Abstract
Replacing sucrose with non-caloric sweeteners is an approach to avoid overweight and diabetes development. Non-caloric sweeteners are classified into either artificial as sucralose or natural as stevia. Both of them have been approved by FDA, but the effects of their chronic consumption are controversial. The present study aimed to evaluate the effects of these two sweeteners, in male and female albino mice, on different blood biochemical parameters, enzymes activities and immunological parameters after 8 and 16 weeks of sweeteners administration. 40.5 mg/ml of sucrose, 5.2 mg/ml of sucralose and 4.2 mg/ml of stevia were dissolved individually in distilled water. Mice were administrated by sweetener's solution for 5 h daily. Male and female mice showed a preference for water consumption with sucralose or stevia. Both of the two sweeteners significantly reduced the hemoglobin level, HCT%, RBCs and WBCs count. After 18 weeks, significant elevations in liver and kidney function enzymes were observed in male and female mice administrated with both non-caloric sweeteners. Histopathological examination in sucralose and stevia administrated groups confirmed the biochemical results; where it revealed a severe damage in liver and kidney sections. While, sucrose administration elevated, only, the levels of ALT, AST and cholesterol in male mice. A vigorous elevation in levels of different immunoglobulin (IgG, IgE and IgA) and pro-inflammatory cytokines (IL-6 and -8), that was accompanied by a significant reduction in level of anti-inflammatory cytokine IL-10, was observed in male and female mice groups administrated with sucralose or stevia. On the other hand, sucrose administration led to an elevation in IgA and reduction in IL-10 levels.
Keywords: Sucrose, Sucralose, Stevia, ALT, AST, Urea, Creatinine, Cytokines, Immunoglobulins
1. Introduction
Sucrose, a disaccharide composed of the monosaccharides fructose and glucose, constitutes 99.7% of normal table sugar. It is not essential in nutrition due to its lack of vitamins and minerals (Mathlouthi and Reiser, 1995). Excessive consumption of sucrose promotes obesity and type II diabetes (Amchra et al., 2018). High consumption of sucrose in drinks and diets has adverse effects on body weight; and may cause other health problems as diabetes and cardiovascular disease (Johnson et al., 2007). Moreover, animal model experiments showed that high consumption of sucrose leads to hyperinsulinemia, hyperglycemia, hypertension, and insulin resistance (Amchra et al., 2018). The American Heart Association limited sugar intake to 30 g (100 calories) per day for average-sized women and 45 g (150 calories) for average-sized men (Mitka, 2009). The last decade saw an intensive elevation in the number of food products that contains non-caloric sweeteners in order to overcome health problems associated with obesity and diabetes. And many research studies have focused on sweetener consumption in obese and diabetic patients. In both cases, the main purpose was to reduce the caloric intake in their usual diet (Gardner et al., 2012).
The increasing ratio of individuals suffering from chronic diseases as diabetes and obesity increased the importance of sugar substitutes as an alternative to sucrose. Sugar substitutes are food additives that duplicate the sweeten taste of sucrose with less or no calorie. It is classified into artificial sweeteners as sucralose, saccharine, aspartame and cyclamate; and natural sweeteners as stevia (Tandel, 2011). The five artificial sweeteners, saccharin, acesulfame, aspartame, neotame, and sucralose, have been approved by FDA. Stevia, a natural low-calorie sweetener, has also been approved (Mattes and Popkin, 2009). The human body responds to these sweeteners in a very complex way. The sweetness of non-nutritive sweeteners is more potent than that of sucrose or high-fructose corn syrup. Their frequent use over stimulates sugar receptors and in turn limits the tolerance for less intensely sweet foods, as fruit, and unsweet foods, as vegetables (Black et al., 1993). Animal studies showed that artificial sweeteners are addictive (Yang, 2010). Lenoir et al. (2007) reported that the intense sweetness of saccharine can surpass cocaine reward, even in drug-sensitized and addicted rats. The study of Mathur et al. (2020) reported that people with type II diabetes who consumed artificial sweeteners had a higher insulin resistance than others. Also, Bueno-Hernández et al. (2019) found that artificial sweeteners, depending on dose and duration of consumption, have a pro-inflammatory effect combined with gastrointestinal disorders. They related that to the elevation of gastrointestinal hormones which control the motility of intestine. Emamat et al. (2020) added that artificial sweeteners had an important role in alteration of intestinal microbiota and dysbiosis.
The use of artificial sweeteners, as sucralose, has been increased due to the health problems related to sucrose. Sucralose, 1,6-dichloro-1,6-dideoxy-β-D-fructofuranosyl-4-chloro-4-deoxy-α-D-galactopyranoside, is a synthetic disaccharide that is produced from sucrose when three hydroxyl groups are replaced by three chlorine atoms. Sucralose has 385–650 more sweetness than sucrose depending upon the specific food application (Schiffman et al., 2008). According to U.S. Food and Drug Administration, the acceptable daily intake (ADI) level for sucralose is 5 mg/kg per day (U.S. FDA, 1998); and according to Scientific Committee on Food of the European Commission, DAI is 15 mg/kg/d (SCF, 2000). Also, sucralose usage is permitted in pregnancy, nursing, with children and patients with medical conditions (Schiffman and Rother, 2013).
However, sucralose chronic consumption has adverse health effects that make its use a subject of controversy (Marti et al., 2008, Greenhill, 2020, Sylvetsky et al., 2020). The hazardous effects of artificial sweeteners have directed the consumers towards natural sweeteners as stevia. Stevia is a sweet glycosides extracted from Stevia rebaudiana (Arumugam et al., 2020). Stevia extract has been used as a sweetener and traditional medicine for several hundred years by local people in South America. Stevia extracts contains a natural non-caloric sweetener known as steviol glycosides. Stevioside (5–10% of total dry weight) and rebaudiauside A (2–4% of total dry weight) are the main steviol glycosides isolated from stevia leaves. Stevioside and rebaudioside A are 250–450 times sweeter than sucrose (Crammer and Ikan, 1986).
The present study aims to evaluate the effects of sucralose as an artificial sweetener and stevia as a natural one on blood biochemical parameters, enzyme activities and immunological parameters in experimental male and female albino mice. Furthermore, liver and kidney sections from all experimental groups will be subjected to histopathological examination. Their effects will be compared to those of sucrose to evaluate their safeness as sugar substitutes.
2. Materials and methods
2.1. Animals
The study was conducted on male and female BALB/c albino mice, 6 weeks age and 18–20 g weight, purchased from Theodor Bilharz Research Institute (TBRI), Giza, Egypt. Mice were hosed in individual cages and allowed to acclimatize for one week, in the animal house environment, before running out the experiment. Experimental protocols were carried out according to the international care and use of laboratory animals’ guidelines and approved by the Institutional Animal Care and Use Committee (IACUC); Cairo University, Faculty of Science, Egypt (CU I F 80 18). Mice groups were maintained under controlled temperature, 21 ± 2 °C, and on 12/12 h light/dark cycle. Standard rat diet (18% crude protein, 5% crude oil, 54% carbohydrates, vitamins, salts and minerals) was allowed ad libitum through the entire experiment.
2.2. Sweeteners
Dose calculation typically requires close attention because of the pharmacokinetics and pharmacodynamic variations between organisms. During the study, allometric scaling let us exchange doses between species. It is usually used for the conversion of doses among species and not within species. It is an empirical approach where drug dose exchange is based on dose-to-body surface normalization. This approach suggests that anatomic, physiologic and biochemical mechanisms between species have some special characteristic when an allometric scale is used for potential differences in the pharmacokinetic/physiological time (Chaturvedi et al., 2001, Rhomberg and Lewandowski, 2006). In this study, Animal equivalent dose (AED) was calculated on the basis of body surface area by multiplying the human dose (mg/kg) by the Km ratio (AED = Human dose X Km ratio) according to Nair and Jacob (2016). Km ratio was obtained from FDA draft guidelines (2005). Sucralose was obtained as sweetal (Hygint pharmaceutical company) and stevia was obtained as SweetLeaf (Wisdom Natural Brands). 40.5 mg/ml of sucrose (S5016), 5.20 mg/ml of sucralose and 4.20 mg/ml of stevia were dissolved individually in distilled water. Mice are nocturnal animals and their water intake is strongly linked to the circadian rhythm of their waking/sleeping behavior (Eckel-Mahan and Sassone-Corsi, 2013). The solutions were placed in the waterers for 5 h (from 7 to 12 pm daily), then replaced with normal drinking water. The body weight, food consumption and volume of daily consumed water with and without sweeteners were determined for all experimental groups at 8 and 16 weeks post administration.
2.3. Experimental design
80 mice (40 each male and female) were divided into eight groups per sex:
Group I: Control group received normal drinking water without sweeteners for 8 weeks,
Group II: Control group received normal drinking water without sweeteners for 16 weeks,
Group III: Mice received sucrose dissolved in drinking water for 8 weeks,
Group IV: Mice received sucrose dissolved in drinking water for 16 weeks,
Group V: Mice received sucralose dissolved in drinking water for 8 weeks,
Group VI: Mice received sucralose dissolved in drinking water for 16 weeks,
Group VII: Mice received stevia dissolved in drinking water for 8 weeks,
Group VIII: Mice received stevia dissolved in drinking water for 16 weeks.
At the end of the experiment, mice were anaesthetized with pentobarbital (80 mg/kg); and blood was collected by direct cardiac puncture (Farid et al., 2020a). Cardiac puncture was advised to extract a single, high quality and large amount of blood from mice model at the terminal stage of the study. Mice should be under terminal anesthesia while gathering blood samples. Correct needle is used for collecting the blood samples from the heart ventricle (Parasuraman et al., 2010). Coagulated blood was centrifuged, for 10 min at 2500 rpm, to separate the serum. Serum aliquots were stored at −80 °C till biochemical and immunological measurement. Heparinized blood was used for measuring haematological parameters.
