Abstract
BACKGROUND:
Long non-coding RNAs (lncRNAs) are a class of transcribed RNA molecules greater than 200 nucleotides in length. Although lncRNAs do not encode proteins, they play numerous functional roles in gene expression regulation. LncRNAs are notably abundant in brain, however, their neural functions remain largely unknown.
METHODS:
We examined the expression of the lncRNA, Gas5, in nucleus accumbens (NAc), a key brain reward region, of adult male mice after cocaine administration. We then performed viral-mediated overexpression of Gas5 in NAc neurons to determine its role in addiction-related behaviors. We also carried out RNA-sequencing to investigate Gas5-mediated transcriptomic changes.
RESULTS:
We demonstrated that repeated short- or long-term cocaine administration decreased expression of Gas5 in NAc. Viral-mediated overexpression of Gas5 in NAc neurons decreased cocaine-induced conditioned place preference. Likewise, Gas5 overexpression led to decreased cocaine intake, decreased motivation and compulsive-like behavior to acquire cocaine, and facilitated extinction of cocaine-seeking behavior. Transcriptome profiling identified numerous Gas5-mediated gene expression changes that are enriched in relevant neural function categories. Interestingly, these Gas5-regulated gene expression changes significantly overlap with chronic cocaine-induced transcriptome alterations, which suggests that Gas5 may serve as an important regulator of transcriptional responses to cocaine.
CONCLUSIONS:
Together, our study demonstrates a novel lncRNA-based molecular mechanism of cocaine action.
Keywords: long non-coding RNA, Gas5, cocaine, addiction, nucleus accumbens, prodynorphin, mouse
INTRODUCTION
Addiction is a neuropsychiatric disorder that is characterized by compulsive drug seeking and taking despite harmful consequences. Repeated use of drugs leads to enduring cellular and molecular changes in the brain’s reward circuitry, particularly in nucleus accumbens (NAc), part of the ventral striatum (1, 2). Accumulating evidence demonstrates that complex drug-induced neuroadaptations in NAc are mediated largely through dynamic regulation of gene expression. Recent studies indicate that epigenetic mechanisms, such as DNA methylation, histone modifications, and certain types of non-coding RNAs (e.g., microRNAs), can modulate expression of gene networks in NAc and other drug reward-associated brain regions, which contributes to drug-induced structural, synaptic, and behavioral plasticity (3, 4).
Over the past decade, next-generation sequencing technologies have advanced our understanding of transcriptomes. One major finding has been that non-protein-coding RNA transcripts dominate the mammalian transcriptome: ~80% of the human genome is transcribed into RNAs, although the large majority are non-coding RNAs that are not translated into proteins (5). In addition to microRNAs, long non-coding RNAs (lncRNAs) are a major class of non-coding RNAs, which are defined as being over 200 nucleotides in length (6, 7). While the functional significance of lncRNAs remains elusive, they appear to play a wide variety of regulatory roles at virtually every stage of gene expression. Strikingly, among the tens of thousands of annotated lncRNAs, about 40% are expressed specifically in brain, which suggests a critical role in neural function (8).
Although lncRNAs comprise an extensive class of transcripts in brain, our understanding of their function remains largely unknown. Accumulating evidence suggests important roles of lncRNAs in nervous system evolution, development, and plasticity (9, 10). LncRNA expression is dynamically regulated during neural development, and is implicated in neural lineage specification and neuron-glia cell fate determination (11). For example, a lncRNA antisense to Dlx1 (Dlx1AS) was found to be upregulated during differentiation of GABAergic neurons and downregulated during oligodendrocyte differentiation (12). Neuronal activity induces expression of certain lncRNAs (13, 14), including many enhancer-associated lncRNAs (15), and aberrant lncRNA expression has been reported in several brain disorders, such as autism, brain tumors, depression, neurodegeneration, and schizophrenia (9, 16–18). A few neural disorder-associated lncRNA functions have been demonstrated through their interaction with specific protein partners. For example, the lncRNA, BACE1-AS, was implicated in a positive feedback loop that drives progression of Alzheimer’s disease through upregulating the sense BACE1 RNA, which is essential for the generation of beta-amyloid and consequent amyloid plaques (19). In contrast, the lncRNA, BDNF-AS, antagonizes BDNF expression and has a negative feedback effect on brain disorders that are associated with reduced levels of BDNF, as has been demonstrated for Huntington’s disease (20). Additionally, the lncRNA, KCNA2-AS, is induced in dorsal root ganglia in response to nerve injury, which negatively regulates expression of the potassium channel subunit KCNA2 and generates symptoms of neuropathic pain (21). Furthermore, lncRNA Gomafu in mouse prefrontal cortex was reported to regulate anxiety-like behaviors (22). It was also found that a novel lncRNA acting as a regulator of the monoamine oxidase A (MAOA) gene in brain mediates impulsive and aggressive behaviors (23). Recently, a primate-specific lncRNA, LINC00473, was identified as a female-specific driver of stress resilience (18).
Given the expansive numbers and diverse types of lncRNA transcripts in brain, we still know very little about their functional roles in drug addiction. A few studies have reported the differential expression of lncRNAs in brains of drug-addicted humans or drug-exposed animals. For example, by use of a customized gene expression microarray, numerous lncRNAs were found to be differentially expressed in mouse NAc after cocaine conditioned place preference (CPP) (24). Several lncRNAs were recognized to be differentially expressed in midbrain of human cocaine users through another custom lncRNA microarray assay (25). And more recently, RNA-sequencing (RNA-seq) was used to identify several differentially expressed lncRNAs in midbrain of human opioid users (26). However, in contrast to the growing recognition of lncRNAs in brain function and other disorders, one major limitation is the lack of functional characterization of lncRNA transcripts within the context of addiction models.
In the current study, we examined expression changes of the lncRNA, Gas5 (growth arrest specific 5), in mouse NAc in response to cocaine administration. The mouse Gas5 gene produces multiple transcript variants and we found one lncRNA isoform, Gas5–209 (ENSMUST00000159663.7), to be specifically decreased in this brain region by cocaine. Therefore, we carried out viral manipulation experiments to investigate the behavioral effects of Gas5 overexpression on cocaine reward and other addiction-like behaviors, and performed transcriptome profiling to better understand its role in cocaine action.
MATERIALS AND METHODS
See Supplemental Material for detailed methods.
Animals
Adult male C57BL/6J mice (8 weeks old) were purchased from Jackson Laboratory and housed on a reverse 12-hour light/dark cycle with food and water available ad libitum. All behavioral experiments including cocaine intraperitoneal (i.p.) injections, cocaine CPP, and intravenous cocaine self-administration (SA), were carried out during the dark cycle. All experimental procedures were approved by the Institutional Animal Care and Use Committees of Florida State University and Icahn School of Medicine at Mount Sinai.
Herpes simplex virus (HSV) and adeno-associated virus (AAV) preparation and stereotaxic surgery
The Gas5 sequence was obtained from ENSEMBL (Gas5–209; ENSMUST00000159663.7, EMSEMBL build GRCm38.p5) and cloned together with EGFP into HSV and AAV2 vectors. Bilateral intra-NAc microinjections were performed as reported previously (27). HSV-EGFP or AAV-EGFP was used as controls, respectively.
RNA isolation and real-time PCR (qPCR)
Total RNA was extracted from NAc tissue using Trizol (Thermo Fisher) according to manufacturer’s protocol. qPCR was performed as previously described (28).
RNA-seq transcriptome profiling
Total RNA (100 ng) was applied to the NEBNext rRNA Depletion Kit (New England Biolabs) and NEBNext Ultra II Directional RNA Library Prep Kit for Illumina (New England Biolabs) for library preparation. RNA-seq libraries were then pooled equimolar and sequenced on a NovaSeq 6000 sequencer (Illumina). The sequencing data were deposited into Gene Expression Omnibus with accession number GSE146005 and analyzed through various bioinformatic algorithms.
Statistical analysis
Student’s t-tests and two-way ANOVAs were used when applicable. All data are presented as mean ± SEM with statistical significance set at p<0.05.
