Abstract
Introduction
Acute pancreatitis (AP) is an inflammatory process of pancreas with varying degree of involvement of regional tissues. The aim of this study was to investigate the potential use of serum cystatin C (Cys-C) for the early and accurate diagnosis of acute kidney injury (AKI) in patients of AP.
Materials and methods
This was a prospective study conducted in 1 year. Total of 215 cases of AP fulfilling the inclusion criteria were enrolled in this study. Patients suffering from chronic pancreatitis, neoplasm, chronic liver disease, and chronic kidney disease were excluded from the study. Diagnosis of AP was based on the Atlanta classification 2012. All patients were classified into a non-AKI group (n = 152) and an AKI group (n = 38) according to the dynamic changes in serum creatinine levels. Serum Cys-C was measured by particle-enhanced immune nephelometric assay.
Results
By univariate logistic regression analysis, body mass index (BMI) (OR = 1.44, 95% CI: 1.23–1.68; p < 0.001), blood urea (OR = 1.15, 95% CI: 1.06–1.23; p < 0.001), Cys-C (OR = 1.04, 95% CI: 1.01–1.07; p < 0.05), serum calcium (OR = 0.59, 95% CI: 0.41–0.86; p < 0.05), and serum lactate dehydrogenase (LDH) (OR = 1.001, 95% CI: 1.0–1.001; p < 0.05) were the significant indicators for AKI in patients with AP. Using multivariate logistic regression analysis, urinary albumin and Cys-C were independent and significant indicators of AKI in patients with AP (OR = 1.026, 95% CI: 1.01–1.07; p < 0.01). Receiver operating characteristic (ROC) curve of serum Cys-C, for AKI in patient with AP could be identified with a sensitivity of 92.06% at specificity of 96.0% [area under the curve (AUC) = 0.96, 95% CI: 0.92–0.98] by baseline serum Cys-C (cutoff value = >32.32 mg/L).
Conclusion
Increase of baseline serum Cys-C was associated with AKI in patients with AP.
How to cite this article
Patel ML, Shyam R, Bharti H, Sachan R, Gupta KK, Parihar A. Evaluation of Serum Cystatin C as an Early Biomarker of Acute Kidney Injury in Patients with Acute Pancreatitis. Indian J Crit Care Med 2020;24(9):777–782.
Keywords: Acute kidney injury, Acute pancreatitis, Cystatin C (Cys-C), Severity
Introduction
Acute pancreatitis (AP) may result in a wide variety of clinical features with a clinical spectrum ranging from meager findings, such as abdominal pain, to one of the cataclysmic deteriorations. Acute pancreatitis patients have a self-limited course, but it may become worse in about 15–20% with progression to multiple organ dysfunction or local complications including necrosis, pseudocyst, and abscesses, and then called severe acute pancreatitis (SAP).1
The pathophysiology of acute kidney injury (AKI) in AP is not well studied. However, a key pathophysiological process involves premature activation of pancreatic enzymes within the acinar cells. This leads to autodigestion of the pancreas and surrounding tissues, triggering a cascade of events that contribute to AKI.2 Acute kidney injury is a known complication of SAP. Overall prevalence of AKI is 7.9% among in hospitalized patients with AP.3 The mortality rate among AKI subgroup was significantly higher (8.8% in AKI group vs 0.7% in non-AKI). Despite the availability of intensive care, AKI is one of the most common causes of death in patients with SAP.3
Kidney disease improving global outcomes (KDIGO) help in providing an accurate and confirmed definition of AKI4 and have thus become standard for diagnostic criteria.5 However, it has certain limitations. First, it causes a problem in diagnosing patients with AKI who have been admitted in medical emergency. Continuous assessment of serum creatinine and urine output for several days is a must for the diagnosis of AKI. Sometimes, the baseline of serum creatinine is unknown and a urinary catheter may not be placed and it may lead to a delay in the diagnosis of AKI and therefore in rendering adequate therapy.6 Second, in spite of damage to kidney, levels of serum creatinine may not rise high enough to fulfil the diagnostic criteria labeling the patient as subclinical AKI.7 All these factors necessitate the need for new biomarkers which may lead to early diagnosis and prediction of AKI.
