Significance
The neuropeptide oxytocin is an important regulator of social behavior and is widely considered to reduce anxiety-related behaviors. However, growing evidence suggests that sometimes oxytocin increases anxiety. How can the same molecule have such different effects on behavior? Here we provide evidence that oxytocin produced outside of the hypothalamus is necessary and sufficient for stress-induced social anxiety behaviors. This suggests that the diverse effects of oxytocin on anxiety-related behaviors are mediated by circuit-specific oxytocin action.
Keywords: anxiety, bed nucleus of the stria terminalis, extended amygdala, California mouse, morpholino
Abstract
Oxytocin increases the salience of both positive and negative social contexts and it is thought that these diverse actions on behavior are mediated in part through circuit-specific action. This hypothesis is based primarily on manipulations of oxytocin receptor function, leaving open the question of whether different populations of oxytocin neurons mediate different effects on behavior. Here we inhibited oxytocin synthesis in a stress-sensitive population of oxytocin neurons specifically within the medioventral bed nucleus of the stria terminalis (BNSTmv). Oxytocin knockdown prevented social stress-induced increases in social vigilance and decreases in social approach. Viral tracing of BNSTmv oxytocin neurons revealed fibers in regions controlling defensive behaviors, including lateral hypothalamus, anterior hypothalamus, and anteromedial BNST (BNSTam). Oxytocin infusion into BNSTam in stress naïve mice increased social vigilance and reduced social approach. These results show that a population of extrahypothalamic oxytocin neurons plays a key role in controlling stress-induced social anxiety behaviors.
Social anxiety disorder (SAD) is one of the most prevalent and debilitating psychiatric disorders across nations (1, 2) and is characterized by a persistent fear or anxiety of unfamiliar social situations or scrutiny by others (3). Available treatments for SAD are ineffective for about half of the patients (4, 5), which highlights the need to identify underlying mechanisms. A key brain region mediating fear and anxiety is the bed nucleus of the stria terminalis (BNST), a complex component of the extended amygdala that is thought to be an important contributor to stress-induced psychiatric disorders (6–8). Compared to healthy controls, individuals diagnosed with SAD show increased phasic activation of the BNST in anticipation of aversive events (9), and altered BNST functional connectivity following unpredictable threats (10). Nonetheless, it is unclear at a mechanistic level how the BNST mediates behavioral phenotypes related to SAD.
One central phenotype contributing to SAD is biased attention to social threat, which can be expressed by 1) attentional avoidance of socially salient information and/or 2) exacerbated vigilance of the social environment (11, 12). Remarkably, social stress can induce both behavioral analogs in rodents (13, 14), providing a unique opportunity to assess underlying mechanisms of behavioral phenotypes associated with SAD. Using the California mouse (Peromyscus californicus), we recently found that stress-induced reductions in social approach and increases in social vigilance are mediated by oxytocin within the BNST. Oxytocin is an important regulator of social behaviors (15–18), and it has been hypothesized that oxytocin increases the salience of both positive and negative social stimuli (19) through distinct neural circuits (20). In female California mice, social defeat induces hyperactivity of oxytocin neurons in medioventral BNST (BNSTmv) up to 10 wk after the last episode of social defeat (21). Pharmacological blockade of oxytocin receptors (OTRs) in the anteromedial BNST (BNSTam), but not the ventral striatum, prevents stress-induced decreases in social approach and increases vigilance (13).
An outstanding question is whether oxytocin produced within the BNST is necessary for stress-induced social deficits. Here we use oligonucleotide analogs to inhibit oxytocin synthesis, viral tracing to identify axonal projections of BNSTmv oxytocin neurons, and site-specific infusion of oxytocin to show that oxytocin produced within the BNST is key in mediating social avoidance and vigilance. Thus, while oxytocin acting in hypothalamic–mesolimbic circuits promotes social approach (22–24), our data in the BNST support the hypothesis that divergent effects of oxytocin on social approach are mediated by discrete brain regions (19, 20, 25).
Results
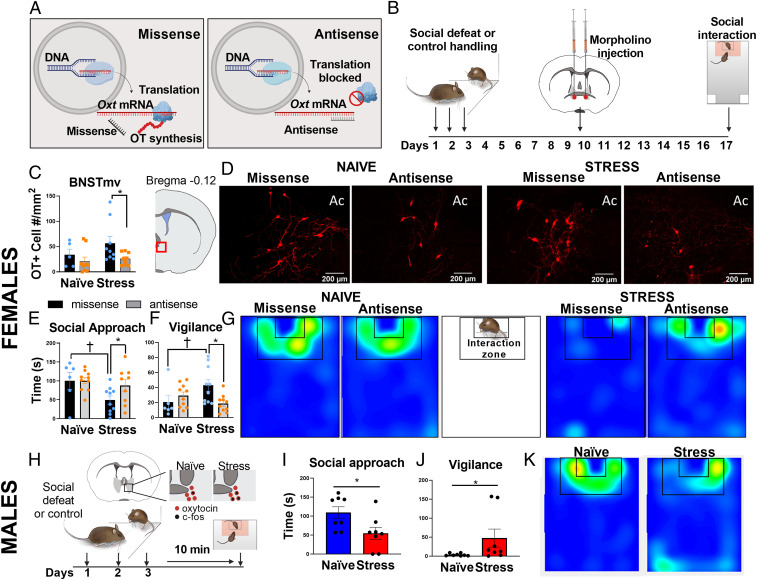
Oxytocin Antisense in BNSTmv Prevents Stress-Induced Changes in Social Approach and Vigilance in Female California Mice.
Social defeat increased oxytocin mRNA and oxytocin/c-fos colocalizations in cells of the BNSTmv in female but not male California mice following a social interaction test (21). These phenotypes were accompanied by reduced social approach and increased vigilance and are independent of estrous stage (13, 26–28). To test whether stress-induced oxytocin synthesis within the BNSTmv is necessary for stress-induced behavior changes, we made a single infusion of vivo-morpholinos targeting Oxt mRNA (antisense) or missense into the BNSTmv (Fig. 1A) of female California mice that were randomly assigned to social defeat or control conditions (Fig. 1B). There was a main effect of antisense to reduce the number of oxytocin immunoreactive cells in the BNSTmv versus missense controls (F1,28 = 5.4, P = 0.02, Fig. 1 C and D). Planned comparisons revealed that compared to missense, antisense reduced oxytocin immunoreactive cells in stressed (P = 0.03, d = 1), but not in control females. There was no evidence of neurotoxicity by morpholino treatment (SI Appendix, Fig. S1B), consistent with previous reports (29). There were no effects of BNST antisense infusions on oxytocin cells in the paraventricular nucleus (PVN, SI Appendix, Fig. S1F), indicating oxytocin knockdown in the BNST was site specific. An alternate schedule of multiple injections of a lower dose of morpholino antisense also reduced oxytocin cell number in the BNSTmv (stress missense vs. stress antisense P = 0.01, d = 1.16, SI Appendix, Fig. S1 H and I).
Fig. 1.
Oxytocin synthesis within BNSTmv is necessary for stress-induced increases in social vigilance and reduced social approach in female California mice. (A) Morpholino oligos inhibit protein synthesis by blocking the translation (29). (B) Experimental timeline of female experiments. (C) Morpholino antisense reduced the number of oxytocin-positive cells detected in BNSTmv compared to missense controls (n = 5 to 10 per group). (D) Representative images of BNSTmv oxytocin (+) cells in naïve and stressed animals receiving missense vs. antisense injections. (E and F) Effects of defeat on behavior in the social interaction test (n = 6 to 10 per group). Defeat reduced social approach and increased social vigilance in missense-treated females but not antisense-treated females. (G) Representative heatmaps for the interaction phase showing reduced time spent in the interaction zone in females receiving missense but not antisense. The lack of yellow/red hotspots in stress/missense reflects the distributed activity of the mouse. (H) Experimental time line of male California mouse experiments. Immediately after a third episode of defeat males show increased cFos/oxytocin colocalizations in the BNSTmv (21). (I) Acute effects of a third episode of defeat on social approach (n = 8 per group). (J) Acute effects of defeat on social approach. (K) Representative heatmaps for the interaction phase showing reduced time spent in the interaction zone in stressed compared to naïve males. *P < 0.05 antisense vs. missense (females) or control vs. stress (male), †P < 0.05 control vs. stress (females). Ac, anterior commissure. Data are available at https://doi.org/10.6084/m9.figshare.12811745.v1.
One week after morpholino injections, female California mice were tested in a social interaction test with a female conspecific. The effect of social defeat on social approach, measured by time spent in the interaction zone, was dependent on antisense treatment (F1,31 = 5.7, P = 0.02, Fig. 1E and Movie S1). Defeat reduced social approach in females receiving missense (P = 0.02, d = 1.1), but not in females receiving antisense. Social approach was negatively correlated with the number of BNST oxytocin cells (r = −0.38, P = 0.01, SI Appendix, Fig. S2A). For social vigilance, effects of stress were blunted by antisense treatment (stress*treatment F1,31 = 6.2, P = 0.02, Fig. 1F). Social vigilance was defined as time spent oriented toward the target mouse while outside of the interaction zone. Stress increased social vigilance in missense-treated females (P = 0.03, d = 0.96) but not antisense-treated females. There were no effects of stress or treatment on approach behavior during the acclimation phase when the target mouse was absent (SI Appendix, Fig. S1C), time spent in center (SI Appendix, Fig. S1D) or distance traveled (SI Appendix, Fig. S1E) during the open field phase. Together, these data show that stress-induced increases in oxytocin synthesis in the BNST are necessary for stress-induced social deficits in female California mice.
Male California Mice Reduced Social Approach and Increased Vigilance Immediately after Defeat.
Although the BNST is sexually dimorphic (30, 31) and known to mediate sex differences in behavior, the population of oxytocin neurons within the BNSTmv is conserved in both sexes. While male California mice do not show social deficits or increased oxytocin/c-fos colocalizations 2 wk after social defeat stress, males show increased oxytocin/c-fos colocalizations in the BNST immediately after a third episode of defeat (21). Thus, we hypothesized that acute social defeat would decrease social approach and increase vigilance in males. When stressed males were tested 10 min after a third episode of defeat (Fig. 1H), social approach was reduced (t14 = 2.46, P = 0.02, d = 1.23, Fig. 1 I and K), and vigilance was increased (t14 = 2.36, P = 0.03, d = 1.18, Fig. 1 J and K) compared to naïve males. There were no effects of defeat on approach behavior when the target mouse was absent (SI Appendix, Fig. S3B) or in the open field (SI Appendix, Fig. S3 C and D). These results implicate oxytocin in the BNST as a potential modulator of social approach in males as well as females. Next, we assessed whether stress effects on the BNST extend to an alternate model species.
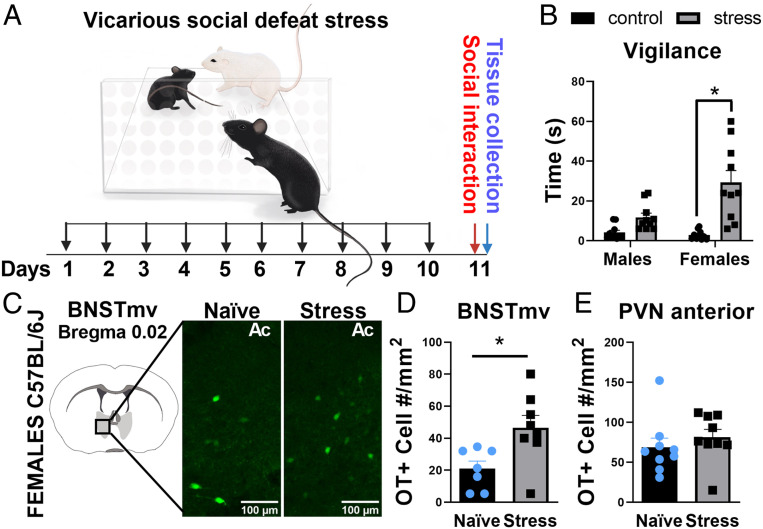
In Female C57BL/6J, Vicarious Social Stress Increases Social Vigilance and BNST Oxytocin Cell Number.
Low aggression in female C57BL/6J mice makes conventional social defeat protocols impractical (but see ref. 32). Vicarious social defeat stress, in which a focal mouse observes social defeat of another mouse, can induce depression- and anxiety-like behaviors in male and female C57BL/6J mice (33, 34). We used the vicarious defeat approach to examine sex-specific effects of stress on male and female C57BL/6J mice in the social interaction test (Fig. 2A). The effects of stress on social vigilance were stronger in females than males (Fig. 2B, sex*stress interaction, F1,36 = 8.2, P = 0.01). Planned comparisons revealed that vicarious defeat increased vigilance in female (P < 0.0001, d = 1.9), but not male mice (P = 0.1, d = 1.4). Based on these results, we conducted immunohistochemical analyses of oxytocin cells in female C57Ll/6J mice. The distribution of oxytocin-positive cells within the BNST was limited to ventral BNST near bregma 0.02 (Fig. 2C), similar to California mice and rats (35). Vicarious stress increased the number of oxytocin cells in the BNST (t14 = 2.721, P = 0.01, d = 1.4, Fig. 2D), but not in the PVN (Fig. 2E), comparable to previous results in female California mice. These data suggest that the impact of social stress on BNST oxytocin neurons is conserved across rodent species.
Fig. 2.
In C57BL/6J mice, vicarious social stress increases social vigilance and BNST oxytocin (OT) cell number in females. (A) Experimental timeline of vicarious defeat stress on behavior and BNSTmv oxytocin cell number. (B) Effects of vicarious social defeat on vigilance behavior (n = 10 per group). Defeat increases vigilance behavior in females, but not males. (C) Representative images of the effects of vicarious social defeat on oxytocin cells in the BNSTmv as visualized by immunohistochemistry. (D and E) Effects of vicarious defeat stress on oxytocin cell number. Stress significantly increased oxytocin cell number in BNST (n = 7 to 8 per group), but not PVN (n = 9 per group). *P < 0.05. Data are available at https://doi.org/10.6084/m9.figshare.12811760.v1.
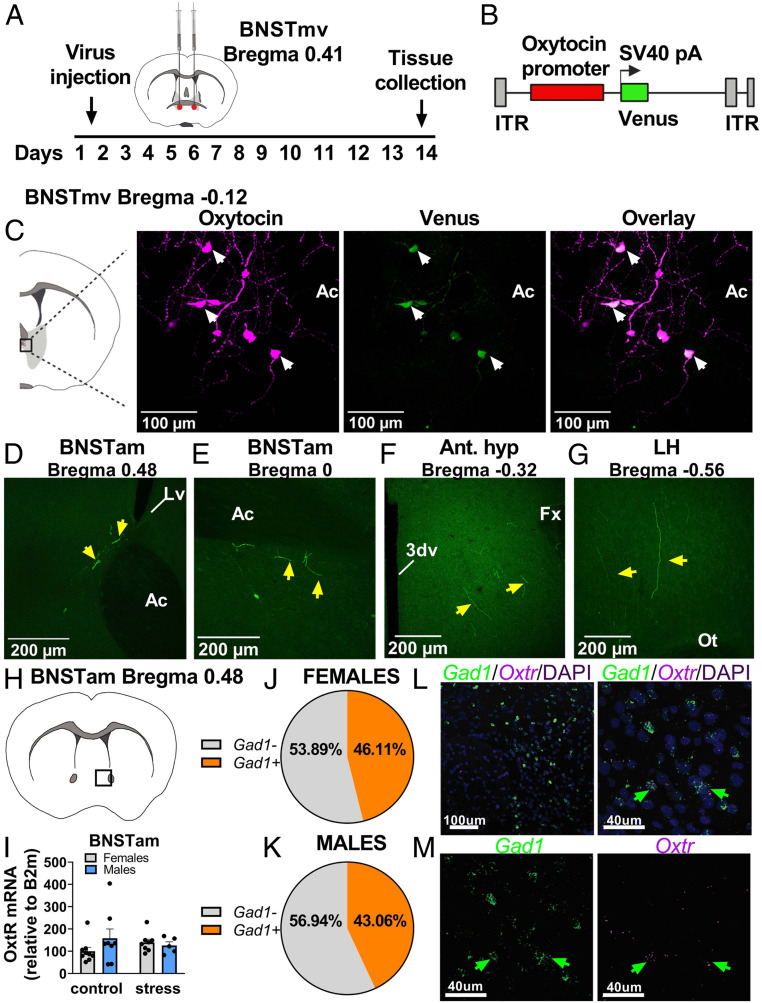
Oxytocin Cells in the BNSTmv Project to the Anteromedial BNST in California Mice.
To identify the anatomical projections of oxytocin neurons in the BNSTmv, we used an adeno-associated virus (AAV) construct expressing Venus under the control of a mouse oxytocin promoter (36) in male and female California mice (OTpr Venus, Fig. 3 A and B). First, we injected the OTpr Venus into BNST and PVN, where previous work shows it expresses specifically in oxytocin neurons (36). Immunohistochemistry in the BNST (Fig. 3C) and the PVN (SI Appendix, Fig. S4A) showed that 63% of oxytocin immunoreactive cells expressed Venus and that all Venus-positive cells coexpressed oxytocin, demonstrating specificity of expression. To identify projections of BNSTmv oxytocin neurons, we infused OTpr Venus exclusively in BNSTmv in a different set of California mice. We found that in both males and females, BNSTmv oxytocin neurons sent fibers to more anterior areas of the BNST, including dorsal (Fig. 3D) and ventral (Fig. 3E) subregions of the BNSTam. We also observed fibers in hypothalamic areas, including anterior (Fig. 3F) and lateral (Fig. 3G) hypothalamus. The identification of fibers in the dorsal BNSTam was notable, as the activation of OTR in dorsal BNSTam is necessary for stress-induced social deficits in female California mice (13). Since BNST oxytocin neurons project to BNSTam in both sexes, we tested whether differences in expression of OTR mRNA in the BNSTam could be a contributor to sex-specific effects of social defeat stress on behavior.
Fig. 3.
Oxytocin cell bodies in BNSTmv send projections to anteromedial BNST. (A) Experimental timeline for viral tracing of BNSTmv oxytocin neurons. (B) AAV construct. (C) Representative images showing colocalization of oxytocin and Venus in the BNSTmv. (D–G) When Venus expression was exclusively limited to BNSTmv neurons, Venus+ fibers were detected in hypothalamic nuclei and anterior BNST, including BNSTam. (H) Diagram showing localization of samples taken for gene expression analyses. (I) There were no differences in Oxtr expression in BNSTam (n = 5 to 8 per group). (J and K) Circle chart representing percentage of Oxtr+/Gad+ cells present in the BNSTam of males and females. (L and M) Representative photomicrographs of Gad1/Oxtr/DAPI fluorescent in situ hybridization in BNSTam. Ac, anterior commissure; Lv, lateral ventricle; 3dv, third ventricle; Fx, fornix; and Ot, optic tract. Data are available at https://doi.org/10.6084/m9.figshare.12811787.v1.
About Half of BNSTam Oxtr Neurons Coexpress Inhibitory Markers in California Mice.
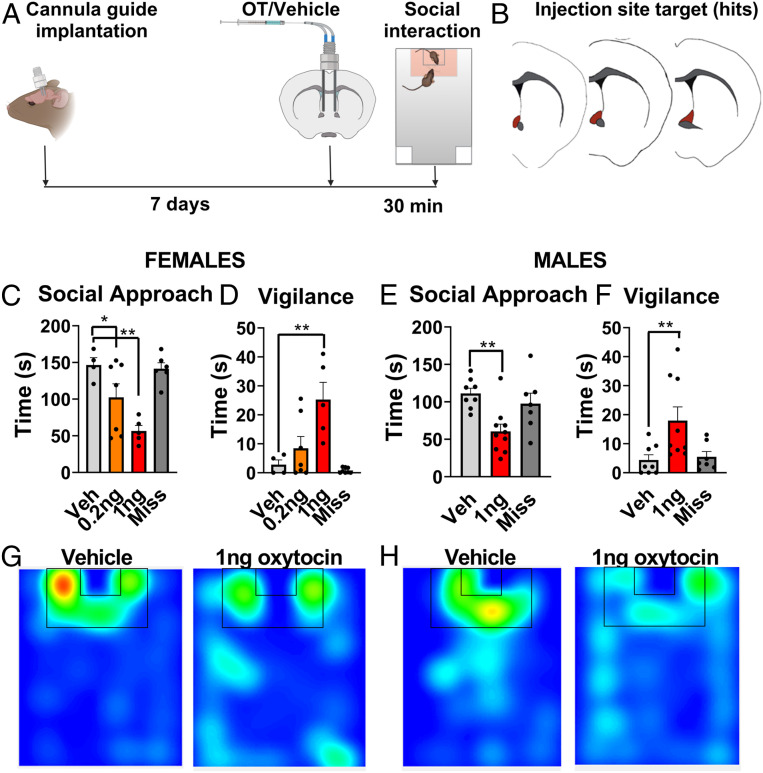
First, we used real-time PCR to assess whether there were basal or stress-induced sex differences in Oxtr expression within the BNSTam. We found no differences (Fig. 3 H and I). These results are consistent with previous autoradiography studies showing no effects of stress on OTR binding in the BNSTam in California mice (13). Next, we employed in situ hybridization using probes against Oxtr, Gad1, and vGlut2 to assess cell types of Oxtr+ in the BNSTam. About half (46% females, 43% males) of the Oxtr+ cells in the BNSTam coexpressed Gad1+ (Fig. 3 J–M). We found no vGlut2+ cells in the BNSTam, although there were vGlut2+ cells in the adjacent lateral septum (SI Appendix, Fig. S4B). We next tested whether administration of oxytocin within the BNSTam is sufficient to reduce social approach and increase vigilance in stress naïve female and male California mice (Fig. 4A).
Fig. 4.
Oxytocin infusion within BNSTam induces social vigilance in stress naïve females and males. (A) Experimental timeline for assessing effects of oxytocin injection in BNSTam. (B) Orange outlines of BNSTam where injections were considered a hit. (C and D) Effects of 0.2 ng or 1 ng injections of oxytocin within BNSTam on female behavior in the social interaction test (n = 5 to 7 per group). Oxytocin dose dependently reduced social approach. (E and F) Effects of 1-ng injection of oxytocin within BNSTam on male behavior in the social interaction test (n = 6 to 9 per group). Oxytocin reduced social approach and increased vigilance in males. (G and H) Representative heatmaps for the interaction phase showing reduced time spent in the interaction zone in naïve males and females receiving oxytocin injections in BNSTam. *P < 0.05, **P < 0.01 vs. vehicle. Data are available at https://doi.org/10.6084/m9.figshare.12811763.v1.
Oxytocin Infusion within the BNSTam Induces Social Vigilance in Stress Naïve Female and Male California Mice.
In stress naïve females, treatment with oxytocin decreased social approach (F3,21 = 9.5, P < 0.001, Fig. 4 B and G). Compared to vehicle, both 0.2 ng (P = 0.03, d = 1.1) and 1.0 ng (P < 0.001, d = 4.8) infusions of oxytocin reduced social approach in a dose-dependent fashion. Treatment with oxytocin also increased social vigilance in females (F3,21 = 8.8, P = 0.001, Fig. 4D). The 1.0 ng (P < 0.001, d = 2.3) but not the 0.2 ng dose increased vigilance in stress naïve females compared to vehicle. There were no effects of oxytocin injections placed outside the BNSTam (miss) on social approach or vigilance. In stress naïve males, oxytocin reduced social approach (F2,21 = 6.7, P < 0.001), with 1.0 ng of oxytocin reducing social approach compared to vehicle (P < 0.001, d = 1.93 Fig. 4 E and H). Similarly, oxytocin also increased social vigilance (F2,21 = 5.3, P = 0.01, Fig. 4F), with 1.0 ng of oxytocin increasing vigilance compared to vehicle (P < 0.001, d = 1.27). Oxytocin receptors in the adjacent nucleus accumbens promote social approach in California mice (24), suggesting that behavioral effects are unlikely to be due to spread of oxytocin to regions adjacent to BNSTam. There were no effects of oxytocin injections placed outside the BNSTam on social approach or vigilance. Together, our results suggest that the oxytocin-dependent regulation of social vigilance by the BNST is intact in both sexes, and that sex differences in behavioral responses to social stress (13, 37) are mediated by sex differences in the release of oxytocin.
Discussion
The social salience hypothesis proposes that oxytocin increases attention and motivational responses to positive and negative social contexts (19, 38), which can account for why oxytocin has both anxiolytic (36, 39–41) and anxiogenic (42–44) effects. Nonetheless, the underlying mechanisms have remained elusive. Here we found that oxytocin, produced and acting within the BNST, drives stress-induced changes in social behavior. Antisense injection in BNSTmv showed that oxytocin synthesis within BNST is necessary for long-term decreases in social approach and increased vigilance folllowing social defeat in female California mice. Viral tracing showed that BNSTmv oxytocin neurons project to the BNSTam, a region in which OTR inhibition is sufficient to prevent stress-induced social deficits (13). Finally, oxytocin infusion into the BNSTam was sufficient to reduce social approach and increase vigilance in stress naïve male and female California mice. These data suggest that stress-sensitive oxytocin neurons located in the BNSTmv project to the BNSTam, where oxytocin acts to drive a social anxiety-related phenotype. These data demonstrate that extrahypothalamic oxytocin synthesis can modulate behavior.
Oxytocin Produced by BNST Neurons Promotes Social Vigilance and Reduces Social Approach.
The BNST modulates anxiety-related behaviors (8, 45), and recent imaging data in humans showed that BNST activity is altered in patients suffering from SAD (9, 10). Inhibition of OTR in the BNST reduces the expression of anxiety-like behaviors (43, 46), but the source of oxytocin was not clear. Using antisense knockdown, we show that oxytocin synthesized in neurons within the BNST is necessary for stress-induced social vigilance and reduced social approach. Although the presence of oxytocin cells in the BNST has been described in multiple species (35, 44, 47), here we demonstrate a behavorial function of these neurons. In previous work, social defeat increased expression of oxytocin mRNA and oxytocin-positive cells within the BNST of female California mice (21), and here we observe similar effects in female C57BL/6J mice. In female California mice, the effects of social defeat on the reactivity of BNST oxytocin neurons (as measured via oxytocin/c-fos colocalizations) are observed between 2 and 10 wk after the last episode of defeat (21). Similarly, the effects of defeat on social approach in females are stronger several weeks after defeat compared to 1 d following defeat (37). In male California mice, social defeat induces an acute increase in activity of oxytocin neurons, but this effect is transitory (21). We observed that immediately after social defeat, male California mice reduced social approach and increased vigilance. This effect is also transitory. An intriguing question is why the long-term effects of defeat on BNST oxytocin neurons are stronger in female California mice compared to males.
One possible molecule involved in female-biased long-term effects of stress is brain derived neurotrophic factor (BDNF), which modulates neuronal plasticity (48). Two weeks after social defeat, female but not male California mice show decreased social approach and increased BDNF protein within the BNST. Selective inhibition of tyrosine-related kinase B receptor (TrkB), an important receptor for BDNF, restores social approach in stressed female California mice (26). Increased BDNF-TrkB signaling may support the effects of oxytocin. In the hypothalamus, oxytocin-dependent maternal behavior is dependent on BDNF-TrkB signaling (49). Enhancement of oxytocin action by BDNF-TrkB signaling could occur through increased long-term potentiation in oxytocin neurons, as seen in other neuron types through pre- and postsynaptic mechanisms (50). Additionally, BDNF could increase synapse formation, which occurs in hypothalamic neurons during the peripartum period resulting in increased excitability (51, 52). These mechanisms might contribute to increased activity of BNST oxytocin neurons in females several weeks after social defeat. In male California mice, the effects of defeat on the activity of oxytocin neurons in the BNST are strong in the short term but, unlike female California mice, they do not persist for weeks. Immediately after a third episode of defeat, oxytocin/c-fos colocalizations in the BNSTmv are elevated in male California mice (21) and C57BL/6J mice (44), suggesting that further investigation of the behavioral function of these neurons in male California mice and C57BL/6J mice is warranted. Next, we explored the descending pathways of BNST oxytocin neurons.
BNST Oxytocin Neurons Project to Nuclei that Control Defensive Behavior and Social Vigilance.
Viral tracing experiments of BNST oxytocin neurons in male and female California mice revealed the presence of fibers originating from oxytocin neurons within the BNST in the anterior hypothalamus, and lateral hypothalamus, consistent with previous tracing experiments (53, 54) that were not cell-type specific. Although we do not know whether BNST oxytocin neurons synapse with neurons within these regions, there is evidence for nonsynaptic, periaxonal, or en passant release of oxytocin (55, 56). Thus, a nucleus containing only fibers of passage could receive oxytocin in the absence of axon terminals. Interestingly, there were many fibers in the BNSTam, which is a key regulator of social anxiety-related behavior. Social anxiety phenotypes are associated with increased activity in the BNSTam (57, 58), and pharmacological inhibition of OTR in the BNSTam restores normative social approach behavior following social defeat (13). Venus-positive fibers originating from the BNSTmv were present in the BNSTam of both sexes, which supports the hypothesis that OTR regulation of social vigilance is intact in male and female California mice. Indeed, infusion of oxytocin into the BNSTam reduced social approach and increased social vigilance in both males and females naïve to social defeat. These results suggest that sex differences in stress-induced vigilance are mediated by sex-specific temporal activation and plasticity of oxytocinergic BNST neurons.
In California mice, about 50% of Oxtr+ cells in the BNSTam coexpress Gad1+, suggesting that oxytocin may act on inhibitory neurons in the BNST, as has been observed in the central nucleus of amygdala (36). Many Oxtr+ cells did not express Gad1+. There were no vGlut2-positive cells observed in the BNSTam, so Oxtr+/Gad1 cells may instead express Gad2 (59) or represent another type of neuronal or nonneuronal cells such as glia (60), which can directly respond to oxytocin and modulate behavior (61). Future single-cell analysis of the BNSTam will be highly informative in understanding how activation of Oxtr modulates social approach and vigilance.
Functional Implications.
Previous work indicated that oxytocin acting in the nucleus accumbens (22, 24), ventral tegmental area (23, 62), prefrontal cortex (63), hypothalamus (64), and central amygdala (65) promotes social approach. However, oxytocin can also reduce social approach and facilitate fear learning toward predictable cues. A key question is how specific brain regions could be distinctly activated considering that hypothalamic oxytocin neurons project widely throughout the brain (56, 66). Our results show that stress-induced intra-BNST oxytocin synthesis facilitates social anxiety-related behaviors. These results do not exclude a role of the PVN for inducing social vigilance, as volume diffusion of oxytocin through extracellular space or the ventricular system gives the PVN an extended reach (67). However, the key role that BNST oxytocin neurons play in long-term changes in female-biased social avoidance and vigilance suggests that these cells may be related to symptoms associated with social anxiety disorder (68) and depression (69), which are more common in women than men. In sum, data in multiple species support an important role of the BNST in social anxiety-related behaviors, and our work shows that oxytocin signaling may be a key underlying mechanism.
Materials and Methods
Experiments in California Mice.
Animals.
All studies were approved by the Institutional Animal Care and Use Committees at UC Davis and UTEP and conformed to NIH guidelines. California mice (P. californicus) were bred at the UC Davis and group housed after weaning in clear polypropylene cages containing sanichip bedding, nestlets, and Enviro-dri. Mice were housed on a 16L:8D light:dark cycle (lights off at 1400 hours), and water and food (Harlan Teklad 2016) were provided ad libitum. All experimental mice were at least 90 d old.
Social defeat stress.
Mice were randomly assigned to control handling or social defeat for 3 consecutive days as described previously (26). Control mice were placed in a clean cage for 7 min. Mice assigned to social defeat were placed in the home cage of an aggressive same-sex mouse. Each episode lasted 7 min or until the resident attacked the focal mouse seven times (whichever occurred first). No animal was physically injured. Immediately after defeat or control handling, mice were returned to their home cage.
Social interaction test.
This test consisted of three phases lasting 180 s each. In the open field phase, the focal mouse was introduced into an empty arena (89 × 63 × 60 cm) (13). In the acclimation phase, an empty wire cage was placed against one wall of the arena. In the interaction phase, an unfamiliar same-sex adult mouse was placed inside the wire cage. Total distance traveled and time spent in 1) the center of the arena (within 8 cm of the sides and within a center zone located 14 cm from the sides) and 2) within 8 cm of the cage (interaction zone) were recorded and analyzed via an automated video-tracking system (Anymaze, Stoelting). Vigilance behavior, defined as time spent with head oriented toward the target mouse while outside of the interaction zone, was manually analyzed by an observer blind to treatment.
Inhibition of oxytocin synthesis within the BNST.
To inhibit oxytocin synthesis, we used vivo-morpholinos (Genetools, LCC), which can prevent translation of a target sequence by blocking the translation initiation complex. The sequence used to block Oxytocin mRNA (antisense) was 5′-TTGGTGTTCTGAGTCCTCGATCC-3′, and 5′-TTCGTCTTCTGACTCCTCCATGC-3′ was used as a missense control. One week after the last episode of control handling or social defeat, animals were randomly assigned to receive one bilateral 0.2-μL injection of 100 pM morpholino missense or antisense aimed a little anterior to medioventral BNST (BNSTmv, anterior-posterior: 0.39, medial-lateral: 1.1, dorsal-ventral: −5.85). These coordinates were selected to minimize chances of spreading to the hypothalamic PVN. Animals were allowed to recover for 1 wk before behavior testing. To confirm successful local inhibition of Oxytocin translation, 40-µm sections of the BNSTmv (three slices per animal) and adjacent PVN (four slices per animal) were stained with an oxytocin antibody (SI Appendix, Table S1). Nissl staining was used to assess tissue integrity (29). For reagents and concentrations used, see SI Appendix, Table S1.
Oxytocin circuit visualization using recombinant AAV (rAAV).
To identify projections of medioventral BNST oxytocin neurons, we used recombinant adeno-associated virus expressing Venus equipped with 2.6K mouse oxytocin promoter (AAV-OTpr-Venus) developed by the V.G. laboratory (36). This construct has been successfully used for anatomical studies of oxytocin circuits in various rodent species including rats (36, 64) and prairie voles (70). Males and females received a bilateral 40-nL injection of AAV-OTpr-Venus (∼1010 copies per µL) into the BNSTmv. We chose to inject the virus anterior to the oxytocin neuron BNST population (AP: 0.41, ML: 1.1, DV: −5.85) to minimize chances of also infecting hypothalamic oxytocin neurons of the PVN. To assess successful anatomical targeting and specificity of infection, immunohistochemistry was used to detect Venus and oxytocin-positive cells in 40-μm brain sections from the BNSTmv and PVN (see SI Appendix, Table S1 for antibodies). The 1-μm z-stack images were then obtained using an Olympus FV1000 laser point-scanning confocal microscope.
Quantitative real-time PCR (qPCR) and fluorescent in situ hybridization.
To assess the effects of social defeat stress on Oxtr expression in the anterior BNST, we used qPCR (SI Appendix, Table S3). Next, to assess whether the cells that express Oxtr in the BNSTam are excitatory or inhibitory, we performed fluorescent in situ hybridization (FISH) studies (ACDBio RNAscope) using probes to detect Oxtr, Gad1, or vGlut based on California mouse mRNA sequences (for full details see SI Appendix, Supplementary Methods). We used an Olympus FV1000 laser point-scanning confocal microscope to collect 20× 1-μm z-stack images. Images where then analyzed for total expression and colocalization of Oxtr/Gad1 or Oxtr/vGlu2 using Fiji.
Effects of exogenous oxytocin infusion within BNSTam.
To test whether oxytocin acting within the BNSTam is sufficient to induce social avoidance and vigilance, we injected one of two doses of synthetic oxytocin (Tocris) into the BNSTam of females and males naïve to stress. Mice were implanted with bilateral guide cannula (Plastics One) using these coordinates: AP: +0.45, ML: ±1.0, DV: +4.6 (13). Mice were single housed after surgery. After 7 d, mice were randomly assigned to receive bilateral 200-nL infusions of either artificial cerebrospinal fluid (aCSF), 0.2 ng (females only), or 1 ng (males and females) of oxytocin using internal guides that projected 1 mm past the guides. Thirty minutes later, each mouse was tested in the social interaction test. Injections were considered hits if they were placed within bregma 0.45 and 0.12 of the BNSTam-based work in rats (71) and a California mouse brain atlas (Fig. 4B, brainmaps.org).
Experiments in C57BL/6J Mice.
Animals.
All studies were approved by the IACUC at UTEP and conformed to NIH guidelines. Eight‐week old female and male C57BL/6J mice (Mus musculus) and retired CD1 male breeder mice (M. musculus) were purchased from Charles River. All mice were housed in standard polypropylene cages containing wood shavings. Experimental C57BL/6 mice were group housed (three to four same-sex animals), while CD1 mice, used as aggressors for vicarious defeat, were single housed. Mice were maintained in a colony room with a 12‐h light/dark cycle (lights on at 7:00 h), 22 °C, and with access to food (Harlan Teklad 7912) and water ad libitum.
Vicarious defeat (C57BL/6J mice).
Male and female mice were randomly assigned to control handling or vicarious social defeat stress as described previously (33). CD1 aggressors were housed in one side of a cage containing clear perforated acrylic glass dividers. Defeated male C57BL/6J mice were placed for 10 min in the same side as the CD1 aggressor, where they were physically attacked. The defeated males were not used for this study. Meanwhile, a male or female C57BL/6J mouse was placed in the neighboring side, experiencing the physical aggression vicariously through visual, olfactory, and auditory cues. Following each defeat episode, vicariously defeated animals were housed for 24 h next to a novel CD1 aggressor until the next stress episode. This was repeated for 10 consecutive days. Control mice were housed in each side of a divided cage (two C57BL/6J mice per cage, same sex) and handled daily. Immediately after the last episode of vicarious defeat, all mice were single housed. The social interaction test and then brain collection was performed 24 h later.
Social interaction test (C57BL/6J mice).
The social interaction test consisted of two phases lasting 150 s each (33). In the first phase (target absent), the animal was placed into an empty arena (40 cm × 40 cm × 40 cm) containing a circular wire cage on one side and allowed to freely explore. In the second phase (target present), an unfamiliar male CD1 mouse was placed in the wire cage. Behavior was recorded via an automated video-tracking system (EthovisionXT; Noldus). Vigilance behavior, defined as time spent with head oriented toward the target mouse while outside of the interaction zone (8-cm-wide corridor surrounding the wire cage), was manually analyzed by an observer blind to treatment.
Oxytocin immunohistochemistry in C57BL/6J mice exposed to vicarious defeat.
To assess whether vicarious defeat induces changes in oxytocin activity within the BNSTmv, 40-µm brain sections of control and stressed females were stained for oxytocin (for antibodies used, see SI Appendix, Table S2).
Statistical analyses (all experiments).
All analyses were done using R software. Two-way ANOVAS were used to analyze effects of morpholino injections on female behavior and oxytocin neuron expression (treatment*stress), as well as the effects of vicarious defeat stress on C57BL/6 mice behavior (sex*stress). t tests were used to assess acute effects of social defeat stress on male California mouse behavior and oxytocin+ cell detection in the BNSTmv of female C57BL/6. Male California mouse vigilance behavior was log transformed for analysis due to heterogeneous variance. Planned comparisons (package lsmeans in R [R Project for Statistical Computing, version 3.0.3, Vienna, Austria], Bonferroni, 0.95 confidence interval) were used if ANOVAS showed significant main or interaction effects. Effect size is reported as Cohen’s d.
Supplementary Material
Acknowledgments
We thank C. J. Clayton for animal care and I. Brust-Mascher for imaging. This work was supported by Becas Chile Comisión Nacional de Investigación Científica y Tecnológica (to N.D.-W.); SC3GM130467 (to S.D.I.); Human Frontiers Science Program Award RGP0019/2015, Deutsche Forschungsgemeinschaft (DFG) Collaborative Research Center (SFB) 1158-2; DFG Grants GR 3619/16-1, GR 3619/4-1, SFB 1158, and Swiss National Science Foundation-DFG grant GR 3619/8-1; and Fritz Thyssen Foundation grant 10.16.2.018 MN (to V.G.); and NIH R01 MH121829-01 (to B.C.T.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2011890117/-/DCSupplemental.
Data Availability.
Data have been deposited in Figshare (10.6084/m9.figshare.12811745, 10.6084/m9.figshare.12811760, 10.6084/m9.figshare.12811787, and 10.6084/m9.figshare.12811763).
References
- 1.Beesdo-Baum K. et al., The natural course of social anxiety disorder among adolescents and young adults. Acta Psychiatr. Scand. 126, 411–425 (2012). [DOI] [PubMed] [Google Scholar]
- 2.Stein D. J. et al.; WHO World Mental Health Survey Collaborators , The cross-national epidemiology of social anxiety disorder: Data from the World mental health survey initiative. BMC Med. 15, 143 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Psychiatric Organization , Diagnostic and Statistical Manual of Mental Disorders (DSM-5), (American Psychiatric Pub, 2013). [Google Scholar]
- 4.Ipser J. C., Kariuki C. M., Stein D. J., Pharmacotherapy for social anxiety disorder: A systematic review. Expert Rev. Neurother. 8, 235–257 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Liebowitz M. R., Gelenberg A. J., Munjack D., Venlafaxine extended release vs placebo and paroxetine in social anxiety disorder. Arch. Gen. Psychiatry 62, 190–198 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Avery S. N., Clauss J. A., Blackford J. U., The human BNST: Functional role in anxiety and addiction. Neuropsychopharmacology 41, 126–141 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goode T. D., Maren S., Role of the bed nucleus of the stria terminalis in aversive learning and memory. Learn. Mem. 24, 480–491 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lebow M. A., Chen A., Overshadowed by the amygdala: The bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol. Psychiatry 21, 450–463 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Figel B. et al., Phasic amygdala and BNST activation during the anticipation of temporally unpredictable social observation in social anxiety disorder patients. Neuroimage Clin. 22, 101735 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clauss J. A., Avery S. N., Benningfield M. M., Blackford J. U., Social anxiety is associated with BNST response to unpredictability. Depress. Anxiety 36, 666–675 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heimberg R. G., Social Phobia: Diagnosis, Assessment, and Treatment, (Guilford Press, 1995). [Google Scholar]
- 12.Spence S. H., Rapee R. M., The etiology of social anxiety disorder: An evidence-based model. Behav. Res. Ther. 86, 50–67 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Duque-Wilckens N. et al., Oxytocin receptors in the anteromedial bed nucleus of the stria terminalis promote stress-induced social avoidance in female California mice. Biol. Psychiatry 83, 203–213 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newman E. L. et al., Fighting females: Neural and behavioral consequences of social defeat stress in female mice. Biol. Psychiatry 86, 657–668 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bosch O. J., Young L. J., Oxytocin and social relationships: From attachment to bond disruption. Curr. Top. Behav. Neurosci. 35, 97–117 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grinevich V., Desarménien M. G., Chini B., Tauber M., Muscatelli F., Ontogenesis of oxytocin pathways in the mammalian brain: Late maturation and psychosocial disorders. Front. Neuroanat. 8, 164 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rogers-Carter M. M. et al., Insular cortex mediates approach and avoidance responses to social affective stimuli. Nat. Neurosci. 21, 404–414 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veenema A. H., Neumann I. D., Central vasopressin and oxytocin release: Regulation of complex social behaviours. Prog. Brain Res. 170, 261–276 (2008). [DOI] [PubMed] [Google Scholar]
- 19.Shamay-Tsoory S. G., Abu-Akel A., The social salience hypothesis of oxytocin. Biol. Psychiatry 79, 194–202 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Steinman M. Q., Duque-Wilckens N., Trainor B. C., Complementary neural circuits for divergent effects of oxytocin: Social approach versus social anxiety. Biol. Psychiatry 85, 792–801 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steinman M. Q. et al., Sex-specific effects of stress on oxytocin neurons correspond with responses to intranasal oxytocin. Biol. Psychiatry 80, 406–414 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dölen G., Darvishzadeh A., Huang K. W., Malenka R. C., Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 501, 179–184 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song Z., Borland J. M., Larkin T. E., O’Malley M., Albers H. E., Activation of oxytocin receptors, but not arginine-vasopressin V1a receptors, in the ventral tegmental area of male Syrian hamsters is essential for the reward-like properties of social interactions. Psychoneuroendocrinology 74, 164–172 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams A. V. et al., Social approach and social vigilance are differentially regulated by oxytocin receptors in the nucleus accumbens. Neuropsychopharmacology 45, 1423–1430 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grinevich V., Knobloch-Bollmann H. S., Eliava M., Busnelli M., Chini B., Assembling the puzzle: Pathways of oxytocin signaling in the brain. Biol. Psychiatry 79, 155–164 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Greenberg G. D. et al., Sex differences in stress-induced social withdrawal: Role of brain derived neurotrophic factor in the bed nucleus of the stria terminalis. Front. Behav. Neurosci. 7, 223 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trainor B. C. et al., Sex differences in stress-induced social withdrawal: Independence from adult gonadal hormones and inhibition of female phenotype by corncob bedding. Horm. Behav. 63, 543–550 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams A. V. et al., Acute inhibition of kappa opioid receptors before stress blocks depression-like behaviors in California mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 86, 166–174 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reissner K. J. et al., Use of vivo-morpholinos for control of protein expression in the adult rat brain. J. Neurosci. Methods 203, 354–360 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allen L. S., Gorski R. A., Sex difference in the bed nucleus of the stria terminalis of the human brain. J. Comp. Neurol. 302, 697–706 (1990). [DOI] [PubMed] [Google Scholar]
- 31.Campi K. L., Jameson C. E., Trainor B. C., Sexual dimorphism in the brain of the monogamous California mouse (Peromyscus californicus). Brain Behav. Evol. 81, 236–249 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takahashi A. et al., Establishment of a repeated social defeat stress model in female mice. Sci. Rep. 7, 12838 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iñiguez S. D. et al., Vicarious social defeat strress induces depression-related outcomes in female mice. Biol. Psychiatry 83, 9–17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warren B. L., Mazei-Robison M., Robison A. J., Iñiguez S. D., Can I get a witness? Using vicarious defeat stress to study mood-related illnesses in traditionally understudied populations. Biolog. Psychiatry 88, 381–391 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DiBenedictis B. T., Nussbaum E. R., Cheung H. K., Veenema A. H., Quantitative mapping reveals age and sex differences in vasopressin, but not oxytocin, immunoreactivity in the rat social behavior neural network. J. Comp. Neurol. 525, 2549–2570 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knobloch H. S. et al., Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 73, 553–566 (2012). [DOI] [PubMed] [Google Scholar]
- 37.Trainor B. C. et al., Sex differences in social interaction behavior following social defeat stress in the monogamous California mouse (Peromyscus californicus). PLoS One 6, e17405 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bartz J. A., Zaki J., Bolger N., Ochsner K. N., Social effects of oxytocin in humans: context and person matter. Trends Cogn. Sci. 15, 301–309 (2011). [DOI] [PubMed] [Google Scholar]
- 39.Blume A. et al., Oxytocin reduces anxiety via ERK1/2 activation: Local effect within the rat hypothalamic paraventricular nucleus. Eur. J. Neurosci. 27, 1947–1956 (2008). [DOI] [PubMed] [Google Scholar]
- 40.Domes G. et al., Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol. Psychiatry 62, 1187–1190 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Kirsch P. et al., Oxytocin modulates neural circuitry for social cognition and fear in humans. J. Neurosci. 25, 11489–11493 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eckstein M. et al., Oxytocin facilitates the sensation of social stress. Hum. Brain Mapp. 35, 4741–4750 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martinon D. et al., Oxytocin receptors in the dorsolateral bed nucleus of the stria terminalis (BNST) bias fear learning toward temporally predictable cued fear. Transl. Psychiatry 9, 140 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nasanbuyan N. et al., Oxytocin-oxytocin receptor systems facilitate social defeat posture in male mice. Endocrinology 159, 763–775 (2018). [DOI] [PubMed] [Google Scholar]
- 45.Fox A. S., Shackman A. J., The central extended amygdala in fear and anxiety: Closing the gap between mechanistic and neuroimaging research. Neurosci. Lett. 693, 58–67 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moaddab M., Dabrowska J., Oxytocin receptor neurotransmission in the dorsolateral bed nucleus of the stria terminalis facilitates the acquisition of cued fear in the fear-potentiated startle paradigm in rats. Neuropharmacology 121, 130–139 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caffé A. R., Van Ryen P. C., Van der Woude T. P., Van Leeuwen F. W., Vasopressin and oxytocin systems in the brain and upper spinal cord of Macaca fascicularis. J. Comp. Neurol. 287, 302–325 (1989). [DOI] [PubMed] [Google Scholar]
- 48.Binder D. K., Scharfman H. E., Brain-derived neurotrophic factor. Growth Factors 22, 123–131 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maynard K. R. et al., BDNF-TrkB signaling in oxytocin neurons contributes to maternal behavior. eLife 7, e33676 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park H., Poo M. M., Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 14, 7–23 (2013). [DOI] [PubMed] [Google Scholar]
- 51.Hatton G. I., Emerging concepts of structure-function dynamics in adult brain: The hypothalamo-neurohypophysial system. Prog. Neurobiol. 34, 437–504 (1990). [DOI] [PubMed] [Google Scholar]
- 52.Stern J. E., Armstrong W. E., Reorganization of the dendritic trees of oxytocin and vasopressin neurons of the rat supraoptic nucleus during lactation. J. Neurosci. 18, 841–853 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Delville Y., De Vries G. J., Ferris C. F., Neural connections of the anterior hypothalamus and agonistic behavior in golden hamsters. Brain Behav. Evol. 55, 53–76 (2000). [DOI] [PubMed] [Google Scholar]
- 54.Dong H.-W., Swanson L. W., Organization of axonal projections from the anterolateral area of the bed nuclei of the stria terminalis. J. Comp. Neurol. 468, 277–298 (2004). [DOI] [PubMed] [Google Scholar]
- 55.Chini B., Verhage M., Grinevich V., The action radius of oxytocin release in the mammalian CNS: From single vesicles to behavior. Trends Pharmacol. Sci. 38, 982–991 (2017). [DOI] [PubMed] [Google Scholar]
- 56.Knobloch H. S., Grinevich V., Evolution of oxytocin pathways in the brain of vertebrates. Front. Behav. Neurosci. 8, 31 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kollack-Walker S., Watson S. J., Akil H., Social stress in hamsters: Defeat activates specific neurocircuits within the brain. J. Neurosci. 17, 8842–8855 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Markham C. M., Norvelle A., Huhman K. L., Role of the bed nucleus of the stria terminalis in the acquisition and expression of conditioned defeat in Syrian hamsters. Behav. Brain Res. 198, 69–73 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Welch J. D. et al., Single-cell multi-omic integration compares and contrasts features of brain cell identity. Cell 177, 1873–1887.e17 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mittaud P., Labourdette G., Zingg H., Guenot-Di Scala D., Neurons modulate oxytocin receptor expression in rat cultured astrocytes: Involvement of TGF-β and membrane components. Glia 37, 169–177 (2002). [DOI] [PubMed] [Google Scholar]
- 61.Wahis J., et al. , Oxytocin acts on astrocytes in the central amygdala to promote a positive emotional state. bioRxiv:2020.02.25.963884 (2020). Posted 26 February 2020.
- 62.Hung L. W. et al., Gating of social reward by oxytocin in the ventral tegmental area. Science 357, 1406–1411 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tan Y. et al., Oxytocin receptors are expressed by glutamatergic prefrontal cortical neurons that selectively modulate social recognition. J. Neurosci. 39, 3249–3263 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tang Y. et al., Social touch promotes interfemale communication via activation of parvocellular oxytocin neurons. Nat. Neurosci. 23, 1125–1137 (2020). [DOI] [PubMed] [Google Scholar]
- 65.Han R. T. et al., Long-term isolation elicits depression and anxiety-related behaviors by reducing oxytocin-induced GABAergic transmission in central amygdala. Front. Mol. Neurosci. 11, 246 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grinevich V., Neumann I. D., Brain oxytocin: How puzzle stones from animal studies translate into psychiatry. Mol. Psychiatry, 10.1038/s41380-020-0802-9 (2020) Published ahead of print. [DOI] [PMC free article] [PubMed]
- 67.Landgraf R., