Abstract
Drug development, from preclinical to clinical studies, is a lengthy and complex process. There is an increased interest in the Kingdom of Saudi Arabia (KSA) to promote innovation, research and local content including clinical trials (Phase I-IV). Currently, there are over 650 registered clinical trials in Saudi Arabia, and this number is expected to increase. An important part of drug development and clinical trials is to assure the safe and effective use of drugs. Clinical pharmacology plays a vital role in informed decision making during the drug development stage as it focuses on the effects of drugs in humans. Disciplines such as pharmacokinetics, pharmacodynamics and pharmacogenomics are components of clinical pharmacology. It is a growing discipline with a range of applications in all phases of drug development, including selecting optimal doses for Phase I, II and III studies, evaluating bioequivalence and biosimilar studies and designing clinical studies. Incorporating clinical pharmacology in research as well as in the requirements of regulatory agencies will improve the drug development process and accelerate the pipeline. Clinical pharmacology is also applied in direct patient care with the goal of personalizing treatment. Tools such as therapeutic drug monitoring, pharmacogenomics and model informed precision dosing are used to optimize dosing for patients at an individual level. In KSA, the science of clinical pharmacology is underutilized and we believe it is important to raise awareness and educate the scientific community and healthcare professionals in terms of its applications and potential. In this review paper, we provide an overview on the use and applications of clinical pharmacology in both drug development and clinical care.
1. Introduction
Drug development is a lengthy, complex and expensive process, starting\from drug discovery to preclinical studies and ultimately structured clinical trials. In the Kingdom of Saudi Arabia (KSA), there is an increased interest to promote innovation, research and local content. A main focus is attracting pharmaceutical companies and contract research organizations (CROs) to conduct clinical trials in KSA (https://www.ic.gov.sa/en/about/press-releases/pharmaus/). Currently, there are more than 650 registered clinical trials in KSA. This number is considered very modest. As a comparison, Poland with a similar gross domestic product (GDP) and population has over 6400 registered clinical trials (https://clinicaltrials.gov/ct2/resultscond=term=cntry=SAstate=city=dist=). There are increased efforts in KSA to increase the number of clinical trials conducted and therefore the number is expected to increase in the near future.
Prior to 2009, clinical trials in KSA were not regulated by any government body local Institutional Review Boards (IRBs) were self-regulating clinical trials conducted at their sites. In 2009, the Saudi Food and Drug Authority (SFDA) established the Clinical Trials Administration. Since 2013, all investigators and sponsors are required to register early-phase clinical trials (Phase I, II and III) with the Saudi Clinical Trials Registry and obtain SFDA approval prior to study initiation(Saudi Food and Drug Authority, 2019).
An important principle of drug development and clinical trials, and its regulation is to ensure the safe and effective use of drugs. This is where clinical pharmacology, playing a vital role in informed decision making during the drug development stage. Clinical pharmacology studies the effect of drugs on humans and includes fields such as pharmacokinetics (PK), pharmacodynamics (PD) and pharmacogenomics (PGx). The fields have grown exponentially over the past two decades (Meibohm and Derendorf, 1997, Birkett et al., 2010, Chien et al., 2005, Miller et al., 2005, Zineh et al., 2017) and are applied in all phases of preclinical and clinical drug development. Specifically in roles such as determining the first-in-human dose, selecting the optimal dose for Phase II and III studies, dosing in special populations, evaluating bioequivalence and biosimilar studies, drug and food interaction studies, as well as in designing and conducting clinical studies (Meibohm and Derendorf, 1997, Chien et al., 2005, Williams and Ette, 2000, Peck, 2017, Tuntland et al., 2014, Zhu et al., 2018, Mehrotra et al., 2016, Aarons et al., 2001, Kuhlmann, 1999, Vozeh et al., 1996, Hughes and Walley, 2001, Lavé et al., 2016). Approximately half of the information provided in the package insert is related to clinical pharmacology (Peck, 2017). It is the responsibility of the clinical pharmacologists to evaluate investigational new drugs.
Many aspects of clinical pharmacology have become more quantitative, as mathematical modeling and simulation is considered to be an integral part of the field and is increasingly used in drug development, described as Model Informed Drug Development or pharmacometrics (Wang et al., 2019, Marshall et al., 2016, Visser et al., 2013). Model Informed Drug Development is used to leverage the data gained from different sources, including real world-data, clinical studies, and preclinical studies to assist in decision making during drug development (Wang et al., 2019, Visser et al., 2013, Marshall et al., 2019, Li et al., 2019, Huang et al., 2013). Model Informed Drug Development is part of the United States (US) FDA’s goals in the Prescription Drug User Fee Amendments of 2017 (https://www.fda.gov/drugs/news-events-human-drugs/cder-conversation-model-informed-drug-development).
Clinical pharmacology can also be applied in direct patient care by personalizing medicine for patients. Tools such as therapeutic drug monitoring (TDM), PGx and Model Informed Precision Dosing can be used to optimize dosing for patients at an individual level (Roberts et al., 2014, Polasek et al., 2019, Polasek et al., 2018, Gonzalez et al., 2017, Perera et al., 2014, Standing, 2017).
Considering these branches of clinical pharmacology from a regulatory, research and industrial perspective, they serve as a powerful tool in optimizing drug safety and efficacy in clinical trials during drug development and patient care. The science of clinical pharmacology is still underutilized in KSA and the aim is to promote and educate healthcare professionals and the scientific community about its potential and applications. In this review paper, we describe the applications of clinical pharmacology in both 1) clinical drug development and in 2) direct clinical care and provide an update about the drug development policies and procedures in Saudi Arabia.
2. Clinical pharmacology applications in clinical drug development
2.1. Phase I studies
The development of a new therapeutic agent is a multi-phase process, presenting ethical, scientific, and economic challenges. The probability of clinical development success is approximately 10% with few drugs surviving beyond clinical trials (Scannell et al., 2012). Phase 1 clinical trials play a crucial role in translating experimental studies in clinical applications and are a critical step in the decision to continue or halt development of promising new treatments. Phase 1 trials, also termed first-in-human studies, are designed to examine investigational new drugs and new combinations or dosing schedules of FDA-approved drugs.
Typically, the target population of a Phase 1 clinical trial consists of healthy volunteers. They represent the ideal population to evaluate the clinical pharmacology, allowing assessment of the drug’s safety profile without the influence or interference of pathological conditions. Clinical studies with healthy volunteers provide basic PK information about a novel drug candidate. They increase the study’s accrual rate and alleviate ethical concerns regarding the enrollment of patients to receive the investigational new drug at sub-therapeutic doses for the purpose of obtaining safety data.
A survey conducted by the British Pharmacological Society suggested that recruiting healthy volunteers for clinical trials is relatively safe. Some participants do experienced life-threatening events but at a very low rate (0.04%) (Orme et al., 1989). This raises an ethical concern about exposing healthy volunteers to risks during early drug development without any potential health benefits. A meta-analysis of non-oncology phase 1 trials found that 34 of the 11,028 healthy subjects experienced serious adverse events, and 50% of these events were not attributed to the study drug or a research procedure; moreover, no deaths or life threatening events were reported (Food and Drug Administration, 2005), indicating that healthy subjects are a suitable population for non-oncology phase 1 studies and it is relatively safe to investigate the safety of drugs on healthy participants (Food and Drug Administration, 2005). However, healthy volunteers are not an appropriate population for oncology drug trials due to the narrow therapeutic index of cytotoxic drugs and potential long-lasting DNA damage. In this case, cancer patients with advanced progression and no response to other treatment are considered a better candidate population to evaluate the dose-toxicity profile, to determine the recommended Phase II dose, to provide preliminary evidence of efficacy, and to identify a specific target population. Major objectives include the assessment of safety and tolerance, characterization of PK and PD of the new therapeutic candidate or novel combination of approved drugs, and the determination of the appropriate dose and schedule for the Phase II studies (Table 1). Phase 0 trials (early Phase1 trial) involve micro-dosing, enabling safer, quicker, and less expensive first-in-human studies by exposing healthy volunteers to sub-therapeutic drug doses.
Table 1.
Selected ongoing Phase1 trials in Saudi Arabia.
| NCT number | Treatment | Study design | Outcomes | Study population |
|---|---|---|---|---|
| NCT02919371 | Sunitinib and bevacizumab | PhaseI/II trial, open label | Safety, efficacy | Patients with advanced renal cell carcinoma |
| NCT03774680 | Cetuximab nanoparticles or oral approved anticancer drug | Phase1, randomized, open-label, parallel assignment | PK | Adult patients with colon cancer or colorectal cancer |
| NCT02628132 | Paclitaxel and durvalumab | Phase I/II trial, open-label | Safety, efficacy | Patients with metastatic triple negative breast cancer |
| Source: ClinicalTrials.gov | ||||
In 2005, the US FDA issued the “Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers” guidelines. It provides researchers with a regulatory framework for estimating the maximum recommended starting dose (MRSD) for healthy volunteers, based on determining the no-observed adverse effects level (NOAEL) in preclinical studies, then using allometric scaling factors to convert mg/kg dosing from animal species to humans to obtain the human equivalent dose (Food and Drug Administration, 2005). Another approach is determining the minimal anticipated biological effect level (MABEL), accepted by the EMA, by integrating all available in vivo and in vitro data for the selection of the safe starting dose and escalation in first-in-human clinical trials (Milton and Horvath, 2009). The NOAEL- or MABEL-derived human relevant dose is adjusted by applying appropriate safety margins to mitigate the risk associated with the first dose given to humans.