RESULTS
Gas5 expression decreases after cocaine exposure
We previously generated an RNA-seq dataset from mouse NAc 24 hours after 7 daily cocaine i.p. injections (28) and observed a selective decrease in levels of one Gas5 transcript, Gas5–209 (ENSMUST00000159663.7, log2 fold change=−0.29, p=0.002). This Gas5 transcript was also most abundant among all Gas5 isoforms expressed in NAc. To confirm this finding, we collected a separate set of NAc tissue 24 hours after 7 daily cocaine i.p. injections and performed qPCR of Gas5–209 and found a nearly 30% decrease (p<0.05, Figure 1A). Furthermore, we examined Gas5–209 expression 1 hour after 7 daily cocaine injections and detected a small but significant ~10% decrease (p<0.05, Figure 1A). To investigate whether the decrease in Gas5–209 is long-lasting, we collected NAc tissue from a cohort of mice 10 days after 28 daily cocaine i.p. injections. Compared to control mice that received saline injections, expression of Gas5–209 was decreased by 16% (p<0.05, Figure 1A). These data suggest a persistent decrease in expression levels of Gas5–209 in NAc following chronic cocaine exposure. Additionally, consistent with reduced Gas5–209 expression after non-contingent i.p. cocaine exposure, we observed decreased expression of Gas5–209 in NAc of mice 24 hours after the reinstatement test of cocaine SA (p<0.001, Figure 1B, Figure S1), which further supports that cocaine negatively regulates Gas5–209 expression in this brain region.
Figure 1. Gas5 expression in nucleus accumbens (NAc) following cocaine exposure.
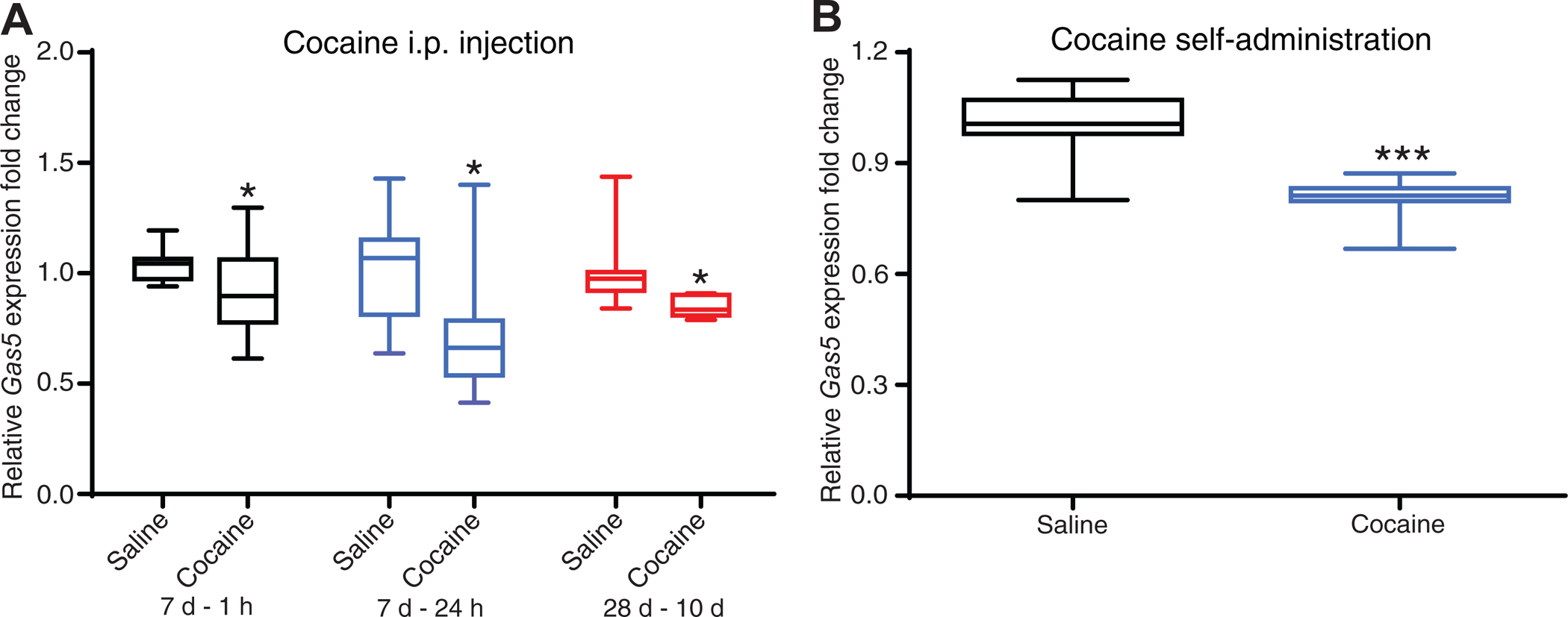
(A) Gas5 expression was significantly decreased in mouse NAc 1 hour or 24 hours after 7 daily cocaine (20 mg/kg) i.p. injections, or 10 days after 28 daily cocaine i.p. injections. *p<0.05, cocaine vs saline, unpaired t-test. 7 days-1 hour (7d-1h), n=11 in saline group, n=12 in cocaine group; 7 days-24 hours (7d-24h), n=11 in each group; 28 days-10 days (28d-10d), n=8 in each group. Data are presented as mean ± SEM. (B) Gas5 expression was decreased in NAc 24 hours after cocaine self-administration (SA) (unpaired t-test, t=4.721, ***p<0.001 compared with that of saline controls). Mice with AAV-EGFP overexpression in NAc were subjected to 7-day cocaine SA under an FR-1 schedule, followed by 5 days of FR-3, after which, a single progressive ration (PR) session was applied. Twenty-four hours after the reinstatement test of cue-induced cocaine/saline-seeking, NAc tissue was collected for qPCR analysis. N=8 in saline SA and 7 in cocaine group. Data are presented as mean ± SEM.
Gas5 regulates cocaine reward behavior
To understand the potential functional role of Gas5–209 expression changes in drug reward, we performed HSV-mediated overexpression of this transcript in NAc neurons (Figure 2A). Compared with the control mice that received HSV-EGFP injections in this region (Figure 2B), we found a 2.24-fold increase in Gas5 expression 72 hours after HSV-Gas5-EGFP injection (p<0.001, Figure 2C). A two-way ANOVA indicated a significant main effect of virus on cocaine-induced CPP (F1,46=4.28, p<0.05, Figure 2D). In contrast to a strong cocaine-induced CPP in HSV-EGFP injected control mice (p<0.001, Figure 2D), animals that received HSV-Gas5-EGFP injections failed to acquire cocaine CPP (p=0.134, Figure 2D) and had significantly lower CPP scores than HSV-EGFP controls (p<0.05). However, there was no difference in general locomotor activity between the two groups (p=0.7291, not significant. Figure S2).
Figure 2. Cocaine conditioned place preference (CPP) after HSV-Gas5-EGFP overexpression in mouse NAc.
