Abstract
Objectives
There are many treatment modalities for myofascial pain, and recent findings reported in the literature highlight the superiority of using local anesthetics as the treatment of choice. The objective of the present study was to compare the effectiveness of two of the most used local anesthetic agents—lidocaine and mepivacaine—in the management of myofascial pain.
Materials and methods
Thirty patients (20 females, 10 males) were randomly assigned to one of two groups: 50% received lidocaine and 50% received mepivacaine. Trigger point injections in the orofacial region were administered 4 times, 10 days between each injection, with 4 weeks of follow-up after the end of the treatment course. Pain levels were recorded using a visual analog scale (VAS) at the time of follow-up and 30 min after injection.
Results
All patients exhibited statistically significant improvement when comparing pre- and post-treatment mean values. Both local anesthetics (i.e., lidocaine and mepivacaine) were similarly effective for the management of myofascial pain (p = 0.875). The mepivacaine-treated group exhibited significantly lower post-injection tenderness than the lidocaine group (p = 0.038). There was no relationship between sex and treatment response. Female and male patients both reported similar responses in terms of VAS scores (p = 0.818).
Conclusion
No drug was superior in the long term; thus, the clinician’s choice can be based on drug availability and patient medical history.
Keywords: Randomized controlled trial, Myofascial pain, Trigger points, Mepivacaine, Lidocaine
Abbreviations: MFP, myofascial pain; MTrPs, myofascial trigger points; VAS, visual analog scale; SPSS, Statistical Package for the Social Sciences
1. Introduction
Myofascial pain (MFP) is a chronic condition characterized by the presence of painful nodes known as trigger points (MTrPs) that are found in the muscle or fascia, causing local or referred tenderness, local twitching of the taught muscle, and neuromuscular dysfunction. (Shah and Gilliams, 2008, Simons, 2004). The prevalence of myofascial pain varies from 30% to 93% among patients with chronic musculoskeletal pain. The estimated mean overall prevalence of active MTrPs has been reported to be 46.1 ± 27.4% (Simons, 1996, Fleckenstein, 2010). Many etiological factors contribute to chronic muscle pain, which negatively affects quality of life and work productivity. Some of the most common factors include incorrect posture continuous overuse of the upper body limbs that lead to fatigue and gradual development of MFP, trauma (sprains or strains), and, ergonomic injuries such as chronic neck pain that dentists experience in the work place (Gerwin, 2010, Lundberg, 1999, Çeliker et al., 2010).
Currently, the management of MFP implies “trigger point inactivation.” This results in decreased pain to enable functional muscle movements and regain muscle strength (Simons, 2004). Needling, with or without active agents, is known to be an effective method of trigger point inactivation (Ay and Evcik, 2010). In fact, dry needling proved to be a superior and safer method compared with systemic analgesics and muscle relaxants taken orally (Pérez, 2015). Recently, studies have shown that patients receiving injection of local anesthesia, compared with dry needling, reported less post-injection tenderness. Moreover, local anesthetic injection proved to be more effective in reducing pain scores in the medium term than dry needling (Kamanli, 2005, Liu, 2015).
Many clinical trials have tested the effect of lidocaine or mepivacaine on the management of MFP (Karadaş, 2013, Frost, 1980, Iwama, 2001). A study by Iwama et al. compared lidocaine versus mepivacaine, both of which were diluted in water, and concluded equal effects (Brockmann, 2014). To our knowledge, however, the present study is the first to compare efficacy of mepivacaine versus lidocaine injection without dilution. The objectives were to test the efficacy of mepivacaine versus lidocaine trigger point injection for the alleviation of local and referred pain, and to reduce post-injection tenderness perceived by patients experiencing orofacial MFP.
2. Materials and methods
2.1. Participant selection
This prospective, randomized controlled study was conducted at the outpatient clinic of a hospital. The objectives were to compare two different local anesthetic substances used for orofacial trigger point injections, with regard to the level of pain 30 min after injection (post-injection tenderness) and long-term chronic pain relief. Informed consent was obtained from each participant before the study. Thirty male and female patients 19–50 years of age, who were diagnosed with orofacial MFP, were recruited for this study.
The inclusion criteria were as follows: primary diagnosis of orofacial MFP, duration of symptoms ≥6 months, and pain level score ≥3 on a visual analog scale (VAS). Individuals taking systemic medications for pain relief, those with fibromyalgia, internal temporomandibular defects, rheumatic or neurological diseases, needle phobia, amide local anesthetic allergy, severe hepatic or cardiovascular disease, patients on anticoagulants and patients undergoing occlusal splint therapy or a physical therapy program were excluded from the study (Food and Drug Administration Revised February, 2010, Food and Drug Administration Revised February, 2010, Clauw, 2014, Simons, 2008).
2.2. Study design
All subjects provided informed consent to participate in the study, which was approved by the Ethics Committee of the College of Dentistry Research Center. Patients were recruited from the oral medicine clinics from September 2015 to April 2016. The diagnosis of active MTrPs and the choice of injection procedure were based on the criteria and method described by Simons et al. Active MTrPs were defined as the sites where painful taut ribbon-shaped or cord-shaped structures were palpable and were accompanied by remote referred pain. (Simons, 2008) Patients were randomly assigned to one of two groups: group 1 (lidocaine 1% without vasoconstrictor); group 2 (mepivacaine 2% without vasoconstrictor). (Yagiela, 1982) The injection volume was 1.8 mL at each injection site. All patients received four injections once every 10 days, with 2 weeks between each injection, performed by one doctor who was blinded to patient allocation (Karadaş, 2013).
Sites were marked by localization of MTrPs in the masseter muscle according to three diagnostic criteria described by Simons et al. (Simons, 1999) Injections were administered to two of the most painful taut bands at each side of the right and left masseter muscles per subject. The injection was administered using a 22-gauge needle in a taut band localized between the thumb and index finger. The investigator injected 0.2 mL of the solution and moved the needle forward and backward, redirected to adjacent trigger points, and eliciting a local twitch response (Hong, 1994). For the purposes of this study, the researchers limited the local twitch response to 4 times at each taut band; thus, each patient received 3.2 mL of the solution. The injected area was compressed firmly for 3 min after injection to achieve hemostasis (Hong, 1994).
The investigator then asked the subject to score treatment efficacy according to a VAS. Pain levels were recorded on a VAS at the time of follow-up, and 30 min after injection (post-injection tenderness). Patients were monitored for 30 min after injection for any signs of complications or allergic reactions. Pain scores according to VAS were recorded as follows: before treatment; 30 min after each injection; and every week from the start of the study until 4 weeks after treatment. A VAS score of 0 indicated no pain and a score of 10 indicated the most severe pain. Data analysis was performed using SPSS version 20.0 (IBM Corporation, Armonk, NY, USA). Two-way analysis of variance (ANOVA) with repeated measures test was used to analyze differences, which were considered to be statistically significant at p < 0.05.
3. Results
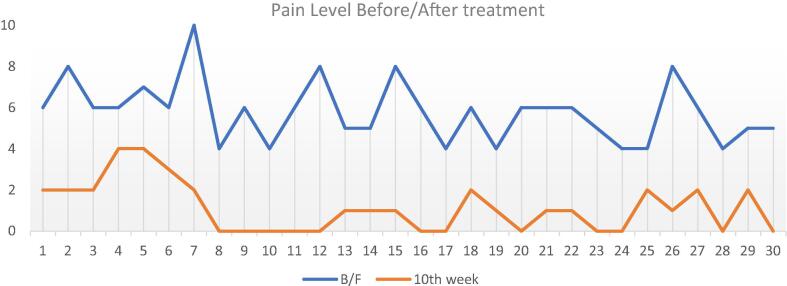
Data were collected and organized, and subsequently analyzed using SPSS version 20. Two-way ANOVA was used to analyze data from the lidocaine and mepivacaine groups, and to compare females with males; statistical significance was set at p < 0.05. There was a total of 30 patients (20 females, 10 males). Both groups demonstrated statistically significant improvement when comparing pre- and post-treatment mean VAS pain scores for both males and females: pre-treatment mean pain score 5.8, and post-treatment score 1.13 (Line graph 1) and (Table 1).
Line Graph. 1.
Y-axis: pain level on the Visual Analogue Scale (VAS) score of 0 indicated no pain and a score of 10 indicated the most severe pain. X-axis: subjects numbered from 1 to 30. It illustrates a significant pain reduction between the pre-treatment (blue line) and the post-treatment (orange line).
Table 1.
Baseline characteristic of participants: pre-treatment and post-treatment pain scores.
| Participant number | Pre-treatment pain score | Post treatment pain score |
|---|---|---|
| 1 | 6 | 2 |
| 2 | 8 | 2 |
| 3 | 6 | 2 |
| 4 | 6 | 4 |
| 5 | 7 | 4 |
| 6 | 6 | 3 |
| 7 | 10 | 2 |
| 8 | 4 | 0 |
| 9 | 6 | 0 |
| 10 | 4 | 0 |
| 11 | 6 | 0 |
| 12 | 8 | 0 |
| 13 | 5 | 1 |
| 14 | 5 | 1 |
| 15 | 8 | 1 |
| 16 | 6 | 0 |
| 17 | 4 | 0 |
| 18 | 6 | 2 |
| 19 | 4 | 1 |
| 20 | 6 | 0 |
| 21 | 6 | 1 |
| 22 | 6 | 1 |
| 23 | 5 | 0 |
| 24 | 4 | 0 |
| 25 | 4 | 2 |
| 26 | 8 | 1 |
| 27 | 6 | 2 |
| 28 | 4 | 0 |
| 29 | 5 | 2 |
| 30 | 5 | 0 |
Statistically significant improvement was detected in both the lidocaine and mepivacaine groups. The mean pain reduction for lidocaine was 4.3 and 5.07 for the mepivacaine group.
At the end of treatment, comparison between lidocaine and mepivacaine revealed that neither of the agents demonstrated superiority according to intergroup comparisons (p = 0.875) (Table 2). Moreover, both female and male patients demonstrated similar VAS scores (p = 0.818) (Table 3).
Table 2.
Comparison between Lidocaine & Mepivacaine. Neither of the agents demonstrated superiority according to intergroup comparisons (P > 0.05).
| Injection type | Mean Difference | Standard Error | Sig.a | 95% Confidence Interval for Differencea |
||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Lidocaine | Mepivacaine | 0.054 | 0.340 | 0.875 | -0.645 | 0.754 |
| Mepivacaine | Lidocaine | -0.054 | 0.340 | 0.875 | -0.754 | 0.645 |
Sig.: Significance probability.
: Dependent variable.
Table 3.
Comparison between male and female groups (P > 0.05). Both female and male patients demonstrated similar VAS scores (p = 0.818).
| Gender | Mean difference | Standard error | Sig.a | 95% Confidence interval for difference a |
||
|---|---|---|---|---|---|---|
| Upper | Lower | |||||
| Male | Female | 0.079 | 0.340 | 0.818 | 0.779 | −0.620 |
| Female | Male | −0.079 | 0.340 | 0.818 | 0.620 | −0.779 |
Sig.: Significance probability.
: Dependent variable.
Using two-way ANOVA to compare the intensity of pain after each course of treatment, postoperative pain associated with mepivacaine was significantly less than that associated with lidocaine according to VAS scores. The mean postoperative pain score according to VAS for the mepivacaine group was 0.9 and 2.4 for the lidocaine group (p = 0.038) (Table 4, Table 5).
Table 4.
ANOVA: Two-Factor without replication. Using two-way ANOVA to compare the intensity of pain after each course of treatment. It showed the post-operative pain associated with Mepivacaine was significantly less than Lidocaine on visual analogue scale (VAS). *ANOVA: analysis of variance
| ANOVA: Two-factor without replication | ||||
|---|---|---|---|---|
| Count | Sum | Average | Variance | |
| Mepivacaine | 4 | 3.733333 | 0.933333258 | 0.305185006 |
| Lidocaine | 4 | 9.6 | 2.4 | 0.154074193 |
Table 5.
Analysis of variance for Lidocaine & Mepivacaine drugs in post-operative pain yielded significant P-value.
|
*ANOVA | ||||||
|---|---|---|---|---|---|---|
| Source of variation | *SS | *df | *MS | F ratio | P-value | F critical |
| Lidocaine & Mepivacaine drugs | 4.302222663 | 1 | 4.302223 | 12.68123 | 0.037791 | 10.12796 |
ANOVA: analysis of variance; SS: Sum of the squares; df: degrees of freedom; MS: Mean Square.
4. Discussion
There are many treatment modalities for MFP, and recent literature findings have highlighted the superiority of local anesthetics as a treatment choice (Liu, 2015). To our knowledge, the present study was the first to measure the effectiveness of two of the most commonly used local anesthetic agents—lidocaine and mepivacaine—in the management of orofacial MFP. The present study aimed to compare two different local anesthetic agents used for orofacial trigger point injections, with regard to the level of pain 30 min after injection (i.e., post-injection tenderness) and long-term chronic pain relief.
Many studies have been done to evaluate the efficacy between lidocaine and normal saline. For instance, Doruk Analan et al. study concluded that lidocaine injections showed better results in reducing symptoms in patients with orofacial MFP than normal saline injections (Doruk Analan et al., 2019).
In addition, Iwama et al. study compared the efficacy of different water-diluted local anesthetics such as lidocaine and mepivacaine for reducing pain in patient with chronic orofacial MFP. The study concluded that may be water-diluted lidocaine or water-diluted mepivacaine injections are beneficial for the treatment of orofacial MFP more than normal saline injections (Iwama et al., 2001).
Results of our study demonstrated that the treatment of MFP using local anesthetics led to effective pain reduction, as reported in previous studies (Kamanli, 2005, de Abreu Venancio, 2009, Karadaş, 2013, Lavelle, 2007). Most hypotheses regarding the development of TrPs suggest that the deprivation of oxygen plays a major role in sustained muscle contracture. As a result, transient hypoxia leads to the development of limited ischemic areas and the release of biochemical substances that are responsible for the development of trigger points and chronic pain. In a study comparing trigger points and unaffected areas, Shah et al. identified many anti-inflammatory mediators such as substance P (Shah, 2008). These anti-inflammatory mediators sensitize nociceptors, causing tissue inflammation and the pain associated with MFP. Local anesthetic injection contributes to inhibition of sodium influx; consequently, no impulses are generated and pain perception is reduced. In addition, local anesthetic agents prevent nociception by inhibiting the release of the neurotransmitter substance P (Li, 1995).
Results of this study revealed no significant difference between the treatment groups (p = 0.875 [n = 30]), with both drugs demonstrating equal effectiveness in pain management. The mean reduction in pain was 5.07 for mepivacaine and 4.3 for lidocaine. Similarly, there was no difference between males and females in terms of pain reduction (p = 0.818).
Our results demonstrated that mepivacaine resulted in significantly lower post-injection tenderness than lidocaine, mean pain score after mepivacaine injection (0.9), and for lidocaine (2.4) (p = 0.03). We hypothesize that this is because mepivacaine has less vasodilating ability than lidocaine, thus a longer duration of anesthesia (Brockmann, 2014, Ekenstam et al., 1957).
While the statistical test yielded no significant difference between the two drugs, further studies on a larger scale with longer follow-up times are recommended. In addition, because no drug was superior, clinicians may consider the availability of the drug as well as the patient’s overall health for the choice of local anesthetic agent (e.g., contraindications to certain local anesthetics, such as allergy) for the treatment of patients diagnosed with MFP.
