Abstract
Cell- based targeted delivery is recently gain attention as a promising platform for delivery of anticancer drug in selective and efficient manner. As a new biotechnology platform, bacterial ghosts (BGs) have novel biomedical application as targeted drug delivery system (TDDS). In the current work, Salmonellas’ BGs was utilized for the first time as hepatocellular cancer (HCC) in-vitro targeted delivery system. Successful BGs loading and accurate analysis of doxorubicin (DOX) were necessary steps for testing the applicability of DOX loaded BGs in targeting the liver cancer cells. Loading capacity was maximized to reach 27.5 µg/mg (27.5% encapsulation efficiency), by incubation of 10 mg BGs with 1 mg DOX at pH 9 in constant temperature (25 °C) for 10 min. In-vitro release study of DOX loaded BGs showed a sustained release (182 h) obeying Higuchi sustained kinetic release model. The death rate (tested by MTT assay) of HepG2 reached to 64.5% by using of 4 μg/ml, while it was about 51% using the same concentration of the free DOX (P value < 0.0001 One-way ANOVA analysis). The proliferative inhibitory concentration (IC50) of the DOX combined formula was 1.328 µg/ml that was about one third of the IC50 of the free DOX (3.374 μg/ml). Apoptosis analysis (tested by flow-cytometry) showed more accumulation in early apoptosis (8.3%) and late apoptosis/necrosis (91%) by applying 1 μg/ml BGs combined DOX, while 1 μg/ml free DOX showed 33.4% of cells in early apoptosis and 39.3% in late apoptosis/necrosis, (P value˃ 0.05: one-way ANOVA). In conclusion, DOX loaded Salmonellas’ BGs are successfully prepared and tested in vivo with promising potential as hepatocellular cancer (HCC) targeted delivery system.
Keywords: Bacterial ghosts, Salmonella, Cell based delivery, Liver cancer, Targeted drug delivery system
1. Introduction
Till date, cancer incidence is the second significant cause of mortality globally (Ferlay et al., 2019). Although chemotherapy treatment for cancer is the main modality, the side-effects of chemotherapeutics have become life-threatening. This is due to non-selective distribution of chemo-molecules resulting in massive destruction in healthy normal cells as well as cancerous ones. Therefore, innovative therapeutic strategies are necessary to be developed for tumor treatment. These approached either passive targeting (depending on the size of leaky vascular fenestration) or active targeting (based on specific biological interaction with target cells (Alanazi et al., 2020). Cell- based targeted delivery is recently gain attention as a promising platform for delivery of anticancer drug in selective and efficient manner. Bacterial ghosts (BGs) as biotechnology platform is considered one of the most advanced site targeting vehicles based on bacterial cell-based targeting strategies (Paukner et al., 2004).
BGs is the cellular intact shell free from most of its cytoplasmic contents that can be obtained first from Gram-negative bacteria by using of bacteriophage PhiX174 lysis gene E (Kwon et al., 2005). Recently, it has also obtained from both Gram-negative and Gram-positive bacteria using critical concentrations of specific chemicals under customized conditions (Amro et al., 2014, Rabea et al., 2018). The retaining of surface properties of the non-living bacteria promotes targeting specific tissues and cells. The uptake of bioengineered probiotic Escherichia coli strain Nissle 1917 (EcN) ghosts by ocular tissues suggested promising BGs based ocular delivery system (Stein et al., 2013). Mannheimia haemolytica were used for targeting DOX toward human colorectal adenocarcinoma cells (Caco-2) (Paukner et al., 2004). Recently, ghosts of E. coli were also used for targeting 5-fluorouracil toward the Caco-2 cells (Youssof et al., 2019). Noticeably, Salmonella was shown to be able to presence in tumor regions selectively. Various studies have shown successful antitumor activity when Salmonella was combined with chemotherapeutic (Jia et al., 2007; Saltzman et al., 2018).
Salmonella could selectively migrate to the tumor region (Wang et al., 2016). This targeting capability was accounted for the following mechanistic reasons involving: serine, aspartate and galactose/ribose receptor on surface of the Salmonella. Those receptors attracted Salmonella toward cancerous cells (Kasinskas and Forbes, 2006). Thus, Salmonella was shown to selectively amasses at a ratio of >1000:1 in tumor tissues in compare with normal tissues (Low et al., 1999). Accordingly, massive numbers of Salmonella were shown to accumulate in tumor area. Despite the ability of living Salmonella in localizing specifically in tumor, its ability to grow out of control and releasing toxins limited its used (Rabea et al., 2018). Therefore, taking the advantageous of ghost bacteria over living Salmonella is mandating its utilization as carrier for the anticancer drugs.
In the present study, non-living Salmonella enterica serovar typhimurium bacterial ghosts (SEG) was effectively produced utilizing tween 80-induced protocol. The main goal of this study was to evaluate SEG as delivery system of Doxorubicin (DOX) as novel anticancer delivery system. The use of targeting (SEG) is therefore hoping to maximizing the efficacy and minimizing doxorubicin’s side effects including sever cardiotoxicity (Bekaii-Saab et al., 2009). Also, factors affecting DOX loading capacity were studied. Pharmaceutical evaluation of DOX loaded SEG was carried out through investigating the DOX releasing behavior and kinetics from SEG. Additionally, antiproliferative activity and apoptosis assay were conducted to assess the in vitro cytotoxicity.
2. Materials and methods
2.1. BGs preparation
BGs were constructed from Salmonella enterica serovar typhimurium ATCC 11331 using the new protocol that prescribed previously (Rabea et al., 2018). Briefly, The Salmonella cells were incubated in Muller-Hinton broth containing 7% v/v tween 80 for 24 h at 37 °C. The grown cells were posed to lactic acid (pH = 3.6). By centrifugation, ghosts’ pellets were separated then washed three times by a sterile solution of half normal saline. Finally, the obtained ghosts were lyophilized and stored for using as a DDS.
2.2. Loading of BGs with doxorubicin
DOX (LC laboratories, Woburn, MA, USA) was loaded into the lyophilized BGs according to reported protocol with optimizing modification (Paukner et al., 2004). Tris HCl buffer solution (pH 9) was used for dissolving DOX powder. Many factors were optimized to reach the maximum loading capacity include: temperature, time of the incubation, DOX concentration, and pH of the DOX buffer solution.
In all trials for testing various factors that affect the loading capacity, 10 mg of lyophilized BGs were incubated with 200 μl DOX solution (5 mg/ml), in a total weight of 1 mg (Except when using different DOX quantities). With constant factors; pH = 7, DOX quantity = 1 mg and incubation period = 10 min, different incubation temperatures (4 °C, 25 °C and 60 °C) were used. In other trials, different times of incubation were tested (10, 20, 30 and 60 min), while constant factors (pH = 7, 25 °C and incubation period = 10 min) were applied. Using constant factors of 25 °C, pH = 7 for 10 min, different DOX quantities were tested (1, 2, 3 and 6 mg). Three different Tris HCl stock buffer solutions were prepared in different pHs: 7, 8 and 9 to be used for dissolving DOX powder and constant factors (25 °C, incubation time = 10 min and DOX quantity = 1 mg) were used.
The loaded BGs were separated by centrifugation at 5000g for 3 min. The obtained pellets were washed by the Tris HCl buffer three times then they were lyophilized. DOX stability has been examined in these previous conditions.
