Abstract
Background: Hereditary hemochromatosis (HH) is a primary iron overload (IO) condition. Absolute telomere length (ATL) is a marker of cellular aging and DNA damage associated with chronic diseases and mortality.
Aim: To evaluate the relationship between ATL and IO in patients with HH.
Methods: Cross-sectional study including 25 patients with HH: 8 with IO and 17 without IO (ferritin < 300 ng/ml) and 25 healthy controls. Inclusion criteria were: age > 18 years, male sex and HH diagnosis. Patients with diabetes or other endocrine and autoimmune diseases were excluded. ATL was measured by real-time PCR.
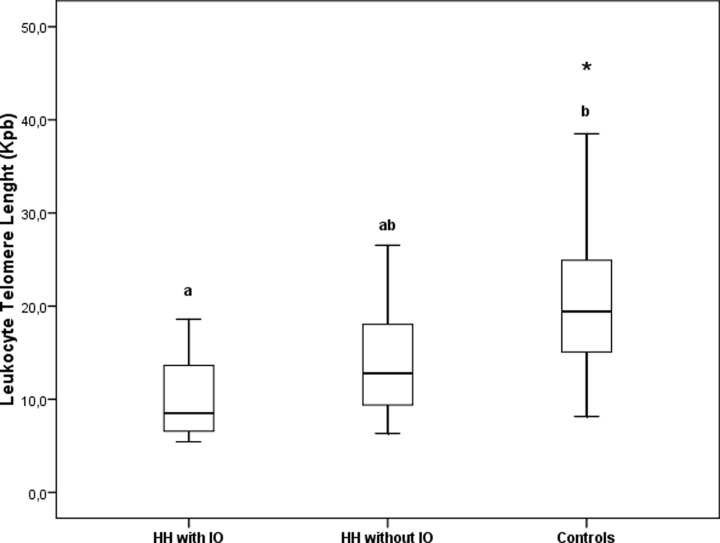
Results: HH patients with IO were older (P<0.001) and showed higher ferritin concentration (P<0.001). Patients with HH, disregarding the iron status, showed higher glucose and body mass index (BMI) than controls (both P<0.01). ATL was shorter in patients with IO than controls [with IO: 8 (6–14), without IO: 13 (9–20), and controls: 19 (15–25) kilobase pairs, P<0.01]; with a linear trend within groups (P for trend <0.01). Differences in ATL remained statistically significant after adjusting by age, BMI and glucose (P<0.05).
Discussion: Patients with IO featured shorter ATL while patients without IO showed only mild alterations vs. controls. Screening for IO is encouraged to prevent iron-associated cellular damage and early telomere attrition.
Keywords: Iron Metabolism, Iron Overload, Telomere Lenght
Introduction
Hereditary hemochromatosis (HH) is a primary condition, which might lead to iron overload (IO) depending on host and ambient factors. Two main types of HH have been described: (1) the recessive type which involves genes that are related to the activation of hepcidin such as the human homeostatic iron regulator (HFE), hemojuvelin (HJV), and hepcidin (HAMP) genes; and (2) the dominant type which involves the hepcidin receptor ferroportin (FPN). Among these, the main gene associated with HH is HFE, located in the short arm of chromosome 6 (6p). The HFE gene encodes a protein that is located on the surface of cells, primarily liver, intestinal and immune cells. This protein interacts with other proteins on the cell surface to detect the amount of iron in the body. When the HFE protein is bound to the transferrin (Tf) receptor (TfR) 1, the receptor cannot bind to Tf preventing the entrance of iron to liver cells. Furthermore, HFE protein regulates the production of hepcidin. Hepcidin is produced by the liver, and it regulates iron absorption from the diet and its release from storage sites in the body. When the HFE protein is not bound to TfR 1, it binds to a group of other proteins that include hepcidin triggering the production of hepcidin. Therefore, binding of HFE protein to TfR 1 represses hepcidin expression. Consequently, current models of iron metabolism regulation propose that highly iron-saturated Tf would release HFE protein from TfR1 increasing iron uptake by cells and hepcidin up-regulation [1,2].
Even if iron is essential for cells, it may also be involved in the generation of reactive oxygen species leading to cellular damage [3–7]. In patients with IO, the hepatocytes, cardiomyocytes and cells from endocrine organs, may be affected [8]. Clinical manifestations of overt IO in patients with HH include altered liver enzymes, hepatocellular carcinoma, osteoarthritis, glucose intolerance and diabetes, sexual hormone disorder, cardiac dysfunction and hyperpigmentation, among others [8]. In fact, IO would decrease insulin secretion and increase β-cell apoptosis via iron-induced oxidative stress [9]. Likewise, oxidative stress would be responsible for the liver damage found in IO patients [10,11]. Moreover, IO would be associated with increased atherosclerotic plaque formation and risk of myocardial infarction [12,13]. On the contrary, iron depletion, the most common and effective treatment for IO patients, would decrease low-density lipoprotein (LDL) oxidation and increase high-density lipoprotein cholesterol (HDL-C) levels [13]. Consistently, prior studies carried out by our group showed that HDL from IO patients present both quantitative and qualitative alterations associated with higher risk of cardiovascular disease [14,15].
