detached MitraClip(s) remained securely fastened to the posterior mitral leaflet; 4) there was no evidence of myocardial erosion or Tendyne dysfunction related to the retained MitraClip(s); and 5) PVLs in our first 2 patients were likely related to TMVI geometry.
Our findings have significant implications for the lifetime management of patients with MR undergoing MitraClip implantation. Patients with residual MR (>2+) following MitraClip placement have a significantly higher rate of mortality at 1 year compared with those with <2+ MR (18) and to date have had limited treatment options. Our work expands on a previous report (19).
With more than 100,000 MitraClips placed to date and reported procedural failure to reduce MR to <2+ rates near 5% (20,21), there is potential for a large patient population eligible for follow-on therapy. This is especially relevant in Europe, as the MitraClip received Conformité Européenne Mark approval before receiving FDA approval in the United States (2008 vs. 2013), and the Tendyne valve recently received Conformité Européenne Mark approval (January 2020). Current treatment options in the United States include placement of an additional MitraClip or surgical excision of a MitraClip followed by surgical mitral valve replacement. Often these therapies are unsuitable because of high transmitral gradients precluding additional MitraClips or because of high or prohibitive operative risk. Herein, we demonstrate the feasibility of electrosurgical MitraClip detachment from the anterior leaflet to recreate a single mitral orifice for TMVI.
An important finding of this study was the overall acute hemodynamic stability of patients during the interval between electrosurgical leaflet laceration and Tendyne valve implantation, similar to other transcatheter electrosurgical valve laceration procedures. There was no escalation of mechanical circulatory support to extracorporeal membrane oxygenation, percutaneous ventricular assist device, cardiopulmonary bypass, or conversion to open surgery. This is likely multifactorial. Immediately following laceration, patients do not have a significant increase in the degree of MR but rather a change from predominantly lateral or medial MR to central MR prior to TMVI. Additionally, the elective use of intra-aortic balloon counterpulsation prior to MitraClip laceration in all cases likely improved patient stability. Despite anterior leaflet laceration, we believe that residual anterior mitral leaflet structures protect against intolerable exacerbation of MR during a limited procedural interval (median 20 min) and allow methodical TMVI. Of note, there was no damage to adjacent structures during laceration.
In this study we demonstrate reliable restoration of a central mitral orifice after MitraClip(s) to allow TMVI and relief of MR without any procedural mortality. Compared with other electrosurgical techniques (retrograde LAMPOON, antegrade LAMPOON, and BASILICA) (11,22) this technique is relatively easier to perform. The procedure is performed using familiar angiographic viewing planes and requires only leaflet laceration but not traversal. Procedural success is also attributable to the design of the Tendyne bioprosthesis, which seals on the native mitral valve annulus and is secured by an apical tether. To date, there are no other available transcatheter mitral valves with similar designs, and whether sufficient leaflet tissue remains to guarantee TMVI anchoring will need to be tested with different prostheses.
Appropriate sizing of the Tendyne prosthesis is critical to ameliorate MR. Prior work has demonstrated an immediate decrease in the size of the mitral annulus following MitraClip implantation (23). Conversely, after electrosurgical laceration of the MitraClip, the mitral annulus immediately increases in diameter. This likely explains the 2 cases of moderate PVL in our series, in contrast to other reports of TMVI using the Tendyne device (17). After this observation was made in the first 2 cases, more oversized valves (septal-lateral) were selected for implantation. Following this change, there was no significant PVL. The long-term implications of moderate PVL following ELASTA-Clip are unknown but may lead to suboptimal clinical outcomes. Annular sizes outside of the currently available Tendyne range remain a limitation at the present time. In this study, there were no cases of left ventricular outflow tract obstruction. This is likely attributable to careful CT-based case selection prior to TMVI.
The long-term implications of a retained MitraClip remain unknown. At 30 days, there did not appear to be any evidence of valve dysfunction attributable to the retained MitraClip. Importantly, in the 2 patients with moderate PVLs, the regurgitant jet was not near to or associated with the retained MitraClip. Although complete MitraClip extraction is theoretically appealing, absent safe and effective off-pump devices, we believe that the risks outweigh potential benefits.
Transcatheter annuloplasty strategies may be a less invasive alternative to ELASTA-Clip with less associated morbidity. All devices remain investigational at this time and may not be anatomically suitable or indicated for patients with residual MR following MitraClip placement. The Cardioband (Edwards Lifesciences, Irvine, California) has been successfully used for this purpose (8). Limitations of this strategy include suboptimal imaging from artifact, immobility of the posterior mitral leaflet following MitraClip implantation, and a requirement for a minimal myocardial thickness. The AccuCinch device (Ancora Heart, Santa Clara, California) is also being implanted in patients with failed MitraClips. Similar to the Cardioband, the device is limited by the need for adequate myocardial thickness and the added difficulty of placing ventricular anchors through a double-orifice mitral valve. The Cerclage mitral annuloplasty system (Transmural Systems, Andover, Massachusetts) is implanted via the coronary sinus through the interventricular septum and allows circumferential compression of the mitral annulus and is undergoing clinical evaluation (NCT03929913). Limitations of the current Cerclage device are the inability to place the device in patients with pacing leads in the coronary sinus.