The loading capacity, the amount of drug loaded per unit weight of BG, was calculated by relating the amount of loaded DOX (to the amount of the BG (weight of bacterial ghost in mg before loading). The following equation was used:
The percentage encapsulation efficiency also was calculated by correlating the amount of DOX loaded (μg to the amount of DOX added μg as the following is the equation used:
2.3. Extraction of the loaded DOX
With the aim of evaluate the BGs loading capacity, the matrix of BGs was disturbed to elaborate the loaded DOX as described before (Alsuwyeh et al., 2018). In brief, 10 mg of lyophilized packed BGs were suspended in 1 ml distilled water then mixed with equal volume of 10% SDS solution and incubated for 10 min at 65 °C. One ml of sulfuric acid (10 mM) were added and further incubated at the above conditions. The suspension was centrifuged in order to obtain the supernatant that was utilized in analysis of DOX.
2.4. The loaded DOX analysis
Quantities of the loaded DOX were analyzed using a new environmentally benign reversed phase high performance chromatography method (RP-HPLC), as formerly described (Alsuwyeh et al., 2018). The technique was evaluated for accuracy, linearity, robustness, precision, selectivity and specificity. This process was carried at 25 °C using Neucleodur 150 mm 4.6 mm RP C8 column that filled with 5 μm static phase filler. The mobile phase comprised of methanol/ethyl acetate (7:3 v/v) in flow rate of 1 ml/min. Using UV mode at wavelength of 480 nm, the drug was detected in. All trials were triplicated and the average of readings has been taken.
2.5. Drug release
In-vitro drug release has completed for both groups: the free DOX and the ghosts combined DOX, using dynamic dialysis method as described before (Ge et al., 2018). In brief, a total volume of 1 ml (4 mg/ml) of free DOX PBS (pH 7.4) solution and of the same volume of ghost combined DOX with a concentration equivalent to the free DOX concentration are placed in dialysis membrane bags. The dialysis bag (MWCO 6–8 kDa, Livingstone, NSW, Australia) was soaked in distilled water for 12 h before use. Each bag was placed in beaker contain 100 ml phosphate buffer saline (PBS) with pH 7.4 and at 37 °C 1 shaking incubator with applying slow shaking (50 rpm). One ml sample was withdrawn from each beaker every predetermined period (0.2, 0.3, 0.5, 1, 2, 3, 4, 5, 6, 12, 24, 48, 72, 96, 182 and 375 h) for analysis by the previously described RP-HPLC. To maintain the sink condition, 1 ml of PBS was added to replace the withdrawn samples. The drug release curves were conducted for both groups between the calculated percentages of released drug versus time. By acquiring the linear regression equations, the most suitable mathematical model was chosen for the kinetics release.
2.6. Assay of antiproliferative activity by measuring HepG2 vitality using MTT assay
The cells of HepG2 ATCC HB-8065 (American Type Cell Culture, VA, USA) were maintained and cultured in DMEM (Dulbecco’s Modified Eagle’s Medium)/ high glucose with 2 mM L-glutathione, 1% penicillin/streptomycin and 10% FBS (Sigma-Aldrich Chemie Gmbh, Taulfkirchen, Germany). The vitality of HepG2 cells was examined after applying escalating concentrations of the free DOX, free ghosts and DOX loaded ghosts. The mitochondrial enzymatic capacity of the live cells was examined for reduction of 3-(2,5-dimethylthiazol-2yl)2,5-diphenyltetrazolium bromide (MTT) into insoluble purple crystals of formazan that can be quantitatively and optically measured (Korashy and El-Kadi, 2004). The cells were incubated at 37 °C for 24 h in a 5% CO2 humidifier incubator Hep G2 [HEPG2] (ATCC® HB?8065™) with various concentrations (0.5 μg, 1 μg, 2 μg and 4 μg) of each group. Afterward, the consumed media were replaced with 200 μl serum free media (SFM) containing MTT (2 mg/ml of PBS - pH 7.2). Further incubation was done in the same above conditions for 3 h. The media were removed by inversion of plates and the formazan crystals were dissolved by gentle shaking for 1 h with 200 μl isopropanol. The color intensity was measured by observing the maximum absorption at 570 nm using BIO-TEK instrument EL 312-e microplate reader (BioTek Instruments Inc., Winooski, VT, USA). The standard curve of MTT has been conducted in order to accurately correlate the optical absorbance with the number of cells. The viable cells percentage has been calculated in relation to 100% to the control cells.
2.7. Analysis of apoptosis by flow cytometry
The comparison between the effects of two increasing concentrations of all tested groups has been achieved by quantifying live, apoptotic, necrotic and late necrotic HepG2 cells using flow cytometry according to what described before (Ashour et al., 2014). The cells were cultured in DMEM/high glucose with 2 mM L-glutathione, 1% penicillin/streptomycin and 10% FBS. The adjusted concentration of cells (1.5 × 106/ml) either treated by two concentrations (0.5 μg/ml and 1 μg/ml) of the three groups, or untreated (control media) and all groups were incubated for 72 h at 37 °C. The cells were centrifuged and washed with PBS before fixation by cold alcohol for 30 min. The collected pellets were resuspended in 1 ml PBS hypotonic fluorochrome solution contains 0.1% sodium citrate, 0.1% Triton X-100 and 50 μg/ml PI (Propidium Iodide) (Thermofisher Scientific Inc. MA, USA) then, suspension was treated in dark with RNAse. The fluorescence of 10,000 PI stained nuclei was measured and the red fluorescence was traversed the light beam and was acquired in FL4 log channel through 675 nm band-pass filter. By using CXP data analysis and acquisition software, the cell cycle phases were analyzed.
In order to distinguish the early apoptosis phase caused by DOX in both forms (free and combined to ghosts) and ghosts as control, assay of annexin V-FITC was applied as detailed in previous protocol (Van Engeland et al., 1996). In brief, the adherent HepG2 cells (1.5 × 106) were washed once with PBS after removal of media. Binding buffer (BioVision Research Products, Mountain view, CA, USA) was added before addition of annexin V-FITC (BioVision Research Products) in final concentration of 3 μg/ml, then the 6-wells plates were incubated for 10 min at room temperature. Then PI (50 μg/ml) was added before further incubation for another 10 min also at room temperature. Washing by binding buffer twice was done aiming to eliminate excess labels. The cells then dethatched using a rubber policeman. Flow cytometry was done immediately to analyze 10,000 events per one sample and then the data were analyzed.
3. Results and discussion
3.1. Production of BGs
The obtained ghosts were intact bacterial shells showing several intra-membranous tunnels (Fig. 1). The optimizing physical and chemical conditions of the incubation led to production of high-quality S. Typhimurium ghosts.
Fig. 1.
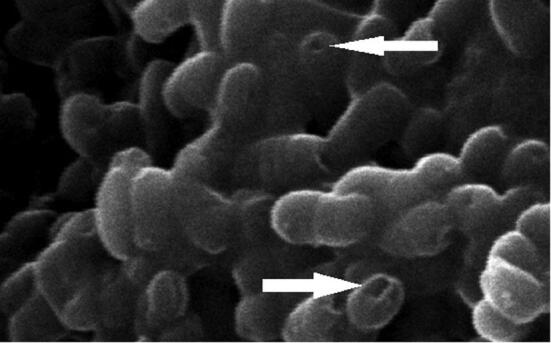
Scanning electron micrograph showing the prepared Salmonella ghosts. The arrows are directed to some surface pores that resulted from incubation of tween 80 with Salmonella culture.
