Abstract
The light-sensitive outer segment organelle of the vertebrate photoreceptor cell is a modified cilium filled with hundreds of flattened “disc” membranes that provide vast light-absorbing surfaces. The outer segment is constantly renewed with new discs added at its base every day. This continuous process is essential for photoreceptor viability. In this review, we describe recent breakthroughs in our understanding of disc morphogenesis, with a focus on the molecular mechanisms responsible for initiating disc formation from the ciliary membrane. We highlight the discoveries that this mechanism evolved from an innate ciliary process of releasing small extracellular vesicles, or ectosomes, and that both disc formation and ectosome release rely on the actin cytoskeleton.
Keywords: vision, photoreceptor, outer segment, cilia, extracellular vesicle, actin cytoskeleton
The photoreceptor outer segment is a highly modified primary cilium
Vertebrate vision begins when light is absorbed by the rod and cone photoreceptor cells of the retina. Rods are highly sensitive to light and primarily responsible for night vision, while cones provide color vision in daylight. Rods and cones contain a specialized organelle, called the photoreceptor outer segment (see Glossary), which is a primary cilium highly modified to serve as an efficient light sensor (Fig. 1). The outer segment contains a microtubular axoneme and is tightly packed with a stack of ~800-2,000 flat disc-shaped membranes, or photoreceptor discs [1] (see [2–4] for reviews). Discs are spaced 32 nm apart [5, 6], which is the length of four tubulin dimers, suggesting that the distance between them is templated by the axoneme. Disc stacks serve to maximize light capture because incoming photons must pass through hundreds of membrane surfaces densely packed with the light-absorbing protein, opsin.
Figure 1. Photoreceptor outer segments are highly modified primary cilia.
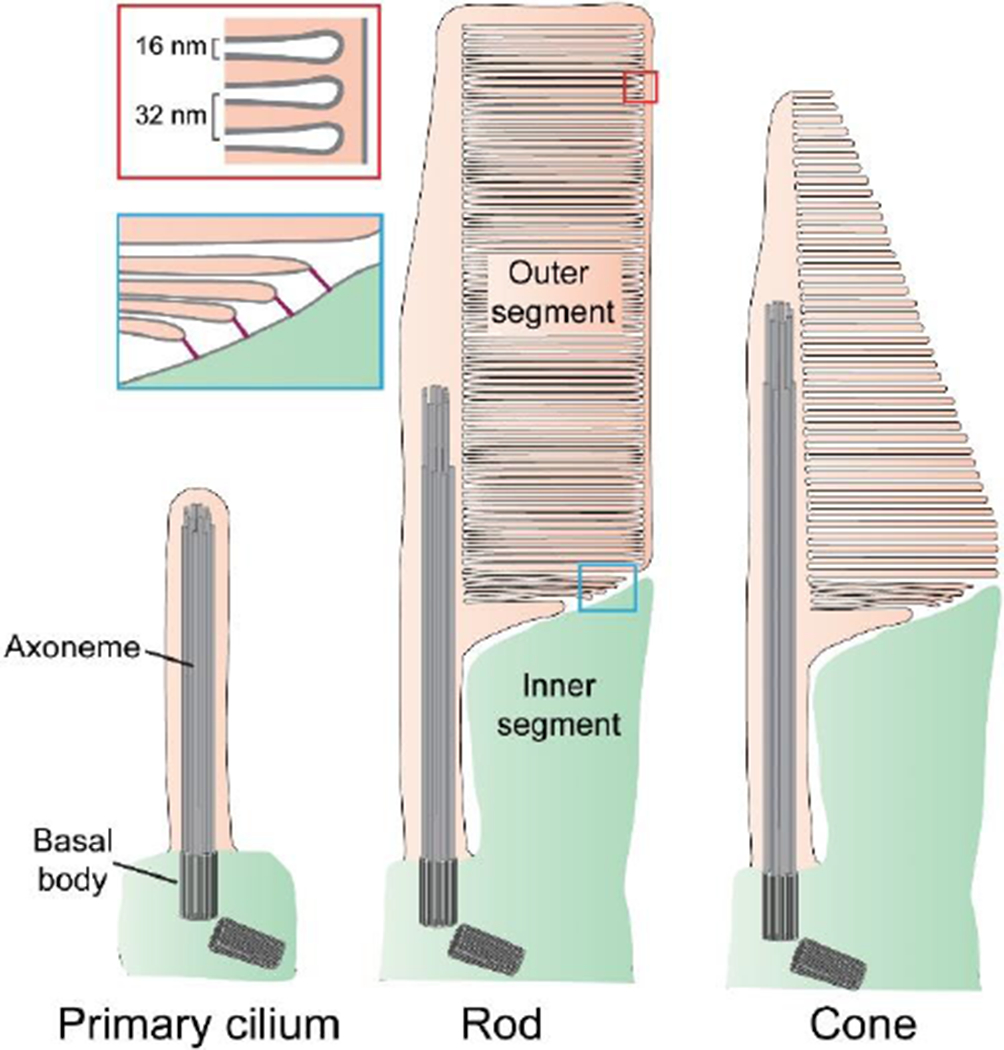
A prototypic primary cilium and outer segments of rod and cone photoreceptor cells are illustrated to highlight their similarity. Each structure is stabilized by a microtubular axoneme extending from a basal body. Photoreceptor discs form at the base of the outer segment as evaginations of the ciliary plasma membrane. In rods, discs eventually enclose inside the outer segment. In cones of lower vertebrates (as illustrated), discs remain as lamellar folds of the plasma membrane. In mammalian cones, discs undergo partial enclosure. The red inset highlights the periodicity of disc spacing. The blue inset illustrates that newly forming discs are connected to the inner segment through linking structures.
Another feature that allows photoreceptors to produce large electrical responses to light is the physical separation of discs from the outer segment plasma membrane, through the process of disc enclosure, which expands the cytosolic space available for rapid longitudinal diffusion of the second messenger, cGMP. Longitudinal cGMP diffusion is further facilitated in many species by indentations of disc rims, known as disc incisures, which are aligned from disc to disc. Together, these specializations allow the propagation of the cGMP signal over a longer distance, ultimately producing a larger electrical response [7]. Outer segment morphology is different between rods and cones (Fig. 1). In rods, discs are completely enclosed. In fish and amphibian cones, discs exist as serial (non-enclosed) folds of the plasma membrane, whereas discs of mammalian cones are partially enclosed. These morphological features contribute to setting the respective light-sensitivities of these photoreceptors: more diffusional space in rods allows them to produce larger electrical responses to identical light stimuli than cones.
Outer segments are continuously renewed by adding new discs at the base and shedding old discs from the tip [8]. This continuous process is critical for maintaining photoreceptor health. Mutations in proteins involved in disc morphogenesis lead to the loss of outer segment integrity, eventually causing photoreceptor cell death and blindness in humans [9]. In this review, we discuss recent developments in our understanding of disc morphogenesis and the striking connection between this process and the formation of extracellular vesicles by the primary cilium. The key points of this review are illustrated in two animations (Movies S1 and S2), which we strongly encourage readers to watch.