Our ELASTA-Clip technique offers a new therapeutic option for patients with recurrent MR and stenosis after MitraClip implantation. Electrosurgical laceration is performed using commercially available devices in an “off-label” fashion. We have now demonstrated a viable treatment strategy for patients for whom there were previously no options. Dedicated devices may reduce procedural complexity and duration in the future.
STUDY LIMITATIONS.
This study reports the experience of electrosurgical laceration of failing MitraClips followed by TMVI in a small series of patients at a single center. All patients received therapy on a compassionate-use basis with FDA off-label use, limiting the generalizability of these results. There was no control group in this compassionate-use study or comparator group of patients who underwent surgical mitral valve replacement.
CONCLUSIONS
Electrosurgical detachment of MitraClip(s) from the anterior leaflet to recreate a single mitral orifice for TMVI is feasible for patients with recurrent MR after edge-to-edge mitral repair. Further evaluation of this approach is warranted in a larger cohort and with different transcatheter mitral valve replacement devices. Dedicated electrosurgery devices may simplify and democratize this procedure.
PERSPECTIVES.
WHAT IS KNOWN?
Patients with severe MR following MitraClip implantation have increased mortality, yet the MitraClip-induced double orifice precludes TMVI.
WHAT IS NEW?
Electrosurgical laceration of a MitraClip allows restoration of a single mitral orifice and TMVI using the Tendyne bioprosthesis.
WHAT IS NEXT?
Larger studies with longer follow-up are needed to define the role of the ELASTA-Clip procedure and the significance of the displaced but retained MitraClip device.
ACKNOWLEDGMENT
The authors thank Lauren Wheeler-Roberts, Kristy Pitts, Elizabeth Charles, Emily Jones, Alex Hall, and Patricia Keegan at Emory for their work in coordinating the compassionate-use protocol; Keshav Kohli at the Georgia Institute of Technology for his assistance with image analysis; and Sam Brenny, Neal Moat, MD, Tom Vilkama, Ihsen Merioua, and Adam Hoyhtya at Abbott for their thoughtful commentary on the manuscript and allowing compassionate use of the Tendyne TMVI system in these patients.
This study was supported by Emory Structural Heart and Valve program intramural funds and by grant Z01-HL006040 from the National Institutes of Health. Dr. Greenbaum is a proctor for Edwards Lifesciences and Medtronic; and has an equity interest in Transmural Systems. Dr. Greenbaum’s employer has research contracts for clinical investigation of transcatheter aortic, mitral, and tricuspid devices from Edwards Lifesciences, Abbott Vascular, Medtronic, and Boston Scientific. Dr. Khan is a proctor for Edwards Lifesciences and Medtronic. Dr. Paone is a consultant and proctor for Edwards Lifesciences. Dr. Grubb is a speaker, proctor, and principal investigator for Edwards Lifesciences; is a speaker, a proctor, and an advisory board member for Boston Scientific; and is a speaker, a proctor, a principal investigator, an advisory board member, and a national principal investigator for Medtronic. Dr. Rogers is a proctor for Edwards Lifesciences and Medtronic. Drs. Khan, Lederman, and Rogers are coinventors on patents, assigned to the National Institutes of Health, on devices for electrosurgical leaflet laceration. Dr. Lederman’s employer has received research support from Edwards Lifesciences for transcatheter modification of the mitral valve. Dr. Babaliaros is a consultant for Edwards Lifesciences and Abbott Vascular; and has an equity interest in Transmural Systems.