3.2. The loaded DOX quantification
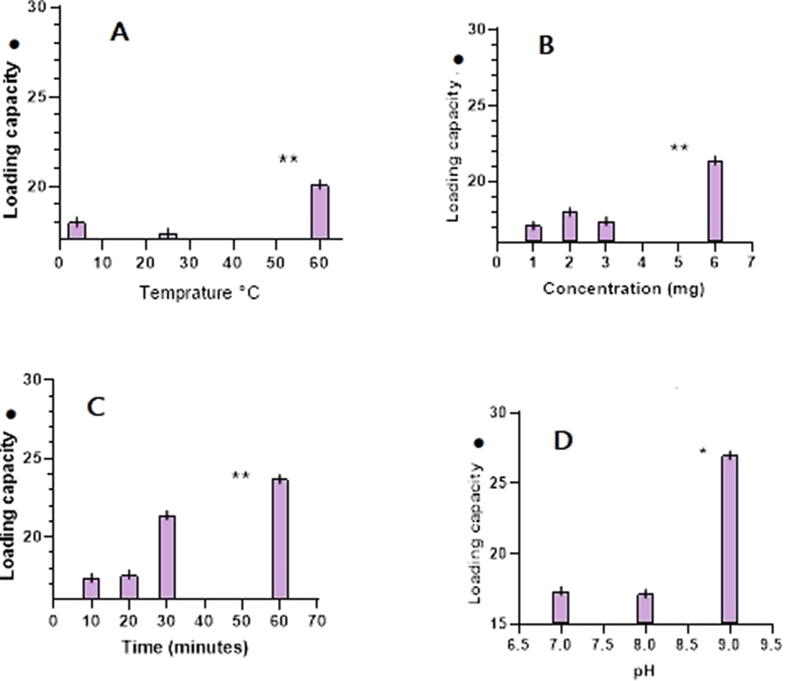
Using temperature of 60 °C achieved 20.1 μg/mg loading capacity with constant factors (pH 7, time = 10 min, DOX = 1 mg) (Fig. 2A). Using 6 mg of DOX achieved 21.4 μg/mg loading capacity with constant factors (pH 7, time = 10 min, temperature = 25 °C) (Fig. 2B). Application of 60 min incubation time achieved 23.7 μg/mg loading capacity with constant factors (pH 7, temperature = 25 °C, DOX = 1 mg) (Fig. 2C). The most effective factor that increased the BGs’ loading capacity was the pH. The highest DOX loading capacity (27.5 μg/mg) was achieved when pH 9 was used in constant factors (time = 10 min, temperature = 25 °C, 1 mg DOX) (Fig. 2D). Comparison with the other factors (temperature, time, and DOX quantity), pH value of 9 achieved significant difference in loading capacity with other individual effecting factors (P-value < 0.01). Also, there were no significant differences among the other factors (temperature, time and DOX quantity) (P-value > 0.01).
Fig. 2.
The effect of various factors on the BGs’ loading capacity: temperature (A), concentration (B), time (C) and pH (D). *: (one sample t-test) P-value < 0.05, ** (one sample t-test): P-value < 0.01. ●: μg/mg (DOX/BG).
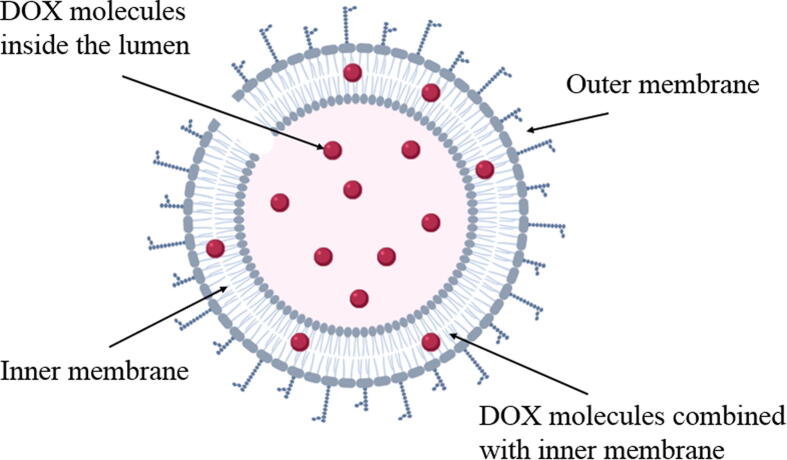
The maximum loading capacity (27.5 μg/mg) of the weight unit of the loaded BG or the maximum encapsulation efficiency (27.5%) are accounted for DOX and this quantity is the sum of encapsulated either inside the ghosts’ lumen or combined to the inner membrane per weight of BG (Fig. 3).
Fig. 3.
Schematic illustration of DOX loading in the salmonella BG.
Different DOX delivery systems other than BGs exhibited variable loaing capacities. The least loaded amount was 0.43% by using the chitosan-polysaccharide polymer- nanoparticles (Janes et al., 2001), while the largest amount that was about 39% using nanoaggregate of o/w emulsion of the synthetic conjugated glycol-chitosan (Nasongkla et al., 2004). Moreover, BGs encapsulation efficiency for DOX was about 10%. This was reported in previous study using a different BG platform (M. haemolytica ghosts) (Paukner et al., 2004). In the present study, 27.5% encapsulation efficiency was achieved with Salmonella BG platform. For more scientific explanation, this could be due to two main reasons. First, the loading parameters were used in the current study. Secondly, type of BGs as platform or BGs preparing method were used. Since the effects of medium temperature and pH on the M. haemolytica ghosts capacity for DOX loading were not examined by Paukner et al. (2004). Although only 10% of DOX quantities were loaded on the M. haemolytica BGs, the confocal laser scanning micrograph showed red fluorescence indicating successful loading of DOX in the used BGs. For the second reason, the BGs loading capacity of calcein (the water soluble marker compound) was only about 2% in E. coli ghosts forcalcein (Paukner et al., 2003, Paukner et al., 2006). For more understanding about its loading, DOX chemical properties, as loading molecules, is important.