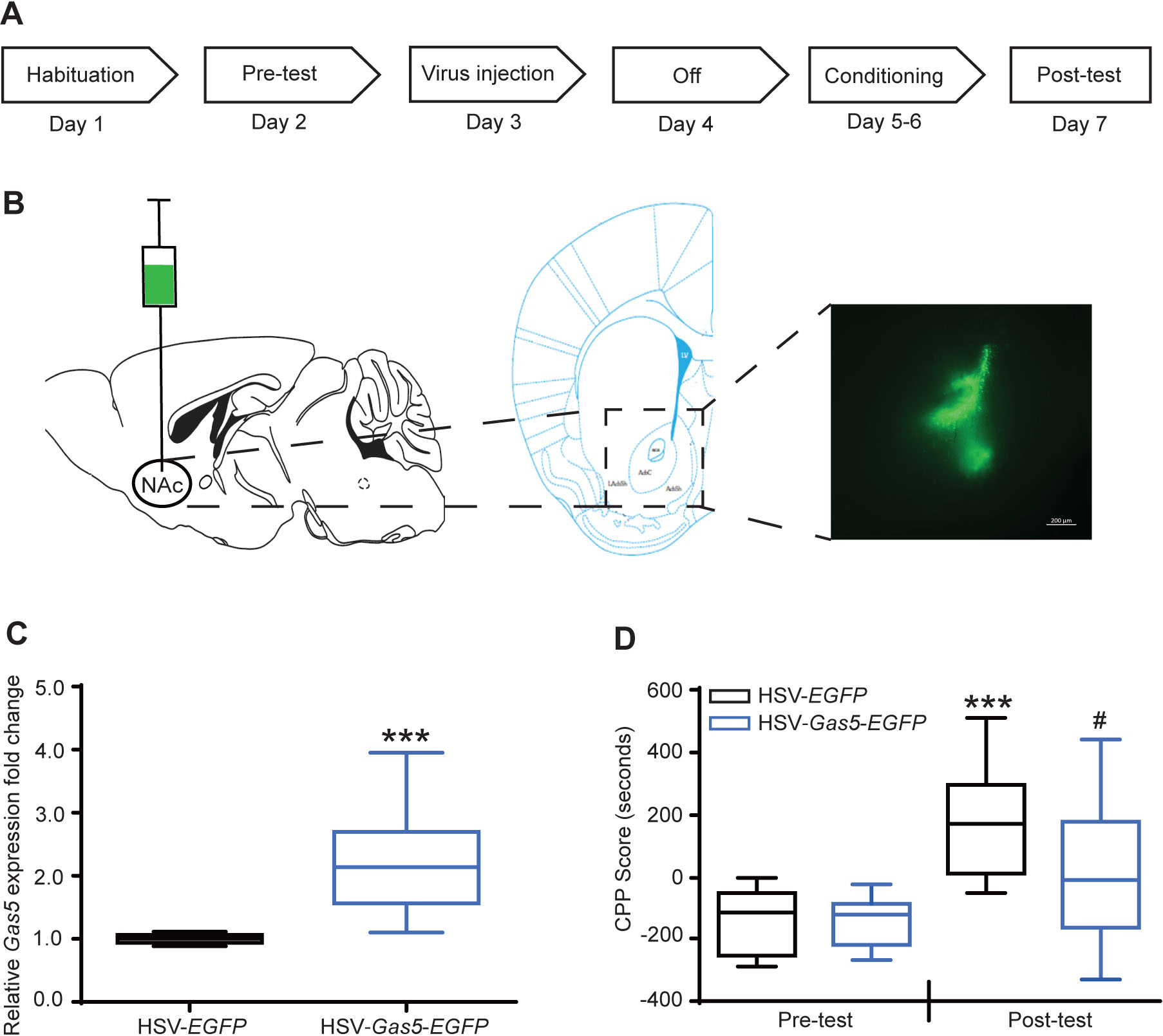
(A) Experimental diagram of cocaine CPP after viral injection. (B) The schematic of stereotaxic viral injection into mouse NAc. A fluorescent image is shown to demonstrate the spread of viral-mediated transgene expression in NAc. Scale bar: 200 μM. (C) Increased expression of Gas5 in NAc 72 hours after HSV-Gas5-EGFP injection. ***p<0.001 compared with HSV-EGFP injected controls (unpaired t-test). N=10 in each group. (D) Gas5 decreased cocaine CPP. A two-way ANOVA indicates the significant main effects of virus on CPP (F1,46=4.28, p<0.05). Tukey’s post hoc comparison shows strong cocaine-induced CPP in the HSV-EGFP injected control group (***, p<0.001, HSV-EGFP post-test vs HSV-EGFP pre-test), while HSV-Gas5-EGFP mice failed to acquire cocaine CPP (p=0.134, HSV-Gas5-EGFP post-test vs HSV-Gas5-EGFP pre-test). Mice in the HSV-Gas5 group showed significantly lower CPP values (#, p<0.05, HSV-Gas5-EGFP post-test vs HSV-EGFP post-test). HSV-EGFP, n=11; HSV-Gas5-EGFP, n=14.
To further assess the role of neuronal Gas5 in cocaine addiction-like behavior, we injected AAV-Gas5-EGFP in NAc and examined the behavioral effects using cocaine SA. Figure 3A shows the timeline and schematic of the entire cocaine SA procedure. We confirmed Gas5 overexpression after AAV-Gas5-EGFP injection (Figure 3B, p<0.001). During acquisition and maintenance of cocaine SA, both AAV-EGFP and AAV-Gas5-EGFP mice reliably self-administered cocaine at a dose of 0.5 mg/kg/infusion under FR-1 and FR-3 schedules (Figure 3C). A repeated-measures two-way ANOVA indicated a significant main effect of session (F6,126=4.806, p<0.001) on average cocaine infusions during the FR-1 training period, indicating escalated cocaine intake as the sessions progressed, but there was no significant main effect of virus during FR-1 (F1,21=1.283, ns: not significant). Across the five FR-3 sessions, there was a gradual restoration of cocaine intake, with significant main effects of both session (F4,76=3.221, p<0.05) and virus (F1,19=4.414, p<0.05) on average cocaine infusions. A subsequent Bonferroni’s post hoc analysis showed significant differences in the average number of cocaine infusions between AAV-EGFP and AAV-Gas5-EGFP mice on day 3 of FR-3 (p<0.05, AAV-Gas5-EGFP vs AAV-EGFP) (Figure 3C). For the active operant response to acquire cocaine (Figure 3D), a main effect of session (F6,126=0.793, ns) or virus (F1,21=0.755, ns) was not detected under FR-1. However, analysis of active operant responses during FR-3 (Figure 3D) revealed a significant main effect of virus (F1,19=5.968, p<0.05) and session (F4,76=3.394, p<0.05). Bonferroni’s post hoc comparisons revealed a significant decrease in active nose-pokes in AAV-Gas5-EGFP mice from sessions 10 (p<0.05, AAV-Gas5-EGFP vs AAV-EGFP) and 11 (p<0.05, AAV-Gas5-EGFP vs AAV-EGFP). In addition to decreased cocaine SA and active responses during FR-3, AAV-Gas5-EGFP mice exhibited a significantly lower breakpoint compared to AAV-EGFP control mice when assessed under a progressive ratio (PR) schedule (p<0.05) (Figure 3E). Although the number of active responses was similar overall between groups across the entire extinction phase, repeated measures two-way ANOVA indicated significant main effects of session (F4,64=7.394, p<0.0001) and virus (F1,16=4.859, p<0.05) on extinction of cocaine SA during the last five days, suggesting that AAV-Gas5-EGFP facilitated extinction (Figure 3F). During the reinstatement test of cue-induced cocaine-seeking (Figure 3G), two-way ANOVA indicated a significant main effect of condition (last day of extinction vs reinstatement) (F1,16=46.57, p<0.0001), but no significant effect between the two groups (F1,16=0.0955, ns). As a control for cocaine SA, a separate cohort of mice was trained to self-administer saline after completion of liquid sucrose SA training. There was no significant difference in saline SA between the AAV-EGFP and AAV-Gas5-EGFP groups (Figure S3), which rules out a potential difference in general learning of operant behaviors required for SA as well as an effect on general locomotor behavior.
Figure 3. Gas5 overexpression in NAc inhibits cocaine self-administration (SA).
