Significance
In many biomaterial systems, plastic deformation of extracellular matrix, degradation, or relaxation of stress are coupled and therefore their effects on cell phenotype are difficult to isolate. Using a polymer plasticizing architecture that specifically decouples irreversible creep from stress relaxation and modulus, network plasticity is shown to have a biphasic relationship with cell spreading.
Keywords: biomaterials, extracellular matrix, viscoelasticity, plasticity, stem cell
Abstract
Mammalian cell morphology has been linked to the viscoelastic properties of the adhesion substrate, which is particularly relevant in biological processes such as wound repair and embryonic development where cell spreading and migration are critical. Plastic deformation, degradation, and relaxation of stress are typically coupled in biomaterial systems used to explore these effects, making it unclear which variable drives cell behavior. Here we present a nondegradable polymer architecture that specifically decouples irreversible creep from stress relaxation and modulus. We demonstrate that network plasticity independently controls mesenchymal stem cell spreading through a biphasic relationship dependent on cell-intrinsic forces, and this relationship can be shifted by inhibiting actomyosin contractility. Kinetic Monte Carlo simulations also show strong correlation with experimental cell spreading data as a function of the extracellular matrix (ECM) plasticity. Furthermore, plasticity regulates many ECM adhesion and remodeling genes. Altogether, these findings confirm a key role for matrix plasticity in stem cell biophysics, and we anticipate this will have ramifications in the design of biomaterials to enhance therapeutic applications of stem cells.
Mammalian cell morphology is a key determinant of function (1, 2), igniting significant interest in understanding how cell spreading is regulated by microenvironmental cues (3, 4). These cues include cell–cell interactions (5), soluble signaling molecules (6), and adhesion to the extracellular matrix (ECM) via transmembrane receptors (7, 8); the physical properties of the ECM, particularly its stiffness (9) and rate of stress relaxation, are known to regulate the ability of a variety of cell types to spread in two-dimensional (10, 11) and three-dimensional (3D) (12) culture, including through remodeling (13, 14). In this report, we explore the role of matrix plasticity in controlling the spread of mesenchymal stem cells (MSCs).
To probe the mechanism through which plasticity impacts cell behavior, a hydrogel system based on alginate was first developed to allow control of network plasticity. Cellular strain on substrates is often not constant, but periodic (15), and can dynamically remodel the surrounding network to cluster adhesion ligands (16). In hydrogels with varying degrees of irreversible deformability, embedded cells would encounter more or less resistance to the dynamic remodeling that is often driven by adherent cells (17). The ability of a cell to remodel its surrounding matrix has been found to be a critical aspect of mechanotransduction (18). Therefore, a substrate’s plasticity, or its ability to irreversibly deform in response to applied stress, is biologically relevant, distinct from applications of constant strain in stress relaxation which indirectly measures remodelability. Previously, it has been shown that stress relaxation behavior can be manipulated by covalently coupling poly(ethylene glycol) (PEG) to alginate and forming ionically cross-linked hydrogels (19), as the PEG spacers facilitate how the calcium cross-links in the hydrogel redistribute the stress by partially uncross-linking (20). Kinetic Monte Carlo (KMC) simulations of a molecular clutch model (21) can be used to predict the cell spreading rate as a function of matrix physical viscoelastic properties. However, in these cases where network plasticity has been previously investigated, the remodelability is coupled with the stress relaxation (22, 23) or network degradation (24), and thus simultaneous changes in Young’s moduli, stress relaxation half-time, ligand density, or mesh size further complicate the identification of major mechanical driving factors. Herein we develop a polymeric architecture with the goal of decoupling remodelability from stress relaxation.
Results
To develop a viscoelastic ECM with independent control over plasticity, we explored changing the binding characteristics of pendant PEG. PEG binding was altered from nonbinding (simple encapsulation) to dynamic and covalent binding by changing the binding chemistry (Fig. 1). To synthesize the alginate, it was functionalized with acetyl groups to allow for the potential to form Schiff bases with primary amines (SI Appendix, Fig. S1). Reactions were verified with both solution and solid-state 1H NMR and 13C NMR, (SI Appendix, Figs. S2–S4). The dynamic bonding nature of the Schiff base formation with a monofunctional PEG-amine was verified by 1H NMR kinetic studies (SI Appendix, Fig. S5), 1H NMR correlation spectroscopy (SI Appendix, Fig. S6), and nuclear Overhauser effect spectroscopy (SI Appendix, Fig. S7) studies probing the proton exchange of the terminal methylene peaks and gel permeation chromatography (GPC), which also demonstrated minimal thermal degradation (SI Appendix, Figs. S8–S9). The dynamic nature of these bonds was also confirmed via demonstration of macroscale self-healing properties of gels fabricated with an additive pendant PEG with a bis-functional primary amine instead of a monofunctional primary amine, to allow it to function as a gel cross-linker (SI Appendix, Fig. S10) (25). We hypothesized the various gels would maintain similar Young’s moduli and stress relaxation values within a given range of stress, while the irreversible plastic deformation would be different (Fig. 1).
