Abstract
Background
Prolonged exposure of free radicals, or known as reactive oxygen species (ROS), in hepatic cells may cause oxidative stress. Without proper treatment, it can induce liver injury and fatal hepatic disease, including cirrhosis. Red betel (Piper crocatum Ruiz and Pav) is one of Indonesia’s medicinal plants that has been known to exhibit antioxidant, anti-inflammatory activities. This study aims to determine hepatoprotective effect of red betel leaves extract (RBLE) towards liver injury.
Method
Hydrogen peroxide-induced HepG2 cells were used as liver injury model·H2O2-induced HepG2 cells were treated with 25 µg/mL and 100 µg/mL RBLE. Several parameters were observed, including TNF-α level through ELISA; necrotic, apoptotic, dead, live cells; and ROS level through flow cytometry analysis; and GPX gene expression through qPCR.
Result
The study showed that treatment with RBLE were able to decrease TNF-α level; necrotic and death cells percentage; as well as ROS level. On the other hand, it were able to increase apoptotic and live cells percentage; as well as GPX gene expression. Low concentration (25 µg/mL) of RBLE treatment exhibited stronger anti-inflammatory activity as it was resulted in the lower TNF-α level and were able to switched hepatic cell death pathway from necrosis to apoptosis as shown by the shifted of apoptotic cells and necrotic cells percentage. This lead to lower death cells and ultimately improve live cells percentage. Meanwhile high concentration of RBLE (100 µg/mL) exhibited stronger antioxidant properties as indicated by lower ROS level and higher GPX gene expression.
Conclusion
Overall, this study was able to demonstrate hepatoprotective effect of RBLE towards liver injury model through its anti-inflammatory and antioxidant activities.
Keywords: Anti-inflammatory, Antinecrotic, Antioxidant, Hepatoprotective, HepG2, Red betel
1. Introduction
Liver is an important organ involved in maintaining homeostasis of the body. It plays a major role in metabolism by processing both endogenous (e.g, cholesterol, fatty acid, proteins, etc.) and exogenous substances (e.g, drugs), convert them into a less-harmful chemicals or eliminate them completely (Ilyas et al., 2016). Prolonged exposure to exogenous substance, such as drugs and alcohol, might causing liver injury. It has been shown that long term or overdose drugs intake could lead to serious liver problems as a result of increased reactive oxygen species (ROS) in hepatic cells, causing oxidative stress (Yoon et al., 2016).
Drugs metabolism has been reported to increase ROS production in hepatic cells. As an unstable atom/molecule, ROS may oxidize important macromolecules resulting in DNA damage, protein damage, and lipid peroxidation; a state referred to as oxidative stress (Pu et al., 2016). ROS has been reported to be involved in many signaling pathway, including inflammation. ROS induce the production of Tumor Necrosis Factor Alpha (TNF-α), a pro-inflammatory cytokine known for its importance as master regulator in balancing between cell survival and cell death. On the other hand, the engagement of TNF-α and its receptor (TNFR) seemed to induce mitochondrial ROS production (Blaser et al., 2016). Oxidative stress-induced inflammation could lead to apoptosis. Under normal condition, endogenous antioxidant enzymes act to balance ROS level, by transforming those free radicals into a less-harmful chemical. Gluthatione peroxidase (GPX) is one of important antioxidant enzymes that transform hydrogen peroxide (H2O2) into water (Ighodaro and Akinloye, 2018).
Untreated liver injury could lead to many fatal diseases. Liver diseases account for 2 million deaths per year worldwide, half of it accounted for cirrhosis. Drug-induced liver injuries are also continue to increase as a major cause of liver disease (Asrani et al., 2019). Many studies has been done to find antioxidant properties in medicinal plants that could be used as a potent candidate for liver injury medication (Jeyadevi et al., 2019, Aara et al., 2020). Red betel (Piper crocatum Ruiz and Pav) is one of Indonesia’s medicinal plants that has been extensively used and known for its antioxidant activity (Kusuma et al., 2017, Lister et al., 2019a, Lister et al., 2019b).
Red betel leaves extract (RBLE) has been reported to contain active compounds, mainly flavonoids, steroids, tannins, saponins, alkaloids, polyphenolics, quinones, and essential oil groups (Wulandari et al., 2018). It has been reported RLBE contain eugenol and hydroxychavicol, a phenolic compound that has antioxidant and anti-inflammatory activities (Dervis et al., 2017). From previous studies, RLBE shown can exhibit antioxidant activities (Lister et al., 2019a); anti-inflammatory and antifungal properties (Misra et al., 2009); and show to have antimigration activity towards metastatic breast cancer (Zulharini et al., 2018), anticancer towards cervical cancer (Widowati et al., 2013).
Therefore, this study aims to determine RBLE as hepatoprotective effect towards liver injury, modeled by H2O2-induced HepG2 cells by in vitro study. The observed parameters of this study include TNF-α level; apoptotic cells, necrotic cells, death cells, and live cells percentage; ROS level; and GPX gene expression.
2. Materials and method
2.1. Piper crocatum extract
Red betel plants (Piper crocatum Ruiz and Pav) were obtained from Pabuaran Cilendek Timur, Bogor, West Java, Indonesia. It was then identified by Herbarium Bogoriense, Botanical Field Research Center for Biology-Indonesian Institute of Science, Bogor, Indonesia. The extraction of red betel leaves was carried out using maceration method. The leaves were dried and mashed (160 g), continued by maceration using distilled ethanol 70% as much as 500 mL. The filtrate was collected every 24 h and the maceration process were repeated until the filtrate became colorless. Then, the collected filtrate was concentrated using evaporator at 50 °C (Zhengzhou Well-known, RE 201D) until rRBLE was obtained and stored at −20 °C (Lister et al., 2019a, Widowati et al., 2017).
2.2. Cell culture and H2O2-induced HepG2
The human hepatocellular carcinoma cells (HepG2, ATCC, HB-8065™) was obtained from Biomolecular and Biomedical Research Center, Aretha Medika Utama, Bandung, Indonesia. It was cultured in Modified Eagle Medium (MEM) (L0416-500, Biowest, Nuaillé, France) that supplemented with 10% (v/v) fetal bovine serum (FBS) (S1810, Biowest, Nuaillé, France), 1% (v/v) nanomycopulitine (LX16, Biowest, Nuaillé, France), and 1% (v/v) antibiotic–antimycotic (15240062, Gibco, Massachusetts, USA). The cells were incubated at 37 °C and 5% CO2 condition. The growth medium were changed every three days. The hepatotoxic model was generated in vitro using H2O2-induced HepG2. Confluent cells are rinsed with PBS and trypsin EDTA (0.25%; 25200072, Gibco, Massachusetts, USA) is added and incubated at 37 °C. Then cells were counted with hemocytometer and seeded in 6 well plates (5 × 105 cells/well). The cells were then incubated in previously described condition for 24 h. The cells, except for negative control group, were induced by 15 mM H2O2 (8.22287.100, Merck, Massachusetts, USA). The cells were divided into several groups, which were: (I) Control (HepG2 without H2O2 induction); (II) Vehicle control (HepG2 cells + DMSO 1%); (III) Liver Injury model (H2O2-induced HepG2); (IV) H2O2-induced HepG2 + RBLE 25 μg/mL; and (V) H2O2-induced HepG2 + RBLE 100 μg/mL. After the addition of each treatment, the cells were again incubated for 24 h. The conditioned medium (CM) were collected and centrifuged at 1600 rpm for 10 min. It was then store at −80 °C for TNF-α level quantification. Cells were also harvested using 0.25% trypsin EDTA (25200072, Gibco, Massachusetts, USA) and stored at −80 °C for further analysis (Lister et al., 2019b, Widowati et al., 2019b; Widowati et al., 2020).