Absolute telomere length (ATL) is related to aging and cellular damage. As a biomarker, it has been associated with insulin resistance, diabetes and cardiovascular diseases [16,17]. Telomeres are complex DNA–protein structures located at the end of eukaryotic chromosomes which shorten with age in all replicating somatic cells [18] and are associated with different pathological conditions [16,19]. In fact, functional telomeres play a key role in the protection of chromosomes against genome instability [20]. Given that iron might promote oxidative stress [21], which could also be a cause of shorter ATL [22], some studies evaluated the association between ATL and markers of iron metabolism. In fact, in a study of 1174 healthy adults, Tf saturation (TfSat), was associated with shorter ATL [23].
The present study aims to evaluate the relationship betweeniron levelsand ATL in male adults diagnosed with HH.
Materials and methods
Subjects and study protocol
Thirty-eight patients with either TfSat > 50% or ferritin concentration > 300 ng/ml were recruited from the Hepatology Division of the University Hospital ‘José de San Martín’ between 2010 and 2012. HH was diagnosed by liver histology compatible with primary IO (iron deposition preferentially within hepatocytes in the periportal region of the hepatic lobule). Among the 38 patients recruited, 13 did not present liver histology compatible with IO and were excluded from the study. Additional inclusion criteria were adult age and male sex. Exclusion criteria were: diabetes, hypothyroidism, cirrhosis, viral hepatitis, HIV infection or cancer. Among the 25 patients with HH included, 8 showed overt IO (TfSat > 50% and ferritin concentration > 300 ng/ml) at the time of the analysis (HH with IO) and the remaining 17 presented TfSat > 50% but ferritin concentration < 300 ng/ml (HH w/o IO). These 17 patients had previously presented IO and had been submitted to a successful phlebotomy to decrease body iron levels. Eight patients were homozygous for the C282Y polymorphism, two were heterozygous for C282Y, one was homozygous for H63D, seven were heterozygous for H63D, one was a compound heterozygote for C282Y/H63D and six were wildtype. Twenty-five age-matched male healthy subjects without clinical or biochemical signs of IO and wildtype for mutations in the HFE gene were also included. A single blood sample from each subject was drawn from the antecubital vein after a 12-h overnight fast. Whole blood was used to determine the complete blood count and an aliquot was stored at −20°C to perform the ATL tests. Serum samples were immediately employed for general biochemical determinations.
The present study was carried out in accordance with the Declaration of Helsinki and its later amendments or comparable ethical standards. The protocol was approved by the Ethical Committees from University Hospital ‘José de San Martín’ and from the School of Pharmacy and Biochemistry, University of Buenos Aires. All patients signed an informed consent to participate in the study.
Anthropometric, analytical and genetic procedures
Height and weight were measured with the subject wearing light clothes and without shoes. Body mass index (BMI) was calculated and BMI categories were defined according to the World Health Organization (WHO) adult definition [24]. Complete blood count was carried out in a LH750 autoanalyzer (Beckman Coulter, Fullerton, CA, U.S.A.). Plasma Tf, apolipoprotein (apo) A-I and apo B concentrations were measured by nephelometry (IMMAGE®, Beckman Coulter, Fullerton, CA, U.S.A.). Ferritin concentration was assayed by an electrochemiluminescence assay (VITROS® ECiQ, Ortho-Clinical Diagnostics, Raritan City, NJ, U.S.A.). Serum levels of iron, glucose, triglycerides and total cholesterol as well as the activities of hepatic enzymes were quantified by standardized methods (Roche Diagnostics, Mannheim, Germany) in a COBAS C501 autoanalyzer (Roche Diagnostics, Mannheim, Germany). LDL-C and HDL-C concentrations were determined by selective precipitation methods.
Measurement of ATL
The ATL measurement was carried out by qPCR as previously described [25], in aStepOne ™ Real-Time PCR System (Applied Biosystems). Genomic DNA was extracted from peripheral blood leukocytes by standard protocols [26]. For each DNA sample, two consecutive reactions were performed: the first one to amplify a fragment of 75 bp of the single copy gene RPLPO (ribosomal protein, large, PO), and the second one for the telomeric sequence. They both were used for standard curve constructions, and allowed the genome/reaction number (S) and the of telomeric sequence/reaction (T) values to be exported from the respective standard curve. The calculation of the T/S ratio resulted in the kbp of telomeric sequence per cell for each individual determination.
Statistical analyses
The Shapiro–Wilks method was employed to assess data distribution. Normally distributed variables were expressed as mean ± SD and skewed-distributed variables as median (interquartile range [IQR]). Differences were analyzed by ANOVA employing Tukey’s post-hoc test; or by Kruskal–Wallis and paired comparison post-hoc test according to normal or skewed data distribution, respectively. Significance was defined as P<0.05 in the two-tailed tests. For all statistical analyses, the software INFOSTAT (GrupoInfostat, Universidad Nacional de Córdoba, Argentina) was employed.