Serum cystatin C (Cys-C) is a cysteine proteinase enzyme inhibitor, and it has low-molecular weight and does not bind to proteins.8 Serum Cys-C is freely filtered through the glomerular membrane and is completely reabsorbed and metabolized through the proximal tubular cells without secretion.9,10 Serum Cys-C is not affected either by inflammation, fever, and or exogenous agents or by muscle mass, gender, or age. Upon mild kidney injury, serum Cys-C begins to increase 24–48 hours before serum Cr increases, and serum Cys-C gradually increases with disease progression.11,12 This early rise of serum Cys-C rise with AKI occurs before rise in serum creatinine, leading to the evaluation of Cys-C as an “early AKI biomarker”. The aim of this study was to investigate the potential use of serum Cys-C for the early and accurate diagnosis of AKI in patients of AP.
Materials and Methods
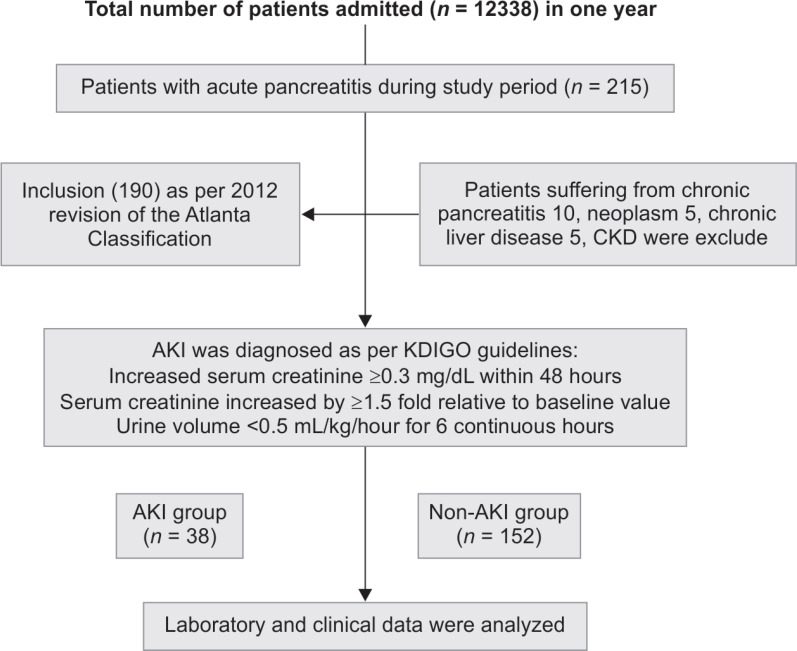
This prospective study was carried out over a period of 1 year from August 2018 to July 2019 in the Department of Medicine in collaboration with the Department of Radiology and Pathology, King George's Medical University, Lucknow, Uttar Pradesh, India. Ethical clearance was obtained from the Institutional Ethics Committee (293/Ethics/R.Cell 18). After written and informed consent, total 215 cases of AP, aged between 15 years and 60 years were recruited for the study. Patients suffering from chronic pancreatitis, neoplasm, chronic liver disease, and chronic kidney disease were excluded from the study. Total 190 patients of AP defined as per Atlanta classification 2012 were enrolled for the study.13 As per Atlanta classification 2012, patients who were fulfilling two out of three criteria including abdominal pain characteristic of AP, serum amylase, and/or lipase levels at least three times the upper limit of normal or characteristic findings of AP on abdominal ultrasonography and/or computed tomography (CT) scan were considered as a case of AP, were included in the study (Flowchart 1).
Flowchart 1.
Flow diagram of patients as per strengthening the reporting of observational studies in epidemiology
Acute kidney injury was diagnosed in accordance with AKIN guideline,5 and the results were as follows: (1) increased serum creatinine ≥0.3 mg/dL within 48 hours; (2) serum creatinine increased by ≥1.5-fold relative to baseline values within 7 days; and (3) urine volume <0.5 mL/kg/hour for 6 continuous hours (Table 1).
Table 1.