Generally, dose-escalation Phase1 trials are designed using the traditional 3 + 3 approach due to its simplicity and ability to detect dose limiting toxicity events occurring in the 15% to 25% range (Iasonos and O'Quigley, 2017). Each cohort consists of three participants who received escalating doses until the maximum tolerated dose (MTD) was reached. If no dose-limiting toxicities (DLTs) are observed during the first treatment cycle of the three participants, the dose is escalated and the second treatment cycle proceeds with a cohort of three subjects (Bjornsson et al., 2003). If one DLT is detected among the previous three participants, the trial recruits three additional participants at this dose level in the first treatment cycle; the trial escalates to the higher dose-level if no additional DLT is found at this dose level (Bjornsson et al., 2003). If two or more DLTs are observed among a cohort of three or six participants at any dose level, the prior dose is defined as the MTD (Bjornsson et al., 2003). After the dose escalation phase is completed and the MTD is determined, investigators enroll participants to receive the MTD. This is termed the dose expansion cohort and is used to elucidate and evaluate the safety of the candidate drug at the maximum tolerated dose, as well as to acquire preliminary evidence of efficacy. There are several limitations associated with the 3 + 3 design, including relatively slow study accrual and a small sample size, which restricts the PK assessment. These limitations led to the emergence of new alternative designs, such as the accelerated titration design.
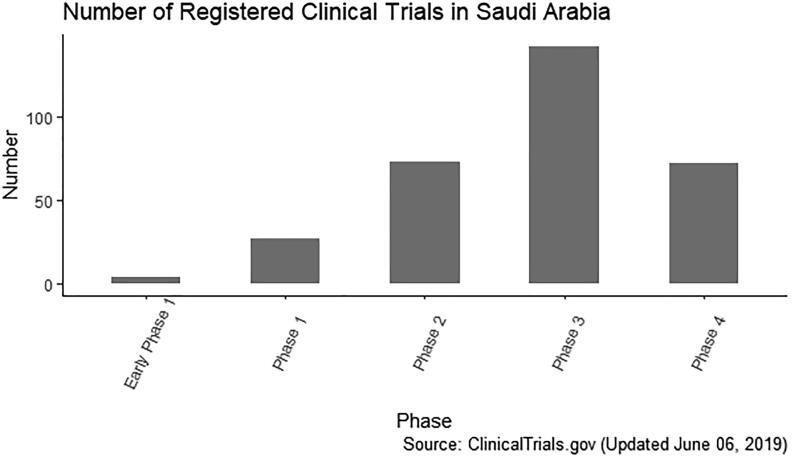
From the data in Table 1 and Fig. 1, it is apparent that few early Phase I/II trials are conducted in KSA. All of these studies are for FDA approved and cancer drugs being evaluated for new indications, meaning none are first in human studies. Major challenges facing first-in-human trials in KSA include a long ethical approval process, recruitment challenges, researchers’ lack of experience, and a limited number of established research sites. Recently, the SFDA approved the first Phase I center in KSA at King Abdullah International Medical Research Center (KAIMRC). The center is expected to start its first phase I trial testing a new MERS-CoV vaccine. This is a milestone achievement and hopefully there will be more centers and early phase clinical trials conducted in KSA. Phase I trials provide significant information guiding the decision to continue or terminate the potential drug’s development. In future, it is critical to encourage national investigators to participate in the early phases of the drug development process for novel therapeutic agents.
Fig. 1.
Number of registered clinical trials in Saudi Arabia.
2.2. Drug-drug interaction and bioequivalence studies
Drug-drug interaction studies are done to evaluate metabolism- and transporter-mediated interactions. The goals are to determine if the investigational drug alters the PK of the other co-administered drugs or vice versa, and whether the observed interaction is clinically significant (Prueksaritanont et al., 2013). Clinical drug-drug interaction studies are conducted after in vitro studies show a potential interaction (Bjornsson et al., 2003). Prospective clinical drug-drug interaction studies are frequently stand-alone studies mostly in healthy volunteers. They do not belong to a particular phase but are conducted early in drug development during Phase I studies. The method to assess drug-drug interaction is to use a strong index perpetrator known to inhibit or induce a particular metabolizing enzyme or transporter. The magnitude of the interaction will depend on the percentage of increase or decrease in plasma concentration or the area under the curve (AUC). As a rule, a 20% change in AUC is considered clinically significant (Prueksaritanont et al., 2013).
Bioequivalence studies are done to compare the systemic exposure profile of the test drug formulation to the reference drug formulation. The rate and extent of absorption of the active ingredient in the test and reference products should not be significantly different and within a pre-specified range determined by the regulatory agency to be considered bioequivalent. The typical study design for a bioequivalence study is a two-period, two-sequence, two-treatment, single dose, crossover study in healthy participants. The total number of samples is 12 to 18 blood samples to adequately estimate the AUC. The rate of absorption is measured by the peak concentration and the extent of absorption is measured by the AUC. The 90% confidence interval for the test to reference ratio of peak concentration and AUC should be within the bioequivalence limits (80–125%) (Chow, 2014, https://www.sfda.gov.sa/en/drug/drug_reg/Regulations/GCC_Guidelines_Bioequivalence.pdf, xxxx).
Currently, most bioequivalence studies are not conducted in Saudi Arabia, but outside the country. The same applies for drug-drug and drug herbal interaction studies. They are rarely performed at clinical centers inside the country. This is important especially for herbal supplements, as their use is common in Saudi Arabia and the type of herbal products used differs compared to other countries. This is due to the lack of centers with experience doing these clinical studies.
2.3. Population pharmacokinetics and pharmacodynamics
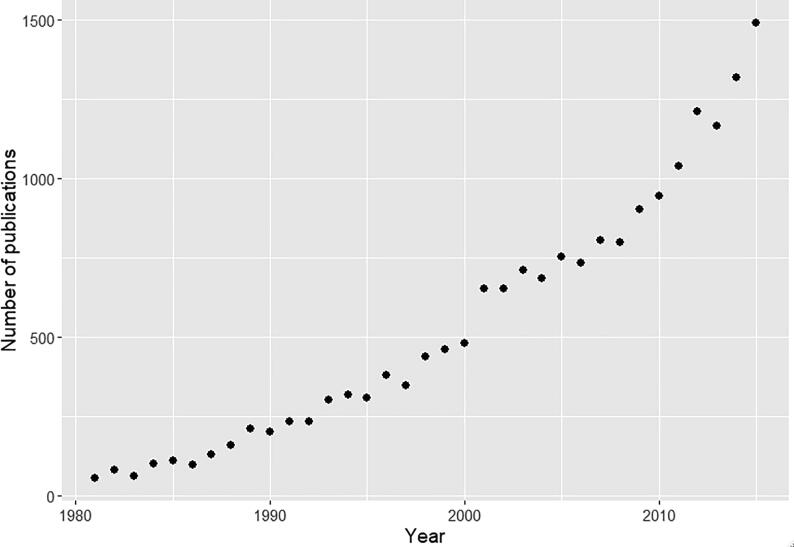
The main application of population PK/PD is during clinical drug development as the process transition from Phase I to II and III. Its use in drug development has become a standard and the majority of recent new drug applications involve it (Lee et al., 2011). Due to its increasing importance, the US FDA issued the first guidelines related to population PK in 1999. Fig. 2 shows the number of publications with the term “population pharmacokinetics” per year from 1980 to 2015.
Fig. 2.
The number of publications with the term “population pharmacokinetics” from 1980 to 2015.
