Abstract
Introduction:
To develop protocols for isolation and culture of human chorionic mesenchymal and trophoblast cells and test their differential responsiveness to oxidative stress
Methods:
Chorion trophoblast cells (CTC) and chorion mesenchymal cells (CMC) were isolated from term fetal membranes by modifying current protocols. Their purity and characteristics were tested using bright field microscopy and after staining for cytokeratin (CK)-7 and vimentin. Cigarette smoke extract (CSE) was used to stimulate cells, and we determined reactive oxygen species (ROS) production using 2′7′-dichlorodihydro-fluorescein assay, stress signaler p38MAPK activation (Western blot) and senescence by flow cytometry. Co-treatment with antioxidant N-acetyl cystine (NAC) either alone or in combination with SB203580 (p38MAPK inhibitor) was used to test oxidative stress (OS)- and p38MAPK-mediated effects.
Results:
The isolation and cell culture protocol used in this study yielded 92% pure CTC and 100% pure CMC. CSE treatment significantly induced ROS production, P-p38MAPK activation, and senescence in both cell types compared to controls. Cotreatment with NAC reduced ROS production and p38MAPK activation, and co-treatment with both NAC and SB203580 reduced senescence. ROS response in CMC was higher than CTC; however, senescence of CTC was 10-fold higher than CMC.
Conclusions:
We introduce approaches for proper isolation and culture of CTC and CMC without any influence or overgrowth of one specific type cell that can confound results. Using this approach, we determined differential effects of CTC and CMC to OS condition seen at term labor. Both CTC and CMC undergo p38MAPK-mediated senescence; however, the rate of senescence is higher in CTC.
Keywords: fetal membranes, trophoblast, preterm birth, oxidative stress, amnion, inflammation senescence
INTRODUCTION
Human amniochorion membranes (fetal membranes) form one of the most important structural components for the intrauterine cavity[1, 2]. Besides protection of the fetus by providing an architectural framework, it also functions as a major organ that performs antimicrobial defense, as well as immune and several endocrine functions[3–5]. Fetal membrane senescence at term is well reported[6, 7], and membrane aging seems to be a predisposing stage for membranes to make an inflammatory contribution to promote parturition[8]. Membranes are thus vital for both pregnancy maintenance and to promote parturition. Membranes are composed of 2 distinct layers: amnion and chorion layers that are separated by a collagen-enriched extracellular matrix (ECM)[3]. A spongy layer in the ECM that is rich in proteoglycans, glycoproteins, and type III collagen separates the amnion from the chorion[9]. Both the single epithelial layer of amnion and the multilayered chorion trophoblast cells are attached to the ECM by a Type IV collagen-rich basement membrane. Scattered in the matrix are amnion and chorion mesenchymal cells[9]. Amnion and chorion layers begin their growth at embryogenesis as 2 independent layers and fuse to form a unit structure by early second trimester of pregnancy[10, 11]. Collectively, the membranes are a rich source of various biomarkers, and the 2 units collectively help in maintaining membrane integrity throughout pregnancy[12, 13]. Functionally, amnion and chorion layers are independent, although data are scarce to delineate their roles in physiologic and pathologic pregnancies.
The amnion layer is highly elastic, as it accommodates stretches and strains during fetal development and amniotic fluid buildup during gestation. The amnion layer slides over the chorion layer, which is relatively thicker and firmer than the amnion[1, 14, 15]. Chorion trophoblasts have a lineage of placental trophoblasts cells; however, they are structurally and functionally independent from placental trophoblast cells[16]. Amnion epithelial and mesenchymal cells are well-studied components of the membrane unit. Based on cellular transition data, we have doubted the origin of mesenchymal cells and postulated that they are likely transient epithelial cells that have transitioned in the stroma[17]. Amnion epithelial cells are shed into amniotic fluid throughout gestation as they proliferate; they often migrate towards stroma through microfractures that develop in the membrane[18]. During this migration, epithelial cells transition into mesenchymal cells and are recycled back as epithelial cells by progesterone and nurturing from amniotic fluid[19]. This cyclic transitions of cells maintains amnion layer integrity[17]. Several reports have indicated that the ratio between epithelial and mesenchymal cells in the amnion layer is 10:1 during pregnancy and in non-labor conditions, and the ratio switches to favor proinflammatory mesenchymal cells at term labor[17, 20–23]. This terminal event helps to enhance membrane and intrauterine inflammation to promote parturition[17]. Avascular amnion membranes, therefore, play a critical structural and functional role during pregnancy and parturition.
The chorion trophoblast layer plays a barrier function, as this layer lines up against maternal decidua. Besides placental-decidual interface, the chorion-decidual segment forms yet another feto-maternal interface[24–28] where the chorion barricades decidual immune cells and immunocompetent decidual cells from infiltrating into the membrane in addition to stopping microbial invasion. The chorion layer is also a rich source of 15-OH prostaglandin dehydrogenase (PGDH) 2, an oxidoreductase that metabolizes prostaglandins and prevents their free passage from one of their sites of production (amnion) to the decidua and myometrium during gestation[29]. Loss of immunoreactive PGDH2 in chorion is a mechanism that allows prostaglandins to reach maternal uterine tissues to initiate labor[30]. Chorion is also a rich source of various endocrine and immune mediators similar to those seen in amnion[24, 31, 32]. Therefore, structurally and functionally, amnion and chorion layers are distinct, and their integrity and unity, respectively, are required to maintain membrane homeostasis needed for maintaining pregnancy.
Although several studies have reported chorion trophoblast cell functions, the origin, characteristics, and fate of chorionic mesenchymal and trophoblasts cells are not studied[31, 33–36]. Our own reported studies are hampered by mixed growth of both trophoblast and fibroblastoid cells[25]. It is unclear if chorion trophoblast can undergo similar transitions as amnion epithelial cells, partly due to technical difficulties in separating the 2 cell populations and the overgrowth of chorion mesenchymal cells that are more vigorous in in vitro culture conditions. Although the messages from any of these studies are not necessarily hampered by the mixed yield of cells, more precise measures, and accurate determinations, as well differential responses from these cell types, can be made by having 2 well isolated and characterized cell populations. The primary objective of this study is to develop a protocol, specifically, modifying existing protocols to isolate chorion mesenchymal cells and trophoblast cells and to test their differential responses, if any, to an OS stimulant.
METHODS
Chorion Cell Isolation:
Placentas were obtained from John Sealy Hospital at The University of Texas Medical Branch (UTMB) at Galveston, TX, USA. No subjects were recruited or consented for this study as we used discarded placentas from normal term not-in-labor cesarean sections (n=5). This study is approved as an exempt protocol for use of discarded and non-identifiable human placental specimens.