Results
Clinical and metabolic characteristics
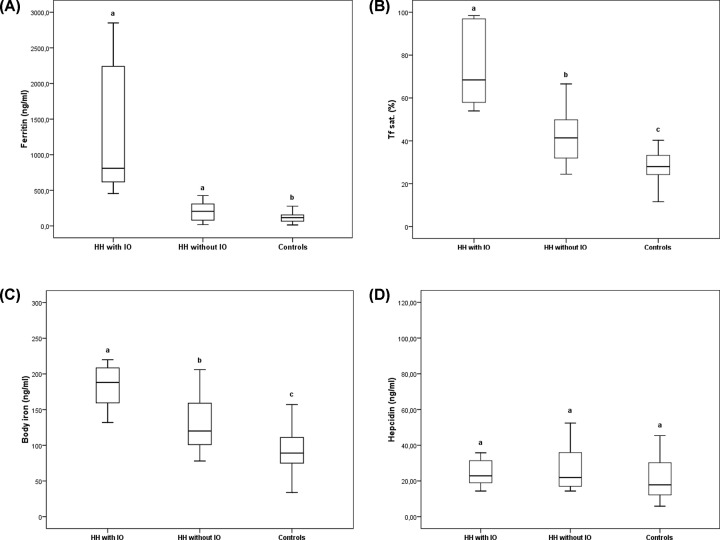
HH patients with overt IO were older (P<0.001) and, therefore, statistical analysis was carried out adjusting by age. Furthermore, HH patients with IO also showed higher AST and ALT activities, as well as ferritin concentration than HH patients without IO or control subjects (P<0.001) (Table 1). In turn, all these parameters were similar in HH patients without IO and controls. Patients with HH, disregarding the iron status, showed higher BMI and glucose levels than the controls (both P<0.01). Finally, TfSat was significantly different in the three groups studied finding the highest values in HH patients with IO and the lowest ones in control subjects (Figure 1). Lipid and lipoprotein profile were similar in all the groups.
Table 1. Clinical and metabolic characteristics from IO patients and control subjects.
| Parameter | HH with IO (n=8) | HH w/o IO (n=17) | Controls (n=25) |
|---|---|---|---|
| Age (y)* | 61 (55–67)1 | 44 (34–55)2 | 49 (35–57)2 |
| BMI (kg/m2)* | 28 ± 31 | 28 ± 21 | 24 ± 22 |
| Glucose (mg/dl)* | 102 (95–114)1 | 95 (90–113)1 | 89 (86–95)2 |
| TG (mg/dl) | 96 (80–133) | 113 (77–158) | 86 (65–114) |
| TC (mg/dl) | 167 ± 32 | 178 ± 38 | 186 ± 30 |
| LDL-C (mg/dl) | 99 ± 20 | 112 ± 35 | 119 ± 28 |
| HDL-C (mg/dl) | 50 ± 13 | 44 ± 8 | 48 ± 11 |
| Apo A–I (mg/dl) | 135 ± 30 | 127 ± 13 | 138 ± 31 |
| Apo B (mg/dl) | 84 ± 20 | 80 ± 32 | 94 ± 19 |
| AST (IU/l)* | 48 (32–57)1 | 32 (22–45)2 | 19 (18–26)2 |
| ALT (IU/l)* | 52 (28–72)1 | 28 (21–34)2 | 20 (17–23)2 |
| Albumin (g/dl) | 4.3 ± 0.3 | 4.4 ± 0.3 | 4.3 ± 0.1 |
| Hepcidin/ferrtin ratio* | 0.03 ± 0.021 | 0.22 ± 0.212 | 0.23 ± 0.172 |
| Tf (mg/dl)* | 203 ± 381 | 248 ± 302 | 249 ± 332 |
Abbreviations: TC, total cholesterol; TG, triglyceride.
* = P<0.01. Different superscript numbers over the mean or the median indicate significantly dissimilar groups.
Figure 1. Iron metabolism in IO patients and control subjects.
Panel A: Ferritin; Panel B: Transferrin Saturation (Transf. Sat.); Panel C: Body Iron; Panel D: Hepcidin. Different letters (a–c) indicate statistically significant differences.
Iron metabolism parameters
HH patient with IO showed higher levels of ferritin, TfSat (%), and body iron plus lower Tf compared with both HH patients without IO and controls. Additionally, HH patients without IO presented higher TfSat (%) and body iron than control subjects. There were no differences among the groups in hepcidin concentration (Figure 1), but hepcidin/ferritin ratio was significantly lower in HH patients with IO (Table 1).
ATL in patients with HH
A linear trend between the groups (P for trend<0.01) was observed. ATL were shorter in patients with overt IO than in controls (Figure 2). Differences in ATL remained statistically significant in a model adjusted by age, BMI and glucose levels (P<0.05).