Discs begin as evaginations of the ciliary membrane
Intrigued by the stunning architecture of the outer segment, biologists have wondered how discs are formed since the 1950s (see [2] for a historical review). In a classic paper, Steinberg and colleagues [10] argued that newly forming discs are evaginations of the ciliary membrane, which increase in size until they reach their final diameter and become enclosed within the outer segment. This model was supported by studies using membrane impermeable lipophilic dyes, which showed that newly forming, but not mature discs, are exposed to the extracellular space (i.e. they are “open”) [11, 12]. An alternative model, primarily based on the observation of small intracellular vesicles at the site of disc formation, postulated that discs are assembled through vesicular fusion inside the outer segment [13–15].
In 2015, three simultaneously published papers decisively confirmed the membrane evagination model of disc morphogenesis [16–18]. Ding and colleagues [16] developed an electron microscopy (EM) tissue processing protocol that optimally preserves newly forming discs and preferentially stains membranes directly exposed to the extracellular space. This was achieved by staining tissue with tannic acid, which poorly penetrates intact membranes. This method labeled newly forming discs much more intensely than mature discs (Fig. 2A), indicating that they are exposed to the extracellular space. Notably, a similar distinction between the staining of open and enclosed discs was recently achieved using different tissue contrasting agents [19].
Figure 2. The outer segment evolved through the retention of ciliary ectosomes.
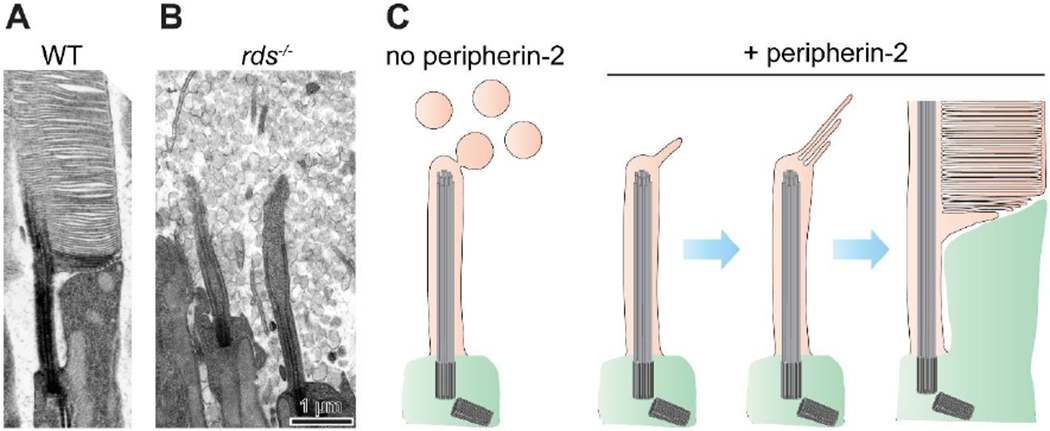
(A) An electron micrograph of an inner-outer segment juncture from a WT mouse rod. The retinas were contrasted with tannic acid to discern between discs exposed to the extracellular space (darkly stained membranes) and those enclosed inside the outer segment (lightly stained membranes). (B) An electron micrograph of a comparable region from an rds−/− mouse lacking peripherin-2 (adapted from [16]). The photoreceptor cilium fails to elaborate an outer segment and resembles a prototypic primary cilium surrounded by massive amounts of ectosomes. (C) A hypothesis on the evolutionary origin of the outer segment. The outer segment evolved through introduction of the photoreceptor-specific protein, peripherin-2, which enabled prototypic photoreceptors to retain ectosomes at the cilium. Subsequent adaptations allowed flattening, elongation and enclosure of retained membrane material.
Volland and colleagues [17] developed another procedure for optimal tissue preservation that combines chemical fixation with high-pressure freezing [20]. They performed 3D electron tomography of newly forming disc membranes in rods of three mammalian species and found that all newly forming discs are continuous with the plasma membrane and exposed to the extracellular space. Interestingly, these tomograms revealed that the newest, not-yet-flattened evaginating disc often contains a narrow membrane tunnel originating from this disc’s basal surface, perhaps initiating the formation of the disc rim.
Burgoyne and colleagues [18] also performed 3D electron tomography on mouse rods and showed that newly forming discs are evaginations of the ciliary membrane. These tomograms revealed the presence of extracellular links connecting the expanding edges of newly forming discs with the photoreceptor inner segment (Fig. 1, blue inset). The authors proposed that these links are formed by the photoreceptor-specific protein protocadherin-21 (a.k.a. cadherin-related family member 1) extending from the disc edge to an unknown partner in the inner segment membrane.
Importantly, none of these three studies observed any vesicles at the site of disc formation, and it was further demonstrated that disc membrane vesiculation at the base of the outer segment is a common artifact of imperfect tissue fixation [16]. Collectively, these publications settled the debate: discs are formed as evaginations of the ciliary membrane and are exposed to the extracellular space until becoming fully enclosed.
Disc formation evolved from the process of ciliary ectosome release
Another cellular process involving plasma membrane evagination is the formation of ectosomes, small ~100-1000 nm vesicles that bud directly from the cell’s plasma membrane into the extracellular space [21]. Remarkably, ectosomes can be released directly from the primary cilium to serve an array of functions [22–26]. Recent literature suggests that ciliary ectocytosis can facilitate cell-to-cell communication [27, 28] or serve to rapidly dispose of material from the cilium, thereby regulating G protein-coupled receptor (GPCR) signaling [29] and cell cycle reentry [30].
Realizing that both disc formation and ciliary ectosome release involve membrane evagination, Salinas and colleagues [31] proposed that discs are modified ectosomes which are retained and flattened at the photoreceptor cilium instead of being released. To test this hypothesis, they revisited the phenotype of the retinal degeneration slow (rds) mouse [32], which lacks peripherin-2 protein [33], the photoreceptor-specific tetraspanin long known to fortify the rims of mature discs [34]. The subretinal space in rds mice is densely packed with extracellular vesicles (Fig. 2B) [35, 36] that contain rhodopsin [37, 38] and appear to originate from the photoreceptor cilium [39]. Salinas and colleagues [31] established that these vesicles are ciliary ectosomes based on their protein composition, size distribution and observations of them directly budding from the photoreceptor cilium. This led to the remarkable conclusion that the outer segment evolutionarily diverged from a primary cilium through the expression of a single protein, which retains ectosomes to expand the ciliary membrane for enhanced light capture (Fig. 2C). Further adaptations, including membrane flattening, stacking and enclosure, allowed the outer segment to evolve into a highly efficient light sensor.
Disc formation is initiated by a branched actin network
Studies in the 1980s revealed the presence of a patch of filamentous actin at the site of disc morphogenesis and nowhere else in the outer segment (Fig. 3A) [40–42]. The functional significance of this actin was shown by treating frog and rabbit retinas with the actin polymerization inhibitor cytochalasin D [43]. This treatment prevented the initiation of new disc formation without stopping the continued delivery of protein and membrane material to the outer segment. As a result, this material was directed to the several nascent discs whose formation was ongoing at the time of treatment, resulting in their uncontrolled overgrowth (Fig. 3B,C).
Figure 3. Branched actin polymerization initiates disc formation.