Chorion trophoblast cultures (CTCs) are traditionally performed using methods detailed by Kliman et al for placental trophoblast cell isolation [37]. Although these methods are ideal for placental trophoblast cell isolation, it is rather tedious to isolate CTCs as it involves gradients that often yield an inconsistent heterogenous mix of cell types. While the gradients were designed to yield distinct populations of cells, the CTCs we generated from isolations using these protocols were often highly contaminated with mesenchymal cells, and these fast-growing cells often overtook the culture. Experiments using these isolated cells often were unreliable, and results varied as there was no consistency in population of cells between preparations. Therefore, we sought an approach that would modify the Kliman method to achieve purer populations of CTCs and CMCs. The method we honed is based on protocols by Kliman and approaches often used in multipotent mesenchymal stem cell isolation [38, 39]. The main difference between the method we use and Kliman-based methods is that where Kliman’s protocols use a series of digestive incubations followed by passing the digested cellular product through a discontinuous gradient, we employ only digestive enzyme incubations to yield 2 distinct cell populations. We further modified our protocol by adding gentle rotation during the 3-hour collagenase incubation. The details of our approach is as follows: fetal membranes were dissected immediately after placental delivery from women (18–40 years old) undergoing elective repeat cesarean section for uncomplicated pregnancies at term (between 37 and 41 weeks of gestation), not in labor. The amnion and chorion layers were manually peeled apart, and the underlying choriodecidua was washed gently with gauze in warm saline to remove all blood and decidua. The chorion layer was then dissected into small pieces (2 cm2), which were incubated at 37°C for 8 minutes in 2.4 U/mL dispase (Sigma, St. Louis, MO) diluted in warmed serum-free DMEM and Ham’s F-12, 50/50 Mix (Mediatech Inc., Manassas, VA). The pieces were then allowed to rest for 8 minutes in pre-warmed DMEM and Ham’s F-12, 50/50 Mix supplemented with 10% Fetal Bovine Serum (FBS). These 2 incubations were repeated 1 more time. The tissue sections were then collected and digested with 0.75 mg/mL collagenase (Sigma) in Hanks Balanced Salt Solutions (HBSS; Mediatech Inc.) for 3 hours at 37°C with agitation. The digested solution was then passed through a 70-μm cell strainer. The filtrate was centrifuged at 3,000 rpm for 10 minutes. The pellet contained the CMCs. The unfiltered remaining tissue was collected and digested in 0.25% Trypsin (Sigma) in PBS for 5 minutes at 37°C. This solution was then passed through a 70-μm cell strainer. The filtered solution was centrifuged at 3,000 rpm for 10 minutes. The pellet contained the CTCs. CMCs were counted and plated in T75 flasks and grown until 80%–90% confluence. The cells were passaged with 0.25% trypsin, 2.21 mM EDTA for 4 minutes at 37°C incubator. The P1 cells were then counted, plated, and treated accordingly. The CMCs were maintained in DMEM and Ham’s F-12, 50/50 Mix supplemented with 10% FBS and antibiotics (100 U/mL penicillin and 100 mg/mL streptomycin, [Sigma]; 5 μg/ml amphotericin B [Sigma]). CTCs were counted, plated in T75 flasks until 80%−90% confluency was obtained. The cells were rinsed with PBS, then treated with TrypLE™ Express (Gibco) for 10–13 minutes at 37°C to passage. The passaged (P1) CTCs were then plated in appropriate tissue culture vessels and treated accordingly. The CTCs were maintained in DMEM and Ham’s F-12, 50/50 Mix supplemented with 0.2% FBS, 0.1 mM 2-mercaptoethanol, 0.5% Penicillin-Streptomycin, 0.3% BSA, 1% ITS-X-supplement, 2 μM CHIR99021, 0.5 μM A83–01, 1 μM SB431542, 1.5 μg/ml L-ascorbic acid, 50 ng/ml EGF, 0.8 mM VPA and 5 μM Y27632[40]. Culture media were replaced every other day.
Immunofluorescence staining for markers of CTC and CMC:
The purity of the CTC and CMC was verified using immunocytochemistry staining for cytokeratin-7 (a biomarker of trophoblast cells) and vimentin (a biomarker of mesenchymal cells). Cells were also stained using a DAPI nuclear stain. Cells grown on glass chamber tissue culture slides were fixed with 4% paraformaldehyde. Cells were then washed, permeabilized with 0.5% Triton-X, and blocked with 3% bovine serum albumin. After blocking, the cells were incubated overnight with primary antibodies at 4°C. Primary antibodies for cytokeratin-7 (ab9021, Abcam, Cambridge, MA), vimentin (ab92547, Abcam), or Alexa Fluor 647 conjugated vimentin (ab195878, Abcam) were used. A secondary antibody with no primary antibody was used as the negative control. Goat anti-mouse secondary antibody Alexa Fluor 594 (Life Technologies, Carlsbad, CA) and donkey anti-rabbit secondary antibody Alexa Fluor 488 (Abcam, Cambridge, MA) were used at dilutions of 1:1000 for 1 hour at room temperature. Cells were counterstained with DAPI. Slides were mounted using MoWiol 4–88 mounting medium and visualized using a Keyence fluorescence microscope (Keyence, Itasca, IL).
Chorion cell stimulation with cigarette smoke extract (CSE):
After isolating 2 distinct cell populations, we further tested responsiveness of these cells to stimulants. We have been using a water-soluble CSE extract that is found to be consistent in producing ROS-associated with OS as is often seen in the intrauterine environment prior to term and preterm labor[41–48]. We have been using CSE as a laboratory reagent to induce OS, and it has been much more effective and reproducible than reagents traditionally used, such as hydrogen peroxide or tumor necrosis factor alpha[45].
Preparation of CSE and stimulation of cells:
To produce water-soluble CSE, smoke from a single lit commercial cigarette (unfiltered Camel™, R.J. Reynolds Tobacco Co, Winston Salem, NC) was bubbled through 25 mL of media, consisting of DMEM/F12 supplemented with 10% FBS. The stock CSE was sterilized using a 0.22-μm Steriflip filter unit (Millipore, Billerica, MA). CSE concentrate was diluted 1:25 for all cell treatments. CTCs and CMCs were stimulated with 1:25 dilutions of CSE in culture media for various experiment-dependent timepoints, described below, at 37°C. In certain experiments, N-acetyl cysteine (NAC, 15mM; Sigma, Cat#A7250), SB203580 (SB; 13mM, Sigma #S8307), or a co-treatment of the two was added to 1:25 CSE to determine the specificity of the OS induction. The antioxidant NAC was used to inhibit ROS production, p38MAPK activation, senescence, and inflammation. SB203580 (SB; 13mM, Sigma #S8307), which is a p38MAPK functional inhibitor, was also used to prevent p38MAPK activation, senescence, and inflammation.