Figure 2. Leukocyte telomere length in healthy controls and IO patients.
Different letters (a-b) indicate statistically significant differences. *P<0.01.
ATL was significantly correlated with BMI (r = −0.37; P<0.05), glucose (r = −0.26; P<0.01), AST (r = −0.36; P<0.05), ALT (r = −0.35, P<0.05), TfSat (r = −0.26; P<0.05), ferritin (r = −0.38; P<0.01), and hepcidin (r = −0.28; P<0.05). Moreover, no association was detected between HFE genotype/allele distribution and ATL. In multivariate analysis, glucose levels remained the only significant predictor of ATL (r2 = 0.32; β = −0.51; P<0.01) in a model that also included age, BMI and ferritin.
Discussion
Telomere shortening can impact tissue homeostasis by impairing cell proliferation and giving rise to genome instability, thus constituting the bases for the development of different pathological conditions [27]. The present study shows that HH patients with overt IO featured significantly shorter ATL while HH patients without IO showed only mild alterations in comparison with healthy controls. The exposition to elevated iron levels over time might be an important factor leading to telomere attrition in HH. The present results highlight the importance of early diagnosis and treatment of IO to preserve ATL.
Excess iron deposition has been shown to be associated with oxidative damage of both circulating biomolecules and cells in a process that accelerates the physiologic changes that naturally occur with aging and leads, among others, to age-related conditions [3–7]. In fact, excess iron accumulation displays multiple toxic effects, which are mostly based on its ability to catalyze the generation of free radicals, resulting in cell death and tissue injury [21]. ROS generation is responsible for the onset of different cellular stress responses such as DNA damage, organelle fragmentation and membrane blebbing. Apart from being directly cytotoxic, iron and free radicals activate inflammatory cells, increasing cytokine production and further generation of ROS [28]. Evidence suggests that free radicals may damage telomere DNA and enhance its shortening [22]. Hence, ATL has been proposed as a marker of cumulative oxidative stress and biological aging [18,22,29].
Apart from the differences observed in the present study in telomere length among the three studied groups, the process of shortening seemed to be associated with the presence of IO. This finding is in agreement with a previous report [30] which showed that iron phenotype and not HFE genotype had a significant effect on telomere length. Patients with IO and, consequently, with the highest values of TfSat and ferritin, showed the shortest telomeres. Furthermore, our study indicates a significant inverse association between TfSat and ferritin levels with telomere length. Similar findings have also been previously reported by Mainou et al. [31], they observed that individuals with high TfSat presented shortened telomere length when compared with those with low TfSat. However, it is important to note that the present study did not include healthy controls and TfSat was the only parameter associated with iron metabolism analyzed in the study. Regarding ferritin levels, Liu et al. [32] observed that telomere length was negatively associated with the presence of high ferritin levels. Nevertheless, in the present study the association between ferritin and ATL was only significant for individuals over 65 years old. Notably, in the present study, ATL was negatively associated with markers of liver damage AST and ALT. This is interesting because the liver is one of the main targets of free radical-induced damage in IO patients [21]. Moreover, in the present study, ATL was inversely correlated with BMI. This finding is consistent with several prior studies carried out by our group which showed increases in BMI and the presence of obesity as major determinants of telomere shortening [33,34].
In addition to oxidative stress, another trait of HH that would contribute to telomere shortening, is inflammation [35,36]. IO promotes liver inflammation which, in tandem with oxidative stress, could contribute to telomere attrition. In particular, iron deposits could directly alter the phenotype of T cells present in the liver leading to changes in cytokine expression [37]. In this regard, it is noteworthy that, as previously mentioned, ATL was negatively associated with ALT and AST levels. Both ALT and AST are considered markers of liver inflammation [38] and would suggest a link between the later and telomere shortening in IO patients.
Remarkably, in the multivariate analysis, glucose levels were the only independent predictor of ATL. This is an interesting finding because it raises the possibility that telomere shortening in IO patients could be the result of alterations in carbohydrate metabolism commonly observed in these patients. Telomere shortening is known to be a feature of diseases that primarily result from alterations in glucose homeostasis such as insulin resistance and diabetes [17]. Nevertheless, to our knowledge, this would be the first study to suggest that impairment in carbohydrate metabolism could be the main factor responsible for telomere shortening in pathologies with high glucose as a secondary trait as is the case with IO. Thus, on these bases, patients with chronic conditions that are frequently associated with hyperglycemia would be also prone to undergo a process of telomere shortening.