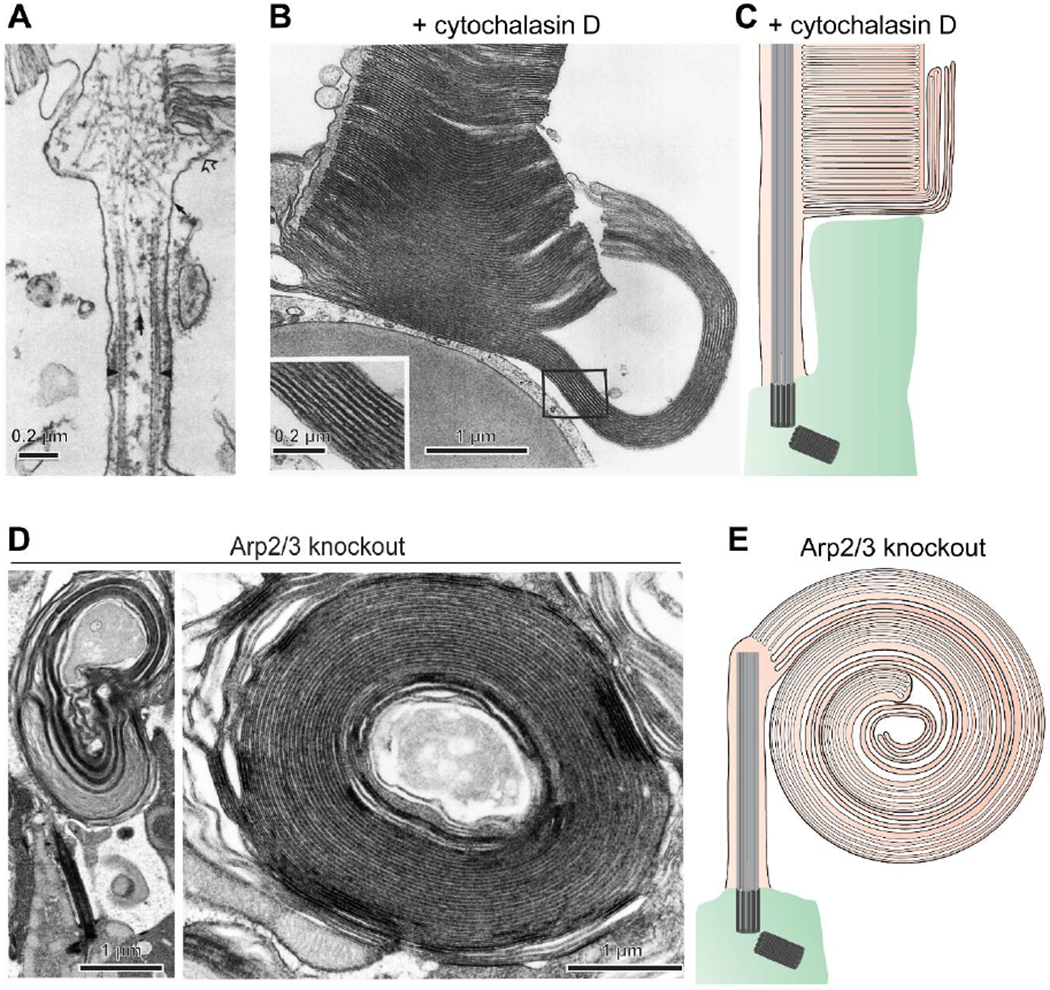
(A) An electron micrograph showing myosin-labeled actin filaments at the site of disc morphogenesis in a rat rod (adapted from [40]). (B) An electron micrograph of a cone from a Xenopus laevis retina acutely treated with the actin polymerization inhibitor cytochalasin D reveals overgrowth of newly forming discs (adapted from [43]). (C) A cartoon illustrating the effect of cytochalasin D treatment. The drug halted the initiation of new disc formation without preventing delivery of new membrane material to the outer segment. This material incorporates into the newly forming discs which were undergoing expansion at the time of treatment, causing their uncontrolled overgrowth. (D) Electron micrographs of overgrown membranes in rods of Arp2/3 knockout mice which have elaborated into large whorls. (E) A cartoon illustrating a massive membrane whorl in the Arp2/3 knockout rod. A permanent halting of the initiation of new disc formation by this knockout causes much more extended membrane outgrowth than an acute cytochalasin D treatment. Images in (C-E) are adapted from [44].
To understand how the actin cytoskeleton is regulated at the site of disc formation, Spencer and colleagues [44] sought to identify proteins located there that catalyze actin nucleation – a critical, rate-limiting step in the formation of actin networks. Following a lead from their proteomic analysis, the authors focused on the Arp2/3 actin nucleation complex, which is unique in its ability to induce the polymerization of branched actin networks (see [45] for review). They generated a rod-specific knockout of an indispensable subunit of this complex, ArpC3, effectively producing an Arp2/3 knockout. This knockout resulted in the complete loss of actin filaments specifically at the outer segment base and prevented the formation of new discs without stopping the delivery of membrane material to the outer segment. As in the case of cytochalasin D treatment [43], Arp2/3 knockout caused massive outgrowth of newly forming disc membranes (Fig. 3D,E). Unlike with acute cytochalasin D treatment, the effect of the Arp2/3 knockout was permanent and, accordingly, produced an even more exaggerated overgrowth of newly forming discs, leading to the formation of massive membrane whorls. This work established that the formation of new discs is initiated by the polymerization of branched actin in a process resembling the formation of lamellipodia in migrating cells [45].
The fact that actin polymerization at the outer segment base is essential for forming new discs poses two fundamental questions. First, what specifies the outer segment base as a site of actin polymerization? Second, what regulates the periodicity of actin polymerization and disassembly during the formation of each new disc?
Whereas the second question remains unanswered, a critical contributor to organizing the actin network at the disc formation site was recently identified by Corral-Serrano and colleagues [46]. They showed that the photoreceptor-specific protein PCARE (Photoreceptor Cilium Actin Regulator; originally called C2orf71) localizes to this site and recruits the actin nucleation promoting factor Wasf3. Nucleation promoting factors are indispensable regulators of actin networks that act in concert with Arp2/3 to form branched actin filaments [45]. The knockout of PCARE results in the loss of both Wasf3 and filamentous actin at the site of disc morphogenesis [46] and leads to the formation of membrane whorls growing from the photoreceptor cilium [47] in a phenotype similar to the Arp2/3 knockout.
These data suggest that PCARE specifies the location at which the actin network is built to perform ciliary membrane evagination. This is consistent with the ciliary targeting of PCARE in cell culture [46, 48]. However, it remains to be determined how PCARE concentrates precisely at the site of disc morphogenesis rather than decorating the entire outer segment or axoneme as it does in cell culture. Another unknown relates to the fact that each actin nucleation promoting factor is typically controlled by a small GTPase, which is known to be Racl in the case of Wasf3 [49]. However, a rod photoreceptor-specific knockout of Racl was reported to have normal outer segments [50], which raises the questions of whether Wasf3 is actually critical for disc formation and whether any small GTPase is involved.
Despite explosive progress in the identification of molecular players responsible for building the actin network during new disc formation, little is known about the proteins responsible for controlling the degree of this network’s expansion and subsequent disassembly. A recent study [51] showed that knockdown of nudC, a regulator of the actin depolymerizing protein cofilin-1 [52], induces a complex phenotype in Xenopus laevis photoreceptors, including overgrown discs at the base of the outer segment. Another recent study reported that the connecting cilium-specific protein RPGR binds to another actin depolymerizing protein, gelsolin [53]. The knockout of either RPGR or gelsolin increases filamentous actin staining at the outer segment base and leads to photoreceptor degeneration. Whether any of these four proteins are directly involved in actin network disassembly at the site of disc formation awaits further validation.