The main advantages of population PK studies include being conducted in the target patient population, not requiring intensive sampling, ability to identify the sources of PK variability, exploring the significance of covariates (e.g. weight, age, genotype, etc.) on PK parameters, and using its parameter estimates to perform simulations for ‘what if’ scenarios (Pillai et al., 2005, Egelund et al., 2011, de Velde et al., 2018, Sheiner and Benet, 1985). This is in contrast with traditional PK studies, which usually required intensive blood sampling, making it difficult to study some patient populations, such as pediatrics, geriatrics, and critically ill patients. The traditional PK analysis is performed in two sequential steps: PK fitting and summary statistics. This method does not distinguish the different sources of variability. Population PK analyzes all the data simultaneously in one step (pharmacostatistical model) using nonlinear mixed-effects regression, providing a better estimation of PK parameters and the associated variabilities. Estimating and understanding the sources of variability is critical in evaluating the safety and efficacy of drugs. In cases where a high inter-individual variability exists, patients can be under- or overdosed (Egelund et al., 2011, Kiang et al., 2012). The main disadvantage of population PK analyses is that they are computationally intensive and require specialized training (Table 2). The population PK approach can also be of limited use if it is not linked with PD or clinical outcomes. Joint population PK/PD models are frequently used in Phase II studies to characterize the drug concentration–response relationships (Chien et al., 2005). This allows the determination of the therapeutic range.
Table 2.
Comparing traditional pharmacokinetics approach to population approach.
| Traditional approach | Population approach | |
|---|---|---|
| Population | Mostly in healthy volunteers | Target population |
| Sampling | Intensive (12–15 samples per subject) | Sparse (1–3 samples) and intensive sampling |
| Design | Needs to be balanced | Mixed designs; studies from different sources, populations, or sites |
| Complexity | Moderate | Computationally intensive and time consuming |
| Analysis approach | Two steps: Nonlinear regression followed by summary statistics | All data at once using nonlinear mixed effects modeling |
| Variability | Minimized by design or selection criteria | More reflective of true population |
| PK-PD exposure response analysis | Cannot be applied | Possible |
Although Phase II studies establish the drug efficacy and safety in a relatively small number of patients, population PK/PD analyses can be applied during this phase to predict the outcomes in a larger population. The analyses are used to determine the optimal dose for the next trial, and the expected effect size to assist in estimating the sample size and design of Phase III studies. This is accomplished by estimating the PK variability in the target population, determining covariates that can influence the PK parameters, and performing exposure–response analyses and clinical trial simulations. Clinical trials can be simulated with a combination of different assumptions and scenarios, such as doses that are beyond those studied in Phase II trials, different sample sizes, and multiple study designs, to assess the probability of success (Hughes and Walley, 2001, Bonate, 2000, Holford et al., 2010, Gal et al., 2018). The simulations provide predictions of the response that can guide the dose selection and study design for Phase III studies. This will decrease the chances of failing for the investigational new drug in Phase III studies due to inappropriate study design, improper effect size, inappropriate dosing, or wrong study population. Simulations support avoiding failures by answering ‘what if’ scenarios through simulating large numbers of virtual patients that are typically ≥ 1000 for each dosing regimen. This allows for estimating the probability of achieving the therapeutic target, while minimizing sub- and supratherapeutic concentrations.
Phase III studies, known as pivotal trials, are conducted with a larger sample size to determine the effectiveness and safety of the study drug on a broader scale (Umscheid et al., 2011).
In Phase III, drug efficacy can be confirmed in diverse populations, as well as allow for dose optimization in special populations. Population PK/PD continues to be used in this Phase to confirm the adequacy of the selected therapeutic dose, potentially determine a therapeutic range for the study drug, and anticipate drug exposure and response when combinations of drugs are used. Additional covariates can be identified and hence minimize unexplained variabilities. This may provide patient-specific dosing based on individual characteristics, such as weight and genotype. The studies provide supportive data for drug registration if successful or explanations for failed clinical trials.
Other applications of population PK analysis include the development of generic products and biosimilar drugs. In some cases, standard noncompartmental analysis is not sufficient to evaluate or design bioequivalence studies, for example for locally acting drugs, drugs with immediate and sustained release products, nanotechnology products and drugs with a long half-life (Fang et al., 2018). Population PK/PD is also an integral part in the development of biosimilar products. Regulatory agencies require that the biosimilar products have a similar PK/PD profile to the reference product (Zhu et al., 2018, Dodds et al., 2013).
2.4. Physiologically-based pharmacokinetic analysis
After drug approval, it is critical to select the right dose in special populations such as pediatrics, geriatrics, renal and hepatic impaired patients, and pregnant patients. Model-based approaches provide an important opportunity to facilitate the determination of the appropriate dose that would balance the benefit and risk for different populations. These models have the capability to evaluate the relationship of drug exposure, treatment outcomes, and various patient-related factors, facilitating the selection of an appropriate dose for an individual patient. The Physiologically Based Pharmacokinetic (PBPK) Modeling Technique provides a platform for the appropriate dose selection in special populations. PBPK modeling allows the integration of drug related and individual-related factors explaining different mechanisms affecting drug disposition and the effect of different physiological and disease states on drug disposition and elimination.
After the development and validation of the PBPK model in healthy participants, the PK of the drug can be investigated in different populations using related parameters. The dose could be extrapolated from one population to another study population. The PBPK model can also be used to investigate the relationship between drug exposure and PD effects (including desired outcomes and/or side effects). This modeling approach is important to determine the safe dose in first-in-human clinical trials based on in vitro and in vivo data compared to including only a few variables in early clinical trials. By adopting the clinical data generated by later clinical trial phases, such as clinical activity, tolerability, and biomarkers, PBPK models can be used to identify the doses that provide the right balance between efficacy and safety and be used in several populations.
Several studies, using PBPK modeling, succeeded to extrapolate the correct dose for a pediatric population in the absence of clinical studies (Mehrotra et al., 2016, Templeton et al., 2018). The role of PBPK modeling in pediatric drug research has been illustrated in several FDA submissions (Leong et al., 2012). It was a fundamental part of these submissions and used in an optimizing study design, recommending starting doses for different age groups (Leong et al., 2012). In addition, the models correctly predict the PK and PD changes in renal and liver impaired patients. The appropriate doses were selected based on the explanation of these changes (Marsousi et al., 2017). For instance, the impact of renal impairment on the downregulation of some enzymes and proteins has been demonstrated using PBPK. Zhao et al. used PBPK models to describe the effect of renal impairment on the PK of three non-renally eliminated drugs, namely repaglinide, telithromycin, and sildenafil (Zhao et al., 2012). They were able to predict the impact of renal impairment on the expression of some metabolizing enzymes and estimate an accurate clearance for these drugs [5]. The models were also used successfully to predict the appropriate dose in special populations such as pregnant and geriatric participants based on the different physiological changes during pregnancy or aging (Howard et al., 2018, Ke et al., 2014). PBPK models have been used for the prediction of the effect of DDIs on the PK of victim drugs. Yoshida et al. surveyed all small molecules of new drugs approved by the FDA between January 2013 and August 2016 to determine the use of PBPK models in the approval and impact on labeling recommendations. A total of 18 products were identified, and in the majority, the models were used to predict the effect of DDIs (Yoshida et al., 2017).
2.5. Pharmacogenomics
The cost of DNA sequencing has decreased due to the development of next-generation sequencing (NGS), whole exome sequencing (WES), and whole genome sequencing (WGS) techniques. The rapid advancement in high throughput genomic technologies has improved the understanding of the role of genetic variations in drug response, accounting for up to 95% of the variability in drug effects and disposition in some cases (Crews et al., 2012). The current shift towards precision medicine is driven by the ability to translate these findings to clinical practice. Integrating PGx approaches in all steps of drug discovery and development can increase the success rate of its approval (Debouck, 2009). At the drug discovery stage, a new chemical entity can be discovered by finding a druggable genome target, which is defined as “a portion of the human genome that is susceptible to pharmacological interaction and simultaneously involved in the pathological mechanisms leading to the disease” (Rask-Andersen et al., 2014). For instance, the proprotein convertase substilisin/kexin type 9 (PCSK9) inhibitors were initially discovered based on findings that a loss-of-function mutation in the PCSK9 gene was associated with a lower plasma concentration of low-density lipoprotein cholesterol (Zhao et al., 2006). In 2015, the first PCSK9 inhibitor was approved by the US FDA. Identifying similar druggable genome targets is relatively easier in populations with a high consanguinity rate, such as the Saudi population, due to a higher probability of children carrying a homozygous loss-of-function mutation (Saleheen et al., 2017, Abu-Elmagd et al., 2015).
The application of PGx in drug development clinical trials have been recognized by several drug regulatory agencies, such as the US FDA and EMA, and guidelines for the submission of genomic information during the drug development process were developed in 2005 (Goodsaid and Papaluca, 2010). The EMA guidance requires a PGx study on the PK of the active substance if the magnitude of the inter-individual variation to drug exposure is so high that it could influence the safety and efficacy of the drug in a genetically variable population (European Medicines Agency, 2012). The US FDA requires a companion diagnostic test to be submitted at the same time of drug approval if it is required for therapeutic selection (Food and Drug Administration, 2013). A PGx test is considered a companion diagnostic if it provides information that is essential for the safe and effective use of a drug as directed on the drug label. The US FDA strongly recommends bio-banking of biological samples from appropriate tissue sources throughout the drug development phases for exploratory analysis, which may be used to provide mechanistic support of drug response and increases the rate of study success. Table 3 shows examples of PGx applications throughout the drug development process, which if considered appropriately, can (1) provide evidence of the presence or absence of a PGx interaction; (2) assess the role of polymorphic pathways in drug metabolism and PK; (3) facilitate an appropriate design for dose–response studies to include or exclude specific groups of patients; (4) inform later clinical trials in all the phases of the drug development process about important stratification and enrichment factors; and (5) assist in compiling informative labeling for the drug under review for regulatory approval (Food and Drug Administration, 2013).