Measurement of ROS:
CTCs and CMCs were grown to 70%–80% confluence in black, clear-bottomed 96-well plates. The cells were loaded with 25μM 2′7′-dichlorodihydro-fluorescein diacetate (H2DCFDA; Invitrogen, Carlsbad, CA) at 37°C for 30 minutes, and cells were exposed to control media or 1:25 CSE media with and without 15mM NAC for up to 4 hours. 100μM hydrogen peroxide (H2O2) was used as a positive control[44]. All treatments were prepared in phenol-red free DMEM and Ham’s F-12, 50/50 Mix (Mediatech, Inc). To determine changes in ROS levels, a baseline fluorometric measurement was taken at time 0, and readings were taken every 10 minutes for the first hour and at 1-hour intervals for a 4-hour period. DCF fluorescence was recorded at 528 nm after excitation at 485 nm in a Bio-Tek® Synergy microplate reader (Bio-Tek®, Winooski, VT). Results are expressed as arbitrary units, calculated using the mean slope of a linear regression of all points within the calculation zone.
Flow cytometry analysis for senescence:
Cells were grown to 70%–80% confluence in 60-mm culture dishes. After 24, 48, and 72 hours of incubation with control media or 1:25 CSE media with and without co-treatments of 15mM NAC + 30μM SB203580 as detailed above, the CTCs and CMCs were analyzed for cell senescence using flow cytometry. Senescence was assessed with the commonly used biomarker senescence-associated-β-Galactosidase (SA-β-Gal), which we have adapted for flow cytometry[25, 45]. Briefly, cells were incubated for 1 hour in complete DMEM and Ham’s F-12, 50/50 Mix medium supplemented with 100nM bafilomycin A1 (baf A1) for 1 hour at 37°C. Without changing media, 5-Dodecanoylaminofluorescein di-b-D-galactopyranoside (C12FDG) was added (final concentration of 10.5μM for CMCs and 15μM for CTCs) and incubated at 37°C for 1 hour. Cells were harvested by trypsinization and centrifugation at 3000 g for 10 minutes at 4°C. The cell pellet was resuspended in 300 μL Coulter DNA Prep Stain (Beckman Coulter, Indianapolis, IN), which contains propidium iodide (PI) to indicate viable and nonviable cells and run immediately on a CytoFlex flow cytometer (Beckman Coulter). Unstained, control CMCs and CTCs were used as negative controls for each gating. Data were analyzed using the Cytexpert software (Beckman Coulter), and cells positive for C12FDG and negative for PI (viable) were considered for analysis.
Western blot analysis:
CTCs and CMCs were grown to 70%–80% confluence in 6-well plates. The cells were treated for 1, 6, and 24 hours with control media or 1:25 CSE with and without 15mM NAC. Cells were lysed in RIPA lysis buffer with freshly added protease and phosphatase inhibitors (0.01%). The lysate was collected after scraping the culture plate, and the insoluble material was removed by centrifugation at 10,000 rpm for 20 minutes at 4°C. The concentration of protein in each lysate was determined by using the BCA protein assay kit (Pierce BCA Protein Assay Kit, Thermo Scientific, Waltham, MA, USA). Equal protein (19 μg) from each sample was loaded onto a 10% SDS-PAGE gel and electrophoresed at 120 V using standard protocols. The resolved proteins were transferred to a PVDF membrane using the Trans-Blot® Turbo™ Transfer System (Bio-Rad Laboratories, Hercules, CA, USA). The membranes were blocked in Tris-Buffered Saline (TBS) containing 0.1% Tween 20 (TBS-T) and 5% skim milk for 2 hours at room temperature. Blots were incubated separately with antibodies against total p38MAPK (Cell Signaling, Danvers, MA, USA, #9212) and phosphorylated (P)-p38MAPK (Cell Signaling, #9211) at 4°C and rocked overnight. Blots were washed 3 times with TBS-T and incubated with appropriate peroxidase-conjugated IgG secondary antibody for 1 hour at room temperature. All blots were reprobed with an antibody to β-actin (Sigma, St. Louis, MO). All blots were developed using chemiluminescence Clarity ECL Western Blotting Substrates (Bio-Rad Laboratories) in accordance with the manufacturer’s recommendations, followed by image detection using a ChemiDoc Imager (Bio-Rad Laboratories). Densitometry was performed using Image Lab software (Bio-Rad Laboratories) to normalize the data for statistical analysis.
Statistical analysis:
For Western and flow cytometry data analysis, we used a repeated measures 2-way ANOVA. Measurements were repeated by both treatment and time factors. Tukey’s multiple comparisons test was performed to compare pairwise treatment effects. All data were analyzed using GraphPad Prism 6 for Windows.
RESULTS
Isolation and characteristics of CTCs and CMCs:
Using a modified approach to existing protocols, we were able to isolate both CTCs and CMCs in their purest forms possible. As shown in Figure 1, after 1 week in culture conditions, CK-7+ cells dominated our CTC preparations with classic trophoblast cell morphology. Our preparation was ~ 92% CK-7+ cells, with the remaining population being both CK-7+/Vimentin+, suggesting that the population of cells may still have some CMCs; however, we were able to limit their growth to a very minimal level (< 8%) even after a week in culture. In Figure 1 (top panel, CTC), we have chosen to show a field with both cell types. The bottom panel in Figure 1 shows classic fibroblastoid cells (CMCs) that are Vimentin+/CK-7−. With this approach, we were able to get distinct populations of cells to specifically study their functional relevance in additional experiments. We used 5 separate placental specimens for this study; however, this protocol is ongoing for various experiments in our lab.
Figure 1: Morphology and markers of chorion trophoblast cells (CTC) and chorion mesenchymal cells (CMC) (n=5).