2.4. Blood picture, blood biochemical parameters, enzyme activities and immunological parameters
Hemoglobin A1c (HbA1c), known as glycosylated or glucosylated hemoglobin, is a minor component of hemoglobin to which glucose is bound. It reflects the average blood glucose levels over the prior 6–8 weeks. Its measurement was used to record the effects of artificial sweeteners on blood glucose level in mice. Its level was determined by mouse kit (E4657). Blood picture (Hb: Hemoglobin, HCT: Hematocrit, RBCS: Red blood cells, WBCS: White blood cells, PLT: Platelet, MCV: mean corpuscular volume, MCH: mean cell hemoglobin, MCHC: mean corpuscular hemoglobin concentration) was evaluated in all experimental groups by an automated counter. Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were assayed by mouse ELISA kit (MBS264717 and MBS450720, respectively). Kidney function parameters were measured by rat urea ELISA kit (MBS751125) and rat creatinine ELISA kit (MBS763433). Oxidative stress biomarkers, superoxide dismutase (SOD) and nitric oxide (NO) were estimated by mouse SOD ELISA kit (MBS034842) and mouse NO ELISA kit (MBS720290). Lipid profiles were determined by Cholesterol (Chol) Assay Kit- high-density lipoproteins (HDL) and low-density lipoproteins (LDL)/VLDL (ab65390) and Triglyceride (TG) Assay Kit (STG-1-NC, Zenbio). Level of serum free fatty acids (FFAs) was detected by Free Fatty Acid Assay Kit - Quantification (ab65341). Circulating lipopolysaccharides (LPS) serum level was measured by mouse LPS ELISA kit (Cat No. MBS700021). All measured immunological parameters were evaluated by sandwich ELISA due to its effectivety and high efficacy in detection according to (Farid et al., 2019; Farid et al., 2020b; Farid et al., 2020c; Farid et al., 2020d). The concentrations of serum interleukin (IL)-6, -8 and -10 were measured by using mouse ELISA kit (ab100712, MBS261967 and ab100697, respectively). Different immunoglobulin (Ig) levels were estimated by Mouse IgG ELISA Kit (ab157719), Mouse IgA ELISA Kit (ab157717) and Mouse IgE ELISA Kit (ab157718).
2.5. Histopathological studied
Liver and kidney from all experimental groups were fixed immediately in 10% neutral buffered formalin followed by the dehydration in different grades of alcohol and clearing in xylol. Organs were embedded in paraffin wax, sectioned at 4–6 μ thick and stained with Haematoxylin and Eosin to be examined microscopically (Farid et al., 2020a).
2.6. Statistical analysis
The results were evaluated by One Way ANOVA test and compared with Duncan's Multiple Range test (DMRT). Results were expressed as mean ± SD and values were considered significant at P < 0.05.
3. Results
3.1. Body weight, food and water (with and without sweeteners) consumption
Sucrose, sucralose and stevia administrated mice groups, male and female, showed a reduction in water consumption without sweeteners when compared to their corresponding control groups (Table 1). Male and female mice groups V, VI, VII and VIII showed an increase in consumption of water with sweeteners (sucralose or stevia) when compared to sucrose administrated groups III and IV. Sucrose, sucralose or stevia administration did not increase the body weight in both male and female groups when compared to control groups I and II. An insignificant decrease in food consumption was observed in male and female mice groups administrated with sucrose, sucralose or stevia at 8 and 16 weeks post administration.
Table 1.
The effects of sucrose, sucralose and stevia administration on body weight, food and water consumption (with and without sweeteners) in male and female albino mice.
| Parameters |
Control groups |
Sucrose |
Sucralose |
Stevia |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Group I | Group II | Group III | Group IV | Group V | Group VI | Group VII | Group VIII | ||
| Male mice | Water consumption (ml/mouse/day) | ||||||||
| Without sweeteners | 4.68 ± 1.5c | 5.47 ± 5.6d | 3.98 ± 9.4b | 3.96 ± 0.3b | 3.03 ± 5.3a | 4.03 ± 2.4b | 3.46 ± 7.1a | 3.89 ± 1.3b | |
| With sweeteners | - | - | 2.14 ± 4.2a | 3.14 ± 6.4b | 3.10 ± 3.6b | 4.56 ± 4.1c | 3.60 ± 0.5b | 4.91 ± 0.3c | |
| Food consumption (g/mouse/day) | 4.31 ± 1.3b | 5.60 ± 4.3c | 3.86 ± 1.9a | 4.98 ± 14.2b | 3.98 ± 4.5a | 5.03 ± 6.6c | 3.65 ± 0.4a | 5.44 ± 1.3c | |
| Body weight (g) | 32.44 ± 0.6a | 37.54 ± 1.6b | 33.04 ± 4.1a | 38.55 ± 3.6b | 34.65 ± 3.1a | 39.63 ± 5.1b | 33.51 ± 1.4a | 39.96 ± 2.6b | |
| Female mice | Water consumption (ml/mouse/day) | ||||||||
| Without sweeteners | 5.59 ± 0.4c | 6.26 ± 0.9d | 4.51 ± 2.1b | 4.01 ± 5.6a | 4.21 ± 4.1a | 4.96 ± 1.9b | 4.36 ± 4.3a | 5.41 ± 0.6c | |
| With sweeteners | – | – | 2.54 ± 5.6a | 3.47 ± 11.1b | 3.94 ± 2.1b | 4.10 ± 4.3c | 4.05 ± 0.8c | 4.81 ± 4.1c | |
| Food consumption (g/mouse/day) | 5.31 ± 0.5a | 6.4 ± 0.7c | 4.92 ± 8.6a | 5.88 ± 5.4b | 4.56 ± 1.1a | 5.96 ± 8.2b | 4.43 ± 5.3a | 5.88 ± 4.4b | |
| Body weight (g) | 33.11 ± 4.0a | 38.47 ± 2.5b | 34.47 ± 12.4a | 39.78 ± 0.6b | 34.57 ± 5.3a | 39.41 ± 0.2b | 34.25 ± 0.8a | 39.81 ± 7.6b | |
Data were presented as mean ± SD. Means followed by the same letter within the same row were not significantly different (P > 0.05), whereas those marked with different ones were significantly different (P < 0.05) using analysis of variance [ANOVA]; Duncan's Multiple Range test (DMRT). Group I and II: Control groups receiving normal drinking water without sweeteners for 8 and 16 weeks, Group III: Mice received sucrose for 8 weeks, group IV: Mice received sucrose for 16 weeks, group V: Mice received sucralose for 8 weeks, Group VI: Mice received sucralose for 16 weeks, Group VII: Mice received stevia for 8 weeks, Group VIII: Mice received stevia for 16 weeks.
3.2. HbA1c level
The levels of HbA1c in control female mice groups I and II were higher than those of control male mice either at 8 or 16 weeks (Fig. 1). The same was observed in sucrose, sucralose and stevia administrated female mice groups (Table 2). Sucralose and stevia administration, in female mice groups, significantly elevated HbA1c after short and long term administration when compared to control group I (group V: 8.08, group VI: 8.11, group VII: 8.70 and group VIII: 8.74 vs group I: 6.58 and group II: 6.96). In male group VI, administration of sucralose for 16 weeks, significantly elevated HbA1c to reach 7.24 ± 0.4 that was higher than those of control groups and other sweetener administrated groups.
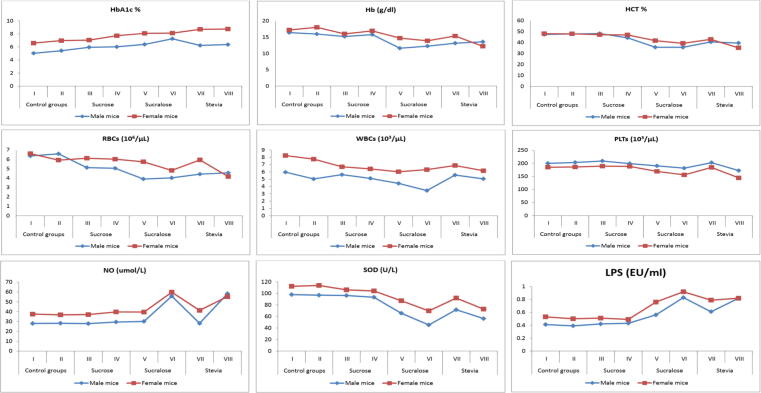
Fig. 1.
The effects of sucrose, sucralose and stevia administration in male and female experimental groups on complete blood picture, NO, SOD and circulating LPS.
Table 2.
The effects of sucrose, sucralose and stevia administration on HbA1c level and complete blood picture in male and female albino mice.
