Abstract
Purpose
To develop and externally validate a multivariate prediction model for the prediction of acute kidney injury (AKI) in COVID-19, based on baseline renal perfusion from contrast-enhanced CT together with clinical and laboratory parameters.
Methods
In this retrospective IRB-approved study, we identified COVID-19 patients who had a standard-of-care contrast-enhanced abdominal CT scan within 5 days of their COVID-19 diagnosis at our institution (training set; n = 45, mean age 65 years, M/F 23/22) and at a second institution (validation set; n = 41, mean age 61 years, M/F 22/19). The CT renal perfusion parameter, cortex-to-aorta enhancement index (CAEI), was measured in both sets. A multivariate logistic regression model for predicting AKI was constructed from the training set with stepwise feature selection with CAEI together with demographical and baseline laboratory/clinical data used as input variables. Model performance in the training and validation set was evaluated with ROC analysis.
Results
AKI developed in 16 patients (35.6%) of the training set and in 6 patients (14.6%) of the validation set. Baseline CAEI was significantly lower in the patients that ultimately developed AKI (P = 0.003). Logistic regression identified a model combining baseline CAEI, blood urea nitrogen, and gender as most significant predictor of AKI. This model showed excellent diagnostic performance for prediction of AKI in the training set (AUC = 0.89, P < 0.001) and good performance in the validation set (AUC 0.78, P = 0.030).
Conclusion
Our results show diminished renal perfusion preceding AKI and a promising role of CAEI, combined with laboratory and demographic markers, for prediction of AKI in COVID-19.
Keywords: Contrast-enhanced CT, Acute kidney injury, COVID-19, Renal perfusion
Introduction
There is increasing evidence that acute kidney injury (AKI) develops commonly in patients with COVID-19 [1], with reported frequencies as high as 37% [2]. COVID-19 patients developing AKI, in conjunction with respiratory symptoms, have a poor prognosis, with a reported 35% mortality [2]. The etiology of AKI in COVID-19 patients is not understood [3, 4]. Possible causes of COVID-19-related AKI include dehydration, hypoperfusion from myocardial dysfunction, immune response dysregulation (cytokine storm), or direct kidney endothelial damage by the SARS-CoV-2 virus [3].
Quantitative kidney imaging performed at baseline at the time of admission may provide information on kidney functional and structural characteristics that precede the development of AKI, thereby aiding in further elucidation of the mechanisms of AKI in COVID-19 as well as in potential prediction of AKI. Ultrasound Doppler-based renal flow and velocity measurements have previously shown promise in terms of prediction of AKI in critically ill, non-COVID-19 patients [5, 6]. Recently, dynamic contrast-enhanced MRI was identified as an early predictor of AKI in a mouse model after administration of toxins [7].
Despite the fact that AKI is a serious complication in COVID-19, reports on the imaging characteristics of the kidney in COVID-19 patients are limited [8, 9]. The goal of our study was to assess the association of baseline kidney contrast-enhanced CT, clinical and laboratory characteristics with the development of AKI in COVID-19 infected patients and to train and externally validate a multivariate logistic regression model combining these characteristics for predicting AKI.
Methods
Study design
This retrospective study was approved by the Institutional Review Boards of both institutions. Using the institutional COVID data repository of Weill Cornell Medicine [10], we identified a training cohort of patients who matched the following inclusion criteria: subjects (1) that received a standard-of-care contrast-enhanced abdominal CT between March 9, 2020 and May 13, 2020; (2) had a positive COVID-19 reverse transcription polymerase chain reaction (RT-PCR) test from a nasopharyngeal sample taken within 5 days from the CT; and (3) were admitted to our hospital no longer than 5 days before or after the CT and (4) had laboratory and vital data recorded within 2 days from the CT. Exclusion criteria were as follows: (1) subjects with end-stage renal disease; (2) history of chronic kidney disease; (3) prior renal transplant; (4) fewer than 2 serum creatinine laboratory results during their COVID-19 infection; and (5) sepsis at baseline due to high prevalence of sepsis-induced AKI [11]. Demographics (age, sex, race) and kidney-related baseline clinical (systolic and diastolic blood pressure) and laboratory (blood urea nitrogen (BUN), potassium and creatinine) data were retrieved from the repository. Body mass index (BMI) and data on comorbidities (previous diagnosis of diabetes, hypertension, heart failure, and coronary artery disease) were also extracted. Intravenous fluid hydration therapy at the time of the CT was also recorded. Estimated glomerular filtration rate (eGFR) measurements were calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [12]. The indications for the abdominal CT scan, including symptoms of nausea, vomiting, and diarrhea which could lead to dehydration, were retrieved from the reports and electronic medical record.