A cycle of actin polymerization and disassembly during new disc formation
Collectively, the findings outlined in the previous section establish a model describing how the initiation of disc morphogenesis is driven by actin, which we illustrate as a step-by-step sequence (Fig. 4 and Movie S1). First, PCARE recruits Wasf3 to the outer segment base where it interacts with Arp2/3 to nucleate the polymerization of branched actin. Actin polymerization pushes out the ciliary membrane to form a new evagination. Before this evagination reaches the final diameter of a mature disc, the actin network is disassembled but the membrane remains protruded and undergoes flattening. Meanwhile, the membrane keeps elongating through continuous incorporation of new membrane material until it reaches its final diameter and becomes enclosed within the outer segment, as described below.
Figure 4. A cycle of actin dynamics during disc formation.
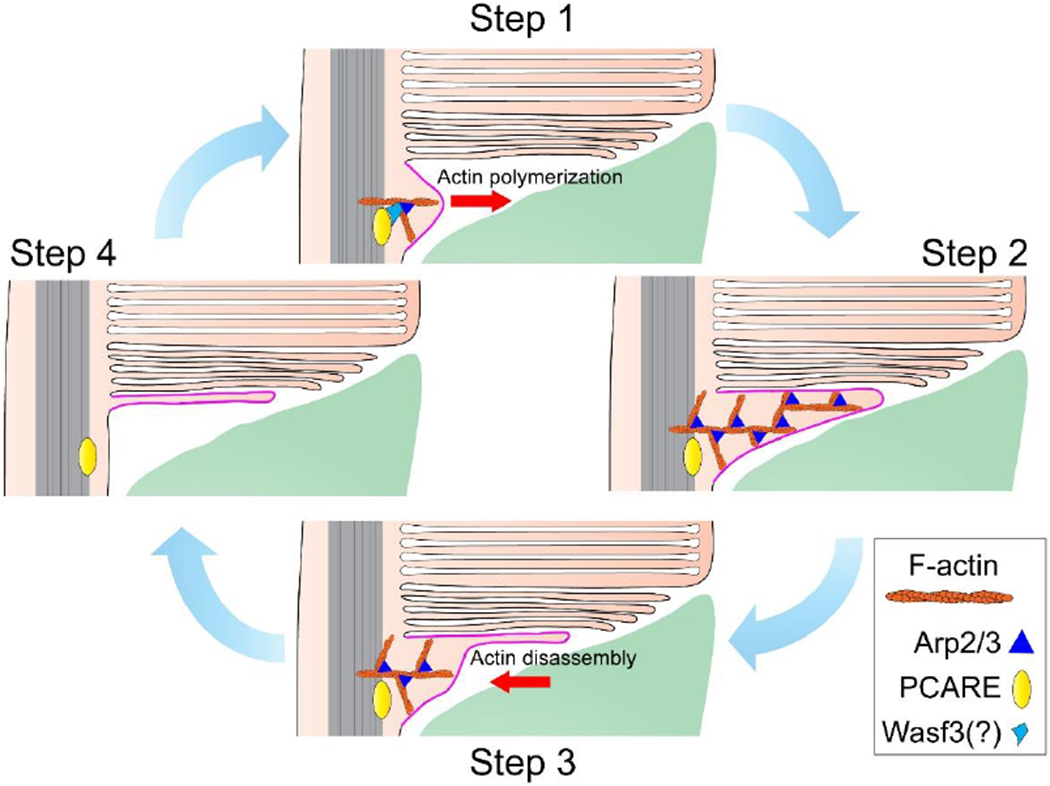
A cycle of actin polymerization/disassembly begins in Step 1 when PCARE recruits an actin nucleation promoting factor, likely Wasf3, to the site of disc morphogenesis. This actin nucleation promoting factor associates with the Arp2/3 complex to nucleate branched actin filaments (F-actin). In Step 2, continued actin polymerization pushes the ciliary membrane outwards. The Arp2/3 complex remains as a structural component of these filaments. In Step 3, while the membrane remains protruded, actin filaments are disassembled and retracted allowing the evaginated membrane to flatten. In Step 4, actin has fully retracted and the initiation step of disc formation is complete. Meanwhile, the newly forming disc continues to elongate through ongoing addition of membrane material. The cartoon is updated from [44]. See also Movie S1 to view the entire process of disc formation.
Actin participates in ciliary ectosome formation
The connection between disc morphogenesis and ciliary ectosome release is not only conceptual but also mechanistic, as both of these processes rely on the action of the actin cytoskeleton. Inhibition of actin polymerization by cytochalasin D prevented the release of ciliary ectosomes from cultured cells [29], just as it prevents the formation of new discs. Furthermore, ciliary ectosome release from these cells was also suppressed by pharmacological inhibition of the Arp2/3 complex [29]. Likewise, actin polymerization is required for the release of ciliary ectosomes in mouse embryonic fibroblasts in a process regulated by the phosphoinositide PI(4,5)P2 [30].
The specific role of actin in ciliary ectosome release may be more complex than merely pushing out the membrane. It was recently proposed that actin may directly participate in the scission of ciliary ectosomes, either by actin ring constriction (like in cytokinesis) or by actin-induced lipid demixing [54]. This hypothesis awaits thorough validation. For instance, constricting actin rings are built from linear actin filaments nucleated by formins rather than branched actin networks built by Arp2/3 [55]. Yet pharmacological inhibition of formin failed to block ciliary ectosome release [29]. Similarly, lipid demixing by branched actin is known to drive membrane endocytosis rather than ectocytosis [56].
Another molecular player likely involved in ciliary ectocytosis is the ESCRT complex, the molecular machinery ubiquitously performing scission of outward budding membranes. Several ESCRT proteins have been identified in ciliary transition zones and ciliary ectosomes in Chlamydomonas and implicated in ciliary ectosome release [23, 57]. An ESCRT-associated protein Alix was found in ectosomes released from both Chlamydomonas cilia [23] and rds mouse photoreceptors [44]. The precise role of ESCRT proteins in ciliary ectosome release remains to be determined. It is also unknown whether the ESCRT complex is required for membrane scission upon ectosome release by rds photoreceptors and how this scission is prevented in normal photoreceptor cells.
The mechanism of disc flattening remains enigmatic
Once the actin filaments driving the formation of a new disc start to disassemble, the evaginating membrane flattens (Steps 3 and 4 in Fig. 4). The molecular mechanism responsible for disc flattening remains unknown. One protein implicated in this process is PRCD (Progressive Rod-Cone Degeneration; a.k.a. photoreceptor disk component PRCD) [58, 59], a small ~6 kDa polypeptide residing specifically in photoreceptor discs [60, 61]. PRCD is anchored in the disc membrane with its C-terminus exposed to the outer segment cytoplasm [62, 63] and is tightly associated with rhodopsin [62]. PRCD immunogold staining [64] led the authors to believe that it is concentrated at the outer segment base. However, this is not corroborated by immunostaining of endogenous [60–62, 64] or epitope-tagged PRCD [62, 63], clearly showing that PRCD is evenly distributed throughout the outer segment length.
The knockout of PRCD results in a peculiar phenotype whereby photoreceptors produce relatively normal outer segments but also release ectosomes (Fig. 5) [61, 64]. Ultrastructural analysis including 3D electron tomography showed that PRCD knockout causes a defect in the flattening of newly forming discs [61]. Newly evaginating discs tend to bulge, yet they manage to eventually flatten, likely releasing their swollen portions as ectosomes. This error-prone disc formation leads to slowly progressing photoreceptor cell death. Despite this recent progress toward understanding PRCD function, the mechanism by which it contributes to disc flattening remains unknown.