Acute kidney injury network criteria
| Stage | Serum creatinine criteria | Urine output criteria |
|---|---|---|
| 1 | ↑ to ≥1.5× baseline or ↑ 0.3 mg/dL from baseline | <0.5 mL/kg/hour ≥6 hours |
| 2 | ↑ to ≥2× baseline | <0.5 mL/kg/hour ≥12 hours |
| 3 | ↑ to ≥3× from baseline or 4 mg/dL with acute ↑ ≥0.5 mg/dL or initiate of RRT irrespective of age at time of initiation | <0.30 mL/kg/hour ≥24 hours Anuria ≥12 hours |
Only one criterion (serum creatinine or urine output) should be fulfilled to quality for a stage
RRT, renal replacement therapy; ↑, increased; ×, times
Patients with mild acute pancreatitis (MAP) had neither local complications nor organ failure. Patients with moderately severe acute pancreatitis (MSAP) had transient organ failure or local complications or both, whereas patients with SAP had persistent organ failure.
After detailed history and physical examination, laboratory investigations were sent at the time of admission. An automated blood-cell analyzer (Abbott Cell Dyn Ruby Hematology Analyzer, Abbott Park, Illinois, USA) was used for routine hematology testing, and an automated clinical biochemistry analyzer (ELITechSelectra ProM, ELITech Group, Puteaux, France) was used for kidney function test, liver function test, serum electrolytes, spot urinary albumin, serum amylase, serum lipase, serum protein, and albumin. Contrast-enhanced pancreatic CT scan was performed after 72 hours of hospitalization.
Serum Cys-C was measured by particle-enhanced immune nephelometric assay (Shanghai Sunred Biological Technology Co. Ltd., China) and its measuring range was 0.6–100 ng/mL. The results of Cys-C were blinded to the medical staffs during the study and did not affect the management of patients.
At the time of discharge/death, patients were graded for severity of disease as having mild, moderately severe, and SAP based on the Atlanta 2012 classification.13
Statistical Analysis
The normality of the continuous variables was tests using the Kolmogorov–Smirnov test and the data for the same are represented as mean ± SD. The categorical variables are presented as percentages. The comparisons for continuous variables were performed using one-way ANOVA and for categorical variables Chi-square test was used. In case of non-normally distributed data, non-parametric analog tests were used. Univariate logistic regression analysis and multiple logistic regression models were used to predict AKI in patients with AP. Receiver operating characteristic (ROC) curve analysis was used to calculate the area under the curve (AUC), sensitivity, and specificity for testing Cys-C as potential marker of AKI in patients with AP. A p value of <0.05 was considered as significant. All analyses were performed using MedCalc Statistical Software version 19.2.6 (MedCalc Software Ltd., Ostend, Belgium) and SPSS software version 19.2.1 (Armonk, New York: IBM Corp.).
Results
Patients’ characteristics are presented in Table 2. There was no statistical difference in the distribution of gender and age. All patients were classified into a non-AKI group (n = 152) and an AKI group (n = 38) according to the dynamic changes in serum creatinine levels. Body mass index (BMI) between the two groups (non-AKI vs AKI) were 23.7 ± 2.0 and 25.8 ± 2.9 which was statistically significant (p < 0.001). In AKI group, mean value of blood urea (31.66 ± 17.08 vs 178.23 ± 82.84), serum creatinine (0.85 ± 1.20 vs 4.12 ± 2.13), and serum Cys-C (29.42 ± 41.56 vs 152.49 ± 117.75) was higher and statistically significant in comparison to non-AKI group (p < 0.001).
Table 2.