DOX is a glycoside antibiotic that consists of tetracyclic quinoid aglycone adriamycinone linked with sugar-amine moiety daunosamine (Arcamone et al., 1972). The binding of amino-sugar moiety of DOX may be established with the charged inner and outer membranes of salmonella ghosts. The overall physiological charge of DOX molecule is single positive (Novotna et al., 2008). This allows DOX binding to the negatively charged fatty acids bound LPS (Lipopolysaccharides) that embedded into Salmonella membranes (Acta et al., 1979, Nikaido, 2003). Also, the reported strong plasma protein binding (about 74%) of DOX (Sieczkowski et al., 2010) may also indicate its firm binding to the outer membrane proteins such as porins and OmpA (Nikaido and Varra, 1985). Furthermore, it could be some other related DOX properties that making the easiness of loading and binding of DOX to Salmonella BGs using the aquatic buffered solution including DOX hydrophilicity, water solubility (~2600 mg/L) (Wong et al., 2009) and preference LPS permeability (Nikaido and Varra, 1985). This is could provide explanation of the effect of studied factors (temperature, concentration, time and pH) on DOX loading. Additionally, the high temperature increased DOX water solubility and led to the membrane LPS permeability. The increased concentration gradient is another factor that may enhance the diffusion of DOX through the biological membranes with special porous architecture. The more time is applied, the more loading is obtained. This indicates that loading of DOX in salmonella ghosts is time dependent within the studied range. The weak polyprotonic acidic nature of DOX (pKa 1 = 7.34 and pka 2 = 9.46) (Wong et al., 2009) keeps it positively charged in the basic pH (8 and 9) that have been used. Consequently, the more intense positive charge molecules exist, the more binding to the membrane motifs may occur. The overall binding of DOX with BGs components presumptively occurred via the hydrogen bonding and ionic bonding, as the DOX molecules have 12 hydrogen ion acceptors and 6 hydrogen ion donor in addition to one positive charge in physiological buffers (ChemAxon, 2019).
3.3. in-vitro DOX (free and ghost combined) release
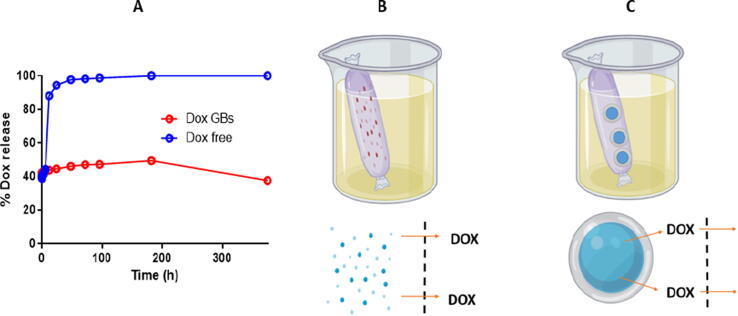
Two different patterns and models were shown by the two groups (Fig. 4). The free DOX was released greater than that of combined to ghost (Fig. 4A). The initial release was calculated after 12 min from the beginning of the experiment. Although the free drug showed almost full release, it showed slower initial release (38.8%) than that of DOX loaded BGs (42%) (not statistically different). The maximum release of DOX from loaded BGs was 47.3% at 96 h, while it was 98.7% form free DOX solution at the same time. After 96 h, the concentrations started to decline as the drug stability was affected (Fig. 4A). Also, the average release of free and BGs-combined DOX were 88% and 43.7% at 12 h, respectively. The in-vitro release of free DOX was 95% at 24 h and it was 44.5% for the BGs-combined DOX at the same time. DOX releases faster from free solution in comparison to loaded BGs. This could be explained that free DOX molecules diffused directly in case of solution (Fig. 4B). Differently, they diffused in two steps in case of BGs (from ghost membrane first and dialysis membrane bag second) (Fig. 4C). There was only one in-vitro release study of DOX from BGs delivery system that reported and DOX released from M. haemolytica ghosts (Paukner et al., 2004). Free DOX was released completely at the tenth hour and only 12.26% of the BGs combined DOX was released at the same time. The more DOX release in the current study may be accounted for the different BG used (M. haemolytica ghosts verses Salmonella enterica). Also, the method of obtaining BG is completely different between the two method leading to different effect on the membrane releasing behavior. Overall, DOX was shown to be release faster from Salmonella BGs than M. haemolytica BGs.
Fig. 4.
DOX release from loaded BGs in comparison to free solution after 16 days (A), releasing of DOX from solution in one step (B), releasing of DOX from loaded BG in two steps (C).
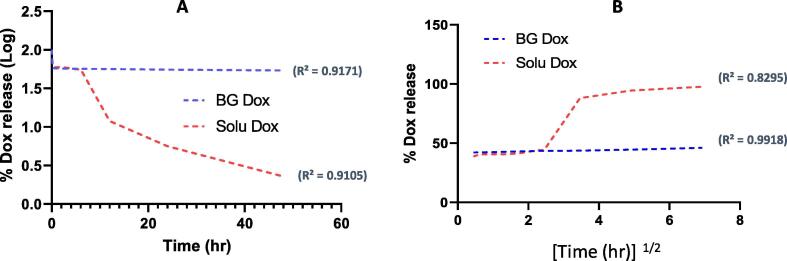
By applying different pharmaceutical kinetic models on the patterns of release of both groups, they fitted more with two different models. The most applicable model has been chosen based on the highest regression coefficient. The free DOX exhibited first order kinetic release according to the obtained regression coefficient (R2) which was 0.91 (Fig. 5A). On the other hand, the DOX loaded BGs showed typical Higuchi model kinetics as R2 = 0.99 (Fig. 5B).
Fig. 5.
Release kinetic of BG-combined DOX and free DOX, First-model kinetic (A) and Higuchi-model kinetic (B).
The first order kinetic model that was exhibited by the free DOX is completely compatible with the fact that the first order kinetic model can be applied on the situation of releasing of delivery system containing water soluble drug within porous materials (dialysis bag) depending on its concentration (Dash et al., 2010, Shaikh et al., 2015). On the other hand, the fitting of the in-vitro release pattern of BGs-DOX combined with Higuchi kinetic model was also referred to the applicability of this model to describe the release of water soluble drug in a modified release pharmaceutical dosage forms, such as biological (BGs) and non-biological (such as liposomes) membranes (Paul, 2011), transdermal systems, and tablet matrix (Brophy and Deasy, 1987, Dash et al., 2010, Higuchi, 1963, Shaikh et al., 2015). The concentration of dissolved drug remains constant during the delivery from the system. In other words, the thermodynamic activity of the drug maintained at (one) even though the decline of the loaded drug from the reservoir. Accordingly, the drug efflux rate remains at its maximum pace excluding the very initial-burst- stage as well as the last –depletion- stage (Paul, 2011).
3.4. Assay of antiproliferative activity
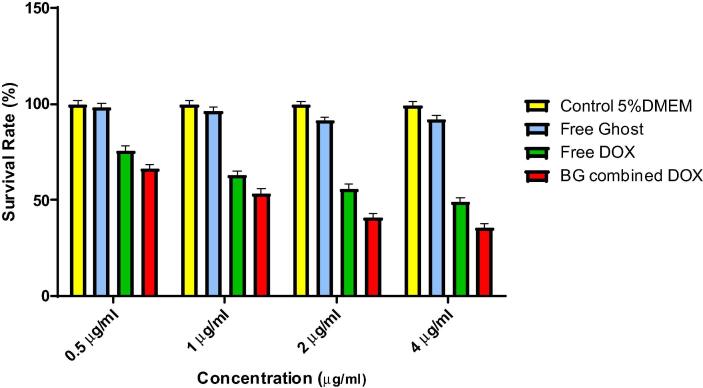
The metabolic capacity varied between different tested groups. All groups showed antiproliferative activity related to its concentration. Though, there was a valuable difference among free DOX group and DOX combined ghosts group as well as between free ghosts group and both groups (free DOX) and (DOX combined ghosts) as well, P value < 0.0001 (One-way ANOVA analysis). All equivalent concentrations of DOX combined ghosts group achieved higher cell death’s percentage than corresponding concentrations of the other two groups, P value < 0.0001 (One-way ANOVA analysis) (Fig. 6).