Figure 5. PRCD is required for the efficient flattening of newly forming discs.
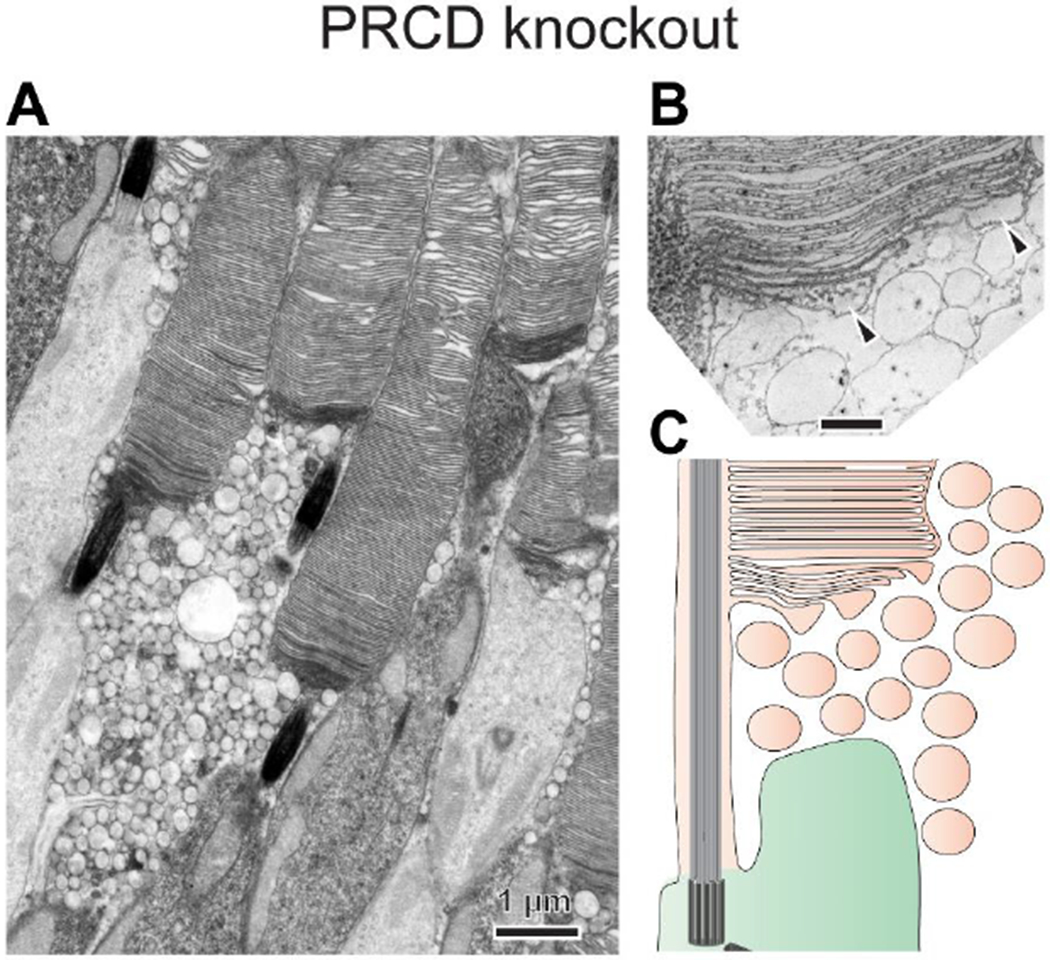
(A) An electron micrograph of PRCD knockout rods reveals the presence of ectosomes accumulated at the outer segment base. (B) An image of a 1 nm z-section from a 3D electron tomogram taken at the base of a PRCD knockout rod outer segment showing that newly forming discs fail to efficiently flatten, forming bulges filled with cytoplasm (marked by arrowheads). These bulges shed as ectosomes before disc enclosure, allowing mature discs to be flat inside the outer segment. (C) An illustration of the PRCD knockout phenotype. Images are adapted from [61].
Another potential contributor to the orderly stacking of flattened discs is rhodopsin. It was proposed that rhodopsin molecules located on apposing extracellular surfaces of evaginating discs engage in adhesive in-trans interactions [65, 66], acting like a “molecular Velcro” leaving essentially no aqueous space between these membranes. A conceptual analogy to this model can be found in chloroplasts, which contain stacked thylakoid membranes for efficient light absorption. These membranes are thought to be laminated together by in-trans electrostatic interactions between apposing trimers of the light-absorbing protein, LHCII [67]. Whether or not rhodopsin functions as Velcro, as well as any significance of its association with PRCD, awaits experimental validation.
Disc enclosure requires complex membrane remodeling
When the expanding membrane of a newly forming disc, in rods of all species and cones of higher vertebrates, reaches its final diameter, it undergoes a process of enclosure within the outer segment plasma membrane, often described as “membrane zipping”. Zipping does not involve membrane fusion, but rather is a complex membrane remodeling process eloquently described in the classic paper by Steinberg and colleagues [10]: The two surfaces of an evagination, [apical and basal], each form one of the surfaces of adjacent discs. The disc rim is initially specified as a region of ciliary membrane between adjacent disc-surface evaginations. This region grows bilaterally around the circumferences of adjacent discs, zippering together the apposed surfaces to form the rim and completed disc. At the same time, it seals the plasma membrane edges of the evaginations, which have become detached from the surfaces (please refer to the 3D animation in Movie S2).
As the hairpin-shaped rim of a zipping disc is formed, it becomes populated by oligomers of peripherin-2 (Fig. 6A,B) [16, 68], suggesting that this protein plays an active role in disc enclosure. Consistent with this idea, peripherin-2 (co-purified with its homologous binding partner ROM1) induces membrane curvature upon reconstitution into lipid membranes [69] and leads to the formation of highly curved intracellular membrane tubules upon expression in cell culture [31, 70, 71]. Intriguingly, these tubules are reminiscent of the tubular invaginations observed in the first newly forming disc [17], implying that peripherin-2 may play a role in the formation of this structure in a photoreceptor. Another important aspect of disc rim formation is that the expression level of peripherin-2 is crucial for achieving normal disc shape and size. A loss of one copy of the peripherin-2 gene, as well as several mutations affecting peripherin-2 expression level, result in the formation of irregularly sized and shaped discs [34].
Figure 6. Peripherin-2 fortifies the disc rim.