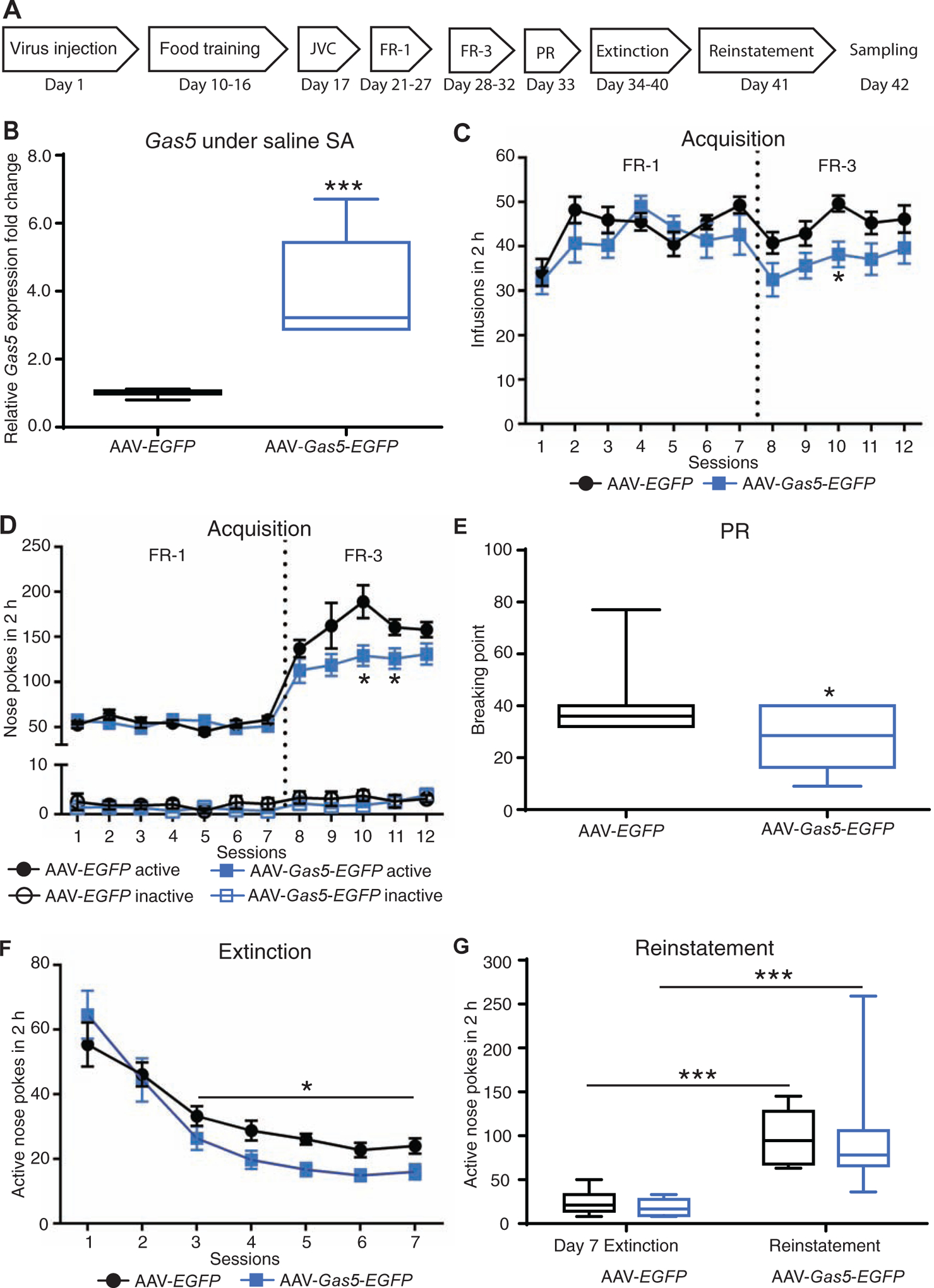
(A) The timeline of the cocaine SA procedure. (B) Validation of Gas5 expression. Twenty-four hours after saline SA, the level of Gas5 was evaluated in NAc of mice injected with either AAV-EGFP or AAV-Gas5-EGFP. AAV-Gas5-EGFP mice showed higher Gas5 expression (***p<0.001). (C) The average cocaine infusions in 2-hour sessions across the 7-day FR-1 and 5-day FR-3 periods. A two-way ANOVA with repeated measures revealed a main effect of virus across the 5-day FR-3 schedule (F1,19=4.41, p<0.05), and Bonferroni’s post hoc comparisons show a significant difference in cocaine SA between the AAV-Gas5-EGFP group and AAV-EGFP control group on the 3rd day of FR-3 (* p<0.05). FR-1, n=10 and 13 for AAV-EGFP group and AAV-Gas5-EGFP group respectively; FR-3, n=8 and 13 for AAV-EGFP group and AAV-Gas5-EGFP group respectively. (D) Operant responses for cocaine SA. The mean numbers of nose-pokes in the active and inactive holes during the acquisition of cocaine SA in 2-hour sessions under FR-1 and FR-3 schedules. Two-way ANOVA with repeated measures revealed a main effect of virus across the 5-day FR-3 schedule (F1,19=5.97, * p<0.05), and Bonferroni’s post hoc comparisons showed a significant decrease in cocaine SA in the AAV-Gas5-EGFP group compared with the AAV-EGFP group on the 3rd and 4th days of FR-3 (* p<0.05). FR-1, n=10 and 13 for the AAV-EGFP group and the AAV-Gas5-EGFP group respectively; FR-3, n=8 and 13 for AAV-EGFP and AAV-Gas5-EGFP mice, respectively. (E) Breakpoint that mice achieved under a progressive ratio (PR) schedule. The AAV-Gas5-EGFP group displayed a significantly lower breakpoint compared with the AAV-EGFP group (* p<0.05). (F) Operant responses during extinction of cocaine SA. The mean number of nose-pokes in the active hole across 7 days of extinction training after cocaine SA are shown. A repeated-measures two-way ANOVA analysis indicated a significant main effect of virus on cocaine extinction across the last 5 days (F1,16=4.859, * p<0.05). n=8 in AAV-EGFP group and n=10 in AAV-Gas5-EGFP group. (G) Reinstatement test of cue-induced cocaine-seeking behavior. n=8 in AAV-EGFP group and n=10 in AAV-Gas5-EGFP group. *** p<0.001 compared with day 7 extinction. Data are presented as mean ± SEM.
Gas5-regulated transcriptome changes and identification of a potential target gene, prodynorphin
To explore the molecular underpinnings of Gas5 action, we performed RNA-seq to profile the NAc transcriptome after neuronal Gas5 overexpression. The sequencing data were of good quality (Table S1, Figure S4). We examined differentially expressed genes (DEGs) in HSV-Gas5-EGFP injected samples compared to control HSV-EGFP samples three days after viral injection, when viral expression is at peak levels. From this analysis, we found numerous DEGs after Gas5 overexpression (downregulated: 1038, upregulated: 673, FDR≤0.05, Figure 4A, Table S2). Some of the top-ranked DEGs are known to be implicated in cocaine addiction, such as Bdnf (log2 Fold Change (log2FC)=−2.97, FDR=7.86 × 10−28), Slc17a6 (log2FC=−1.54, FDR=2.80 × 10−17), Homer2 (log2FC=−0.93, FDR=9.36 × 10−17), and Nr4a2 (logFC=−3.54, FDR=5.08 × 10−32), to name a few. To further investigate the underlying functional implications of Gas5-mediated transcriptome regulation, we evaluated enriched KEGG pathways using an over-representation analysis. We found many meaningful over-represented neural pathways, which include: glutamatergic synapse (Enrichment Ratio (ER)=3.66, FDR=7.01 × 10−8), GABAergic synapse (ER=3.80, FDR=1.06 × 10−6), neuroactive ligand-receptor interaction (ER=2.169, FDR=2.70 × 10−5), and cholinergic synapse (ER=2.84, FDR=1.16 × 10−4) (Figure 4B, Table S3).
Figure 4. Gene expression changes after HSV-Gas5-EGFP overexpression in NAc.
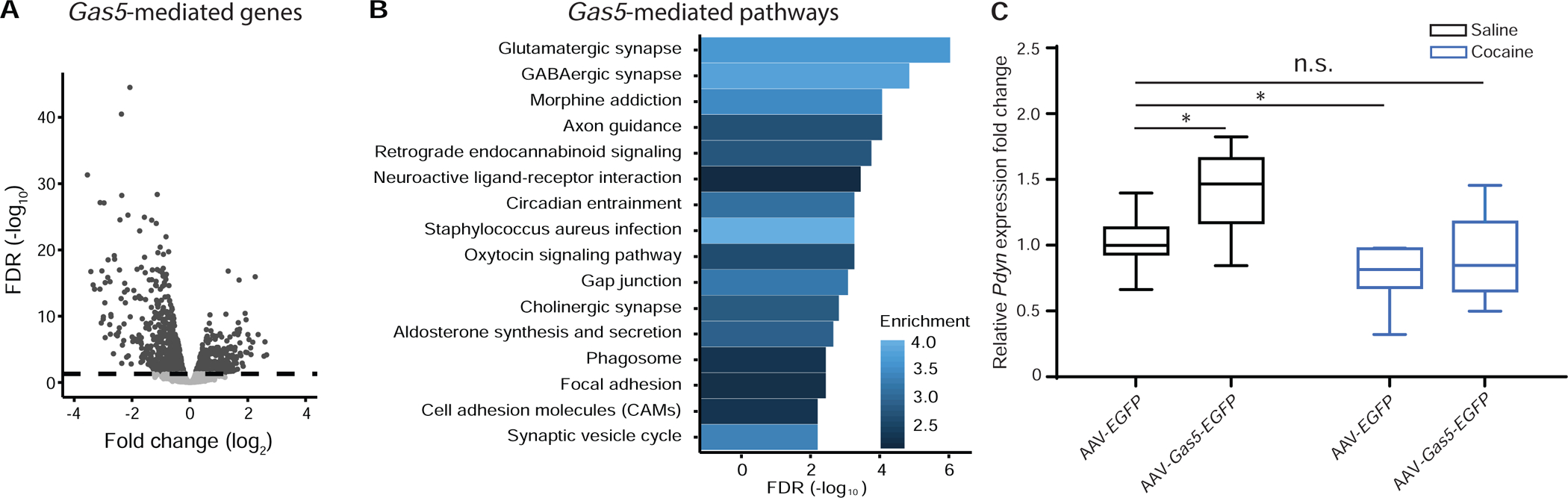
(A) Volcano plot visualizing normalized gene expression values in log2FC (x axis), where resulting FDR values are −log10 transformed (y axis). The dashed line represents FDR=0.05. (B) Bar plot representing significantly enriched KEGG pathways resulting from HSV-Gas5-EGFP overexpression. The color gradient for each bar represents the enrichment ratio (observed/expected) with the degree of blue color indicating increasing enrichment. (C) Cocaine SA suppressed the expression of Pdyn. AAV-Gas5-EGFP promoted Pdyn expression under basal conditions (p<0.05, AAV-Gas5-EGFP saline vs AAV-EFGP saline), while abstinence from cocaine SA decreased Pdyn expression (p<0.05, AAV-EFGP cocaine vs AAV-EFGP saline). In addition, AAV-Gas5-EGFP normalized the cocaine-induced decrease in Pdyn expression back to basal expression levels relative to the AAV-EGFP saline group. AAV-EGFP saline, n=8; AAV-Gas5-EGFP saline, n=6; AAV-EGFP cocaine, n=7; AAV-Gas5-EGFP cocaine, n=12. * p<0.05, n.s., not significant. Data are presented as mean ± SEM.