As far as we know, our study is the first to show that IO patients who underwent successful treatment aimed to reduce body iron levels and, therefore, systemic oxidative stress [39,40], present ATL values statistically similar to those of healthy controls. This is consistent with previous findings, both in vivo and in vitro, that indicate a positive effect of antioxidant therapy on telomere length [41,42]. Previous studies showed that the addition of N-acetylcysteine to both human lymphocytes exposed to irradiation and human astrocytoma cells infected with the human immunodeficiency virus decreases the rate of telomere attrition [43,44]. Similarly, serum drawn from subjects under a low-saturated fat diet showed the slowest rate of telomere shortening when added to the culture medium of human umbilical vein endothelial cells [45]. Furthermore, several studies reported an increase in telomere length after antioxidant supplementation in animal models [46,47]. Moreover, different double-blind, randomized placebo-controlled studies suggested that supplementation with ω-3 fatty acids could slow telomere attrition in both overweight patients and older-age individuals [48,49]. Similarly, Zhu et al. [50] observed a significant decrease in telomere attrition in overweight individuals after 4 months of vitamin D supplementation.
The fact that iron depletion could decrease telomere attrition is noteworthy given that the latter has been associated with shorter lifespan as well as a wide variety of age-related diseases and conditions, including cardiovascular disease, diabetes, insulin resistance and hypertension [30,51–54]. Regarding cardiovascular disease, telomere length has been proposed as a predictor of coronary heart disease events [55,56] and cardiovascular disease-related mortality [57,58]. In fact, telomere length would be associated with subclinical atherosclerosis markers such as carotid artery intima media thickness [59,60] and carotid artery calcification [51,61].
In summary, we observed an association between ATL and IO overt condition. HH patients with overt IO featured significantly shorter ATL, while HH patients without IO showed similar ATL when compared with healthy controls.
Conclusion
In conclusion, overt IO was associated with shorter ATL in patients with HH. Screening for IO is encouraged to prevent iron-associated cellular damage and early telomere attrition. ATL could provide prognostic information for IO-related complications in patients with HH.
Abbreviations
- ALT
alanine aminotransferase
- Apo
apolipoprotein
- AST
aspartate aminotransferase
- ATL
absolute telomere length
- BMI
body mass index
- HDL-C
high-density lipoprotein cholesterol
- HFE
homeostatic iron regulator
- HH
hereditary hemochromatosis
- IO
iron overload
- LDL
low-density lipoprotein
- Tf
transferrin
- TfR
Tf receptor
- TfSat
Tf saturation
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the University of Buenos Aires [grant number UBACyT CB23]; the CONICET [grant number PIP 516]; and the ANPCyT [grant number PICT 2016-2018].
Author Contribution
Maximilino Martin carried out most of the biochemical assays detailed in the paper and contributed to the writing of the manuscript. Andrea Millan, Florencia Ferraro, Walter Tetzlaff, Ezequiel Lozano Chiappe, Eliana Botta and Marcelo Castro carried out the remaining part of the biochemical assays detailed in the paper. Laura Boero, Jorge Rey, Jorge Daruich, Gustavo Frechtel, Tomas Meroño, Gloria Cerrone and Fernando Brites contributed to the writing of the manuscript.
References
- 1.Pantopoulos K. (2008) Function of the hemochromatosis protein HFE: lessons from animal models. World J. Gastroenterol. 14, 6893–6901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katsarou M., Papasavva M., Latsi R. and Drakoulis N. (2019) Hemochromatosis: hereditary hemochromatosis and HFE gene. Vitam. Horm. 110, 201–222 [DOI] [PubMed] [Google Scholar]
- 3.Pra D., Franke S.I.R., Henriques J.A.P. and Fenech M. (2012) Iron and genome stability: an update. Mutat. Res. 733, 92–99 [DOI] [PubMed] [Google Scholar]
- 4.Xu J., Marzetti E., Seo A.Y., Kim J.S., Prolla T.A. and Leeuwenburgh C. (2010) The emerging role of iron dyshomeostasis in the mitochondrial decay of aging. Mech. Ageing Dev. 131, 487–493 10.1016/j.mad.2010.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zecca L., Casella L., Albertini A., Bellei C., Zucca F.A., Engelen M. et al. (2008) Neuromelanin can protect against iron-mediated oxidative damage in system modeling iron overload of brain aging and Parkinson's disease. J. Neurochem. 106, 1866–1875 [DOI] [PubMed] [Google Scholar]