Fig. 1.
Schematic depicting how the PEG spacer binding characteristics are proposed to influence hydrogel plasticity in response to cyclical forces. By incorporating pendant PEG to alginate that is either bound covalently, dynamically through Schiff base formation, or simply encapsulated (nonbinding), the irreversible plastic deformations can be controlled with respect to the Young’s modulus and stress relaxation. PEG less constrained to the alginate is proposed to lead to an increase in the force loading of calcium cross-links and thus enhanced irreversible reorganization of the network. In covalent-bound PEG gels, the network responds similarly in response to a singular stress on the network, but upon repeated events, the PEG spacers cannot rearrange to provide optimal plastic behavior. Nonbinding PEG is free-floating and can reorganize in response to stress while the dynamic-binding PEG has intermediate mobility. Individual PEG chains are labeled in the first column to demonstrate chain mobility.
Mechanical characterization revealed that plasticity could be altered independently of initial moduli and stress relaxation behavior with variations in PEG binding. Gels formed with nonbound, covalently attached PEG and dynamic PEG were shown to have similar initial Young’s moduli, as determined by rheology (Fig. 2A) and nanoindentation (SI Appendix, Fig. S11). Consistent with these results, the Young’s modulus of alginate hydrogels with covalently attached pendant PEG was primarily attributed to the polymer molecular weight of the PEG and the concentration of divalent cation (26). Interestingly, the use of PEG-amine species of different molecular weights did not impact the Young’s moduli, which further supports findings in the literature that PEG plasticizers were not substantially load-bearing in covalently bound PEG hydrogels (26). Chain length was also found to have minimal impact on the modulus within the range of stress and chain length utilized, consistent with literature demonstrating viscoelastic effects were dominated more by PEG density than chain length (26). Stress relaxation was measured by holding a constant 40% strain on the hydrogels (Fig. 2B), resulting in overlapping stress–strain curves regardless of the binding characteristic of the PEG (Fig. 2C), demonstrating similar stress relaxation behavior. The stress–strain response similarity among samples was maintained up to strains exceeding 100% (SI Appendix, Fig. S12), which are above typical strains exerted by MSCs (27). Stress relaxation behavior at different strains was also similar (SI Appendix, Fig. S13), and repeated stress relaxation tests revealed alterations in gel stress relaxation behavior with additional cycles, but in a very similar manner for all three types of hydrogels (SI Appendix, Fig. S14), with minimal leaching of the nonbound PEG (SI Appendix, Fig. S15). To measure the plasticity of the hydrogels, we performed repeated creep–relaxation tests (Fig. 2D) and calculated the degree of plasticity as the total irreversible creep normalized by the total creep (Fig. 2E) (23). The encapsulated nonbinding PEG gels were the most remodelable, which increased with successive cycles. Gels with PEG covalently bound to the alginate polymer backbone exhibited the least amount of plasticity, and the hydrogels where PEG bonded dynamically with the alginate backbone exhibited intermediate plasticity, falling between the behavior of free soluble PEG and covalently bound PEG. The hydrogels exhibited stress-enhanced plasticity, with clear delineations in creep behavior from 2 Pa to 40 Pa of stress (Fig. 2F), with the dynamic hydrogels showing plasticity similar to collagen gels (23). At biologically relevant stresses, these data indicate that changing the binding characteristics of the PEG affects the creep independently of stress relaxation and Young’s modulus, likely because stress relaxation within the network is dominated by calcium cross-link reorganization (20). The initial network reorganization is similar for constant strains, independent of PEG binding dynamics, but upon repeated perturbations the data suggest the PEG spacers rearrange according to the most favorable thermodynamic state, leading to differences in the plasticity becoming more apparent (SI Appendix, Fig. S16)
Fig. 2.
Modulating the plasticity of alginate hydrogels independently of initial elastic modulus and stress relaxation. (A) Rheological data quantifying the initial Young’s modulus of the various calcium-cross-linked hydrogels. Rheological measurements of five biological replicates. All subsequent experiments were conducted using 10-kDa PEG. (B) Rheological test profile of the stress relaxation assay. (C) The stress relaxation behavior of hydrogels with different incorporation of PEG spacers, analyzed at a strain of 40% with significant overlap. (D) Rheological test profile of the repeated creep assay to probe hydrogel plasticity. (E) Repeated creep studies conducted at 10 Pa for gels with differential incorporation of PEG. (F) The ratio of irreversible to reversible strain due to creep plotted as a function of stress for different PEG incorporation approaches.