2.3. Total protein measurements
The quantification of total protein was performed to determine TNF-α level by mg protein. Total protein measurement was conducted using Quick Start Bradford 1x Dye Reagent (5000205, Biorad, California, US) according to manufacturer’s protocol with BSA as standard. The result was read at at 595 nm using spectrophotometer (Multiskan™ GO Micro plate Spectrophotometer, Thermo Fisher Scientific, Waltham, USA) (Ernst and Zor, 2010; Widowati et al., 2019b).
2.4. Quantification of TNF-α level
TNF-α level quantification was performed using ELISA assay according to the manufacturer’s protocol (430204, BioLegend, California, US). Optical density (OD) were measured at 450 nm and 570 nm using spectrophotometer (Laksmitawati et al., 2016; Widowati et al., 2018a).
2.5. Apoptotic, necrotic, death, and live cells assay
PBS was used to wash the cells from each group, then it was harvested using trypsin EDTA. Then it was centrifuged and counted using hemacytometer. To 5 × 105 accounted cells, Annexin V binding buffer (130-092-820, Miltenyi Biotec, Bergisch Gladbach, Germany) as much as 500 μL, Anti-FITC (130-048-701, Miltenyi Biotec, Bergisch Gladbach, Germany) as much as 5 μL, and Propidium Iodide (130-093-233, Miltenyi Biotec, Bergisch Gladbach, Germany) as much as 5 μL were added and continued by incubation in the dark at 4 °C. The HepG2 cells apoptotic, necrotic, death, and live cells percentage were measured by MACSquant Analyzer 10 (Miltenyi Biotec, Bergisch Gladbach, Germany) (Widowati et al., 2018a; Widowati et al., 2020).
2.6. Reactive oxygen species assay
Flow cytometry using a DCF-DA fluorescent probe (invitrogen) were used for detection of the intracellular ROS levels. Four different groups were prepared, which were: (I) Negative control (untreated HepG2 cells); (II) Liver injury model (H2O2-induced HepG2 cells); (III) H2O2-induced HepG2 cells + RBLE 25 µg/mL; and IV) HepG2 cells + RBLE 100 µg/mL. HepG2 cells at density of 25 × 104 cells/0.5 mL Facs buffer (2.5% FBS in PBS) were incubated with 20 µM DCF-DA at 37 °C for 45 min. After that, group III and group IV were treated with 25 and 100 µg/mL, respectively, and were incubated for 4 h. The intracellular ROS levels were analyzed using Miltenyi Flow Cytometer (MAQS quant). The analyzed fluorescence values were expressed as a percentage relative to control group (Widowati et al., 2014; Prahastuti et al., 2019).
2.7. GPX gene expression level
The harvested HepG2 cells were processed for RNA isolation for further assay. RNA isolation was conducted using Aurum™ Total RNA mini Kit Bio-Rad 732-6820, according to manufacturer’s protocol, continued by cDNA synthesis using iScript Reverse Transcription Supermix for RT-PCR (170-8841, Biorad, California, US). The GPX gene expression level along with the constitutively expressed β-actin gene were analyzed using RT-qPCR (Thermo Scientific, PikoReal 96), with SsoFast Evagreen Supermix (172-5200, Biorad, California, US). The qPCR process were carried out through 7 min pre-denaturation at 95 °C; continued by 40 cycles of 30 sec of denaturation at 95 °C, 40 sec of annealing at 60 °C, and 60 sec of elongation at 72 °C. Gene expression was measured as relative copy number compared to control using ΔΔCT method (Widowati et al., 2018a, Widowati et al., 2018b, Afifah et al., 2019). The primer sequences of GPX and β-actin gene used in this study can be seen at Table 1 also the concentration and purity of RNA can be seen at Table 2.
Table 1.
Primer sequences.
| Gene Symbols | Primer Sequences (5′ to 3′) Upper strand: Sense Lower strand: Antisense |
Annealing (0C) | Cycle | Reference |
|---|---|---|---|---|
| β-Actin | 5′-TCTGGCACCACACCTTCTACAATG-3′ | 60 | 40 | Widowati et al., 2018a, Widowati et al., 2018b |
| 5′-AGCACAGCCTGGATAGCAACG-3′ | ||||
| GPX | 5′-CCAAGCTCATCACCTGGTCT-3′ | 60 | 40 | Fang et al. (2010) |
| 5′-TCGATGTCAATGGTCTGGAA-3′ | ||||
Table 2.
Concentration and Purity of RNA.
| Sample | RNA concentration (ng/µL) | RNA purity (λ 260/λ 280 nm) |
|---|---|---|
| Negative control | 75.70 | 2.3810 |
| Positive control (H2O2-induced HepG2) | 96.40 | 2.4264 |
| H2O2-induced HepG2 + RBLE 25 µg/mL | 67.20 | 2.4168 |
| H2O2-induced HepG2 + RBLE 100 µg/mL | 88.65 | 2.4068 |
2.8. Statistical analysis
All the experiment was done in triplicate and the data were presented as mean ± standard deviation. Statistical analysis was conducted using SPSS software (version 23.0) (IBM Corp, 2015). The significant differences between treatments and controls were determined using the Student’s T-Test or Mann Whitney U according to normality of the data at significance level 95% (α = 0.05).
3. Results
In this study, liver injury condition was modeled by H2O2-induced human hepatocellular carcinoma (HepG2) cells in vitro·H2O2 is one of the reactive oxygen species (ROS) molecules that in excess concentration could induced oxidative stress (Vilema-Enríquez et al., 2016). ROS are atoms or molecule that have unpair electrons, which usually unstable and highly reactive, that can initiate lipid peroxidation, cause trigger inflammation response, DNA damage, and induce apoptosis, resulting in injury (Li et al., 2015). Liver injury, caused by inflammation, could be detected by several indication, including TNF-α level; apoptotic, necrotic, dead, and live cells percentage; ROS level; and GPX gene expression in cells.
3.1. TNF-α level
TNF-α is one of the most important cytokines that plays a major role in the pro-inflammatory cytokines cascade production (Laksmitawati et al., 2016). It is rapidly released in time of trauma, wound, oxidative stress, or after bacterial infection (Parameswaran & Patial, 2010). The concentration of TNF-α level in hepatic cells determined its damaging or protective effect on liver injury. Previous study has also shown that TNF-α is involved in regulating proliferation and cell death of hepatocytes (Zhao et al., 2020).
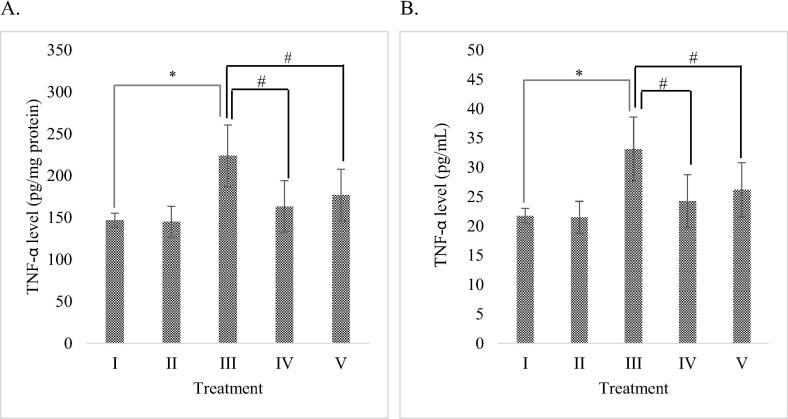
Based on the result, DMSO as extract solvent didn’t increase or decrease the TNF-α level significantly (P > 0.05) compare to normal cell without any inducer. It means DMSO wasn’t hepatotoxicity to HepG2 cells. The effect of RBLE towards TNF-α level of H2O2-induced HepG2 cells are shown in Fig. 1. The highest TNF-α level was exhibited by liver injury model, indicating that inflammation took place and liver damage was successfully induced by H2O2 in HepG2 cells. Treatment with RBLE resulting in a relatively lower TNF-α level compared to liver injury model. TNF-α level was reduced significantly in H2O2-induced HepG2 cells treated with 25 μg/mL RBLE. This result shows that RBLE has potential in suppressing TNF-α production, thus can be beneficial in liver damage treatment.
Fig. 1.
Effect of RBLE towards TNF-α Level of H2O2-induced HepG2 cells. (A) TNF-α level (pg/mg protein) on H2O2-induced HepG2 cells. (B) TNF-α level (pg/mL) on H2O2-induced HepG2 cells. *Data is presented as mean ± standard deviation. (I): Control (untreated HepG2 cells); (II): Vehicle control (HepG2 cells + DMSO 1%); (III) LI model (Liver Injury model: H2O2-induced HepG2 cells); (IV) RBLE25 (H2O2-induced HepG2 cells + RBLE 25 μg/mL); (V) RBLE100 (H2O2-induced HepG2 cells + RBLE 100 μg/mL). Single star sign (*) marks statistical difference between control group and Liver Injury (LI) model group at 0.05 significance level, while single hashtag symbol (#) marks statistical difference for the treatments groups compared to Liver Injury (LI) model group at 0.05 significance level.
3.2. Apoptotic, necrotic, live cells percentage
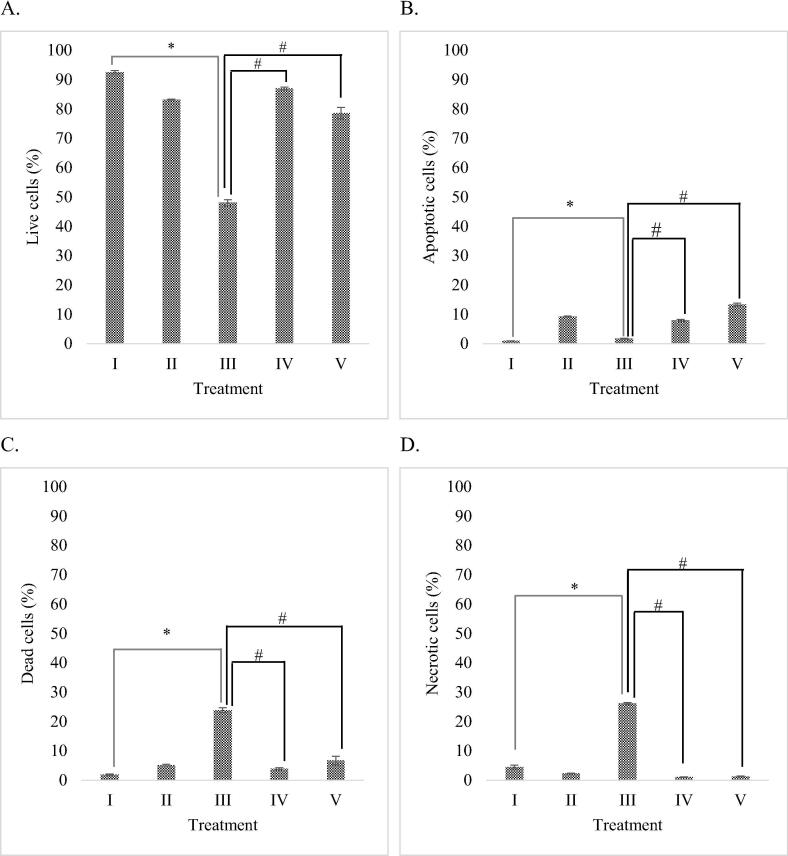
Apoptotic activities of RBLE towards H2O2-induced HepG2 cells were analyzed using flow cytometry. The analysis reveals the percentage of live cells, apoptotic cells, dead cells, as well as necrotic cells. Results of flow cytometry analysis are shown in Fig. 2.
Fig. 2.
Effect of RBLE towards apoptosis inducing activity of H2O2-induced HepG2 cells. (A) Live cells (%); (B) Apoptotic cells (%); (C) Dead cells (%); (D) Necrotic cells (%). *Data is presented as mean ± standard deviation. (I): Control (untreated HepG2 cells); (II): Vehicle control (HepG2 cells + DMSO 1%); (III) LI model (Liver Injury model; H2O2-induced HepG2 cells); (IV) RBLE25 (H2O2-induced HepG2 cells + RBLE 25 μg/mL); (V) RBLE100 (H2O2-induced HepG2 cells + RBLE 100 μg/mL). Single star sign (*) marks statistical difference between control and Liver Injury (LI) model group at 0.05 significance level, single hashtag (#) marks statistical difference for treatment groups compared to Liver Injury (LI) model group at 0.05 significance level.
Based on the result, DMSO didn’t increase or decrease the percentage of live cells, apoptotic cells, dead cells, as well as necrotic cells significantly (P > 0.05) compare to normal cell without any inducer. It means DMSO wasn’t hepatotoxicity to HepG2 cells. Based on the result presented in Fig. 2, it is shown that treatment with RBLE significantly reduced necrosis, switched the cell death pathway to apoptosis instead. It is evident that apoptotic cells in the RBLE treatment groups were significantly higher than the liver injury model, with the highest concentration of RBLE resulted in a higher apoptotic index. Overall, treatment with RBLE were able to increased live cells percentage and reduced dead cells percentage in the liver injury model.