- 6.Dunaief J. (2006) Iron induced oxidative damage as a potential factor in age-related macular degeneration: The Cogan Lecture. Invest. Ophthalmol. Vis. Sci. 47, 4660–4664 10.1167/iovs.06-0568 [DOI] [PubMed] [Google Scholar]
- 7.Quintana C., Bellefqih S., Laval J.Y., Guerquin-Kern J.L., Wu T.D., Avila J. et al. (2006) Study of the localization of iron, ferritin, and hemosiderin in Alzheimer’s disease hippocampus by analytical microscopy at the subcellular level. J. Struct. Biol. 153, 42–54 10.1016/j.jsb.2005.11.001 [DOI] [PubMed] [Google Scholar]
- 8.Siddique A. and Kowdley K.V. (2012) Review article: the iron overload syndromes. Aliment. Pharmacol. Ther. 35, 876–893 10.1111/j.1365-2036.2012.05051.x [DOI] [PubMed] [Google Scholar]
- 9.Simcox J. and McClain D.A. (2013) Iron and diabetes risk. Cell Metab. 17, 329–341 10.1016/j.cmet.2013.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Datz C., Muller E. and Aigner E. (2017) Iron overload and non-alcoholic fatty liver disease. 2017. Miner. Endocrinol. 42, 173–183 [DOI] [PubMed] [Google Scholar]
- 11.Huan H., Yang Q., Zeyu Z., Zelong W., Dan L., Zhangping L. et al. (2018) Dual action of vitamin C in iron supplement therapeutics for iron deficiency anemia: prevention of liver damage induced by iron overload. Food Funct. 9, 5390. [DOI] [PubMed] [Google Scholar]
- 12.Corti M., Gaziano M. and Hennekens C.H. (1997) Iron status and risk of cardiovascular disease. Ann. Epidemiol. 7, 62–68 10.1016/S1047-2797(96)00112-3 [DOI] [PubMed] [Google Scholar]
- 13.Sullivan J. (1999) Iron therapy and cardiovascular disease. Kidney Int. Suppl. 69, 135–137 10.1046/j.1523-1755.1999.055Suppl.69135.x [DOI] [PubMed] [Google Scholar]
- 14.Meroño T., Gómez Rosso L., Sorroche P., Boero L., Arbelbide J. and Brites F. (2011) High risk of cardiovascular disease in iron overload patients. Eur. J. Clin. Invest. 41, 479–486 10.1111/j.1365-2362.2010.02429.x [DOI] [PubMed] [Google Scholar]
- 15.Meroño T., Brites F., Dauteuille C., Lhomme M., Menafra M., Arteaga A. et al. (2015) Metabolic alterations, HFE gene mutations and atherogenic lipoprotein modifications in patients with primary iron overload. Clin. Sci. (Lond.) 128, 609–618 10.1042/CS20140300 [DOI] [PubMed] [Google Scholar]
- 16.Haycock P.C., Heydon E.E., Kaptoge S., Butterworth A.S., Thompson A. and Willeit P. (2014) Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ 349, g4227 10.1136/bmj.g4227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verhulst S., Dalgård C., Labat C., Kark J.D., Kimura M., Christensen K. et al. (2016) A short leucocyte telomere length is associated with development of insulin resistance. Diabetologia 59, 1258–1265 10.1007/s00125-016-3915-6 [DOI] [PubMed] [Google Scholar]
- 18.Babizhayev M.A., Savel’yeva E.L., Moskvina S.N. and Yegorov Y.E. (2011) Telomere length is a biomarker of cumulative oxidative stress, biologic age, and an independent predictor of survival and therapeutic treatment requirement associated with smoking behavior. Am. J. Ther. 18, 209–226 10.1097/MJT.0b013e3181cf8ebb [DOI] [PubMed] [Google Scholar]
- 19.Martínez P. and Blasco M.A. (2017) Telomere-driven diseases and telomere-targeting therapies. J. Cell Biol. 216, 875–887 10.1083/jcb.201610111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Sullivan R.J. and Karlseder J. (2010) Telomeres: protecting chromosomes against genome instability. Nat. Rev. Mol. Cell Biol. 11, 171–181 10.1038/nrm2848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bacon B.R. and Britton R.S. (1989) Hepatic injury in chronic iron overload. Role of lipid peroxidation. Chem. Biol. Interact. 70, 183–226 10.1016/0009-2797(89)90045-8 [DOI] [PubMed] [Google Scholar]
- 22.von Zglinicki T. (2000) Role of oxidative stress in telomere length regulation and replicative senescence. Ann. N.Y. Acad. Sci. 908, 99–110 10.1111/j.1749-6632.2000.tb06639.x [DOI] [PubMed] [Google Scholar]
- 23.Shin C. and Baik I. (2017) Transferrin saturation concentrations associated with telomeric ageing: a population-based study. Br. J. Nutr. 117, 1693–1701 10.1017/S0007114517001696 [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization (2020) https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight [Google Scholar]
- 25.O’Callaghan N. and Fenech M. (2011) A quantitative pcr method for measuring absolute telomere length. Biol. Proced. Online. 13, 3 10.1186/1480-9222-13-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray M. and Thompson W.F. (1980) Rapid isolation of high molecular 611 weight plant DNA. Nucleic Acids Res. 8, 4321–4325 10.1093/nar/8.19.4321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henriques C.M. and Ferreira M.G. (2012) Consequences of telomere shortening during lifespan. Curr. Opin. Cell Biol. 24, 804–808 10.1016/j.ceb.2012.09.007 [DOI] [PubMed] [Google Scholar]