Table 3.
Pharmacogenomics applications across drug development phases.
| Phase | Applications area | Example |
|---|---|---|
| Pre-clinical |
|
|
| 0 |
|
|
| I |
|
|
| II |
|
|
| III |
|
|
| IV |
|
|
It is known that there are significant differences in the prevalence of adverse drug events and drug efficacy between geographic populations, which could be due to genetic variations (Wilson et al., 2001). For instance, in comparison to the European and African populations, the Saudi population had a significantly higher frequency of SLCO1B1*5 and CYP2D6*17; which are associated with simvastatin-induced myopathy and metabolisms of several neuropsychiatric medications, respectively (Mizzi et al., 2016). The same study has also showed small differences in allele frequencies within CYP2C19 and CYP2C9 between Saudi and European populations. In addition, we recently demonstrated the association of VKORC1 promoter variant’s with warfarin weekly dose and the time to reach the stable International Normalized Ratio (INR) (Al Ammari et al., 2020). Other variants in very important pharmacogenes such as UGT1A; DPYD, and NAT2 were also reported in the Saudi population which warrant further consideration during drug approval and post approval process (Alkharfy et al., 2017, Bukhari et al., 2019, Al-Shaqha et al., 2015).
This population-specific difference have received greater attention by drug regulatory agencies, with potential implications for the drug approval process (Regulating pharmacogenomics, 2019). The adaption of the PGx approach could be cost effective as shown by Verbelen et al, who reviewed 44 economic investigations of the cost effectiveness of a PGx -informed strategy for 10 drugs. Of all the studies, 57% concluded in favor of PGx testing, 30% were cost effective and 27% cost saving (Verbelen et al., 2017).
3. Application in clinical care
Clinical pharmacology can ensure the safe and effective use of medication in clinical care by evaluating new drugs for the institutional pharmacy and therapeutic committees, performing therapeutic drug monitoring (TDM), and applying genotype-guided dosing and dosing in special populations. In addition, model informed precision dosing, a new field incorporating different specialties within clinical pharmacology, takes into account several factors (e.g. demographic, genetic, disease, and environmental factors) to select the optimal dose to maximize efficacy and minimize toxicity (Polasek et al., 2018, Darwich et al., 2017, Neely, 2017). The disciplines are summarized shortly.
3.1. Therapeutic drug monitoring (TDM)
TDM is a clinical tool used to individualize therapy for patients. It is usually applied to drugs that have a narrow therapeutic index and/or high between-subject variability. Other important factors to consider are the availability of rapid and cost-effective drug assays and an understanding of the concentration–response relationship. TDM is routinely performed for vancomycin, aminoglycosides, tacrolimus, cyclosporine, phenytoin, and valproic acid. There is an increased interest to expand the use of TDM for other drugs to guide the dose adjustment decision. This is particularly important for antimicrobials and anti-cancer drugs, such as anti-HIV drugs, antifungals, beta-lactams, antituberculous drugs, busulfan, and tyrosine kinase inhibitors (Alsultan and Peloquin, 2014, Owusu Obeng et al., 2014, Ashbee et al., 2014, Yu et al., 2014, Russell and Kangarloo, 2008, Roberts et al., 2012). These drugs are used for life-threatening diseases, usually without a clear PD or clinical parameter to evaluate a patient’s response to therapy. Although the criteria used for TDM applies to several of these drugs, TDM is still not routinely used with the main challenge being assay availability.
It is also important to improve our approach in TDM (de Velde et al., 2018). The traditional approach was to monitor the concentration (usually a trough concentration) using a single sample and compare that to a reference range. This approach has several limitations, including the use of simplistic PK models and other relevant patient information, low accuracy of some drug assays, laboratory variability in reporting and the difficulty of measuring the drug or the metabolites within a clinically useful timeframe. An alternative approach is to perform TDM using specialized computer software or model informed precision dosing (Neely and Jelliffe, 2008, Polasek et al., 2019).
3.2. Pharmacogenomics in clinical practice
Pharmacogenetic-guided treatment can personalize medicine and improve medication efficacy and safety. It is expected that personalized medicine will reshape healthcare delivery in the future (Haycox et al., 2014). There are key PGx databases providing valuable resources and clinical guidelines, such as the Pharmacogenomics Knowledge Base (PharmGKB), with high-quality clinical information and various annotations for a wide range of medication (Whirl-Carrillo et al., 2012). Another example is the Clinical Pharmacogenetics Implementation Consortium (CPIC), a shared project between PharmGKB and the Pharmacogenomics Research Network who has published over 35 peer-reviewed guidelines to incorporate PGx information in routine clinical practice (Caudle et al., 2014, https://cpicpgx.org/guidelines, 2019). The Dutch Pharmacogenetics Working Group (DPWG) also provides genotype-based therapeutic and dose recommendations and assists in the integration in a computerized system for drug prescription and automated medication surveillance (Swen et al., 2008, Swen et al., 2011). These initiatives and projects emphasize the need for developing a national project to assess and implement a pharmacogenetic-based treatment approach.
Several studies evaluated the importance of PGx for specific drugs such as abacavir, clopidogrel, codeine, and carbamazepine (Wang et al., 2016, Li-Wan-Po et al., 2010, Ned Mmsc, 2010, Ferrell and McLeod, 2008, Martin et al., 2014). The US FDA approved the labeling of 213 drugs with PGx markers and issued black box warnings for nine (Table 4). Substantial evidence of PGx interaction, especially for these medications, necessitate considering PGx dosing guidelines before prescribing for the patient. As an example, the CPIC recommends considering an alternative medication for abacavir for a carrier of HLA-B*57:01, due to the significantly increased risk of drug hypersensitivity (Martin et al., 2014).
Table 4.
Table of Pharmacogenomic Biomarkers in Drug Labeling FDA.
| Drug | Therapeutic Area | Biomarker | Labeling Sections |
|---|---|---|---|
| Abacavir | Infectious Diseases | HLA-B | Boxed Warning, Dosage and Administration, Contraindications, Warnings and Precautions |
| Carbamazepine | Neurology | HLA-B | Boxed Warning, Warnings, Precautions |
| Clopidogrel | Cardiology | CYP2C19 | Boxed Warning, Warnings and Precautions, Clinical Pharmacology |
| Codeine | Anesthesiology | CYP2D6 | Boxed Warning, Warnings and Precautions, Use in Specific Populations, Patient Counseling Information |
| Lenalidomide | Hematology | Chromosome 5q | Boxed Warning, Indications and Usage, Adverse Reactions, Use in Specific Populations, Clinical Studies |
| Pegloticase | Rheumatology | G6PD | Boxed Warning, Contraindications, Warnings and Precautions, Patient Counseling Information |
| Rasburicase (1) | Oncology | G6PD | Boxed Warning, Contraindications, Warnings and Precautions |
| Rasburicase (2) | Oncology | CYB5R | Boxed Warning, Contraindications, Warnings and Precautions |
| Sodium Nitrite | Toxicology | Nonspecific (Congenital Methemoglobinemia) | Boxed Warning, Warnings and Precautions |
| Tramadol | Anesthesiology | CYP2D6 | Boxed Warning, Warnings, Precautions, Use in Specific Populations, Clinical Pharmacology |
Table is based on data reported on the FDA website: https://www.fda.gov/drugs/science-research-drugs/table-pharmacogenomic-biomarkers-drug-labeling.
Only a few studies, generally with a small sample size, investigated the impact of genetic factors on the drug response in the Saudi population. The Saudi population has a unique distribution of genetic variants within ADME-related genes, reported in a study that genotyped 600 healthy Saudi adults for 1936 genetic variants using the Affymetrix Drug Metabolizing Enzymes and Transporters (DMET) platform (Mizzi et al., 2016, Wakil et al., 2015). The study highlighted some of the actionable pharmacogenetic variants that are more predominant in the Saudi population. For example, variants within SLCO1B1, associated with statin toxicity, were common in the Saudi population, while variants within CYP2D6 (CYP2D6*4/*4F/*4G/*4H/*10 that influence the metabolism of several psychotropic drugs, were significantly lower. Interestingly, the study reported that the average warfarin dose is lower in Saudis (35.8 mg/week) compared to Europeans (37.8 mg/week, p < 0.0001) (Mizzi et al., 2016). Further studies, designed to assess the value of genotype-guided dosing in clinical practice, are required. The ethical aspects of conducting PGx in clinical practice are part of the dilemmas of implementing genomics in routine clinical practice, which is neither new nor specific to PGx. The ethical issues are related to respect for person autonomy, privacy, confidentiality, informed consent, justice, and equality (Marx-Stölting, 2007). It is essential to consider all the ethical consequences to develop appropriate guidelines and policies before the adaptation of PGx in clinical practice.