Differences in gene expression in undifferentiated MSCs (SI Appendix, Fig. S17) encapsulated in the gels of varying plasticity for 3 d was next assessed, using a NanoString messenger RNA (mRNA) analysis focused on ECM-related genes. Three days was selected to specifically measure the effects of plasticity prior to differences in cell proliferation and significant nascent ECM deposition (SI Appendix, Fig. S18) (18). A NanoString mRNA analysis, through a panel of 770 ECM-related genes, showed significant nonbiased heat-map segregation of differential expression (DE) based on gel plasticity (Fig. 3A). Cells within the dynamic and covalent hydrogels were closer in relationship than those encapsulated in nonbinding hydrogels, which aligned with the differences in respective plasticity values measured at 10 Pa. Strikingly, the greatest percentage of DE genes were associated with adhesion and matrix remodeling, with cytokine and chemokine signaling genes also significantly impacted (Fig. 3B). The latter is consistent with recent findings that cytokine/chemokine expression in MSCs is mechanosensitive (28). Gene enrichment analysis confirmed that the matrix plasticity had significant effects on a wide array of cell functions, with cell spreading/matrix interactions figuring prominently (Fig. 3C). Other viscoelasticity-sensitive pathways, such as phosphatidylinositol 3-kinase, Janus kinase/signal transducers and activators of transcription (JAK-STAT), and transforming growth factor (TGF)-β have been demonstrated to affect MSC differentiation in fibrotic diseases (29); we found these genes to change expression based on hydrogel plasticity. Volcano plots comparing nonbinding to covalent gels demonstrated that cell spreading, ECM, and actin cytoskeleton-mediating genes such as Samsn1 and Met were dramatically down-regulated (Fig. 3D), while integrin-related cell adhesion and communication genes such as Thy1 were up-regulated in the dynamic vs. nonbinding (Fig. 3E). Differences between cells encapsulated within dynamic and covalent gels were more nuanced, with more cell adhesion and matrix remodeling genes up-regulated but collagen and some inflammatory cytokines down-regulated (Fig. 3F).
Fig. 3.
Network plasticity effects on matrix remodeling and adhesion mRNA expression. Cells were encapsulated in alginate hydrogels and incubated for 72 h before mRNA isolation and analysis via NanoString of matrix remodeling and adhesion-associated mRNA. (A) Tree heat map of mRNA expression of five biological replicates of MSCs incubated in each of the three ECMs. (B) Pie chart representing the percentage of DE genes belonging to the most represented family classifications across the three different matrix plasticity conditions. (C) Enrichment analysis of DE genes at different ECM plasticity, measured at 10 Pa of stress. DE was defined as at least a ±2× change and false discovery rate <0.05. (D) Volcano plot of DE genes comparing the nonbinding to a baseline of covalent hydrogels, (E) dynamic to a baseline covalent, and (F) dynamic to baseline nonbinding gels, with statistically-significant DE with P < 0.05 colored in blue. All data are shown with mean ± SD.
The analysis of gene expression led us to next examine the impact of plasticity on cell spreading, due to its widespread impact on expression of genes related to adhesion and matrix interactions. The aspect ratio of MSCs (SI Appendix, Fig. S19) encapsulated in alginate hydrogels was found to be regulated by hydrogel plasticity, with the greatest degree of spreading in gels of intermediate plasticity (Fig. 4 A and B). However, there were not significant changes in nuclear shape (Fig. 4C) or cell volume (Fig. 4D), which suggests that the various presentations of PEG were not causing changes in osmotic pressure, with alterations in spreading secondary to the volume changes. Changes in cellular spreading corresponded with focal adhesion formation, with intermediate plasticity hydrogels leading to more frequent and larger focal adhesions (Fig. 4E). Quantification of nearest-neighbor distances for each FAK cluster’s center of mass confirmed that focal adhesions were more clustered in the gels with dynamically bound PEG (Fig. 4F). To determine if the effect of hydrogel plasticity was dependent on the Young’s moduli of the gels, we next investigated the spreading of cells in gels exhibiting moduli of 1.1, 3.6, and 8.0 kPa, respectively, while maintaining a similar tan(delta) (SI Appendix, Fig. S20). The gel plasticity only impacted cell spreading at the intermediate modulus (Fig. 4G). At lower moduli, the cells did not spread well and remained globular, even at moderate levels of ECM plasticity. At higher moduli, the cells all had similarly high cell aspect ratios. The nuclear aspect ratio and cell volume also followed similar trends, where the nucleus became more elongated with cell spreading and cell volume was inversely related as typical with spreading cells (SI Appendix, Fig. S21). These findings suggest that the modulus can dominate the impact of plasticity on cell spreading outside of specific domains.
Fig. 4.
Cell spreading and focal adhesion clustering are enhanced with moderate network plasticity. (A) Representative maximum intensity projection images of MSCs encapsulated within 3.6-kPa (initial modulus) alginate gels with different plasticity. Green represents actin, blue represents the nucleus, and red represents FAK kinase. Confocal images were taken after 3 d in culture. (B) Quantification of the cell aspect ratio, as defined by the 3D rectangular bounding box’s longest side divided by its shortest side. (C) Quantification of the nuclear aspect ratio, as defined by the 3D rectangular bounding box’s longest side divided by its shortest side, showing no significant difference between the conditions. (D) Cellular volume as measured per cell using Imaris Image Analysis software. (E) Representative images of focal adhesions between different binding PEG. (Scale bar, 5 µm.) (F) Histograms of focal adhesion spacing in 3D space, as based on the nearest neighbor distances for k = 4 neighbors. ANOVA analysis resulted in P < 0.001. (G) Cell spreading in gels of Young’s moduli of 1.1, 3.4, and 8 kPa, respectively, as measured by nanoindentation and varying levels of plasticity. *P < 0.1, **P < 0.01, ***P < 0.001, n.s., not significant.