- 28.Nakamura T., Naguro I. and Ichijo H. (2019) Iron homeostasis and iron-regulated ROS in cell death, senescence and human diseases. Biochim. Biophys. Acta Gen. Subj. 1863, 1398–1409 10.1016/j.bbagen.2019.06.010 [DOI] [PubMed] [Google Scholar]
- 29.Shammas M. (2011) Telomeres, lifestyle, cancer, and aging. Curr. Opin. Clin. Nutr. Metab. Care 14, 28–34 10.1097/MCO.0b013e32834121b1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mainous A.G. III, Wright R.U., Hulihan M.M., Twal W.O., McLaren C.E., Diaz V.A. et al. (2013) Telomere length and elevated iron: the influence of phenotype and HFE genotype. Am. J. Hematol. 88, 492–496 10.1002/ajh.23438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mainous A.G. III, Wright R.U., Hulihan M.M., Twal W.O., McLaren C.E., Diaz V.A. et al. (2014) Elevated transferrin saturation, health-related quality of life and telomere length. Biometals 27, 135–141 10.1007/s10534-013-9693-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu B., Sun Y., Xu G., Snetselaar L.G., Ludewig G., Wallace R.B. et al. (2019) Association between body iron status and leukocyte telomere length, a biomarker of biological aging, in a nationally representative sample of US adults. Acad. Nutr. Diet. 119, 617–625 10.1016/j.jand.2018.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gielen M., Hageman G.J., Antoniou E.E., Nordfjall K., Mangino M., Balasubramanyam M. et al. (2018) Body mass index is negatively associated with telomere length: a collaborative cross-sectional meta-analysis of 87 observational studies. Am. J. Clin. Nutr. 108, 453–475 10.1093/ajcn/nqy107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iglesias Molli A., Panero J., Dos Santos P.C., González C.D., Vilariño J., Sereday M. et al. (2017) Metabolically healthy obese women have longer telomere length than obese women with metabolic syndrome. PLoS ONE 12, e0174945 10.1371/journal.pone.0174945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kordinas V., Ioannidis A. and Chatzipanagiotou S. (2016) The telomere/telomerase system in chronic inflammatory diseases. Cause or effect? Genes (Basel) 3, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J., Rane G., Dai X., Shanmugam M.K., Arfuso F., Samy R.P. et al. (2016) Ageing and the telomere connection: an intimate relationship with inflammation. Ageing Res. Rev. 25, 55–69 10.1016/j.arr.2015.11.006 [DOI] [PubMed] [Google Scholar]
- 37.Hübscher S.G. (2003) Iron overload, inflammation and fibrosis in genetic haemochromatosis. J. Hepatol. 38, 521–525 10.1016/S0168-8278(03)00078-3 [DOI] [PubMed] [Google Scholar]
- 38.McGill M. (2016) The past and present of serum aminotransferases and the future of liver injury biomarkers. EXCLI J. 15, 817–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Houglum K., Ramm G.A., Crawford D.H., Witztum J.L., Powell L.W. and Chojkier M. (1997) Excess iron induces hepatic oxidative stress and transforming growth factor beta1 in genetic hemochromatosis. Hepatology 26, 605–610 10.1002/hep.510260311 [DOI] [PubMed] [Google Scholar]
- 40.Kaito M., Iwasa M., Kobayashi Y., Fujita N., Tanaka H., Gabazza E.C. et al. (2006) Iron reduction therapy by phlebotomy reduces lipid peroxidation and oxidative stress in patients with chronic hepatitis C. J. Gastroenterol. 41, 921–922 10.1007/s00535-006-1871-5 [DOI] [PubMed] [Google Scholar]
- 41.Glade M. and Meguid M. (2015) Oxidative telomere attrition, nutritional antioxidants and biological aging. Nutrition 31, 1447–1451 10.1016/j.nut.2015.05.018 [DOI] [PubMed] [Google Scholar]
- 42.Reichert S. and Stier A. (2017) Does oxidative stress shorten telomeres in vivo? A review. Biol Lett. 13, pii:20170463 10.1098/rsbl.2017.0463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pereboeva L., Westin E., Patel T., Flaniken I., Lamb L., Klingelhutz A. et al. (2013) DNA damage responses and oxidative stress in dyskeratosiscongenita. PLoS ONE 8, e76473 10.1371/journal.pone.0076473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pollicita M., Muscoli C., Sgura A., Biasin A., Granato T., Masuelli L. et al. (2009) Apoptosis and telomeres shortening related to HIV-1 induced oxidative stress in an astrocytoma cell line. BMC Neurosci. 10, 51 10.1186/1471-2202-10-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marin C., Delgado-Lista J., Ramirez R., Carracedo J., Caballero J., Perez-Martinez P. et al. (2012) Mediterranean diet reduces senescence-associated stress in endotelial cells. Age (Dordr.) 34, 1309–1316 10.1007/s11357-011-9305-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Badás E., Martínez J., Rivero de Aguilar Cachafeiro J., Miranda F., Figuerola J. and Merino S. (2015) Ageing and reproduction: antioxidant supplementation alleviates telomere loss in wild birds. J. Evol. Biol. 28, 896–905 10.1111/jeb.12615 [DOI] [PubMed] [Google Scholar]