The characteristics of subjects
| Characteristics | Non-AKI group (n = 152) | AKI group (n = 38) | p value |
|---|---|---|---|
| Age (years) | 35.8 ± 14.03 | 38.26 ± 11.08 | 0.31 |
| Gender (M/F) | 84/68 | 22/16 | 0.41 |
| BMI (kg/m2) | 23.7 ± 2.0 | 25.8 ± 2.9 | <0.001 |
| Blood urea (mg/dL) | 31.66 ± 17.08 | 178.23 ± 82.84 | <0.001 |
| S. creatinine (mg/dL) | 0.85 ± 1.20 | 4.12 ± 2.13 | <0.001 |
| Cys-C (ng/mL) | 29.42 ± 41.56 | 152.49 ± 117.75 | <0.001 |
| S. amylase (U/L) | 437.12 ± 546.5 | 615.8 ± 495.22 | <0.05 |
| S. lipase (U/L) | 278.05 ± 465.32 | 424.45 ± 623.72 | 0.11 |
| FBS (mg/dL) | 128.01 ± 69.75 | 128.10 ± 55.63 | 0.99 |
| S. bilirubin (mg/dL) | 1.58 ± 2.41 | 1.85 ± 3.08 | 0.56 |
| SGOT (U/L) | 82.90 ± 169.45 | 59.67 ± 66.10 | 0.41 |
| SGPT (U/L) | 57.45 ± 87.07 | 42.71 ± 75.63 | 0.34 |
| Urine albumin (μg/L) | 200 ± 104.65 | 410 ± 156.48 | <0.01 |
| Mortality | 1 (0.6%) | 5 (13.1%) | <0.01 |
Significantly higher values of BMI, blood urea, and Cys-C were observed in patients with AKI as compared to patients without AKI
The number of patients with MAP, MSAP, and SAP were 69 (36.3%), 40 (22.1%), and 79 (41.5%), respectively. There were 38 (20%) patients who had developed AKI according to the AKIN guideline. The number of patients with AKI stage I, II, and III was 8 (21%), 9 (23.6%), and 21 (55.2%) respectively (Table 3).
Table 3.
Number of AKI with stages in acute pancreatitis
| Stage of AKI | AKI at presentation (n = 38) (20.0%) | |||
|---|---|---|---|---|
| Mild acute pancreatitis (MAP) [n = 69 (36.3%)] | Moderately severe acute pancreatitis (MSAP) [n = 42 (22.10%)] | Severe acute pancreatitis (SAP) [n = 79 (41.5%)] | Total number of cases (n = 190) | |
| Grade I | – | 8 (21%) | – | |
| Grade II | – | – | 9 (23.6%) | |
| Grade III | – | – | 21 (55.2%) | |
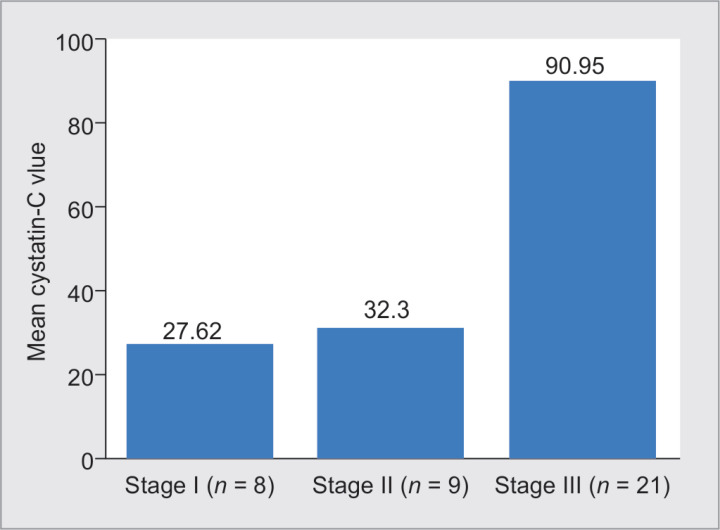
The Spearman's correlation coefficient between Cys-C and the stages of AKI was observed to be 27.62 ± 16.65 for stage I AKI, 32.30 ± 20.49 for stage II AKI and 90.95 ± 156.58 for stage III AKI which shows that increase in the stage of AKI was associated with higher values of Cys-C (Table 4 and Fig. 1).
Table 4.
Correlation of Cys-C with grades of AKI
| Stage of AKI | Cys-C |
|---|---|
| Stage I (AKI = 1) | 27.62 ± 16.65 (15.58–65.76) |
| Stage II (AKI = 2) | 32.30 ± 20.49 (23.19–86.35) |
| Stage III (AKI = 3) | 90.95 ± 156.58 (20.79–731) |
The Spearman's correlation coefficient between Cys-C and the stages of AKI was observed to be 0.27 (p < 0.01), which shows that increase in the stage of AKI was associated with higher values of Cys-C. Table 6 also shows the increasing trend of mean value of Cys-C with stage of AKI
Fig. 1.
