Significance
The pH of the global ocean is decreasing due to the absorption of anthropogenically emitted CO2, causing ocean acidification (OA). OA negatively impacts marine shellfish and threatens the continuing economic viability of molluscan shellfish aquaculture, a global industry valued at more than 19 billion USD. We identify traits linked to growth and lipid regulation that contribute tolerance to OA in abalone aquaculture, with broader implications for adaptation efforts in other shellfish species. We also identify evolved heritable variation for physiological resilience to OA that may be exploited in commercial and restoration aquaculture breeding programs to offset the negative consequences of continuing climate change.
Keywords: climate resilience, aquaculture, genetic variation, global environmental change, lipid regulation
Abstract
Ocean acidification (OA) poses a major threat to marine ecosystems and shellfish aquaculture. A promising mitigation strategy is the identification and breeding of shellfish varieties exhibiting resilience to acidification stress. We experimentally compared the effects of OA on two populations of red abalone (Haliotis rufescens), a marine mollusc important to fisheries and global aquaculture. Results from our experiments simulating captive aquaculture conditions demonstrated that abalone sourced from a strong upwelling region were tolerant of ongoing OA, whereas a captive-raised population sourced from a region of weaker upwelling exhibited significant mortality and vulnerability to OA. This difference was linked to population-specific variation in the maternal provisioning of lipids to offspring, with a positive correlation between lipid concentrations and survival under OA. This relationship also persisted in experiments on second-generation animals, and larval lipid consumption rates varied among paternal crosses, which is consistent with the presence of genetic variation for physiological traits relevant for OA survival. Across experimental trials, growth rates differed among family lineages, and the highest mortality under OA occurred in the fastest growing crosses. Identifying traits that convey resilience to OA is critical to the continued success of abalone and other shellfish production, and these mitigation efforts should be incorporated into breeding programs for commercial and restoration aquaculture.
The pH of the global ocean is decreasing due to the absorption of anthropogenically emitted atmospheric carbon dioxide (CO2), a process termed ocean acidification (OA) (1, 2). This process negatively impacts the growth, calcification, reproduction, and survival of calcifying shellfish (3–8). Additional acidification over the coming decades (9) threatens the long-term economic viability of commercial molluscan shellfish production (10), which is valued globally at more than 19 billion US dollars (11) and is considered a more sustainable alternative to terrestrial animal protein (12, 13).
The commercial production of molluscs requires the culturing of diverse life history phases, including larval and juvenile stages (i.e., “seed production”) (14). These life history phases are particularly sensitive to OA impacts (4–8). Significant uncertainty remains regarding how these sensitivities will affect shellfish production, especially since commercially cultured taxa show different vulnerabilities to OA (15). Variation in responses to OA is likely a complex function of differences in natural selection regimes (15–17), sensitivities among different early-life history stages (18), and in the nature and strength of transgenerational effects (19). As such, there is a critical need to understand how, and in which contexts, these factors contribute to OA impacts on molluscan seed production.
Red abalone (Haliotis rufescens) is a popular seafood and culturally iconic marine gastropod native to the west coast of North America (20, 21). Once abundant populations of this species supported both commercial and recreational fisheries, but with recent widespread population collapses (22, 23), commercial aquaculture now serves as the only source for abalone in the United States. OA poses a broad threat to the sustainable commercial and restoration aquaculture of this and other abalone species worldwide (5, 24–29). During early life, the nonfeeding (lecithotrophic) larvae of abalone depend on maternally provisioned energy reserves, much of which occurs in the form of yolk lipids (30), which support numerous physiological processes (31). Yolk lipids remaining at the end of the swimming phase likely play a critical role in determining how OA affects abalone seed success after larval settlement.
Along the west coast of North America, acidification rates are among the highest in the global surface ocean (32). Regional wind and currents drive coastal upwelling of deep-oceanic, low pH waters during spring and summer months (33), thereby periodically exposing coastal habitats (including regions of aquaculture activity) to extremely low pH (34, 35). Ocean circulation models predict that the intensity, frequency, and duration of these events will increase rapidly in the coming decades, outpacing many other regions (35–37), highlighting the need for industry adaptation and management options for this region in particular. Moreover, upwelling intensity is not uniform along the coast (34), and the resulting mosaic of strong and weak upwelling regions might have selected for variable sensitivities to OA in resident populations (38).
In this work, we sought to identify abalone traits that affect resilience to OA and that may be harnessed to support continuing productive aquaculture in the face of OA. Our experiments contrasted the responses to OA of early-stage abalone from two populations in California; a low pH, strong upwelling zone population (Van Damme State Park, CA; VD) and a higher pH, weaker upwelling zone population (Santa Barbara, CA; SB) (39). VD animals were wild-collected, whereas SB animals were aquaculture-raised, fourth-generation descendants of wild-collected abalone from the Santa Barbara Channel. We hypothesized that VD animals would be resilient to the effects of OA, given their historical exposure to low pH conditions. Families from each population were created and cultured from embryos to 3 mo of age (SI Appendix, Fig. S1) in an experimental CO2 manipulation apparatus (38). Experiments focused on survival and growth in two life history stages that encompass the period of highest mortality for red abalone (40); the swimming, larval phase (lasting 7 d post-fertilization, or “DPF”) through the juvenile post-settlement stage (97 DPF). After this critical bottleneck, mortality rates steeply decline to nominal levels during the market grow-out period (typically 3–4 y). Survival and growth were therefore assessed during this critical initial developmental window. Total lipid content of red abalone larvae was also quantified at 4 DPF, 7 DPF, and in newly settled spat (10 DPF) from random samples of individuals (see Materials and Methods and SI Appendix for full methodology).