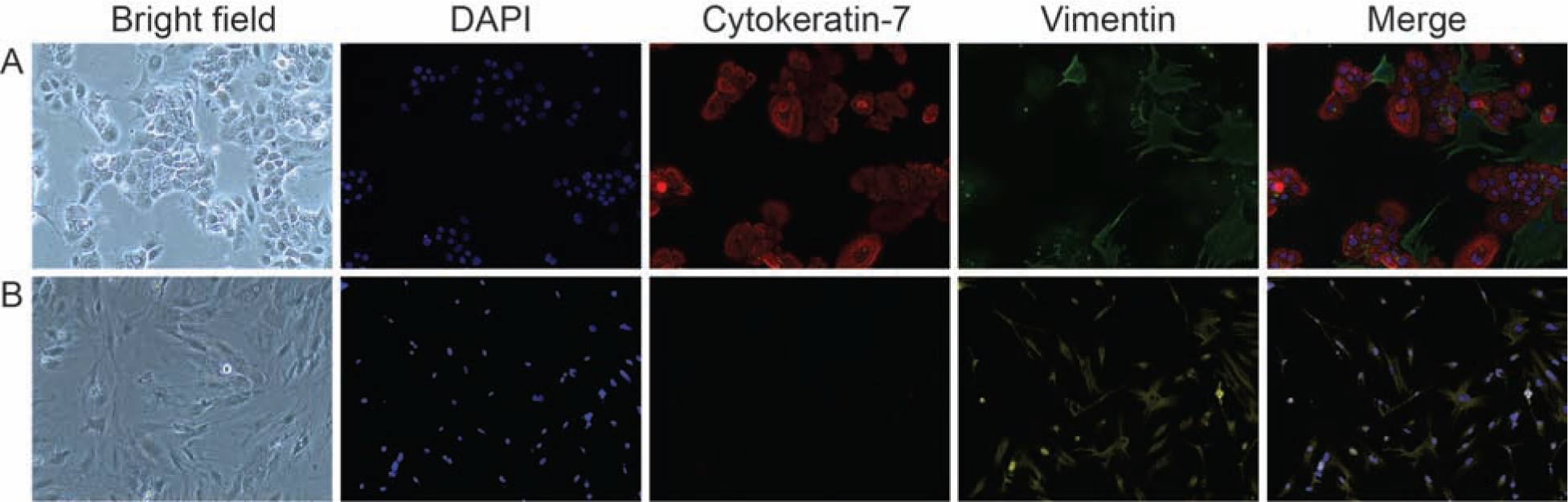
A: Chorion trophoblast cells—Bright field images of CTC, DAPI nuclear staining (blue), CK-7 (red), vimentin (green), and merged image.
B: Chorion mesenchymal cells—Bright field images of CMC, DAPI nuclear staining (blue), CK-7 (red), vimentin (green), and merged image.
CSE-induced ROS production in CTCs and CMCs:
To determine differences, if any between CTCs and CMCs in responding to a stimulus, CSE-treated cells were subjected to ROS measurement. Both CTCs and CMCs generated ROS within 2 minutes of stimulation with CSE as a measure of relative fluorescence of substrate H2DCF. As shown in Figures 2A and B, both CTCs and CMCs produced ROS that was almost 1.5-fold higher than control. Co-treatment with the antioxidant NAC substantially reduced ROS from both cell types, confirming CSE-mediated OS of cells. To note, the amount of ROS by CMCs is a full fold higher than CTCs, confirming prior reports by Kendal-Wright et al[20] and Sun et al[21] that fetal membrane ECM mesenchymal cells react much more rigorously to stimulants to cause higher proinflammatory and oxidative stress responses.
Figure 2: CSE induces increased ROS levels in chorion trophoblast cells (CTC) and chorion mesenchymal cells (CMC) (n=5).
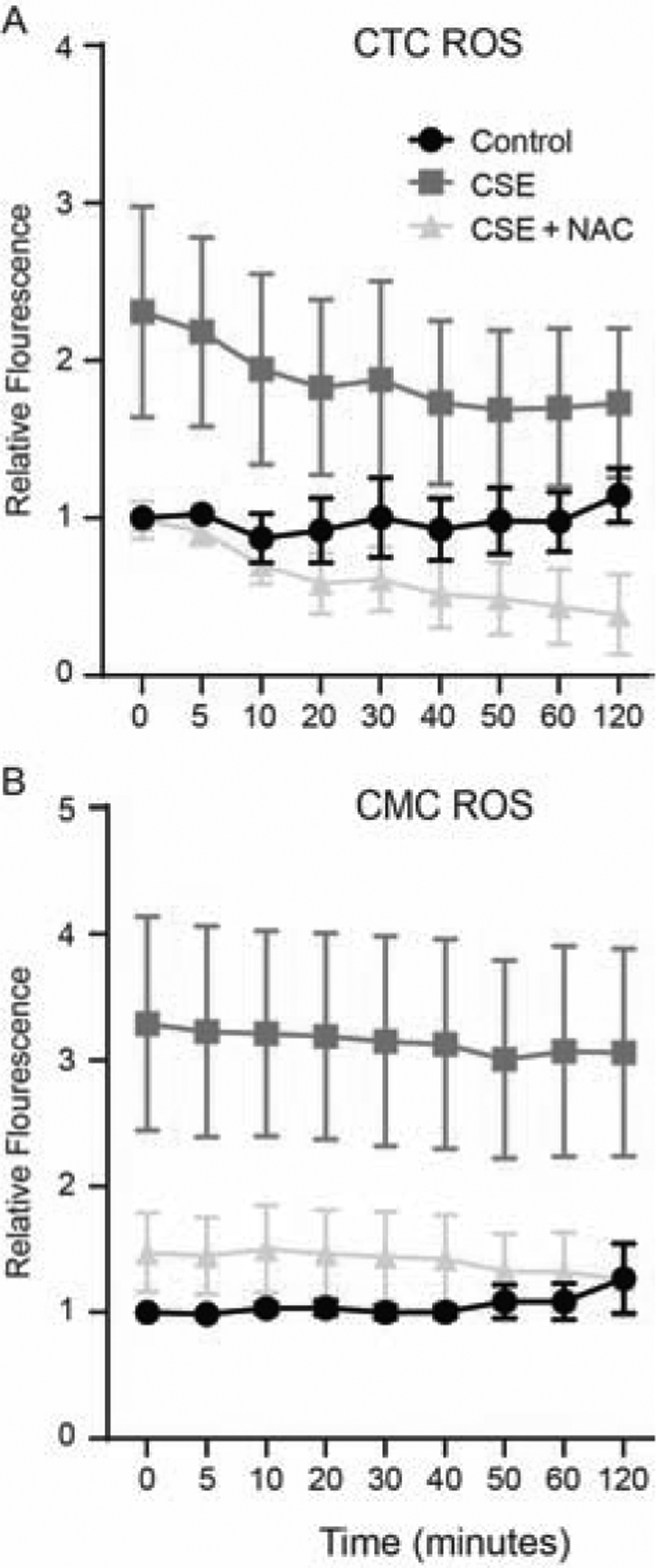
A: Chorion trophoblast cells—Relative fluorescence was higher after CSE treatment of CTC compared to control and was significantly reduced by co-treatment with N-acetyl cysteine.
B: Chorion mesenchymal cells—Relative fluorescence was higher after CSE treatment of CMC compared to control and was significantly reduced by co-treatment with antioxidant N-acetyl cysteine.