Bar diagram showing mean Cys-C with stage and number of AKI
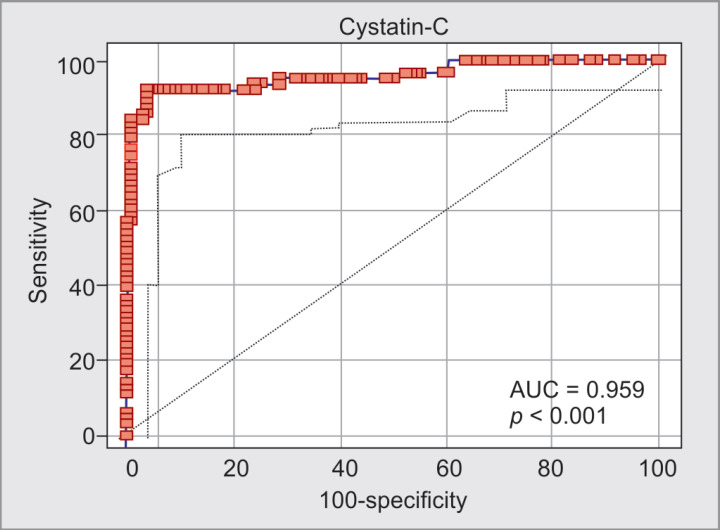
By univariate logistic regression analysis, BMI (OR = 1.44, 95% CI: 1.23–1.68; p < 0.001), blood urea (OR = 1.15, 95% CI: 1.06–1.23; p < 0.001), Cys-C (OR = 1.04, p < 0.05), serum calcium (OR = 0.59, p < 0.05), and serum LDH (OR = 1.001, 95% CI: 1.0–1.002; p < 0.05) were the significant indicators for AKI in patients with AP. Using multivariate logistic regression analysis, urinary albumin and Cys-C were independent and significant indicators of AKI in patients with AP (OR = 1.026, 95% CI: 1.01–1.07; p < 0.01) (Tables 5 and 6). Receiver operating characteristic curve of serum Cys-C, for AKI in patient with AP could be identified with a sensitivity of 92.06% at specificity of 96.0% (AUC = 0.96, 95% CI: 0.92–0.98) by baseline serum Cys-C (cutoff value = >32.32 mg/L) (Fig. 2).
Table 5.
Indicators for AKI in patient with acute pancreatitis by univariate logistic regression analysis
| Characteristics | Odds ratio | 95% CI | p value |
|---|---|---|---|
| Age (years) | 1.01 | 0.98–1.04 | 0.36 |
| Gender (M/F) | 1.27 | 0.58–2.87 | 0.57 |
| BMI (kg/m2) | 1.44 | 1.23–1.68 | <0.001 |
| B urea (mg/dL) | 1.15 | 1.06–1.23 | <0.001 |
| S. creatinine (mg/dL) | 1.04 | 1.02–1.06 | <0.001 |
| Urinary albumin (μg/L) | 1.06 | 1.02–1.10 | <0.001 |
| Cys-C (ng/mL) | 10.04 | 5.01–16.07 | <0.01 |
| S. amylase (U/L) | 1.01 | 1.0–1.01 | 0.048 |
| S. lipase (U/L) | 1.0 | 1.0–1.01 | 0.12 |
| S. bilirubin (mg/dL) | 1.04 | 0.91–1.17 | 0.56 |
| SGOT (U/L) | 0.99 | 0.99–1.01 | 0.41 |
| SGPT (U/L) | 0.98 | 0.97–1.01 | 0.34 |
| S. calcium (mg/dL) | 0.59 | 0.41–0.86 | <0.05 |
| S. LDH (mg/dL) | 1.001 | 1.0–1.002 | <0.0.5 |
By univariate logistic regression analysis BMI (OR = 1.44, p < 0.001), blood urea (OR = 1.15, p < 0.001), Cys-C (OR = 10.04, p < 0.05), serum calcium (OR = 0.59, p < 0.05), and serum LDH (OR = 1.001, p < 0.05) were the significant indicators for AKI in patients with acute pancreatitis
Table 6.