Fig. 6.
The antiproliferative activities of different tested groups of different concentrations (0.5, 1, 2, and 4 μg/ml); free ghost, free DOX, C: BG-combined DOX in addition to the control group (5% DMEM), (One-way ANOVA) P value < 0.0001.
The tested concentrations in the combined DOX were equivalent to those of free DOX ones. Interestingly, DOX combined ghost group was the most efficient antiproliferative tested dosage forms on HepG2 cells. The equivalent concentrations of 0.5 μg/ml and 4 μg/ml were enough to kill 33.66% and 64.5% of the cancerous cells respectively in comparison to almost 0% death in the control group (5% Dulbecco’s Modified Eagle’s Media, DMEM). On the other hand, 0.5 μg/ml and 4 μg/ml of the free DOX group killed 24.28% and 51% of the cells, respectively (Fig. 6).
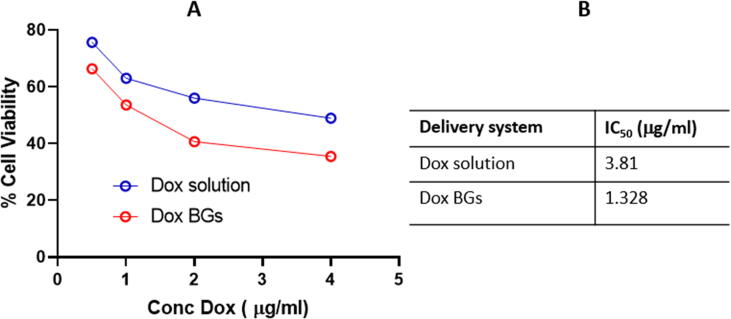
Although the antiproliferative activity of free ghosts was unanticipated, it showed limited antiproliferative activity. The maximum tested concentration of free ghost group (4 μg/ml) only killed 7.85% of the malignant cells population. The cancer cells proliferative inhibitory concentration (IC50) of the DOX combined BGS was (1.328 μg/ml), which is about one third of the IC50 of the free DOX (3.81 μg/ml), (Fig. 7). This indicates that loading DOX in BGs enhances its cytotoxicity.
Fig. 7.
Cell viability of free DOX and BG-combined DOX ghost (A) and IC50 (B).
3.5. Apoptosis analysis by flow cytometry
The effects of different groups on the HepG2 phases of life cycle revealed significant variability. The effect of free ghosts group was very comparable to the control group (5% DMEM). None of the two groups (control and free ghosts) showed noticeable apoptotic or necrotic effects (Fig. 8, Fig. 9). The quantified cells that were shown as annexin V-FITC negative/ PI negative were counted as live cells (non-apoptotic), while those revealed as annexin V-FITC positive/ PI positive were counted as late apoptotic or necrotic cells. The cells showed annexin V-FITC negative/PI positive were counted as necrotic cells and the cells that appeared annexin V-FITC positive/ PI negative were counted as early necrotic cells.
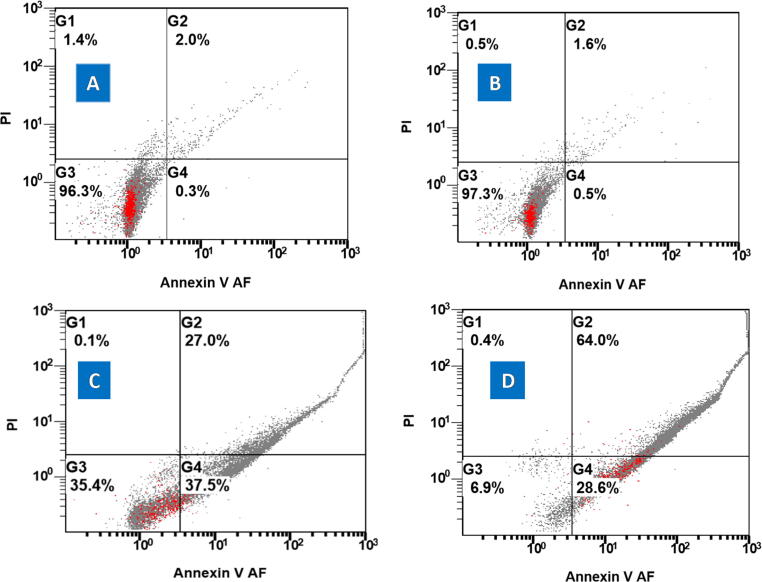
Fig. 8.
The apoptosis and/or necrosis activities of: (A) control group (5%DMEM), (B) free ghost group (0.5 μg/ml), (C) free DOX (0.5 μg/ml), and (D) ghost combined DOX (0.5 μg/ml) on the HepG2 cells.
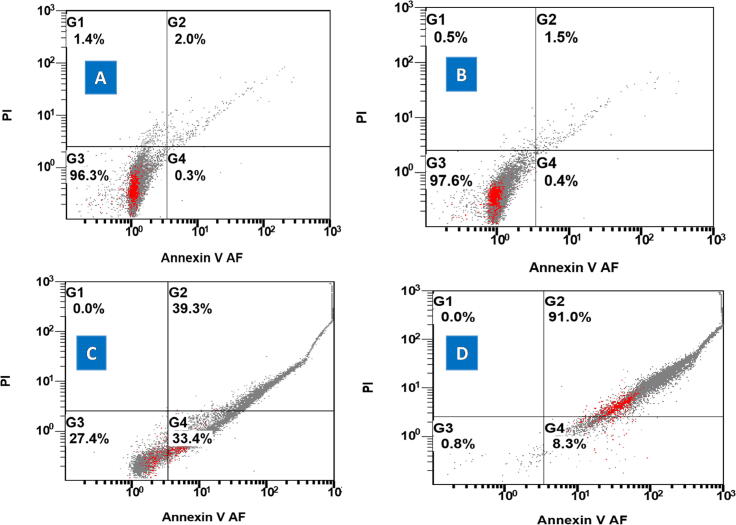
Fig. 9.
The apoptosis and/or necrosis activities of (A) control group (5%DMEM), (B) free ghost group (1 μg/ml), (C) free DOX (1 μg/ml), and (D) ghost combined DOX (1 μg/ml) on the HepG2 cells.
The effects of both free DOX and BGs-combined DOX groups were consistent with MTT assay results. Both groups obviously affected the HEPG2 cells’ life cycles proportionally to the increased concentrations. The cells were accumulated in the early apoptosis/necrotic phase in (37.5%) and in the late apoptosis/necrotic phase in (27%) by using of 0.5 μg/ml of free DOX. By using of 1 μg/ml free DOX, 33.4% of cells did early apoptosis and 39.3% did late apoptosis/necrosis (Fig. 8, Fig. 9C).
The DOX combined ghosts showed more shifting cells toward late apoptosis/necrosis in comparison to that of free DOX. Data showed that early apoptosis (28.6%) and late apoptosis/necrosis (64%) was observed by applying 0.5 μg/ml of DOX. Also, it showed more accumulation in late apoptosis/necrosis (91%) than early apoptosis (8.3%) by applying 1 μg/ml of DOX combined ghosts (Fig. 8, Fig. 9D). The statistical analysis using ANOVA exposed a significant difference between the tested groups: ghosts combined DOX and free DOX (P value ˃ 0.05: one-way ANOVA). Also, it shows significant difference between the free ghost’s group/control (5% DMEM) and ghosts combined DOX and free DOX groups (P value ˃ 0.01: one-way ANOVA).
