Abstract
Sesamin, a major lignin isolated from sesame (Sesamum indicum) seeds and sesame oil, is known to possess antioxidant and anti-inflammatory properties. Several studies have revealed that oxidative stress and inflammation play a major role in a variety of cardiovascular diseases (CVDs). This comprehensive review summarizes the evidence on the effects of sesamin on CVD and its risk factors, principally due to its antioxidant properties. Specifically, this review highlights the mechanisms underlying the anti-hypertensive, anti-atherogenic, anti-thrombotic, anti-diabetic, and anti-obesity, lipolytic effects of sesamin both in vivo and in vitro, and identifies the signaling pathways targeted by sesamin and its metabolites. The data indicates that RAS/MAPK, PI3K/AKT, ERK1/2, p38, p53, IL-6, TNFα, and NF-κB signaling networks are all involved in moderating the various effects of sesamin on CVD and its risk factors. In conclusion, the experimental evidence suggesting that sesamin can reduce CVD risk is convincing. Thus, sesamin can be potentially useful as an adjuvant therapeutic agent to combat CVD and its multitude of risk factors.
Keywords: Sesamin, CVD, Hypertension, Atherosclerosis, Thrombosis, Diabetes, Obesity
1. Background
Sesame (Sesamum indicum) seeds have long been used as a traditional health food in East Asia and its oil has been an important component of natural Indian remedies and Chinese medicine to increase energy and prevent aging (Mahendra Kumar and Singh, 2015). Sesame is rich in a variety of lignans, such as sesamin, episesamin, sesamol, sesamolin, and γ-tocopherol, which are a group of bioactive phenolic compounds also found in flax, barley, buckwheat, millet, oats, rye, nuts, and legumes (Jeng and Hou, 2005). However, they are most abundant in sesame seed, with sesamin as the major isomer (1520 ± 6.8 μmol/100 g) (Liu et al., 2006, Liang et al., 2015). Raw sesame oil contains 0.5–1.1% sesamin and 0.2–0.6% sesamolin that contribute to the medicinal properties of the oil, since these lignans are said to possess antioxidant activities (Kumar and Singh, 2015). A variety of biochemical actions have been attributed to sesamin, such as the specific inhibition of Δ5-desaturation of (n-6) fatty acids that aids in blocking the formation of pro-inflammatory prostaglandins, protection against ethanol and carbon tetrachloride-induced liver damage, enhancing the bioavailability of γ-tocopherol, and suppressive effects against induced carcinogenesis in animals (Peñalvo et al., 2005). Although the exact mechanism of some of these effects is unknown, it is proposed that the role of sesamin is proactive and that its metabolites are responsible for the physiological effects (Peñalvo et al., 2005). A study by Nakai and colleagues illustrates that sesamin undergoes oxidative demethylenation of methylene dioxyphenyls to catechol or methoxycatechol in rat liver, which are important moieties that contribute to antioxidant activities (Nakai et al., 2003). Moreover, other studies illustrate that sesamin is a precursor of the mammalian lignans enterodiol and enterolactone, which are found to have protective effects against diseases such as breast cancer (Peñalvo et al., 2005, Liu et al., 2006). Sesamin has also been shown to lower blood pressure, reduce serum lipid, and inhibit absorption and synthesis of cholesterol in rats (Kumar and Singh, 2015).
With the multitude of potential health impacts of sesamin, this review aims to assess the scientific evidence on the effects of sesamin on CVD and its risk factors in particular. These risk factors include the roles of sesamin in hypertension, progression of atherosclerosis, thrombosis, hypercholesterolemia, obesity, inflammation and diabetes. This paper emphasizes the molecular pathways involved and analyzes the mechanisms of action of sesamin, which bring about these properties.
2. Effects of sesamin on hypertension
The Center of Disease Control and Prevention (CDC) states that as of 2016 one in three American adults has high blood pressure. This translates to 75 million adults, of which only 54% have their blood pressure under control (High blood pressure fact sheet, 2016). Eating a healthy diet with less salt, being physically active, maintaining a healthy weight, and limiting the amount of alcohol intake are some lifestyle changes that can help control high blood pressure. In addition to lifestyle changes, medications such as thiazide diuretics, angiotensing converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), calcium channel blockers, β-blockers, central α‐agonists, direct vasodilators, and direct renin inhibitors (DRIs) can be utilized to keep blood pressure in check (Houston, 2013). Natural compounds, such as food lignans, flavonoids, vitamins, and minerals with anti-hypertensive properties have also been examined as potential therapeutic approaches to combat hypertension.
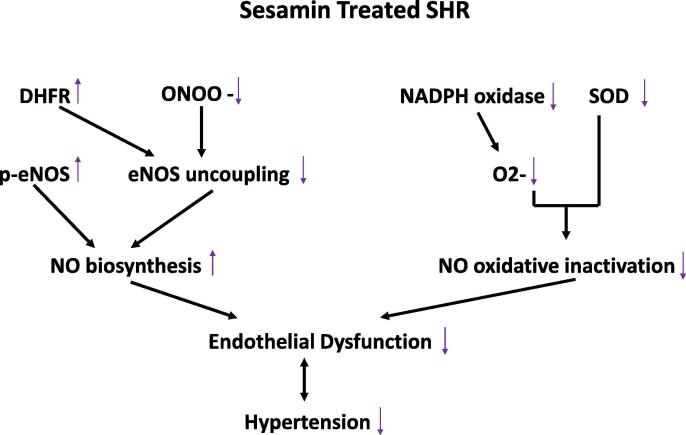
Several in vitro and in vivo studies illustrate the anti-hypertensive effects of sesamin. A study by Li and colleagues shows that administration of sesamin (50 mg and 100 mg) for 4 weeks decreases the right ventricular systolic pressure and mean pulmonary arterial pressure in monocrotaline induced hypertensive rats (Li et al., 2015). The mechanism of action is related to the sesamin-induced inhibition of the expression of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase isoforms NOX2 and NOX4, and malondialdehyde (MDA) content and an increase in total antioxidant capacity (T-AOC) (Li et al., 2015). The anti-hypertensive effects of sesamin is also noted in a study using a two kidney, one clip renovascular hypertensive rat model. Results illustrate a decrease in systolic blood pressure after 4 weeks of sesamin administration (Kita et al., 1995). To investigate the potential mechanism of action, a study using the same model of hypertensive rats has shown that an 8-week administration of sesamin decreases systolic blood pressure, improves acetylcholine induced vasorelaxation, and enhances nitric oxide (NO) bioactivity in the aorta by upregulating the expression of endothelial nitric oxide synthase (eNOS), decreasing MDA content, and reducing nitrotyrosine and NADPH oxidase subunit p47phox (Kong et al., 2009). In addition, sesamin is found to have a dose-dependent inhibitory effect on NADPH oxidase subunit p22phox (Zhang et al., 2013). Decreasing nitrotyrosine and upregulating dihydrofolate reductase (DHFR) suppresses eNOS uncoupling. This suppression, along with the downregulation of p47phox reduces NO oxidative inactivation and decreases superoxide anion production, enhancing NO bioactivity in aortas and reducing hypertension (Kong et al., 2015a). This mechanism of action is described in Fig. 1, which illustrates the effects of sesamin treatment in spontaneously hypertensive rats (SHR), as adapted from a study by Kong and colleagues (Kong et al., 2015a).
Fig. 1.
The mechanism of action by which sesamin enhances NO bioactivity in the aortas of spontaneously hypertensive rats (SHR). The sesamin-mediated suppression of eNOS uncoupling, via regulation of DHFR and ONOO- levels, coupled with the stimulatory effect of sesamin on p-eNOS increases NO biosynthesis to relieve endothelial dysfunction and hypertension. Sesamin also inhibits NADPH oxidase, subsequently decreasing superoxide levels and NO oxidative inactivation, contributing to the suppression of endothelial dysfunction and hypertension (adapted from Kong et al., 2015a).
The anti-hypertensive effects of sesamin are also attributed to its metabolites’ radical scavenging ability, which is shown to induce endothelium-dependent vasorelaxation (Nakano et al., 2006). Systolic blood pressure and the production of aortic superoxide (O2−) are positively correlated. Therefore, the antioxidant activity of sesamin may potentially contribute to its anti-hypertensive properties (Nakano et al., 2002). In fact, studies using DOCA salt-induced hypertensive rats confirm the ability of sesamin in inhibiting superoxide production (Nakano et al., 2002, Nakano et al., 2003, Nakano et al., 2004, Nakano et al., 2006, Nakano et al., 2008) and improve vasodilator responses (Matsumura et al., 2000) contributing to its anti-hypertensive effects. Sesamin-mediated amelioration of DOCA salt-induced hypertension strongly suggests its utility as a prophylactic treatment in the development of hypertension (Matsumura et al., 1995). Further investigation on the anti-hypertensive effects of sesamin on salt-loaded and unloaded stroke-prone spontaneously hypertensive rats (SHRSP) reports that sesamin significantly suppresses the development of hypertension (215 ± 4 vs. 180 ± 4 mmHg) and efficiently maintains suppression in the salt-loaded group. However, there is no significant suppression in the salt unloaded group (Matsumura et al., 1998). Since sesamin feeding appears more effective as an anti-hypertensive regimen in salt-loaded SHRSP than in unloaded SHRSP, it is considered more useful as a prophylactic treatment in the malignant status of hypertension (Matsumura et al., 1998). It is important to note that the anti-hypertensive effects of sesamin are not limited to salt-induced hypertensive animal models. Indeed, sesamin administration to streptozotocin (STZ)-induced diabetic rats significantly ameliorates the effect on blood pressure in rats with type 1 diabetes (Thuy et al., 2017).
Sesamin has also been investigated as a potential inhibitor to the synthesis of 20-hydroxyeicosatetraenoic acid (20-HETE), a metabolite of arachidonic acid (AA). 20-HETE has been shown to interact with the renin-angiotensin system (RAS) and alter the regulation of blood pressure, contributing to the pathogenesis of hypertension (Hoopes et al., 2015). Angiotensin II and endothelin-1 induce the synthesis and release of 20-HETE, which in turn mediates the vasoconstrictor and pressor effects of angiotensin II by activating the RAS/MAP kinase pathway, mediating hypertrophy and hypertension (Hoopes et al., 2015). Thus, the inhibition of 20-HETE can hinder the development of angiotensin II-dependent hypertension. Moreover, 20-HETE sensitizes vascular smooth muscles to a variety of vasoconstrictor stimuli, such as angiotensin II, phenylephrine, and endothelin, and plays a role in the development of endothelial dysfunction through decreased synthesis and accelerated degradation of NO (Hoopes et al., 2015). In vitro, sesamin is shown to prevent the synthesis of 20-HETE in human liver and renal microsomes with IC50 < 20 μmol/L (Wu et al., 2009). Furthermore, in a randomized, controlled crossover trial administration of 25 g of sesame per day (approximately 50 mg of sesame lignin) to overweight men and women (n = 33) for 5 weeks decreases plasma and urinary 20-HETE by 28% and 32%, respectively. However, blood pressure is not affected (Wu et al., 2009). Accordingly, sesamin is found to decrease the concentration of endothelin-1 in human umbilical vein endothelial cells (HUVECs) in a dose-dependent manner (Lee et al., 2004). In addition to its inhibitory effects of endothelin-1, sesamin is also observed to increase the concentration of NO, induce eNOS mRNA and protein expression, and NOS activity (Lee et al., 2004). Ultimately, the enhancement of endothelium-dependent vasorelaxation is one of the important mechanisms of the in vivo anti-hypertensive effects of sesamin (Nakano et al., 2006).
The anti-hypertensive properties of sesamin is also observed in humans. A double-blind, crossover, placebo-controlled trial has investigated the effects of sesamin administration on blood pressure in mildly hypertensive middle aged men and women. Twelve subjects are administered capsules containing 60 mg of sesamin for 4 weeks. At the end of the trial, systolic blood pressure decreases from 137.6 ± 2.2 to 134.1 ± 1.7 mmHg, and diastolic blood pressure from 87.7 ± 1.3 to 85.8 ± 1.0 mmHg (Miyawaki et al., 2009). Epidemiological studies suggest a 2–3 mmHg decrease in blood pressure can reduce the rate of CVDs. Thus, the slight reduction of systolic and diastolic blood pressure by sesamin may be significant enough to decrease the risk of developing CVDs (Miyawaki et al., 2009). The anti-hypertensive effects of sesamin are also examined in patients with rheumatoid arthritis, who have a higher risk of developing CVDs (Helli et al., 2016). Results show that 200 mg/day sesamin supplementation for 6 weeks significantly decreases systolic blood pressure, adding to the plethora of evidence illustrating the anti-hypertensive effects of sesamin (Helli et al., 2016). The reported in vitro and in vivo anti-hypertensive effects of sesamin are summarized in Table 1.
Table 1.