The external validation set consisted of a cohort of COVID-19 patients matching the same eligibility criteria at Columbia University Medical Center. The inclusion dates of contrast-enhanced CT exams were between April 1, 2020 and April 30, 2020 for the validation set. For the validation set, only variables that were identified as significant predictors in the multivariate model built in the training set were recorded, as well as dynamic creatinine values in order to establish the diagnosis of AKI.
AKI in both the training and validation set was defined according to the Kidney Disease: Improving Global Outcomes (KDIGO) criteria [13]. According to those criteria, AKI is diagnosed when an increase in serum creatinine by 0.3 mg/dl is observed within 48 h or when serum creatinine increases at least 1.5 times from its baseline value within 7 days.
CT acquisition
A routine single-phase contrast-enhanced CT of the abdomen and pelvis was performed in all subjects. Iohexol (Omnipaque 300, GE Healthcare) was injected at a dose of 1.5 ml/kg (maximum 150 ml) at an injection rate of 3 ml/s, followed by a 50 ml saline flush at the same injection rate. The CT scan was performed on systems from the same vendor (GE Healthcare; scanner type Optima CT660, Revolution EVO or Discovery CT750). CT images were acquired at 70 s after injection. Tube voltage was 100–140 kVp and images were reconstructed to a slice thickness of 2.5–5 mm. None of the patients had baseline eGFR < 30 ml/min/1.73 m2 at the time of the CT exam, as this is a relative contraindication for injection of iodinated contrast material [14].
CT analysis
Oval-shaped single-slice regions of interest (ROIs) were placed in the renal cortex of both kidneys at the mid-pole and in the aorta above the renal bifurcation using the PACS system. The sizes of the ROI in the cortex and aorta were approximately 0.3 cm2 and 0.9 cm2, respectively. The perfusion parameter cortex-to-aorta enhancement index (CAEI) was calculated by dividing the mean Hounsfield units (HU) in the cortex by mean HU in the aorta, as described previously [15]. A mean CAEI for each patient was calculated by averaging the cortex-to-aorta enhancement index values for each kidney.
Statistical analysis
Univariate analysis (either Mann–Whitney U tests or chi-squared tests based on type of variable) was performed to evaluate differences in CAEI, laboratory, and clinical parameters between patients who developed AKI and those that did not develop AKI in the training cohort. The correlation between continuous variables was assessed using the Spearman analysis. Multivariate logistic regression analysis with stepwise feature selection was performed to develop a prediction model for AKI in the training set. Separate models were built using all variables (demographical, vital, laboratory, and CT data) and using all variables except the CT data as input, in order to evaluate the potential additional value of CT for the prediction of AKI. Model performance was evaluated using ROC analysis and compared using likelihood ratio tests. The performance of the optimal model, defined as the model reaching highest area-under-the-curve (AUC) for AKI prediction in the training set, was evaluated in the validation set using ROC analysis. Statistical analyses were performed in MATLAB (version R2019b, MathWorks, Natick, MA, USA) and SPSS (version 20, IBM, Armonk, NY, USA). A two-sided p-value of 0.05 was considered significant.
Results
Patient characteristics of training cohort and validation cohort
Forty-five patients (M/F 22/13, average age 65 years) were identified for the training cohort. In 23 (51%) of the patients, the CT was indicated due to one or more GI symptoms [abdominal pain, n = 15 (33.3%), nausea, n = 2 (4.4%), vomiting, n = 8 (17.8%), diarrhea, n = 2 (4.4%)]. Baseline eGFR ranged from 30 to 127 ml/min/1.73 m2. Of the 45 included patients, 16 (35.6%) developed AKI over the course of their illness. For these 16 patients developing AKI, mean serum creatinine rose from 1.15 to 1.92 mg/dl and mean eGFR dropped from 59.6 to 34.6 ml/min/1.73 m2. Average time between initial PCR COVID-19 diagnosis and peak serum creatinine in the AKI patients was 9 days (range 1–41 days).
The external validation cohort consisted of 41 patients (M/F 22/19, average age 61 years). In 28 of these patients (68.3%), indication for the CT scan included GI symptoms [abdominal pain, n = 25 (61.0%); nausea, n = 6 (14.6%), vomiting, n = 6 (14.6%), diarrhea, n = 4 (9.8%)]. Baseline eGFR in the validation set ranged between 30 and 163 ml/min/1.73 m2. Six of the patients (14.6%) developed AKI. In these six patients, mean serum creatinine increased from 1.05 to 2.58 mg/dl and mean eGFR decreased from 74.4 to 40.4 ml/min/1.73 m2. In this validation cohort, the average time between COVID-19 diagnosis and peak serum creatinine in AKI patients was 4 days (range 2—9 days).