3.3. Reactive oxygen species assay
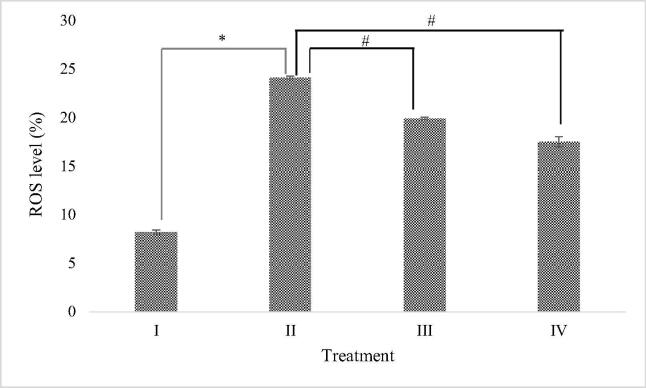
The effect of RBLE towards ROS level of H2O2-induced HepG2 cells are shown in Fig. 3. It was evident that liver injury model exhibited the highest level of ROS (24.1 ± 2.54%), indicating that liver damage was successfully induced. Treatment with RBLE, both 25 μg/mL and 100 μg/mL, were able to significantly reduce ROS level compared to liver injury model (19.9 ± 0.14% and 17.49 ± 0.52%, respectively). It was shown that ROS level was gradually decreased along with the increase of RBLE concentration. Even though it was still far from the normal cells condition, which is shown by the control group, this result indicates that RBLE has a potential as ROS scavenger.
Fig. 3.
Effect of RBLE towards ROS percentage of H2O2-induced HepG2 cells. *Data is presented as mean ± standard deviation. (I): Control (untreated HepG2 cells); (II) LI model (Liver Injury model; H2O2-induced HepG2 cells); (III) RBLE25 (H2O2-induced HepG2 cells + RBLE 25 μg/mL); (IV) RBLE100 (H2O2-induced HepG2 cells + RBLE 100 μg/mL). Single star sign (*) marks statistical difference between control group and Liver Injury (LI) model group and at 0.05 significance level, single hashtag (#) marks statistical difference in treatment groups compared to Liver Injury (LI) model group at 0.05 significance level.
3.4. GPX gene expression level
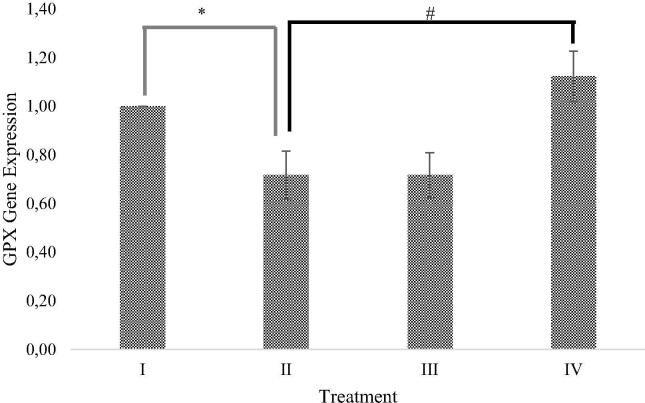
The effect of RBLE towards GPX gene expression in H2O2-induced HepG2 cells are shown in Fig. 4. Based on this study, it was shown that induction of H2O2 were resulting in GPX gene expression decrease, as an oxidative damage result. Treatment with RBLE 100 μg/mL was could significantly increased GPX gene expression (1.55 ± 0.10) relative to liver injury model. However, treatment with 25 μg/mL were not able to increase GPX gene expression (0.66 ± 0.11).
Fig. 4.
Effect of RBLE towards GPX gene expression of H2O2-induced HepG2 cells. *Data is presented as mean ± standard deviation. (I): Control (untreated HepG2 cells); (II): LI model (Liver Injury model; H2O2-induced HepG2 cells); (III) RBLE25 (H2O2-induced HepG2 cells + RBLE 25 μg/mL); (IV): RBLE100 (H2O2-induced HepG2 cells + RBLE 100 μg/mL). Single star sign (*) marks statistical difference for control group compared to Liver Injury (LI) model group 0.05 significance level, single hashtag (#) marks statistical difference for treatment groups compared to Liver Injury (LI) model group at 0.05 significance level.
4. Discussion
RBLE mainly contains flavonoids, tannins, saponins, and alkaloid (Maslikah et al., 2016). Ethanolic extract of red betel leaves that obtained by Soxhlet extraction has been reported to contain neophytadiene, propionic acid, and elemicin (Anugrahwati et al., 2016). Based on Gas Chromatography-Mass Spectrometry (GCMS) analysis on several varieties of piper betel plants, it has been known that piper betel leaves extract possess at least 10 different phytochemicals, mainly eugenol and hydroxychavicol (Begam et al., 2018). A study from Lister et al., 2019a has demonstrated that RBLE along with its compound, hydroxychavicol and eugenol, have antioxidant activity as indicated by their ability in scavenging DPPH and H2O2; as well as reducing ABTS and FRAP.
The production of ROS inside of cells are tightly regulated by enzymes, and are usually produced in low/moderate concentration. Excess ROS and failure decomposition of free radicals by antioxidant enzymes may lead to liver injury (Ismail et al., 2010, Kaur et al., 2019). The hydrogen peroxide induction clearly induced oxidative stress in hepatic cells as the result shown an increase in ROS level of H2O2-induced HepG2 cells (24.10 ± 2.54%). Hydrogen peroxide could passes freely through the plasma membrane and act as an intracellular ROS major source. In normal condition, H2O2 would be transformed into water by glutathione peroxidases by using it to oxidize reduce GSH into gluthatione disulfide (GSSG). In case of excessive ROS level, the hydrogen peroxide could be transformed into a highly reactive hydroxyl radical (HO*) with the presence of transition metals such as iron (Fe2+) and copper (Cu+). These free radicals could directly induce DNA damage and lipid peroxidation resulting in oxidative stress (Shimizu et al., 2012, Zhang et al., 2018). Our study revealed that RBLE treatment were able to significantly decreased ROS level, even though it was still far from normal condition (Fig. 3). This result supported previous study which stated that RBLE possess antioxidant activities that might be beneficial for liver injury medication. It seemed that RBLE affects ROS level in a dose-dependent manner, as higher concentration resulted in lower ROS level (Lister et al., 2019a, Lister et al., 2019b).