Results
Lipid Concentrations and Relationship to Survival.
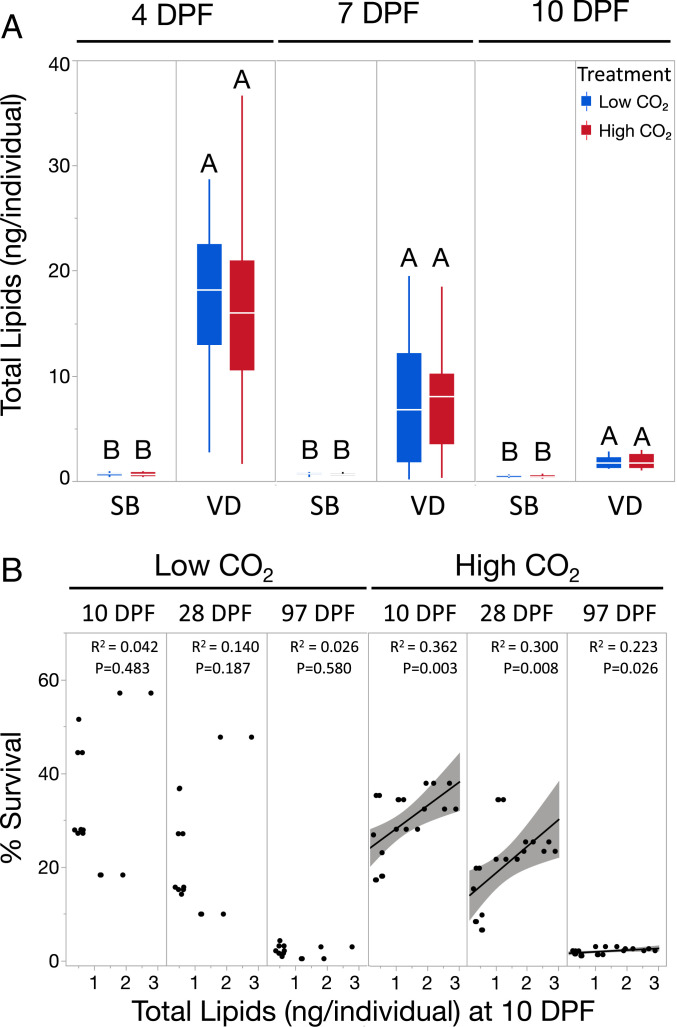
Population and CO2 treatment did not affect larval survival during the initial swimming phase, while lipid provisioning and utilization strategies varied significantly between populations. During the larval phase at both 4 DPF and 7 DPF, F1-generation animals from SB (hereafter SB-F1) showed very low lipid concentrations (Fig. 1A). In contrast, VD-F1 larvae exhibited lipid concentrations that were up to 29× greater than SB-F1 (Fig. 1A). Lipid concentrations were greater in VD-F1 than SB-F1 for all pairwise combinations (P < 0.0001). CO2 did not affect larval lipid concentration in either population. Immediately post-settlement (10 DPF), VD-F1 abalone exhibited over 3× the lipid concentration of SB-F1 animals under low CO2 and 4× the lipid concentration of SB-F1 animals under high CO2 (all P < 0.0001, Fig. 1A), indicating that this difference in provisioning carried over from swimming larvae into the settled, early-juvenile, feeding phase. No differences were observed between CO2 treatments within populations. When aggregated across F1 experiments, post-settlement larval lipid concentration at 10 DPF was a significant predictor of survival at 10 DPF (P = 0.0031), 28 DPF (P = 0.0083), and 97 DPF (P = 0.026) under high CO2, but was not a significant predictor at any time point under low CO2 (Fig. 1B), highlighting the strong relationship between energy reserves during earliest development and eventual survival success for abalone under OA.
Fig. 1.
Population differences in larval lipid concentration and relationship to survival in red abalone. (A) Box plots of lipid concentrations assessed across developmental time points indicate that abalone sourced from an upwelling region at VD exhibit significantly elevated larval lipid concentrations throughout the larval phase at 4 d post-fertilization (DPF), 7 DPF, and post-settlement at 10 DPF as compared to larvae from SB (all P < 0.0001). Shared letters indicate group means that are not significantly different based on means comparisons of least squares group estimates assessed separately at each time point (P > 0.05). (B) Under high CO2, post-settlement lipid concentration was a significant predictor of survival across post-settlement time points while this trend was not observed under low CO2. Shaded areas surrounding regression slopes represent 95% confidence bands. Lipid data presented taken from combined results on VD-F1 and SB-F1 animals.