Effects of sesamin on hypertension.
| Reference | Experimental Model | Dosage | Administration Mode | Administration Duration | Experimental N | Response |
|---|---|---|---|---|---|---|
| Animal Models | ||||||
| Kita et al. (1995) | Sprague-Dawley rats with two-kidney, one-clip hypertension | 1 w/w% (10 g/kg) |
Oral | 4 weeks | 11 | Suppression of kidney clip-induced increase in systolic BP (42 mmHg decrease) |
| Matsumura et al. (1995) | Sprague-Dawley rats with DOCA salt-induced hypertension | 1 w/w% (10 g/kg) |
Oral | 5 weeks | 8 | Suppression of DOCA salt-induced increase in systolic BP (46 mmHg decrease) |
| Matsumura et al. (1998) | Stroke-prone spontaneously hypertensive rats | 1 w/w% (10 g/kg) |
Oral | 24 weeks | 7–9 | Suppression of systolic BP increase, 15–30 mmHg decrease in systolic BP |
| Matsumura et al. (2000) | Sprague-Dawley rats with DOCA salt-induced hypertension | 1 w/w% (10 g/kg) |
Oral | 5 weeks | 8 | Suppression of DOCA salt-induced increase in systolic BP (28 mmHg decrease) |
| Noguchi et al. (2001) | Stroke-prone spontaneously hypertensive rats | 1000 mg/kg | Oral | 5 weeks | 3 | Decrease in systolic BP by sesamin treatment (20 mmHg decrease), vitamin E treatment (31 mmHg decrease), and sesamin + vitamin E treatment (52 mmHg decrease) |
| Nakano et al. (2003) | Sprague-Dawley rats with DOCA salt-induced hypertension | 0.1 w/w% (1 g/kg), 1 w/w% (10 g/kg) |
Oral | 5 weeks | 14 | Dose-dependent decrease in systolic BP (0.1 w/w% diet − 29.2 mmHg decrease, 1 w/w% diet − 46 mmHg decrease) |
| Nakano et al. (2008) | Sprague-Dawley rats with DOCA salt-induced hypertension | 1 w/w% (10 g/kg) |
Oral | 5 weeks | 6 | Suppression of DOCA salt-induced increase in systolic BP (36.8 mmHg decrease), NADPH activity, and mRNA expression of p22phox, gp91phox, and Nox1 |
| Kong et al. (2009) | Sprague-Dawley rats with two-kidney, one-clip hypertension | 60, 120 mg/kg | Oral | 8 weeks | 7 | Decrease in systolic BP by 11% (60 mg treatment) and 17% (120 mg treatment) |
| Li et al. (2015) | Sprague-Dawley rats with monocrotaline-induced pulmonary hypertension | 50, 100 mg/kg | Oral | 4 weeks | 12 | Decrease in right systolic ventricular pressure and mean arterial pressure |
| Kong et al. (2015a) | Spontaneously hypertensive rats | 80, 160 mg/kg | Oral | 8 weeks | 10 | Decrease in systolic BP by 12% (80 mg treatment) and 16% (160 mg treatment) |
| Thuy et al. (2017) | Sprague-Dawley rats with STZ-induced type 1 diabetes | 50, 100, 200 mg/kg | Oral | 4 weeks | 5 | Increase in systolic BP and diastolic BP in STZ-induced rats (50 mg, 17/8.2 mmHg increase, 100 mg, 37.8/14.7 mmHg increase, 200 mg, 38.6/17.5 mmHg increase) |
| Human Subjects | ||||||
| Miyawaki et al. (2009) | Japanese, middle-aged, mildly hypertensive patients | 10 mg/capsule. 3 capsules twice per day |
Oral | 4 weeks | 12–13 | Decrease in systolic BP by 3.5 mmHg and diastolic BP by 1.9 mmHg |
| Helli et al. (2016) | Iranian, overweight (BMI 25–35), middle-aged, RA patients | 200 mg per day | Oral | 6 weeks | 22 | Decrease in systolic BP by 4.3 mmHg and diastolic BP by 1.0 mmHg |
3. Effects of sesamin on atherosclerosis
Atherosclerosis is a chronic inflammatory disease that is primarily driven by the accumulation of low-density lipoprotein (LDL)-cholesterol and lipoprotein particles, followed by active inflammatory processes in particular areas of disturbed non laminar flow at branch points in arteries (Pahwa and Jialal, 2020). Atherosclerosis contributes to the development of CVDs, such as ischemic heart disease, which increases the risk of myocardial infarction (Pahwa and Jialal, 2020). It has been reported that about 735,000 Americans have a heart attack annually, 75% of which are caused by atherosclerotic lesion (or plaque) ruptures. Atherosclerosis also increases the risk of having a stroke, as the lesion formation in arteries carrying blood to the brain can lead to ischemic stroke (Pahwa and Jialal, 2020). The most common risk factors are diets high in saturated fats, hypercholesterolemia, hypertension, cigarette smoking, diabetes mellitus, age, male gender, obesity, and sedentary lifestyle. These risk factors cause endothelial injury and lead to endothelial dysfunction, increasing the adhesiveness of the endothelium to leukocytes or platelets, and the formation of vasoactive molecules, cytokines, and growth factors. If this inflammatory response persists, smooth muscle cells migrate, proliferate, and intermix with an area of inflammation forming an intermediate lesion (Ross, 1999). This migration is controlled by angiotensin II, platelet derived growth factors (PDGF) and insulin-like growth factors (IGF) (Pahwa and Jialal, 2020). The lesion grows as the continued inflammatory response attracts more lymphocytes and monocyte-derived macrophages, which proliferate and absorb modified ApoB lipoprotein to form foam cells, in which cholesterol accumulates. Activation of these cells leads to further release of cytokines, chemokines, growth factors, and hydrolytic enzymes, inducing more damage and causing necrosis of endothelial tissue (Ross, 1999). Therefore, cycles of accumulation of macrophages and lymphocytes, migration and proliferation of smooth muscle cells, and fibrous tissue formation cause the lesion to grow into the lumen of arteries and hinder blood flow.
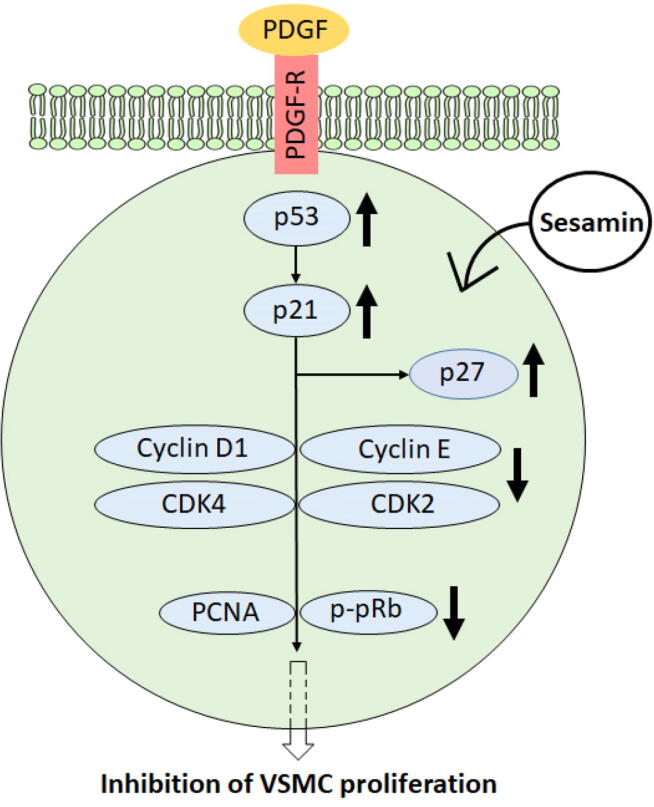
It is evident that inflammatory responses play a crucial role in the formation of atherosclerotic lesions. Sesame oil has been shown to reduce atherosclerotic lesions, triglycerides, plasma cholesterol, and LDL-cholesterol levels in low-density lipoprotein receptor-deficient (LDLR−/−) mice through induction of various genes involved in cholesterol metabolism and reverse cholesterol transport (RCT) and reduction of inflammatory cytokine expression (Narasimhulu et al., 2015). To specify the lignans involved in this anti-inflammatory response, and thus anti-atherogenic effects of sesame oil, further investigation has been conducted using isolated lignans. Sesamin is found to decrease atherosclerotic lesion formation in ApoE−/− mice, a common model of atherosclerosis by 40%, although this does not appear to be statistically significant (Loke et al., 2010). For future investigation, we suggest that the anti-atherogenic effects of sesamin should be assessed in LDLR−/− mice, a diet-induced model of atherosclerosis. This suggestion stems from the observation that ApoE−/− mice display severe hypercholesterolemia and atherosclerotic lesion formation whether fed a chow diet or an atherogenic diet (Plump et al., 1992), while LDLR−/− mice display less profound hypercholesterolemia and atherosclerotic lesion formation only when they are fed an atherogenic diet (Ishibashi et al., 1994). We expect the anti-atherogenic effects of sesamin to be more pronounced in LDLR−/− mice compared to ApoE−/− mice. We also suggest that the potential anti-atherogenic effects of sesamin be examined in Watanabe heritable hyperlipidemic rabbits, which can serve as a great choice of an animal model to mimic the profile of familial hypercholesterolemia and atherosclerosis in humans. Moreover, Hirata and colleagues show that administration of sesamin and its stereoisomers to hypercholesterolemic patients reduces LDL-C levels, and thus the risk of developing atherosclerosis (Hirata et al., 1996). Results show that 1–10 µM episesamin interferes with tumor necrosis factor α (TNFα)-induced activation of human and mouse vascular smooth muscle cells (VSMCs) by interrupting the activation of extracellular signal-regulated kinases (ERK1/2), protein kinase B (AKT), nuclear factor-kappa B (NF-κB), and vascular cell adhesion molecule (VCAM-1) (Freise and Querfeld, 2014). Similarly, sesamin reduces PDGF-BB-induced growth and proliferation of human, mouse, and rat VSMCs by disabling the activation of MAPK and PI3K pathways, and decreasing oxidative stress through induction of heme oxygenase-1 (HO-1) expression (Freise et al., 2015). Furthermore, sesamin and episesamin decrease the expression and secretion of two key matrix metalloproteinases, MMP-2 and MMP-9 (Freise and Querfeld, 2014), which promote the migration and proliferation of VSMCs contributing to the formation and development of atherosclerotic lesions (Li et al., 1996, Katsuda and Kaji, 2003 Wågsäter et al., 2011). The potency of inhibiting VSMCs proliferation is determined at 1, 5, and 10 µM sesamin, which provides an inhibition percentage of 49.8 ± 22.0%, 74.6 ± 19.9%, and 87.8 ± 13.0%, respectively, illustrating a dose-dependent inhibition (Han et al., 2015). Sesamin also significantly reduces PDGF-induced DNA synthesis, and halts cell cycle progression in the G0/G1 to S phase. Fig. 2 illustrates the mechanism by which sesamin administration leads to inhibited expression of cyclin D1, cyclin E, cyclin-dependent kinase 2 (CDK2), cyclin-dependent kinase 4 (CDK4), and proliferating cell nuclear antigen (PCNA) as well as reduced phosphorylation of ritnoblastoma protein (pRb), while upregulating the expression of p53, p21, and p27 (Han et al., 2015).
Fig. 2.
The mechanism of action underlying the anti-proliferative action of sesamin on PDGF-induced VSMCs. Sesamin upregulates p53, leading to the upregulation of p21 and eventually p27. This leads to the suppression of cyclin D1, cyclin E, CDK2, and CDK4. In turn, the levels of PCNA and p-pRb are reduced, inhibiting VSMC proliferation (adapted from Han et al., 2015).
We have previously shown that treating macrophages with sesame oil leads to a significant enhancement of peroxisome proliferator-activated receptor γ1 (PPARγ1) and liver X receptor α (LXRα) expression and transcriptional activity in a MAPK-dependent manner (Majdalawieh and Ro, 2015). Indeed, other studies have demonstrated that sesamin (25–100 μM) upregulates PPARγ1 and LXRα expression and transcriptional activity in macrophage via MAPK signaling, leading to enhanced macrophages cholesterol efflux (Liu et al., 2014, Majdalawieh and Ro, 2014). PPARγ1 and LXRα are important nuclear receptors involved in macrophage cholesterol homeostasis and inflammation. Hence, another mechanism by which sesamin induces anti-atherogenic effects is through its ability to improve macrophage cholesterol efflux, preventing the formation of foam cells. Upon their activation, PPARγ1 and LXRα induce various ABC transporters as well as ApoE. In turn, excess cholesterol is transferred from macrophages to high-density lipoproteins (HDLs) to initiate RCT (Majdalawieh and Ro, 2010). Detailed experimental data pertaining to the ability of sesamin to regulate cholesterol metabolism and maintain macrophage cholesterol homeostasis as well as the underlying molecular mechanisms and signal transduction pathways involved in such regulation have been recently analyzed and reviewed (Majdalawieh et al., 2020).