Among the DEGs after HSV-Gas5-EGFP injection, we found that prodynorphin (Pdyn) expression was increased (Table S2). Pdyn is the endogenous ligand for the kappa opioid receptor (KOR), which, when stimulated, produces aversive, dysphoric effects in humans and animals (29, 30). KOR agonists have been shown to block locomotor and rewarding effects of cocaine in rodents (31, 32) and non-human primates (33). To further explore our finding, we examined Pdyn expression in NAc by qPCR after AAV-Gas5-EGFP injection and cocaine SA. A two-way ANOVA revealed significant main effects of both virus (F1,29=7.36, p<0.05) and drug (F1,29=12.57, p<0.01) on Pdyn gene expression in NAc. In AAV-EGFP-injected mice, Pdyn levels were decreased 24 hours after cocaine SA reinstatement testing, relative to their saline counterparts (p<0.05, Tukey’s post hoc comparison, AAV-EGFP, cocaine vs AAV-EGFP, saline, Figure 4C). This was consistent with a previous report showing decreased Pdyn expression in NAc after cocaine SA in rats (34). Furthermore, consistent with the HSV-Gas5-EGFP-induced increase in Pdyn expression, AAV-Gas5-EGFP injection in NAc also elevated Pdyn levels (p<0.05, AAV-Gas5-EGFP, saline vs AAV-EGFP, saline). Moreover, the ability of chronic cocaine to decrease Pdyn expression in NAc was blunted by Gas5 overexpression, with Pdyn expression brought to near baseline levels compared to EGFP-Sal mice (p=0.525, AAV-Gas5-EGFP, cocaine vs AAV-EGFP, saline, Figure 4C). These results suggest that the Gas5-mediated induction of Pdyn, and the resulting dysphoria, may contribute to the decrease in cocaine CPP and SA observed in our Gas5-overexpressing mice.
Gas5 may serve as a key regulator of cocaine-induced transcriptional changes
To further explore the role of Gas5 in cocaine-induced transcriptional alterations, we tested potential overlapping gene expression between Gas5 overexpression and chronic cocaine administration. For the latter, we utilized a published NAc RNA-seq dataset generated 24 hours after 7 daily cocaine i.p. injections (28). By intersecting DEGs from both RNA-seq datasets, we found that there are 39 DEGs shared between the two (Figure 5A). Furthermore, we identified a significant overlap between genes downregulated by Gas5 overexpression and genes upregulated after chronic cocaine administration (Gas5-down DEGs vs cocaine-up DEGs: odds ratio=194.06, p-value=8.52 × 10−52), and a marginally significant overlap between DEGs in the concordant down-regulated direction (Gas5-down DEGs vs cocaine-down DEGs: odds ratio=19.78, p-value=7.52 × 10−4; Figure 5B. Table S4). To test overall transcriptome signatures between Gas5-mediated transcriptional changes and chronic cocaine-induced transcriptional changes, a rank-rank hypergeometric overlay (RRHO) analysis was performed, which revealed significant discordant expression signatures (Figure 5C). The significant discordant expression signature between Gas5-repressed genes (Gas5-down) and chronic cocaine-upregulated genes (cocaine-up) represents an overlap of 423 genes. To elucidate the biological significance of these overlapping transcriptome profiles, we looked for over-represented KEGG pathways. Interestingly, many of the over-represented pathways from Gas5 overexpression were also found in this analysis, such as glutamatergic synapse (ER=8.57, FDR=4.97 × 10−8), dopaminergic synapse (ER=5.31, FDR=6.89 × 10−4), GABAergic synapse (ER=5.92, FDR=0.0026), neuroactive ligand-receptor interaction (ER=3.38, FDR=0.0022), and cholinergic synapse (ER=4.61, FDR=0.0095) (Figure 5D, Table S5). Considering together that chronic cocaine decreases Gas5 expression, and the significant overlap between upregulated genes after chronic cocaine and downregulated genes after Gas5 overexpression, our analysis suggests that Gas5 may serve as a major regulator of cocaine-induced transcriptional alterations.
Figure 5. Comparison of transcriptome profiles between chronic cocaine administration and Gas5 overexpression in NAc.
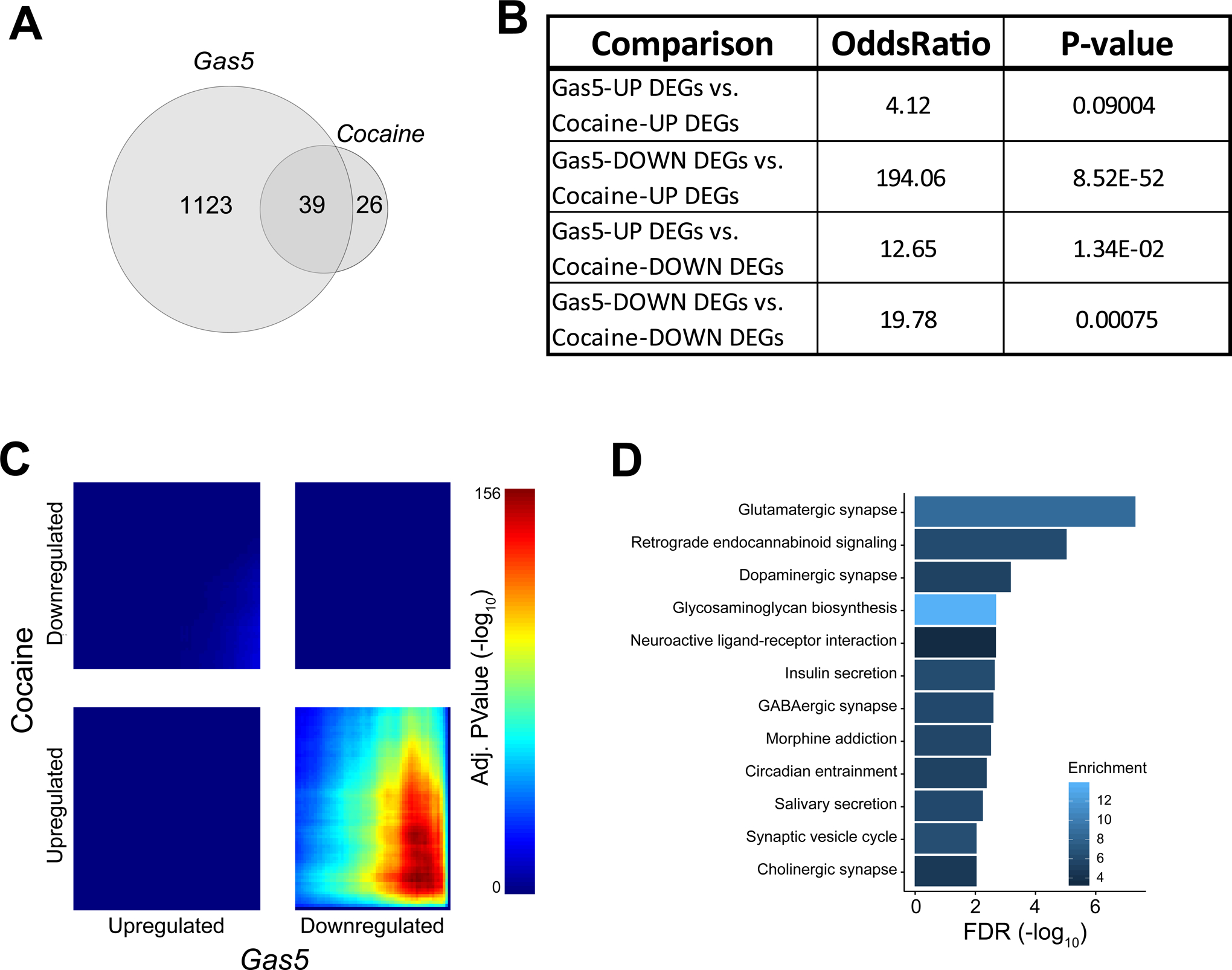
(A) Venn diagram representing the number of overlapping differentially expressed genes (DEGs) in mouse NAc after HSV-Gas5-EGFP and chronic cocaine i.p. administration. (B) The table represents results from Fisher’s exact tests after analyzing concordant and discordant DEGs with log2FC≤−0.5 or ≥0.05 cut-offs for the effect, and p-value≤0.05 as the cut-off for significance from both datasets. (C) RRHO (rank-rank hypergeometric overlap) map compares NAc transcriptomes after HSV-mediated Gas5 overexpression and chronic cocaine administration. The comparison is made in a whole-transcriptome and threshold-free manner, where pixels represent overlapping genes. The color of each pixel represents the Benjamini-Yekutieli adjusted −log10(p-value) of a hypergeometric test, with warmer colors representing more significant results, indicating either discordant (upper left, bottom right) or concordant (upper right, bottom left) expression profiles. (D) Bar plot representing significantly enriched KEGG pathways from significant RRHO signatures with the whole annotated transcriptome as background. The color gradient for each bar represents the enrichment ratio (observed/expected), with the degree of blue color showing increasing enrichment.