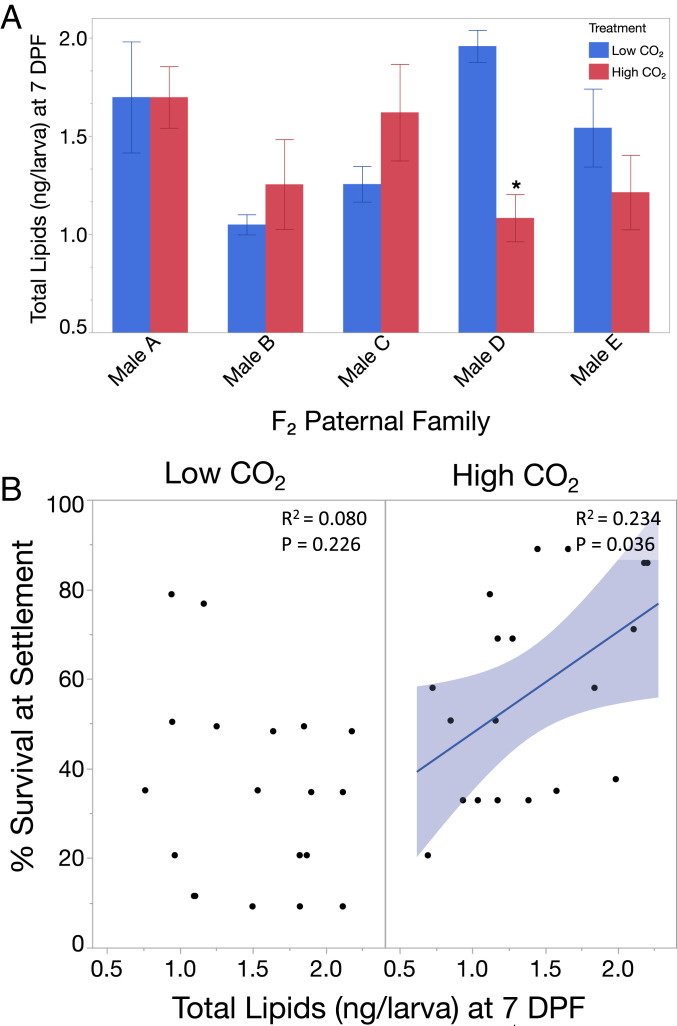
We examined the persistence of these differences into the next generation by raising SB-F1 abalone to maturity and spawning them. In the resulting F2-generation larvae (SB-F2), we observed differences in lipid metabolism at the level of individual fathers (Fig. 2A). This metabolic variation was associated with differential susceptibility to mortality under high CO2 (Fig. 2B). As with the SB-F1 generation, survival of SB-F2 animals during the larval phase was not affected by CO2 treatment (SI Appendix, Fig. S3). SB-F2 had higher starting lipid concentrations at 4 DPF than SB-F1 (P < 0.0001), and these concentrations declined across the period of larval development to 7 DPF (P < 0.0001). At the level of individual paternal crosses, we observed suggestive evidence of a three-way CO2 × time × paternal family interaction (P = 0.055), wherein total lipid concentrations declined during larval development when measured at 4 DPF vs. 7 DPF in some SB-F2 paternal family crosses under high CO2. This variation in lipid drawdown appeared to impact post-settlement survival of SB-F2 lineages under OA, as lipid concentration at 7 DPF was a significant predictor of survival at settlement under high CO2 (P = 0.0357) (Fig. 2B). Additionally, under high CO2, post-settlement lipid concentration was a significant predictor of survival to 28 DPF and 97 DPF, as observed in the aggregate SB-F1–generation response, whereas, under low CO2, no relationship was observed (SI Appendix, Fig. S2). This suggests that larval lipid metabolism in the SB population is a potentially heritable trait, which varies among individual paternal lineages, leading to differential survival outcomes under high CO2 stress.
Fig. 2.
SB-F2 paternal family lipid drawdown and relation to survival of red abalone under high CO2. (A) During the swimming larval phase, SB-F2–generation paternal families exhibited a three-way CO2 × time × paternal family interaction (P = 0.055), where under high CO2 the lipid content of larvae from some paternal lineages declined from 4 DPF to 7 DPF whereas in others it did not. This suggests that lipid regulation under high CO2 is a variable, and potentially heritable, paternally influenced trait. Asterisks indicate a significant decline (P < 0.05) in total lipid concentration at 7 DPF under high CO2. (B) Under high CO2, total lipid concentration at the end of the swimming larval phase (7 DPF) was a positive predictor of survival at settlement (P = 0.0357) in experiments on SB-F2–generation animals. Under low CO2, no relationship was observed, suggesting that lipid metabolism under high CO2 significantly influences susceptibility of lineages to post-settlement mortality. Shaded area surrounding regression slope represents 95% confidence band.
Differences in Survival and Growth between Populations and Among Families.
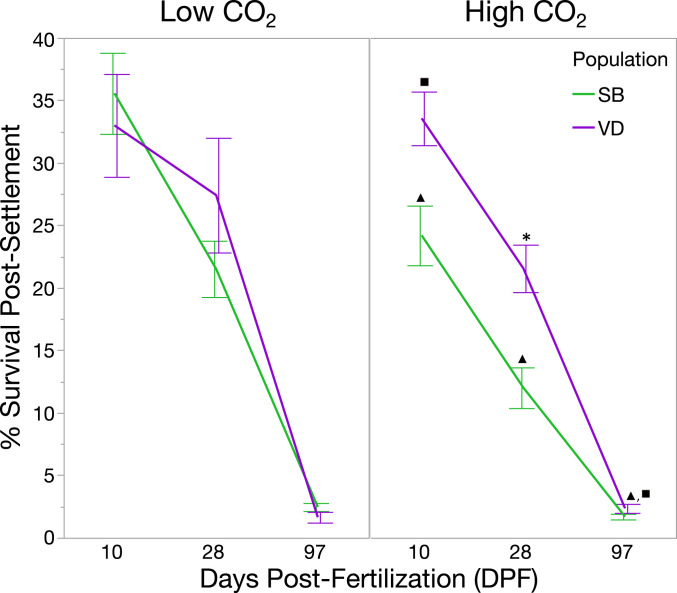
Post-settlement survival in SB-F1 animals was significantly impacted by OA, whereas VD-F1 showed resilience to this stressor. Relative to low CO2, SB-F1 abalone exhibited declines in survival under high CO2 at all time points, with mean reductions of 33% at 10 DPF (P = 0.0047), 50% at 28 DPF (P = 0.0008) and 43% at 97 DPF (P = 0.0276) (Fig. 3). Post-settlement survival of VD-F1 animals did not differ between high and low CO2 at any time point. Post-settlement survival was affected by CO2 (P = 0.0005) and population (P = 0.0353), with significant interactions of population × CO2 (P = 0.0391), CO2 × time (P = 0.0019), population × time (P = 0.0212), and suggestive evidence of a three-way interaction of CO2 × population × time (P = 0.0588).