To better understand the influence of plasticity on cell spreading, a variation on the molecular clutch model previously developed to explain the impact of elastic moduli and stress relaxation on cell spreading was constructed (21). The molecular clutch model predicts that the cell spreading speed is correlated with the stress relaxation timescale, showing enhancement at intermediate viscosities within a Maxwell model, and we explored whether the model would also predict enhancement at intermediate plasticity. The standard linear model for viscoelasticity was adapted by introducing a sliding frictional element to explicitly characterize the irreversible strain (Fig. 5A), and the relationship between cell spreading speed and the plasticity of the ECM was probed via KMC (SI Appendix, Fig. S22). Although the model previously developed by Shenoy and coworkers considers one-dimensional spreading (21), here we also applied this approach to experimental results in 3D. The cell-spreading speeds of the MSCs in the different plasticity networks were observed first by live cell imaging of individual cells (Movies S1 and S2) and then determined by fixing large population of cells at various time points (SI Appendix, Fig. S23) and imaging using lightsheet microscopy. As expected from the previous analysis of spreading at 72 h, the cell-spreading speed was highest at the intermediate hydrogel plasticity. Subsequently, close agreement was found between the predictions of the model and the experimental results, with both demonstrating a maximal rate of spreading for a given ECM, at an optimal amount of network plasticity (Fig. 5B). A fourth type of hydrogel, which utilizes a bis-amine dynamic-binding 10-kDa PEG demonstrating intermediate plasticity between dynamic and covalent hydrogels, was used in this analysis to better compare experiments to the model. For materials with lower plasticity, the spreading speed reaches an asymptote that is likely dominated by the elastic confinement of cells, presumably because the time scales for network reorganization are much slower than actin retrograde flow (30). Materials with higher viscoplasticity also have a slower spreading speed, likely as more focal adhesions form in this case (SI Appendix, Fig. S24), and the finite recruitment of adhesion ligands within the ECM inhibits spreading and polarization (24). Strikingly, it has been shown that collagen, despite occupying a complex and heterogenous class of material, has plasticity values ranging from 0.16 to 0.4 (23), which fall within the optimal plasticity for cell spreading, according to the modeling.
Fig. 5.
Relating ECM plasticity to the molecular clutch theory. (A) Schematic describing how viscoplasticity in hydrogels was modeled by incorporating a sliding frictional element into a generalized Maxwell model. (B) Plot of the plasticity of the modified alginate hydrogels (left to right: covalent, dynamic-bis, dynamic, and nonbinding) versus the corresponding experimental cell spreading speeds, with an overlay in red of a KMC simulation of the modified motor-clutch model (n = 50). Spreading of cells in (C) nonbinding, (D) dynamic, and (E) covalent-binding PEG with increasing amounts of blebbistatin. (F) Schematic of the biphasic relationship of matrix plasticity with cell spreading according to molecular clutch theory with the red dots representative of experimental conditions. (G) Interpretation of the impact of adding blebbistatin to cells in various hydrogels; the molecular clutch loading rate is decreased with blebbistatin, shifting the conditions left along the curve. Red dots in F and G represent experimental cell spreading data from C–E. YAP nuclear translocation in cells within the (H) nonbinding, (I) dynamic, and (J) covalent-binding PEG with addition of various concentrations of blebbistatin to the culture media. *P < 0.1, **P < 0.01, n.s., not significant.
To test the impact of actin–myosin contraction in this setting, MSCs in the various hydrogels were treated with blebbistatin, and the spreading was again analyzed in relation to the hydrogel plasticity. The spreading of cells in the nonbinding gels was not appreciably impacted by blebbistatin treatment (Fig. 5C), while cells in the dynamic-binding PEG gels exhibited a small decrease in spreading with increasing blebbistatin inhibition (Fig. 5D). Strikingly, cells in the covalent gels spread to a greater extent with increasing blebbistatin (Fig. 5E), which also corresponded to decreases in the cell volume (SI Appendix, Fig. S25). While the plasticity of the networks was previously noted to not affect the cell volume (Fig. 4D), changing the contractility may alter cell volume due to an inability of the cells to fully respond metabolically to the cell stretching in this condition (18). In the molecular clutch model, cell spreading is predicted to first increase due to strain-stiffening effects of plasticity but then drop off as the bound clutches become saturated. The spreading data with blebbistatin treatment suggests that cells in the dynamic-bound PEG networks with intermediate plasticity are initially near the optimal molecular clutch loading rate for cell spreading, so treatment with blebbistatin decreases the cell spreading rate and focal adhesions (Fig. 5 F and G). Cells in the nonbinding pendant PEG hydrogels exist to the far left of the optimum, and reducing the molecular clutch loading is expected to minimally change spreading. Cells in the least plastic gels are interpreted to be to the right of the optimum point, and blebbistatin treatment would move toward the optimum, increasing cell spreading (Fig. 5 F and G). The molecular clutch model also suggests that development of focal adhesions and cell spreading are related metrics (11), and analysis of focal contacts under these conditions largely confirmed the model predictions, as mature focal adhesions significantly increased in the least plastic gels at 15 µM blebbistatin (SI Appendix, Fig. S25).