Indicators for AKI in patient with acute pancreatitis by multiple logistic regression analyses
| Characteristics | Odds ratio | 95% CI | p value |
|---|---|---|---|
| Urinary albumin | 1.06 | 1.02–1.10 | <0.01 |
| Cys-C | 10.026 | 4.01–15.07 | <0.01 |
Using multivariate logistic regression analysis, urinary albumin and Cys-C were independent and significant indicators of AKI in patients with acute pancreatitis (OR = 10.026, p < 0.01)
Fig. 2.
ROC showing AKI in patients with acute pancreatitis with a sensitivity of 92.06% at specificity of 96.0% (AUC = 0.96, 95% CI: 0.92–0.98) by baseline serum Cys-C (cutoff value = >32.32 ng/mL)
Discussion
Acute kidney injury is one of the known systemic complications in patients with AP.14 Our study result shows that prevalence of AKI among AP around 20%, which is lower than the previously reported prevalence of 4 to 42%.12,15–17 Possible explanations behind uprising AP-related hospitalization rate could be a number of factors, such as the obesity18 and alcohol consumption,19,20 gallstone-related AP,21 and biliary pancreatitis, respectively.
The causes for AKI in the patients with SAP can be multifactorial. The clinical symptoms associated with AP, such as vomiting and the loss of appetite, result in fluid depletion. In the course of AP, toxins, free radicals, cytokines, and other inflammatory mediators are released to the circulation, which lead to endothelial dysfunction and increased permeability of blood vessels,22 which further exacerbates the hypovolemia. The systemic inflammatory response causes the constriction of blood vessels and the stimulation of baroreceptors. The redistribution of body fluids and the shift toward the third space decrease the intravascular volume. These mechanisms lead to hypoperfusion of the kidneys. Additionally, the intra-abdominal pressure increases due to ascites and sometimes hemorrhages, leading to the development of abdominal compartment syndrome and the decline in renal perfusion. Ischemia and oxygen deficiency lead to the impairment of renal function.16,23
In our study, out of 190 patients 38 (20%) developed AKI. Male patients were much prone to AKI (11.5%) in comparison to female (8.4%). Our study result is comparable to the study performed by other author showed that 18 (7.6%) patients had developed AKI. There was a higher percentage of AKI in males (10.9%) than in females (3.7%). This result was consistent with previous report,24 suggesting that male patients with AP were more prone to develop AKI.
Acute kidney injury is the most common cause of mortality in SAP patients.25,26 Studies showed that ≥50% of deaths occur in SAP patients within the first week of AP.27 Thus, early interventions of AKI are urgently needed for the patients in this critical stage. Majority of the SAP with AKI group patients in our study had stage III according to the KDIGO guidelines [n = 21, (55.2%)], followed by stage II [n = 9, (23.6%)] and stage I [n = 8, (21%)] in MSAP group. In the study by Zhou et al.,25 majority were AKIN stage III 34.3% followed by AKIN stage II 18.4%. Patients with AKI had significantly higher mortality when compared to non-AKI group (13.1 vs 0.6%; p < 0.01). Prognosis of the SAP patients with AKI is influenced by the presence of diabetes, alcohol use, severity grading of AKI, and the severity of AP. These findings were in concordance with the study by Kumar et al.16
In our study, liver function indexes [total protein, albumin, total bilirubin, direct bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP)] had no differences between AP patients with AKI and without AKI. These results suggested that liver function parameter not be adopted to predict AKI in patients with AP. Furthermore, the high level of blood urea, serum Cr, and serum uric acid were found in patients with AKI. Our study result is supported by other study where liver function indexes might not be adopted to predict AKI in patients with AP.24
In our study by univariate logistic regression analysis, BMI (OR = 1.44, p < 0.001), blood urea (OR = 1.15, p < 0.001), Cys-C (OR = 10.04, p < 0.05), serum calcium (OR = 0.59, p < 0.05), and serum LDH (OR = 1.001, p < 0.05) were the significant indicators for AKI in patients with AP. By multivariate logistic regression analysis, urinary albumin and Cys-C were independent and significant indicators of AKI in patients with AP (OR = 10.026, p < 0.01). In both type of analysis, serum Cys-C was the strong indicator of AKI.