Fig. 3.
Differences in survival between populations of red abalone under low and high CO2. Under low CO2, abalone from different source populations performed similarly across post-settlement time points, whereas under high CO2, abalone sourced from an upwelling region at VD showed elevated survival compared to SB at all time points, with 32% greater survival at 97 DPF. Under high CO2, SB abalone exhibited a significant decline in survival compared to low CO2 at all time points, whereas VD abalone showed no difference in survival between high and low CO2 at any time point. Asterisks and squares indicate group mean differences between populations at a given time point under high CO2, assessed at P > 0.05 and P > 0.07, respectively. Triangles indicate a difference at a given time point within the SB population across treatment levels assessed at (P > 0.05). Error bars are ±1 SE.
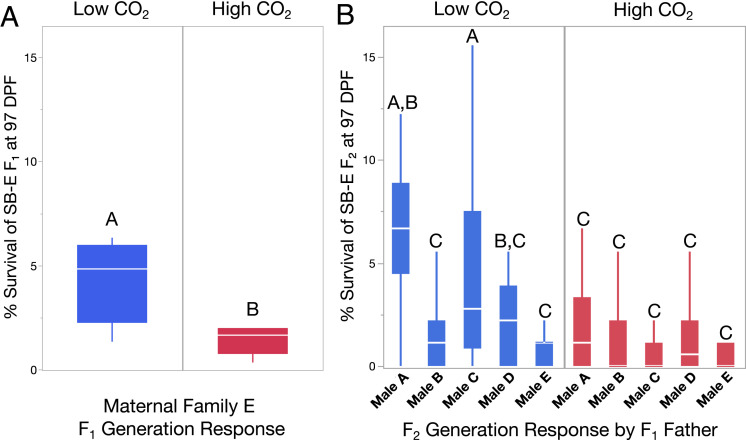
Within the SB-F1 population, some maternal families were more sensitive to OA than others, whereas all VD-F1 families were equally resilient to OA (P < 0.0001; SI Appendix, Fig. S4). At 97 DPF, mean survival was reduced across SB-F1 maternal families under high CO2 compared to low CO2 (P = 0.0025), with survival reduced by 67% in the most affected family (family SB-F1-E, Fig. 4A, P = 0.0038). In contrast, no declines in survival were observed among maternal VD-F1 families under high CO2, indicating that this population was well adapted to high CO2 conditions.
Fig. 4.
Comparison of F1- and F2-generation response of red abalone to high CO2. (A) Under high CO2, Santa Barbara family SB-E-F1 exhibited a 67% decline in survival. (B) This trend repeated in experiments on SB-E-F2 descendants after a generation in a common garden environment, with an aggregate 68% decline in survival under high CO2 in the subsequent generation. When subdivided into paternal family responses, a significant paternal family × CO2 interaction was observed in the F2 generation (P < 0.0001). Shared letters indicate group means that are not significantly different based on means comparisons of least squares group estimates (P > 0.05).
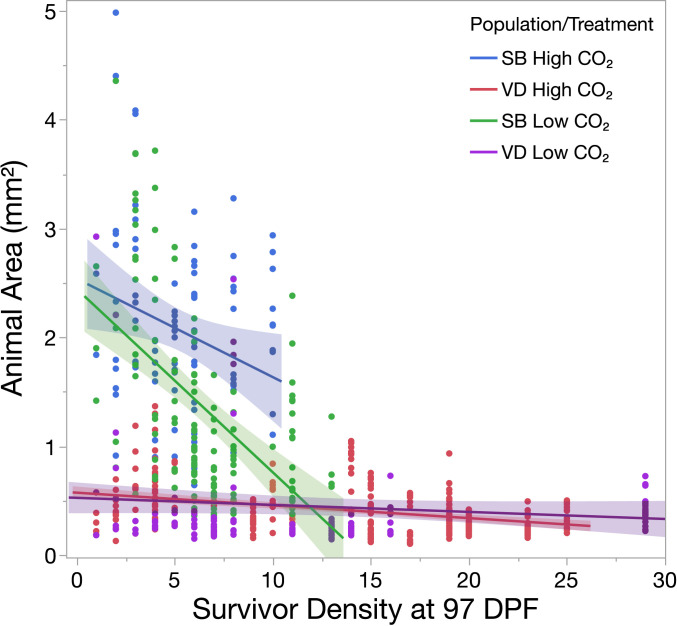
While VD-F1 abalone experienced less mortality under high-CO2 conditions, these animals grew more slowly than the SB-F1 population (Fig. 5). We observed negative density dependence in both populations, where the average size of animals in experimental units was negatively associated with the number of survivors in those units (P < 0.0001). However, this relationship varied by population (population × density, P = 0.0003), with a dampened influence on size in VD-F1 compared to SB-F1 (all VD-F1 vs. SB-F1 pairwise comparisons, P < 0.0001) indicating a substantially lower effect of density on growth in the VD-F1 population.
Fig. 5.
Differences in sizes attained by red abalone populations under low and high CO2. Abalone size was negatively associated with the density of survivors in experimental units at 97 DPF (P < 0.0001). A significant density × population interaction was also observed, where the size of slower growing VD abalone was less affected by density than the SB population (P < 0.001). Shaded areas surrounding regression slopes represent 95% confidence bands.