3.3. Model informed precision dosing
Though PGx and TDM are useful clinical tools for dose optimization, the optimal approach is to incorporate all relevant information about the drug’s PK/PD/PGx, using Model Informed Precision Dosing employs PKPD modeling and simulation (M&S) techniques in clinical care to optimize dose selection for an individual patient. This optimal dose is most likely to be associated with improved therapeutic outcomes, including optimal efficacy and minimal undesired events (Darwich et al., 2017, Polasek et al., 2018, Tängdén et al., 2017, Neely and Jelliffe, 2010, Leroux et al., 2016, Neely et al., 2016, Neely et al., 2015). The capacity of model informed precision dosing to yield an optimal dose is due to the inherent ability of PKPD techniques to account for between-subject variability (Collins and Varmus, 2015). The implications of between-subject variability is noticed in clinical practice as one dose result in a range of drug concentrations and responses in a population (Polasek et al., 2019). Between-subject variability is attributed to drug-related factors (such as drug-drug interactions and concurrent use of medications), PGx, and patient’s physiological and demographic factors (age, obesity, severity of diseases, and presence of comorbidities) (Polasek et al., 2019, Polasek et al., 2018).
Dose regimens established during drug development are usually derived from small-scale clinical trials, with a controlled and homogenous study sample. To maintain simplicity and avoid confusion in clinical practice, these regimens are communicated in a simple static format and as a function of a limited number of predictive variables (e.g. nomograms, tables, and formulas) (Polasek et al., 2018, Jadhav et al., 2015). This oversimplified approach limits the amount of information available, forcing clinicians to rely on local guidance or make inferences (in cerebro modeling) (Darwich et al., 2017, Jadhav et al., 2015) and discrepancies in dose adjustments may arise. A survey investigating the daily practices of a French network of neonatal intensive care units, reported 25 different cefotaxime dosage regimens (Leroux et al., 2015). Though simple dose adjustment methods, such as scaling by weight or body surface area, may suffice, it is unlikely that such methods can sufficiently manage the complexity of the problem (Standing, 2017).
In contrast to in cerebro dose adjustments, model informed precision dosing capitalize on the advancement in computational power to use available data appropriately, including in vitro, demographics, and physiological data (Darwich et al., 2017, Neely, 2017). Dosing with model informed precision dosing approach is more precise and advantageous (Neely and Jelliffe, 2010, Størset et al., 2015, Neely et al., 2018, Fukudo et al., 2009). For example, it can addresses special populations by adjusting for maturation and/or alterations in physiological functions such as pediatrics or critically ill patients (Roberts et al., 2014). An additional advantage of the model is allowing for simple dose modifications as a function of multiple predictive variables, such as weight, creatinine clearance, and postnatal age. The model also offer the possibility of studying multiple simulated scenarios when it may not be possible to conduct a clinical trial due to ethical, time, or cost-related challenges (Neely, 2017). For example, Monte Carlo simulations can provide insight about the probability of attaining a specific therapeutic target, guiding the dosing and choice of drug (Tängdén et al., 2017). Finally, some PKPD modeling techniques implemented in model informed precision dosing, such as machine learning and Bayesian priors, can learn and improve consistently, allowing for a progressive improvement in precision (Darwich et al., 2017, Standing, 2017). Using model informed precision dosing allows for rapid achievement of the optimal dose (Neely and Jelliffe, 2010, Størset et al., 2015).
Currently, the application of model informed precision dosing in clinical care is available for numerous drugs through various platforms, such as InsightRX, BestDose, DoseMe, Precise PK, PK-PD Compass, and Virtual Twin. The techniques used to construct models implemented in such platforms vary and may include Bayesian forecasting, PBPK, and quantitative systems pharmacology. Although a large body of literature is available related to the ability of Bayesian forecasting to predict observed individual and population drug concentrations retrospectively, large-scale prospective clinical trials evaluating full-scale utility and cost-effectiveness are still lacking (Darwich et al., 2017, Neely, 2017). Though modeling and simulation approaches are routinely used by the US FDA and pharmaceutical industry to select the doses of new drugs, evaluate drug-drug interactions, and identify the spectrum of drug response in patients at higher risk, using model informed precision dosing in healthcare remains a promising approach to be meticulously explored (Gonzalez et al., 2017, Jadhav et al., 2015).
4. Summary
Clinical pharmacology holds the promise of improving patient care, drug development and drug regulation. Our recommendation is to expand the role and scope of clinical pharmacology and to educate more scientist in this field.
In clinical practice, we are moving away from a ‘one-size-fits-all’ approach to personalized medicine, as there is a need to individualize treatment based on patient related differences, such as demographic information and genetic variability. As modern clinical care implements precision medicine, the integration and utilization of PGx and model informed precision dosing in clinical practice will become essential. Both PGx and model informed precision dosing can contribute significantly to improve clinical outcomes and reduce the cost of care.
When it comes to conducting clinical trials, there are still significant room for improvement. KSA conducts only 0.21% of global clinical studies, more than the other Arab states excluding Egypt. The contribution of KSA in terms of clinical studies does not reflect the resources available in the country and necessitate the establishment of research groups to identify issues and create a sustainable infrastructure for clinical research.
A growing number of sponsors are investing in the KSA healthcare system due to the level of development of the healthcare system, the large annual expenditure on healthcare, the availability of world-class medical facilities, the presence of nationally and internationally trained investigators, and the rapid growth of the pharmaceutical industry. Yet several challenges impede the progression of clinical research in KSA, such as insufficient funding, inadequate clinical pharmacology training, lengthy ethical approval process, the difficulty of recruiting study participants, and lack of experienced researchers conducting clinical trials. We recommend establishing a Saudi Clinical Practice Consortium, responsible for conducting clinical studies and establishing clinical guidelines tailored to each population. In addition, this consortium would be responsible for providing specific recommendations to each population as well as identifying and validating genetic biomarkers in regional sites.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aarons L., Karlsson M.O., Mentré F. Role of modelling and simulation in Phase I drug development. Eur. J. Pharm. Sci. 2001;13:115–122. doi: 10.1016/s0928-0987(01)00096-3. [DOI] [PubMed] [Google Scholar]