CSE induces p38MAPK activation in CTCs and CMCs:
We have seen p38MAPK activation when these 2 cell populations were examined together. Using Western blot analysis, we report that CSE causes significant activation of p38MAPK (Fig 3A) in CTCs within 1 hour compared to control (P<0.05). Significantly higher expression was also evident at 6 hours and was not significant at 24 hours. In CMCs, p38MAPK activation was not different at 1 hour compared to control (P=ns) but reached significance at 6 hours (P< 0.05) and plateaued at 24 hours (P=ns) (Fig 3B). NAC co-treatment reduced p38MAPK, suggesting that the activation of p38MAPK is in response to oxidative-stress-induced signaling.
Figure 3: Time-dependent activation (Phosphorylation) of p38MAPK chorion trophoblast cells (CTC) and chorion mesenchymal cells (CMC) (representative Western blot analysis from n=5).
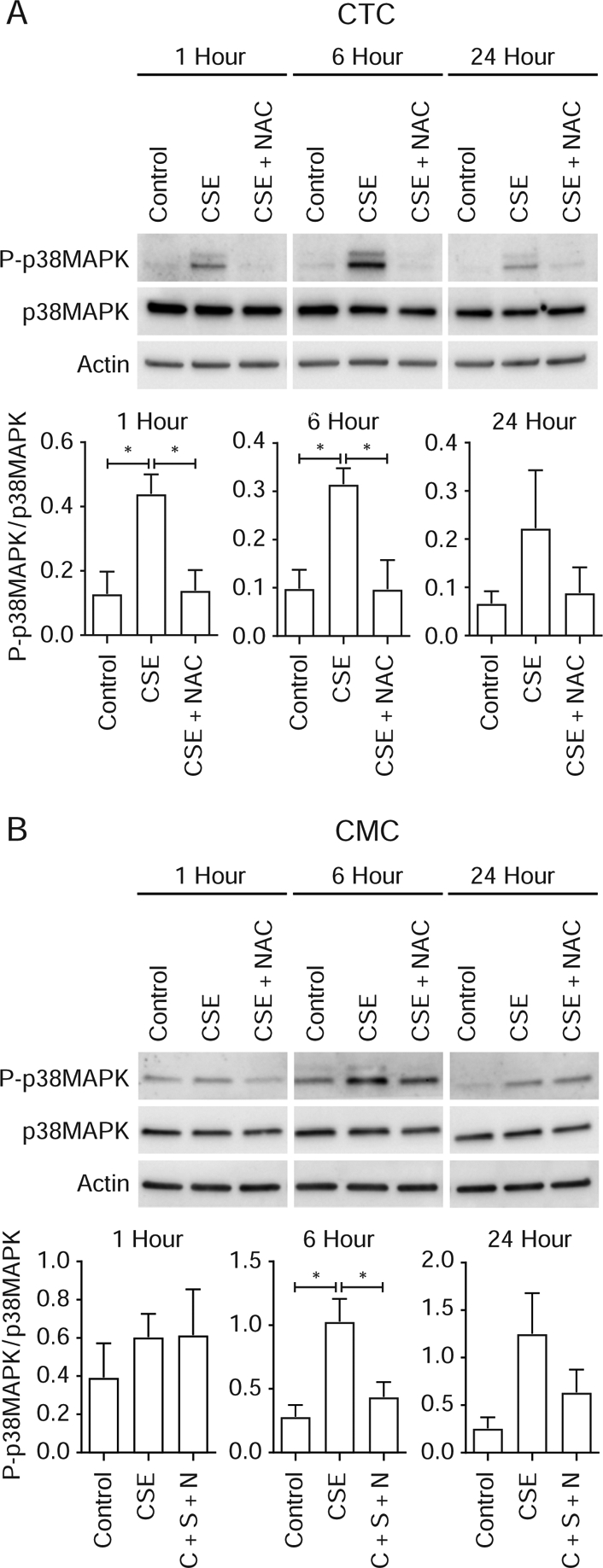
A: Chorion trophoblast cells—CSE increased P-p38MAPK after 1 and 6 hours of stimulation with CSE compared to controls, and this activation was reduced by co-treatment with antioxidant N-acetyl cysteine. Although higher at 24 hours, P-p38MAPK was not statistically significant.
B: Chorion mesenchymal cells—CSE induced no change in P-p38MAPK levels at 1 hour; however, P-p38MAPK was increased after 6 hours of stimulation compared to controls, and this activation was reduced by co-treatment with antioxidant N-acetyl cysteine. Although higher at 24 hours, it was not statistically significant.
CSE induces senescence in CTC and CMC:
Because OS-induced p38MAPK is shown to cause fetal membrane senescence, we examined this effect. As shown in Figures 4A and B, CSE caused significant increase in senescence at 48 hours compared to control (Figure 4A) and was maintained at 72 hours. The number of senescent cells were 6- and 5-folds higher after CSE treatment at both 48 and 72 hours, respectively, compared to control. Similarly, CMCs also showed a significantly higher number of senescent cells at both 48 and 72 hours compared to control (both P< 0.05). The number of senescent cells were 4- and 2.5-fold higher than controls. In both these cell types, co-treatment with NAC and SB503280 reduced the number of senescent cells, suggesting the likely pathway to senescence is oxidative-stress-induced p38MAPK activation. To note, although significantly higher than control, the number of senescent CMCs were much fewer than CTCs.
Figure 4: Time-dependent senescence associated β-Galactosidase (SA-β-Gal) activation in chorion trophoblast cells (CTC) and chorion mesenchymal cells (CMC) (representative western blot analysis from n=5).