Reduction of 3- (4, 5-Dimethylthiazol-2-yl) -2,5-diphenyltetrazolium bromide (MTT) used in this study is the most commonly used method measuring the cellular proliferation and cytotoxicity (Liu et al., 2002). Measuring apoptosis activity, which was applied in this study, is another way for indicating cellular vitality and their proliferation activity (Koopman et al., 1997). Detection and quantification of apoptotic cells can be achieved using flow cytometry. It was use to quantify the fluorescence labelled annexin V-a subfamily of annexin proteins- FITC which was bonded to the externalized plasma membrane phosphatidylserine (Zhang et al., 1997). The apoptotic cells can be distinguished from necrotic cells and then they can bequantified using PI. The fluorescent agent can penetrate damageding cells but cannot penetrate intact cells binds to DNA and RNA fragments released from either necrotic or late apoptotic cells (Riccardi and Nicoletti, 2006, Sawai and Domae, 2011).
The only reported DOX delivery system to Caco-2 cell line stated the superiority of DOX-M. haemolytica ghosts combined over the free DOX. At 100 ng/ml concentration of free DOX and 16 h incubation, the Caco-2 cell vitality was 66.1 ± 3.3%. On the other hand, the equivalent concentration of BGs-DOX combined achieved vitality percentage of 16.8 ± 4.3% indicating that at least two orders of magnitude greater antiproliferative activity of BGs-DOX combined than the free DOX. The empty ghosts showed a slight antiproliferative activity. The cancer cell line proliferation was investigated using the ability of 5-Brom- 2-deoxyuridin (BrdU) to correlate DNA synthesis (Paukner et al., 2004).
The Salmonella enterica serovar typhimurium BGs loaded by DOX in an equivalent concentration (max used concentration) of 4 μg/ml killed 64.5% of the HepG-2 cells after incubation for 24 h. On the other hand, the free DOX killed only 51.1% while the empty BGs killed 7.9%. These results showed three times less toxic dose of BGs-combined DOX (IC 50% = 1.328 μg/ml) than the free DOX (IC 50% = 3.374 μg/ml) indicating safer use of Salmonella’s BGs loaded DOX. The high difference between the apoptotic effects of 1 μg (max used concentration) of the BGs-DOX combined (91% late apoptosis/ early necrosis and 8.3% early apoptosis) and the free DOX apoptotic effects (39.3% late apoptosis/early necrosis and 33.4% early necrosis) was consistent with the difference cytotoxic activities between both.
DOX suffers from lack of selectivity to tumor site leading to sever side effects such cardiotoxicity. Also, it suffers from cellular uptake challenges including multidrug resistance originating from the P-glycoprotein and slow penetration due to interaction with lipid membrane. Thus, it has been given by hepatic artery route to increase its efficacy (Tam, 2013). The response rate of hepatocellular is low with high recurrence of tumors. Finding novel delivery systems to slow the growth of advanced liver cancer and exhibit less severe side effects is needed.
The higher cytotoxic activity and apoptotic effects of Salmonella’s BGs-combined DOX may refer to some reasons. First: high ability of adherence, invasion, and affinity of salmonella cells to hepatic cells (Peterson, 1989, Lajarin et al., 1996). Second, The synergistic effect of salmonella with DOX as an antiproliferative agent in hepatic cancer cells (Li et al., 2012). Thirdly, free DOX faces cellular permeability challenges such as multidrug resistance which is originating from the P-glycoprotein and topoisomerase II resistance (Patil et al., 2008). Furthermore, free DOX has slow cellular penetration which was attributed to an electrostatic interaction between doxorubicin and lipid molecules in the cell membrane. (Aminipour et al., 2020).
The hepatic cellular uptake of Salmonella BG, that preserve most external surface properties, was mainly achieved by invasion of salmonella cells. The invasion mechanism of salmonella to the hepatic cells has been correlated to some intrinsic factors which called type III secretion systems (TTSS) and fusion proteins. Such factors are responsible for salmonellas’ virulence and animal host infections. (Geddes et al., 2007, Li et al., 2012). This strong adherence and invasion established the concept of targeting liver cells by DOX-BGs combined. Additionally, Salmonella is well-known to preferentially accumulate within tumors, especially in the nonhypoxic and hypoxic regions. Even some Salmonella strains is being evaluated in a phase I clinical trial in humans (Mi et al., 2019).
The localization of bacteriobots (S. typhimurium attached to a microstructure) in the dissected tumor masses was confirmed in in-vitro and in-vivo tests (Park et al., n.d). Moreover, salmonella was successfully used as therapeutic and gene delivery vectors indicating its capabilities to release them intracellular (Pawelek et al., 1997). This way in vitro releasing study was performed in the current work. The DOX release and delivery within the hepatic cells most probably was accomplished by simple diffusion through BG membrane and tunnels after phagocytosis of loaded BGs by the hepatic cancer cells (Kudela et al., 2008). Such delivery can be indicated by the significant difference of higher antipriliferative activity of BG-DOX loaded than free DOX in the same concentration. Cytotoxic activity has been reported for Salmonella typhimurium strain 7207 lx as they accumulated in cancer cells cytoplasm killing about 40% of HepG-2 cells population and induced apoptosis after long period of incubation (34 days) (Li et al., 2012). The reported activity was reconciled with the current finding of the cytotoxic activity of Salmonella’s BGs alone that reached about 7.9% and slight apoptosis induction (1.6%) (Fig. 6, Fig. 8B, respectively).
In conclusion, the simply prepared BGs of Salmonella were successfully loaded by DOX. Moreover, the utilization of such novel DDS is offering a sustained release (Higuchi kinetic pattern) and the release was over period of four hours. Also, the cell based delivery showed superior antiproliferative and apoptosis activities over the free DOX in the HepG2 cell line. More investigation about cellular trafficking of DOX through ghost bacteria membrane is required to explore the advantageous of BG as delivery system. The new delivery system will be tested in the future in animal models bearing liver cancer.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This Project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, Award Number (14-NAN-561-02).
Footnotes
Peer review under responsibility of King Saud University.