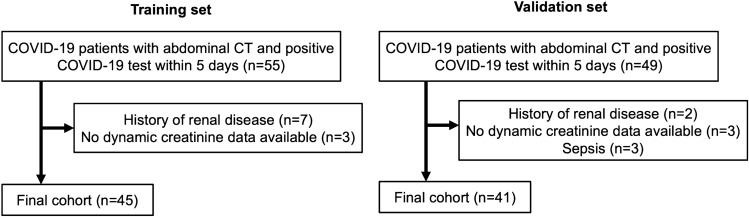
Flowcharts of patient inclusion in the training and validation cohorts are shown in Fig. 1.
Fig. 1.
Flowchart of patient inclusion
Differences in baseline characteristics in patients that developed AKI
Table 1 shows characteristics of the training cohort by AKI status. Baseline CT perfusion as measured by perfusion parameter CAEI was significantly lower in the patients that ultimately developed AKI (P = 0.003; Fig. 2).
Table 1.
Demographical, clinical, and CT characteristics of the entire training cohort (n = 45), patients in the training cohort who did not develop AKI (n = 29) and patients in the training cohort who developed AKI (n = 16)
| All ( n = 45) | No AKI ( n = 29) | AKI ( n = 16) | P# | |
|---|---|---|---|---|
| Age (years) | 65 (24–97) | 61 (24–97) | 72 (44–97) | 0.027 |
| Gender | 0.175 | |||
| Male | 23 (51%) | 17 (58.6%) | 6 (37.5%) | |
| Female | 22 (49%) | 12 (41.4%) | 10 (62.5%) | |
| Race | ||||
| Black | 6 (13.3%) | 4 (13.8%) | 2 (12.5%) | 0.995 |
| White | 7 (15.6%) | 4 (13.8%) | 3 (18.8%) | |
| Asian | 6 (13.3%) | 4 (13.8%) | 2 (12.5%) | |
| Multiracial | 14 (31.1%) | 9 (31.0%) | 5 (31.3%) | |
| Unknown | 12 (26.7%) | 8 (27.6%) | 4 (25.0%) | |
| BMI (kg/m2) | 25.6 (16.1–42.5) | 25.5 (16.1–42.5) | 25.6 (16.6–40.4) | 0.742 |
| GI symptoms | 23 (51.1%) | 17 (58.6%) | 6 (37.5%) | 0.175 |
| Comorbidities | ||||
| Diabetes | 13 (28.9%) | 8 (27.6%) | 5 (31.3%) | 0.795 |
| Hypertension | 26 (57.8%) | 16 (55.2%) | 10 (62.5%) | 0.634 |
| Heart failure | 5 (11.1%) | 0 (0%) | 5 (31.3%) | 0.001 |
| Coronary artery disease | 6 (13.3%) | 3 (10.3%) | 3 (18.8%) | 0.427 |
| None of the above | 17 (37.8%) | 12 (41.3%) | 5 (31.3%) | 0.502 |
| Kidney function | ||||
| Baseline Serum creatinine (mg/dl) | 0.98 (0.30–1.96) | 0.89 (0.30–1.79) | 1.15 (0.44–1.96) | 0.022 |
| Peak post CT creatinine (mg/dl) | 1.31 (0.64–3.93) | 0.97 (0.64–1.79) | 1.92 (1.07–3.93) | < 0.001 |
| Baseline BUN (mg/dl) | 21.6 (5.0–112.0) | 16.7 (5.0–39.0) | 30.4 (11.0–112.0) | 0.003 |
| Baseline BUN/creatinine | 22.7 (8.3–57.2) | 19.9 (8.3–50.0) | 27.6 (10–57.1) | 0.112 |
| Baseline potassium (mEq/l) | 4.1 (3.0–5.7) | 4.1 (3.0–5.7) | 4.1 (3.0–5.2) | 0.749 |
| Baseline eGFR (ml/min/1.73 m2) | 76.6 (30.1–127.0) | 86.0 (42.0–127.0) | 59.6 (30.1–102.6) | 0.003 |
| Lowest post CT eGFR (ml/min/1.73 m2) | 64.2 (10.0–122.6) | 80.5 (37.0–122.6) | 34.6 (10.0–61.9) | < 0.001 |
| Vital signs | ||||
| Baseline systolic blood pressure (mmHg) | 132 (97–196) | 136 (111–187) | 123 (97–196) | 0.012 |
| Baseline diastolic blood pressure (mmHg) | 81 (55–131) | 84 (66–105) | 75 (55–131) | 0.004 |
| IV fluid hydration at time of CT | 21 (47%) | 13 (45%) | 8 (50%) | 0.739 |
| CT perfusion | ||||
| CAEI (%) | 101 (64–141) | 107 (76–141) | 89 (64–140) | 0.003 |
Note Data are presented as number (percentage) for categorical variables and as mean (range) for continuous variables
#The p-values result from comparison between the no AKI vs. AKI cohort using chi-squared tests for categorical variables and Mann–Whitney U tests for continuous variables
AKI acute kidney injury, BMI body mass index, BUN blood urea nitrogen, CAEI cortex-to-aorta enhancement index, eGFR estimated glomerular filtration rate, GI gastrointestinal