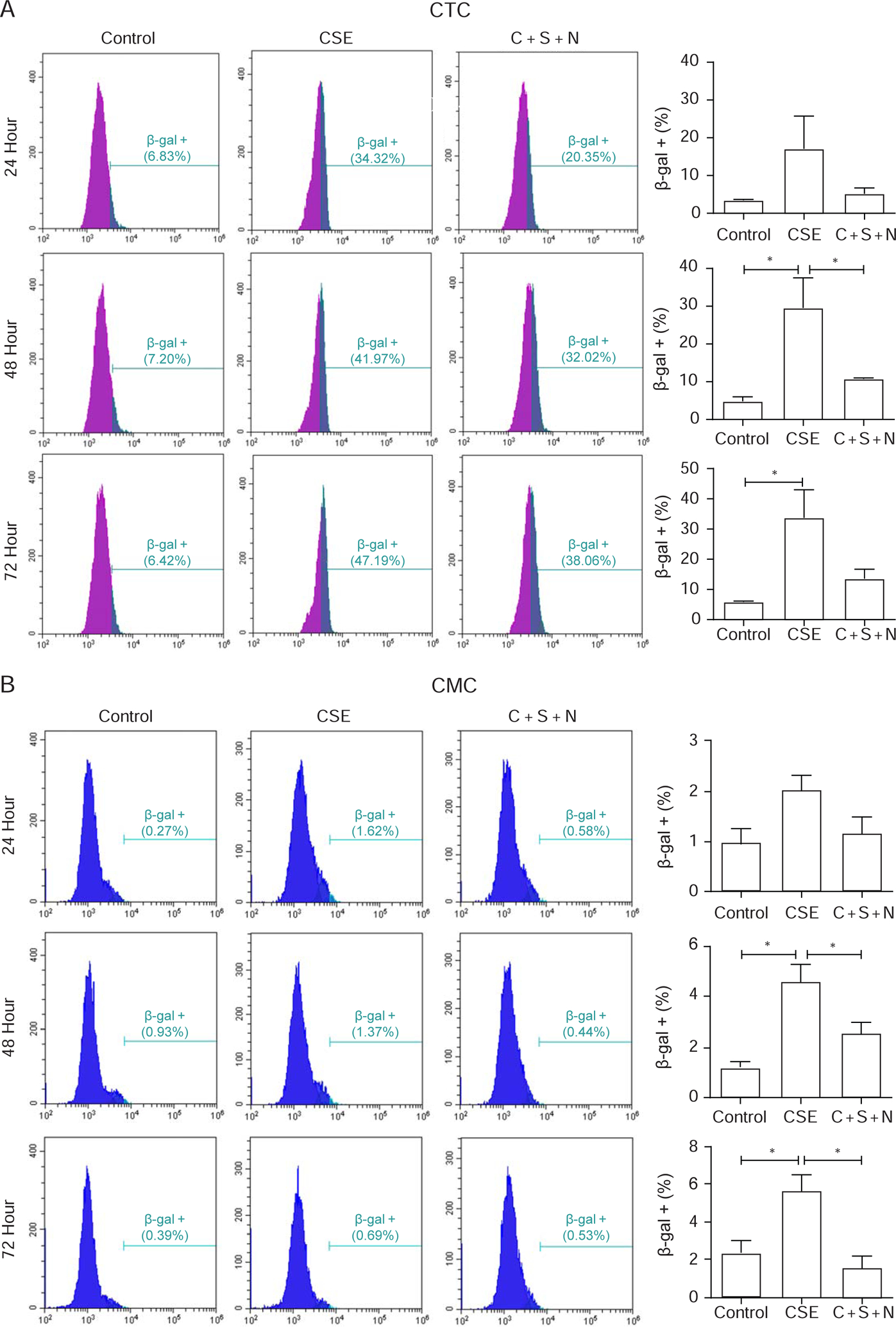
A: Chorion trophoblast cells—CSE significantly increased SA-β-Gal activity in CTC after 48 and 72 hours and was reduced by co-treatment with SB530280 (S) and N-acetyl cysteine (N). No effect was seen after 24 hours.
B: Chorion mesenchymal cells—CSE significantly increased SA-β-Gal activity in CMC after 48 and 72 hours and was reduced by co-treatment with SB530280 (S) and N-acetyl cysteine (N). No effect was seen after 24 hours.
DISCUSSION
The primary purpose of this study is to introduce an isolation and culture approach for human chorion trophoblast and chorion mesenchymal cells. As shown in Figure 1, we were successful in developing an approach that yielded > 90% pure chorion trophoblast cells and 100% pure chorion mesenchymal cells. Although, CTC cultures still have a small percentage of CMCs, we were able to restrict their overgrowth and their likely impact on CTC function. Using this culture approach, we demonstrate that like amnion cells, both CTCs and CMCs undergo senescence through p38MAPK-mediated signaling pathway, OS can be reduced by treatment with antioxidant NAC, and senescence can be minimized by reducing OS with NAC and p38MAPK activation using SB203580 treatment. CSE-induced OS and p38MAPK activation also involves DNA damage and generation of oxidized Guanine bases (data not shown). This is similar to what we have reported in amnion epithelial cells and mesenchymal cells [44, 49].
Like amnion mesenchymal cells, CMCs respond to OS-inducing stimulus with higher production of ROS than CTCs and activate p38MAPK; however, the downstream effect, such as senescence, is not as pronounced as in CTCs. This is partly due to their stem-cell-like capabilities and capacity to withstand the onslaught to protect the membrane ECM. Although they are more rigorous in their inflammatory and OS response than epithelial cells, their self-destruction is not typical, as their persistence is needed for tissue remodeling to overcome any adverse events during pregnancy. We have already reported the transition property of amnion mesenchymal cells to become amnion epithelial cells and promote membrane wound healing capacity[19, 50]. Such remodeling by mesenchymal cells requires stemness (ability to proliferate, transition, and migrate); hence, they are much sturdier and resilient than epithelial or trophoblast cells. We have not examined if these 2 cell types are capable of transitioning under OS conditions, and that is beyond the scope of this work. As mentioned in the introduction, microfractures are seen extending from the amnion epithelium to the chorion trophoblast layer, and these are likely sites of tissue remodeling[18]. We speculate that such transitions are also likely in this layer for rebuilding degraded collagen and damaged cells. Vacuolated chorion laeve cells line the top part of the trophoblast layer and are proximal to the basement membrane of the chorion[51]. They are a unique cell population among the trophoblast layer, rich in lipid droplets and pinocytotic vesicles, along with numerous intracellular filaments and desmosomes[51]. These cells are considered the counterpart to placental trophoblast where maternal-fetal transport of materials can occur. Microfractures are often seen in this region, and laeve cells may therefore also represent an aging population of chorion trophoblast where active remodeling of tissue may take place. Therefore, cyclic transition of cells to replace old with new cells may take place in this region. As stated in the introduction, in the amnion region, the mesenchymal cell transition to epithelium is done by progesterone-mediated c-MYC activation[17]. Because chorion is one of the richest sources and producers of progesterone in the membranes, endogenous production of progesterone may be utilized by mesenchymal cells to rebuild lost cells and maintain its thickness[52]. These transition and remodeling theories in chorion membrane are not yet demonstrated and, hence, are speculative.
Senescence in the chorion layer has been shown before as well[25, 53, 54]. In this study, we further demonstrate a differential senescence effect where trophoblast cells are more prone to senescence under OS conditions, which helps to clarify data in our prior publication where we showed senescence of cells in a mixed population of mesenchymal and trophoblast cells[25]. Feng et al have shown that senescence of chorion cells is mediated by the downregulation of PGRMC1, a membrane receptor component that mediated P4’s responsiveness. We have recently elaborated on this data and report that in fetal membranes, PGRMC1 is not the only P4 receptor, but cell-type and stimulant-dependent differential expression of both PGRMC1 and 2 that- erase can regulate P4’s function (manuscript under review). In the mesenchymal cells it is primarily PGRMC2 and in CTC it is both PGRMC1 and 2. It is expected that both receptors and their functions may be required to accommodate loads of P4 produced by the trophoblast layers. Although the precise function of P4 is unclear in CTs, it is likely that P4 and its receptors are the key components of the antiinflammatory immune barrier function between the decidua and fetal membranes. Differential gene silencing approaches are needed to better understand the specific functional contributions of each of these receptors in CTCs.