In F2-generation abalone, SB-F2 paternal family lineages showed significant reductions in survival under high CO2 at 97 DPF (Fig. 4B, P < 0.0001). These SB-F2 lineages were descended from family SB-E-F1 reared in low CO2 conditions and exhibited a comparable mortality response to the cumulative impact observed in SB-E-F1 in the prior generation (Fig. 4). Particularly large declines in survival were observed in paternal families SB-A-F2 (73%, P < 0.0001) and SB-C-F2 (80%, P < 0.0001). Post-settlement survival of SB-F2 was affected by CO2 (P = 0.0221), paternal identity (P < 0.0001), and the interaction terms of time × CO2 (P < 0.0001), time × paternal identity (P < 0.0001), CO2 × paternal identity (P < 0.0001), and the three-way interaction of paternal identity × time × CO2 (P < 0.0001), demonstrating that individual paternal SB-F2 lines were differentially susceptible to OA stress.
No difference in the size of survivors was observed among CO2 treatments or family groups in the SB-F2 generation. However, we observed a 42% reduction in the average CaCO3 shell mass in SB-F2 generation abalone cultured under high CO2 compared to those raised under low CO2 (P = 0.0039), as well as variation in this response by paternal family (paternal family × CO2 interaction, P = 0.0409). The average organic mass of these animals was 27% lower under high CO2 (P = 0.085), and there was suggestive evidence of a paternal family × CO2 interaction (P = 0.0627). When shell areas were regressed against either CaCO3 mass or organic mass under high and low CO2, we observed differences in the slopes of these relationships (CaCO3, P < 0.0001; organic, P = 0.0129), where slopes were shallower under high CO2. This indicated that, while SB-F2 animals superficially achieved equivalent sizes under high and low CO2, juveniles raised under high CO2 were significantly lighter and altered in their compositions (SI Appendix, Fig. S5).
Tradeoffs Underlying Sensitivity to OA.
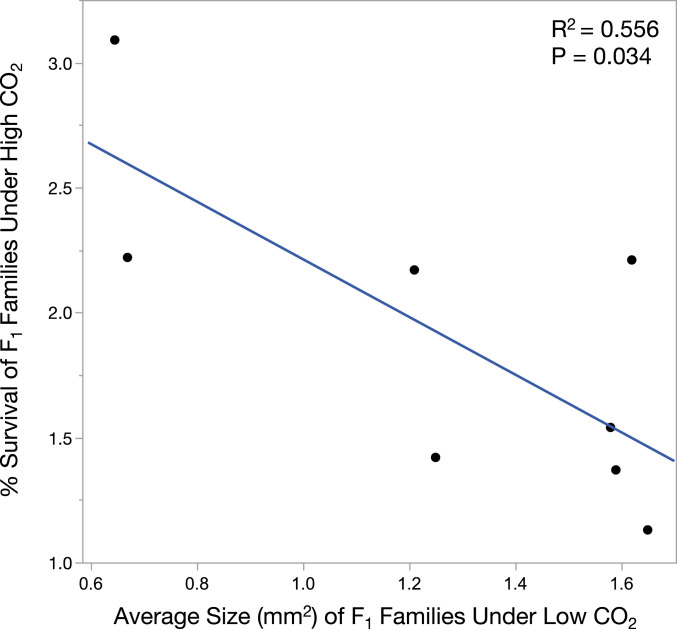
When contrasting the growth performance of all maternal F1 family groups tested, we observed a negative relationship between the average size attained by family crosses under low CO2 and the survival of that same family under high CO2 (P = 0.0338, Fig. 6). This result is consistent with a compensatory tradeoff, where faster growing lineages under low CO2 conditions are those most likely to experience elevated mortality under high CO2.
Fig. 6.
Potential tradeoff between growth rate and mortality in maternal families of red abalone under high CO2. Cumulatively across all maternal F1 families examined, a negative relationship was observed between the average size of crosses attained under low CO2 and the average survival of that cross under high CO2, suggesting that slower growing families are more resilient to the effects of OA.
Discussion
In this multigenerational study of OA effects on abalone, we demonstrated that mortality under OA is strongly correlated with differences in lipid provisioning and metabolism and that variation in traits associated with lipid regulation are observed at population, maternal, and paternal genetic levels. Low lipid concentrations were linked to significant mortality and vulnerability to high CO2 in animals sourced from the weaker upwelling environment in Santa Barbara, and this pattern persisted in F2-generation abalone. These results suggest that the negative effects of OA on red abalone populations are mediated in part by evolutionary differences arising from the interaction of this stressor with underlying genetic variation for lipid regulation.
Unlike in other marine molluscs with planktotrophic (feeding) larvae (7, 41), our results indicate that abalone larvae exposed to high CO2 experienced delayed impacts, as there were no differences in survival among treatments or populations during the larval swimming phase. However, clear differences in lipid metabolism were observed between populations during the swimming phase. These differences strongly structured eventual survival (Fig. 1B), even at the fine scale of individual F2 parents (Fig. 2), highlighting the importance of differential maternal provisioning and heritable variation in lipid metabolism as critical factors influencing vulnerability to OA. These results emphasize the importance of carryover effects of larval condition on juvenile performance (4, 42), an increasingly vital consideration for abalone and other shellfish producers under OA.