ROS are tightly regulated by various enzymes and proteins, such as superoxide dismutase (SOD), catalase (CAT), and GPX under normal conditions (Shehat and Tigno-Aranjuez, 2019). Glutathione peroxidase is an enzyme family responsible in converting hydrogen peroxide (H2O2) into less harmful chemicals, mainly water. There are at least 8 isoforms of GPX, 1 to 8, present in cells. GPX1 is the most abundant form of the enzyme and present virtually in cells. GPX is also referred to as selenocysteine peroxidases as most of it depends on selenium for its activity (Ighodaro and Akinloye, 2018). The production of GPX enzymes are controlled by GPX gene family expressions that have been known to be regulated by several mechanisms, including oxidative stress (Lubos et al., 2011). The oxidative stress caused by excessive intracellular H2O2 has been well documented resulted in decrease of GPX enzymes activity and increase gene expression as a consequent of ROS activity (Bak et al., 2014). However, H2O2 exposure at very high concentration (15 mM) resulted in decrease of GPX gene expression as exhibited by the liver injury model in this study (Fig. 4). Migue et al. (2009) had reported that acute liver injury condition, caused by exposure of H2O2 at high concentration in HeLa cells, decreased Se:GPx enzymes activity. Our results suggested that acute liver injury condition might also play role in down-regulating GPX gene expression (Migue et al., 2009). Treatment with RBLE at high concentration (100 μg/mL) resulted in a significant increase of GPX gene expression, even higher than the normal cells. It seemed the antioxidants properties of RBLE helped in decreasing free radicals level inside of cells to a tolerable level and thus inducing the normal antioxidant response.
Previous studies have shown that inflammation and oxidative stress are interconnected process and involved in many pathological conditions including liver injury, cardiovascular, and kidney, as well as cancer (Biswas et al., 2007, Reuter et al., 2010, Ambade and Mandrekar, 2012, García et al., 2017). Cross-talk between ROS and TNF-α seemed to be the key factors on how these phenomenon could be interconnected. Li et al. (2015) was reported that the pro-inflammatory cytokine shown to play a major role in liver damage initiation. Intracellular ROS activate NF-kB protein and subsequently increase TNF-α expression. On the other hand, TNF/TNFR engagement induce JNK signaling pathway that lead to mitochondrial ROS production through its binding with SAB protein. TNF-α also activates Caspase8, ROMO1, and BclXl proteins that reduce mitochondrial membrane potential, inducing more ROS production. This mitochondrial ROS induce intrinsic signaling pathway of apoptosis (Sabio and Davis, 2014; Blaser et al., 2016). In this study, RBLE treatment showed its potential as anti-inflammatory agent by successfully decreased TNF-α level in H2O2-induced HepG2 cells. Lower RBLE concentration showed stronger anti-inflammatory effect as it was resulting in significantly lower TNF-α level (163.51 ± 30.54 pg/mg protein) compared to higher concentration (176.80 ± 30.96 pg/mg protein) (Fig. 1).
Hepatocellular death is considered as an important process involved in the liver injury progression. Several types of cell deaths, whether controlled or not, have been reported to take place side by side in the development of the injury. Apoptosis, necrosis, and necroptosis are the types of cell deaths that involved in the process (Luedde et al., 2014, Weng et al., 2015).
Apoptosis is one of programmed cell death phenomenon known by several characteristics including cells shrinkage and DNA fragmentation. The generation of ROS is one of the effective mechanism which leads to apoptosis (Kaur et al., 2020a). Apoptotic cells are engulfed by macrophage to prevent intercellular component leakage, thus not affecting neighbor cells. There are two possible pathway of apoptosis that has been identified in mammalian cells, which are intrinsic and extrinsic pathways. Intrinsic pathways, also known as mitochondrial pathway, is regulated mainly by Bcl2 proteins family. Bcl2 inhibits Bac/Bax activation that responsible in inducing cytochrome c release from mitochondria. In case of apoptotic stimulus, such as oxidative stress, Bac and Bax are activated resulting in cytochrome c release. Cytochrome c together with Apaf-1 and Caspase9 assembling an apoptosome which then activates Caspase3 who act as the executioner. It has been reported, caspases play a crucial role in maintaining the homeostasis through the regulation of inflammation and cell death (Kaur et al., 2020b). Meanwhile, extrinsic pathway is triggered by the binding between death factors (such as TNF-α and FasL) to its receptor. In hepatic cells, this interaction activates Caspase8 and inhibits Bcl 2 protein, subsequently inducing intrinsic cell death pathway as described previously (Nagata, 2018).
In this study, it was shown that induction of H2O2 was causing cell deaths through necrosis (26.15 ± 0.31%) rather than apoptosis (1.81 ± 0.12%) in liver injury model (Fig. 2). This seemed to be the case as the induction of H2O2 was conducted in high concentration, thus triggered acute liver injury instead of chronic condition. Many studies have revealed that necrosis is an important process involved in acute liver injury cases (Weng et al., 2015, Luedde et al., 2014). High amount of free radicals might induce mitochondrial permeability transition (MPT) and thus opening mitochondrial pore that lead to mitochondrial swelling. This condition affects adenosine triphosphate (ATP) availability, as the amount of functional mitochondria were also decrease. It has been known that lack of energy source, in this case ATP, could switch apoptosis pathway to necrosis. Depletion of ATP subsequently causing failure of ATP-dependent ion pumps, thus triggering cell and organelles swelling and induce the formation of membrane “blebs” leading to cell rupture (Duprez et al., 2009, Luedde et al., 2014).
In this study, treatment with RBLE successfully shifted hepatic cell death pathway from necrosis to apoptosis. It was shown that necrotic cells percentage were significantly decreased, while the apoptotic cells percentage of RBLE treatment groups were significantly higher than liver injury model. This result demonstrated RBLE properties as anti-inflammatory agent, as apoptosis has known to trigger lower inflammation response compared to necrosis (Luedde et al., 2014). RBLE treatment were also able to decrease dead cells percentage, and subsequently improve cells condition as shown by the significant increase of live cells percentage.
Based on this study, it was evident that RBLE demonstrated its hepatoprotective effect through its antioxidant and anti-inflammatory activities. RBLE has been proven to contain many phytochemicals that could be potential to be used for liver injury medication. RBLE compouds, Eugenol and hydroxychavicol, have been studied for their antioxidant activities. Eugenol (4-allyl-2-methoxyphenol) is a phenolic compound belongs to class of phenylpropanoids (Barboza et al., 2018). It is widely known for its pharmacological properties including antioxidant, anti-inflammatory, antitumor, and antibacterial activity (Kim et al., 2003, Hamed et al., 2012, Dervis et al., 2017). Hydroxychavicol has been suggested as antimutagenic, anticarcinogenic, antioxidant, anti-inflammatory, and chemopreventive agent (Ali et al., 2010). As the most abundant compounds of RBLE, eugenol and hydroxychavicol, is suggested to be the reason of RBLE antioxidant and anti-inflammatory activities in this study. Their free radicals scavenging ability, as demonstrated by Lister et al., 2019a, Lister et al., 2019b previously, seemed to neutralize HO* in H2O2-HepG2 cells and thus improving cells condition under oxidative stress. Based on our study and literature review, we proposed a mechanism on how RBLE could act as hepatoprotective agent in liver injury (Fig. 5).