In summary, we present an approach that is much more suitable to yield CTCs and CMCs for further studies. Like amnion, fetal membrane chorion is susceptible to OS, and the cellular contributions by each chorion cell type to senescence and senescence-associated inflammation may vary. The type of response to different pregnancy associated stimuli or pregnancy-associated risk factors from the chorion membrane may not be generalizable. A better understanding of how each cell type responds is critical in understanding their contributions to membrane integrity as well as membrane pathology.
HIGHLIGHTS.
A novel isolation and culture approach for chorion trophoblast and mesenchymal cells of the fetal membrane has been developed.
Chorion mesenchymal cells are much more responsive to oxidative stress than trophoblast cells.
Although both cells activate p38MAPK in response to oxidative stress, mesenchymal cells are noticeably more resistant to senescence than chorion trophoblast cells.
Approaches described in this report will enable investigators to grow these cells independently and study their functional properties.
Acknowledgement—
The authors would like to thank Dr. Lauren Richardson and Dr. Samantha Sheller-Miller, postdoctoral fellows in the Menon lab, for their assistance with statistical analysis of data, imaging, and flow cytometry. We also thank Talar Kechichian, MS, lab manager in the Menon lab, for support with Western blots.
Funding—This study is funded by an NIH/NICHD grant to R Menon (R03HD098469)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: Authors report no conflict of interest.
REFERNCES
- 1.Menon R and Moore JJ, Fetal Membranes, Not a Mere Appendage of the Placenta, but a Critical Part of the Fetal-Maternal Interface Controlling Parturition. Obstet Gynecol Clin North Am, 2020. 47(1): p. 147–162. [DOI] [PubMed] [Google Scholar]
- 2.Bryant-Greenwood GD, The extracellular matrix of the human fetal membranes: structure and function. Placenta, 1998. 19(1): p. 1–11. [DOI] [PubMed] [Google Scholar]
- 3.Menon R, Richardson LS, and Lappas M, Fetal membrane architecture, aging and inflammation in pregnancy and parturition. Placenta, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gomez-Lopez N, et al. , Normal and premature rupture of fetal membranes at term delivery differ in regional chemotactic activity and related chemokine/cytokine production. Reprod. Sci, 2013. 20(3): p. 276–284. [DOI] [PubMed] [Google Scholar]
- 5.Hunt JS and Fishback JL, Amniochorion: immunologic aspects--a review. Am J Reprod Immunol, 1989. 21(3–4): p. 114–8. [DOI] [PubMed] [Google Scholar]
- 6.Behnia F, et al. , Chorioamniotic Membrane Senescence: A Signal for Parturition? 1. Am. J. Obstet. Gynecol, 2015. [DOI] [PubMed] [Google Scholar]
- 7.Menon R, et al. , Placental membrane aging and HMGB1 signaling associated with human parturition. Aging (Albany NY), 2016. 8(2): p. 216–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menon R, Initiation of human parturition: signaling from senescent fetal tissues via extracellular vesicle mediated paracrine mechanism. Obstet Gynecol Sci, 2019. 62(4): p. 199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strauss JF 3rd, Extracellular matrix dynamics and fetal membrane rupture. Reprod Sci, 2013. 20(2): p. 140–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox H and Faulk WP, The placenta as an experimental model. Clin. Endocrinol. Metab, 1981. 10(1): p. 57–72. [DOI] [PubMed] [Google Scholar]
- 11.Watson AJ, The cell biology of blastocyst development. Mol Reprod Dev, 1992. 33(4): p. 492–504. [DOI] [PubMed] [Google Scholar]
- 12.Stalberg C, et al. , Anti-inflammatory Elafin in human fetal membranes. J Perinat Med, 2016. [DOI] [PubMed] [Google Scholar]
- 13.Menon R NN, Bredeson S Polettini J, Fetal Membranes: Potential Source of Preterm Birth Biomarkers, in General Methods in Biomarker Research and Their Applications, Preedy PVB Vr, Editor 1907, Springer Science Publisher; p. 483–529. [Google Scholar]
- 14.Calvin SE and Oyen ML, Microstructure and mechanics of the chorioamnion membrane with an emphasis on fracture properties. Ann N Y Acad Sci, 2007. 1101: p. 166–85. [DOI] [PubMed] [Google Scholar]
- 15.Hoyes AD, Structure and function of the amnion. Obstet Gynecol Annu, 1975. 4: p. 1–38. [PubMed] [Google Scholar]
- 16.Florio P, et al. , Human placenta, chorion, amnion and decidua express different variants of corticotropin-releasing factor receptor messenger RNA. Placenta, 2000. 21(1): p. 32–7. [DOI] [PubMed] [Google Scholar]
- 17.Richardson LS, Taylor RN, and Menon R, Reversible EMT and MET mediate amnion remodeling during pregnancy and labor. Sci Signal, 2020. 13(618). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richardson LS, et al. , Discovery and Characterization of Human Amniochorionic Membrane Microfractures. Am J Pathol, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richardson L and Menon R, Proliferative, Migratory, and Transition Properties Reveal Metastate of Human Amnion Cells. Am J Pathol, 2018. 188(9): p. 2004–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sato BL, et al. , Human amnion mesenchymal cells are pro-inflammatory when activated by the Toll-like receptor 2/6 ligand, macrophage-activating lipoprotein-2. Placenta, 2016. 44: p. 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun K and Myatt L, Enhancement of glucocorticoid-induced 11beta-hydroxysteroid dehydrogenase type 1 expression by proinflammatory cytokines in cultured human amnion fibroblasts. Endocrinology, 2003. 144(12): p. 5568–77. [DOI] [PubMed] [Google Scholar]
- 22.Wang W, et al. , Involvement of GR and p300 in the induction of H6PD by cortisol in human amnion fibroblasts. Endocrinology, 2012. 153(12): p. 5993–6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun K, et al. , Glucocorticoids induce cytosolic phospholipase A2 and prostaglandin H synthase type 2 but not microsomal prostaglandin E synthase (PGES) and cytosolic PGES expression in cultured primary human amnion cells. J Clin Endocrinol Metab, 2003. 88(11): p. 5564–71. [DOI] [PubMed] [Google Scholar]