As YAP has been described as a key player in mechanotransduction (7, 8, 11), its nuclear translocation was analyzed to explore the relationship of gel plasticity with classic mechanotransduction pathways, and to further probe the impact of inhibiting contractility. YAP translocation into the nucleus is often indicative of transcriptional changes in cells in response to mechanotransduction, and it is often coupled to substrate rigidity that either aids or hinders force transmission to the nucleus (31). YAP translocation had a biphasic relationship to network plasticity, with highest levels of YAP translocation in the intermediate plasticity gels in the absence of blebbistatin treatment (Fig. 5 H–J); this effect was similar to that observed for cell spreading (SI Appendix, Fig. S26). To determine the impact of actin–myosin contractility on mechanotransduction, cells were subsequently cultured in the various gels with different concentrations of blebbistatin (SI Appendix, Fig. S27). In the most plastic gels, YAP translocation was not impacted by blebbistatin treatment (Fig. 5H). YAP translocation decreased in cells in the intermediate plasticity hydrogels with increasing concentrations of blebbistatin (Fig. 5I). In contrast, YAP nuclear translocation was enhanced in the least plastic gels as the blebbistatin concentration was increased (Fig. 5J). This was consistent with DE analysis showing TGF-β and matrix metallopeptidases (MMP) were positively correlated with YAP, as well as several other homeostatic, developmental, and ECM-associated mRNA (Fig. 3 E–G).
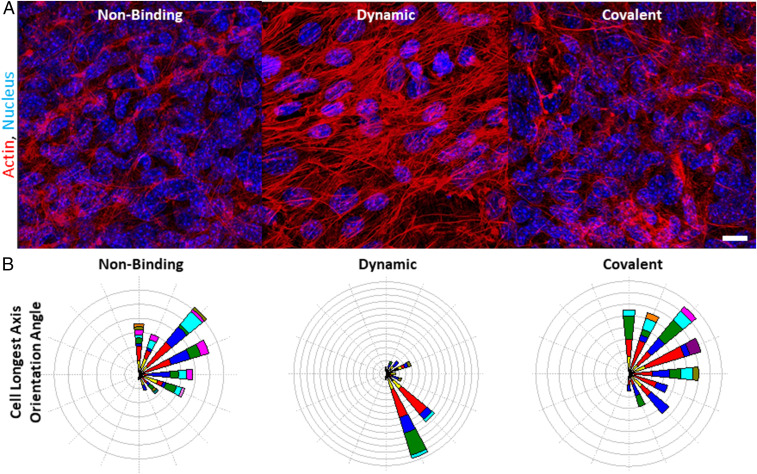
Under longer-term incubation of MSCs inside these hydrogels of differential plasticity, we also observed cell-spreading differences to continue at 21 d of encapsulation (Fig. 6A). Not only were the cells embedded in the intermediate plasticity hydrogel initially the most spread, but they also continued this trend for weeks later. At the intermediate plasticity, far more stress fibers formed and the angular orientation was significantly more aligned with other cells in the surrounding matrix (Fig. 6B). Also, previous results demonstrated more MMP and proliferative expression in the dynamic-binding hydrogels compared to more Col4a5 and proteinase inhibitors such as Serpinh1 in the nonbinding and covalent conditions after only 72 h of incubation (Fig. 3 E–G). This suggests that the initial interactions between cells and the ECM prior to large-scale protein and matrix deposition may play an important role in priming cell-spreading behavior that continues to influence cellular remodeling and function at later times.
Fig. 6.
Long-term effects of ECM plasticity on stress fiber orientation. (A) Maximum intensity projection of a confocal image of MSCs incubated in hydrogels with different matrix plasticity for 21 d. Actin was stained in red with phalloidin and DAPI stained the nucleus in blue. (Scale bar, 18 μm.) (B) Polar plot histograms of actin fiber orientation angle as analyzed in Imaris of z-stacks. From a range of 0° to 180° with respect to the imaging frame, the actin stress fibers’ longest axis was matched to an angle of best fit.