References
- Acta J.B.B., Schindler M., Osborn M.J. Interaction of divalent cations and polymyxin B with. Am. Chem. Soc. 1979;18(20):4425–4430. doi: 10.1021/bi00587a024. [DOI] [PubMed] [Google Scholar]
- Alanazi Saleh A., Harisa Gamaleldin I., Badran Mohamed M., Haq Nazrul, Radwan Awwad A., Kumar Ashok, Shakeel Faiyaz, Alanazi Fars K. Cholesterol-conjugate as a new strategy to improve the cytotoxic effect of 5-fluorouracil on liver cancer: impact of liposomal composition. Curr. Drug Deliv. 2020;17:1. doi: 10.2174/1567201817666200211095452. [DOI] [PubMed] [Google Scholar]
- Alsuwyeh A.A., Alanazi F., Shakeel F., Salem-Bekhit M.M., Haq N. Estimation of anti-neoplastic drug doxorubicin in bacterial ghost matrix by New “Environmentally Benign” RP-HPLC method: a step towards sustainable development of pharmaceutical industry. Arab. J. Sci. Eng. 2018;43(1):181–190. doi: 10.1007/s13369-017-2664-2. [DOI] [Google Scholar]
- Aminipour Z., Khorshid M., Keshvari H., Bonakdar S., Wagner P., Van der Bruggen B. Passive permeability assay of doxorubicin through model cell membranes under cancerous and normal membrane potential conditions. Eur. J. Pharm. Biopharm. 2020;146:133–142. doi: 10.1016/j.ejpb.2019.10.011. [DOI] [PubMed] [Google Scholar]
- Amro A.A., Salem-Bekhit M.M., Alanazi F.K. Plackett-Burman randomization method for Bacterial Ghosts preparation form E. coli JM109. Saudi Pharmaceut. J. 2014;22(3):273–279. doi: 10.1016/j.jsps.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcamone F., Cassinelli G., Franceschi G., Penco S., Pol C., Redaelli S., Selva A. International Symposium on Adriamycin. 1972. Structure and physicochemical properties of adriamycin (Doxorubicin) pp. 9–22. [DOI] [Google Scholar]
- Ashour A.E., Abd-Allah A.R., Korashy H.M., Attia S.M., Alzahrani A.Z., Saquib Q., Rishi A.K. Thymoquinone suppression of the human hepatocellular carcinoma cell growth involves inhibition of IL-8 expression, elevated levels of TRAIL receptors, oxidative stress and apoptosis. Mol. Cell. Biochem. 2014;389(1–2):85–98. doi: 10.1007/s11010-013-1930-1. [DOI] [PubMed] [Google Scholar]
- Bekaii-Saab T., Markowitz J., Prescott N., Sadee W., Heerema N., Wei L., Villalona-Calero M. A multi-institutional phase II study of the efficacy and tolerability of lapatinib in patients with advanced hepatocellular carcinomas. Clin. Cancer Res. 2009;15(18):5895–5901. doi: 10.1158/1078-0432.CCR-09-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brophy M.R., Deasy P.B. Application of the Higuchi model for drug release from dispersed matrices to particles of general shape. Int. J. Pharm. 1987;37(1–2):41–47. doi: 10.1016/0378-5173(87)90008-1. [DOI] [Google Scholar]
- ChemAxon, 2019. ChemAxon - Software Solutions and Services for Chemistry & Biology. Retrieved February 4, 2019, from https://chemaxon.com/products/calculators-and-predictors#polarization.
- Dash S., Murthy P., Nath L., Chourdhury P. Kinetic modelling of drug release from controlled drug delivery systems. Polish Pharm. Soc. 2010;67(3):217–223. doi: 10.2307/3237001. [DOI] [PubMed] [Google Scholar]
- Ferlay J., Colombet M., Soerjomataram I., Mathers C., Parkin D.M., Piñeros M., Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer. 2019;144(8):1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- Ge Z., Ma R., Xu G., Chen Z., Zhang D., Wang Q., Ma W. Development and in vitro release of isoniazid and rifampicin-loaded bovine serum albumin nanoparticles. Med. Sci. Monit. 2018;24:473–478. doi: 10.12659/MSM.905581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi T. Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J. Pharm. Sci. 1963;52(12):1145–1149. doi: 10.1002/jps.2600521210. [DOI] [PubMed] [Google Scholar]
- Janes K.A., Fresneau M.P., Marazuela A., Fabra A. Chitosan nanoparticls as drug delivery systems for doxorubicin. J. Control. Release. 2001;73:255–267. doi: 10.1016/s0168-3659(01)00294-2. [DOI] [PubMed] [Google Scholar]
- Jia L.J., Wei D.P., Sun Q.M., Huang Y., Wu Q., Hua Z.C. Oral delivery of tumor-targeting Salmonella for cancer therapy in murine tumor models. Cancer Sci. 2007;98:1107–1112. doi: 10.1111/j.1349-7006.2007.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasinskas R.W., Forbes N.S. Salmonella typhimurium specifically chemotax and proliferate in heterogeneous tumor tissue in vitro. Biotechnol. Bioeng. 2006;94:710–721. doi: 10.1002/bit.20883. [DOI] [PubMed] [Google Scholar]
- Koopman, G., Reutelingsperger, C.P.M., Kuijten, G.A.M., Keehnen, R.M.J., Pals, S.T., & Van Oers, M.H.J., 1997. RAPID COMMUNICATION Annexin V for Flow Cytometric Detection of Phosphatidylserine Expression on B Cells Undergoing Apoptosis. Retrieved from http://www.bloodjournal.org. [PubMed]
- Korashy H.M., El-Kadi A.O.S. Differential effects of mercury, lead and copper on the constitutive and inducible expression of aryl hydrocarbon receptor (AHR)-regulated genes in cultured hepatoma Hepa 1c1c7 cells. Toxicology. 2004;201(1–3):153–172. doi: 10.1016/j.tox.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Kudela P., Paukner S., Mayr U.B., Cholujova D., Kohl G., Schwarczova Z., Lubitz W. Effective gene transfer to melanoma cells using bacterial ghosts. Cancer Lett. 2008;262(1):54–63. doi: 10.1016/j.canlet.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Kwon S.R., Yoon K.N., Sung K.K., Dong S.K., Kim K.H. Generation of Edwardsiella tarda ghosts by bacteriophage PhiX174 lysis gene E. Aquaculture. 2005;250(1–2):16–21. doi: 10.1016/j.aquaculture.2005.02.052. [DOI] [Google Scholar]
- Lajarin F., Rubio G., Galvez J., Garcia-Peñarrubia P. Adhesion, invasion and intracellular replication of Salmonella typhimurium in a murine hepatocyte cell line. Effect of cytokines and LPS on antibacterial activity of hepatocytes. Microb. Pathog. 1996;21(5):319–329. doi: 10.1006/mpat.1996.0065. [DOI] [PubMed] [Google Scholar]
- Li B., He H., Zhang S., Zhao W., Li N., Shao R. Salmonella typhimurium strain SL7207 induces apoptosis and inhibits the growth of HepG2 hepatoma cells in vitro and in vivo. Acta Pharm. Sinica B. 2012;2(6):562–568. doi: 10.1016/j.apsb.2012.10.006. [DOI] [Google Scholar]
- Liu Y., Peterson D.A., Kimura H., Schubert D. Mechanism of Cellular 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) Reduction. J. Neurochem. 2002;69(2):581–593. doi: 10.1046/j.1471-4159.1997.69020581.x. [DOI] [PubMed] [Google Scholar]