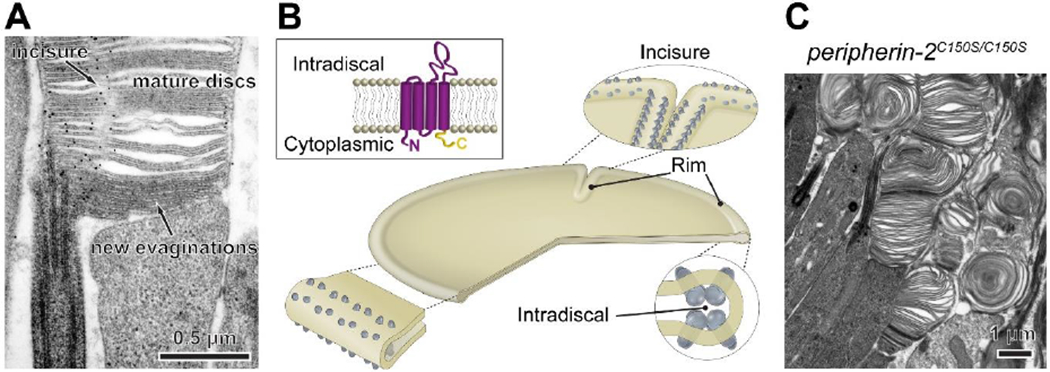
(A) Immunogold labeling shows that, in newly forming discs, peripherin-2 localizes only to the disc rims adjacent to the axoneme and is absent from the elongating disc edges. In mature discs, peripherin-2 localizes to the entire disc rim, including incisures. Shown is an uncropped version of an image from [16]. (B) Illustration of peripherin-2 localization in a disc. Long, self-assembling chains of peripherin-2 oligomers extend circumferentially throughout the disc rim to form and maintain its hairpin-like shape. Peripherin-2 oligomers similarly regulate the formation of disc incisures and fortify their rims. The image is modified from [71]. The rectangular inset illustrates the organization of the peripherin-2 molecule. The tetraspanin body (purple) is critical for supporting disc rim structure, while the C-terminus (yellow) is essential for preventing the release of ciliary ectosomes. (C) The C150S mutation of peripherin-2, which prevents its high-order oligomerization, causes major outer segment abnormalities in knockin mice. Tannic acid staining reveals the presence of both darkly stained (open) and lightly stained (enclosed) membrane structures, suggesting that high-order peripherin-2 oligomerization is not essential for disc enclosure.
The two critical functions of peripherin-2 are performed by different parts of its molecule: membrane curvature is induced by its tetraspanin core, whereas the retention of ciliary ectosomes described above is achieved by its C-terminus (Fig. 6B, inset) [31]. In photoreceptors, genetic replacement of peripherin-2’s tetraspanin core with that of ROM1 (leaving the C-terminus of peripherin-2 for ectosome retention) leads to a disruption of the hairpin-shaped disc rim structure [72].
The role of peripherin-2 oligomerization in supporting disc rim structure was shown in studies of mice and frogs expressing its C150S mutant [71, 73–77], shown to prevent high-order peripherin-2 oligomerization In vitro [78, 79]. Introduction of this mutation causes severe disruption of outer segment structure in mice (Fig. 6C) [73–75, 77]. Transgenic expression of the C150S mutant in frogs disrupts the continuity of disc rims and incisures [71] (the latter also noted in [76]). This result led to the proposal that long, self-assembling oligomeric chains of peripherin-2, up to 100 nm in length, extend around the disc rim circumference to maintain its shape and continuity [71].
In rods, disc enclosure culminates in membrane scission, which completely separates each mature disc from the surrounding plasma membrane. Conceptually, disc scission resembles an endocytic event, such as budding of a vesicle from the plasma membrane into the cytoplasm. It is not known whether peripherin-2 participates in membrane scission, however, a recent study found that disruption of its oligomerization in the C150S knockin mouse does not prevent disc enclosure [77]. As shown in Fig. 6C, the majority of dysmorphic outer segment membranes produced by rods of this mouse are enclosed. Still, the entire cohort of proteins involved in the process of disc enclosure remains to be determined.
Concluding remarks
The past five years have been marked by rapid advances in our understanding of how the light-sensitive photoreceptor outer segment is built. Reconfirming that photoreceptor discs are formed through an evagination of the ciliary membrane stimulated newfound interest in elucidating the underlying mechanisms and sparked the conceptual connection between disc formation and ciliary ectosome release. We have now gained a general appreciation of how new discs are initiated through the expansion of branched actin filaments. However, much remains to be learned about what controls the cyclic nature of this never-ending process, as well as the subsequent steps of disc maturation: flattening, expansion and enclosure (see Outstanding Questions). Equipped by new ultrastructural techniques and the growing availability of mouse models, the field is poised to continue making great strides in this direction.
Outstanding questions.
Each photoreceptor cell produces a new disc about every 20 minutes. What cellular signal controls the timing of disc formation?
Actin polymerization pushes out the ciliary membrane to initiate disc formation. How and when is this actin depolymerized?
How does the disc assume and maintain its flat shape?
How does the disc become enclosed inside the outer segment?
Discs are formed in a process that evolved from ciliary ectocytosis. What is the full extent of overlap between the molecular mechanisms underlying these processes?
Is it possible to trace at which point each step of disc morphogenesis, from ectosome retention to disc enclosure, arose in the evolution of ciliary photoreceptors?
Supplementary Material
Highlights:
Light capturing “disc” membranes of vertebrate photoreceptor cells are formed as serial evaginations of the ciliary membrane.
Many primary cilia release extracellular vesicles called ectosomes.
The photoreceptor cilium also has an innate ability to release ectosomes, but they are retained by the photoreceptor-specific protein peripherin-2. Retained ectosomes are transformed into discs through membrane flattening, expansion and enclosure.
Both ciliary ectosomes and photoreceptor discs rely on branched actin networks nucleated by the Arp2/3 complex for their formation.
Acknowledgments:
The authors are grateful to S.M. Gospe and M.A. Cady (Duke University), K.J. Verhey (University of Michigan), J.C. Besharse (Medical College of Wisconsin), G.J. Pazour and M.W. Stuck (University of Massachusetts) for critically reading the manuscript; J.-D. Ding (Duke University) for capturing an image used in Figure 6A; A.F.X. Goldberg (Oakland University) for kindly providing an image used in Figure 6B. Computer animations were created by D.G. Toth (MrDanToth@gmail.com). The authors are supported by EY012859 (V.Y.A.), EY030451 (V.Y.A.), EY005722 (V.Y.A.), EY025558 (W.J.S.), EY029929 (T.R.L.), EY025732 (J.N.P.) and an Unrestricted Award from Research to Prevent Blindness Inc. (Duke University).
Glossary
- Photoreceptor outer segment
A sensory cilium emanating from rod and cone photoreceptor cells that is responsible for generating an electrical response upon light excitation.
- Photoreceptor inner segment
A compartment of the photoreceptor cell responsible for protein and lipid synthesis and energy production.
- Photoreceptor disc
A flattened membrane vesicle (in rods) or a lamella (in cones) densely packed with light sensing molecules. Hundreds of discs are stacked within a single outer segment to maximize the efficiency of light capture.
- Disc enclosure
A membrane remodeling process that separates a newly forming disc from the outer segment plasma membrane.
- Disc incisure
A deep recess in the rim of a photoreceptor disc. Disc incisures are aligned along the disc stack to increase the longitudinal diffusion of cytoplasmic molecules within the outer segment. Depending on the species, an outer segment can contain between one and over two dozen incisures.
- Rhodopsin
A light-sensitive G protein-coupled receptor that serves as the visual pigment in rod photoreceptors.
- Ectosome (also known as microvesicle)
A type of extracellular vesicle that is produced by ectocytosis, i.e. direct budding from the plasma or ciliary membrane. Ectosomes should not be confused with exosomes, which are formed inside the cell in multivesicular bodies and subsequently released en masse by exocytosis upon fusion of a multivesicular body with the plasma membrane. Ectosomes are typically larger in size than exosomes: 100-1,000 nm vs. 50-150 nm.
- Actin nucleation
The rate-limiting step of actin cytoskeleton assembly whereby actin nucleating proteins stabilize the formation of an actin trimer that can subsequently be elongated into a filament. The Arp2/3 actin nucleator catalyzes the formation of branched actin filaments, whereas linear actin filaments are typically nucleated by formins.