Discussion
These findings implicate matrix plasticity as an important regulator of MSC biology broadly and cell spreading more specifically. Herein, we created a system of PEG-alginate hydrogels with tunable binding kinetics, and cell spreading was shown to be enhanced at intermediate plasticity conditions that are similar to natural collagen gels. The use of the PEG moiety as a tunable plasticizing agent showed several advantages over previously published artificial ECM systems in that the initial Young’s modulus and stress relaxation were able to be independently controlled with respect to the plasticity. Due to the unique architecture of the system, by simply tuning the binding kinetics of the PEG pendant group to the alginate backbone the initial Young’s modulus and the stress relaxation were not significantly affected within a given stress range, even under successive stress relaxation probes. Under cycles of constant strain, the differences in plasticity were apparent, likely as it is a closer measurement of network remodeling as compared to the corollary energy dissipation in stress relaxation. As the PEG pendant groups are non-load-bearing (19, 20), they may be able to facilitate molecular mobility of the alginate chains depending on the binding constraints, while not significantly affecting osmotic compression due to the lower PEG concentrations used (32). Also, by changing only the PEG terminus to affect the binding kinetics with alginate, the system minimizes the possibility for differential chemical influences, especially compared to the larger macromolecular structure and amount of proteins present in the serum-enriched incubation media.
The focus in this study on the ratio of irreversible to reversible deformation in response to creep, we believe, is a biologically relevant metric. This is likely to be particularly important in the initial stages of cell contact with matrices, when the cells are initially probing their environment, although our data also demonstrate the initial effects are maintained over longer periods of time. In native ECM where the network mechanical properties often are dominated by the properties of collagen (23), an intermediate amount of plasticity may reflect the matching of the network remodeling time scale with that of retrograde actin flow and integrin binding (21). This possibility is consistent with the findings of our KMC simulations. However, a limitation of this modeling approach is that it assumes the plasticity and modulus remain constant with time and cell spreading, though with successive remodeling of the ECM network this is unlikely. Even in the nonbinding PEG case the plasticizing effects of the pendant PEG does not go on indefinitely, as the system will inevitably reach a point of strain-induced phase separation. Our results are also consistent with past experimental results (10) analyzing YAP translocation in the context of viscoelasticity; however, the ability to alter YAP translocation while matrix moduli and stress relaxation remain constant indicates that matrix plasticity specifically is a significant controlling factor that may be an underlying mechanical metric involved in stress relaxation mechanotransduction.
In conclusion, a synthetic artificial ECM hydrogel system has been developed that can independently tune network plasticity from stress relaxation and modulus and be used to demonstrate the role of plasticity in cell spreading. The relationship between network plasticity and cell spreading was modeled through KMC simulations, and these support the role of plasticity in spreading. Differential expression analysis of mRNA also coincided with previously reported RNA-sequencing data for cells incubated in hydrogels with differential stress relaxation data for certain gene networks like TGF-β and Wnt yet differed in others such as JAK-STAT, suggesting different mechanisms of mechanotransduction. This enhanced understanding of how cells can reshape their surrounding matrix, and how their surrounding matrix directs their behavior, will likely be important in not only the design of new biomaterials but also in understanding disease states that involve changes in the ECM.
Materials and Methods
Synthesis and Characterization of Functionalized Alginate.
The following chemicals were obtained from Sigma Aldrich: tert-butyl acetoacetate (98%), 1-allyl-3methylimidaxolium chloride (AMIMCl), N-hydroxysulfosuccinimide-sodium (NHS) (98%), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC) (98%), and methanol (99.9%). PEG species were obtained from Laysan Bio, and GGGGRGDSP (98.13%) was obtained from Peptide 2.0. I-1G alginate was obtained from Kimica.
Two types of alginate polymers were synthesized, alginates with covalently coupled PEG and ones without. For all materials, the starting alginate was first acetylated, followed by conjugation of Arg–Gly–Asp (RGD). From there, the alginates were aliquoted and separately reacted with PEG through EDC/NHS coupling to produce the covalent-bound PEG, or later dissolved and mixed with PEG with terminal amines to produce the dynamic-bound PEG. Nonbinding PEG was formulated by mixing the functionalized alginate with carboxylic acid terminal PEG. The alginate (2.33 g) was dissolved in 50 g of AMIMCl at 110 °C and 30 mL of tert-butyl acetoacetate was added dropwise for 45 min. The reaction was then cooled to room temperature and washed in dry methanol. Alginate was purified by dialysis and then conjugated with 758 mg of RGD, followed by 61.3 mg of PEG in the covalent case, and purified again by dialysis and subsequent carbon black sterile filtration and lyophilization. With the acetoacetate functionality along the backbone of the alginate, PEG with terminal amine groups were mixed with the alginate and after 45 min of mixing form a Schiff base. This affords a degree of acetate functionalization of ∼15%, as determined by GPC and NMR (SI Appendix, Figs. S1–S9). GPC experiments were run on a Malvern Viscotek 270max GPC equipped with a GPCmax solvent and sample delivery module, an Eldex Ch-150 temperature-controlled column holder, a VE 3580 refractive index detector, a viscotek 270 Dual Detector featuring intrinsic viscosity (IV-DP) and right-angle light scattering. Samples were dissolved in 0.1 M NaNO3 buffer solution at 7 mg/mL, and 200 µL of sample was injected. Separation was through two TSK-gel columns (G4000PWXL and G3000PWXL) and molecular weight calculations were based on pullulan standards. High-resolution 1H NMR spectra were obtained in deuterium oxide using a Varian Unity-600 (600 MHz) NMR spectrometer, and solid-state NMR spectra were obtained through Spectral Data Services. RGD ligand density was determined to be 150 µM via 1H NMR.