Examining the effects of sesamin on endothelial leukocyte adhesion molecules provides further insight into the mechanism(s) of action that sesamin utilizes to induce anti-atherogenic effects. A study by Wu and colleagues demonstrates that pretreatment of TNFα-treated human aortic endothelial cells (HAECs) with 10 µM and 100 µM sesamin reduces intercellular cell adhesion molecule-1 (ICAM-1) expression by 35% and 70%, respectively (Wu et al., 2010). This is achieved by suppressing the increase in human antigen R (HuR) translocation, and its interaction with the 3′UTR of ICAM-1, induced by TNFα, significantly reducing monocyte binding to TNFα-stimulated HAECs. (Wu et al., 2010). The inhibitory effects of sesamin on ICAM-1 expression are also mediated by downregulation of ERK1/2 and p38. Moreover, in vivo analysis of aortas of apolipoprotein E-deficient (ApoE−/−) mice shows that sesamin attenuates intimal thickening and ICAM-1 expression (Wu et al., 2010). In an in vivo study, Japanese white rabbits are separated into three groups: model group (high cholesterol diet without sesamin), sesamin group (high cholesterol diet with 4 mg/day sesamin), and a control group (normal diet). Blood sample analysis after 5 and 8 weeks of sesamin administration reveals a significant reduction in total cholesterol (TC) and LDL-C compared to the model group. Furthermore, pathological examination of the aorta after the 8 week period demonstrates the aorta intima is thinner, the amount of macrophage in atherosclerotic lesions is lower, and mRNA expression levels of VCAM-1 is reduced in the sesamin group (Guan and Wang, 2009a). In a related study, the same researchers demonstrate that sesamin administration to Japanese white rabbits relieves pathological changes and suppresses VCAM-1 expression by 27.59% in aortas (Guan and Wang, 2009b). The significance of ICAM-1 and VCAM-1 suppression by sesamin can be understood by investigating their roles in atherosclerotic lesion formation and progression. Both ICAM-1 and VCAM-1 are endothelial cell adhesion molecules of the immunoglobulin (Ig) gene superfamily that play a role in the adhesion of monocytes to the endothelium (Cybulsky et al., 2001). Although they are structurally similar, they differ in their pattern of regulation (Ley and Huo, 2001). ICAM-1 regulates the recruitment of monocytes into the atherosclerosis-prone areas of blood vessels (Galkina and Ley, 2007), while VCAM-1 is involved in the initiation of atherosclerotic lesion formation (Cybulsky et al., 2001). The suppression of these cell adhesion molecules, specifically ICAM-1, protects from the progression of atherosclerotic lesion formation and delays the progression of atherosclerosis (Collins et al., 2000, Kitagawa et al., 2002). Therefore, the introduction of sesamin in diet may decrease the risk of developing atherosclerotic lesions. The reported in vitro and in vivo anti-atherogenic effects of sesamin are summarized in Table 2.
Table 2.
Effects of sesamin on atherosclerosis.
| Reference | Experimental Model | Dosage | Administration Mode | Administration Duration | Experimental N | Response |
|---|---|---|---|---|---|---|
| Cells and Cell Lines | ||||||
| Wu et al. (2010) | HAECs | 5, 10, 25, 50, 100 µM |
N/A | 24 h | 3 | - Decrease in ICAM-1 mRNA and protein expression through inhibition of cytoplasmic human antigen R (HuR) translocation and HuR-ICAM-1 mRNA interaction - Downregulation of ERK1/2 and p38 signaling |
| Liu et al. (2014) | RAW264.7 macrophages | 0.1, 1, 10 µM | N/A | 6, 24 h | 3–4 | - Decrease in intracellular cholesterol level, increase in HDL-mediated cholesterol efflux - Increase in expression of PPARγ1, LXRα and ABCG1 - Increase in PPARγ1 transcriptional activity - No effect on cholesterol uptake |
| Majdalawieh and Ro (2014) | Chinese hamster ovary (CHO) cell line Peritoneal macrophages |
25, 50, 75, 100 μM 25, 50, 75, 100 μM |
N/A N/A |
24 h 24 h |
4 4 |
- Increase in PPARγ1 and LXRα expression and transcriptional activity - Improvement of macrophage cholesterol efflux |
| Freise and Querfeld (2014) | Human and Mouse VSMCs | 0.1, 0.5, 1, 5, 10 µM episesamin |
N/A | 24 h | 3–12 | - Decrease in basal and TNFα-induced proliferation and migration of VSMCs - Inhibition of ERK1/2 and AKT activity - Suppression of basal and TNFα-induced expression and secretion of MMP-2 & MMP-9 mRNA - Inhibition of NF-κB activity - Attenuation of TNFα- and H2O2-induced oxidative stress |
| Freise et al. (2015) | Human VSMCs Mouse VSMCs Rat VSMCs |
1, 2, 5, 10 µM episesamin and sesamin |
N/A | 24–36 h 20–24 h 48–96 h |
4 | Decrease in basal and platelet-derived growth factor (PDGF)-BB induced proliferation and migration of VSMCs |
| Han et al. (2015) | Rat aortic VSMCs | 1, 5, 10 μM | N/A | 24 h | 3 | - Inhibition of cyclin D1, cyclin E, CDK2, CDK4, and PCNA expression - Suppression of pRb phosphorylation - Upregulation of p53, p21, and p27 expression |
| Animal Models | ||||||
| Guan and Wang (2009a) | Japanese white rabbits | 4 mg per day | Oral | 8 weeks | 6 | - Decrease in LDL levels - Thinner aorta intima - Lower macrophage amount in atherosclerotic lesions Decrease in VCAM-1 expression |
| Guan and Wang (2009b) | Japanese white rabbits | Unknown | Unknown | 8 weeks | 6 | - Decrease in LDL levels - Thinner aorta intima - Lower macrophage amount in atherosclerotic lesions - Decrease in VCAM-1 expression by 27.59% |
| Loke et al. (2010) | ApoE−/− mice | 64 mg/kg | Oral | 20 weeks | 25 | Decrease in atherosclerotic lesion formation by 40% |
| Wu et al. (2010) | ApoE−/− mice | 0.5 w/w% (5 g/kg) |
Oral | 11 weeks | 12 | - Decrease in ICAM-1 protein expression - Reduction of tunica intima in the aorta |
| Human Subjects | ||||||
| Hirata et al. (1996) | Hypercholesterolemia patients | 3.6 mg/ capsule (9 capsules per day for 4 weeks, 18 capsules per day for the next 4 weeks) |
Oral | 8 weeks | 6 | Decrease in TC, LDL, and ApoB levels |
4. Effects of sesamin on thrombosis
Atherosclerotic lesion rupture is a primary trigger for the development of arterial thrombosis (Mackman, 2008). Arterial thrombosis mainly occurs due to platelet aggregation. When an atherosclerotic lesion ruptures, platelets are recruited to the area through the interaction of platelet cell surface receptors with collagen and von Willebrand factor (Mackman, 2008). After this initial recruitment, additional platelets aggregate to the area and result in the growth of the thrombus. Subsequently, thrombin, a protease, cleaves the platelet receptor protease activated receptor 1 (PAR1) stimulating platelets to release granule contents, which further promotes platelet recruitment, aggregation, and activation (Mackman, 2008). Leukocytes also play an important role in thrombogenesis (Madamanchi et al., 2005). Various agonists influence the formation of platetelet-leukocyte aggregates, which enhance thrombin generation. One such factor is leukocyte-released superoxide (Madamanchi et al., 2005). Thrombin is thus an important component of the coagulation cascade that ultimately leads to formation of fibrin from fibrinogen, which stabilizes the structure of the thrombus (Mackman, 2008, Ku et al., 2013). Tissue factor initiates the coagulation cascade upon its exposure to blood (Mackman, 2008). Moreover, it promotes the procoagulant activities of leukocytes, specifically monocytes/macrophages (Madamanchi et al., 2005). It has also been observed that polymorphonuclear leukocytes regulate the expression of tissue factor by mononuclear cells via reactive oxygen species (ROS), establishing that oxidative stress plays an important role in pathogenesis of arterial thrombosis (Madamanchi et al., 2005). Unlike arterial thrombi, venous thrombi, which lead to deep vein thrombosis, are rich in fibrin and erythrocytes. These thrombi can lead to a pulmonary embolism that is considered to be the third leading cause of cardiovascular-associated death, following myocardial infarction and stroke, which are primarily caused by acute arterial thrombosis (Mackman, 2008). Anti-thrombotic drugs have been developed which target platelets, fibrin, and other various components of the coagulation cascade. However, due to their side effects such as heamorrhaging, they are not readily used (Mackman, 2008). Therefore, natural compounds with antioxidant and anti-thrombotic effects are being investigated.
The effects of sesamin on thrombogenesis have been examined, primarily due to its antioxidant properties. In a study conducted by Noguchi and colleagues, stroke-prone spontaneously hypertensive rats (SHRSP) are separated into four groups: vitamin E group (1000 mg α-tocopherol/kg diet), sesamin group (1000 mg sesamin/kg diet), vitamin E + sesamin group (1000 mg α-tocopherol/kg plus 1000 mg sesamin/kg diet), and control group (Noguchi et al., 2001). The thrombotic tendency is measured using the number of helium-neon laser pulses required to form an occlusive thrombus. After 5 weeks of administration, the number of laser pulses required to induce a thrombus increases in the sesamin, vitamin E, and sesamin plus vitamin E groups. Therefore, administration of sesamin alone or in combination with vitamin E seems to induce anti-thrombotic effects (Noguchi et al., 2001). Following the same approach, a similar experiment examines the effect of a variety of sesame seed wholegrains and extracts on the formation of thrombi. Mice have free access to a Western high-fat diet containing 5% sesame seed wholegrains (roasted and crushed) for 12 weeks. Moreover, the purified ingredients are administered intra-arterially (sesamin: 1 and 10 mmol/L; sesamolin: 0.1 and 1 mmol/L; sesamol: 0.01, 0.1, and 1 mmol/L) and orally (sesamin: 3 mmol/L; sesamolin: 3 mmol/L; sesamol: 1 or 3 mmol/L). Helium-neon laser measurements indicate that all three purified ingredients exhibit anti-thrombotic activities with sesamol as the most effective ingredient, followed by sesamolin, and sesamin. Moreover, of the whole grain varieties, Col/Chichibu/Maruteru-2/1995 and T016 show anti-thrombotic effects, while 00037803 is prothrombotic (Kinugasa et al., 2011). Utlizing an alternative approach by investigating the anti-coagulant properties of sesamin and epi-sesamin, Ku and colleagues monitor the effect of these stereoisomers on the activity of cell-based thrombin, activated blood coagulation factor X (FXa), activated partial thromboplastin time (aPTT), prothrombin time (PT), plasminogen activator inhibitor type 1 (PAI-1) and tissue-type plasminogen activator (t-PA) expression (Ku et al., 2013). Observations from this study demonstrate that epi-sesamin is a more potent anti-coagulant, and therefore, an anti-thrombotic agent, as it prolongs aPTT and PT, prevents the production of thrombin and FXa, reduces thrombin catalyzed platelet aggregation in mice, and inhibits TNFα-induced secretion of PAI-1 in HUVECs (Ku et al., 2013). The anti-thrombotic effects of sesamin are not extensively investigated. However, the reported findings seem to point to the role of sesamin in decreasing the risk of thrombus formation. The reported in vitro and in vivo anti-thrombotic effects of sesamin are summarized in Table 3.
Table 3.
Effects of sesamin on thrombosis.
| Reference | Experimental Model | Dosage | Administration Mode | Administration Duration | Experimental N | Response |
|---|---|---|---|---|---|---|
| Cell and Cell Lines | ||||||
| Ku et al. (2013) | HUVECs | 0.5, 1, 2, 5, 10, 20, 50 µM | N/A | 10 min − 18 h | 3 | Negligible effect on coagulation, bleeding time, thrombin production and activity, FXa production and activity, platelet aggregation, fibrin polymerization, and plasminogen activator inhibitor type 1 (PAI-1)/tissue-type plasminogen activator (t-PA) ratio |
| Animal Models | ||||||
| Noguchi et al. (2001) | Stroke-prone spontaneously hypertensive rats | 1000 mg/kg | Oral | 5 weeks | 3 | Increase in the number of He-Ne laser pulses required to induce thrombosis |
| Kinugasa et al. (2011) | C57BL/6 mice | 1, 10 mmol/L 3 mmol/L (7.7 mg/kg) |
Intra-arterial Oral |
10 min 2 h |
7 (1 mmol/L), 6 (10 mmol/L) 6 |
Increase in the number of He-Ne laser pulses required to induce thrombosis |
| Ku et al. (2013) | ICR mice | 7 µg | Intravenous | 1 hr | 5 | Negligible effect on coagulation, bleeding time, thrombin production and activity, FXa production and activity, platelet aggregation, fibrin polymerization, and plasminogen activator inhibitor type 1 (PAI-1)/tissue-type plasminogen activator (t-PA) ratio |
5. Effects of sesamin on diabetes
Diabetes mellitus is one of the most prominent causes of death globally, with a mortality rate of about 1.6 million individuals worldwide, and is considered the third highest risk factor for global premature mortality due to hyperglycaemia and hyperglycaemic-induced oxidative stress and inflammation (Oguntibeju, 2019). Diabetes is a metabolic disorder which occurs when the body is unable to control blood glucose levels, either due to the impaired production of insulin (Type 1) or impaired response to insulin (Type 2) (Oguntibeju, 2019). The liver, skeletal muscles, and adipose tissue are the main tissues affected by insulin resistance. This impaired response to insulin leads to a deficient uptake and phosphorylation of glucose by skeletal muscles and defective synthesis of glycogen, in addition to an increased uptake of free fatty acids and insufficient oxidation in mitochondria (Sala and Zorzano, 2016). This leads to an accumulation of triglycerides in myofibers. Therefore, the development of type 2 diabetes is linked with low grade chronic inflammation, contributing to insulin resistance and oxidative stress that play a role in pathogenesis of diabetic complications (Oguntibeju, 2019). In 2017, it was reported that 46 million individuals were diabetic in North America and the Caribbean, and currently data from the International Diabetes Federation (IDF) suggests that there are about 415 million diabetic patients worldwide with a prevalence rate of 8.8% (Oguntibeju, 2019). Moreover, it is estimated that by 2040 this number will increase to 642 million, with type 2 diabetes as the major type of diabetes (Oguntibeju, 2019).