- ESCRT complexes (Endosomal Sorting Complexes Required for Transport)
Cytosolic protein complexes which facilitate membrane budding and scission in a direction away from the cytoplasm.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sjostrand FS (1953) The ultrastructure of the outer segments of rods and cones of the eye as revealed by the electron microscope. J Cell Physiol 42 (1), 15–44. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg AF et al. (2016) Molecular basis for photoreceptor outer segment architecture. Prog Retin Eye Res 55, 52–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearring JN et al. (2013) Protein sorting, targeting and trafficking in photoreceptor cells. Prog Retin Eye Res 36, 24–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wensel TG et al. (2016) Structural and molecular bases of rod photoreceptor morphogenesis and disease. Prog Retin Eye Res 55, 32–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nickell S et al. (2007) Three-dimensional architecture of murine rod outer segments determined by cryoelectron tomography. J Cell Biol 177 (5), 917–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilliam JC et al. (2012) Three-dimensional architecture of the rod sensory cilium and its disruption in retinal neurodegeneration. Cell 151 (5), 1029–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gross OP et al. (2012) Spatiotemporal cGMP dynamics in living mouse rods. Biophysical Journal 102 (8), 1775–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young RW and Bok D (1969) Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol 42 (2), 392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartong DT et al. (2006) Retinitis pigmentosa. Lancet 368 (9549), 1795–809. [DOI] [PubMed] [Google Scholar]
- 10.Steinberg RH et al. (1980) Disc morphogenesis in vertebrate photoreceptors. J Comp Neurol 190 (3), 501–8. [DOI] [PubMed] [Google Scholar]
- 11.Laties AM et al. (1976) Procion yellow: a marker dye for outer segment disc patency and for rod renewal. Experimental Eye Research 23 (2), 139–48. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto B and Besharse JC (1985) Light and temperature modulated staining of the rod outer segment distal tips with Lucifer yellow. Investigative Ophthalmology & Visual Science 26 (5), 628–35. [PubMed] [Google Scholar]
- 13.Tokuyasu K and Yamada E (1959) The fine structure of the retina studied with the electron microscope. IV. Morphogenesis of outer segments of retinal rods. J Biophys Biochem Cytol 6, 225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obata S and Usukura J (1992) Morphogenesis of the photoreceptor outer segment during postnatal development in the mouse (BALB/c) retina. Cell and Tissue Research 269 (1), 39–48. [DOI] [PubMed] [Google Scholar]
- 15.Chuang JZ et al. (2007) SARA-regulated vesicular targeting underlies formation of the light-sensing organelle in mammalian rods. Cell 130 (3), 535–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding JD et al. (2015) Discs of mammalian rod photoreceptors form through the membrane evagination mechanism. J Cell Biol 211 (3), 495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volland S et al. (2015) Three-dimensional organization of nascent rod outer segment disk membranes. Proc Natl Acad Sci U S A 112 (48), 14870–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burgoyne T et al. (2015) Rod disc renewal occurs by evagination of the ciliary plasma membrane that makes cadherin-based contacts with the inner segment. Proc Natl Acad Sci U S A 112 (52), 15922–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Datta P et al. (2019) The myosin-tail homology domain of centrosomal protein 290 is essential for protein confinement between the inner and outer segments in photoreceptors. J Biol Chem 294 (50), 19119–19136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Volland S and Williams DS (2018) Preservation of Photoreceptor Nanostructure for Electron Tomography Using Transcardiac Perfusion Followed by High-Pressure Freezing and Freeze-Substitution. Adv Exp Med Biol 1074, 603–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalra H et al. (2016) Focus on extracellular vesicles: introducing the next small big thing. Int J Mol Sci 17 (2), 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wood CR et al. (2013) The cilium secretes bioactive ectosomes. Curr Biol 23 (10), 906–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Long H et al. (2016) Comparative analysis of ciliary membranes and ectosomes. Curr Biol 26 (24), 3327–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silva M et al. (2017) Cell-specific alpha-tubulin isotype regulates ciliary microtubule ultrastructure, intraflagellar transport, and extracellular vesicle biology. Curr Biol 27 (7), 968–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szempruch AJ et al. (2016) Extracellular vesicles from trypanosoma brucei mediate virulence factor transfer and cause host anemia. Cell 164 (1-2), 246–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akella JS et al. (2020) Ciliary Rab28 and the BBSome negatively regulate extracellular vesicle shedding. eLife 9, e50580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J and Barr MM (2018) Cell-cell communication via ciliary extracellular vesicles: clues from model systems. Essays Biochem 62 (2), 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J et al. (2020) Release and targeting of polycystin-2-carrying ciliary extracellular vesicles. Curr Biol 30 (13), R755–R756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nager AR et al. (2017) An actin network dispatches ciliary GPCRs into extracellular vesicles to modulate signaling. Cell 168 (1-2), 252–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phua SC et al. (2017) Dynamic remodeling of membrane composition drives cell cycle through primary cilia excision. Cell 168 (1-2), 264–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salinas RY et al. (2017) Photoreceptor discs form through peripherin-dependent suppression of ciliary ectosome release. J Cell Biol 216 (5), 1489–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Nie R et al. (1978) A new H-2-linked mutation, rds, causing retinal degeneration in the mouse. Tissue Antigens 12 (2), 106–8. [DOI] [PubMed] [Google Scholar]
- 33.Travis GH et al. (1989) Identification of a photoreceptor-specific mRNA encoded by the gene responsible for retinal degeneration slow (rds). Nature 338 (6210), 70–3. [DOI] [PubMed] [Google Scholar]
- 34.Stuck MW et al. (2016) PRPH2/RDS and ROM-1: Historical context, current views and future considerations. Prog Retin Eye Res 52, 47–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen AI (1983) Some cytological and initial biochemical observations on photoreceptors in retinas of rds mice. Invest Ophthalmol Vis Sci 24 (7), 832–43. [PubMed] [Google Scholar]
- 36.Jansen HG and Sanyal S (1984) Development and degeneration of retina in rds mutant mice: electron microscopy. J Comp Neurol 224 (1), 71–84. [DOI] [PubMed] [Google Scholar]
- 37.Nir I and Papermaster DS (1986) Immunocytochemical localization of opsin in the inner segment and ciliary plasma membrane of photoreceptors in retinas of rds mutant mice. Invest Ophthalmol Vis Sci 27 (5), 836–40. [PubMed] [Google Scholar]
- 38.Usukura J and Bok D (1987) Changes in the localization and content of opsin during retinal development in the rds mutant mouse: immunocytochemistry and immunoassay. Exp Eye Res 45 (4), 501–15. [DOI] [PubMed] [Google Scholar]
- 39.Jansen HG et al. (1987) Development and degeneration of retina in rds mutant mice: ultraimmunohistochemical localization of opsin. Exp Eye Res 44 (3), 347–61. [DOI] [PubMed] [Google Scholar]
- 40.Arikawa K and Williams DS (1989) Organization of actin filaments and immunocolocalization of alpha-actinin in the connecting cilium of rat photoreceptors. J Comp Neurol 288 (4), 640–6. [DOI] [PubMed] [Google Scholar]