DISCUSSION
LncRNAs are emerging as important components of gene regulatory networks in concert with other key molecules, such as epigenetic regulators and transcription factors. LncRNAs have been implicated in numerous cellular processes, including gene transcription, RNA processing, and chromatin modifications (6, 7). However, the functional role of lncRNAs in psychiatric disorders, especially drug addiction, remains largely unknown. Gas5 is a lncRNA that has been implicated in a myriad of biological and behavioral functions, including embryonic stem cell self-renewal and pluripotency (35, 36), axonal demyelination (37), ischemic stroke (38), tumor suppression (39), novelty-induced behaviors (11), and aggression (40). The mouse Gas5 gene produces 25 transcript variants, with five being characterized as lncRNAs (Ensembl). We found that the transcript Gas5–209 (ENSMUST00000159663.7) is decreased in NAc after cocaine administration. This isoform is one of the shorter Gas5 transcripts, measuring 346 nucleotides in length. It is also one of the most abundantly expressed Gas5 transcripts in NAc based on our published RNA-seq data (28). In the present study, we confirmed a decrease in Gas5–209 expression that was seen in the RNA-seq dataset, and demonstrated this decrease in NAc after both short-term and long-term cocaine exposure. Furthermore, this decrease in Gas5 expression was observed 10 days after a 28-day chronic cocaine administration regimen, suggesting long-lasting effects of cocaine on Gas5 expression. An earlier microarray screen of lncRNA expression in response to methamphetamine exposure in rat primary cortical neuron culture also found Gas5 to be differentially expressed (41). We subsequently employed viral-mediated Gas5 overexpression in NAc and found that it antagonized cocaine CPP. We next demonstrated that cocaine SA was also significantly attenuated upon Gas5 overexpression in NAc of mice under an FR-3 schedule of reinforcement, suggesting that Gas5 impaired the intensity of incentive motivation for taking cocaine when more effort was required. Likewise, mice with Gas5 overexpression displayed lower break points under a PR schedule, suggesting that Gas5 reduced motivation and compulsivity for acquiring cocaine. Mice that overexpress Gas5 in NAc also exhibited greater cocaine extinction behavior. Taken together, our study supports the notion that Gas5 in NAc attenuates motivation for cocaine, presumably by reducing its rewarding value as supported by our CPP findings.
With the broad application of still evolving next-generation sequencing techniques, the human and mouse genomes are estimated to have around 50,000 lncRNA genes, which far exceeds the number of protein-coding genes. Among lncRNAs, about 40% are expressed specifically in brain, which underscores the importance of studying their influence on neural function (8). Notably, lncRNAs express in a cell-type and brain-region specific manner. For example, by analyzing in situ hybridization data from the Allen Brain Atlas, it was found that most lncRNAs are located in distinct brain regions, cell types, or subcellular components (42). Moreover, using RNA-seq, it was recognized that lncRNAs are differentially expressed across various cortical layers (43) and brain regions (18). It is therefore likely that large numbers of lncRNAs escaped detection in previous RNA-seq experiments performed in bulk tissue due to highly specific expression patterns in cell subtypes or low levels of overall expression as well. Moreover, though many lncRNAs are polyadenylated, a significant portion of lncRNAs persist as non-polyadenylated transcripts, which renders them undetectable upon poly-A based RNA-seq profiling. Moreover, identification of antisense lncRNAs requires strand-specific RNA-seq, which is still not a routine technique. Therefore, it will be important to further study lncRNAs in brain with cell-type and brain-region specificity under appropriate experimental designs in the future.
Although growing numbers of lncRNAs have been identified and annotated in the nervous system, with a few being implicated in specific brain disorders, such substances abuse (24–26, 41), a major limitation of existing studies is a lack of lncRNA functional characterization. It should also be noted that our knowledge of the contribution of lncRNAs to the nervous system remains unclear based on the numerous discordant findings between in vitro and in vivo studies of the same lncRNA. For example, knockdown of the highly expressed lncRNA Malat1 in cultured hippocampal neurons decreased synaptic density, and its overexpression increased synaptic density (44), along with associated changes in gene expression and alternative splicing. However, Malat1 knockout mice demonstrated no apparent developmental or viability phenotype. Depletion of Malat1 does not affect global gene expression, splicing factor levels, or alternative splicing in vivo (45, 46). A similar discrepancy was observed for Gomafu (also known as MIAT), a lncRNA with demonstrated roles in retinal development, stem cell differentiation, alternative splicing, and possibly schizophrenia (22, 47). Gomafu knockout mice displayed no overt developmental phenotypes, although they were hyperactive, which was further enhanced when treated with methamphetamine (48). These discordant results could be explained by context-specific roles of lncRNAs or by developmental compensation. Nevertheless, these findings warrant a careful characterization of lncRNA function when designing experiments in vitro and in vivo.
Over the past decade, lncRNA transcripts have emerged as critical factors to exert diverse regulatory roles across a variety of cellular processes. Although the functions of the majority of lncRNAs are still unresolved, a common emerging theme is that lncRNAs form ribonucleic acid-protein complexes to influence gene expression (6, 7). For example, lncRNAs can serve as decoys by attaching to DNA-binding proteins to preclude their access to target DNA. LncRNAs may also function as scaffolds to bring protein partners together into a complex. As well, lncRNAs can act as guides to bind to a protein and target it to a particular region of the genome. Additional lncRNA mechanisms are likely to be discovered, but the diversity of the presently known protein partners (epigenetic molecules, chromatin modifiers, transcription factors, splicing factors) of lncRNA-protein complexes demonstrates that lncRNAs are a multifaceted class of regulatory molecules.
Gas5 was isolated originally from NIH 3T3 cells and shown to accumulate in growth-arrested cells and produce a spliced lncRNA (49). Gas5 was previously shown to serve as a decoy for glucocorticoid receptors (GRs) through its hairpin sequence motif, which resembles the DNA-binding site of GRs (50), and thereby prevents GRs from regulating transcription of their target genes. However, following RNA-seq of NAc after Gas5 overexpression, we did not detect appreciable alterations of GR signaling-related pathways. We also did not detect expression changes of several known GR-responsive genes by qPCR (data not shown). These observations suggest that Gas5 may act through GR-independent mechanisms in NAc in response to cocaine.