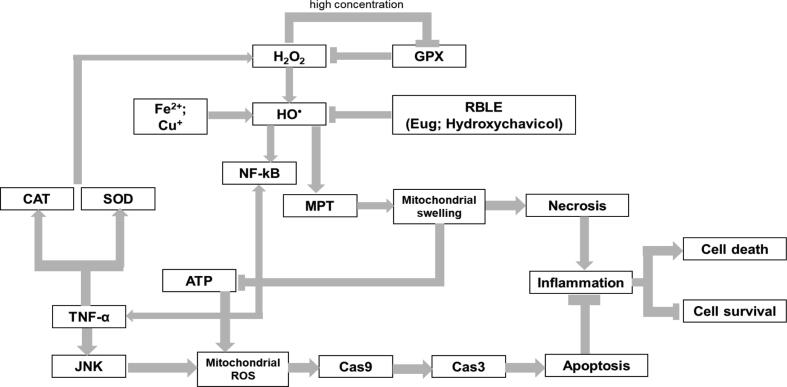
Fig. 5.
Proposed mechanism on how RBLE could act as a hepatoprotective agent in liver injury model·H2O2 is transformed into hydroxyl radical (HO*) through Fenton reaction with the presence of transition metal such as iron and copper. Under normal condition, H2O2 is neutralized by Glutathione Peroxidase (GPX) enzymes activity through oxidation of GSH into GSSG. It seemed that excessive H2O2 lead to down regulation of GPX gene expression. Hydroxyl radical activates NFkB that lead to TNF-α production. TNF/TNFR engagement induces production of catalase (CAT) and superoxide dismutase (SOD) and thus increasing extracellular H2O2. Meanwhile, TNF-α also induced JNK signaling pathways, resulting in increased mitochondrial ROS and lead to activation of Caspase 9 and apoptosis executioner, Caspase3. On the other hand, high amount of HO* triggers mitochondrial permeability transition (MPT), causing mitochondria to swell, and suppress ATP production. This pathway lead to unregulated cell death, necrosis; increase inflammation; and promote cell death. Lack of ATP also affects apoptosis pathway and switch it into necrosis instead. If this condition left untreated, liver injury could take place. Treatment with RBLE could neutralized HO* in cells and thus lowering necrosis, shifted the cells death pathway to apoptosis which induce lower inflammation response instead. Subsequently, RBLE treatment could increase the survival of hepatic cells.
5. Conclusion
RBLE treatment could decrease TNF-α level, necrotic cells percentage, dead cells percentage, and ROS level leading to higher apoptotic and live cells percentage; as well as increase GPX gene expression in H2O2-induced liver injury model. Low concentration of RBLE (25 μg/mL) exhibited best anti-inflammatory properties as it had the lowest TNF-α level and increased live cells percentage by shifting cell death pathway from necrosis to apoptosis. While high concentration of RBLE resulted in lowest ROS level and highest GPX gene expression, indicating its antioxidant properties.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We gratefully acknowledge the financial and facilities support provided by the Research Institutions and Community Service of Universitas Prima Indonesia. Medan, Indonesia for research grant 2018 and also Aretha Medika Utama Biomolecular and Biomedical Research Center, Bandung, Indonesia for providing research methodology and laboratory facilities. We would also extend our gratitude to Hanna Sari Widya Kusuma, Seila Arumwardana, Dwi Surya Artie, Rr. Anisa Siwianti, Dewani Tediana Yusepany, Kamila Yashfa Gunawan, Ika Adhani Sholihah, and Jenifer Kim Aviani from Aretha Medika Utama-Biomolecular and Biomedical Research Center, Bandung, Indonesia for their technical support.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aara A., Chappidi V., Ramadas M.N. Antioxidant activity of eugenol in Piper betel leaf extract. J. Fam. Med. Primary Care. 2020;9(1):327–331. doi: 10.4103/jfmpc.jfmpc_809_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afifah E., Mozef T., Sandra F., Arumwardana S., Rihibiha D.D., Nufus H., Rizal R., Amalia A., Bachtiar I., Murti H., Widowati W. Induction of matrix metalloproteinases in chondrocytes by interleukin IL-1β as an osteoarthritis model. J. Math. Fundam. Sci. 2019;51(2):103–111. [Google Scholar]
- Ali I., Khan F.G., Suri K.A., Gupta B.D., Satti N.K., Dutt P., Afrin F., Qazi G.N., Khan I.A. In vitro antifungal activity of hydroxychavicol isolated from Piper betle L. Ann. Clin. Microbiol. Antimicrobials. 2010;9(1):1–9. doi: 10.1186/1476-0711-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambade A., Mandrekar P. Oxidative stress and inflammation: essential partners in alcoholic liver disease. Int. J. Hepatol. 2012;2012:1–9. doi: 10.1155/2012/853175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anugrahwati M., Purwaningsih T., Manggalarini J.A., Alnavis N.B., Wulandari D.N., Pranowo H.D. Extraction of ethanolic extract of red betel leaves and its cytotoxicity test on HeLa cells. Procedia Eng. 2016;148:1402–1407. [Google Scholar]
- Asrani S.K., Devarbhavi H., Eaton J., Kamath P.S. Burden of liver diseases in the world. J. Hepatol. 2019;70(1):151–171. doi: 10.1016/j.jhep.2018.09.014. [DOI] [PubMed] [Google Scholar]
- Bak M.J., Jeong W.S., Kim K.B. Detoxifying effect of fermented black ginseng on H2O2-induced oxidative stress in HepG2 cells. Int. J. Mol. Med. 2014;34(6):1516–1522. doi: 10.3892/ijmm.2014.1972. [DOI] [PubMed] [Google Scholar]
- Barboza J.N., da Silva Maia Bezerra Filho C., Silva R.O., Medeiros J.V.R., de Sousa D.P. An overview on the anti-inflammatory potential and antioxidant profile of eugenol. Oxidat. Med. Cell. Longevity. 2018;2018:1–9. doi: 10.1155/2018/3957262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begam K.M.F., Ravichandran P., Manimekalai V. Phytochemical analysis of some selected varieties of Piper betle L. Int. J. Curr. Pharm. Res. 2018;10(2):89–93. [Google Scholar]
- Biswas S.K., Lopes De Faria J.B., Biswas S.K., Lopes De Faria J.B. Which comes first: renal inflammation or oxidative stress in spontaneously hypertensive rats? Free Radical Res. 2007;41(2):216–224. doi: 10.1080/10715760601059672. [DOI] [PubMed] [Google Scholar]
- Blaser H., Dostert C., Mak T.W., Brenner D. TNF and ROS crosstalk in inflammation. Trends Cell Biol. 2016;26(4):249–261. doi: 10.1016/j.tcb.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Dervis E., Yurt Kilcar A., Medine E.I., Tekin V., Cetkin B., Uygur E., Muftuler F.Z.B. In vitro incorporation of radioiodinated eugenol on adenocarcinoma cell lines (Caco2, MCF7, and PC3) Cancer Biother. Radiopharm. 2017;32(3):75–81. doi: 10.1089/cbr.2017.2181. [DOI] [PubMed] [Google Scholar]
- Duprez L., Vanlangenakker N., Festjens N., Van Herreweghe F., Berghe T.V., Vandenabeele P. Essentials of Apoptosis. Humana Press; Totowa, NJ: 2009. Necrosis: molecular mechanisms and physiological roles; pp. 599–633. [Google Scholar]