Sesamin has been shown to possess both antioxidant and anti-inflammatory effects. Therefore, its effect has been investigated on diabetes mellitus and its associated complications. Treatment of spontaneously diabetic mice (KK-Ay) with sesamin significantly decreases fasting plasma glucose, insulin, triglyceride, cholesterol, free fatty acid, MDA content, and glycosylated plasma proteins. Furthermore, it improves the insulin-binding capacity to liver crude plasma membrane, thus ameliorating insulin resistance (Hong et al., 2012). To investigate the effects of sesamin in humans, type 2 diabetic patients are administered 200 mg of sesamin per day for 8 weeks. Upon completion, anthropometric measurements and blood sample examination illustrate that sesamin supplementation significantly reduces fasting blood sugar (FBS), glycated hemoglobin (HbA1c), TNFα, interleukin 6 (IL-6), hip and waist circumference, and body adiposity index (BAI). Moreover, adiponectin levels are significantly increased (Mohammad Shahi et al., 2017). These results suggest that sesamin can induce beneficial effects on glycemic status, inflammatory factors, and body composition. Similarly, sesamin supplementation of hyperlipidemia rats cause the same inhibitory effect on FBS and HbA1c. Sesamin also reduces pancreatic insulin and somatostatin levels to prevent β-cell exhaustion, and improves insulin sensitivity (An and Zhang, 2010).
One of the precursors to the development of diabetes is the overconsumption of foods highly concentrated in fats. However, co-administration of sesamin with high-fat foods ameliorates some of the adverse effects of this type of diet. For example, when sesamin is consumed by Zucker (fa/fa) rats supplied with a high-fat diet, the increase in fasting blood glucose and concentration of white adipose tissue is ameliorated. Furthermore, sesamin supplementation increases the activity of acyl-CoA dehydrogenase (ACAD) and decreases the levels of glucose-6-phosphatase (G6Pase) (Fujiwara et al., 2006). This decline in G6Pase reduces the dephosphorylation of glucose-6-phosphate (G6P) to glucose, thus decreasing the concentration of blood glucose. Furthermore, sesamin seems to have beneficial effects on the complications associated with type 2 diabetes. Certain diabetes-associated complications, such as atherosclerosis, coronary artery disease, and nephropathy occur due to increased synthesis of inflammatory cytokines induced by advanced glycated end products (AGEs), which are proteins, lipids, or end products, such as Schiff base and Amadori products that are non-enzymatically glycated upon exposure to sugars (Oguntibeju, 2019). Sesamin significantly reduces the increase in type 2 diabetes-associated pro-inflammatory cytokines, such as TNFα and IL-6 (Zakerzadeh et al., 2014). Moreover, sesamin prevents the impairment of mitochondrial function in skeletal muscles observed in diabetic individuals, and thus ameliorates the decline in their exercise capacity (Takada et al., 2015). Sesamin achieves this by inhibiting the increased activity of NADPH oxidase and production of superoxide anions, thus reducing oxidative stress in skeletal muscles. Furthermore, sesamin ameliorates the decrease in citrate synthase activity, an important enzyme in the tricarboxylic acid cycle, which plays a critical role in exercise capacity of skeletal muscles (Takada et al., 2015). AGEs also play a role in the production of ROS, which induce oxidative stress (Oguntibeju, 2019). Oxidative stress is involved in the pathogenesis of CVDs, hence why diabetic patients also have a higher risk of developing cardiovascular complications (Oguntibeju, 2019). A study investigating the effect of sesamin on peroxidation levels of diabetic blood samples concludes that the antioxidant properties of sesamin can potentially protect plasma, LDL, and erythrocyte membrane from oxidation, and thus reduce the risk of developing coronary heart disease (Dhar et al., 2005). Moreover, sesamin is found to possess cardioprotective properties (Thuy et al., 2017). A 4 week administration of sesamin (100 and 200 mg/kg body weight) to STZ-induced type 1 diabetic rats decreases blood glucose levels, and significantly improves heart rate and blood pressure. Furthermore, the anti-inflammatory effect of sesamin helps to improve the myocardial damage associated with diabetes by improving the disorder in the myofiber arrangements (Thuy et al., 2017). Finally, sesamin is also observed to restore the altered QT interval in diabetics, thus revealing that sesamin has a positive effect in improving cardiovascular dysfunction in STZ-induced type 1 diabetic rats (Thuy et al., 2017). The sesamin-induced reduction of blood glucose levels, as observed in this study, can be attributed to its protective effects on pancreatic β-cells (Thuy et al., 2017). Investigating the effect of sesamin on NIT-1 pancreatic β-cells damaged by STZ demonstrates that sesamin can improve insulin secretion after treatment of β-cells with STZ. Moreover, sesamin protects these cells from STZ-induced oxidative damage by increasing the content of reduced glutathione (GSH), and activity of superoxide dismutase (SOD) and glutathione peroxidase (GSHpx). Sesamin also ameliorates the increase in NO induced damage by decreasing the activity of nitric oxide synthase (NOS) and inducible NOS (iNOS) (Lei et al., 2012). This protective effect is observed on AGE induced β-cell damage as well. AGEs increase the production of ROS in these cells, which promote apoptotic pathways and cell death (Kong et al., 2015b). In vitro (MIN6 cell line) and in vivo (C57BL/6J mice) experiments reveal that sesamin induces an antioxidant defense mechanism by downregulating the expression of p22phox and p67phox NADPH oxidase subunits, and decreasing the activity of NADPH oxidase, thus reducing the production of ROS in these cells (Kong et al., 2015b). The reported in vitro and in vivo anti-diabetic effects of sesamin are summarized in Table 4.
Table 4.
Effects of sesamin on diabetes.
| Reference | Experimental Model | Dosage | Administration Mode | Administration Duration | Experimental N | Response |
|---|---|---|---|---|---|---|
| Cells and Cell Lines | ||||||
| Lei et al. (2012) | STZ-challenged pancreatic β NIT-1 cell line | 100, 200, 400 µg/mL | N/A | 24 h | 10 | - Protection against STZ-induced cell death - Improvement of insulin secretion in STZ-challenged pancreatic β NIT-1 cells |
| Kong et al. (2015b) | Pancreatic MIN6 β-cell line |
50, 100 μM | N/A | 2 h | 4 | Amelioration of AGE-induced β-cell dysfunction and apoptosis |
| Animal Models | ||||||
| Fujiwara et al. (2006) | Zucker (fa/fa) rats | 0.02 w/w% (0.2 g/kg), 0.05 w/w% (0.5 g/kg) |
Oral | 40 days | Unknown | - Reduction in fasting plasma glucose, leptin, and mRNA levels of G6Pase - Increase in ACAD activity |
| An et al. (2010) | Hyperlipidemia Sprague-Dawley rats | Unknown | Unknown | 7 weeks | Unknown | - Reduction in serum TC, TG, LDL, ApoB, and insulin levels - Increase in HDL-C and ApoA levels - Inhibition of pancreatic insulin and somatostatin levels - Improvement in insulin sensitivity |
| Hong et al. (2012) | Spontaneously diabetic mice (KK-Ay) |
50, 100 mg/kg | Oral | 2 weeks | 10 | - Decrease in blood glucose, glycated serum protein, and serum insulin - Increase in liver glycogen content |
| Kong et al. (2015b) | C57BL/6J mice | 160 mg/kg | Oral | 4 weeks | 6 | Amelioration of AGE-induced β-cell dysfunction and apoptosis |
| Takada et al. (2015) | C57BL/6J mice | 0.2 w/w% (2 g/kg) (5 mg/day) |
Oral | 8 weeks | 6–10 | Attenuation of blood insulin and serum lipid increase caused by high-fat diet |
| Thuy et al. (2017) | Sprague-Dawley rats with STZ-induced type 1 diabetes | 50, 100, 200 mg/kg | Oral | 4 weeks | 5 | Decrease in blood glucose level by 17% (50 mg treatment), 30% (100 mg treatment), and 26% (200 mg treatment) |
| Human Subjects | ||||||
| Mohammad Shahi et al. (2016) | Type 2 diabetic patients | 200 mg per day | Oral | 8 weeks | 24 | - Decrease in serum levels of fasting blood sugar (FBS) and glycated hemoglobin (HbA1c) - Increase in serum levels of adiponectin |
6. Effects of sesamin on obesity
According to the Center of Disease Control and Prevention (CDC) in 2017–2018, 42.4% of the population of the United States was obese (CDC, 2020a, CDC, 2020b). This is mostly due to a sedentary lifestyle, and the consumption of high-fat foods (Chinnala et al., 2014). Obesity is related to a variety of metabolic and non metabolic complications, such as type 2 diabetes, dyslipidemia, athersclerosis, hypertension, and other CVDs (Chinnala et al., 2014, Raeisi-Dehkordi et al., 2018). The scarcity of synthetic drugs against obesity, with sufficient efficacy and minimum side effects, has prompted researchers to shift to medicinal herbs and natural compounds for the treatment of obesity (Chinnala et al., 2014). It has been reported that foods highly concentrated in antioxidants, such as flaxseeds, cranberry, and green tea may be helpful in the management of obesity, such that high levels of antioxidants may lead to weight loss (Raeisi-Dehkordi et al., 2018).
Sesame seed and its oil as well as sesamin posses antioxidative properties. Therefore, their effects on obesity have been studied. Sesame seed is found to exhibit a high binding activity to adenosine A1 receptors, which appears to correlate with lipolytic activity when blocked (Yuliana et al., 2011). A study by Bigoniya and colleagues investigates the effect of sesame seed cake (SSC), which is the residue left after sesame oil extraction, on obesity in rats fed with a high-fructose diet (Bigoniya et al., 2012). The exact content of sesamin in sesame seeds depends on the extraction method, technical specifications, and instrumentation (Dar and Arumugam, 2013). It is reported that sesamin content in 100 g of sesame seeds is 1800 mg (i. e. 18 mg/g) (Hemalatha and Ghafoorunissa, 2004). The administration of 2 g/kg (i.e. ~36 mg sesamin/kg) and 4 g/kg (i.e. ~72 mg sesamin/kg) of SSC for 30 days ameliorates weight gain by 1.84% and 2.34% respectively. Moreover, SSC administration significantly reduces the weight of the liver and kidney, suggesting the prevention of fat deposition out of the muscle. Similar results are obtained in a study administering 200 mg/kg and 400 mg/kg methanolic extract of S. indicum Linn. (MESI) to rats fed with a high-fat diet. After 40 days it is reported that MESI administration significantly reduces the body weight and food intake of the high-fat diet fed rats, indicating that MESI posseses weight reducing and hypophagic properties (Chinnala et al., 2014).