Nanoindentation.
Mechanical testing was performed using an Agilent G200 nanoindenter with a 90° diamond conical probe tip with a 50-μm radius (DCMII; Micro Star Technologies) to enable measurement of bulk properties. The tip area function was calibrated using fused quartz, and a punch diameter of 45.153 μm was calculated at a 5-μm precompression depth. A 5 × 5 array of indents with 200-μm spacing was generated.
Rheology.
Rheology measurements were made with an AR-G2 stress-controlled rheometer (TA Instruments). Alginate solution (800 µL) was immediately mixed with 3% CaSO4 (200 µL) slurry and was deposited onto the surface of a 20-mm rough-surface plate and immediately brought the 20-mm rough 1° cone geometry down to a gap of 1 mm. The mechanical properties were then measured following a gelation time of 45 min.
Encapsulating MSCs in Hydrogels.
Cell-containing gels were formed by mixing cell suspensions with 800 µL of 1.6% volume to mass ratio the variously modified Kimica alginates to afford a density of 2.23 million cells per mL. This suspension was then mixed with 200 µL of 3% CaSO4 slurry via female–female luerlock couplers and immediately cast in 1-mm-thick disks and punched out into 8-mm-diameter circles after 45 min of gelation. The disks were then transferred to well plates and immersed in media.
NanoString Analysis.
Cell-laden hydrogels were decross-linked in 1 mL of 5 mM EDTA for 5 min with vigorous mixing and spun down at 1,400 rpm for 5 min to aspirate the alginate solution. The pelleted cells were then ruptured using RNA lysis buffer and immediately incubated with the NanoString IO360 kit primers according to protocol. The analysis was of the NanoString readout were analyzed using the NanoString nCounter nSolver 4.0 software (NanoString MAN-C0019-08) with the NanoString Advanced Analysis Module 2.9 plugin (NanoString MAN-10,030-03) following the NanoString Gene Expression Data Analysis Guidelines (NanoString MAN-C0011-04). The Advanced Analysis Module 2.0 software uses open-source R programs for QC, normalization, DE analysis, pathway scoring, and gene-set enrichment analysis.
Immunohistochemistry.
First, the gels were removed from the well plates and placed into new wells containing phosphate-buffered saline (PBS) and subsequently rinsed. The gels were then fixed in 4% paraformaldehyde at 37 °C for 45 min. Gels were then washed five times quickly with calcium-containing PBS (cPBS) and three times for 5 min with cPBS. The gels were then stained following standard immunohistochemistry protocols. The following reagents were used: YAP antibody (4912; Cell Signaling), Focal Adhesion Kinase (40794; Abcam), Prolong Glass antifade reagent with DAPI (Invitrogen), AF-488 Phalloidin to stain actin (Invitrogen), and goat anti-rabbit IgG AF 647 (Invitrogen).
Confocal Imaging.
A Zeiss Airyscan LSM 880 confocal microscope with superresolution was used to image individual cells one at a time. Z-stacks were imaged of 40 µm × 40 µm × 40 µm with a 63× objective, and a z-stack spacing of 0.15 µm, as per the calculated Huyggens limit. Approximately 50 cells were imaged for each condition.
Image Analysis.
For measurements of cell aspect ratio, the data were analyzed in Imaris 9.2 from z-stacks obtained by a Zeiss LSM 880 + Airyscan microscope. The longest axis in 3D was measured for each cell, and the bounding-box width of the cell perpendicular to that axis was measured as the shortest axis. The ratios were then recorded to note cell spreading. Focal adhesions were analyzed in Imaris using the “Spots” feature, and the coordinates were input into MATLAB for nearest-neighbor analysis for a k = 4. A Zeiss Lighsheet Z1 was used to image entire hydrogel sections and cellular measurements were calculated through Arivis Vision4D. YAP translocation was measured by comparing the fluorescent intensity of the YAP channel colocalized with the DAPI stain to the intensity in the immediate cytoplasm within 5 μm of the nuclear envelope.
Numerical Methods.
The KMC simulations based on analytical solutions to ordinary differential equations to characterize cell spreading were adapted from prior literature (21) and similarly performed in MATLAB.
Supplementary Material
Acknowledgments
This work was supported by the NIH (R01DE013033, R01EB023287) and the US Army Medical Research Acquisition Activity (W81XXWH-16-1-0784). We thank K. H. Vining and A. Elosegui-Artola for their invaluable discussions and feedback, D. S. Richardson at the Harvard Center for Biological Imaging for help with imaging, M. Lewandowski at the Wyss Institute for help with GPC, and S. Huang for help with NMR.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2008801117/-/DCSupplemental.
Data Availability.
All study data are included in the paper and SI Appendix.