| Parameters |
Control groups |
Sucrose |
Sucralose |
Stevia |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Group I | Group II | Group III | Group IV | Group V | Group VI | Group VII | Group VIII | ||
| Male mice | HbA1c% | 5.02 ± 1.28a | 5.41 ± 2.1a | 5.94 ± 0.9a | 6.01 ± 2.5a | 6.38 ± 1.9a | 7.24 ± 0.4b | 6.22 ± 1.1a | 6.36 ± 0.8a |
| Hb (g/dl) | 16.44 ± 3.03b | 15.99 ± 2.3b | 15.21 ± 2.91b | 15.81 ± 5.4b | 11.62 ± 2.5a | 12.26 ± 1.6a | 13.16 ± 3.2a | 13.58 ± 2.2a | |
| HCT (%) | 47.21 ± 7.46b | 47.71 ± 4.16b | 48.11 ± 7.8b | 44.21 ± 1.9b | 35.61 ± 7.37a | 35.54 ± 4.7a | 40.43 ± 8.3a | 39.44 ± 6.3a | |
| RBCs (106/μL) | 6.36 ± 1.8b | 6.59 ± 8.3b | 5.12 ± 0.9b | 5.05 ± 0.8b | 3.90 ± 0.9a | 4.02 ± 0.5a | 4.42 ± 1a | 4.56 ± 0.7a | |
| WBCs (103/μL) | 5.96 ± 1.35b | 5.03 ± 3.5b | 5.62 ± 0.86b | 5.11 ± 0.7b | 4.42 ± 0.96ab | 3.44 ± 1a | 5.56 ± 1.2b | 5.04 ± 0.9b | |
| PLTS (103/μL) | 200.04 ± 5.7a | 203.47 ± 2.5a | 209.02 ± 4.7a | 199.21 ± 5.4a | 190.45 ± 2.5a | 181.63 ± 5.3a | 202.62 ± 3.2a | 172.41 ± 4.7a | |
| MCV (fl) | 90.76 ± 1.6a | 91.02 ± 2.5a | 94.54 ± 9.9a | 93.41 ± 8.4a | 91.16 ± 3.6a | 88.48 ± 0.8a | 91.90 ± 4.4a | 86.28 ± 2.9a | |
| MCH (pg) | 30.91 ± 1.3a | 31.00 ± 6.4a | 29.42 ± 3.2a | 29.14 ± 1.2a | 29.73 ± 5.4a | 30.14 ± 0.5a | 29.12 ± 1.1a | 29.65 ± 1.1a | |
| MCHC (g/dl) | 31.32 ± 1.4a | 31.40 ± 4.2a | 31.84 ± 3.3a | 30.04 ± 1.3a | 30.76 ± 1.17a | 29.21 ± 0.4a | 30.94 ± 1.5a | 29.06 ± 0.8a | |
| Female mice | HBA1c% | 6.58 ± 1.8a | 6.96 ± 0.1a | 7.04 ± 0.6a | 7.71 ± 4.2ab | 8.08 ± 1.3b | 8.11 ± 0.7b | 8.70 ± 1.6bc | 8.74 ± 0.4bc |
| Hb (g/dl) | 17.24 ± 2.8b | 18.06. ± 5.4b | 16.05 ± 2.6b | 16.98 ± 1.3b | 14.72 ± 3.2a | 13.90 ± 1.5a | 15.38 ± 2.1a | 12.22 ± 1.2a | |
| HCT (%) | 48.00 ± 5.7b | 47.87 ± 1.6b | 47.07 ± 1.2b | 46.88 ± 0.6b | 41.60 ± 6.5a | 39.18 ± 3.7a | 42.80 ± 3.9a | 35.06 ± 3.6a | |
| RBCs (106/μL) | 6.61 ± 1.7c | 5.92 ± 1.9c | 6.12 ± 5.1c | 6.02 ± 4.9c | 5.74 ± 1.9b | 4.82 ± 1.8a | 5.94 ± 1.51b | 4.16 ± 0.4a | |
| WBCs (103/μL) | 8.24 ± 1.5b | 7.75 ± 0.5b | 6.70 ± 1.3a | 6.41 ± 0.8a | 6.02 ± 1.9a | 6.31 ± 1.1a | 6.88 ± 1.4a | 6.16 ± 0.7a | |
| PLTS (103/μL) | 185.20 ± 14.1d | 186.20 ± 4.2d | 189.30 ± 6.1d | 188.40 ± 5.7d | 169.20 ± 8.3c | 155.80 ± 12b | 184.60 ± 4.4c | 144.20 ± 13.9a | |
| MCV (fl) | 88.12 ± 1.4a | 87.41 ± 9.3a | 90.47 ± 8.1a | 89.06 ± 7.2a | 88.58 ± 2.5a | 91.96 ± 0.7a | 88.41 ± 1.1a | 88.18 ± 1a | |
| MCH (pg) | 30.91 ± 1.3a | 30.63 ± 6.8a | 28.01 ± 4.3a | 29.01 ± 0.1a | 30.90 ± 1.3a | 32.01 ± 0.7a | 30.91 ± 1.3a | 30.42 ± 0.5a | |
| MCHC (g/dl) | 30.44 ± 1.1a | 30.04 ± 4.4a | 29.99 ± 5.5a | 30.09 ± 3.2a | 30.62 ± 1.1a | 29.06 ± 0.9a | 30.16 ± 1.2a | 29.20 ± 0.4a | |
Data were presented as mean ± SD. Means followed by the same letter within the same row were not significantly different (P > 0.05), whereas those marked with different ones were significantly different (P < 0.05) using analysis of variance [ANOVA]; Duncan's Multiple Range test (DMRT). Group I and II: Control groups receiving normal drinking water without sweeteners for 8 and 16 weeks, Group III: Mice received sucrose for 8 weeks, group IV: Mice received sucrose for 16 weeks, group V: Mice received sucralose for 8 weeks, Group VI: Mice received sucralose for 16 weeks, Group VII: Mice received stevia for 8 weeks, Group VIII: Mice received stevia for 16 weeks.
3.3. Complete blood picture
Sucrose administration did not affect hemoglobin levels, in both male and female mice, either on short or long term (Fig. 1). While sucralose administration caused a strong drop in hemoglobin (Hb) levels in male groups V and VI (11.62 ± 2.5 and 12.26 ± 1.6, respectively) when compared to control groups I and II. Stevia administration for 16 weeks caused a significant reduction in hemoglobin level of female group VIII (12.22 ± 1.2) more than that of male group VIII (13.58 ± 2.2). In male and female mice groups, Hematocrit (HCT) was significantly reduced after sucralose and stevia administration, either for 8 or 16 weeks, in comparison to control groups and sucrose administrated groups III and IV. Sucrose administration, in male groups III and VI, showed no significant change in red blood cells (RBCs) count (5.12 ± 0.9 and 5.05 ± 0.8, respectively); the same was observed among sucrose administrated female groups III and VI (6.12 ± 5.1 and 6.02 ± 4.9, respectively) when compared to female control groups I and II (6.61 ± 1.7 and 5.92 ± 1.9). In male groups, sucralose or stevia administration significantly reduced RBCs count in comparison to male control groups (group V: 3.90 ± 0.9, group VI: 4.02 ± 0.5, group VII: 4.42 ± 1 and group VIII: 4.56 ± 0.7 vs group I: 6.36 ± 1.8 and group II: 6.59 ± 8.3). On the other hand, sucralose or stevia administration in female groups caused a gradual decrease in RBCs count from 8 to 16 weeks of administration (group V: 5.74 ± 1.9, group VI: 4.82 ± 1, group VII: 5.94 ± 1.51 and group VIII: 4.16 ± 0.4 vs group I: 6.61 ± 1.7 and group II: 5.92 ± 1.9). White blood cells (WBCs) count in all female mice groups was higher than their corresponding male mice groups. Sucrose administration in male groups III and VI did not show any significant change in WBCs count when compared to male control groups (group III: 5.62 ± 0.86, group VI: 5.11 ± 0.7 vs group I: 5.96 ± 1.35 and group II: 5.03 ± 3.5); while its administration in their corresponding female groups induced a significant reduction in WBCs count (group III: 6.70 ± 1.3, group VI: 6.41 ± 0.8 vs group I: 8.24 ± 1.5 and group II: 7.75 ± 0.5). Sucralose administration, in male groups only, showed a gradual decrease in WBCs count to reach 4.42 ± 0.96 and 3.44 ± 1 after 8 and 16 weeks of administration, respectively. Stevia administration, either for short or long term in male mice groups VII and VIII, did not showed any change in WBCs count in comparison to control groups and sucrose administrated groups III and VI. Stevia administration in female mice groups VII and VIII showed a significant decrease in WBCs count (6.88 ± 1.4 and 6.16 ± 0.7, respectively) when compared to female control groups (8.24 ± 1.5 and 7.75 ± 0.5); and no change when compared to sucrose or sucralose administrated female mice groups. Male control groups showed platelets count higher than that of female control groups. Platelets (PLT) count, in long term administrated male groups with sucralose or stevia, showed insignificant decrease when compared to control groups (group VI: 181.63 ± 5.3, group VIII: 172.41 ± 4.7 vs group II: 203.47 ± 2.5). Short term administration of sucralose, in female group V, showed a significant reduction in platelets count (169.20 ± 8.3) that continued to reach 155.80 ± 12 after 16 weeks of administration. Stevia administration in female group VII for 8 weeks did not affect platelets count; but after 16 weeks of administration a significant drop was observed in comparison to female control group I (group VII: 184.60 ± 4.4, group VIII: 144.20 ± 13.9 vs group II: 186.20 ± 4.2). No significant changes were observed in mean corpuscular volume (MCV), mean cell hemoglobin (MCH) or mean corpuscular hemoglobin concentration (MCHC) among experimental groups for male and female mice (Table 2).
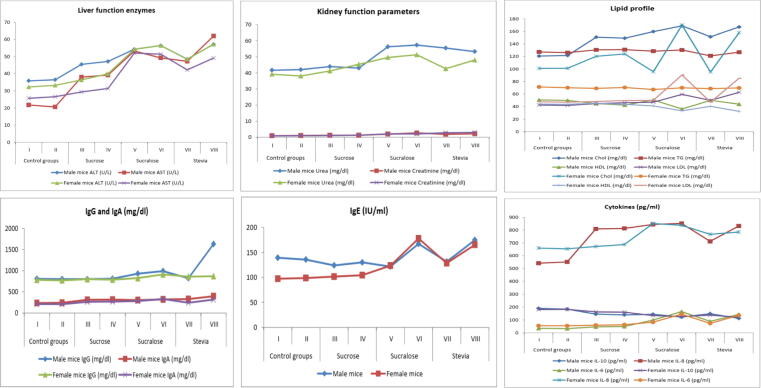
3.4. Liver function enzymes
Sucrose administration increased alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels in male mice groups III and IV in a significant way; while no significant change was observed upon their administration in female mice groups (Table 3, Fig. 2). Short and long term administration of sucralose, in both male and female mice groups, significantly elevated ALT and AST levels when compared to their corresponding control groups or sucrose administrated groups III and IV. Stevia administration for 8 weeks significantly increased ALT level, in both male (48.44 ± 9.5) and female (48.44 ± 9.1) mice group VII; this increase continued to reach the highest level among all experimental groups (57.14 ± 3.6 and 56.14 ± 2.6 for male and female mice group VIII, respectively). AST level, in stevia administrated female group VII (42.21 ± 1.6) and VIII (49.08 ± 0.7), was significantly higher than that of control groups I and II (25.62 ± 2.5 and 26.55 ± 19.4) and sucrose administrated groups III and IV (29.30 ± 3.2 and 31.31 ± 0.4, respectively); but significantly lower than that of sucralose administrated groups (52.01 ± 5.2 and 51.34 ± 0.8 for group V and VI, respectively).
Table 3.