- 47.Tarry-Adkins J., Blackmore H.L., Martin-Gronert M.S., Fernandez-Twinn D.S., McConnell J.M., Hargreaves I.P. et al. (2013) Coenzyme Q10 prevents accelerated cardiac aging in a rat model of poor maternal nutrition and accelerated postnatal growth. Mol. Metab. 2, 480–490 10.1016/j.molmet.2013.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kiecolt-Glaser J., Epel E., Belury M., Andridge R., Lin J., Glaser R. et al. (2013) Omega-3 fatty acids, oxidative stress, and leukocyte telomere length: a randomized controlled trial. Brain Behav. Immun. 28, 16–24 10.1016/j.bbi.2012.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O'Callaghan N., Parletta N., Milte C.M., Benassi-Evans B., Fenech M. and Howe P.R. (2014) Telomere shortening in elderly individuals with mild cognitive impairment may be attenuated with ω-3 fatty acid supplementation: a randomized controlled pilot study. Nutrition 30, 489–491 10.1016/j.nut.2013.09.013 [DOI] [PubMed] [Google Scholar]
- 50.Zhu H., Guo D., Li K., Pedersen-White J., Stallmann-Jorgensen I., Huang Y. et al. (2012) Increased telomerase activity and vitamin d supplementation in overweight africanamericans. Int. J. Obes. (Lond.) 36, 10 10.1038/ijo.2011.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mainous A.G. III, Codd V., Diaz V.A., Schoepf U.J., Everett C.J., Player M.S. et al. (2010) Leukocyte telomere length and coronary artery calcification. Atherosclerosis 210, 262–267 10.1016/j.atherosclerosis.2009.10.047 [DOI] [PubMed] [Google Scholar]
- 52.Adaikalakoteswari A., Balasubramanyam M. and Mohan V. (2005) Telomere shortening occurs in Asian Indian Type 2 diabetic patients. Diabetes Med. 22, 1151–1156 10.1111/j.1464-5491.2005.01574.x [DOI] [PubMed] [Google Scholar]
- 53.Demissie S., Levy D., Benjamin E.J., Cupples L.A., Gardner J.P., Herbert A. et al. (2006) Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell 5, 325–330 10.1111/j.1474-9726.2006.00224.x [DOI] [PubMed] [Google Scholar]
- 54.Kark J., Nassar H., Shaham D., Sinnreich R., Goldberger N., Aboudi V. et al. (2013) Leukocyte telomere length and coronary artery calcification in Palestinians. Atherosclerosis 229, 363–368 10.1016/j.atherosclerosis.2013.05.030 [DOI] [PubMed] [Google Scholar]
- 55.Brouilette S., Moore J.S., McMahon A.D., Thompson J.R., Ford I., Shepherd J. et al. (2007) Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet 369, 107–114 10.1016/S0140-6736(07)60071-3 [DOI] [PubMed] [Google Scholar]
- 56.Fitzpatrick A., Kronmal R.A., Gardner J.P., Psaty B.M., Jenny N.S., Tracy R.P. et al. (2007) Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am. J. Epidemiol. 165, 14–21 10.1093/aje/kwj346 [DOI] [PubMed] [Google Scholar]
- 57.Fitzpatrick A., Kronmal R.A., Kimura M., Gardner J.P., Psaty B.M., Jenny N.S. et al. (2011) Leukocyte telomere length and mortality in the Cardiovascular Health Study. J. Gerontol. A Biol. Sci. Med. Sci. 66, 421–429 10.1093/gerona/glq224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bakaysa S., Mucci L.A., Slagboom P.E., Boomsma D.I., McClearn G.E., Johansson B. et al. (2007) Telomere length predicts survival independent of genetic influences. Aging Cell 6, 769–774 10.1111/j.1474-9726.2007.00340.x [DOI] [PubMed] [Google Scholar]
- 59.O'Donnell C., Demissie S., Kimura M., Levy D., Gardner J.P., White C. et al. (2008) Leukocyte telomere length and carotid artery intimal medial thickness: the Framingham Heart Study. Arterioscler Thromb. Vasc. Biol. 28, 1165–1171 10.1161/ATVBAHA.107.154849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Meyer T., Rietzschel E.R., De Buyzere M.L., Langlois M.R., De Bacquer D., Segers P. et al. (2009) Systemic telomere length and preclinical atherosclerosis: the Asklepios Study. Eur. Heart J. 30, 3074–3081 10.1093/eurheartj/ehp324 [DOI] [PubMed] [Google Scholar]
- 61.Kroenke C., Pletcher M.J., Lin J., Blackburn E., Adler N., Matthews K. et al. (2012) Telomerase, telomere length, and coronary artery calcium in black and white men in the CARDIA study. Atherosclerosis 220, 506–512 10.1016/j.atherosclerosis.2011.10.041 [DOI] [PubMed] [Google Scholar]