After settlement, energy should not be limiting for commercially raised abalone, which are typically provided a diet of cultured benthic diatoms. However, our results suggest that the energy and nutrition provided by this food source was insufficient to counteract the negative effects of OA on the SB population post-settlement. Polyunsaturated fatty acids (PUFA) are essential to the maintenance of metabolic functions in developing marine molluscs (43), with PUFA being the most digestible lipid form for abalone as compared to saturated fatty acids (SFA) and monounsaturated fatty acids (44). Recent work suggests that OA can reduce the ratio of PUFA:SFA in diatoms, with resulting declines in the fatty acid composition, growth, and egg production of copepods fed this diet (45). Given these impacts of OA on the digestibility and energetic value of diatoms, maternally provisioned energy reserves likely serve as a critical buffer for aquaculture-raised abalone under OA conditions, as we observed that these starting resources boosted survival under OA throughout the early-juvenile phase (97 DPF).
Tradeoffs between Growth and Vulnerability to OA.
In our experimental comparisons of animals sourced from a strong vs. weaker upwelling environment, animals from the strong upwelling population (VD) retained a roughly fourfold greater concentration of lipids after completing the larval phase, which may largely explain the resilience to OA observed during post-larval growth in this population (Figs. 1 and 3). Despite this advantage, increased survival under OA appears to be associated with a sharply reduced growth rate (Fig. 5). VD animals were 70% and 80% smaller at 3 mo of age under low and high CO2, respectively, as compared to SB crosses raised at equivalent densities. Given that aquaculture operators typically select for faster growing animals with quicker times to market to reduce stock loads, this outcome may reflect farm selection for rapid growers in the SB population. When the average size attained by F1 maternal family crosses under low CO2 is regressed against the mortality response observed by those same family groups under high CO2, a clear tradeoff appears to operate where the fastest growing families are those most susceptible to mortality under high CO2 (Fig. 6). This growth/OA-susceptibility tradeoff has been postulated for wild populations of the oyster Ostrea lurida, where slow shell building may lessen the energetic burden of acidification (46). Similarly, in studies of the purple sea urchin Strongylocentrotus purpuratus, reallocation of energy under a low basal metabolic rate allowed for tolerance of OA stress (47). Authors of this study concluded that heritable variation in metabolic rates and linked variation in the ability to allocate ATP could strongly influence resilience to OA and the adaptive potential of populations.
We also demonstrate such variation in abalone, with trait divergence indicated at the scale of populations within a single species. Our evidence suggests that in the case of abalone, traits enabling rapid growth show a negative correlation with traits needed for OA resistance during early development, in contrast to findings from commercially selected oysters in Australia (17). While it is unknown as to whether this relationship may hold true for other wild upwelling/nonupwelling populations, by selecting for rapid growers, aquaculture operators may inadvertently be selecting for genotypes vulnerable to OA. This relationship could have significant implications for continuing seed production success and the viability of abalone aquaculture.
It is tempting to assume that rapidly growing SB lineages that survive early OA stress will maintain these growth rates to adulthood. However, this conclusion does not account for our observation that equivalently sized SB-F2 animals (shell area) had dramatically reduced shell (total CaCO3) and body masses (total organic mass) per unit area under high CO2 (SI Appendix, Fig. S5). As a result, SB-F2 animals raised under high CO2 were lighter and potentially weaker with less mass to withstand environmental stress during grow-out (48) and an uncertain long-term growth and commercial viability. Further investigation of this relationship in OA-tolerant VD animals is necessary to determine the mechanisms responsible for these results and the generality of this effect on abalone growth.
Breeding Programs for OA Resilience.
Results from our experiments on SB-F2–generation animals indicate that, during the larval phase, lipid metabolism is potentially a paternally inherited trait. We found that paternal lineages crossed with a common mother exhibited differential drawdown of equivalently provisioned maternal yolk. Greater yolk drawdown during the larval phase translated to elevated mortality at settlement in families with low larval lipid concentrations at 7 DPF (Fig. 2). These findings suggest that paternal lineages could therefore be selected and bred for lipid metabolism performance in this and other shellfish species. Follow-up studies with greater genetic replication are needed to confirm this outcome and to determine if the enhanced maternal lipid provisioning of the VD population is a widespread and heritable characteristic of abalone populations sourced from strong upwelling zones. If so, these important genetic variants should be integrated into commercial and conservation breeding programs for abalone and should also be identified in other invertebrate taxa raised for aquaculture such as limpets, some giant clams, sea cucumbers, and other species possessing various yolk-dependent lecithotrophic larval forms. Such traits could play a critical role in breeding programs to enhance the resilience of commercial lines, whereby OA-tolerant varieties could be maintained at farms and utilized during particularly strong upwelling seasons. These variants could also be utilized in the culture of depleted species for supplementation and wild restoration in an increasingly acidic ocean. As such, we recommend continuing rigorous examinations of ways to improve the quality of eggs, larvae, juveniles, and settlement diets to build essential resilience into the aquaculture of molluscan shellfish, which will be critically threatened by OA in the coming decades.
Materials and Methods
Abalone were cultured under mean conditions of 14.2 °C at either low CO2 (450 µatm, pHT 8.00, Ωaragonite = 2) or high CO2 (1,080 µatm, pHT 7.65, Ωaragonite = 0.99) (SI Appendix, Table S1) characteristic of nonupwelling vs. upwelling pH values experienced along the California coast. Animals were cultured from embryos to 3 mo of age in an experimental CO2 manipulation apparatus housed at the University of California, Davis, Bodega Marine Laboratory (38). Survival in experimental replicates was visually quantified across experiments during the larval phase at 4 and 7 DPF and after settlement at 10, 28, and 97 DPF (see SI Appendix, Fig. S1 for outline and phasing of experiments). This time course of early development captured critical phases of metamorphosis from yolk-dependent, swimming larvae to feeding, benthic juveniles. During the larval phase and immediately post-settlement, animals were analyzed for total lipid content. At the conclusion of experiments, abalone were photographed and sized using digital imaging. Surviving SB-F1 abalone were then raised for 616 d and induced to spawn. Experiments were repeated on the resulting SB-F2 animals. At the conclusion of the SB-F2 experiment, size and total organic and carbonate masses of individuals was measured. See SI Appendix for additional methodological details.
Supplementary Material
Acknowledgments
We thank L. Heidenreich, E. Hubbard, K. Magaña, N. Sippl-Swezey, C. Catton, M. Heard, G. Ayad, T. Leung, S. Garcia, P. Shukla, S. Merolla, B. Walker, H. Weinberger, S. Bashevkin, T. Winquist, A. Broffman, C. Vines, and M. Portales for assistance with laboratory work and experimental maintenance during the course of this research. B. Gaylord provided useful comments on the manuscript and we thank the reviewers of this article for their expertise and thoughtful insight. We also thank the California Department of Fish and Wildlife for the contribution of wild broodstock collected from Van Damme State Park. Funding for this research was provided by National Oceanic and Atmospheric Administration Small Business Innovation Research Contracts WC-133R-15-CN-0072 and WC-133R-16-CN-0105 to The Cultured Abalone Farm, California Sea Grant R/HCME-10, and the California Conservation Genomics Project.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2006910117/-/DCSupplemental.
Data Availability.
Data files underying the presented analyses are available via the Dryad digital data repository (https://doi.org/10.25338/B8XK8R).
References
- 1.Caldeira K., Wickett M. E., Anthropogenic carbon and ocean pH. Nature 425, 365 (2003). [DOI] [PubMed] [Google Scholar]
- 2.Feely R. A. et al., Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science 305, 362–366 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Kroeker K. J., Kordas R. L., Crim R. N., Singh G. G., Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol. Lett. 13, 1419–1434 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Hettinger A. et al., Persistent carry-over effects of planktonic exposure to ocean acidification in the Olympia oyster. Ecology 93, 2758–2768 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Byrne M. et al., Unshelled abalone and corrupted urchins: Development of marine calcifiers in a changing ocean. Proc. Biol. Sci. 278, 2376–2383 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffith A. W., Gobler C. J., Transgenerational exposure of North Atlantic bivalves to ocean acidification renders offspring more vulnerable to low pH and additional stressors. Sci. Rep. 7, 11394 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barton A., Hales B., Waldbusser G. G., Langdon C., Feely R. A., The Pacific oyster, Crassostrea gigas, shows negative correlation to naturally elevated carbon dioxide levels: Implications for near-term ocean acidification effects. Limnol. Oceanogr. 57, 698–710 (2012). [Google Scholar]
- 8.Gazeau F. et al., Impact of elevated CO2 on shellfish calcification. Geophys. Res. Lett. 34, L07603 (2007). [Google Scholar]
- 9.Caldeira K., Wickett M., Ocean model predictions of chemistry changes from carbon dioxide emissions to the atmosphere and ocean. J. Geophys. Res. C Oceans 110, 1–12 (2005). [Google Scholar]
- 10.Cooley S. R., Lucey N., Kite-Powell H., Doney S. C., Nutrition and income from molluscs today imply vulnerability to ocean acidification tomorrow. Fish. 13, 182–215 (2012). [Google Scholar]
- 11.Food and Agriculture Organization of the United Nations ,“The State of World Fisheries and Aquaculture” (FAO, Rome, 2010). [Google Scholar]
- 12.Gentry R. R. et al., Mapping the global potential for marine aquaculture. Nat. Ecol. Evol. 1, 1317–1324 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Froehlich H. E., Runge C. A., Gentry R. R., Gaines S. D., Halpern B. S., Comparative terrestrial feed and land use of an aquaculture-dominant world. Proc. Natl. Acad. Sci. U.S.A. 115, 5295–5300 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Utting S. D., Spencer B. E., “The hatchery culture of bivalve mollusc larvae and juveniles” (Vol. 68, Leaflet Laboratory of Ministry of Agriculture, Fisheries and Food, Directorate of Fisheries Research, 1991).