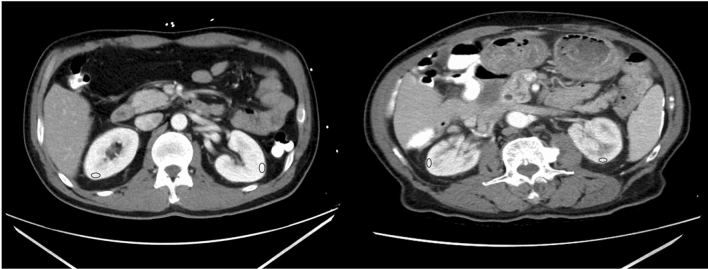
Fig. 2.
Baseline contrast-enhanced CT image of (left) a 54-year old male COVID-19 patient who did not develop AKI and (right) an 89-year old male COVID-19 patient who developed AKI. ROIs in the renal cortex are shown in blue. ROIs in the aorta were drawn at the level of the renal bifurcation, and are thus not displayed in these images. The relative enhancement in the cortex of the kidneys of the patient that developed AKI was lower than in the patient that did not develop AKI (CAEI 0.75 vs. 1.01), indicative of reduced blood flow
In terms of baseline kidney function laboratory measurements, serum creatinine and BUN were significantly higher in patients that developed AKI (P = 0.022 and P = 0.003, respectively), while eGFR was significantly lower (P = 0.003). Baseline potassium was not significantly different between the groups (P = 0.749). Both baseline systolic and diastolic blood pressure were significantly lower in patients that developed AKI (P = 0.012 and P = 0.004, respectively).
With respect to demographics, age was significantly higher in the AKI group (P = 0.027), while gender and race were not significantly different between the AKI vs. no AKI groups (P = 0.175 and P = 0.995, respectively). BMI was also not significantly different between the groups (P = 0.742).
The presence of GI symptoms (nausea, vomiting, and diarrhea) at time of the CT scan was also not significantly different between the groups (P = 0.175).
The majority of the patients (n = 28, 62.2%) had one or more comorbidities. Hypertension was the most prevalent comorbidity (57.8% of all patients). However, the presence of previous history of hypertension was not significantly different between the patients that developed AKI vs. the patients who did not (P = 0.634). Previous diagnosis of diabetes or coronary artery disease also did not affect the development of AKI in our cohort (P = 0.795 and P = 0.427, respectively). Five of the patients had previous diagnosis of heart failure, and all these patients ultimately developed AKI, leading to a highly significant difference in prevalence of heart failure between the groups (P = 0.001).
Twenty-one patients (46.7%) received intravenous fluids at the time of the CT scan. The intravenous hydration status at time of the CT did however not significantly affect the development of AKI (P = 0.739).
Figure 3 shows the correlation matrix between continuous demographical, clinical and CT parameters. Most of the correlations were weak to moderate (absolute r < 0.6), indicating complementary value of the assessed variables, except for a strong negative correlation between eGFR and creatinine (r = -0.837, P < 0.001) and a strong positive correlation between diastolic and systolic blood pressure (r = 0.634, P < 0.001).
Fig. 3.
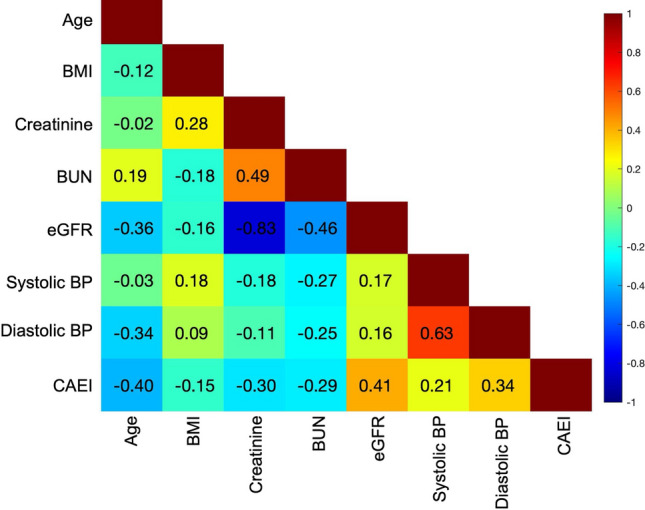
Matrix of Spearman correlation coefficients between demographical, clinical, and CT parameters in the training cohort
Prediction of AKI in training cohort
A logistic regression model combining the CT parameter CAEI, baseline BUN, and gender was found to be the most predictive for AKI (p-value of model < 0.001).