Drugs and natural compounds that posses weight-reducing properties elicit their effects by several mechanisms including suppression of lipogenesis, reduction in fat absorption by the gastrointerstinal tract, and activation of apoptosis in adipocytes (Raeisi-Dehkordi et al., 2018). Pancreatic triacylglycerol lipase (PTL) is a major enzyme responsible for the digestion and absorption of dietary fat. PTL hydrolyzes lipids into monoglycerols and free fatty acids in the duodenum, which are then packed into micelle and absorbed into the lymphatic system through lacteals. Blocking of PTL with tetrahydrolipstatin has been utilized in the management of obesity, however has reported several side effects (Badmaev et al., 2015). S. indicum extract (70%) has been found to inhibit the action of PTL by 15% at 1 µg/mL and 17.5% at 100 µg/mL, suggesting that it may possess weight reducing properties (Badmaev et al., 2015). However, it is important to note a discrepancy in PTL inactiviation between the low and high dose. In fact, S. indicum extract is revealed to possess a dual role in its action on pancreatic lipase activity. For example, the co-administration of 1 µg/mL S. indicum extract (70%) with 10 µg/mL Coleus forskohlii extract (98%) (which has a PTL activity inhibition rate of 7.4%) has resulted in a 9.6% inhibition of PTL activity, rather than the expected 22.5% inhibition (Badmaev et al., 2015). Therefore, it is concluded that S. indicum extract enhances the inhibition of PTL activity in a lower dose range and moderates PTL inhibition in a higher dose range. The sesame extract’s dual action means that it can be added to anti-obesity therapies which target PTL activity as a safety measure and to prevent side effects due to excessive inhibition of PTL (Badmaev et al., 2015). S. indicum extract, as well as sesamin, are also considered to be an important component of hepatothermic therapy (HT) of obesity (McCarty, 2001). HT is a regimen that attempts to simultaneously decrease respiratory quotient and boost basal metabolism, thus supporting fat loss and reducing serum free fatty acids (McCarty, 2001). This therapy maximizes the capacity for fatty acid oxidation in hepatocytes, and drives hepatic thermogenic mechanisms. Sesamin administration doubles mitochondrial mass, which proportionally increases carnitine palmitoyl transferase-1 (CPT-1) expression, thus increasing hepatic beta oxidation of fatty acids (McCarty, 2001). Sesamin-mediated increase in gene expression of CPT-1 and many other enzymes involved in fatty acid oxidation occurs through the activation of peroxisome proliferator-activated receptor α (PPARα) (Ide et al., 2001). Sesamin not only increases lipolytic enzyme activity, but also decreases the activity of lipogenic enzymes, such as fatty acid synthase (FAS), by down-regulating the gene expression of sterol regulatory element bing protein-1 (SREBP-1) (Ide et al., 2001). Sesamin also acts as an antagonist to liver X receptor (LXRα) and pregnane X receptor (PXR) ameliorating drug induced hepatic lipogenesis and potentially helping in the treatment of non alcoholic fatty liver disease (NAFLD) (Tai et al., 2019). It inhibits hepatic lipogenesis partially through the activation of adenosine monophosphate-activated protein kinase (AMPK) and inhibition of SREBP-1c expression (Tai et al., 2019).
When investigating the potential effect of the epimer of sesamin, (+)-episesamin, it is revealed that episesamin is responsible for inhibition of adipogenesis (Freise et al., 2013). The presence of episesamin during hormone induced differentiation of 3T3-L1 preadipocytes reduces the phosphorylation of ERK1/2 and β-catenin as well the protein expression of PPARγ, and increases the expression of inducible nitric oxide synthase (iNOS) (Freise et al., 2013). This reduces the accumulation of intracellular lipid droplets and decreases protein expression of glucose transporter 4 (GLUT-4) and vascular endothelial growth factor. A reduction in stored lipid droplets is also observed upon the treatment of mature adipocytes with episesamin. Moreover, episesamin seems to induce proaptotic enzymes caspases 3 and 7. Thus, episesamin can reduce the proliferation and differentiation of preadipocytes into mature adipocytes, and induce lipolytic as well as apoptotic cascades in mature adipocytes (Freise et al., 2013). The reported in vitro and in vivo anti-obesity, lipolytic effects of sesamin are summarized in Table 5.
Table 5.
Effects of sesamin on obesity.
| Reference | Experimental Model | Dosage | Administration Mode | Administration Duration | Experimental N | Response |
|---|---|---|---|---|---|---|
| Cell and Cell Lines | ||||||
| Freise et al. (2013) | 3 T3-L1 preadipocytes | 5, 10, 20, 40 µM episesamin | N/A | 24 h | 12 | - Reduction in the accumulation of lipid droplets - Inhibition of GLUT-4 and vascular endothelial growth factor expression in preadipocytes - Reduction in intracellular lipid droplets and induced apoptosis in mature adipocytes |
| Badmaev et al. (2015) | Pancreatic lipase assay | 0.1, 1, 10, 100 µg/mL | N/A | 15 min | 2 | Inhibition of pancreatic triacylglycerol lipase (PTL) activity by S. indicum extract |
| Tai et al. (2019) | Human hepatocellular carcinoma cell line (HepG2) Human intestinal cell line (LS174T) Hepatoma cell line (HepaRG) |
20, 40 μM 20, 40 μM 5, 10 μM |
N/A N/A N/A |
24 h 24 h 2 weeks |
4 3 3 |
-Sesamin reduced valproate-and rifampin-induced hepatic lipogenesis - Sesamin selectively induces reverse cholesterol transport- (RCT-) related genes in LS174Tcell -Sesamin inhibits the mRNA and protein expression of T0901317-induced LXRα downstream gene |
| Animal Models | ||||||
| Ide et al. (2001) | Sprague-Dawley rats | 0.1 w/w% (1 g/kg), 0.2 w/w% (2 g/kg), 0.4 w/w% (4 g/kg) |
Oral | 15 days | 7–8 | Suppression of hepatic FAS expression |
| Chinnala et al. (2014) | Sprague-Dawley rats | 200, 400 mg/kg | Oral | 40 days | 6 | Decrease in body weight and food intake |
7. Anti-inflammatory effects of sesamin
It is well known that inflammation is a major biological process involved in the development of CVD and its risk factors. Several in vitro and in vivo studies have demonstrated an anti-inflammatory role of sesamin in various inflammatory conditions. For instance, sesamin treatment has been shown to inhibit the secretion of proinflammatory cytokines including IL-1β, IL-6, iNOS, and TNFα in different cell types (Freise et al., 2012, Kong et al., 2014, Xu et al., 2015, Ahmad et al., 2016, Lin et al., 2019, Zhao et al., 2019). Moreover, sesamin treatment is accompanied by a down-regulation of inflammatory matrix metalloproteases (MMPs) including MMP-1, MMP-2, MMP-3, MMP-9, and MMP-13 (Freise et al., 2012, Harikumar et al., 2010, Xu et al., 2015, Lin et al., 2019). Sesamin is also shown to inhibit the expression of LPS-induced macrophage-derived chemokines (MDCs) (e.g. interferon γ (IFNγ)-inducible protein 10 (IP-10/CXCL10)) in human primary monocytes and THP-1 cells (Hsieh et al., 2014). The anti-inflammatory effects of sesamin have also been observed in different in vivo models (Cui et al., 2010, Lin et al., 2014, Ahmad et al., 2016, Zhang et al., 2016, Li et al., 2016, Fan et al., 2017, Rousta et al., 2018, Zhao et al., 2019, Bai et al., 2019, Lin et al., 2019, Sayhan et al., 2019 Ali et al., 2020). Consistently, sesamin has been shown to suppress a series of inflammatory markers associated with type-II diabetes and rheumatoid arthritis in humans (Mohammad Shahi et al., 2017, Helli et al., 2019). Sesamin seems to exert its inhibitory effects on inflammation via epigenetic regulation as well as modulation of several signaling pathways involving ER (Hsieh et al., 2014), PPARα (Hsieh et al., 2014, Zhang et al., 2016), PPARγ (Majdalawieh and Ro, 2014), ERK-1/2 (Chung et al., 2010, Cui et al., 2010, Akl et al., 2012, Majdalawieh and Ro, 2014, Bai et al., 2019), p38 MAPK (Jeng et al., 2005, Hsieh et al., 2014, Lee et al., 2011, Xu et al., 2015, Li et al., 2016, Lin et al., 2019), JNK (Akl et al., 2012, Akl et al., 2013, Ma et al., 2014, Fan et al., 2017, Lin et al., 2019), COX-2 (Hsieh et al., 2011, Lin et al., 2019), and NF-κB (Jeng et al., 2005, Cui et al., 2010, Harikumar et al., 2010, Lee et al., 2011, Freise et al., 2012, Akl et al., 2012, Akl et al., 2013, Kong et al., 2014, Lin et al., 2014, Xu et al., 2015, Li et al., 2016, Fan et al., 2017, Lin et al., 2019). Detailed experimental data pertaining to the anti-inflammatory effects of sesamin as well as the underlying molecular mechanisms and signal transduction pathways involved in such regulation has been recently analyzed and reviewed (Majdalawieh et al., 2017). Hence, the anti-hypertensive, anti-atherogenic, anti-thrombotic, anti-diabetic, anti-obesity properties of sesamin can be attributed, at least in part, to its anti-inflammatory effects and its ability to regulate the inflammatory status.
8. Potential toxicity and adverse health effects of sesamin
The cytotoxic effects of sesamin are investigated by utilizing different toxicity tests. In an in vivo analysis of toxicity of sesamin, it is concluded that doses up to 280 mg/kg/day are safe and do not lead to cytotoxicity (Rogi et al., 2011). Hori and colleagues have evaluated the genotoxicity of sesamin through several tests, such as a bacterial reverse mutation assay (Ames test), a chromosomal aberration test in cultured Chinese hamster lung cells (CHL/IU), a bone marrow micronucleus (MN) test in Crlj:CD1 (ICR) mice, and a comet assay using the liver of Sprague-Dawley rats (Hori et al., 2011). Sesamin shows a negative result in the Ames test with and without S9 mix. Moreover, in the in vitro chromosomal aberration test, sesamin does not induce chromosomal aberrations without the presence of S9 mix. However, structural abnormalities are detected at cytotoxic concentrations in the presence S9 mix. The in vivo bone marrow MN test and comet assay indicate that sesamin has no genotoxic activity, as oral administration of up to 2 g/kg does not cause a significant increase in the percentage of micronucleated polychromatic erythrocytes or in the percent DNA in the comet tails (Hori et al., 2011). To further investigate the possible toxic effects of sesamin, cell viability tests are performed using acid phosphatase (ACP) activity assay in HepG2 and LS174T cells (Lim et al., 2012). HepG2 and LS174T cells are exposed to sesamin (10, 20, 30, and 40 µM) and cell viability is assessed. Sesamin causes mild cytotoxicity. However, even at the highest concentration, cell viability remains at 80%. Similarly, in another study, Hs68 cells (human skin fibroblasts) treated with 5, 10, 25, and 50 µM sesamin are shown to exhibit a 95% cell viability (Lin et al., 2019). The same study reveals that sesamin treatment for 10 weeks does not cause skin toxicity in BALB/cAnN.Cg-Foxn1nu/CrlNarl mice. Moreover, Wu and colleagues demonstrate that treatment of HAECs with up to 100 µM sesamin for 24 h does not impact cell viability (Wu et al., 2010). Pretreatment of human prostate cancer cell line (PC3) with sesamin for 1 hr also shows no toxic impact of sesamin (Xu et al., 2015). Sesamin is considered to be a low-moderate hazardous material. It is considered a methylenedioxybenzene synergist, which when swallowed may cause loss of appetite, vomiting, diarrhea, inflamed bowel with bleeding, bleeding from the lung, wasting, and possible central depression. Moreover, sesamin (originally known as piperonyl butoxide, PBO) can interfere with the metabolism of hormones, which may damage humeral organs such as the thyroid, adrenal, and pituitary glands. Sesamin can cause eye and skin irritation, and when inhaled, it may be irritating to the mucous membranes and upper respiratory tract. It is predicted that the oral lethal dose of sesamin in humans is 5.15 g/kg (Takahashi et al., 1994).
9. Proposed preclinical and clinical investigation
Considering the huge lack of preclinical and clinical studies pertaining to the effects of sesamin on CVD and its risk factors, we herein propose well-designed, randomized, placebo-controlled clinical studies that may aid in clinically establishing these links between the reported experimental anti-hypertensive, anti-atherogenic, anti-thrombotic, anti-diabetic, and anti-obesity effects of sesamin. In one investigation, age-matched patients of hypertension, atherosclerosis, thrombosis, type 2 diabetes, and/or obesity can be divided into five groups. Group I patients can receive one of the common drugs used to treat the corresponding medical condition (e.g. hypertension), group II can receive the common drug plus sesamin (50 mg per day for 8 weeks, oral), group III can receive the common drug plus sesamin (200 mg per day for 8 weeks, oral), group IV can receive the common drug plus sesamin (50 mg per day for 16 weeks, oral), while group V can receive the common drug plus sesamin (200 mg per day for 16 weeks, oral). The prognosis and clinical parameters of the corresponding medical condition in all patients can be evaluated during and after the clinical trial. We also propose a longitudinal prevention study to evaluate the potential preventive effects of sesamin against hypertension, atherosclerosis, thrombosis, type 2 diabetes, and/or obesity. To this end, age- and gender-matched healthy individuals (age > 50 years) can be divided into four groups. Group I subjects will receive a placebo (control) (1–2 years, oral), group II subjects will receive sesamin (50 mg per day for 1–2 years, oral), group III subjects will receive sesamin (100 mg per day for 1–2 years, oral), and group IV subjects will receive sesamin (200 mg per day for 1–2 years, oral). All participants will be closely and systematically monitored with regard to the clinical parameters of the corresponding medical condition. Such studies may be very helpful in understanding the role of sesamin in the prevention and treatment of the aforementioned medical conditions in preclinical and clinical settings.