- Abu-Elmagd M., Assidi M., Schulten H.J. Individualized medicine enabled by genomics in Saudi Arabia. BMC Med. Genomics. 2015;8(Suppl 1):S3. doi: 10.1186/1755-8794-8-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Ammari M., AlBalwi M., Sultana K. The effect of the VKORC1 promoter variant on warfarin responsiveness in the Saudi WArfarin Pharmacogenetic (SWAP) cohort. Sci. Rep. 2020;10:11613. doi: 10.1038/s41598-020-68519-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkharfy K.M., Jan B.L., Afzal S. Prevalence of UDP-glucuronosyltransferase polymorphisms (UGT1A6∗2, 1A7∗12, 1A8∗3, 1A9∗3, 2B7∗2, and 2B15∗2) in a Saudi population. Saudi Pharm J. 2017;25:224–230. doi: 10.1016/j.jsps.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shaqha W.M., Alkharfy K.M., Al-Daghri N.M., Mohammed A.K. N-acetyltransferase 1 and 2 polymorphisms and risk of diabetes mellitus type 2 in a Saudi population. Ann Saudi Med. 2015;35:214–221. doi: 10.5144/0256-4947.2015.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsultan A., Peloquin C.A. Therapeutic drug monitoring in the treatment of tuberculosis: an update. Drugs. 2014;74:839–854. doi: 10.1007/s40265-014-0222-8. [DOI] [PubMed] [Google Scholar]
- Ashbee H.R., Barnes R.A., Johnson E.M., Richardson M.D., Gorton R., Hope W.W. Therapeutic drug monitoring (TDM) of antifungal agents: guidelines from the British Society for Medical Mycology. J. Antimicrob. Chemother. 2014;69:1162–1176. doi: 10.1093/jac/dkt508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkett D., Brøsen K., Cascorbi I. Clinical pharmacology in research, teaching and health care: Considerations by IUPHAR, the International Union of Basic and Clinical Pharmacology. Basic Clin. Pharmacol. Toxicol. 2010;107:531–559. doi: 10.1111/j.1742-7843.2010.00602.x. [DOI] [PubMed] [Google Scholar]
- Bjornsson T.D., Callaghan J.T., Einolf H.J. The conduct of in vitro and in vivo drug-drug interaction studies: a Pharmaceutical Research and Manufacturers of America (PhRMA) perspective. Drug Metab. Dispos. 2003;31:815–832. doi: 10.1124/dmd.31.7.815. [DOI] [PubMed] [Google Scholar]
- Bonate P.L. Clinical trial simulation in drug development. Pharm. Res. 2000;17:252–256. doi: 10.1023/a:1007548719885. [DOI] [PubMed] [Google Scholar]
- Bukhari N., Azam F., Alfawaz M., Zahrani M. Identifying a Novel DPYD polymorphism associated with severe toxicity to 5-FU chemotherapy in a Saudi patient. Case Rep Genet. 2019;2019:5150725. doi: 10.1155/2019/5150725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudle K.E., Klein T.E., Hoffman J.M. Incorporation of pharmacogenomics into routine clinical practice: the Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline development process. Curr. Drug Metab. 2014;15:209–217. doi: 10.2174/1389200215666140130124910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien J.Y., Friedrich S., Heathman M.A., de Alwis D.P., Sinha V. Pharmacokinetics/Pharmacodynamics and the stages of drug development: role of modeling and simulation. AAPS J. 2005;7:E544–E559. doi: 10.1208/aapsj070355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow S.C. Bioavailability and bioequivalence in drug development. Wiley Interdiscip. Rev. Comput. Stat. 2014;6:304–312. doi: 10.1002/wics.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F.S., Varmus H. A new initiative on precision medicine. New Engl. J. Med. 2015;372:793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews K.R., Hicks J.K., Pui C.H., Relling M.V., Evans W.E. Pharmacogenomics and individualized medicine: translating science into practice. Clin. Pharmacol. Ther. 2012;92:467–475. doi: 10.1038/clpt.2012.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwich A.S., Ogungbenro K., Vinks A.A. Why has model-informed precision dosing not yet become common clinical reality? lessons from the past and a roadmap for the future. Clin. Pharmacol. Ther. 2017;101:646–656. doi: 10.1002/cpt.659. [DOI] [PubMed] [Google Scholar]
- Darwich A.S., Ogungbenro K., Vinks A.A. Why has model-informed precision dosing not yet become common clinical reality? lessons from the past and a roadmap for the future. Clin. Pharmacol. Ther. 2017;101:646–656. doi: 10.1002/cpt.659. [DOI] [PubMed] [Google Scholar]
- de Velde F., Mouton J.W., de Winter B.C.M., van Gelder T., Koch B.C.P. Clinical applications of population pharmacokinetic models of antibiotics: Challenges and perspectives. Pharmacol. Res. 2018;134:280–288. doi: 10.1016/j.phrs.2018.07.005. [DOI] [PubMed] [Google Scholar]
- Debouck C. Integrating genomics across drug discovery and development. Toxicol. Lett. 2009;186:9–12. doi: 10.1016/j.toxlet.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Dodds M., Chow V., Markus R., Pérez-Ruixo J.J., Shen D., Gibbs M. The use of pharmacometrics to optimize biosimilar development. J. Pharm. Sci. 2013;102:3908–3914. doi: 10.1002/jps.23697. [DOI] [PubMed] [Google Scholar]
- Egelund E.F., Barth A.B., Peloquin C.A. Population pharmacokinetics and its role in anti-tuberculosis drug development and optimization of treatment. Curr. Pharm. Des. 2011;17:2889–2899. doi: 10.2174/138161211797470246. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency, 2012. Guideline on the Use of Pharmacogenetic Methodologies in the Pharmacokinetic Evaluation of Medicinal Products. www.ema.europa.eu. Accessed December 17, 2018.
- Fang L., Kim M.J., Li Z. Model-informed drug development and review for generic products: summary of FDA public workshop. Clin. Pharmacol. Ther. 2018;104:27–30. doi: 10.1002/cpt.1065. [DOI] [PubMed] [Google Scholar]
- Ferrell P.B., McLeod H.L. Carbamazepine, HLA-B*1502 and risk of Stevens-Johnson syndrome and toxic epidermal necrolysis: US FDA recommendations. Pharmacogenomics. 2008;9:1543–1546. doi: 10.2217/14622416.9.10.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration, 2005 Jul. Guidance for industry: estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. Center for Drug Evaluation and Research (CDER), 7.
- Food and Drug Administration. Clinical Pharmacogenomics: Premarket Evaluation in Early-Phase Clinical Studies and Recommendations for Labeling. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM337169.pdf. Accessed December 17, 2018.
- Fukudo M., Yano I., Shinsako K. Prospective evaluation of the bayesian method for individualizing Tacrolimus dose early after living-donor liver transplantation. J. Clin. Pharmacol. 2009;49:789–797. doi: 10.1177/0091270009333853. [DOI] [PubMed] [Google Scholar]
- Gal J., Milano G., Ferrero J.M. Optimizing drug development in oncology by clinical trial simulation: Why and how? Brief Bioinform. 2018;19:1203–1217. doi: 10.1093/bib/bbx055. [DOI] [PubMed] [Google Scholar]
- Gonzalez D., Rao G.G., Bailey S.C. Precision dosing: public health need, proposed framework, and anticipated impact. Clin. Transl. Sci. 2017;10:443–454. doi: 10.1111/cts.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodsaid F., Papaluca M. Evolution of biomarker qualification at the health authorities. Nat. Biotechnol. 2010;28:441–443. doi: 10.1038/nbt0510-441. [DOI] [PubMed] [Google Scholar]
- Haycox A., Pirmohamed M., McLeod C., Houten R., Richards S. Through a glass darkly: economics and personalised medicine. Pharmacoeconomics. 2014;32:1055–1061. doi: 10.1007/s40273-014-0190-6. [DOI] [PubMed] [Google Scholar]
- Holford N., Ma S.C., Ploeger B.A. Clinical trial simulation: a review. Clin. Pharmacol. Ther. 2010;88:166–182. doi: 10.1038/clpt.2010.114. [DOI] [PubMed] [Google Scholar]
- Howard M., Barber J., Alizai N., Rostami-Hodjegan A. Dose adjustment in orphan disease populations: the quest to fulfill the requirements of physiologically based pharmacokinetics. Expert Opin. Drug Metab. Toxicol. 2018;14:1315–1330. doi: 10.1080/17425255.2018.1546288. [DOI] [PubMed] [Google Scholar]
- https://clinicaltrials.gov/ct2/results?cond=&term=&cntry=SA&state=&city=&dist=. Accessed June 17, 2019.
- https://cpicpgx.org/guidelines/. Accessed April 7, 2019.
- https://www.fda.gov/drugs/news-events-human-drugs/cder-conversation-model-informed-drug-development. Accessed April 17, 2019.
- https://www.ic.gov.sa/en/about/press-releases/pharmaus/. Accessed May, 5, 2019.
- https://www.sfda.gov.sa/en/drug/drug_reg/Regulations/GCC_Guidelines_Bioequivalence.pdf.