- Ernst O., Zor T. Linearization of the Bradford protein assay. JoVE-J. Vis. Exp. 2010;38:1–7. doi: 10.3791/1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang W., Goldberg M.L., Pohl N.M., Bi X., Tong C., Xiong B., Koh T.J., Diamond A.M., Yang W. Functional and physical interaction between the selenium-binding protein 1 (SBP1) and the glutathione peroxidase 1 selenoprotein. Carcinogenesis. 2010;31(8):1360–1366. doi: 10.1093/carcin/bgq114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García N., Zazueta C., Aguilera-Aguirre L. Oxidative stress and inflammation in cardiovascular disease. Oxid. Med. Cell. Longevity. 2017;2017:1–2. doi: 10.1155/2017/5853238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamed S.F., Sadek Z., Edris A. Antioxidant and antimicrobial activities of clove bud essential oil and eugenol nanoparticles in alcohol-free microemulsion. J. Oleo Sci. 2012;61(11):641–648. doi: 10.5650/jos.61.641. [DOI] [PubMed] [Google Scholar]
- Ighodaro O.M., Akinloye O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018;54(4):287–293. [Google Scholar]
- Ilyas U., Katare D.P., Aeri V., Naseef P.P. A review on hepatoprotective and immunomodulatory herbal plants. Pharmacogn. Rev. 2016;10(19):66–70. doi: 10.4103/0973-7847.176544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail N.A., Okasha S.H., Dhawan A., Abdel-Rahman A.O., Shaker O.G., Sadik N.A. Antioxidant enzyme activities in hepatic tissue from children with chronic cholestatic liver disease. Saudi J. Gastroenterol.: Off. J. Saudi Gastroenterol. Assoc. 2010;16(2):90–94. doi: 10.4103/1319-3767.61234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyadevi R., Ananth D.A., Sivasudha T. Hepatoprotective and antioxidant activity of Ipomoea staphylina Linn. Clin. Phytosci. 2019;5(1):1–11. [Google Scholar]
- Kaur S., Kumar A., Thakur S., Kumar K., Sharma R., Sharma A., Singh P., Sharma U., Kumar S., Landi M., Brestič M., Kaur S.J. Antioxidant, antiproliferative and apoptosis-inducing efficacy of fractions from Cassia fistula L. leaves. Antioxidants. 2020;9(2):173–204. doi: 10.3390/antiox9020173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S., Pandit K., Chandel M., Kaur S. Antiproliferative and apoptogenic effects of Cassia fistula L. n-hexane fraction against human cervical cancer (HeLa) cells. Environ. Sci. Pollut. Res. Int. 2020;26:1–17. doi: 10.1007/s11356-020-08916-9. [DOI] [PubMed] [Google Scholar]
- Kaur S., Sharma D., Singh A.P., Kaur S. Amelioration of hepatic function, oxidative stress, and histopathologic damages by Cassia fistula L. fraction in thioacetamide-induced liver toxicity. Environ. Sci. Pollut. Res. 2019;26(29):29930–29945. doi: 10.1007/s11356-019-06158-y. [DOI] [PubMed] [Google Scholar]
- Kim S.S., Oh O.J., Min H.Y., Park E.J., Kim Y., Park H.J., Han Y.N., Lee S.K. Eugenol suppresses cyclooxygenase-2 expression in lipopolysaccharide-stimulated mouse macrophage RAW264. 7 cells. Life Sci. 2003;73(3):337–348. doi: 10.1016/s0024-3205(03)00288-1. [DOI] [PubMed] [Google Scholar]
- Kusuma S.A.F., Sumiwi S.A., Riska D.A.M. Effect of red Piper betle leaf (Piper crocatum Ruiz and Pav.) ethanolic extract on plasma biochemical and hematological parameters in vivo. J. Pharm. Res. 2017;11:9–12. [Google Scholar]
- Laksmitawati D.R., Prasanti A.P., Larasinta P., Syauta G.A., Hilda R., Ramadaniati H.U., Widyastuti A., Karami N., Afni M., Rihibiha D.D., Kusuma H.S.W., Widowati W. Anti-inflammatory potential of gandarusa (Gendarussa vulgaris Nees) and soursoup (Annona muricate L) extracts in LPS stimulated-macrophage cell (RAW264.7) J. Natural Remed. 2016;16(2):73–81. [Google Scholar]
- Li S., Tan H.Y., Wang N., Zhang Z.J., Lao L., Wong C.W., Feng Y. The role of oxidative stress and antioxidants in liver diseases. Int. J. Mol. Sci. 2015;16(11):26087–26124. doi: 10.3390/ijms161125942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister I.N.E., Ginting C.N., Girsang E., Armansyah A., Marpaung H.H., Sinaga A.P.F., Handayani R.A.S., Rizal R. Antioxidant properties of red betel (Piper crocatum) leaf extract and its compounds. J. Natural Remed. 2019;19(4):198–205. [Google Scholar]
- Lister I.N.E., Ginting C.N., Girsang E., Amansyah A., Chiuman L., Yanti N.L.W.E., Rizal R., Widowati W. Hepatoprotective effect of eugenol on acetaminophen-induced hepatotoxicity in HepG2 cells. J. Phys. Conf. Ser. 2019;1374(012009):1–7. [Google Scholar]
- Lubos E., Loscalzo J., Handy D.E. Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011;15(7):1957–1997. doi: 10.1089/ars.2010.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luedde T., Kaplowitz N., Schwabe R.F. Cell death and cell death responses in liver disease: mechanisms and clinical relevance. Gastroenterology. 2014;147(4):765–783. doi: 10.1053/j.gastro.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslikah S., Lestari S.R., Wulandari N. Active compounds of red betel (Piper crocatum) extract for safe antioxidant as cytotoxicity test revealed. Int. J. ChemTech Res. 2016;9(4):513–520. [Google Scholar]
- Migue F., Augusto A.C., Gurgueira S.A. Effect of acute vs chronic H2O2-induced oxidative stress on antioxidant enzyme activities. Free Radic. Res. 2009;43(4):340–347. doi: 10.1080/10715760902751894. [DOI] [PubMed] [Google Scholar]
- Misra P., Kumar A., Khare P., Gupta S., Kumar N., Dube A. Pro-apoptotic effect of the landrace bangla mahoba of Piper betle on Leishmania donovani may be due to the high content of eugenol. J. Med. Microbiol. 2009;58(8):1058–1066. doi: 10.1099/jmm.0.009290-0. [DOI] [PubMed] [Google Scholar]
- Nagata S. Apoptosis and clearance of apoptotic cells. Annu. Rev. Immunol. 2018;36:489–517. doi: 10.1146/annurev-immunol-042617-053010. [DOI] [PubMed] [Google Scholar]