10. Conclusions
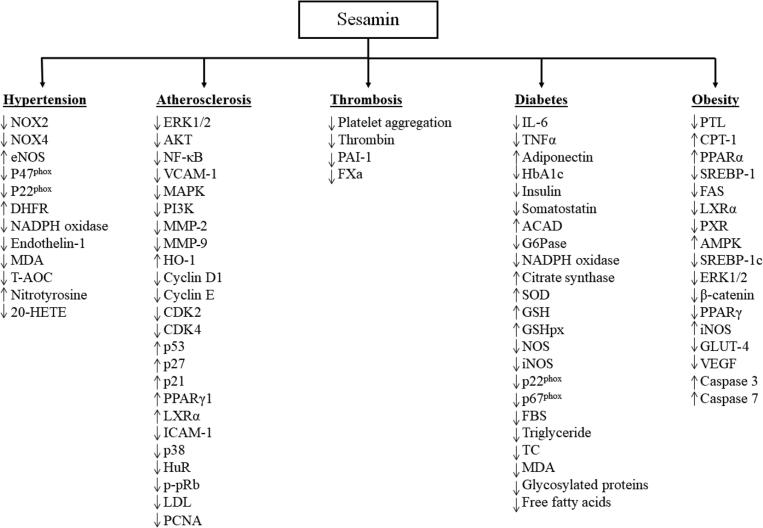
This paper demonstrates that sesamin induces its effects on hypertension, atherosclerosis, thrombosis, obesity, and diabetes through multiple pathways with the antioxidant and anti-inflammatory properties of sesamin being the predominant means that underlie these effects. Fig. 3 provides a summary of the main regulatory effects of sesamin on proteins and signaling factors involved in hypertension, atherosclerosis, thrombosis, diabetes, and obesity. By inhibiting the production of ROS, sesamin enhances NO bioactivity in blood vessels, therefore ameliorating endothelial dysfunction and hypertension, decreasing vascular inflammatory response, and altering the progression of atherosclerotic lesion formation and thrombosis. Sesamin can also impede the development of type-II diabetes by protecting pancreatic β-cells. Sesamin also regulates adipogenesis and obesity by inhibiting the absorption of fat from the gastrointestinal tract, increasing the activity of lipolytic enzymes, decreasing the activity of lipogenic enzymes, preventing the differentiation of preadipocytes into mature adipocytes, inducing apoptosis in mature adipocytes, and reducing lipid droplets in mature adipocytes. The reported effects of sesamin seems to be mediated via the RAS/MAPK, PI3K/AKT, ERK1/2, p38, p53, IL-6, TNFα, and NF-κB signaling pathways. Given that most studies were performed using multiple cell lines and animal models, further research has to be conducted using humans to better underscore the effectiveness of sesamin in combating CVD and its risk factors. It is well known that natural compounds can be effective adjuvants to therapy with fewer side effects than conventional therapies. Therefore, it would also be beneficial to determine clinically whether high doses of sesamin taken as supplements or as regular dietary doses are required to induce a therapeutic response.
Fig. 3.
A summary of the main regulatory effects of sesamin on proteins and signaling factors involved in hypertension, atherosclerosis, thrombosis, diabetes, and obesity. The upward-pointing arrows indicate positive regulation, while downward-pointing arrows indicate negative regulation.
Funding
We acknowledge the support by the Open Access Program from the American University of Sharjah. This paper represents the opinions of the authors and does not mean to represent the position or opinions of the American University of Sharjah.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahmad S., ElSherbiny N.M., Jamal M.S., Alzahrani F.A., Haque R., Khan R., Zaidi S.K., AlQahtani M.H., Liou G.I., Bhatia K. Anti-inflammatory role of sesamin in STZ induced mice model of diabetic retinopathy. J. Neuroimmunol. 2016;295–296:47–53. doi: 10.1016/j.jneuroim.2016.04.002. [DOI] [PubMed] [Google Scholar]
- Akl M.R., Ayoub N.M., Abuasal B.S., Kaddoumi A., Sylvester P.W. Sesamin synergistically potentiates the anticancer effects of γ-tocotrienol in mammary cancer cell lines. Fitoterapia. 2013;84:347–359. doi: 10.1016/j.fitote.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Akl M.R., Ayoub N.M., Sylvester P.W. Mechanisms mediating the synergistic anticancer effects of combined γ-tocotrienol and sesamin treatment. Planta Med. 2012;78(16):1731–1739. doi: 10.1055/s-0032-1315302. [DOI] [PubMed] [Google Scholar]
- Ali B.H., Al Salam S., Al Suleimani Y., Al Za'abi M., Ashique M., Manoj P., Sudhadevi M., Al Tobi M., Nemmar A. Ameliorative effect of sesamin in cisplatin-induced nephrotoxicity in rats by suppressing inflammation, oxidative/nitrosative stress, and cellular damage. Physiol. Res. 2020;69(1):61–72. doi: 10.33549/physiolres.934142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An J.B., Zhang R.J. Effects of sesamin on lipid metabolism in hyperlipidemia rats. J. Xi'an Jiaotong Univ. (Med. Sci.) 2010;31:67–70. [Google Scholar]
- Badmaev V., Hatakeyama Y., Yamazaki N., Noro A., Mohamed F., Ho C.T., Pan M.H. Preclinical and clinical effects of Coleus forskohlii, Salacia reticulata and Sesamum indicum modifying pancreatic lipase inhibition in vitro and reducing total body fat. J. Funct. Foods. 2015;15:44–51. [Google Scholar]
- Bai X., Gou X., Cai P., Xu C., Cao L., Zhao Z., Huang M., Jin J. Sesamin enhances Nrf2-mediated protective defense against oxidative stress and inflammation in colitis via AKT and ERK activation. Oxid Med Cell Longev. 2019;2019:2432416. doi: 10.1155/2019/2432416. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bigoniya P., Nishad R., Singh C.S. Preventive effect of sesame seed cake on hyperglycemia and obesity against high fructose-diet induced type 2 diabetes in rats. Food Chem. 2012;133(4):1355–1361. [Google Scholar]
- CDC, 2020. Facts about Hypertension. Centers for Disease Control and Prevention.
- CDC, 2020. Obesity is a Common, Serious, and Costly Disease. Centers for Disease Control and Prevention.
- Chinnala K.M., Elsani M.M., Mekala S.K. Evaluation of antiobesity activity of Sesamum indicum Linn. in high fat diet induced obesity in rats. Int. J. Phytopharmacol. 2014;5(3):179–182. [Google Scholar]
- Chung B.H., Lee J.J., Kim J.D., Jeoung D., Lee H., Choe J., Ha K.S., Kwon Y.G., Kim Y.M. Angiogenic activity of sesamin through the activation of multiple signal pathways. Biochemical and Biophysical Research Communications. 2010;391(1):254–260. doi: 10.1016/j.bbrc.2009.11.045. [DOI] [PubMed] [Google Scholar]
- Collins R.G., Velji R., Guevara N.V., Hicks M.J., Chan L., Beaudet A.L. P-selectin or intercellular adhesion molecule (ICAM)-1 deficiency substantially protects against atherosclerosis in apolipoprotein e-deficient mice. J. Exp. Med. 2000;191(1):189–194. doi: 10.1084/jem.191.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y., Hou X., Chen J., Xie L., Yang L., Le Y. Sesamin inhibits bacterial formylpeptide-induced inflammatory responses in a murine air-pouch model and in THP-1 human monocytes. J. Nutr. 2010;140(2):377–381. doi: 10.3945/jn.109.117804. [DOI] [PubMed] [Google Scholar]
- Cybulsky M.I., Iiyama K., Li H., Zhu S., Chen M., Iiyama M., Davis V., Gutierrez-Ramos J.C., Connelly P.W., Milstone D.S. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J. Clin. Investig. 2001;107(10):1255–1262. doi: 10.1172/JCI11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar A.A., Arumugam N. Lignans of sesame: purification methods, biological activities and biosynthesis - a review. Bioorg. Chem. 2013;50:1–10. doi: 10.1016/j.bioorg.2013.06.009. [DOI] [PubMed] [Google Scholar]
- Dhar P., Chattopadhyay K., Bhattacharyya D., Ghosh S. Antioxidative effect of sesame lignans in diabetes mellitus blood: An in vitro study. J. Oleo Sci. 2005;54(1):39–43. doi: 10.5650/jos.56.19. [DOI] [PubMed] [Google Scholar]
- Fan D., Yang Z., Yuan Y., Wu Q.Q., Xu M., Jin Y.G., Tang Q.Z. Sesamin prevents apoptosis and inflammation after experimental myocardial infarction by JNK and NF-κB pathways. Food Funct. 2017;8(8):2875–2885. doi: 10.1039/c7fo00204a. [DOI] [PubMed] [Google Scholar]
- Freise C., Querfeld U. The lignan (+)-episesamin interferes with TNF-α-induced activation of VSMC via diminished activation of NF-κB, ERK1/2 and AKT and decreased activity of gelatinases. Acta Physiol. 2014;213(3):642–652. doi: 10.1111/apha.12400. [DOI] [PubMed] [Google Scholar]
- Freise C., Sommer K., Querfeld U. Protective effects of the polyphenols (+)-episesamin and sesamin against PDGF-BB-induced activation of vascular smooth muscle cells are mediated by induction of haem oxygenase-1 and inhibition of mitogenic signalling. J. Funct. Foods. 2015;18:586–597. [Google Scholar]
- Freise C., Trowitzsch-Kienast W., Erben U., Seehofer D., Kim K.Y., Zeitz M., Reuhl M., Somasundaram R. (+)-Episesamin inhibits adipogenesis and exerts anti-inflammatory effects in 3T3-L1 (pre)adipocytes by sustained wnt signaling, down-regulation of PPARγ and induction of iNOS. J. Nutrit. Biochem. 2013;24(3):550–555. doi: 10.1016/j.jnutbio.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Freise C., Trowitzsch-Kienast W., Ruehl M., Erben U., Seehofer D., Kim K.Y., Zeitz M., Somasundaram R. (+)-Episesamin exerts anti-neoplastic effects in human hepatocellular carcinoma cell lines via suppression of nuclear factor-kappa B and inhibition of MMP-9. Invest. New Drugs. 2012;30(6):2087–2095. doi: 10.1007/s10637-011-9762-x. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y., Okamura Y., Iwamura M., Ikemoto S., Ono Y., Kiso Y., Seyama Y. Sesamin reduced blood glucose concentrations in Zucker fatty rat. Atheroscler. Suppl. 2006;7 454–454. [Google Scholar]
- Galkina E., Ley K. Vascular adhesion molecules in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2007;27(11):2292–2301. doi: 10.1161/ATVBAHA.107.149179. [DOI] [PubMed] [Google Scholar]
- Guan L.K., Wang S.I. Effects of sesamin on the plaques formation and mRNA expression of VCAM-1 in experimental models of rabbit atherosclerosis. Modern Prev. Med. 2009;15 [Google Scholar]
- Guan L.K., Wang S.I. Effects of sesamin on the formation of atheromatous plaque and the expression of VCAM-1 in atherosclerotic rabbit. Shandong Med. J. 2009;36 [Google Scholar]
- Han J.H., Lee S.G., Jung S.H., Lee J.J., Park H.S., Kim Y.H., Myung C.S. Sesamin inhibits PDGF-mediated proliferation of vascular smooth muscle cells by upregulating p21 and p27. J. Agric. Food. Chem. 2015;63(33):7317–7325. doi: 10.1021/acs.jafc.5b03374. [DOI] [PubMed] [Google Scholar]
- Harikumar K.B., Sung B., Tharakan S.T., Pandey M.K., Joy B., Guha S., Krishnan S., Aggarwal B.B. Sesamin manifests chemopreventive effects through the suppression of NF-kappa B-regulated cell survival, proliferation, invasion, and angiogenic gene products. Mol. Cancer Res. 2010;8(5):751–756. doi: 10.1158/1541-7786.MCR-09-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helli B., Mowla K., Mohammadshahi M., Jalali M.T. Effect of sesamin supplementation on cardiovascular risk factors in women with rheumatoid arthritis. J. Am. Coll. Nutr. 2016;35(4):300–307. doi: 10.1080/07315724.2015.1005198. [DOI] [PubMed] [Google Scholar]
- Helli B., Shahi M.M., Mowla K., Jalali M.T., Haghighian H.K. A randomized, triple-blind, placebo-controlled clinical trial, evaluating the sesamin supplement effects on proteolytic enzymes, inflammatory markers, and clinical indices in women with rheumatoid arthritis. Phytother. Res. 2019;33(9):2421–2428. doi: 10.1002/ptr.6433. [DOI] [PubMed] [Google Scholar]
- Hemalatha S., Ghafoorunissa S.A. Lignans and tocopherols in Indian sesame cultivars. J. Am. Oil Chem. Soc. 2004;81:467–470. [Google Scholar]
- Hirata F., Fujita K., Ishikura Y., Hosoda K., Ishikawa T., Nakamura H. Hypocholesterolemic effect of sesame lignan in humans. Atherosclerosis. 1996;122(1):135–136. doi: 10.1016/0021-9150(95)05769-2. [DOI] [PubMed] [Google Scholar]