The effects of sucrose, sucralose and stevia administration on serum levels of liver and kidney functions parameters, oxidative stress biomarkers and lipid profile in male and female albino mice.
| Parameters |
Control groups |
Sucrose |
Sucralose |
Stevia |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Group I | Group II | Group III | Group IV | Group V | Group VI | Group VII | Group VIII | ||
| Male mice | ALT (U/L) | 35.81 ± 6.36a | 36.42 ± 6.6a | 45.41 ± 6.79b | 47.10 ± 7.2b | 54.24 ± 2.3c | 56.38 ± 1.9c | 48.44 ± 9.5b | 57.14 ± 3.6c |
| AST (U/L) | 21.76 ± 6.5a | 20.63 ± 4.5a | 37.98 ± 5.6b | 39.18 ± 6.2b | 53.22 ± 21.6 cd | 49.18 ± 0.4c | 47.21 ± 2.7c | 61.92 ± 1.6e | |
| Urea (mg/dl) | 41.68 ± 5.7a | 42.03 ± 0.8a | 43.96 ± 4.0a | 43.01 ± 4.1a | 56.3 ± 14.9b | 57.34 ± 3.6b | 55.52 ± 9.8b | 53.32 ± 4.8b | |
| Creatinine (mg/dl) | 0.90 ± 1.43a | 1.03 ± 4.3a | 1.18 ± 0.08a | 1.20 ± 0.1a | 1.91 ± 0.4ab | 2.60 ± 6.5b | 1.81 ± 0.3ab | 2.12 ± 7.1b | |
| NO (umol/L) | 28.01 ± 1.16a | 28.21 ± 6.1a | 27.88 ± 4.2a | 29.44 ± 3.5a | 30.12 ± 1.1a | 55.82 ± 3.7b | 28.16 ± 6.9a | 58.3 ± 5.6b | |
| SOD (U/L) | 97.80 ± 1.16d | 96.85 ± 5.4d | 96.19 ± 2.5d | 93.24 ± 5.3d | 65.46 ± 1.5c | 45.18 ± 1.6a | 71.61 ± 3.3c | 56.11 ± 2.7b | |
| Chol (mg/dl) | 120.61 ± 18.73a | 121.52 ± 8.4.a | 150.84 ± 13.1b | 149.14 ± 1.3b | 159.56 ± 54.3c | 168.74 ± 10.7d | 151.34 ± 35.2b | 166.90 ± 16d | |
| TG (mg/dl) | 127.21 ± 13.6a | 126.08 ± 8.6a | 130.51 ± 10.5a | 130.91 ± 1.4a | 128.44 ± 38.8a | 130.30 ± 25.9a | 120.96 ± 25.4a | 126.74 ± 13a | |
| HDL (mg/dl) | 50.64 ± 10.26c | 49.85 ± 6.7c | 44.81 ± 6.8c | 42.46 ± 5.2b | 50.51 ± 6.7c | 36.26 ± 5.5a | 49.92 ± 6.8c | 43.84 ± 7.4b | |
| LDL (mg/dl) | 42.55 ± 5.79a | 42.01 ± 7.5a | 44.72 ± 11.5a | 46.11 ± 2.3a | 46.98 ± 40.8a | 59.43 ± 6.4b | 49.83 ± 29.3a | 62.71 ± 10b | |
| FFAs (mmol/L) | 0.62 ± 0.1a | 0.64 ± 0.2a | 0.59 ± 0.4a | 0.60 ± 0.9a | 0.94 ± 0.1b | 1.01 ± 0.5b | 0.86 ± 0.3b | 0.9 ± 0.6b | |
| Liver TG (mg/g) | 105.16 ± 0.65a | 103.44 ± 0.4a | 102.41 ± 1.3a | 103.21 ± 3.1a | 100.78 ± 0.8a | 101.36 ± 2.7a | 108.67 ± 0.5a | 102.77 ± 6.7a | |
| Liver Chol (mg/g) | 87.13 ± 4.03a | 89.46 ± 0.6a | 95.77 ± 2.4b | 100.41 ± 1.1b | 101.49 ± 5.2b | 121.77 ± 9.2c | 105.11 ± 2.9b | 130.43 ± 11.4c | |
| Female mice | ALT (U/L) | 32.22 ± 3.6a | 33.20 ± 7.6a | 36.32 ± 7.9a | 39.92 ± 5.1a | 54.24 ± 23.3c | 56.38 ± 1.9c | 48.44 ± 9.1b | 56.14 ± 2.6c |
| AST (U/L) | 25.62 ± 2.5a | 26.55 ± 19.4a | 29.30 ± 3.2a | 31.31 ± 0.4a | 52.01 ± 5.2c | 51.34 ± 0.8c | 42.21 ± 1.6b | 49.08 ± 0.7c | |
| Urea (mg/dl) | 39.18 ± 3.6a | 38.07 ± 2.6a | 41.23 ± 0.6a | 45.34 ± 6.1ab | 49.62 ± 6.1b | 51.32 ± 2.9b | 42.61 ± 1.6a | 48.02 ± 2.8b | |
| Creatinine (mg/dl) | 0.82 ± 1.4a | 0.91 ± 7.4a | 1.01 ± 4.4a | 1.21 ± 4.6a | 2.06 ± 1.2b | 2.04 ± 0.8b | 2.71 ± 1.5c | 2.91 ± 0.4c | |
| NO (umol/L) | 37.48 ± 6.6a | 36.77 ± 4.3a | 37.03 ± 6.1a | 39.74 ± 7.6a | 39.62 ± 1.6a | 59.78 ± 3.2b | 41.31 ± 1.6a | 55.32 ± 3.1b | |
| SOD (U/L) | 112.14 ± 1.1d | 113.63 ± 12.4d | 106.03 ± 2.4d | 104.14 ± 7.3d | 87.14 ± 1.17b | 69.61 ± 4.1a | 91.80 ± 1.6c | 72.66 ± 2.3b | |
| Chol (mg/dl) | 100.91 ± 7.2a | 101.13 ± 4.9a | 120.22 ± 5.2b | 124.04 ± 4.9b | 95.62 ± 23.7a | 170.05 ± 8.4d | 95.21 ± 1.6a | 157.92 ± 9.3c | |
| TG (mg/dl) | 71.34 ± 7.8a | 70.03 ± 7.6a | 69.03 ± 0.4a | 70.43 ± 4.8a | 67.21 ± 16.8a | 69.92 ± 7.2a | 68.62 ± 1.6a | 69.68 ± 14.5a | |
| HDL (mg/dl) | 44.06 ± 3.1b | 43.65 ± 6.1b | 45.36 ± 1.6b | 43.61 ± 1.3b | 41.22 ± 5.9b | 33.34 ± 3.5a | 40.52 ± 1.6b | 32.41 ± 4.1a | |
| LDL (mg/dl) | 47.61 ± 2.5a | 46.97 ± 0.5a | 48.11 ± 4.8a | 49.54 ± 0.4a | 49.90 ± 14.5a | 90.48 ± 4.9b | 46.98 ± 0.3a | 85.16 ± 6.4b | |
| FFAs (mmol/L) | 0.59 ± 0.3a | 0.54 ± 0.4a | 0.56 ± 0.1a | 0.58 ± 0.6a | 0.99 ± 0.6c | 1.1 ± 0.6c | 0.73 ± 0.4b | 0.83 ± 0.2b | |
| Liver TG (mg/g) | 77.65 ± 1.1a | 75.33 ± 0.9a | 76.53 ± 4.1a | 74.69 ± 2.4a | 77.98 ± 3.1a | 71.43 ± 0.8a | 77.44 ± 3.4a | 78.56 ± 6.1a | |
| Liver Chol (mg/g) | 55.43 ± 2.0a | 58.96 ± 0.4a | 56.47 ± 3.2a | 59.41 ± 2.3a | 77.23 ± 0.9b | 94.33 ± 1.3c | 81.42 ± 1.9b | 110.74 ± 7.1d | |
Data were presented as mean ± SD. Means followed by the same letter within the same row were not significantly different (P > 0.05), whereas those marked with different ones were significantly differed (P < 0.05) using analysis of variance [ANOVA]; Duncan's Multiple Range test (DMRT). Group I and II: Control groups receiving normal drinking water without sweeteners for 8 and 16 weeks, Group III: Mice received sucrose for 8 weeks, group IV: Mice received sucrose for 16 weeks, group V: Mice received sucralose for 8 weeks, Group VI: Mice received sucralose for 16 weeks, Group VII: Mice received stevia for 8 weeks, Group VIII: Mice received stevia for 16 weeks.
Fig. 2.
The effects of sucrose, sucralose and stevia administration in male and female experimental groups on liver function enzymes, kidney function parameters, lipid profile, immunoglobulins and cytokines.
3.5. Kidney function parameters
Sucrose administration in male and female mice groups III and VI did not affect urea and creatinine levels either on short or long term (Table 3, Fig. 2). Sucralose administration, in male and female groups V and VI, significantly elevated urea and creatinine levels when compared to their corresponding control groups. The effect of stevia on urea level, after 8 weeks of administration, was more obvious in male group than in female ones (55.52 ± 9.8 and 42.61 ± 1.6, respectively); the same was observed after 18 weeks of administration. Creatinine level, in female groups VII and VIII (2.71 ± 1.5 and 2.91 ± 0.4, respectively), was higher than their corresponding male groups (1.81 ± 0.3 and 2.12 ± 7.1 for group VII and VIII, respectively).
3.6. Free radicles and antioxidant enzymes
Levels of free radicle and antioxidant were higher in female groups than in their corresponding male groups (Fig. 1). Short and long term administration of sucrose and short term administration of both stevia and sucralose did not affect nitric oxide (NO) level in both male and female groups (Table 3). Only, long term administration of sucralose or stevia significantly elevated NO level in both male and female groups VI and VIII. A significant reduction in Superoxide dismutase (SOD) level was observed after 8 weeks of administration of sucralose or stevia in both male and female groups; however the reduction effect of sucralose was higher than that of stevia. Male group VI, long term sucralose administrated group, recorded the lowest SOD level (45.18 ± 1.6) among all experimental groups.
3.7. Lipid profile
Sucrose administration for 8 and 16 weeks significantly elevated cholesterol (Chol) level in both male (150.84 ± 13.1 and 149.14 ± 1.3 for groups III and IV, respectively) and female groups (120.22 ± 5.2 and 124.04 ± 4.9 for groups III and IV, respectively) when compared to their corresponding controls. Long term administration of sucralose and stevia significantly increased cholesterol level in female groups VI and VIII (170.05 ± 8.4 and 157.92 ± 9.3, respectively) when compared to short term administrated groups V and VII (95.62 ± 23.7 and 95.21 ± 1.6, respectively). In male mice groups, administration of sucralose or stevia for 8 weeks significantly elevated cholesterol level; and this elevation continued to reach 168.74 ± 10.7 and 166.90 ± 16 after 16 weeks. No significant changes were observed in triglycerides (TG) level among all male or female groups (Fig. 2). Long term administration of sucralose or stevia significantly decreased serum high-density lipoproteins (HDL) level and increased serum low-density lipoproteins (LDL) level in male and female mice groups VI and VIII (Table 3). Levels of free fatty acids in sucralose and stevia administrated groups, either on short or long term, were significantly higher than those of control or sucrose administrated groups (Table 3). Levels of liver triglycerides and cholesterol in male mice groups were higher than their corresponding in female mice groups. No significance differences were detected in liver triglycerides levels among all experimental groups. Both sucralose and stevia administration, for 16 weeks, significantly elevated liver cholesterol level in both male and female mice groups.