- 24.Bukowski R, et al. , Onset of human preterm and term birth is related to unique inflammatory transcriptome profiles at the maternal fetal interface. PeerJ, 2017. 5: p. e3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin J, et al. , Oxidative stress induces p38MAPK-dependent senescence in the feto-maternal interface cells. Placenta, 2018. 67: p. 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar D, et al. , A new methodology to measure strength of adherence of the fetal membrane components, amnion and the choriodecidua. Placenta, 2009. 30(6): p. 560–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kusuma GD, et al. , Isolation and Characterization of Mesenchymal Stem/Stromal Cells Derived from Human Third Trimester Placental Chorionic Villi and Decidua Basalis. Methods Mol Biol, 2018. 1710: p. 247–266. [DOI] [PubMed] [Google Scholar]
- 28.Presicce P, et al. , IL-1 signaling mediates intrauterine inflammation and chorio-decidua neutrophil recruitment and activation. JCI Insight, 2018. 3(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giannoulias D, et al. , Localization of prostaglandin H synthase, prostaglandin dehydrogenase, corticotropin releasing hormone and glucocorticoid receptor in rhesus monkey fetal membranes with labor and in the presence of infection. Placenta, 2005. 26(4): p. 289–97. [DOI] [PubMed] [Google Scholar]
- 30.Van Meir CA, et al. , Immunoreactive 15-hydroxyprostaglandin dehydrogenase (PGDH) is reduced in fetal membranes from patients at preterm delivery in the presence of infection. Placenta, 1996. 17(5–6): p. 291–297. [DOI] [PubMed] [Google Scholar]
- 31.Poisner AM, et al. , Localization of renin in trophoblasts in human chorion laeve at term pregnancy. Endocrinology, 1981. 109(4): p. 1150–5. [DOI] [PubMed] [Google Scholar]
- 32.King AE, et al. , Innate immune defences in the human uterus during pregnancy 6. Placenta, 2007. 28(11–12): p. 1099–1106. [DOI] [PubMed] [Google Scholar]
- 33.Allen TK, et al. , The Effect of Progestins on Tumor Necrosis Factor alpha-Induced Matrix Metalloproteinase-9 Activity and Gene Expression in Human Primary Amnion and Chorion Cells In Vitro 1. Anesth. Analg, 2015. 120(5): p. 1085–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li W, et al. , Enhancement of cortisol-induced 11beta-hydroxysteroid dehydrogenase type 1 expression by interleukin 1beta in cultured human chorionic trophoblast cells. Endocrinology, 2006. 147(5): p. 2490–5. [DOI] [PubMed] [Google Scholar]
- 35.Fariha MM, et al. , Human chorion-derived stem cells: changes in stem cell properties during serial passage. Cytotherapy, 2011. 13(5): p. 582–93. [DOI] [PubMed] [Google Scholar]
- 36.Dunand C, et al. , Endocrine gland-derived endothelial growth factor (EG-VEGF) is a potential novel regulator of human parturition. Biol Reprod, 2014. 91(3): p. 73. [DOI] [PubMed] [Google Scholar]
- 37.Kliman HJ, et al. , Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology, 1986. 118(4): p. 1567–82. [DOI] [PubMed] [Google Scholar]
- 38.Bailo M, et al. , Engraftment potential of human amnion and chorion cells derived from term placenta. Transplantation, 2004. 78(10): p. 1439–48. [DOI] [PubMed] [Google Scholar]
- 39.Soncini M, et al. , Use of highly sensitive mitochondrial probes to detect microchimerism in xenotransplantation models. Xenotransplantation, 2006. 13(1): p. 80–5. [DOI] [PubMed] [Google Scholar]
- 40.Okae H, et al. , Derivation of Human Trophoblast Stem Cells. Cell Stem Cell, 2018. 22(1): p. 50–63 e6. [DOI] [PubMed] [Google Scholar]
- 41.Ayad MT, Taylor BD, and Menon R, Regulation of p38 mitogen-activated kinase-mediated fetal membrane senescence by statins. Am J Reprod Immunol, 2018: p. e12999. [DOI] [PubMed] [Google Scholar]
- 42.Menon R and Fortunato SJ, Distinct pathophysiologic pathways induced by in vitro infection and cigarette smoke in normal human fetal membranes. Am J Obstet Gynecol, 2009. 200(3): p. 334 e1–8. [DOI] [PubMed] [Google Scholar]
- 43.Menon R, et al. , Histological evidence of oxidative stress and premature senescence in preterm premature rupture of the human fetal membranes recapitulated in vitro. Am J Pathol, 2014. 184(6): p. 1740–51. [DOI] [PubMed] [Google Scholar]
- 44.Menon R, et al. , Senescence of Primary Amniotic Cells via Oxidative DNA Damage. PLoS. One, 2013. 8(12): p. e83416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dixon CL, et al. , A distinct mechanism of senescence activation in amnion epithelial cells by infection, inflammation, and oxidative stress. Am J Reprod Immunol, 2018. 79(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheller S, et al. , Amnion-Epithelial-Cell-Derived Exosomes Demonstrate Physiologic State of Cell under Oxidative Stress. PLoS One, 2016. 11(6): p. e0157614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Polettini J, Richardson LS, and Menon R, Oxidative stress induces senescence and sterile inflammation in murine amniotic cavity. Placenta, 2018. 63: p. 26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Polettini J, et al. , Expression profiles of fetal membrane nicotinamide adenine dinucleotide phosphate oxidases (NOX) 2 and 3 differentiates spontaneous preterm birth and pPROM pathophysiologies. Placenta, 2014. [DOI] [PubMed] [Google Scholar]
- 49.Richardson L, et al. , Oxidative Stress-Induced TGF-beta/TAB1-mediated p38MAPK activation in human amnion epithelial cells. Biol Reprod, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Richardson L, et al. , Amnion membrane organ-on-chip: an innovative approach to study cellular interactions. FASEB J, 2019. 33(8): p. 8945–8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yeh IT, O’Connor DM, and Kurman RJ, Vacuolated cytotrophoblast: a subpopulation of trophoblast in the chorion laeve. Placenta, 1989. 10(5): p. 429–38. [DOI] [PubMed] [Google Scholar]
- 52.Mitchell B, et al. , Local modulation of progesterone production in human fetal membranes. J Clin Endocrinol Metab, 1982. 55(6): p. 1237–9. [DOI] [PubMed] [Google Scholar]
- 53.Feng L, et al. , Roles of Progesterone Receptor Membrane Component 1 in Oxidative Stress-Induced Aging in Chorion Cells. Reprod Sci, 2019. 26(3): p. 394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gomez-Lopez N, et al. , Preterm labor in the absence of acute histologic chorioamnionitis is characterized by cellular senescence of the chorioamniotic membranes. Am J Obstet Gynecol, 2017. 217(5): p. 592 e1–592 e17. [DOI] [PMC free article] [PubMed] [Google Scholar]