- Hong L., Yi W., Liangliang C., Juncheng H., Qin W., Xiaoxiang Z. Hypoglycaemic and hypolipidaemic activities of sesamin from sesame meal and its ability to ameliorate insulin resistance in KK-Ay mice. J. Sci. Food Agric. 2012;93(8):1833–1838. doi: 10.1002/jsfa.5974. [DOI] [PubMed] [Google Scholar]
- Hoopes S.L., Garcia V., Edin M.L., Schwartzman M.L., Zeldin D.C. Vascular actions of 20-HETE. Prostaglandins Other Lipid Mediat. 2015;120:9–16. doi: 10.1016/j.prostaglandins.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori H., Takayanagi T., Kamada Y., Shimoyoshi S., Ono Y., Kitagawa Y., Shibata H., Nagao M., Fujii W., Sakakibara Y. Genotoxicity evaluation of sesamin and episesamin. Mutat. Res. 2011;719(1–2):21–28. doi: 10.1016/j.mrgentox.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Houston M. Nutrition and nutraceutical supplements for the treatment of hypertension: Part I. J. Clin. Hypertension. 2013;15(10):752–757. doi: 10.1111/jch.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh C.C., Kuo C.H., Kuo H.F., Chen Y.S., Wang S.L., Chao D., Lee M.S., Hung C.H. Sesamin suppresses macrophage-derived chemokine expression in human monocytes via epigenetic regulation. Food Funct. 2014;5(10):2494–2500. doi: 10.1039/c4fo00322e. [DOI] [PubMed] [Google Scholar]
- Hsieh P.F., Hou C.W., Yao P.W., Wu S.P., Peng Y.F., Shen M.L., Lin C.H., Chao Y.Y., Chang M.H., Jeng K.C. Sesamin ameliorates oxidative stress and mortality in kainic acid-induced status epilepticus by inhibition of MAPK and COX-2 activation. J. Neuroinflammation. 2011;8:57. doi: 10.1186/1742-2094-8-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide T., Ashakumary L., Takahashi Y., Kushiro M., Fukuda N., Sugano M. Sesamin, a sesame lignan, decreases fatty acid synthesis in rat liver accompanying the down-regulation of sterol regulatory element binding protein-1. BBA. 2001;1534(1):1–13. doi: 10.1016/s1388-1981(01)00167-6. [DOI] [PubMed] [Google Scholar]
- Ishibashi S., Goldstein J.L., Brown M.S., Herz J., Burns D.K. Massive xanthomatosis and atherosclerosis in cholesterol-fed low density lipoprotein receptor-negative mice. J. Clin. Invest. 1994;93:1885–1893. doi: 10.1172/JCI117179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeng K.C.G., Hou R.C.W. Sesamin and sesamolin: nature’s therapeutic lignans. Curr. Enzym. Inhib. 2005;1:11–20. [Google Scholar]
- Jeng K.C., Hou R.C., Wang J.C., Ping L.I. Sesamin inhibits lipopolysaccharide-induced cytokine production by suppression of p38 mitogen-activated protein kinase and nuclear factor-kappaB. Immunol. Lett. 2005;97(1):101–106. doi: 10.1016/j.imlet.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Katsuda S., Kaji T. Atherosclerosis and extracellular matrix. J. Atherosclerosis Thrombosis. 2003;10(5):267–274. doi: 10.5551/jat.10.267. [DOI] [PubMed] [Google Scholar]
- Kinugasa C., Naemura A., Hyodo K., Nakai Y., Katsuta M., Yamamoto J. Experimental antithrombotic effects of sesame seed whole grains and extracts. Blood Coagul. Fibrinolysis. 2011;22(6):526–531. doi: 10.1097/MBC.0b013e328347b085. [DOI] [PubMed] [Google Scholar]
- Kita S., Matsumura Y., Morimoto S., Akimoto K., Furuya M., Oka N., Tanaka T. Antihypertensive effect of sesamin. II. Protection against two-kidney, one-clip renal hypertension and cardiovascular hypertrophy. Biol. Pharm. Bull. 1995;18(9):1283–1285. doi: 10.1248/bpb.18.1283. [DOI] [PubMed] [Google Scholar]
- Kitagawa K., Matsumoto M., Sasaki T., Hashimoto H., Kuwabara K., Ohtsuki T., Hori M. Involvement of ICAM-1 in the progression of atherosclerosis in APOE-knockout mice. Atherosclerosis. 2002;160(2):305–310. doi: 10.1016/s0021-9150(01)00587-1. [DOI] [PubMed] [Google Scholar]
- Kong X., Li W., Guo L., Zhang J., Chen X., Liu W., Yang J. Sesamin enhances nitric oxide bioactivity in aortas of spontaneously hypertensive rats. Therapeutic Adv. Cardiovasc. Dis. 2015;9(5):314–324. doi: 10.1177/1753944715586178. [DOI] [PubMed] [Google Scholar]
- Kong X., Ma M.Z., Zhang Y., Weng M.Z., Gong W., Guo L.Q., Zhang J.X., Wang G.D., Su Q., Quan Z.W., Yang J.R. Differentiation therapy: sesamin as an effective agent in targeting cancer stem-like side population cells of human gallbladder carcinoma. BMC Complement. Altern. Med. 2014;14:254. doi: 10.1186/1472-6882-14-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X., Wang G.D., Ma M.Z., Deng R.Y., Guo L.Q., Zhang J.X., Yang J.R., Su Q. Sesamin ameliorates advanced glycation end products-induced pancreatic β-cell dysfunction and apoptosis. Nutrients. 2015;7(6):4689–4704. doi: 10.3390/nu7064689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X., Yang J.R., Guo L.Q., Xiong Y., Wu X.Q., Huang K., Zhou Y. Sesamin improves endothelial dysfunction in renovascular hypertensive rats fed with a high-fat, high-sucrose diet. Eur. J. Pharmacol. 2009;620(1–3):84–89. doi: 10.1016/j.ejphar.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Ku S.K., Kim J.A., Han C.K., Bae J.S. Antithrombotic activities of epi-sesamin in vitro and in vivo. Am. J. Chin. Med. 2013;41(6):1313–1327. doi: 10.1142/S0192415X13500882. [DOI] [PubMed] [Google Scholar]
- Lee C.C., Chen P.R., Lin S., Tsai S.C., Wang B.W., Chen W.W., Tsai C.E., Shyu K.G. Sesamin induces nitric oxide and decreases endothelin-1 production in HUVECs: Possible implications for its antihypertensive effect. J. Hypertens. 2004;22(12):2329–2338. doi: 10.1097/00004872-200412000-00015. [DOI] [PubMed] [Google Scholar]
- Lee C.C., Liu K.J., Wu Y.C., Lin S.J., Chang C.C., Huang T.S. Sesamin inhibits macrophage-induced vascular endothelial growth factor and matrix metalloproteinase-9 expression and proangiogenic activity in breast cancer cells. Inflammation. 2011;34(3):209–221. doi: 10.1007/s10753-010-9226-z. [DOI] [PubMed] [Google Scholar]
- Lei H., Han J., Wang Q., Guo S., Sun H., Zhang X. Effects of sesamin on streptozotocin (STZ)-induced NIT-1 pancreatic β-cell damage. Int. J. Mol. Sci. 2012;13(12):16961–16970. doi: 10.3390/ijms131216961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K., Huo Y. VCAM-1 is critical in atherosclerosis. J. Clin. Investig. 2001;107(10):1209–1210. doi: 10.1172/JCI13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Piao H., Zheng M., Jin Z., Zhao L., Yan G. Sesamin attenuates allergic airway inflammation through the suppression of nuclear factor-kappa B activation. Exp Ther Med. 2016;12(6):4175–4181. doi: 10.3892/etm.2016.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Gao Y., Li S., Yang J. Effect of sesamin on pulmonary vascular remodeling in rats with monocrotaline-induced pulmonary hypertension. Zhongguo Zhong Yao Za Zhi. 2015;40(7):1355–1361. [PubMed] [Google Scholar]
- Li Z., Li L., Zielke H.R., Cheng L., Xiao R., Crow M.T., Stetler-Stevenson W.G., Froehlich J., Lakatta E.G. Increased expression of 72-kd type IV collagenase (MMP-2) in human aortic atherosclerotic lesions. Am. J. Pathol. 1996;148(1):121–128. [PMC free article] [PubMed] [Google Scholar]
- Liang Y.T., Chen J., Jiao R., Peng C., Zuo Y., Lei L., Liu Y., Wang X., Ma M.Y., Huang Y., Chen Z.Y. Cholesterol-lowering activity of sesamin is associated with down-regulation on genes of sterol transporters involved in cholesterol absorption. J. Agric. Food. Chem. 2015;63(11):2963–2969. doi: 10.1021/jf5063606. [DOI] [PubMed] [Google Scholar]
- Lim Y.P., Ma C.Y., Liu C.L., Lin Y.H., Hu M.L., Chen J.J., Hung D.Z., Hsieh W.T., Huang J.D. Sesamin: a naturally occurring lignan inhibits CYP3A4 by antagonizing the pregnane X receptor activation. Evid Based Complem. Alternat. Med. 2012;2012 doi: 10.1155/2012/242810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.H., Shen M.L., Zhou N., Lee C.C., Kao S.T., Wu D.C. Protective effects of the polyphenol sesamin on allergen-induced T(H)2 responses and airway inflammation in mice. PLoS ONE. 2014;9(4) doi: 10.1371/journal.pone.0096091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T.Y., Wu P.Y., Hou C.W., Chien T.Y., Chang Q.X., Wen K.C., Lin C.Y., Chiang H.M. Protective effects of sesamin against UVB-induced skin inflammation and photodamage in vitro and in vivo. Biomolecules. 2019;9(9):479. doi: 10.3390/biom9090479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Saarinen N.M., Thompson L.U. Sesamin is one of the major precursors of mammalian lignans in sesame seed (Sesamum indicum) as observed in vitro and in rats. J. Nutr. 2006;136(4):906–912. doi: 10.1093/jn/136.4.906. [DOI] [PubMed] [Google Scholar]
- Liu N., Wu C., Sun L., Zheng J., Guo P. Sesamin enhances cholesterol efflux in RAW264.7 macrophages. Molecules. 2014;19(6):7516–7527. doi: 10.3390/molecules19067516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loke W.M., Proudfoot J.M., Hodgson J.M., McKinley A.J., Hime N., Magat M., Stocker R., Croft K.D. Specific dietary polyphenols attenuate atherosclerosis in apolipoprotein E-knockout mice by alleviating inflammation and endothelial dysfunction. Arterioscler. Thromb. Vasc. Biol. 2010;30(4):749–757. doi: 10.1161/ATVBAHA.109.199687. [DOI] [PubMed] [Google Scholar]
- Ma J.Q., Ding J., Zhang L., Liu C.M. Hepatoprotective properties of sesamin against CCl4 induced oxidative stress-mediated apoptosis in mice via JNK pathway. Food Chem. Toxicol. 2014;64:41–48. doi: 10.1016/j.fct.2013.11.017. [DOI] [PubMed] [Google Scholar]
- Mackman N. Triggers, targets and treatments for thrombosis. Nature. 2008;451(7181):914–918. doi: 10.1038/nature06797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madamanchi N.R., Hakim Z.S., Runge M.S. Oxidative stress in atherogenesis and arterial thrombosis: The disconnect between cellular studies and clinical outcomes. J. Thromb. Haemost. 2005;3(2):254–267. doi: 10.1111/j.1538-7836.2004.01085.x. [DOI] [PubMed] [Google Scholar]
- Mahendra Kumar C., Singh S.A. Bioactive lignans from sesame (Sesamum indicum L.): Evaluation of their antioxidant and antibacterial effects for food applications. J. Food Sci. Technol. 2015;52(5):2934–2941. doi: 10.1007/s13197-014-1334-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdalawieh A.F., Dalibalta S., Yousef S.M. Effects of sesamin on fatty acid and cholesterol metabolism, macrophage cholesterol homeostasis and serum lipid profile: A comprehensive review. Eur. J. Pharmacol. 2020;885 doi: 10.1016/j.ejphar.2020.173417. [DOI] [PubMed] [Google Scholar]
- Majdalawieh A.F., Massri M., Nasrallah G.K. A comprehensive review on the anti-cancer properties and mechanisms of action of sesamin, a lignan in sesame seeds (Sesamum indicum) Eur. J. Pharmacol. 2017;815:512–521. doi: 10.1016/j.ejphar.2017.10.020. [DOI] [PubMed] [Google Scholar]