5. Conclusions
Both lidocaine and mepivacaine injection into the trigger point were effective in relieving pain among patients with MFP, with mepivacaine yielding results comparable to those of lidocaine. Although there was less postoperative pain associated with mepivacaine in the short term, it had the same long-term effect as lidocaine. Clinicians have the choice to use either of these two drugs, taking into consideration the patient’s general health and drug availability.
Acknowledgements
This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-track Research Funding Program.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Hamad Albagieh, Email: halbageah@ksu.edu.sa.
Ashwag Aloyouny, Email: ayaloyouny@pnu.edu.sa.
Nojoud Alshehri, Email: 437202792@student.ksu.edu.sa.
Noor Alsammahi, Email: nyalsammahi@pnu.edu.sa.
Dima Almutrafi, Email: dbalmatrafi@psmmc.med.sa.
References
- Shah J.P., Gilliams E.A. Uncovering the biochemical milieu of myofascial trigger points using in vivo microdialysis: an application of muscle pain concepts to Myofascial Pain syndrome. J. Bodywork Movement Therap. 2008;12(4):371–384. doi: 10.1016/j.jbmt.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Simons D.G. Review of enigmatic MTrPs as a common cause of enigmatic musculoskeletal pain and dysfunction. J. Electromyogr. Kinesiol. 2004;14(1):95–107. doi: 10.1016/j.jelekin.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Simons D.G. Clinical and etiological update of myofascial pain from trigger points. J. Musculoskeletal Pain. 1996;4(1–2):93–122. [Google Scholar]
- Fleckenstein J. Discrepancy between prevalence and perceived effectiveness of treatment methods in Myofascial Pain syndrome: results of a cross-sectional, nationwide survey. BMC Musculoskeletal Disorders. 2010;11(1):32. doi: 10.1186/1471-2474-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerwin R. Myofascial pain syndrome: here we are, where must we go? J. Musculoskeletal Pain. 2010;18(4):329–347. [Google Scholar]
- Lundberg U. Psychophysiological stress responses, muscle tension, and neck and shoulder pain among supermarket cashiers. J. Occup. Health Psychol. 1999;4(3):245. doi: 10.1037//1076-8998.4.3.245. [DOI] [PubMed] [Google Scholar]
- Çeliker R., Atalay A., Guven Z. Health-related quality of life in patients with Myofascial Pain syndrome. Curr. Pain Headache Rep. 2010;14(5):361–366. doi: 10.1007/s11916-010-0141-7. [DOI] [PubMed] [Google Scholar]
- Ay S., Evcik D. Comparison of injection methods in Myofascial Pain syndrome: a randomized controlled trial. Clin. Rheumatol. 2010;29(1):19–23. doi: 10.1007/s10067-009-1307-8. [DOI] [PubMed] [Google Scholar]
- Pérez L.M.G. Deep dry needling of trigger points located in the lateral pterygoid muscle: efficacy and safety of treatment for management of myofascial pain and temporomandibular dysfunction. Medicina oral, patología oral y cirugía bucal Ed inglesa. 2015;20(3):9. doi: 10.4317/medoral.20384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamanli A. Comparison of Lidocaine injection, botulinum toxin injection, and dry needling to trigger points in Myofascial Pain syndrome. Rheumatol. Int. 2005;25(8):604–611. doi: 10.1007/s00296-004-0485-6. [DOI] [PubMed] [Google Scholar]
- de Abreu Venancio Botulinum toxin, Lidocaine, and dry-needling injections in patients with Myofascial Pain and headaches. CRANIO®. 2009;27(1):46–53. doi: 10.1179/crn.2009.008. [DOI] [PubMed] [Google Scholar]
- Karadaş Ö. Lidocaine injection of pericranial myofascial trigger points in the treatment of frequent episodic tension-type headache. J Headach Pain. 2013;14(1):1–8. doi: 10.1186/1129-2377-14-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavelle E.D. Myofascial trigger points. Anesthesiology clinics. 2007;25(4):841–851. doi: 10.1016/j.anclin.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Liu L. Effectiveness of dry needling for myofascial trigger points associated with neck and shoulder pain: a systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2015;96(5):944–955. doi: 10.1016/j.apmr.2014.12.015. [DOI] [PubMed] [Google Scholar]
- Frost F.A. A control, double-blind comparison of Mepivacaine injection versus saline injection for Myofascial Pain. The Lancet. 1980;315(8167):499–501. doi: 10.1016/s0140-6736(80)92761-0. [DOI] [PubMed] [Google Scholar]
- Iwama H. Water-diluted local anesthetic for trigger-point injection in chronic myofascial pain syndrome: evaluation of types of local anesthetic and concentrations in water. Reg. Anesth. Pain Med. 2001;26(4):333–336. doi: 10.1053/rapm.2001.24672. [DOI] [PubMed] [Google Scholar]
- Brockmann W.G. Mepivacaine: a closer look at its properties and current utility. General dentistry. 2014;62:70–75. [PubMed] [Google Scholar]
- Food and Drug Administration Revised February 2010. Xylocaine (Lidocaine Hcl) accessed November 2015.