- Parameswaran N., Patial S. Tumor necrosis factor-α signaling in macrophages. Crit. Reviews™ Eukaryotic Gene Expr. 2010;20(2):87–103. doi: 10.1615/critreveukargeneexpr.v20.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prahastuti S., Hidayat M., Tania S., Widowati W., Amalia A., Qodariah R.L., Rizal R., Kusuma H.S.W., Khoiriyah Z. Ethanol extract of jati belanda (Guazuma ulmifolia L.) as therapy for chronic kidney disease in in vitro model. J. Reports Pharmaceutic. Sci. 2019;8(2):229–235. [Google Scholar]
- Pu S., Ren L., Liu Q., Kuang J., Shen J., Cheng S., Zhang Y., Jiang W., Zhang Z., Jiang C., He J. Loss of 5-lipoxygenase activity protects mice against paracetamol-induced liver toxicity. Br. J. Pharmacol. 2016;173(1):66–76. doi: 10.1111/bph.13336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter S., Gupta S.C., Chaturvedi M.M., Aggarwal B.B. Oxidative stress, inflammation, and cancer: how are they linked? Free Radical Biol. Med. 2010;49(11):1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabio G., Davis R.J. TNF and MAP kinase signalling pathways. Semin. Immunol. 2014;26(3):237–246. doi: 10.1016/j.smim.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehat M.G., Tigno-Aranjuez J. Flow cytometric measurement of ROS production in macrophages in response to FcγR cross-linking. J. Visual. Exp. 2019;145:1–15. doi: 10.3791/59167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu I., Shimamoto N., Saiki K., Furujo M., Osawa K. Lipid peroxidation in hepatic fibrosis. Lipid Peroxidat. 2012;2012:483–492. [Google Scholar]
- Vilema-Enríquez G., Arroyo A., Grijalva M., Amador-Zafra R.I., Camacho J. Molecular and cellular effects of hydrogen peroxide on human lung cancer cells: potential therapeutic implications. Oxid. Med. Cell. Longev. 2016;2016:1–12. doi: 10.1155/2016/1908164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng H.L., Cai X., Yuan X., Liebe R., Dooley S., Li H., Wang T.L. Two sides of one coin: massive hepatic necrosis and progenitor cell-mediated regeneration in acute liver failure. Front. Physiol. 2015;6:178–190. doi: 10.3389/fphys.2015.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widowati W., Wijaya L., Wargasetia T.L., Bachtiar I., Yellianty Y., Laksmitawati D.R. Antioxidant, anticancer and apoptosis-inducing effects of Piper extracts in HeLa cells. J. Exp. Integr. Med. 2013;3(3):225–230. [Google Scholar]
- Widowati W., Widyanto R.M., Husin W., Ratnawati H., Laksmitawati D.R., Setiawan B., Nugrahenny D., Bachtiar I. Green tea extract protects endothelial progenitor cells from oxidative insult through reduction of intracellular reactive oxygen species activity. Iran. J. Basic Med. Sci. 2014;17(9):702–709. [PMC free article] [PubMed] [Google Scholar]
- Widowati W., Jasaputra D.K., Sumitro S.B., Widodo M.A., Mozef T., Rizal R., Kusuma H.S.W., Laksmitawati D.R., Murti H., Bachtiar I., Faried A. Effect of interleukins (IL-2, IL-15, IL-18) on receptors activation and cytotoxic activity of natural killer cells in breast cancer cell. Afr. Health Sci. 2020;2(2):822–832. doi: 10.4314/ahs.v20i2.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widowati W., Prahastuti S., Ekayanti N.L.W., Munshy U.Z., Kusuma H.S.W., Wibowo S.H.B., Amalia A., Widodo W., Rizal R. Anti-inflammation assay of black soybean extract and its compounds on lipopolysaccharide-induced RAW 264.7 cell. J. Physics: Conf. Series. 2019;1374(012052):1–11. [Google Scholar]
- Widowati W., Sumitro S.B., Jasaputra D.K., Onggowidjaja P., Rihibiha D.D., Widodo M.A., Afifah E., Kusuma H.S.W., Rizal R., Amalia A., Murti H., Bachtiar I. Effects of conditioned medium of co-culture IL-2 induced NK cells and human wharton’s jelly mesenchymal stem cells (hWJMSCs) on apoptotic gene expression in a breast cancer cell line (MCF-7) J. Math. Fundam. Sci. 2019;51(3):205–224. [Google Scholar]
- Widowati W., Rani A.P., Hamzah R.A., Arumwardana S., Afifah E., Kusuma H.S.W., Rihibiha D.D., Nufus H., Amalia A. Antioxidant and antiaging assays of Hibiscus sabdariffa extract and its compounds. Nat. Prod. Sci. 2017;23(3):192–200. [Google Scholar]
- Widowati W., Jasaputra D.K., Sumitro S.B., Widodo M.A., Afifah E., Rizal R., Rihibiha D.D., Kusuma H.S., Murti H., Bachtiar I., Faried A. Direct and indirect effect of TNF-α and IFNγ toward apoptosis in breast cancer cells. Mol. Cell. Biomed. Sci. 2018;2(2):60–69. [Google Scholar]
- Widowati W., Afifah E., Mozef T., Sandra F., Rizal R., Amalia A., Arinta Y., Bachtiar I., Murti H. Effects of insulin-like growth factor-induced wharton jelly mesenchymal stem cells toward chondrogenesis in an osteoarthritis model. Iran. J. Basic Med. Sci. 2018;21(7):745–752. doi: 10.22038/IJBMS.2018.28205.6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulandari N., Meiftasari A., Fadliyah H., Jenie R.I. Red betel leaves methanolic extract (Piper crocatum Ruiz and Pav.) increases cytotoxic effect of doxorubicin on WiDr colon cancer cells through apoptosis induction. Indonesian J. Cancer Chemoprevent. 2018;9(1):1–8. [Google Scholar]
- Yoon E., Babar A., Choudhary M., Kutner M., Pyrsopoulos N. Acetaminophen-induced hepatotoxicity: A comprehensive update. J. Clin. Translat. Hepatol. 2016;4(2):131–142. doi: 10.14218/JCTH.2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Wang N., Xu Y., Tan H.Y., Li S., Feng Y. Molecular mechanisms involved in oxidative stress-associated liver injury induced by Chinese herbal medicine: An experimental evidence-based literature review and network pharmacology study. Int. J. Mol. Sci. 2018;19(9):2745–2774. doi: 10.3390/ijms19092745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Jiang J., Jing Y., Liu W., Yang X., Hou X., Gao L., Wei L. The concentration of tumor necrosis factor-α determines its protective or damaging effect on liver injury by regulating Yap activity. Cell Death Dis. 2020;11(1):1–13. doi: 10.1038/s41419-020-2264-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulharini M., Sutejo I.R., Fadliyah H., Jenie R.I. Methanolic extract of red betel leaves (Piper crocatum Ruiz & Pav) perform cytotoxic effect and antimigration activity toward metastatic breast cancer. Indonesian J. Cancer Chemoprevent. 2018;8(3):94–100. [Google Scholar]