Although the Cys-C might be influenced by non-renal factors, such as age, gender, and race. In our study, baseline serum Cys-C concentration was significantly increased in AP patients with AKI compared to that without AKI, suggesting that the increased baseline serum Cys-C might be associated with AKI in patients with AP. Furthermore, baseline serum Cys-C is an independent and significant indicator for AKI in patients with AP according to the multivariate logistic regression analysis. Acute kidney injury in patients with AP could be identified with a sensitivity of 92.06% at specificity of 96.0% (AUC = 0.96, 95% CI: 0.92–0.98) by baseline serum Cys-C (cutoff value = >32.32 ng/mL) in our study. Our study results were compatible to other study where sensitivity of 88.9% at specificity of 100% (AUC = 0 948, 95% CI: 0.879–1.000) by serum baseline Cys-C (cutoff value = 1.865 mg/L).24
Limitations of Our Study
First, this was a single-center study, whether our findings can be extended to the general population remains in doubt. Second, because of the relatively small sample size of AKI patients, statistical significance in our study should be interpreted with caution.
Conclusion
Our finding suggested that baseline serum Cys-C can be a biomarker for AKI in patients with AP. Measurement of baseline serum Cys-C might be used as an early indicator, predicting AKI in patients with AP and could be used routinely in clinical laboratories.
Footnotes
Source of support: Self-funding
Conflict of interest: None
References
- 1.Swaroop VS, Chari ST, Clain JE. Severe acute pancreatitis. JAMA. 2004;291(23):2865–2868. doi: 10.1001/jama.291.23.2865. DOI: [DOI] [PubMed] [Google Scholar]
- 2.Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: bench to the bedside. Gastroenterology. 2007;132(3):1127–1151. doi: 10.1053/j.gastro.2007.01.055. DOI: [DOI] [PubMed] [Google Scholar]
- 3.Devani K, Charilaou P, Radadiya D, Brahmbhatt B, Young M, Reddy C. Acute pancreatitis: trends in outcomes and the role of acute kidney injury in mortality- a propensity-matched analysis. Pancreatology. 2018;18(8):870–877. doi: 10.1016/j.pan.2018.10.002. DOI: [DOI] [PubMed] [Google Scholar]
- 4.Kidney Disease: Improving Global Outcomes (KDIGO). Acute kidney injury work group: KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012;2(suppl:):1S–138S. [Google Scholar]
- 5.Mehta RL, Kellum JA, Shah SV, Pickering RP, Kerridge BT, Ruan WJ, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;1(2):R31. doi: 10.1186/cc5713. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.di Somma S, Magrini L, de Berardinis B, Marino R, Ferri E, Moscatelli P, et al. Additive value of blood neutrophil gelatinase-associated lipocalin to clinical judgement in acute kidney injury diagnosis and mortality prediction in patients hospitalized from the emergency department. Crit Care. 2013;17(1):R29. doi: 10.1186/cc12510. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nickolas TL, Schmidt-Ott KM, Canetta P, Forster C, Singer E, Sise M, et al. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: a multicentre prospective cohort study. J Am Colle Cardiol. 2012;59(3):246–255. doi: 10.1016/j.jacc.2011.10.854. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levey S, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. Amore accuratemethodtoestimate glomerular filtration rate from serum creatinine: a new prediction equation,. Ann Internal Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. DOI: [DOI] [PubMed] [Google Scholar]
- 9.Dharnidharka VR, Kwon C, Stevens G. Serumcystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002;40(2):221–226. doi: 10.1053/ajkd.2002.34487. DOI: [DOI] [PubMed] [Google Scholar]
- 10.Randers E, Erlandsen EJ. Serum cystatin C as an endogenous marker of the renal function—a review. Clin Chem Laborat Med. 1999;37(4):389–395. doi: 10.1515/CCLM.1999.064. DOI: [DOI] [PubMed] [Google Scholar]