- Food and Drug Administration Revised February 2010.Carbocaine (Mepivacaine HCl) accessed November 2015.
- Clauw D.J. Fibromyalgia: a clinical review. JAMA. 2014;311(15):1547–1555. doi: 10.1001/jama.2014.3266. [DOI] [PubMed] [Google Scholar]
- Simons D.G. New views of myofascial trigger points: etiology and diagnosis. Arch. Phys. Med. Rehabil. 2008;89(1):157–159. doi: 10.1016/j.apmr.2007.11.016. [DOI] [PubMed] [Google Scholar]
- Yagiela J.A. Mechanism of epinephrine enhancement of Lidocaine-induced skeletal muscle necrosis. J. Dent. Res. 1982;61(5):686–690. doi: 10.1177/00220345820610051301. [DOI] [PubMed] [Google Scholar]
- Simons, D.G., et.al., 1999. Travell & Simons' Myofascial Pain and dysfunction: upper half of body (Vol. 1). Lippincott Williams & Wilkins.
- Hong C.Z. LIDOCAINE INJECTION VERSUS DRY NEEDLING TO MYOFASCIAL TRIGGER POINT: The Importance of the Local Twitch Response. Am. J. Phys. Med. Rehabil. 1994;73(4):256–263. doi: 10.1097/00002060-199407000-00006. [DOI] [PubMed] [Google Scholar]
- Hong C.Z. Considerations and recommendations regarding myofascial trigger point injection. J. Musculoskeletal Pain. 1994;2(1):29–59. [Google Scholar]
- Doruk Analan P., Aslan H., Tok Umay S. A comparison of the effects of lidocaine and saline injection on pain, disability, and shear-wave elastography findings in patients with myofascial trigger points. Cyprus J. Med. Sci. 2019;4(2):103–109. doi: 10.5152/cjms.2019.709. [DOI] [Google Scholar]
- Iwama H., Ohmori S., Kaneko T., Watanabe K. Water-diluted local anesthetic for trigger-point injection in chronic myofascial pain syndrome: evaluation of types of local anesthetic and concentrations in water. Reg. Anesth. Pain. Med. 2001;26(4):333–336. doi: 10.1053/rapm.2001.24672. [DOI] [PubMed] [Google Scholar]
- Shah J.P. Biochemicals associated with pain and inflammation are elevated in sites near to and remote from active myofascial trigger points. Arch. Phys. Med. Rehabil. 2008;89(1):16–23. doi: 10.1016/j.apmr.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Li Y.M. Local anesthetics inhibit substance P binding and evoked increases in intracellular Calcium sup 2+ J. Am. Soc. Anesthesiologists. 1995;82(1):166–173. doi: 10.1097/00000542-199501000-00021. [DOI] [PubMed] [Google Scholar]
- AF Ekenstam, B., et al., 1957. Local anaesthetics: I. N-alkyl pyrrolidine and N-alkyl piperidine carboxylic acid amides. Acta Chem Scand, 11, pp.1183–90.