- Huang S.M., Abernethy D.R., Wang Y., Zhao P., Zineh I. The utility of modeling and simulation in drug development and regulatory review. J. Pharm. Sci. 2013;102:2912–2923. doi: 10.1002/jps.23570. [DOI] [PubMed] [Google Scholar]
- Hughes D.A., Walley T. Economic evaluations during early (phase II) drug development: a role for clinical trial simulations? Pharmacoeconomics. 2001;19:1069–1077. doi: 10.2165/00019053-200119110-00001. [DOI] [PubMed] [Google Scholar]
- Iasonos A., O'Quigley J. Sequential monitoring of Phase I dose expansion cohorts. Stat. Med. 2017;36:204–214. doi: 10.1002/sim.6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav P.R., Cook J., Sinha V. A proposal for scientific framework enabling specific population drug dosing recommendations. J. Clin. Pharmacol. 2015;55:1073–1078. doi: 10.1002/jcph.579. [DOI] [PubMed] [Google Scholar]
- Ke A.B., Rostami-Hodjegan A., Zhao P., Unadkat J.D. Pharmacometrics in pregnancy: An unmet need. Annu. Rev. Pharmacol. Toxicol. 2014;54:53–69. doi: 10.1146/annurev-pharmtox-011613-140009. [DOI] [PubMed] [Google Scholar]
- Kiang T.K., Sherwin C.M., Spigarelli M.G., Ensom M.H. Fundamentals of population pharmacokinetic modelling: modelling and software. Clin. Pharmacokinet. 2012;51:515–525. doi: 10.2165/11634080-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Kuhlmann J. Alternative strategies in drug development: clinical pharmacological aspects. Int. J. Clin. Pharmacol. Ther. 1999;37:575–583. [PubMed] [Google Scholar]
- Lavé T., Caruso A., Parrott N., Walz A. Translational PK/PD modeling to increase probability of success in drug discovery and early development. Drug Discov Today Technol. 2016;21–22:27–34. doi: 10.1016/j.ddtec.2016.11.005. [DOI] [PubMed] [Google Scholar]
- Lee J.Y., Garnett C.E., Gobburu J.V. Impact of pharmacometric analyses on new drug approval and labelling decisions: a review of 198 submissions between 2000 and 2008. Clin. Pharmacokinet. 2011;50:627–635. doi: 10.2165/11593210-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Leong R., Vieira M.L., Zhao P. Regulatory experience with physiologically based pharmacokinetic modeling for pediatric drug trials. Clin. Pharmacol. Ther. 2012;91:926–931. doi: 10.1038/clpt.2012.19. [DOI] [PubMed] [Google Scholar]
- Leroux S., Zhao W., Bétrémieux P., Pladys P., Saliba E., Jacqz-Aigrain E. Therapeutic guidelines for prescribing antibiotics in neonates should be evidence-based: a French national survey. Arch. Dis. Child. 2015;100:394–398. doi: 10.1136/archdischild-2014-306873. [DOI] [PubMed] [Google Scholar]
- Leroux S., Jacqz-Aigrain E., Biran V. Clinical utility and safety of a model-based patient-tailored dose of vancomycin in neonates. Antimicrob. Agents Chemother. 2016;60:2039–2042. doi: 10.1128/AAC.02214-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Han H., Wang J. Model informed drug development and regulation in china: challenges and opportunities. CPT Pharmacometrics Syst. Pharmacol. 2019;8:59–61. doi: 10.1002/psp4.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li-Wan-Po A., Girard T., Farndon P., Cooley C., Lithgow J. Pharmacogenetics of CYP2C19: functional and clinical implications of a new variant CYP2C19*17. Br. J. Clin. Pharmacol. 2010;69:222–230. doi: 10.1111/j.1365-2125.2009.03578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall S.F., Burghaus R., Cosson V. Good practices in model-informed drug discovery and development: practice, application, and documentation. CPT Pharmacometrics Syst. Pharmacol. 2016;5:93–122. doi: 10.1002/psp4.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall S., Madabushi R., Manolis E. Model-informed drug discovery and development: current industry good practice and regulatory expectations and future perspectives. CPT Pharmacometrics Syst. Pharmacol. 2019;8:87–96. doi: 10.1002/psp4.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsousi N., Desmeules J.A., Rudaz S., Daali Y. Usefulness of PBPK modeling in incorporation of clinical conditions in personalized medicine. J. Pharm. Sci. 2017;106:2380–2391. doi: 10.1016/j.xphs.2017.04.035. [DOI] [PubMed] [Google Scholar]
- Martin M.A., Hoffman J.M., Freimuth R.R. Clinical Pharmacogenetics Implementation Consortium Guidelines for HLA-B Genotype and Abacavir Dosing: 2014 update. Clin. Pharmacol. Ther. 2014;95:499–500. doi: 10.1038/clpt.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx-Stölting L. Pharmacogenetics and ethical considerations: why care? Pharmacogenomics J. 2007;7:293–296. doi: 10.1038/sj.tpj.6500425. [DOI] [PubMed] [Google Scholar]
- Mehrotra N., Bhattaram A., Earp J.C. Role of quantitative clinical pharmacology in pediatric approval and labeling. Drug Metab. Dispos. 2016;44:924–933. doi: 10.1124/dmd.116.069559. [DOI] [PubMed] [Google Scholar]
- Meibohm B., Derendorf H. Basic concepts of pharmacokinetic/pharmacodynamic (PK/PD) modelling. Int. J. Clin. Pharmacol. Ther. 1997;35:401–413. [PubMed] [Google Scholar]
- Miller R., Ewy W., Corrigan B.W. How modeling and simulation have enhanced decision making in new drug development. J. Pharmacokinet Pharmacodyn. 2005;32:185–197. doi: 10.1007/s10928-005-0074-7. [DOI] [PubMed] [Google Scholar]
- Milton M.N., Horvath C.J. The EMEA guideline on first-in-human clinical trials and its impact on pharmaceutical development. Toxicol. Pathol. 2009;37:363–371. doi: 10.1177/0192623309332997. [DOI] [PubMed] [Google Scholar]
- Mizzi C., Dalabira E., Kumuthini J. A European spectrum of pharmacogenomic biomarkers: implications for clinical pharmacogenomics. PLoS One. 2016;11:e0162866. doi: 10.1371/journal.pone.0162866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ned Mmsc Phd R.M., 2010. Genetic testing for CYP450 polymorphisms to predict response to clopidogrel: current evidence and test availability. Application: pharmacogenomics. PLoS Curr. 2. [DOI] [PMC free article] [PubMed]
- Neely M. Scalpels not hammers. Clin. Pharmacol. Ther. 2017;101:368–372. doi: 10.1002/cpt.593. [DOI] [PubMed] [Google Scholar]
- Neely M. Scalpels not hammers: The way forward for precision drug prescription. Clin. Pharmacol. Ther. 2017;101:368–372. doi: 10.1002/cpt.593. [DOI] [PubMed] [Google Scholar]
- Neely M., Jelliffe R. Practical therapeutic drug management in HIV-infected patients: use of population pharmacokinetic models supplemented by individualized Bayesian dose optimization. J. Clin. Pharmacol. 2008;48:1081–1091. doi: 10.1177/0091270008321789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely M., Jelliffe R. Practical, individualized dosing: 21st century therapeutics and the clinical pharmacometrician. J. Clin. Pharmacol. 2010;50:842–847. doi: 10.1177/0091270009356572. [DOI] [PubMed] [Google Scholar]
- Neely M.N., Kato L., Youn G. Prospective trial on the use of trough concentration versus area under the curve to determine therapeutic vancomycin dosing. Antimicrob. Agents Chemother. 2018;62 doi: 10.1128/AAC.02042-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely M., Margol A., Fu X. Achieving target voriconazole concentrations more accurately in children and adolescents. Antimicrob. Agents Chemother. 2015;59:3090–3097. doi: 10.1128/AAC.00032-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely M., Philippe M., Rushing T. Accurately achieving target Busulfan exposure in children and adolescents with very limited sampling and the BestDose software. Ther. Drug Monit. 2016;38:332–342. doi: 10.1097/FTD.0000000000000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme M., Harry J., Routledge P., Hobson S. Healthy volunteer studies in Great Britain: the results of a survey into 12 months activity in this field. Br. J. Clin. Pharmacol. 1989;27:125–133. doi: 10.1111/j.1365-2125.1989.tb05342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owusu Obeng A., Egelund E.F., Alsultan A., Peloquin C.A., Johnson J.A. CYP2C19 Polymorphisms and Therapeutic Drug Monitoring of Voriconazole: Are We Ready for Clinical Implementation of Pharmacogenomics? Pharmacotherapy. 2014 doi: 10.1002/phar.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck R. The pharmaceutical industry needs more clinical pharmacologists. Br. J. Clin. Pharmacol. 2017;83:2343–2346. doi: 10.1111/bcp.13370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera V., Dolton M.J., McLachlan A.J., Carr V.J., Day R.O. Pharmacometrics: an underused resource in Australian clinical research. Med. J. Aust. 2014;200:82–83. doi: 10.5694/mja13.10663. [DOI] [PubMed] [Google Scholar]
- Pillai G.C., Mentré F., Steimer J.L. Non-linear mixed effects modeling - from methodology and software development to driving implementation in drug development science. J. Pharmacokinet Pharmacodyn. 2005;32:161–183. doi: 10.1007/s10928-005-0062-y. [DOI] [PubMed] [Google Scholar]
- Pingault J.B., O'Reilly P.F., Schoeler T., Ploubidis G.B., Rijsdijk F., Dudbridge F. Using genetic data to strengthen causal inference in observational research. Nat. Rev. Genet. 2018;19:566–580. doi: 10.1038/s41576-018-0020-3. [DOI] [PubMed] [Google Scholar]
- Polasek T.M., Shakib S., Rostami-Hodjegan A. Precision dosing in clinical medicine: present and future. Expert Rev. Clin. Pharmacol. 2018;11:743–746. doi: 10.1080/17512433.2018.1501271. [DOI] [PubMed] [Google Scholar]
- Polasek T.M., Rayner C.R., Peck R.W., Rowland A., Kimko H., Rostami-Hodjegan A. Toward dynamic prescribing information: codevelopment of companion model-informed precision dosing tools in drug development. Clin. Pharmacol. Drug Dev. 2018 doi: 10.1002/cpdd.638. [DOI] [PubMed] [Google Scholar]
- Polasek T.M., Rostami-Hodjegan A., Yim D.S. What Does it Take to Make Model-Informed Precision Dosing Common Practice? Report from the 1st Asian Symposium on Precision Dosing. AAPS J. 2019;21:17. doi: 10.1208/s12248-018-0286-6. [DOI] [PubMed] [Google Scholar]
- Polasek T.M., Rostami-Hodjegan A., Yim D.-S. Springer; 2019. What Does it Take to Make Model-Informed Precision Dosing Common Practice? Report from the 1st Asian Symposium on Precision Dosing. [DOI] [PubMed] [Google Scholar]
- Prueksaritanont T., Chu X., Gibson C. Drug-drug interaction studies: regulatory guidance and an industry perspective. AAPS J. 2013;15:629–645. doi: 10.1208/s12248-013-9470-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rask-Andersen M., Masuram S., Schiöth H.B. The druggable genome: Evaluation of drug targets in clinical trials suggests major shifts in molecular class and indication. Annu. Rev. Pharmacol. Toxicol. 2014;54:9–26. doi: 10.1146/annurev-pharmtox-011613-135943. [DOI] [PubMed] [Google Scholar]
- Regulating pharmacogenomics: An overview of developments in various countries and industry response to regulatory initiatives - A report for Health Canada. http://www.gbcbiotech.com/farmacogenomica/normatividad/health/Regulating%20pharmacogenomics%20report%20for%20Health%20Canada.pdf. Accessed April 2, 2019.