ABBREVIATIONS AND ACRONYMS
- CT
computed tomography
- ELASTA-Clip
electrosurgical laceration and stabilization of MitraClip
- FDA
U.S. Food and Drug Administration
- IQR
interquartile range
- MR
mitral regurgitation
- PVL
perivalvular leak
- TMVI
transcatheter mitral valve implantation
Footnotes
Dr. Babaliaros’s employer has research contracts for clinical investigation of transcatheter aortic, mitral, and tricuspid devices from Edwards Lifesciences, Abbott Vascular, Medtronic, and Boston Scientific. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Takayuki G, Sören S, Kristin R, et al. Surgical revision of failed percutaneous edge-to-edge mitral valve repair: lessons learned. Interact Cardiovasc Thorac Surg 2019;28:900–7. [DOI] [PubMed] [Google Scholar]
- 2.Alozie A, Westphal B, Kische S, et al. Surgical revision after percutaneous mitral valve repair by edge-to-edge device: when the strategy fails in the highest risk surgical population. Eur J Cardiothorac Surg 2014;46:55–60. [DOI] [PubMed] [Google Scholar]
- 3.Geidel S, Schmoeckel M. Impact of failed mitral clipping on subsequent mitral valve operations. Ann Thorac Surg 2014;97:56–63. [DOI] [PubMed] [Google Scholar]
- 4.Geidel S, Wohlmuth P, Schmoeckel M. Survival prediction in patients undergoing open-heart mitral valve operation after previous failed MitraClip procedures. Ann Thorac Surg 2016;101:952–8. [DOI] [PubMed] [Google Scholar]
- 5.Kreidel F, Alessandrini H, Wohlmuth P, Schmoeckel M, Geidel S. Is surgical or catheter-based interventions an option after an unsuccessful Mitral Clip? Semin Thorac Cardiovasc Surg 2018;30:152–7. [DOI] [PubMed] [Google Scholar]
- 6.Mazur P, Mok S, Krishnaswamy A, Kapadia S, Navia JL. Mitral valve surgery following failed MitraClip implantation. J Card Surg 2017;32: 14–25. [DOI] [PubMed] [Google Scholar]
- 7.Mkalaluh S, Szczechowicz M, Karck M, Weymann A. Failed MitraClip therapy: surgical revision in high-risk patients. J Cardiothorac Surg 2019;14:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Latib A, Ancona MB, Ferri L, et al. Percutaneous direct annuloplasty with Cardioband to treat recurrent mitral regurgitation after MitraClip implantation. J Am Coll Cardiol Intv 2016;9(18):e191–2. [DOI] [PubMed] [Google Scholar]
- 9.Neuss M, Schau T, Isotani A, Pilz M, Schöpp M, Butter C. Elevated mitral valve pressure gradient after MitraClip implantation deteriorates long-term outcome in patients with severe mitral regurgitation and severe heart failure. J Am Coll Cardiol Intv 2017;10:931–9. [DOI] [PubMed] [Google Scholar]
- 10.Babaliaros VC, Greenbaum AB, Khan JM, et al. Intentional percutaneous laceration of the anterior mitral leaflet to prevent outflow obstruction during transcatheter mitral valve replacement: firstin-human experience. J Am Coll Cardiol Intv 2017;10:798–809. [Google Scholar]
- 11.Khan JM, Babaliaros VC, Greenbaum AB, et al. Anterior leaflet laceration to prevent ventricular outflow tract obstruction during transcatheter mitral valve replacement. J Am Coll Cardiol 2019; 73:2521–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan JM, Rogers T, Schenke WH, et al. Intentional laceration of the anterior mitral valve leaflet to prevent left ventricular outflow tract obstruction during transcatheter mitral valve replacement: pre-clinical findings. J Am Coll Cardiol Intv 2016;9:1835–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan JM, Lederman RJ, Sanon S, et al. Transcatheter mitral valve replacement after transcatheter electrosurgical laceration of Alfieri stitch (ELASTIC): first-in-human report. J Am Coll Cardiol Intv 2018;11:808–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan JM, Rogers T, Greenbaum AB, et al. Transcatheter electrosurgery: state-of-the-art review. J Am Coll Cardiol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stone GW, Adams DH, Abraham WT, et al. Clinical trial design principles and endpoint definitions for transcatheter mitral valve repair and replacement: part 2: endpoint definitions: a consensus document from the Mitral Valve Academic Research Consortium. Eur Heart J 2015;36:1878–91. [DOI] [PubMed] [Google Scholar]
- 16.Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 2011;123: 2736–47. [DOI] [PubMed] [Google Scholar]
- 17.Muller DWM, Farivar RS, Jansz P, et al. Transcatheter mitral valve replacement for patients with symptomatic mitral regurgitation: a global feasibility trial. J Am Coll Cardiol 2017;69: 381–91. [DOI] [PubMed] [Google Scholar]
- 18.Tabata N, Weber M, Sugiura A, et al. Impact of the leaflet-to-annulus index on residual mitral regurgitation in patients undergoing edge-to-edge mitral repair. J Am Coll Cardiol Intv 2019; 12:2462–72. [DOI] [PubMed] [Google Scholar]
- 19.Sorajja P, Bae R, Gössl M, et al. Complementary transcatheter therapy for mitral regurgitation. J Am Coll Cardiol 2019;73:1103–4. [DOI] [PubMed] [Google Scholar]
- 20.Obadia JF, Messika-Zeitoun D, Leurent G, et al. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med 2018;379:2297–306. [DOI] [PubMed] [Google Scholar]
- 21.Stone GW, Lindenfeld J, Abraham WT, et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med 2018;379: 2307–18. [DOI] [PubMed] [Google Scholar]
- 22.Khan JM, Greenbaum AB, Babaliaros VC, et al. The BASILICA trial: prospective multicenter investigation of intentional leaflet laceration to prevent TAVR coronary obstruction. J Am Coll Cardiol Intv 2019;12:1240–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donmez E, Salcedo EE, Quaife RQ, Burke JM, Gill EA, Carroll JD. The acute effects of edge-to-edge percutaneous mitral valve repair on the shape and size of the mitral annulus and its relation to mitral regurgitation. Echocardiography 2019;36:732–41. [DOI] [PubMed] [Google Scholar]