- Low K.B., Ittensohn M., Le T., Platt J., Sodi S., Amoss M. Lipid A mutant Salmonella with suppressed virulence and TNFα induction retain tumor-targeting in vivo. Nat. Biotechnol. 1999;17:37. doi: 10.1038/5205. [DOI] [PubMed] [Google Scholar]
- Mi Z., Feng Z.-C., Li C., Yang X., Ma M.-T., Rong P.-F. Salmonella-mediated cancer therapy: an innovative therapeutic strategy. J. Cancer. 2019;10 doi: 10.7150/jca.32650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasongkla N., Shuai X., Ai H., Weinberg B.D., Pink J., Boothman D.A., Gao J. cRGD-functionalized polymer micelles for targeted doxorubicin delivery. Angew. Chem. Int. Ed. 2004;43(46):6323–6327. doi: 10.1002/anie.200460800. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. MMBR. 2003;67(4):593–656. doi: 10.1128/MMBR.67.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Varra M. Molecular basis of bacterial outer membrane permeability. Microbiol. Mol. Biol. Rev. 1985;67(1):593–656. doi: 10.1128/mmbr.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotna R., Wsol V., Xiong G., Maser E. Inactivation of the anticancer drugs doxorubicin and oracin by aldo-keto: Reductase (AKR) 1C3. Toxicol. Lett. 2008;181(1):1–6. doi: 10.1016/j.toxlet.2008.06.858. [DOI] [PubMed] [Google Scholar]
- Park, S. J., Park, S.-H., Cho, S., Kim, D.-M., Lee, Y., Ko, S. Y., Park, S. (n.d.). New paradigm for tumor theranostic methodology using bacteria-based microrobot. https://doi.org/10.1038/srep03394. [DOI] [PMC free article] [PubMed]
- Patil R.R., Guhagarkar S.A., Devarajan P.V. Engineered nanocarriers of doxorubicin: A current update. Crit. Rev. Ther. Drug Carrier Syst. 2008;25:1–61. doi: 10.1615/CritRevTherDrugCarrierSyst.v25.i1.10. [DOI] [PubMed] [Google Scholar]
- Paukner S., Kohl G., Jalava K., Lubitz W. Sealed bacterial ghosts - Novel targeting vehicles for advanced drug delivery of water-soluble substances. J. Drug Target. 2003;11:151–161. doi: 10.1080/10611860310001593366. [DOI] [PubMed] [Google Scholar]
- Paukner S., Kohl G., Lubitz W. Bacterial ghosts as novel advanced drug delivery systems: Antiproliferative activity of loaded doxorubicin in human Caco-2 cells. J. Control. Release. 2004;94(1):63–74. doi: 10.1016/j.jconrel.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Paukner S., Stiedl T., Kudela P., Bizik J., Al Laham F., Lubitz W. Bacterial ghosts as a novel advanced targeting system for drug and DNA delivery. Expert Opin. Drug Deliv. 2006;3(1):11–22. doi: 10.1517/17425247.3.1.11. [DOI] [PubMed] [Google Scholar]
- Paul D.R. Elaborations on the Higuchi model for drug delivery. Int. J. Pharm. 2011;418(1):13–17. doi: 10.1016/j.ijpharm.2010.10.037. [DOI] [PubMed] [Google Scholar]
- Pawelek J.M., Low K.B., Bermudes3 D. Tumor-targeted Salmonella as a Novel Anticancer Vector1. Cancer Res. 1997;57 [PubMed] [Google Scholar]
- Peterson W.L. Salmonella bacterial adherence and penetration of mucosal cells: Inducing role of the epithelium. Gastroenterology. 1989;97(October):1055–1056. doi: 10.1016/0016-5085(89)91523-0. [DOI] [PubMed] [Google Scholar]
- Rabea S., Salem-Bekhit M.M., Alanazi F.K., Yassin A.S., Moneib N.A., Hashem A.E.M. A novel protocol for bacterial ghosts’ preparation using tween 80. Saudi Pharm. J. 2018;26(2):232–237. doi: 10.1016/J.JSPS.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccardi C., Nicoletti I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat. Protoc. 2006;1(3):1458–1461. doi: 10.1038/nprot.2006.238. [DOI] [PubMed] [Google Scholar]
- Saltzman D., Augustin L., Leonard A., Mertensotto M., Schottel J. Low dose chemotherapy combined with attenuated Salmonella decreases tumor burden and is less toxic than high dose chemotherapy in an autochthonous murine model of breast cancer. Surgery. 2018;163:509–514. doi: 10.1016/j.surg.2017.09.036. [DOI] [PubMed] [Google Scholar]
- Sawai H., Domae N. Discrimination between primary necrosis and apoptosis by necrostatin-1 in Annexin V-positive/propidium iodide-negative cells. Biochem. Biophys. Res. Commun. 2011;411(3):569–573. doi: 10.1016/j.bbrc.2011.06.186. [DOI] [PubMed] [Google Scholar]
- Shaikh H.K., Kshirsagar R.V., Patil S.G. Mathematical models for drug release characterization: A review. World J. Pharm. Pharm. Sci. 2015;4(4):324–338. [Google Scholar]
- Sieczkowski E., Lehner C., Ambros P.F., Hohenegger M. Double impact on p-glycoprotein by statins enhances doxorubicin cytotoxicity in human neuroblastoma cells. Int. J. Cancer. 2010;126(9):2025–2035. doi: 10.1002/ijc.24885. [DOI] [PubMed] [Google Scholar]
- Stein E., Inic-Kanada A., Belij S., Montanaro J., Bintner N., Schlacher S., Barisani-Asenbauer T. In vitro and in vivo uptake study of Escherichia coli nissle 1917 bacterial ghosts: Cell-based delivery system to target ocular surface diseases. Invest. Ophthalmol. Vis. Sci. 2013;54(9):6326–6333. doi: 10.1167/iovs.13-12044. [DOI] [PubMed] [Google Scholar]
- Tam K. The Roles of Doxorubicin in Hepatocellular Carcinoma. Admet & Dmpk. 2013;1(3):29–44. doi: 10.5599/admet.1.3.7. [DOI] [Google Scholar]
- Van Engeland M., Ramaekers F.C.S., Schutte B., Reutelingsperger C.P.M. A novel assay to measure loss of plasma membrane asymmetry during apoptosis of adherent cells in culture. Cytometry. 1996;24(2):131–139. doi: 10.1002/(SICI)1097-0320(19960601)24:2<131::AID-CYTO5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Wang C.Z., Kazmierczak R.A., Eisenstark A. Strains, mechanism, and perspective: salmonella-based cancer therapy. Int. J. Microbiol. 2016;2016:5678702. doi: 10.1155/2016/5678702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong I.L.K., Chan K.F., Ka H.T., Chi Y.L., Zhao Y., Tak H.C., Chow L.M.C. Modulation of multidrug resistance protein 1 (MRP1/ABCC1)-mediated multidrug resistance by bivalent apigenin homodimers and their derivatives. J. Med. Chem. 2009;52(17):5311–5322. doi: 10.1021/jm900194w. [DOI] [PubMed] [Google Scholar]
- Youssof Abdullah M.E., Alanazi Fars K., Salem-Bekhit Mounir M., Shakeel Faiyaz, Haq Nazrul. Bacterial ghosts carrying 5-fluorouracil: a Novel biological carrier for targeting colorectal cancer. AAPS PharmSciTech. 2019;20:48. doi: 10.1208/s12249-018-1249-z. [DOI] [PubMed] [Google Scholar]
- Zhang G., Gurtu V., Kain S.R., Yan G. Annexin V-FITC Binding Assay. Biotechniques. 1997;25(September):525–531. doi: 10.2144/97233pf01. https://www.biotechniques.com/multimedia/archive/00011/97233pf01_11715a.pdf Retrieved from. [DOI] [PubMed] [Google Scholar]