- 11.National Kidney Foundation. K/DOQI clinical practice guideline to define chronic kidney disease: evaluation, classification and stratification. Am J Kidney Dis. 2001;39:S1–S266. [PubMed] [Google Scholar]
- 12.Kong L, Santiago N, Han TQ, Zhang SD. Clinical characteristics and prognosticfactors of severe acute pancreatitis. World J Gastroenterol. 2004;10(22):3336–3338. doi: 10.3748/wjg.v10.i22.3336. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis-2012: revision of the Atlanta classification and definition by international consensus. Gut. 2013;62(1):102–111. doi: 10.1136/gutjnl-2012-302779. DOI: [DOI] [PubMed] [Google Scholar]
- 14.Ljutic D, Piplovic-Vukovic T, Raos V, Andrews P. Acute renal failure as acomplication of acute pancreatitis. Ren Fail. 1996;18(4):629–633. doi: 10.3109/08860229609047687. DOI: [DOI] [PubMed] [Google Scholar]
- 15.Lin HY, Lai JI, Lai YC, Lin PC, Chang SC, Tang GJ. Acute renal failure in severepancreatitis: a population-based study. Ups J Med Sci. 2011;116(2):155–159. doi: 10.3109/03009734.2010.547636. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar R, Pahwa N, Jain N. Acute kidney injury in severe acute pancreatitis: an experience from a tertiary care center. Saudi J Kidney Dis Transplant. 2015;26(1):56–60. doi: 10.4103/1319-2442.148734. DOI: [DOI] [PubMed] [Google Scholar]
- 17.Herrera Gutierrez ME, Seller Perez G, de La Rubia De Gracia C, Chaparro Sanchez MJ, Nacle Lopez B. Acute renal failure profile and prognostic value insevere acute pancreatitis. Med Clin. 2000;115(19):721–725. doi: 10.1016/S0025-7753(00)71674-5. DOI: [DOI] [PubMed] [Google Scholar]
- 18.Seidell JC, Halberstadt J. The global burden of obesity and the challenges of prevention. Ann Nutr Metab. 2015;66(Suppl 2:):7–12. doi: 10.1159/000375143. DOI: [DOI] [PubMed] [Google Scholar]
- 19.Roberts SE, Akbari A, Thorne K, Atkinson M, Evans PA. The incidence of acute pancreatitis: impact of social deprivation, alcohol consumption, seasonal and demographic factors. Aliment Pharmacol Ther. 2013;38(5):539–548. doi: 10.1111/apt.12408. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, et al. Prevalence of 12-month alcohol use, high-risk drinking, and dsm-iv alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the national epidemiologic survey on alcohol and related conditions. JAMA Psychiatry. 2017;74(9):911–923. doi: 10.1001/jamapsychiatry.2017.2161. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144(6):1252–1261. doi: 10.1053/j.gastro.2013.01.068. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dumnicka P, Maduzia D, Ceranowicz P, Olszanecki R, Drożdż R, Kuśnierz-Cabala B. The interplaybetween inflammation, coagulation and endothelial injury in the early phase of acute pancreatitis: clinicalimplications. Int J Mol Sci. 2017;18(2):354. doi: 10.3390/ijms18020354. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manokaran S, Edwin FM, Srinivasaprasad N, Suren S. A study of acute kidney injury in severe acute pancreatitis in a tertiary care hospital from South India. IOSR J Dent Med Sci. 2018;17:45–48. [Google Scholar]
- 24.Chai X, Huang HB, Feng G, Cao YH, Cheng QS, Li SH, et al. Baseline serum cystatin C is a potential predictor for acute kidney injury in patients with acute pancreatitis. Dis Markers. 2018. 8431219. DOI: [DOI] [PMC free article] [PubMed]
- 25.Zhou J, Li Y, Tang Y, Liu F, Yu S, Zhang L, et al. Effect of acute kidney injury on mortality and hospital stay in patient with severe acute pancreatitis. Nephrology. 2015;20(7):485–491. doi: 10.1111/nep.12439. DOI: [DOI] [PubMed] [Google Scholar]
- 26.Petejova N, Martinek A. Acute kidney injury following acute pancreatitis: a review. Biomedical Papers. 2013;157(2):105–113. doi: 10.5507/bp.2013.048. DOI: [DOI] [PubMed] [Google Scholar]
- 27.Mole DJ, Olabi B, Robinson V, Garden OJ, Parks RW. Incidence of individual organ dysfunction in fatal acute pancreatitis: analysis of 1024 death records. HPB. 2009;11(2):166–170. doi: 10.1111/j.1477-2574.2009.00038.x. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]