We searched for transcription factor binding motifs within the sequence of the Gas5–209 transcript and identified four potential binding sites for SMAD, a transcription factor previously implicated in cocaine action (34, 51). It would be interesting to explore if and how SMAD signaling is involved in Gas5 actions. The enrichment of Gas5 target genes in glutamatergic and other neural transmission pathways also suggests its functional role in synaptic transmission that deserves future investigation. To gain further molecular insight, we found numerous gene expression changes in NAc in response to Gas5 overexpression, which suggests that Gas5 may have a broad regulatiory effect on gene expression. Moreover, these Gas5-dependent DEGs are selectively enriched in multiple relevant pathyways, such as various neurotransmitter actions, axon guidance, and addiction. Intriguingly, more than half (60%) of chronic cocaine-induced DEGs were also regulated by Gas5 overexpression in this brain region. Among these genes, we found most to be downregulated by Gas5 and upregulated by cocaine. Given the repressive role of cocaine on Gas5 expression, and Gas5’s repressive effect on behavioral responses to cocaine, we propose that Gas5 serves predominantly as a negative regulator of cocaine-induced changes in gene expression which underlies a negative feedback loop between Gas5 and cocaine action. However, we should emphasize that, while a majority of cocaine-regulated transcripts are also affected by Gas5 overexpression, the opposite is not true: Gas5 overexpression altered the transcription of a large number of RNAs that are not affected by cocaine. The role of Gas5 in transcriptional regulation in NAc may, therefore, be viewed as far broader than simply opposing cocaine action.
Future studies are needed to better understand Gas5-cocaine interactions as well as to identify the potential protein partners of Gas5 with which it targets specific genes including those also influenced by cocaine. Presently, very little is known about how specific lncRNAs are guided to selective sites in the genome. Our expectation is that the development of methods (52, 53) to map the genomic binding sites of lncRNAs within identified neuronal cell types in vivo will facilitate our understanding of the influence of Gas5 on cocaine addiction.
Supplementary Material
KEY RESOURCES TABLE
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Organism/Strain | Mouse: C57BL/6J, male | The Jackson Laboratory | RRID:IMSR_JAX:000664 | |
| Viral Strain | HSV-Gas5-EGFP | Dr. Rachael Neve at MGH | N/A | Gas5 driven by IE45 promoter, EGFP driven by CMV promoter |
| Viral Strain | HSV-EGFP | Dr. Rachael Neve at MGH | N/A | EGFP driven by CMV promoter |
| Viral Strain | AAV2-Gas5-EGFP | VectorBuilder Inc | VB170715–1030 | Gas5 driven by CMV promoter, EGFP driven by EF1A promoter |
| Viral Strain | AAV2-EGFP | VectorBuilder Inc | VB170715–1030 | EGFP driven by EF1A promoter |
| Chemical Compound or Drug | Cocaine | Sigma-Aldrich | C5776 | |
| Commercial Assay Or Kit | Zymo RNA Clean and Concentrate Kit | Zymo Research | 11–352B | |
| Commercial Assay Or Kit | Trizol | Thermo Fisher | 15596026 | |
| Commercial Assay Or Kit | Agilent RNA 6000 Nano kit | Agilent | 5067–1511 | |
| Commercial Assay Or Kit | qScript cDNA Supermix | Quanta | 101414–106 | |
| Commercial Assay Or Kit | PerfeCTa SYBR Green SuperMix | Quanta | 101414–168 | |
| Commercial Assay Or Kit | Agilent DNA 1000 Kit | Agilent | 5067–1504 | |
| Commercial Assay Or Kit | KAPA Library Quantification Kit | Roche Sequencing Solutions | 7960140001 | |
| Sequence-Based Reagent | NEBNext rRNA Depletion Kit | NEB | E6350 | |
| Sequence-Based Reagent | NEBNext Ultra II Directional RNA Library Prep Kit for Illumina | NEB | E7765 | |
| Sequence-Based Reagent | NEBNext Multiplex Oligos for Illumina | NEB | E7600 | |
| Deposited Data; Public Database | GSE146005 | NCBI GEO Datasets | RRID:SCR_005012; https://www.ncbi.nlm.nih.gov/gds | |
| Deposited Data; Public Database | KEGG PATHWAY Database | https://doi.org/10.1093/nar/gkw1092 | RRID:SCR_018145; https://www.genome.jp/kegg/pathway.html | |
| Software; Algorithm | Med-PC V | Med Associates Inc. | SOF-736 | |
| Software; Algorithm | GraphPad Prism 6 | GraphPad Software | N/A | |
| Software; Algorithm | STAR software | https://doi.org/10.1093/bioinformatics/bts635 | RRID:SCR_015899; https://github.com/alexdobin/STAR | |
| Software; Algorithm | FeatureCounts software | https://doi.org/10.1093/bioinformatics/btt656 | RRID:SCR_012919; http://bioinf.wehi.edu.au/featureCounts/ | |
| Software; Algorithm | R Project for Statistical Computing software | N/A | RRID:SCR_001905; http://www.r-project.org/ | |
| Software; Algorithm | EdgeR R software package | https://doi.org/10.1093/bioinformatics/btp616 | RRID:SCR_012802; http://bioconductor.org/packages/edgeR/ | |
| Software; Algorithm | Ggplot2 R software package | N/A | RRID:SCR_014601; https://cran.r-project.org/web/packages/ggplot2/index.html | |
| Software; Algorithm | WebGestalt: WEB-based GEne SeT AnaLysis Toolkit | https://doi.org/10.1093/nar/gkz401 | RRID:SCR_006786; http://www.webgestalt.org/ | |
Acknowledgements and Disclosure
This work was supported by National Institutes of Health grants (DP1DA046587 and R01DA046720 to J.F. and P01DA047233 and R37DA007359 to E.J.N). The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nestler EJ (2005): Is there a common molecular pathway for addiction? Nat Neurosci. 8:1445–1449. [DOI] [PubMed] [Google Scholar]
- 2.Koob GF, Volkow ND (2016): Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry.3:760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng J, Nestler EJ (2013): Epigenetic mechanisms of drug addiction. Curr Opin Neurobiol. 23:521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robison AJ, Nestler EJ (2011): Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci. 12:623–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernstein BE, Birney E, Dunham I, Green ED, Gunter C, Snyder M (2012): An integrated encyclopedia of DNA elements in the human genome. Nature. 489:57–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rinn JL, Chang HY (2012): Genome regulation by long noncoding RNAs. Annu Rev Biochem. 81:145–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mercer TR, Mattick JS (2013): Structure and function of long noncoding RNAs in epigenetic regulation. Nat Struct Mol Biol. 20:300–307. [DOI] [PubMed] [Google Scholar]
- 8.Briggs JA, Wolvetang EJ, Mattick JS, Rinn JL, Barry G (2015): Mechanisms of Long Non-coding RNAs in Mammalian Nervous System Development, Plasticity, Disease, and Evolution. Neuron. 88:861–877. [DOI] [PubMed] [Google Scholar]
- 9.Andersen RE, Lim DA (2018): Forging our understanding of lncRNAs in the brain. Cell Tissue Res. 371:55–71. [DOI] [PubMed] [Google Scholar]
- 10.Ng SY, Lin L, Soh BS, Stanton LW (2013): Long noncoding RNAs in development and disease of the central nervous system. Trends in genetics : TIG. 29:461–468. [DOI] [PubMed] [Google Scholar]
- 11.Mercer TR, Qureshi IA, Gokhan S, Dinger ME, Li G, Mattick JS, et al. (2010): Long noncoding RNAs in neuronal-glial fate specification and oligodendrocyte lineage maturation. BMC Neurosci. 11:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinger ME, Amaral PP, Mercer TR, Pang KC, Bruce SJ, Gardiner BB, et al. (2008): Long noncoding RNAs in mouse embryonic stem cell pluripotency and differentiation. Genome Res. 18:1433–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ataman B, Boulting GL, Harmin DA, Yang MG, Baker-Salisbury M, Yap EL, et al. (2016): Evolution of Osteocrin as an activity-regulated factor in the primate brain. Nature. 539:242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pruunsild P, Bengtson CP, Bading H (2017): Networks of Cultured iPSC-Derived Neurons Reveal the Human Synaptic Activity-Regulated Adaptive Gene Program. Cell Rep. 18:122–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim TK, Hemberg M, Gray JM, Costa AM, Bear DM, Wu J, et al. (2010): Widespread transcription at neuronal activity-regulated enhancers. Nature. 465:182–U165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan SK, Pastori C, Penas C, Komotar RJ, Ivan ME, Wahlestedt C, et al. (2018): Serum long noncoding RNA HOTAIR as a novel diagnostic and prognostic biomarker in glioblastoma multiforme. Mol Cancer. 17:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pastori C, Wahlestedt C (2012): Involvement of long noncoding RNAs in diseases affecting the central nervous system. RNA Biol. 9:860–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Issler O, van der Zee YY, Ramakrishnan A, Wang J, Tan C, Loh YE, et al. (2020): Sex-Specific Role for the Long Non-coding RNA LINC00473 in Depression. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faghihi MA, Modarresi F, Khalil AM, Wood DE, Sahagan BG, Morgan TE, et al. (2008): Expression of a noncoding RNA is elevated in Alzheimer’s disease and drives rapid feed-forward regulation of beta-secretase. Nature medicine. 14:723–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie Y, Hayden MR, Xu B (2010): BDNF overexpression in the forebrain rescues Huntington’s disease phenotypes in YAC128 mice. J Neurosci. 30:14708–14718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao X, Tang Z, Zhang H, Atianjoh FE, Zhao JY, Liang L, et al. (2013): A long noncoding RNA contributes to neuropathic pain by silencing Kcna2 in primary afferent neurons. Nat Neurosci. 16:1024–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spadaro PA, Flavell CR, Widagdo J, Ratnu VS, Troup M, Ragan C, et al. (2015): Long Noncoding RNA-Directed Epigenetic Regulation of Gene Expression Is Associated With Anxiety-like Behavior in Mice. Biol Psychiatry. 78:848–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Labonte B, Abdallah K, Maussion G, Yerko V, Yang J, Bittar T, et al. (2020): Regulation of impulsive and aggressive behaviours by a novel lncRNA. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bu Q, Hu Z, Chen F, Zhu R, Deng Y, Shao X, et al. (2012): Transcriptome analysis of long non-coding RNAs of the nucleus accumbens in cocaine-conditioned mice. J Neurochem. 123:790–799. [DOI] [PubMed] [Google Scholar]
- 25.Bannon MJ, Savonen CL, Jia H, Dachet F, Halter SD, Schmidt CJ, et al. (2015): Identification of long noncoding RNAs dysregulated in the midbrain of human cocaine abusers. J Neurochem. 135:50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saad MH, Rumschlag M, Guerra MH, Savonen CL, Jaster AM, Olson PD, et al. (2019): Differentially expressed gene networks, biomarkers, long noncoding RNAs, and shared responses with cocaine identified in the midbrains of human opioid abusers. Sci Rep. 9:1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng J, Shao N, Szulwach KE, Vialou V, Huynh J, Zhong C, et al. (2015): Role of Tet1 and 5-hydroxymethylcytosine in cocaine action. Nature neuroscience. 18:536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng J, Wilkinson M, Liu X, Purushothaman I, Ferguson D, Vialou V, et al. (2014): Chronic cocaine-regulated epigenomic changes in mouse nucleus accumbens. Genome Biol. 15:R65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hurd YL (1996): Cocaine effects on dopamine and opioid peptide neural systems: implications for human cocaine abuse. NIDA Res Monogr. 163:94–116. [PubMed] [Google Scholar]
- 30.Carlezon WA Jr., Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, et al. (1998): Regulation of cocaine reward by CREB. Science. 282:2272–2275. [DOI] [PubMed] [Google Scholar]
- 31.Heidbreder CA, Goldberg SR, Shippenberg TS (1993): The kappa-opioid receptor agonist U-69593 attenuates cocaine-induced behavioral sensitization in the rat. Brain research. 616:335–338. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki T, Shiozaki Y, Masukawa Y, Misawa M, Nagase H (1992): The role of mu- and kappa-opioid receptors in cocaine-induced conditioned place preference. Jpn J Pharmacol. 58:435–442. [DOI] [PubMed] [Google Scholar]
- 33.Spealman RD, Bergman J (1992): Modulation of the discriminative stimulus effects of cocaine by mu and kappa opioids. J Pharmacol Exp Ther. 261:607–615. [PubMed] [Google Scholar]
- 34.Gancarz AM, Wang ZJ, Schroeder GL, Damez-Werno D, Braunscheidel KM, Mueller LE, et al. (2015): Activin receptor signaling regulates cocaine-primed behavioral and morphological plasticity. Nat Neurosci. 18:959–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu C, Zhang Y, Wang Q, Xu Z, Jiang J, Gao Y, et al. (2016): Long non-coding RNA GAS5 controls human embryonic stem cell self-renewal by maintaining NODAL signalling. Nat Commun. 7:13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tu J, Tian G, Cheung HH, Wei W, Lee TL (2018): Gas5 is an essential lncRNA regulator for self-renewal and pluripotency of mouse embryonic stem cells and induced pluripotent stem cells. Stem Cell Res Ther. 9:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun D, Yu Z, Fang X, Liu M, Pu Y, Shao Q, et al. (2017): LncRNA GAS5 inhibits microglial M2 polarization and exacerbates demyelination. EMBO reports. 18:1801–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen F, Zhang L, Wang E, Zhang C, Li X (2018): LncRNA GAS5 regulates ischemic stroke as a competing endogenous RNA for miR-137 to regulate the Notch1 signaling pathway. Biochemical and biophysical research communications. 496:184–190. [DOI] [PubMed] [Google Scholar]
- 39.Gao ZQ, Wang JF, Chen DH, Ma XS, Wu Y, Tang Z, et al. (2017): Long non-coding RNA GAS5 suppresses pancreatic cancer metastasis through modulating miR-32–5p/PTEN axis. Cell & bioscience. 7:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feldker DE, Datson NA, Veenema AH, Proutski V, Lathouwers D, De Kloet ER, et al. (2003): GeneChip analysis of hippocampal gene expression profiles of short- and long-attack-latency mice: technical and biological implications. Journal of neuroscience research. 74:701–716. [DOI] [PubMed] [Google Scholar]
- 41.Xiong K, Long L, Zhang X, Qu H, Deng H, Ding Y, et al. (2017): Overview of long non-coding RNA and mRNA expression in response to methamphetamine treatment in vitro. Toxicology in vitro : an international journal published in association with BIBRA. 44:1–10. [DOI] [PubMed] [Google Scholar]
- 42.Mercer TR, Dinger ME, Sunkin SM, Mehler MF, Mattick JS (2008): Specific expression of long noncoding RNAs in the mouse brain. Proc Natl Acad Sci U S A. 105:716–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Belgard TG, Marques AC, Oliver PL, Abaan HO, Sirey TM, Hoerder-Suabedissen A, et al. (2011): A transcriptomic atlas of mouse neocortical layers. Neuron. 71:605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bernard D, Prasanth KV, Tripathi V, Colasse S, Nakamura T, Xuan Z, et al. (2010): A long nuclear-retained non-coding RNA regulates synaptogenesis by modulating gene expression. EMBO J. 29:3082–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eissmann M, Gutschner T, Hammerle M, Gunther S, Caudron-Herger M, Gross M, et al. (2012): Loss of the abundant nuclear non-coding RNA MALAT1 is compatible with life and development. RNA Biol. 9:1076–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang B, Arun G, Mao YS, Lazar Z, Hung G, Bhattacharjee G, et al. (2012): The lncRNA Malat1 is dispensable for mouse development but its transcription plays a cis-regulatory role in the adult. Cell Rep. 2:111–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barry G, Briggs JA, Vanichkina DP, Poth EM, Beveridge NJ, Ratnu VS, et al. (2014): The long non-coding RNA Gomafu is acutely regulated in response to neuronal activation and involved in schizophrenia-associated alternative splicing. Mol Psychiatry. 19:486–494. [DOI] [PubMed] [Google Scholar]
- 48.Ip JY, Sone M, Nashiki C, Pan Q, Kitaichi K, Yanaka K, et al. (2016): Gomafu lncRNA knockout mice exhibit mild hyperactivity with enhanced responsiveness to the psychostimulant methamphetamine. Sci Rep. 6:27204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schneider C, King RM, Philipson L (1988): Genes specifically expressed at growth arrest of mammalian cells. Cell. 54:787–793. [DOI] [PubMed] [Google Scholar]
- 50.Kino T, Hurt DE, Ichijo T, Nader N, Chrousos GP (2010): Noncoding RNA gas5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Science signaling. 3:ra8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walker DM, Cates HM, Loh YE, Purushothaman I, Ramakrishnan A, Cahill KM, et al. (2018): Cocaine Self-administration Alters Transcriptome-wide Responses in the Brain’s Reward Circuitry. Biol Psychiatry. 84:867–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chu C, Qu K, Zhong FL, Artandi SE, Chang HY (2011): Genomic maps of long noncoding RNA occupancy reveal principles of RNA-chromatin interactions. Molecular cell. 44:667–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simon MD, Wang CI, Kharchenko PV, West JA, Chapman BA, Alekseyenko AA, et al. (2011): The genomic binding sites of a noncoding RNA. Proc Natl Acad Sci U S A. 108:20497–20502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