- Majdalawieh A.F., Ro H.S. PPARγ1 and LXRα face a new regulator of macrophage cholesterol homeostasis and inflammatory responsiveness, AEBP1. Nucl. Receptor Signaling. 2010;8 doi: 10.1621/nrs.08004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdalawieh A.F., Ro H.S. The anti-atherogenic properties of sesamin are mediated via improved macrophage cholesterol efflux through PPARγ1-LXRα and MAPK signaling. Int. J. Vitam. Nutr. Res. 2014;84(1–2):79–91. doi: 10.1024/0300-9831/a000195. [DOI] [PubMed] [Google Scholar]
- Majdalawieh A.F., Ro H.S. Sesamol and sesame (Sesamum indicum) oil enhance macrophage cholesterol efflux via up-regulation of PPARγ1 and LXRα transcriptional activity in a MAPK-dependent manner. Eur. J. Nutr. 2015;54(5):691–700. doi: 10.1007/s00394-014-0747-3. [DOI] [PubMed] [Google Scholar]
- Matsumura Y., Kita S., Morimoto S., Akimoto K., Furuya M., Oka N., Tanaka T. Antihypertensive effect of sesamin. I. Protection against deoxycorticosterone acetate-salt-induced hypertension and cardiovascular hypertrophy. Biol. Pharm. Bull. 1995;18(7):1016–1019. doi: 10.1248/bpb.18.1016. [DOI] [PubMed] [Google Scholar]
- Matsumura Y., Kita S., Ohgushi R., Okui T. Effects of sesamin on altered vascular reactivity in aortic rings of deoxycorticosterone acetate-salt-induced hypertensive rat. Biol. Pharm. Bull. 2000;23(9):1041–1045. doi: 10.1248/bpb.23.1041. [DOI] [PubMed] [Google Scholar]
- Matsumura Y., Kita S., Tanida Y., Taguchi Y., Morimoto S., Akimoto K., Tanaka T. Antihypertensive effect of sesamin. III. Protection against development and maintenance of hypertension in stroke-prone spontaneously hypertensive rats. Biol. Pharm. Bull. 1998;21(5):469–473. doi: 10.1248/bpb.21.469. [DOI] [PubMed] [Google Scholar]
- McCarty M. Hepatothermic therapy of obesity: rationale and an inventory of resources. Med. Hypotheses. 2001;57(3):324–336. doi: 10.1054/mehy.2000.1322. [DOI] [PubMed] [Google Scholar]
- Miyawaki T., Aono H., Toyoda-Ono Y., Maeda H., Kiso Y., Moriyama K. Antihypertensive effects of sesamin in humans. J. Nutr. Sci. Vitaminol. 2009;55(1):87–91. doi: 10.3177/jnsv.55.87. [DOI] [PubMed] [Google Scholar]
- Mohammad Shahi M., Zakerzadeh M., Zakerkish M., Zarei M., Saki A. Effect of sesamin supplementation on glycemic status, inflammatory markers, and adiponectin levels in patients with type 2 diabetes mellitus. J. Dietary Suppl. 2017;14(1):65–75. doi: 10.1080/19390211.2016.1204404. [DOI] [PubMed] [Google Scholar]
- Nakai M., Harada M., Nakahara K., Akimoto K., Shibata H., Miki W., Kiso Y. Novel antioxidative metabolites in rat liver with ingested sesamin. J. Agric. Food. Chem. 2003;51(6):1666–1670. doi: 10.1021/jf0258961. [DOI] [PubMed] [Google Scholar]
- Nakano D., Itoh C., Ishii F., Kawanishi H., Takaoka M., Kiso Y., Tsuruoka N., Tanaka T., Matsumura Y. Effects of sesamin on aortic oxidative stress and endothelial dysfunction in deoxycorticosterone acetate-salt hypertensive rats. Biol. Pharm. Bull. 2003;26(12):1701–1705. doi: 10.1248/bpb.26.1701. [DOI] [PubMed] [Google Scholar]
- Nakano D., Itoh C., Takaoka M., Kiso Y., Tanaka T., Matsumura Y. Antihypertensive effect of sesamin. IV. Inhibition of vascular superoxide production by sesamin. Biol. Pharm. Bull. 2002;25(9):1247–1249. doi: 10.1248/bpb.25.1247. [DOI] [PubMed] [Google Scholar]
- Nakano D., Kurumazuka D., Nagai Y., Nishiyama A., Kiso Y., Matsumura Y. Dietary sesamin suppresses aortic NADPH oxidase in DOCA salt hypertensive rats. Clin. Exp. Pharmacol. Physiol. 2008;35(3):324–326. doi: 10.1111/j.1440-1681.2007.04817.x. [DOI] [PubMed] [Google Scholar]
- Nakano D., Kwak C.J., Fujii K., Ikemura K., Satake A., Ohkita M., Takaoka M., Ono Y., Nakai M., Tomimori N., Kiso Y., Matsumura Y. Sesamin metabolites induce an endothelial nitric oxide-dependent vasorelaxation through their antioxidative property-independent mechanisms: Possible involvement of the metabolites in the antihypertensive effect of sesamin. J. Pharmacol. Exp. Therapeut. 2006;318(1):328–335. doi: 10.1124/jpet.105.100149. [DOI] [PubMed] [Google Scholar]
- Nakano D., Takaoka M., Kiso Y., Matsumura Y. Antihypertensive effect of sesamin. Vas. Dis. Prevent. 2004;1:233–241. [Google Scholar]
- Narasimhulu C.A., Selvarajan K., Litvinov D., Parthasarathy S. Anti-atherosclerotic and anti-inflammatory actions of sesame oil. J. Med. Food. 2015;18(1):11–20. doi: 10.1089/jmf.2014.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T., Ikeda K., Sasaki Y., Yamamoto J., Seki J., Yamagata K., Nara Y., Hara H., Kakuta H., Yamori Y. Effects of vitamin E and sesamin on hypertension and cerebral thrombogenesis in stroke-prone spontaneously hypertensive rats. Hypertens. Res. 2001;24(6):735–742. doi: 10.1291/hypres.24.735. [DOI] [PubMed] [Google Scholar]
- Oguntibeju O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019;11(3):45–63. [PMC free article] [PubMed] [Google Scholar]
- Pahwa, R., Jialal, I., 2020. Atherosclerosis. StatPearls Publishing. Treasure Island (FL). Available from: https://www.ncbi.nlm.nih.gov/books/NBK507799/. [PubMed]
- Peñalvo J.L., Heinonen S.M., Aura A.M., Adlercreutz H. Dietary sesamin is converted to enterolactone in humans. J. Nutr. 2005;135(5):1056–1062. doi: 10.1093/jn/135.5.1056. [DOI] [PubMed] [Google Scholar]
- Plump A.S., Smith J.D., Hayek T., Aalto-Setala K., Walsh A., Verstuyft J.G., Rubin E.M., Breslow J.L. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- Raeisi-Dehkordi H., Mohammadi M., Moghtaderi F., Salehi-Abargouei A. Do sesame seed and its products affect body weight and composition? A systematic review and meta-analysis of controlled clinical trials. J. Funct. Foods. 2018;49:324–332. [Google Scholar]
- RogiT T.N., Ono Y., Kiso Y. The mechanism underlying the synergetic hypocholesterolemic effect of sesamin and α-tocopherol in rats fed a high-cholesterol diet. J. Pharmacol. Sci. 2011;115(3):408–416. doi: 10.1254/jphs.10287fp. [DOI] [PubMed] [Google Scholar]
- Rousta A.M., Mirahmadi S.M., Shahmohammadi A., Nourabadi D., Khajevand-Khazaei M.R., Baluchnejadmojarad T., Roghani M. Protective effect of sesamin in lipopolysaccharide-induced mouse model of acute kidney injury via attenuation of oxidative stress, inflammation, and apoptosis. Immunopharmacol. Immunotoxicol. 2018;40(5):423–429. doi: 10.1080/08923973.2018.1523926. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis - an inflammatory disease. N. Engl. J. Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Sala D., Zorzano A. Impact of type 2 diabetes on skeletal muscle mass and quality. In: Mauricio D., editor. Molecular Nutrition and Diabetes. Academic Press; 2016. pp. 73–85. [Google Scholar]
- Sayhan M.B., Oguz S., Salt Ö., Can N., Ozgurtas T., Yalta T.D. Sesamin ameliorates mucosal tissue injury of mesenteric ischemia and reperfusion in an experimental rat model. Arch. Med. Sci. 2019;15(6):1582–1588. doi: 10.5114/aoms.2017.68535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai T.S., Tien N., Shen H.Y., Chu F.Y., Wang C.C.N., Lu C.H., Yu H.I., Kung F.P., Chuang H.H., Lee Y.R., Chang H.Y., Lim Y.P. Sesamin, a naturally occurring lignan, inhibits ligand-induced lipogenesis through interaction with liver x receptor alpha (LXRα) and pregnane x receptor (PXR) Evidence-Based Complemen. Alternat. Med. 2019;2019:1–17. doi: 10.1155/2019/9401648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada S., Kinugawa S., Matsushimam S., Takemoto D., Furihata T., Mizushima W., Fukushima A., Yokota T., Ono Y., Shibata H., Okita K., Tsutsui H. Sesamin prevents decline in exercise capacity and impairment of skeletal muscle mitochondrial function in mice with high-fat diet-induced diabetes. Exp. Physiol. 2015;100(11):1319–1330. doi: 10.1113/EP085251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi O., Oishi S., Fujitani T., Tanaka T., Yoneyama M. Chronic toxicity studies of piperonyl butoxide in F344 rats: induction of hepatocellular carcinoma. Fundam. Appl. Toxicol. 1994;22(2):293–303. doi: 10.1006/faat.1994.1033. [DOI] [PubMed] [Google Scholar]
- Thuy T.D., Phan N.N., Wang C.Y., Yu H.G., Wang S.Y., Huang P.L., Do Y.Y., Lin Y.C. Novel therapeutic effects of sesamin on diabetes-induced cardiac dysfunction. Mol. Med. Rep. 2017;15(5):2949–2956. doi: 10.3892/mmr.2017.6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wågsäter D., Zhu C., Björkegren J., Skogsberg J., Eriksson P. MMP-2 and MMP-9 are prominent matrix metalloproteinases during atherosclerosis development in the Ldlr-/-Apob100/100 mouse. Int. J. Mol. Med. 2011;28(2):247–253. doi: 10.3892/ijmm.2011.693. [DOI] [PubMed] [Google Scholar]
- Wu J.H.Y., Hodgson J.M., Clarke M.W., Indrawan A.P., Barden A.E., Puddey I.B., Croft K.D. Inhibition of 20-hydroxyeicosatetraenoic acid synthesis using specific plant lignans: In vitro and human studies. Hypertension. 2009;54(5):1151–1158. doi: 10.1161/HYPERTENSIONAHA.109.139352. [DOI] [PubMed] [Google Scholar]
- Wu W.H., Wang S.H., Kuan I.I., Kao Y.S., Wu P.J., Liang C.J., Chien H.F., Kao C.H., Huang C.J., Chen Y.L. Sesamin attenuates intercellular cell adhesion molecule-1 expression in vitro in TNF-alpha-treated human aortic endothelial cells and in vivo in apolipoprotein-E-deficient mice. Mol. Nutr. Food Res. 2010;54(9):1340–1350. doi: 10.1002/mnfr.200900271. [DOI] [PubMed] [Google Scholar]
- Xu P., Cai F., Liu X., Guo L. Sesamin inhibits lipopolysaccharide-induced proliferation and invasion through the p38-MAPK and NF-κB signaling pathways in prostate cancer cells. Oncol. Rep. 2015;33(6):3117–3123. doi: 10.3892/or.2015.3888. [DOI] [PubMed] [Google Scholar]
- Yuliana N.D., Iqbal M., Jahangir M., Wijaya C.H., Korthout H., Kottenhage M., Kim H.K., Verpoorte R. Screening of selected Asian spices for anti obesity-related bioactivities. Food Chem. 2011;126(4):1724–1729. doi: 10.1016/j.foodchem.2010.12.066. [DOI] [PubMed] [Google Scholar]
- Zakerzadeh M., Mohammadshahi M., Zakerkish M., Saki A. The effects of sesamin supplementation on serum levels of inflammatory markers in type 2 diabetes mellitus; a double blinded, randomized clinical trial patients with. Nutr. Food Sci. Res. 2014;1(1) 268–268. [Google Scholar]
- Zhang J., Yang J., Chen G., Tang L., Li W., Yang H., Kong X. Sesamin ameliorates arterial dysfunction in spontaneously hypertensive rats via downregulation of NADPH oxidase subunits and upregulation of eNOS expression. Acta Pharmacol. Sin. 2013;34(7):912–920. doi: 10.1038/aps.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Yu Y., Hu S., Zhang J., Yang H., Han B., Cheng Y., Luo X. Sesamin ameliorates hepatic steatosis and inflammation in rats on a high-fat diet via LXRα and PPARα. Nutr. Res. 2016;36(9):1022–1030. doi: 10.1016/j.nutres.2016.06.015. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Wang Q., Jia M., Fu S., Pan J., Chu C., Liu X., Liu X., Liu Z. (+)-Sesamin attenuates chronic unpredictable mild stress-induced depressive-like behaviors and memory deficits via suppression of neuroinflammation. J. Nutr. Biochem. 2019;64:61–71. doi: 10.1016/j.jnutbio.2018.10.006. [DOI] [PubMed] [Google Scholar]