- 41.Chaitin MH et al. (1984) Actin in the photoreceptor connecting cilium: immunocytochemical localization to the site of outer segment disk formation. J Cell Biol 99 (1 Pt 1), 239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vaughan DK and Fisher SK (1987) The distribution of F-actin in cells isolated from vertebrate retinas. Exp Eye Res 44 (3), 393–406. [DOI] [PubMed] [Google Scholar]
- 43.Williams DS et al. (1988) Disruption of microfilament organization and deregulation of disk membrane morphogenesis by cytochalasin D in rod and cone photoreceptors. J Comp Neurol 272 (2), 161–76. [DOI] [PubMed] [Google Scholar]
- 44.Spencer WJ et al. (2019) Photoreceptor disc membranes are formed through an Arp2/3-dependent lamellipodium-like mechanism. Proc Natl Acad Sci U S A 116 (52), 27043–27052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blanchoin L et al. (2014) Actin dynamics, architecture, and mechanics in cell motility. Physiol Rev 94 (1), 235–63. [DOI] [PubMed] [Google Scholar]
- 46.Corral-Serrano JC et al. (2020) PCARE and WASF3 regulate ciliary F-actin assembly that is required for the initiation of photoreceptor outer segment disk formation. Proc Natl Acad Sci U S A 117 (18), 9922–9931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kevany BM et al. (2015) Animals deficient in C2Orf71, an autosomal recessive retinitis pigmentosa-associated locus, develop severe early-onset retinal degeneration. Hum Mol Genet 24 (9), 2627–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nishimura DY et al. (2010) Discovery and functional analysis of a retinitis pigmentosa gene, C2ORF71. Am J Hum Genet 86 (5), 686–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen Z et al. (2010) Structure and control of the actin regulatory WAVE complex. Nature 468 (7323), 533–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haruta M et al. (2009) Depleting Rac1 in mouse rod photoreceptors protects them from photo-oxidative stress without affecting their structure or function. Proc Natl Acad Sci U S A 106 (23), 9397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boitet ER et al. (2019) NudC regulates photoreceptor disk morphogenesis and rhodopsin localization. FASEB J 33 (8), 8799–8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang C et al. (2016) NudC regulates actin dynamics and ciliogenesis by stabilizing cofilin 1. Cell Res 26 (2), 239–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Megaw R et al. (2017) Gelsolin dysfunction causes photoreceptor loss in induced pluripotent cell and animal retinitis pigmentosa models. Nat Commun 8 (1), 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nachury MV and Mick DU (2019) Establishing and regulating the composition of cilia for signal transduction. Nat Rev Mol Cell Biol 20, 389–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Glotzer M (2005) The molecular requirements for cytokinesis. Science 307 (5716), 1735–9. [DOI] [PubMed] [Google Scholar]
- 56.Romer W et al. (2010) Actin dynamics drive membrane reorganization and scission in clathrin-independent endocytosis. Cell 140 (4), 540–53. [DOI] [PubMed] [Google Scholar]
- 57.Diener DR et al. (2015) Proteomic analysis of isolated ciliary transition zones reveals the presence of ESCRT proteins. Curr Biol 25 (3), 379–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zangerl B et al. (2006) Identical mutation in a novel retinal gene causes progressive rod-cone degeneration in dogs and retinitis pigmentosa in humans. Genomics 88 (5), 551–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spencer WJ and Arshavsky VY (2019) PRCD is a small disc-specific rhodopsin-binding protein of unknown function. Adv Exp Med Biol 1185, 531–535. [DOI] [PubMed] [Google Scholar]
- 60.Skiba NP et al. (2013) Proteomic identification of unique photoreceptor disc components reveals the presence of PRCD, a protein linked to retinal degeneration. J Proteome Res 12 (6), 3010–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spencer WJ et al. (2019) PRCD is essential for high-fidelity photoreceptor disc formation. Proc Natl Acad Sci U S A 116 (26), 13087–13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spencer WJ et al. (2016) Progressive rod-cone degeneration (PRCD) protein requires N-terminal S-acylation and rhodopsin binding for photoreceptor outer segment localization and maintaining intracellular stability. Biochemistry 55 (36), 5028–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Murphy J and Kolandaivelu S (2016) Palmitoylation of progressive rod-cone degeneration (PRCD) regulates protein stability and localization. J Biol Chem 291 (44), 23036–23046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Allon G et al. (2019) PRCD is concentrated at the base of photoreceptor outer segments and is involved in outer segment disc formation. Hum Mol Genet 28 (24), 4078–4088. [DOI] [PubMed] [Google Scholar]
- 65.Miyaguchi K et al. (1992) Topography of opsin within disk and plasma membranes revealed by a rapid-freeze deep-etch technique. J Neurocytol 21 (11), 807–19. [DOI] [PubMed] [Google Scholar]
- 66.Hubbell WL et al. (2003) Rhodopsin structure, dynamics, and activation: a perspective from crystallography, site-directed spin labeling, sulfhydryl reactivity, and disulfide cross-linking. Adv Protein Chem 63, 243–90. [DOI] [PubMed] [Google Scholar]
- 67.Pribil M et al. (2014) Structure and dynamics of thylakoids in land plants. J Exp Bot 65 (8), 1955–72. [DOI] [PubMed] [Google Scholar]
- 68.Arikawa K et al. (1992) Localization of peripherin/rds in the disk membranes of cone and rod photoreceptors: relationship to disk membrane morphogenesis and retinal degeneration. J Cell Biol 116 (3), 659–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kevany BM et al. (2013) Structural and functional analysis of the native peripherin-ROM1 complex isolated from photoreceptor cells. J Biol Chem 288 (51), 36272–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Milstein ML et al. (2017) An inducible amphipathic helix within the intrinsically disordered C terminus can participate in membrane curvature generation by peripherin-2/rds. J Biol Chem 292 (19), 7850–7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Milstein ML et al. (2020) Multistep peripherin-2/rds self-assembly drives membrane curvature for outer segment disk architecture and photoreceptor viability. Proc Natl Acad Sci U S A 117 (8), 4400–4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Conley SM et al. (2019) Prph2 initiates outer segment morphogenesis but maturation requires Prph2/Rom1 oligomerization. Hum Mol Genet 28 (3), 459–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chakraborty D et al. (2009) Differential requirements for retinal degeneration slow intermolecular disulfide-linked oligomerization in rods versus cones. Hum Mol Genet 18 (5), 797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chakraborty D et al. (2010) Differences in RDS trafficking, assembly and function in cones versus rods: insights from studies of C150S-RDS. Hum Mol Genet 19 (24), 4799–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zulliger R et al. (2018) Oligomerization of Prph2 and Rom1 is essential for photoreceptor outer segment formation. Hum Mol Genet 27 (20), 3507–3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Loewen CJ et al. (2003) The role of subunit assembly in peripherin-2 targeting to rod photoreceptor disk membranes and retinitis pigmentosa. Mol Biol Cell 14 (8), 3400–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lewis TR et al. (2020) Photoreceptor disc enclosure occurs in the absence of normal peripherin-2/rds oligomerization. Front Cell Neurosci 14 (92), 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goldberg AF et al. (1998) Cysteine residues of photoreceptor peripherin/rds: role in subunit assembly and autosomal dominant retinitis pigmentosa. Biochemistry 37 (2), 680–5. [DOI] [PubMed] [Google Scholar]
- 79.Loewen CJ and Molday RS (2000) Disulfide-mediated oligomerization of Peripherin/Rds and Rom-1 in photoreceptor disk membranes. Implications for photoreceptor outer segment morphogenesis and degeneration. J Biol Chem 275 (8), 5370–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