in which gender is a binary variable (1 for females and 0 for males). Standardized odds ratios of the model variables are given in Table 2. An AUC of 0.89 (95% CI 0.78–1; P < 0.001) was observed for prediction of AKI in the training cohort using this model, with sensitivity and specificity of 87.5% and 82.8%, respectively.
Table 2.
Standardized odds ratios of the variables in the prediction model for AKI
| Variable | Standardized odds ratio | 95% CI | P |
|---|---|---|---|
| CAEI | 0.258 | (0.08–0.84) | 0.024 |
| BUN | 8.051 | (1.3–48.9) | 0.023 |
| Gender | 3.191 | (1.16–8.79) | 0.025 |
BUN blood urea nitrogen, CAEI cortex-to-aorta enhancement index, CI confidence interval
CAEI alone showed an AUC of 0.77 (95% CI 0.61–0.92) for prediction of AKI, with sensitivity and specificity of 72.4% and 75.0%, respectively.
Modeling excluding CAEI as an input parameter yielded a significant model using a combination of BUN and gender:
For this model, an AUC of 0.83 (95% CI 0.69–.97) was found, with sensitivity of 79.3% and specificity of 75%.
Likelihood ratio tests indicated that the model combining CAEI, BUN, and gender significantly outperformed the model combining BUN and gender (P = 0.008) and the use of CAEI only (P < 0.001), and was thus selected as optimal model for prediction of AKI.
External validation of AKI prediction model
The trained model for AKI prediction showed good diagnostic performance for prediction of AKI in the validation cohort with an AUC of 0.78 (95% CI 0.64–0.92; P = 0.030) and sensitivity and specificity of 83.3% and 77.1%, respectively.
Discussion
The ratio of renal cortical signal intensity to aorta signal intensity on contrast-enhanced CT is an indicator of renal perfusion known as the cortex-to-aorta enhancement index (CAEI). We found that CAEI at the time of COVID-19 diagnosis was significantly reduced in patients who ultimately developed AKI. We also observed that a prediction model combining the CAEI feature with BUN and patient gender achieved a high AUC of 0.89 for predicting AKI in the training cohort. The model also showed good performance in an external validation cohort with AUC = 0.78. Our study thus demonstrates the potential role of imaging perfusion measurements for risk assessment of AKI in patients admitted with COVID-19 and suggests that reduced renal perfusion in COVID-19 precedes full-fledged AKI. While a large-scale study has identified risk factors of AKI in COVID-19 [16], our study adds imaging for prediction of this serious complication.
CAEI is a quantitative parameter reflecting microcirculation in the cortex [15]. Therefore, the reduction in CAEI is most likely related to a reduction in cortical blood flow, which may be due to systemic reasons including reduced cardiac output. This hypothesis is further supported by the lower baseline systolic and diastolic blood pressures in patients that developed AKI. We also found that patients with heart failure had significantly increased risk of development of AKI, although AKI also developed in patients without previous diagnosis of heart failure or other comorbidities. The risk factors identified in our study differed from a previous study that identified CKD, elevated serum potassium, and male gender as risk factors for AKI in COVID-19 [16]. The discrepancy in results is most likely related to the fact that we excluded patients with CKD from our study, in order to evaluate possible risk factors of COVID-19-related AKI in a cohort of patients without any history of kidney-related issues. Patients with CKD often have hyperkalemia [17], which may explain the identification of serum potassium as risk factor in the previous study. We did not see any significant effects of gender on the prevalence of AKI, which may be related to the small sample size in our study. Further studies in larger cohorts of patients are needed to evaluate the influence of gender in the development of AKI in COVID-19, while controlling for other gender-related covariates such as the prevalence of several comorbidities, including heart failure, hypertension, coronary artery disease and diabetes, which have all shown to be gender-dependent [18].