- 15.Ellis R. P., Urbina M. A., Wilson R. W., Lessons from two high CO2 worlds—Future oceans and intensive aquaculture. Glob. Change Biol. 23, 2141–2148 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofmann G. E. et al., Exploring local adaptation and the ocean acidification seascape—Studies in the California current large marine ecosystem. Biogeosciences 11, 1053–1064 (2014). [Google Scholar]
- 17.Fitzer S. C. et al., Selectively bred oysters can alter their biomineralization pathways, promoting resilience to environmental acidification. Glob. Change Biol. 25, 4105–4115 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Byrne M., “Impact of ocean warming and ocean acidification on marine invertebrate life history stages: Vulnerabilities and potential for persistence in a changing ocean” in Oceanography and Marine Biology: An Annual Review, Vol. 49, Gibson R. N., Atkinson J. A., Gordon J. D. M., Smith I. P., Hughes D. J., Eds. (CRC Press, Boca Raton, FL, 2011), pp. 1–42. [Google Scholar]
- 19.Lee Y. H., Jeong C. B., Wang M., Hagiwara A., Lee J. S., Transgenerational acclimation to changes in ocean acidification in marine invertebrates. Mar. Pollut. Bull. 153, 111006 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Leighton D. L., The Biology and Culture of the California Abalones, (Dorrance Publishing Company, 2000). [Google Scholar]
- 21.Field L. W., Abalone Tales: Collaborative Explorations of Sovereignty and Identity in Native California, (Duke University Press, 2008). [Google Scholar]
- 22.Rogers-Bennett L., Catton C. A., Marine heat wave and multiple stressors tip bull kelp forest to sea urchin barrens. Sci. Rep. 9, 15050 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogers-Bennett L., Haaker P. L., Huff T. O., Dayton P. K., Estimating baseline abundances of abalone in California for restoration. California Cooperative Oceanic Fisheries Investigations Reports 43, 97–111 (2002). [Google Scholar]
- 24.Crim R. N., Sunday J. M., Harley C. D. G., Elevated seawater CO2 concentrations impair larval development and reduce larval survival in endangered northern abalone (Haliotis kamtschatkana). J. Exp. Mar. Biol. Ecol. 400, 272–277 (2011). [Google Scholar]
- 25.Li J. et al., The detrimental effects of CO2-driven chronic acidification on juvenile Pacific abalone (Haliotis discus hannai). Hydrobiologia 809, 297–308 (2018). [Google Scholar]
- 26.Boch C. A. et al., Effects of current and future coastal upwelling conditions on the fertilization success of the red abalone (Haliotis rufescens). ICES J. Mar. Sci. 74, 1125–1134 (2017). [Google Scholar]
- 27.Wessel N. et al., Effect of CO2–induced ocean acidification on the early development and shell mineralization of the European abalone (Haliotis tuberculata). J. Exp. Mar. Biol. Ecol. 508, 52–63 (2018). [Google Scholar]
- 28.Zippay M. L., Hofmann G. E., Effect of pH on gene expression and thermal tolerance of early life history stages of red abalone (Haliotis rufescens). J. Shellfish Res. 29, 429–439 (2010). [Google Scholar]
- 29.Guo X., Huang M., Pu F., You W., Ke C., Effects of ocean acidification caused by rising CO2 on the early development of three mollusks. Aquat. Biol. 23, 147–157 (2015). [Google Scholar]
- 30.Moran A. L., Manahan D. T., Energy metabolism during larval development of green and white abalone, Haliotis fulgens and H. sorenseni. Biol. Bull. 204, 270–277 (2003). [DOI] [PubMed] [Google Scholar]
- 31.Laudicella V. A., Whitfield P. D., Carboni S., Doherty M. K., Hughes A. D., Application of lipidomics in bivalve aquaculture, a review. Rev. Aquac. 12, 678–702 (2019). [Google Scholar]
- 32.Gruber N. et al., Rapid progression of ocean acidification in the California Current System. Science 337, 220–223 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Feely R. A., Sabine C. L., Hernandez-Ayon J. M., Ianson D., Hales B., Evidence for upwelling of corrosive “acidified” water onto the continental shelf. Science 320, 1490–1492 (2008). [DOI] [PubMed] [Google Scholar]
- 34.Chan F. et al., Persistent spatial structuring of coastal ocean acidification in the California Current System. Sci. Rep. 7, 2526 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang D., Gouhier T. C., Menge B. A., Ganguly A. R., Intensification and spatial homogenization of coastal upwelling under climate change. Nature 518, 390–394 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Sydeman W. J. et al., Climate change and wind intensification in coastal upwelling ecosystems. Science 345, 77–80 (2014). [DOI] [PubMed] [Google Scholar]
- 37.Bakun A. et al., Anticipated effects of climate change on coastal upwelling ecosystems. Curr. Clim. Change Rep. 1, 85–93 (2015). [Google Scholar]
- 38.Swezey D. S. et al., Plastic responses of bryozoans to ocean acidification. J. Exp. Biol. 220, 4399–4409 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Feely R. A. et al., Chemical and biological impacts of ocean acidification along the west coast of North America. Estuar. Coast. Shelf Sci. 183, 260–270 (2016). [Google Scholar]
- 40.“Nutrition and growth of abalone” in Handbook of Culture of Abalone and Other Marine Gastropods, Hahn K. O., Ed. (CRC Press, Boca Raton, FL, 1989), pp. 135–156. [Google Scholar]
- 41.Thomsen J. et al., Naturally acidified habitat selects for ocean acidification-tolerant mussels. Sci. Adv. 3, e1602411 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phillips N. E., Effects of nutrition-mediated larval condition on juvenile performance in a marine mussel. Ecology 83, 2562–2574 (2002). [Google Scholar]
- 43.Soudant P., Marty Y., Moal J., Masski H., Samain J. F., Fatty acid composition of polar lipid classes during larval development of scallop Pecten maximus (L.). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 121, 279–288 (1998). [Google Scholar]
- 44.Dunstan G. A., Brown M. R., Maguire G. B., Volkman J. K., Formulated feeds for newly settled juvenile abalone based on natural feeds (diatoms and crustose coralline algae). FRDC Project No. 1996/386, CSIRO Marine Research, Hobart, Tas. (Australia) (2002)
- 45.Rossoll D. et al., Ocean acidification-induced food quality deterioration constrains trophic transfer. PLoS One 7, e34737 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waldbusser G. G. et al., Slow shell building, a possible trait for resistance to the effects of acute ocean acidification. Limnol. Oceanogr. 61, 1969–1983 (2016). [Google Scholar]
- 47.Pan T. C. F., Applebaum S. L., Manahan D. T., Experimental ocean acidification alters the allocation of metabolic energy. Proc. Natl. Acad. Sci. U.S.A. 112, 4696–4701 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gaylord B. et al., Functional impacts of ocean acidification in an ecologically critical foundation species. J. Exp. Biol. 214, 2586–2594 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data files underying the presented analyses are available via the Dryad digital data repository (https://doi.org/10.25338/B8XK8R).