- Roberts J.A., Norris R., Paterson D.L., Martin J.H. Therapeutic drug monitoring of antimicrobials. Br. J. Clin. Pharmacol. 2012;73:27–36. doi: 10.1111/j.1365-2125.2011.04080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J.A., Abdul-Aziz M.H., Lipman J. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect. Dis. 2014;14:498–509. doi: 10.1016/S1473-3099(14)70036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J.A., Abdul-Aziz M.H., Lipman J. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect. Dis. 2014;14:498–509. doi: 10.1016/S1473-3099(14)70036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J.A., Kangarloo S.B. Therapeutic drug monitoring of busulfan in transplantation. Curr. Pharm. Des. 2008;14:1936–1949. doi: 10.2174/138161208785061382. [DOI] [PubMed] [Google Scholar]
- Saleheen D., Natarajan P., Armean I.M. Human knockouts and phenotypic analysis in a cohort with a high rate of consanguinity. Nature. 2017;544:235–239. doi: 10.1038/nature22034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saudi Food and Drug Authority. Drug Guidelines. https://www.sfda.gov.sa/en/drug/drug_reg/Pages/default.aspx. Accessed April 2, 2019.
- Scannell J.W., Blanckley A., Boldon H., Warrington B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat. Rev. Drug Discov. 2012;11:191–200. doi: 10.1038/nrd3681. [DOI] [PubMed] [Google Scholar]
- Sheiner L.B., Benet L.Z. Premarketing observational studies of population pharmacokinetics of new drugs. Clin. Pharmacol. Ther. 1985;38:481–487. doi: 10.1038/clpt.1985.212. [DOI] [PubMed] [Google Scholar]
- Standing J.F. Understanding and applying pharmacometric modelling and simulation in clinical practice and research. Br. J. Clin. Pharmacol. 2017;83:247–254. doi: 10.1111/bcp.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standing J.F. Understanding and applying pharmacometric modelling and simulation in clinical practice and research. Br. J. Clin. Pharmacol. 2017;83:247–254. doi: 10.1111/bcp.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Størset E., Åsberg A., Skauby M. Improved tacrolimus target concentration achievement using computerized dosing in renal transplant recipients–A Prospective, randomized study. Transplantation. 2015;99:2158–2166. doi: 10.1097/TP.0000000000000708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swen J.J., Wilting I., de Goede A.L. Pharmacogenetics: from bench to byte. Clin. Pharmacol. Ther. 2008;83:781–787. doi: 10.1038/sj.clpt.6100507. [DOI] [PubMed] [Google Scholar]
- Swen J.J., Nijenhuis M., de Boer A. Pharmacogenetics: from bench to byte–an update of guidelines. Clin. Pharmacol. Ther. 2011;89:662–673. doi: 10.1038/clpt.2011.34. [DOI] [PubMed] [Google Scholar]
- Tängdén T., Martin V.R., Felton T. The role of infection models and PK/PD modelling for optimising care of critically ill patients with severe infections. Intensive Care Med. 2017;43:1021–1032. doi: 10.1007/s00134-017-4780-6. [DOI] [PubMed] [Google Scholar]
- Templeton I.E., Jones N.S., Musib L. Pediatric dose selection and utility of PBPK in determining dose. AAPS J. 2018;20:31. doi: 10.1208/s12248-018-0187-8. [DOI] [PubMed] [Google Scholar]
- Tuntland T., Ethell B., Kosaka T. Implementation of pharmacokinetic and pharmacodynamic strategies in early research phases of drug discovery and development at Novartis Institute of Biomedical Research. Front. Pharmacol. 2014;5:174. doi: 10.3389/fphar.2014.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umscheid C.A., Margolis D.J., Grossman C.E. Key concepts of clinical trials: a narrative review. Postgrad. Med. 2011;123:194–204. doi: 10.3810/pgm.2011.09.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbelen M., Weale M.E., Lewis C.M. Cost-effectiveness of pharmacogenetic-guided treatment: are we there yet? Pharmacogenomics J. 2017;17:395–402. doi: 10.1038/tpj.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser S.A., Manolis E., Danhof M., Kerbusch T. Modeling and simulation at the interface of nonclinical and early clinical drug development. CPT Pharmacometrics Syst. Pharmacol. 2013;2:e30. doi: 10.1038/psp.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vozeh S., Steimer J.L., Rowland M. The use of population pharmacokinetics in drug development. Clin. Pharmacokinet. 1996;30:81–93. doi: 10.2165/00003088-199630020-00001. [DOI] [PubMed] [Google Scholar]
- Wakil S.M., Nguyen C., Muiya N.P. The Affymetrix DMET Plus platform reveals unique distribution of ADME-related variants in ethnic Arabs. Dis. Markers. 2015;2015:542543. doi: 10.1155/2015/542543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhao X., Lin J. Association between CYP2C19 loss-of-function allele status and efficacy of clopidogrel for risk reduction among patients with minor stroke or transient ischemic attack. JAMA. 2016;316:70–78. doi: 10.1001/jama.2016.8662. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhu H., Madabushi R., Liu Q., Huang S.M., Zineh I. Model-informed drug development: current US regulatory practice and future considerations. Clin. Pharmacol. Ther. 2019;105:899–911. doi: 10.1002/cpt.1363. [DOI] [PubMed] [Google Scholar]
- Whirl-Carrillo M., McDonagh E.M., Hebert J.M. Pharmacogenomics knowledge for personalized medicine. Clin. Pharmacol. Ther. 2012;92:414–417. doi: 10.1038/clpt.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P.J., Ette E.I. The role of population pharmacokinetics in drug development in light of the Food and Drug Administration's 'Guidance for Industry: population pharmacokinetics'. Clin. Pharmacokinet. 2000;39:385–395. doi: 10.2165/00003088-200039060-00001. [DOI] [PubMed] [Google Scholar]
- Wilson J.F., Weale M.E., Smith A.C. Population genetic structure of variable drug response. Nat. Genet. 2001;29:265–269. doi: 10.1038/ng761. [DOI] [PubMed] [Google Scholar]
- Yoshida K., Budha N., Jin J.Y. Impact of physiologically based pharmacokinetic models on regulatory reviews and product labels: Frequent utilization in the field of oncology. Clin. Pharmacol. Ther. 2017;101:597–602. doi: 10.1002/cpt.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Steeghs N., Nijenhuis C.M., Schellens J.H., Beijnen J.H., Huitema A.D. Practical guidelines for therapeutic drug monitoring of anticancer tyrosine kinase inhibitors: focus on the pharmacokinetic targets. Clin. Pharmacokinet. 2014;53:305–325. doi: 10.1007/s40262-014-0137-2. [DOI] [PubMed] [Google Scholar]
- Zhao P., MeL Vieira, Grillo J.A. Evaluation of exposure change of nonrenally eliminated drugs in patients with chronic kidney disease using physiologically based pharmacokinetic modeling and simulation. J. Clin. Pharmacol. 2012;52:91S–108S. doi: 10.1177/0091270011415528. [DOI] [PubMed] [Google Scholar]
- Zhao Z., Tuakli-Wosornu Y., Lagace T.A. Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote. Am. J. Hum. Genet. 2006;79:514–523. doi: 10.1086/507488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P., Sy S.K.B., Skerjanec A. Application of pharmacometric analysis in the design of clinical pharmacology studies for biosimilar development. AAPS J. 2018;20:40. doi: 10.1208/s12248-018-0196-7. [DOI] [PubMed] [Google Scholar]
- Zineh I., Abernethy D., Hop C. Improving the tools of clinical pharmacology: Goals for 2017 and beyond. Clin. Pharmacol. Ther. 2017;101:22–24. doi: 10.1002/cpt.530. [DOI] [PubMed] [Google Scholar]