The reduced renal blood flow and blood pressure observed in our AKI cohort may also be caused by dehydration as reflected by the prognostic value of BUN [19]. The prolonged fever and gastrointestinal symptoms that many COVID-19 infected patients experience could lead to significant dehydration, leading to pre-renal AKI [20]. As many of the patients received the abdominal CT because of abdominal pain, dehydration may have played an important role in the development of AKI in our patient cohort. Nevertheless, the presence of GI symptoms at the time of the CT scan was not significantly different in patients that ultimately developed AKI in our study. In addition, while almost half of the patients received intravenous fluids at the time of the CT scan, the hydration therapy status at time of CT did not predict AKI in our cohort. Further studies are thus needed to evaluate the potential role of dehydration on AKI in COVID-19.
The logistic regression modeling identified the combination of CAEI along with gender and BUN as optimal predictor for AKI. CAEI and BUN reflect measures of kidney perfusion and function, respectively. The addition of gender to the model could be due to a previous observation that the CAEI parameter is associated with gender [15] and adding gender to the model may correct for this effect. Interestingly, eGFR was not selected as optimal predictor in the multivariate modeling, suggesting that CAEI is more predictive than eGFR for AKI. While CAEI reflects flow, eGFR is a composite measurement of renal blood flow and filtration fraction [19]. In early phases of stress to the kidney, the kidneys may compensate to the reduced blood flow by increasing the filtration fraction [19]. A renal blood flow parameter, such as CAEI, may thus be an earlier predictor of renal stress compared to eGFR. This corresponds to previous observations in a mouse model exposed to toxins, in which DCE-MRI enhancement parameters were found to change earlier as a result from the kidney injury compared to laboratory kidney function measurements [7].
The good performance in the external validation cohort indicates generalizability of this AKI prediction model. An interesting observation was a lower incidence of AKI development in the validation cohort vs. the training cohort (14.6% vs. 35.6%). The frequency of AKI in the training set was similar to a large-scale analysis of AKI, in which a frequency of 36.6% was reported [2]. A meta-analysis of AKI in COVID-19 has shown that the reported incidence of AKI in COVID-19 varies widely [21]. Further studies are needed to evaluate whether the differences in reported AKI frequencies are attributable to purely statistical or inclusion criteria variation, or due to other factors such as differences in patient populations served by different hospitals or differences in patient management routines.
The prediction of AKI using baseline characteristics may have substantial clinical utility. Although further research is needed on the management of AKI in COVID-19, guidelines for monitoring of development AKI in critically ill patients include regular monitoring of serum creatinine and urine output [22]. In addition, hemodynamic monitoring can be applied, as hypotension and reduced cardiac output reduce renal perfusion and may result in AKI. More frequent monitoring could possibly be employed for those patients at risk of AKI based on their baseline characteristics. In addition, the fluid status of those patients needs be adequately monitored, and fluid replacement should be applied where necessary.
Contrast-enhanced abdominal CT, with its cost and radiation exposure, may not be the method of choice for screening COVID-19 patients for risk of AKI. Furthermore, injection of CT-iodinated contrast in patients with compromised renal function is controversial, although recent analyses in large cohorts of patients have shown negligible risk of AKI after contrast-enhanced CT [23, 24]. Nevertheless, the CAEI measurements may be employed in patients that already have a clinical indication for contrast-enhanced abdominal CT. Non-contrast imaging methods for renal blood flow measurements could potentially also be utilized. In particular, the MRI arterial spin labeling (ASL) method is promising for quantitative measurements of renal perfusion without contrast injection [25].
Our study had several limitations. First, the sample size of both the training and validation sets was small, since contrast-enhanced abdominal CT is not routinely performed in COVID-19 patients. Second, due to the retrospective design, the CT acquisition was performed on different systems. However, the CAEI measurements reflecting the ratio between cortex and aorta attenuation values may partially mitigate effects of differences in acquisition parameters on absolute HU in CT. Lastly, also due to the retrospective design, analysis was based on a standard-of-care clinical single-phase CT scan. The CAEI parameter is strongly dependent on the phase of the acquisition, because of different contrast dynamics in the cortex and aorta. In our study, the imaging was performed at a fixed time post injection, allowing for consistent analysis across our cohort. Nevertheless, for translatability of the technique, dynamic contrast-enhanced CT or MR analysis allowing for quantitative renal blood flow estimation may provide more robust measurements of renal perfusion [26].
In conclusion, our study demonstrates that simple CAEI measurements from contrast-enhanced CT at baseline are significantly lower in COVID-19 patients that ultimately develop AKI indicating that reduced renal perfusion precedes the diagnosis of AKI. In addition, a model of CAEI, in conjunction with BUN and gender, showed good diagnostic performance for prediction of AKI in COVID-19, both in the training and in the validation set. Our results thus indicate a promising role of quantitative imaging of renal perfusion, combined with laboratory and demographic markers, for prediction of AKI in COVID-19 patients.