3.8. Immunological parameters
A significant elevation in levels of circulating LPS was observed in both male and female mice groups, administrated with sucralose or stevia, for 8 weeks (Fig. 1). This elevation continued to reach the highest levels after 16 weeks of administration. No significant change was observed in sucrose administrated groups III and IV. Sucrose administration did not affect serum IgG or IgE levels either in male or female mice groups III and IV when compared to their corresponding control groups I and II (Table 4, Fig. 2). IgA level, after short or long term of sucrose administration, was significantly elevated in both male and female mice groups III and IV. Short term administration of sucralose significantly elevated IgG level in both male and female group V (929.01 ± 13.4 and 822.66 ± 17.7, respectively); this elevation continued to reach 990.00 ± 15.8 and 912.21 ± 14.4 after 16 week of administration. Significant elevations in levels of IgE (167.44 ± 11.1 and 178.10 ± 8.9) and IgA (318.70 ± 4.2, 331.52 ± 3.9) were observed in male and female group VI, respectively. Long term administration of stevia significantly increased IgG, IgE and IgA levels in male (1630.21 ± 4.8, 174.88 ± 16.8 and 396.50 ± 13.3) and female (869.21 ± 2.2, 165.52 ± 1.9 and 316.6 ± 6.2) group VIII. In male mice, IL-10 level was reduced after 8 weeks administration of sucrose, sucralose and stevia (145.91 ± 8.95, 144.51 ± 18.8 and 148.71 ± 10.4, respectively) in comparison to control group I (189.62 ± 9.9). The lowest IL-10 level was recorded in male and female group VIII (113.18 ± 8.9 and 121.43 ± 9.7, respectively). Pro-inflammatory cytokines, IL-6 and −8, levels were significantly increased with sucralose and stevia administration either on short or long term (Table 4). However, IL-6 and -8 levels were higher in female groups than in male ones. The effect of sucrose administration on pro-inflammatory cytokines was, only, observed in male group III and IV; no significant difference was observed among female groups I, II, III and IV (Fig. 2).
Table 4.
The effects of sucrose, sucralose and stevia administration on serum levels of circulating LPS, different immunoglobulins and cytokines in male and female albino mice.
| Parameters |
Control groups |
Sucrose |
Sucralose |
Stevia |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Group I | Group II | Group III | Group IV | Group V | Group VI | Group VII | Group VIII | ||
| Male mice | LPS (EU/ml) | 0.41 ± 3.6 a | 0.39 ± 1.2 a | 0.42 ± 9.1 a | 0.43 ± 0.3 a | 0.56 ± 2.6b | 0.83 ± 11.2c | 0.61 ± 8.8b | 0.82 ± 4.3c |
| IgG (mg/dl) | 807.32 ± 9.3a | 799.08 ± 6.1a | 800.24 ± 8.3a | 809.04 ± 4.8a | 929.01 ± 13.4b | 990.00 ± 15.8b | 820.48 ± 7.4a | 1630.21 ± 4.8c | |
| IgE (IU/ml) | 139.40 ± 16.1a | 135.71 ± 0.1a | 124.08 ± 14.3a | 130.09 ± 3.3a | 121.76 ± 5.7a | 167.44 ± 11.1b | 131.56 ± 12.2a | 174.88 ± 16.8b | |
| IgA (mg/dl) | 234.72 ± 1.3a | 240.91 ± 5.2a | 310.14 ± 5.6b | 315.98 ± 3.1b | 304.38 ± 14.3b | 318.70 ± 4.2b | 328.92 ± 3.2c | 396.50 ± 13.3d | |
| IL-10 (pg/ml) | 189.62 ± 9.9d | 184.01 ± 11.4d | 145.91 ± 8.95c | 140.02 ± 2.4c | 144.51 ± 18.8c | 123.04 ± 28.9b | 148.71 ± 10.4c | 113.18 ± 8.9a | |
| IL-8 (pg/ml) | 542.20 ± 8.9a | 552.14 ± 8.4a | 808.52 ± 8.7c | 812.71 ± 5.5c | 843.29 ± 2.6d | 851.28 ± 16.9d | 711.91 ± 28.3b | 831.83 ± 9.4d | |
| IL-6 (pg/ml) | 34.65 ± 0.1a | 32.88 ± 10.5a | 46.15 ± 0.2b | 49.51 ± 0.2b | 99.47 ± 1.6c | 165.47 ± 0.2e | 89.74 ± 0.1c | 143.61 ± 0.4d | |
| LPS (EU/ml) | 0.53 ± 10.1 a | 0.50 ± 2.6 a | 0.51 ± 6.1 a | 0.49 ± 7.6 a | 0.76 ± 4.4b | 0.92 ± 2.9d | 0.79 ± 7.4b | 0.82 ± 11.9c | |
| Female mice | IgG (mg/dl) | 782.51 ± 19.1a | 770.04 ± 10.8a | 798.40 ± 0.3a | 786.74 ± 3.1a | 822.66 ± 17.7b | 912.21 ± 14.4c | 859.31 ± 1.6b | 869.21 ± 2.2b |
| IgE (IU/ml) | 97.38 ± 48.1a | 98.74 ± 8.4a | 101.55 ± 4.4a | 104.47 ± 6.4a | 123.63 ± 12.3b | 178.10 ± 8.9c | 128.60 ± 1.6b | 165.52 ± 1.9c | |
| IgA (mg/dl) | 210.06 ± 11.2a | 208.41 ± 1.4a | 263.19 ± 5.1c | 268.22 ± 7.1c | 282.12 ± 31.8c | 331.52 ± 3.9d | 241.1 ± 1.6b | 316.6 ± 6.2d | |
| IL-10 (pg/ml) | 180.81 ± 2.1d | 182.23 ± 1.4d | 162.92 ± 7.6c | 160.63 ± 3.6c | 134.24 ± 14.6b | 127.42 ± 14.6a | 137.74 ± 2.1b | 121.43 ± 9.7a | |
| IL-8 (pg/ml) | 659.84 ± 2.1a | 654.41 ± 8.1a | 672.54 ± 4.1a | 687.91 ± 5.9a | 850.31 ± 13.9c | 836.92 ± 1.1c | 766.91 ± 5.9b | 784.69 ± 1.6b | |
| IL-6 (pg/ml) | 55.15 ± 0.7a | 54.66 ± 5.5a | 59.41 ± 1.7a | 62.85 ± 2.5a | 83.07 ± 0.1b | 145.47 ± 0.2d | 73.78 ± 1.4b | 136.42 ± 0.4c | |
Data were presented as mean ± SD, Means followed by the same letter within the same row were not significantly different (P > 0.05), whereas those marked with different ones were significantly differed (P < 0.05) using analysis of variance [ANOVA]; Duncan's Multiple Range test (DMRT). Group I and II: Control groups receiving normal drinking water without sweeteners for 8 and 16 weeks, Group III: Mice received sucrose for 8 weeks, group IV: Mice received sucrose for 16 weeks, group V: Mice received sucralose for 8 weeks, Group VI: Mice received sucralose for 16 weeks, Group VII: Mice received stevia for 8 weeks, Group VIII: Mice received stevia for 16 weeks.
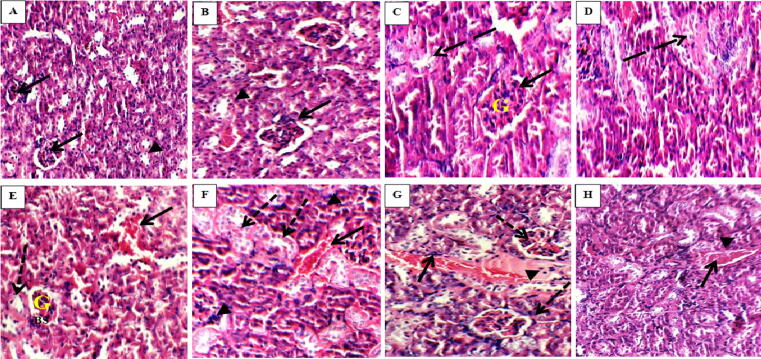
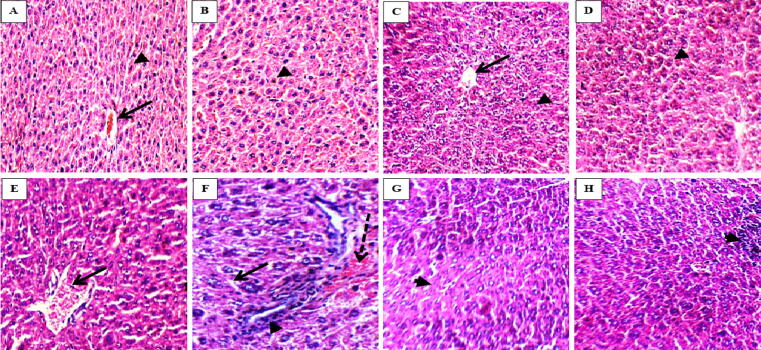
3.9. Histopathological result
Our results showed no significant differences in histopathological sections of both sexes. Where, the severe damage upon stevia or sucralose administration was the same in male groups and their corresponding female ones. Therefore, we chose to present one figure for both male and female mice groups. Kidney section of control groups, either I or II, showed normal renal corpuscles with average sized glomeruli and renal tubules (Fig. 3; A and B). Sucrose administrated groups III and IV showed comparable results to control groups I and II, where no hemorrhage or lymphocyte infiltration was observed (Fig. 3; C and D). On the other hand, sucralose administration severely affected the kidney of group V. Where, Fig. 3(E) showed small sized glomeruli (G) with wide Bowman's space (BS) and areas of hemorrhage. Long and short term sucralose administrated group V and VI showed proximal tubules with markedly edematous epithelial lining and loss of brush borders (Fig. 3; E and F). Group VII, short term stevia administrated group, showed renal corpuscles with average glomeruli with a few inflammatory infiltrate (Fig. 3, G). These changes continued until 16 weeks of stevia administration with the appearance of congested blood vessels in group VIII (Fig. 3H). Liver section of control groups (I and II) and sucrose administered groups (III and IV) showed normal hepatic architecture with hepatocytes arranged in single cell cords with average central vein (Fig. 4; A, B, C and D). Group V, short term sucralose administrated group, showed liver sections with loss of hepatic architecture. Long term administration of sucralose in group VI revealed apoptotic hepatocyte, intra-lobular inflammatory infiltrate and area of hemorrhage (Fig. 4, F). Loss of hepatic architecture with intra-lobular inflammatory infiltrate was observed in group VII and VIII (Fig. 4 G and H).
Fig. 3.
Haematoxylin and eosin mice kidney sections showing average tubule (arrow heads) and average glomeruli (arrows) in control groups I and II (A and B; X200 and X400, respectively); average tubule (dashed arrow) and average sized glomeruli (arrows) in group III (C, X400); average tubule (dashed arrow) with no hemorrhage or inflammatory infiltrate in group IV (D, X400), small sized glomeruli (G) with wide Bowman's space (BS), areas of hemorrhage (arrow) and markedly edematous epithelial lining with loss of brush borders (dashed arrow) in group V (E, X400); areas of hemorrhage (arrow) with inflammatory infiltrate (arrow head) and markedly edematous epithelial lining with loss of brush borders (dashed arrow) in group VI (F, X400); average glomeruli (dashed arrows) and congested blood vessels (arrow) with inflammatory infiltrate (arrow head) in group VII (G, X400); congested blood vessels (arrow) with inflammatory infiltrate (arrow head) in group VIII (H, X400).
Fig. 4.
Haematoxylin and eosin mice liver sections showing average central veins (arrow) and average hepatocytes arranged in single cell cords (arrow head) in groups I, II, III and IV (A, B, C and D, respectively; X400); loss of hepatic architecture with dilated central vein (arrow) in group V (E, X400); apoptotic hepatocyte (arrow) with intra-lobular inflammatory infiltrate (arrow heads) with area of hemorrhage (dashed arrow) in group VI (F, X400); loss of hepatic architecture with intra-lobular inflammatory infiltrate (arrow heads) in group VII and VIII (G and H, X400).
4. Discussion
Our results showed that stevia and sucralose administration negatively affected both male and female mice. The idea of using both sexes was to prove our hypothesis about the harmful effects of non-caloric sweeteners either artificial or natural. If we used one sex only, we would miss the effects in the other one. The difference in the levels of measured parameters, between male and female mice, can be attributed to the difference in the metabolism of both sexes in addition to the female estrus cycle. This study reported that, male and female mice groups showed a preference for water consumption with sucralose and stevia; a phenomenon that can be explained by: 1- sucralose and stevia are many times sweeter than sucrose, 2- male and female mice became addicted to the intense sweet taste of sucralose and stevia. Chandrashekar et al. (2006) reported that the sweet taste perception depends on two G-protein-coupled subunit receptors on the tongue. The sweet test stimulation of these receptors provokes a sensation that most mammals, especially rodents, find intensely rewarding (Sclafani, 2004). Lenoir et al. (2007) allowed rats to choose between saccharin sweetened water and cocaine (a highly addictive substance); they found that 94% of rats preferred the sweet taste of saccharin. Shil et al. (2020) added that non-caloric sweeteners like sucralose negatively affected the intestinal epithelium through these sweet taste receptors. Wang et al. (2016) showed that sucralose administration increased food consumption by direct stimulation of sweet taste receptors and by indirect stimulation of taste-independent neuronal mechanisms. Several large scale prospective cohort studies found a positive correlation between weight gain and chronic artificial sweetener use. In the 1980 s, San Antonio Heart Study examined 3,682 adults over 7–8 year period and reported an increase in the body mass index with dose dependence of artificial sweeteners (Fowler et al., 2008). However, our study showed that Sucralose and stevia are considered non-caloric sweetener, they did not change the body weight of either male or female mice.
The present study showed that sucrose administration did not affect HbA1c level, but prolonged administration of sucralose or stevia significantly elevated its level especially in female groups. These results are contradicted with other studies on diabetic rats; as Chang et al. (2005) reported that stevia increase insulin sensitivity and Jeppesen et al. (2002) showed the anti-hyperglycemic effects of stevia. These contradictory results can be explained by the different physiologic conditions between diabetic models and healthy one. Our study was carried out in healthy male and female mice and not diabetic ones. Our results were confirmed by the previous study of Suez et al. (2004), who reported that artificial sweeteners (saccharin, sucralose or aspartame) consuming mice groups developed a marked significant glucose intolerance (P < 0.001). Also, Rosales-Gómez et al. (2018) showed that stevia has increased glycaemia and the HOMA index, evidencing insulin resistance, in healthy mice. Also, Becker et al. (2020) showed that stevia changed the gut microbiota as did saccharin when consumed with a high fat diet.
According to our results, a reduction in hemoglobin level and HCT % in male and female mice groups with a drop in RBCs, WBCs and platelets' count were observed upon short and long term administration of sucralose or stevia. However, these adverse effects were not seen in sucrose administrated groups. Sucralose and stevia, especially after 16 weeks of administration in male and female mice, significantly elevated levels of liver function enzymes (ALT and AST), urea, creatinine, cholesterol, LDL and free fatty acids. While, sucrose administration elevated levels of ALT, AST and cholesterol levels in male mice only. Both of sucralose or stevia administration led to oxidative stress in male and female mice that was obvious by the elevated level of NO and the reduced level of SOD. These alteration in kidney and liver function parameters in sucralose and stevia administrated groups were confirmed by the histopathological results. Where, kidney section of sucralose administrated groups V and VI showed small sized glomeruli with wide Bowman's space, proximal tubules with markedly edematous epithelial lining, loss of brush borders and areas of hemorrhage. Also, short term sucralose administrated group V showed liver section with loss of hepatic architecture; and long term administrated group VI revealed apoptotic hepatocyte, intra-lobular inflammatory infiltrate and an area of hemorrhage. The adverse effects of sucralose can be attributed to the fact that sucralose is not metabolized by the intestinal bacteria, not absorbed or transported through intestinal epithelia and excreted through the kidney in urine (Knight et al., 2011). Dhurandhar et al. (2018) reported that liver of sucralose treated rats showed markable changes indicating its toxic effects. Also, Helal et al. (2019) noticed an elevation in the levels of AST and ALT, serum creatinine and urea in rat receiving sucralose.
Our results reported that kidney sections of group VII, short term stevia administrated group, showed renal corpuscles with average glomeruli and a few inflammatory infiltrate. These changes continued until the 16 weeks of stevia administration with the appearance of congested blood vessels in group VIII. Loss of hepatic architecture with intra-lobular inflammatory infiltrate was observed in liver sections of stevia administrated groups VII and VIII. The adverse effects of stevia administration, in our study, were controversial to other studies that showed the hepatoprotective and anti-oxidant effect of Stevia rebaudiana leaf extract in experimentally induced liver injury in rats (Ramos-Tovar et al., 2018). This can be explained by other studies that examined the pharmacokinetics of stevioside and were in agreement with our results. Stevioside is a hydrophilic glycoside with a high molecular weight that is not absorbed in the intestine; where the gastric juice and digestive enzymes fail to degrade it (Koyama et al., 2003). But, the intestinal flora of rats (Wingard et al., 1980), mice (Hutapea et al., 1997), pigs (Geuns et al., 2003) and humans (Koyama et al., 2003) convert stevioside into steviol. Geuns et al. (2007) reported that after 3 days following a consumption of 750 mg/day of stevioside in human volunteers, no measurable amount of stevioside was detected in the feces of all subjects. Instead, free steviol was found (Geuns et al., 2007). In 1986, Nakayama and his collages were the first to examine the pharmacokinetics of stevioside where they used administrated male wister rats by 125 mg/kg of 3H-stevioside through oral route. After 8 h, the maximum radioactivity level in blood reached 4.8 μg/ml with organ accumulation in small and large bowel. Cardoso et al. (1996) reported stevioside accumulation in liver, intestine and kidney; and added that the highest accumulation was in liver. Steviol was the major metabolite in bile by using high performance liquid chromatography. However, the removal and excretion of steviol by liver negatively affects the bile metabolism. Obviously, chronic stevia consumption puts stress on liver that results in an elevation in ALT, AST and altered bile output. Bueno-Hernández et al. (2019) reported that stevia and its derivatives have elevated the percentage of liver fat. Steviol, a toxic stevioside metabolite, is reabsorbed from the intestine to the blood circulation and accumulated in the kidney to be excreted in urine. The body begins to step up urination to facilitate its removal; a process known as diuresis. Moreover, Panichkul et al., 1988, Toskulkao et al., 1994a, Toskulkao et al., 1994b) reported that rats administrated by 4.1 g/kg of stevioside suffered from nephrotoxicity that was obvious by the elevation in levels of blood urea nitrogen and serum creatinine. Toskulkao et al. (1994b) added that the proximal tubules were the site for stevioside accumulation because of the histopathological changes found in it. Taking into consideration that the proximal tubules are the site of elimination of xenobiotics (drugs, food additives and environmental pollutants) via organic ions transporters (Pritchard and Miller, 1993) and any disturbance in this system will reduce xenobiotics clearance.
In the present study, immunological measurement showed an elevation in IgA level and a reduction in IL-10 level upon sucrose administration in both male and female mice. On the other hand, the adverse effects of sucralose and stevia were obvious in levels of immunoglobulins and cytokines. Where, their consumption has led to a vigorous increase in levels of different immunoglobulin (IgG, IgE and IgA) and pro-inflammatory cytokines (IL-6 and -8). This was accompanied by a significant reduction in level of the anti-inflammatory cytokine IL-10.
The mucosal surface of colon hosts >100 trillion of microbes that plays an important role in innate immune system (Ley et al., 2006, Kamada et al., 2013). Under normal conditions, colonocytes form a barrier through which fluid and electrolyte are transported and serve as an innate immune sensor of intestinal flora (Sartor, 2008). This is accomplished by the interaction between toll-like receptors (TLRs) of colonocytes and bacterial derived antigens as lipopolysaccharides (LPS). This TLRs stimulation leads to the activation of NF-κB signaling pathway and subsequent secretion of pro-inflammatory cytokines as IL-8. However several factors, as food additives, can reshape these microbes (Ley et al., 2006, Nicholson et al., 2012) and disrupt these intestinal barriers leading to LPS translocation via the portal vein to liver. In liver, the hepatic innate system will be activated leading to the secretion of pro-inflammatory cytokines (Crispe, 2009). Thus, the high elevation in LPS levels observed in our study with sucralose or stevia administration can be explained by the intestinal barrier disruption and bacterial reshaping. It was found that sucralose inhibited the growth of certain intestinal microbes (Omran et al., 2013) and altered rat gut microbiota leading to inflammatory lymphocyte infiltration (Abou-Donia et al., 2008)
Several studies reported the anti-inflammatory effect of stevioside in vitro and in vivo (Boonkaewwan et al., 2008). The increased level of pro-inflammatory cytokines in this study can be attributed to the accumulation of stevioside in liver and kidney leading to their damage that was obvious by the elevation in liver and kidney function parameters; and the increased oxidative stress that results from high level of NO and low level of the anti-oxidant enzyme SOD. Also, several studies showed that stevia chronic consumption can decrease the beneficial intestinal bacteria and promotes the unhealthy one. Where, Deniņa et al. (2014) reported that the growth of Lactobacillus reuteri strains, a beneficial intestinal bacteria that forms lactic acid, was inhibited by stevia sweeteners stevioside and rebaudioside A. Sehar et al. (2008) found that stevioside increased the proliferation of T and B lymphocytes and added that stevioside is a potent stimulator of humoral and cellular immune response.
In conclusion, non-caloric sweeteners either artificial (sucralose) or natural (stevia) hide several risks to their consumers. They are responsible for: 1- increasing glycaemia in spite of their lack of calories, 2- increasing liver enzymes due to the intestinal flora reshaping, 3- elevation of urea and creatinine levels, 4- reduction of the anti-inflammatory cytokines and elevation of the pro-inflammatory cytokines secretion. On the other hand, sucrose is a caloric sweetener with some risks of course; but it is safer than sucralose or stevia. So we recommend not using sucralose or stevia and decreasing the used daily dose of sucrose instead.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that: there is no conflict of interest.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abou-Donia M.B., El-Masry E.M., Abdel-Rahman A.A., McLendon R.E., Schiffman S.S. Splenda alters gut microflora and increases intestinal p-glycoprotein and cytochrome P-450 in male rats. J. Toxicol. Environ. Health A. 2008;71:1415–1429. doi: 10.1080/15287390802328630. [DOI] [PubMed] [Google Scholar]
- Amchra F.Z., Al Faiz C., Chaouqi S., Khiraoui A., Benhmimou A., Guedira T. Effect of Stevia rebaudiana, sucrose and aspartame on human health: A comprehensive review. J. Med. Plants Stud. 2018;6(1):102–108. [Google Scholar]
- Arumugam B., Subramaniam A., Alaguraj P. Stevia as a Natural sweetener: A Review. Cardiovasc. Hematol. Agents. Med. Chem. 2020 doi: 10.2174/1871525718666200207105436. [DOI] [PubMed] [Google Scholar]
- Becker S.L., Chiang E., Plantinga A., Carey H.V., Suen G., Swoap S.J. Effect of stevia on the gut microbiota and glucose tolerance in a murine model of diet-induced obesity. FEMS Microbiol. Ecol. 2020;96(6):fiaa079. doi: 10.1093/femsec/fiaa079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black R.M., Leiter L.A., Anderson G.H. Consuming aspartame with and without taste: differential effects on appetite and food intake of young adult males. Physiol. Behav. 1993;53(3):459–466. doi: 10.1016/0031-9384(93)90139-7. [DOI] [PubMed] [Google Scholar]
- Boonkaewwan C., Ao M., Toskulkao C., Rao M.C. Specific immunomodulatory and secretory activities of stevioside and steviol in intestinal cells. J. Agric. Food Chem. 2008;56(10):3777–3784. doi: 10.1021/jf072681o. [DOI] [PubMed] [Google Scholar]
- Bueno-Hernández N., Vázquez-Frías R., Abreu Y Abreu A.T., Almeda-Valdés P., Barajas-Nava L.A., Carmona-Sánchez R.I., Chávez-Sáenz J., Consuelo-Sánchez A., Espinosa-Flores A.J., Hernández-Rosiles V., Hernández-Vez G., Icaza-Chávez M.E., Noble-Lugo A., Romo-Romo A., Ruiz-Margaín A., Valdovinos-Díaz M.A., Zárate-Mondragón F.E. Review of the scientific evidence and technical opinion on noncaloric sweetener consumption in gastrointestinal diseases. Rev. Gastroenterol. Mex. 2019;84(4):492–510. doi: 10.1016/j.rgmx.2019.08.001. [DOI] [PubMed] [Google Scholar]
- Cardoso V.N., Barbosa M.F., Muramoto E., Mesquita C.H., Almeida M.A. Pharmacokinetic studies of 131I-stevioside and its metabolites. Nucl. Med. Biol. 1996;23(1):97–100. doi: 10.1016/0969-8051(95)02072-1. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J., Hoon M.A., Ryba N.J., Zuker C.S. The receptors and cells for mammalian taste. Natur. 2006;444(7117):288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- Chang J.-C., Wu M.C., Liu I.-M., Cheng J.-T. Increase of insulin sensitivity by stevioside in fructose-rich chow-fed rats. Horm. Metab. Res. 2005;37(10):610–616. doi: 10.1055/s-2005-870528. [DOI] [PubMed] [Google Scholar]
- Chaturvedi P.R., Decker C.J., Odinecs A. Prediction of pharmacokinetic properties using experimental approaches during early drug discovery. Curr. Opin. Chem. Biol. 2001;5:452–463. doi: 10.1016/s1367-5931(00)00228-3. [DOI] [PubMed] [Google Scholar]
- Crammer B., Ikan R. Sweet glycosides from the stevia plant. Chem. Br. 1986;22:915–917. [Google Scholar]
- Crispe I.N. The liver as a lymphoid organ. Annu. Rev. Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- Deniņa I., Semjonovs P., Fomina A., Treimane R., Linde R. The influence of stevia glycosides on the growth of Lactobacillus reuteri strains. Lett. Appl. Microbiol. 2014;58(3):278–284. doi: 10.1111/lam.12187. [DOI] [PubMed] [Google Scholar]
- Dhurandhar D., Bharihoke V., Kalra S. A histological assessment of effects of sucralose on liver of albino rats. Morphologie. 2018;102:197–204. doi: 10.1016/j.morpho.2018.07.003. [DOI] [PubMed] [Google Scholar]
- Eckel-Mahan K., Sassone-Corsi P. Metabolism and the Circadian Clock Converge. Physiol. Rev. 2013;93(1):107–135. doi: 10.1152/physrev.00016.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emamat H., Ghalandari H., Tangestani H., Abdollahi A., Hekmatdoost A. Artificial sweeteners are related to non-alcoholic fatty liver disease: Microbiota dysbiosis as a novel potential mechanism. EXCLI J. 2020;19:620–626. doi: 10.17179/excli2020-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farid A., Amadou M., Gehan S. Treatment potential of Aloe Vera gel in Gairdia Intestinalis infected albino rats. Journal of the Egyptian Society of Parasitology. 2020;50(1):160–166. doi: 10.12816/jesp.2020.88827. [DOI] [Google Scholar]
- Farid A., Eissa R., Nada A., El Amir M., El Amir A. Serology of the systemic lupus erythematosus in egyptian patients. Journal of the Egyptian Society of Parasitology. 2019;49:403–406. doi: 10.21608/jesp.2019.68159. [DOI] [Google Scholar]
- Farid A., Kamel D., Montaser S., Ahmed M., El Amir M., El Amir A. Synergetic role of senna and fennel extracts as antioxidant, anti-inflammatory and anti-mutagenic agents in irradiated human blood lymphocyte cultures. Journal of Radiation Research and Applied Sciences. 2020;13:191–199. doi: 10.1080/16878507.2020.1723948. [DOI] [Google Scholar]
- Farid A., Kamel D., Montaser S., Ahmed M., El Amir M., El Amir A. Assessment of antioxidant, immune enhancement, and antimutagenic efficacy of fennel seed extracts in irradiated human blood cultures. Journal of Radiation Research and Applied Sciences. 2020;13:260–266. doi: 10.1080/16878507.2020.1728963. [DOI] [Google Scholar]
- Farid A., Tawfik A., Elsioufy B., Safwat G. Narrow Band Ultraviolet B Therapy Deactivates Th1/Th17 Pathway and Activates Th2 Cytokines Secretion in Egyptian Psoriatic Arthritis Patients. Journal of Radiation Research and Applied Sciences. 2020;13:356–361. doi: 10.1080/16878507.2020.1742443. [DOI] [Google Scholar]
- Fowler S.P., Williams K., Resendez R.G., Hunt K.J., Hazuda H.P., Stern M.P. Fueling the obesity epidemic? Artificially sweetened beverage use and long-term weight gain. Obesity (Silver Spring) 2008;16(8):1894–1900. doi: 10.1038/oby.2008.284. [DOI] [PubMed] [Google Scholar]
- Gardner C., Wylie-Rosett J., Gidding S.S., Steffen L.M., Johnson R.K., Reader D., Lichtenstein A.H. Nonnutritive sweeteners: Current use and health perspectives: A scientific statement from the American heart association and the American diabetes association. Circulation. 2012;126(4):509–519. doi: 10.1161/CIR.0b013e31825c42ee. [DOI] [PubMed] [Google Scholar]
- Geuns J.M.C., Buyse J., Vankeirsbilck A., Temme E.H.M. Metabolism of stevioside by healthy subjects. Exp. Biol. Med. 2007;232(1):164–173. [PubMed] [Google Scholar]
- Geuns J.M., Augustijns P., Mols R., Buyse J.G., Driessen B. Metabolism of stevioside in pigs and intestinal absorption characteristics of stevioside, rebaudioside A and steviol. Food Chem. Toxicol. 2003;41(11):1599–1607. doi: 10.1016/s0278-6915(03)00191-1. [DOI] [PubMed] [Google Scholar]
- Greenhill C. Metabolic effects of sucralose. Nat. Rev. Endocrinol. 2020;16(5):256–257. doi: 10.1038/s41574-020-0348-6. [DOI] [PubMed] [Google Scholar]
- Helal E.G.E., Al-Shamrani A., Abdelaziz M.A., El-Gamal M.S. Comparison between the effect of sucralose and sodium saccharin on some physiological parameters in male albino rats. Egyp. J. Hospital Med. 2019;74(7):1552–1558. [Google Scholar]
- Hutapea A.M., Toskulkao C., Buddhasukh D., Wilairat P., Glinsukon T. Digestion of stevioside, a natural sweetener, by various digestive enzymes. J. Clin. Biochem. Nutr. 1997;23(3):177–186. [Google Scholar]
- Jeppesen P.B., Gregersen S., Alstrup K.K., Hermansen K. Stevioside induces antihyperglycaemic, insulinotropic and glucagonostatic efects in vivo: Studies in the diabetic GotoKakizaki (GK) rats. Phytomedicine. 2002;9(1):9–14. doi: 10.1078/0944-7113-00081. [DOI] [PubMed] [Google Scholar]
- Johnson R.J., Segal M.S., Sautin Y., Nakagawa T., Feig D.I., Kang D.H., Gersch M.S., Benner S., Sánchez-Lozada L.G. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am. J. Clin. Nutrit. 2007;86:899–906. doi: 10.1093/ajcn/86.4.899. [DOI] [PubMed] [Google Scholar]
- Kamada N., Seo S.U., Chen G.Y., Nunez G. Role of the gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol. 2013;13:321–335. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- Knight J., Jiang J., Wood K.D., Holmes R.P., Assimos D.G. Oxalate and sucralose absorption in idiopathic calcium oxalate stone formers. Urology. 2011;78(2):475.e9–475.e13. doi: 10.1016/j.urology.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama E., Kitazawa K., Ohori Y., Izawa O., Kakegawa K., Fujino A., Ui M. In vitro metabolism of the glycosidic sweeteners, stevia mixture and enzymatically modified stevia in human intestinal microflora. Food Chem. Toxicol. 2003;41(3):359–374. doi: 10.1016/s0278-6915(02)00235-1. [DOI] [PubMed] [Google Scholar]
- Lenoir M., Serre F., Cantin L., Ahmed S.H. Intense sweetness surpasses cocaine reward. PLoS ONE. 2007;2(8):e698. doi: 10.1371/journal.pone.0000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley R.E., Peterson D.A., Gordon J.I. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Marti N., Funes L.L., Saura D., Micol V. An update on alternative sweeteners. Int. Sugar J. 2008;110:425–429. [Google Scholar]
- Mathlouthi M., Reiser P. first ed. Springer Science and Business Media; 1995. Sucrose: Properties and applications. [Google Scholar]
- Mathur K., Agrawal R.K., Nagpure S., Deshpande D. Effect of artificial sweeteners on insulin resistance among type-2 diabetes mellitus patients. J. Family Med. Prim. Care. 2020;9(1):69–71. doi: 10.4103/jfmpc.jfmpc_329_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattes R.D., Popkin B.M. Nonnutritive sweetener consumption in humans: effects on appetite and food intake and their putative mechanisms. Am. J. Clin. Nutr. 2009;89:1–14. doi: 10.3945/ajcn.2008.26792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitka M. AHA: Added sugar not so sweet. J. Am. Med. Assoc. 2009;302:1741–1742. doi: 10.1001/jama.2009.1534. [DOI] [PubMed] [Google Scholar]
- Nair A.B., Jacob S. A simple practice guide for dose conversion between animals and human. J. Basic. Clin. Pharm. 2016;7(2):27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K., Kasahara D., Yamamoto F. Absorption, distribution, metabolism and excretion in rats. J. Food Hyg. Soc. Jpn. 1986;27:1–8. [Google Scholar]
- Nicholson J.K., Holmes E., Kinross J., Burcelin R., Gibson G., Jia W., Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012;3366:1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- Omran A., Ahearn G., Bowers D., Swenson J., Coughlin C. Metabolic effects of sucralose on environmental bacteria. J. Toxicol. 2013;2013:372986. doi: 10.1155/2013/372986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panichkul T., Glinsukon T., Buddhasukh D., Cheuychit P., Pimolsri U. The plasma levels of urea nitrogen, creatinine and uric acid and urine volume in rats and hamsters treated with stevioside. Thai. J. Toxicol. 1988;4:47–52. [Google Scholar]
- Parasuraman S., Raveendran R., Kesavan R. Blood sample collection in small laboratory animals. J. Pharmacol. Pharmacother. 2010;1(2):87–93. doi: 10.4103/0976-500X.72350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard J.B., Miller D.S. Mechanisms mediating renal secretion of organic anions and cations. Physiol. Rev. 1993;73(4):765–796. doi: 10.1152/physrev.1993.73.4.765. [DOI] [PubMed] [Google Scholar]
- Ramos-Tovar E., Hernández-Aquino E., Casas-Grajales S., Buendia-Montaño L.D., Galindo-Gómez S., Camacho J., Tsutsumi V., Muriel P. Stevia prevents acute and chronic liver injury induced by carbon tetrachloride by blocking oxidative stress through nrf2 upregulation. Oxid. Med. Cell Longev. 2018;2018:3823426. doi: 10.1155/2018/3823426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhomberg L.R., Lewandowski T.A. Methods for identifying a default cross-species scaling factor. Hum. Ecol. Risk. Assess. 2006;12:1094–1127. [Google Scholar]
- Rosales-Gómez C.A., Martínez-Carrillo B.E., Reséndiz-Albor A.A., Ramírez-Durán N., Valdés-Ramos R., Mondragón-Velásquez T., Escoto-Herrera J.A. Chronic consumption of sweeteners and its effect on glycaemia, cytokines, hormones, and lymphocytes of GALT in CD1 mice. Biomed. Res. Int. 2018;2018:1345282. doi: 10.1155/2018/1345282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor R.B. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134(2):577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- Schiffman S.S., Rother K.I. Sucralose, a synthetic organochlorine sweetener: overview of biological issues. J. Toxicol. Environ. Health, Part B. 2013;16:399–451. doi: 10.1080/10937404.2013.842523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman S.S., Sattely-Miller E.A., Bishay I.E. Sensory properties of neotame: Comparison with other sweeteners. In: Weerasinghe D.K., DuBois G.E., editors. Sweetness and sweeteners: Biology, chemistry and psychophysics. Oxford University Press; New York, NY: 2008. pp. 511–529. [Google Scholar]
- Scientific Committee on Food, 2000. Opinion of the Scientific Committee on Food on Sucralose (adopted by the SCF on 7 September 2000). SCF/CS/ADDS/EDUL/190 Final 12/9/2000. European Commission. Health & Consumer Protection Directorate-General.
- Sclafani A. Oral and postoral determinants of food reward. Physiol. Behav. 2004;81(5):773–779. doi: 10.1016/j.physbeh.2004.04.031. [DOI] [PubMed] [Google Scholar]
- Sehar I., Kaul A., Bani S., Pal H.C., Saxena A.K. Immune up regulatory response of a non-caloric natural sweetener, stevioside. Chem. Biol. Interact. 2008;173(2):115–121. doi: 10.1016/j.cbi.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Shil A., Olusanya O., Ghufoor Z., Forson B., Marks J., Chichger H. Artificial sweeteners disrupt tight junctions and barrier function in the intestinal epithelium through activation of the sweet taste receptor, T1R3. Nutrients. 2020;12(6):1862. doi: 10.3390/nu12061862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suez J., Korem T., Zeevi D., Zilberman-Schapira G., Thaiss C.A., Maza O., Israeli D., Zmora N., Gilad S., Weinberger A., Kuperman Y., Harmelin A., Kolodkin-Gal I., Shapiro H., Halpern Z., Segal E., Elinav E. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2004;514:181–186. doi: 10.1038/nature13793. [DOI] [PubMed] [Google Scholar]
- Sylvetsky A.C., Sen S., Merkel P., Dore F., Stern D.B., Henry C.J., Cai H., Walter P.J., Crandall K.A., Rother K.I., Hubal M.J. Consumption of Diet soda sweetened with sucralose and acesulfame-potassium alters inflammatory transcriptome pathways in females with overweight and obesity. Mol. Nutr. Food Res. 2020;64(11):e1901166. doi: 10.1002/mnfr.201901166. [DOI] [PubMed] [Google Scholar]
- Tandel K.R. Sugar substitutes: Health controversy over perceived benefits. J. Pharmacol. Pharmacother. 2011;2(4):236–243. doi: 10.4103/0976-500X.85936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toskulkao C., Deechakawan W., Leardkamolkarn V., Glinsukon T., Buddhasukh D. The low calorie natural sweetener stevioside: nephrotoxicity and its relationship to urinary enzyme excretion in the rat. Phytother. Res. 1994;8(5):281–286. [Google Scholar]
- Toskulkao C., Deechakawan W., Temcharoen P., Buddhasukh D., Glinsukon T. Nephrotoxic effects of stevioside and steviol in rat renal cortical slices. J. Clin. Biochem. Nutr. 1994;16(2):123–131. [Google Scholar]
- U.S. Food and Drug Administration (FDA), 1998. Food additives permitted for direct addition to food for human consumption; sucralose. 21CFR Part 172 [Docket No. 87F-0086]. Fed. Reg. 63(64), 16417–16433.
- USFDA . US Food and Drug Administration; Rockville, MD: 2005. Guidance for Industry: Estimating the Maximum Safe Starting Dose in Adult Healthy Volunteer. [Google Scholar]
- Wang Q.P., Lin Y.Q., Zhang L., Wilson Y.A., Oyston L.J., Cotterell J., Qi Y., Khuong T.M., Bakhshi N., Planchenault Y., Browman D.T., Lau M.T., Cole T.A., Wong A.C., Simpson S.J., Cole A.R., Penninger J.M., Herzog H., Neely G.G. Sucralose promotes food intake through NPY and a neuronal fasting response. Cell Metab. 2016;24(1):75–90. doi: 10.1016/j.cmet.2016.06.010. [DOI] [PubMed] [Google Scholar]
- Wingard R.E., Brown J.P., Enderlin F.E., Dale J.A., Hale R.L., Seitz C.T. Intestinal degradation and absorption of the glycosidic sweeteners stevioside and rebaudioside A. Experientia. 1980;36(5):519–520. doi: 10.1007/BF01965774. [DOI] [PubMed] [Google Scholar]
- Yang Q. Gain weight by “going diet?” Artificial sweeteners and the neurobiology of sugar cravings. Yale J. Biol. Med. 2010;83(2):101–108. [PMC free article] [PubMed] [Google Scholar]