References
- 1.Vining K. H., Mooney D. J., Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell Biol. 18, 728–742 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker B. M., Chen C. S., Deconstructing the third dimension: How 3D culture microenvironments alter cellular cues. J. Cell Sci. 125, 3015–3024 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang W. Y., Davidson C. D., Lin D., Baker B. M., Actomyosin contractility-dependent matrix stretch and recoil induces rapid cell migration. Nat. Commun. 10, 1186 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Q. et al., Extracellular matrix scaffolding guides lumen elongation by inducing anisotropic intercellular mechanical tension. Nat. Cell Biol. 18, 311–318 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Ferreira S. A. et al., Neighboring cells override 3D hydrogel matrix cues to drive human MSC quiescence. Biomaterials 176, 13–23 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pardo-Pastor C. et al., Piezo2 channel regulates RhoA and actin cytoskeleton to promote cell mechanobiological responses. Proc. Natl. Acad. Sci. U.S.A. 115, 1925–1930 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oria R. et al., Force loading explains spatial sensing of ligands by cells. Nature 552, 219–224 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Bennett M. et al., Molecular clutch drives cell response to surface viscosity. Proc. Natl. Acad. Sci. U.S.A. 115, 1192–1197 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engler A. J., Sen S., Sweeney H. L., Discher D. E., Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Elosegui-Artola A. et al., Mechanical regulation of a molecular clutch defines force transmission and transduction in response to matrix rigidity. Nat. Cell Biol. 18, 540–548 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Elosegui-Artola A. et al., Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell 171, 1397–1410.e14 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Das R. K., Gocheva V., Hammink R., Zouani O. F., Rowan A. E., Stress-stiffening-mediated stem-cell commitment switch in soft responsive hydrogels. Nat. Mater. 15, 318–325 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Arnoldini S. et al., Novel peptide probes to assess the tensional state of fibronectin fibers in cancer. Nat. Commun. 8, 1793 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brauer E. et al., Collagen fibrils mechanically contribute to tissue contraction in an in vitro wound healing scenario. Adv. Sci. (Weinh.) 6, 1801780 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonakdar N. et al., Mechanical plasticity of cells. Nat. Mater. 15, 1090–1094 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Kechagia J. Z., Ivaska J., Roca-Cusachs P., Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol. 20, 457–473 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Doyle A. D., Yamada K. M., Mechanosensing via cell-matrix adhesions in 3D microenvironments. Exp. Cell Res. 343, 60–66 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loebel C., Mauck R. L., Burdick J. A., Local nascent protein deposition and remodelling guide mesenchymal stromal cell mechanosensing and fate in three-dimensional hydrogels. Nat. Mater. 18, 883–891 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaudhuri O. et al., Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 15, 326–334 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao X., Huebsch N., Mooney D. J., Suo Z., Stress-relaxation behavior in gels with ionic and covalent crosslinks. J. Appl. Phys. 107, 63509 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gong Z. et al., Matching material and cellular timescales maximizes cell spreading on viscoelastic substrates. Proc. Natl. Acad. Sci. U.S.A. 115, E2686–E2695 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wisdom K. M. et al., Matrix mechanical plasticity regulates cancer cell migration through confining microenvironments. Nat. Commun. 9, 4144 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nam S., Lee J., Brownfield D. G., Chaudhuri O., Viscoplasticity enables mechanical remodeling of Matrix by cells. Biophys. J. 111, 2296–2308 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madl C. M. et al., Maintenance of neural progenitor cell stemness in 3D hydrogels requires matrix remodelling. Nat. Mater. 16, 1233–1242 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wojtecki R. J., Meador M. A., Rowan S. J., Using the dynamic bond to access macroscopically responsive structurally dynamic polymers. Nat. Mater. 10, 14–27 (2011). [DOI] [PubMed] [Google Scholar]
- 26.Nam S., Stowers R., Lou J., Xia Y., Chaudhuri O., Varying PEG density to control stress relaxation in alginate-PEG hydrogels for 3D cell culture studies. Biomaterials 200, 15–24 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurpinski K., Chu J., Hashi C., Li S., Anisotropic mechanosensing by mesenchymal stem cells. Proc. Natl. Acad. Sci. U.S.A. 103, 16095–16100 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mao A. S. et al., Programmable microencapsulation for enhanced mesenchymal stem cell persistence and immunomodulation. Proc. Natl. Acad. Sci. U.S.A. 116, 15392–15397 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Darnell M. et al., Material microenvironmental properties couple to induce distinct transcriptional programs in mammalian stem cells. Proc. Natl. Acad. Sci. U.S.A. 115, E8368–E8377 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee H. P., Gu L., Mooney D. J., Levenston M. E., Chaudhuri O., Mechanical confinement regulates cartilage matrix formation by chondrocytes. Nat. Mater. 16, 1243–1251 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cosgrove B. D. et al., N-cadherin adhesive interactions modulate matrix mechanosensing and fate commitment of mesenchymal stem cells. Nat. Mater. 15, 1297–1306 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo M. et al., Cell volume change through water efflux impacts cell stiffness and stem cell fate. Proc. Natl. Acad. Sci. U.S.A. 114, E8618–E8627 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the paper and SI Appendix.