Funding
This study received support from NewYork-Presbyterian Hospital (NYPH) and Weill Cornell Medical College (WCMC), including the Clinical and Translational Science Center (CTSC) (UL1 TR000457) and Joint Clinical Trials Office (JCTO).
Data availability
Available upon reasonable request.
Compliance with ethical standards
Consent to participate
The requirement for informed consent was waived by the institutional review board.
Ethical approval
This study was approved by the institutional review board.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Stefanie J. Hectors, Email: sjh4002@med.cornell.edu
Martin R. Prince, Email: map2008@med.cornell.edu
References
- 1.Batlle D, Soler MJ, Sparks MA, Hiremath S, South AM, Welling PA, Swaminathan S, Covid, Ace2 in Cardiovascular L, Kidney Working G Acute Kidney Injury in COVID-19: Emerging Evidence of a Distinct Pathophysiology. J Am Soc Nephrol. 2020 doi: 10.1681/ASN.2020040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, Hazzan AD, Fishbane S, Jhaveri KD, Northwell C-RC, the Northwell Nephrology C-RC Acute Kidney Injury in Patients Hospitalized with Covid-19. Kidney Int. 2020 doi: 10.1016/j.kint.2020.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Ronco C, Reis T, Husain-Syed F. Management of acute kidney injury in patients with COVID-19. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naicker S, Yang CW, Hwang SJ, Liu BC, Chen JH, Jha V. The Novel Coronavirus 2019 epidemic and kidneys. Kidney Int. 2020;97(5):824–828. doi: 10.1016/j.kint.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhi HJ, Zhao J, Nie S, Ma YJ, Cui XY, Zhang M, Li Y. Prediction of acute kidney injury: the ratio of renal resistive index to semiquantitative power Doppler ultrasound score-a better predictor?: A prospective observational study. Medicine (Baltimore) 2019;98(21):e15465. doi: 10.1097/MD.0000000000015465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darmon M, Bourmaud A, Reynaud M, Rouleau S, Meziani F, Boivin A, Benyamina M, Vincent F, Lautrette A, Leroy C, Cohen Y, Legrand M, Morel J, Terreaux J, Schnell D. Performance of Doppler-based resistive index and semi-quantitative renal perfusion in predicting persistent AKI: results of a prospective multicenter study. Intensive Care Med. 2018;44(11):1904–1913. doi: 10.1007/s00134-018-5386-3. [DOI] [PubMed] [Google Scholar]
- 7.Privratsky JR, Wang N, Qi Y, Ren J, Morris BT, Hunting JC, Johnson GA, Crowley SD. Dynamic contrast-enhanced MRI promotes early detection of toxin-induced acute kidney injury. Am J Physiol Renal Physiol. 2019;316(2):F351–F359. doi: 10.1152/ajprenal.00416.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Z, Wu M, Yao J, Guo J, Liao X, Song S, Li J, Duan G, Zhou Y, Wu X, Zhou Z, Wang T, Hu M, Chen Z, Fu Y, Lei C, Dong H, Xu C, Hu Y, Han M, Zhou Y, Jia H, Chen X, Yan J. Caution on kidney dysfunctions of COVID-19 patients. medRxiv. 2020 doi: 10.1101/2020.02.08.20021212. [DOI] [Google Scholar]
- 9.Basara Akin I, Altay C, Eren Kutsoylu O, Secil M. Possible radiologic renal signs of COVID-19. Abdom Radiol (NY) 2020 doi: 10.1007/s00261-020-02671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sholle ET, Kabariti J, Johnson SB, Leonard JP, Pathak J, Varughese VI, Cole CL, Campion TR., Jr Secondary Use of Patients' Electronic Records (SUPER): An Approach for Meeting Specific Data Needs of Clinical and Translational Researchers. AMIA Annu Symp Proc. 2017;2017:1581–1588. [PMC free article] [PubMed] [Google Scholar]
- 11.Zarjou A, Agarwal A. Sepsis and acute kidney injury. J Am Soc Nephrol. 2011;22(6):999–1006. doi: 10.1681/ASN.2010050484. [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kellum JA, Lameire N, Group KAGW Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1) Crit Care. 2013;17(1):204. doi: 10.1186/cc11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davenport MS, Khalatbari S, Dillman JR, Cohan RH, Caoili EM, Ellis JH. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material. Radiology. 2013;267(1):94–105. doi: 10.1148/radiol.12121394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szczurowska A, Guzinski M, Krajewski W, Kaminska D, Koscielska-Kasprzak K, Arruza Echevarria A, Malkiewicz B, Debinski P, Mazanowska O, Klinger M, Sasiadek M. Preoperative Computed Tomography Parameters and Deterioration of Remaining Kidney Function in Living Donors. Transplant Proc. 2018;50(6):1597–1601. doi: 10.1016/j.transproceed.2018.03.118. [DOI] [PubMed] [Google Scholar]
- 16.Chan L, Chaudhary K, Saha A, Chauhan K, Vaid A, Baweja M, Campbell K, Chun N, Chung M, Deshpande P, Farouk SS, Kaufman L, Kim T, Koncicki H, Lapsia V, Leisman S, Lu E, Meliambro K, Menon MC, Rein JL, Sharma S, Tokita J, Uribarri J, Vassalotti JA, Winston J, Mathews KS, Zhao S, Paranjpe I, Somani S, Richter F, Do R, Miotto R, Lala A, Kia A, Timsina P, Li L, Danieletto M, Golden E, Glowe P, Zweig M, Singh M, Freeman R, Chen R, Nestler E, Narula J, Just AC, Horowitz C, Aberg J, Loos RJF, Cho J, Fayad Z, Cordon-Cardo C, Schadt E, Levin MA, Reich DL, Fuster V, Murphy B, He JC, Charney AW, Bottinger EP, Glicksberg BS, Coca SG, Nadkarni GN. Acute Kidney Injury in Hospitalized Patients with COVID-19. medRxiv. 2020 doi: 10.1101/2020.05.04.20090944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakhoul GN, Huang H, Arrigain S, Jolly SE, Schold JD, Nally JV, Jr, Navaneethan SD. Serum Potassium, End-Stage Renal Disease and Mortality in Chronic Kidney Disease. Am J Nephrol. 2015;41(6):456–463. doi: 10.1159/000437151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerdts E, Regitz-Zagrosek V. Sex differences in cardiometabolic disorders. Nat Med. 2019;25(11):1657–1666. doi: 10.1038/s41591-019-0643-8. [DOI] [PubMed] [Google Scholar]
- 19.Smith JH, Robinson S, Pearcy M. Renal responses to exercise, heat and dehydration. J Appl Physiol. 1952;4(8):659–665. doi: 10.1152/jappl.1952.4.8.659. [DOI] [PubMed] [Google Scholar]
- 20.Gabarre P, Dumas G, Dupont T, Darmon M, Azoulay E, Zafrani L. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med. 2020 doi: 10.1007/s00134-020-06153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brienza N, Puntillo F, Romagnoli S, Tritapepe L. Acute Kidney Injury in Coronavirus Disease 2019 Infected Patients: A Meta-Analytic Study. Blood Purif. 2020 doi: 10.1159/000509274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Acute KDIGOK, Group. KIW KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012;2:1–138. doi: 10.1038/kisup.2012.1. [DOI] [Google Scholar]
- 23.Aycock RD, Westafer LM, Boxen JL, Majlesi N, Schoenfeld EM, Bannuru RR (2018) Acute Kidney Injury After Computed Tomography: A Meta-analysis. Ann Emerg Med 71 (1):44–53 e44. doi:10.1016/j.annemergmed.2017.06.041 [DOI] [PubMed]
- 24.Gorelik Y, Yaseen H, Heyman SN, Khamaisi M. Negligible Risk of Acute Renal Failure Among Hospitalized Patients After Contrast-Enhanced Imaging With Iodinated Versus Gadolinium-Based Agents. Invest Radiol. 2019;54(5):312–318. doi: 10.1097/RLI.0000000000000534. [DOI] [PubMed] [Google Scholar]
- 25.Nery F, Buchanan CE, Harteveld AA, Odudu A, Bane O, Cox EF, Derlin K, Gach HM, Golay X, Gutberlet M, Laustsen C, Ljimani A, Madhuranthakam AJ, Pedrosa I, Prasad PV, Robson PM, Sharma K, Sourbron S, Taso M, Thomas DL, Wang DJJ, Zhang JL, Alsop DC, Fain SB, Francis ST, Fernandez-Seara MA. Consensus-based technical recommendations for clinical translation of renal ASL MRI. MAGMA. 2020;33(1):141–161. doi: 10.1007/s10334-019-00800-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang K, Ferguson CM, Abumoawad A, Saad A, Textor SC, Lerman LO. A modified two-compartment model for measurement of renal function using dynamic contrast-enhanced computed tomography. PLoS One. 2019;14(7):e0219605. doi: 10.1371/journal.pone.0219605. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Available upon reasonable request.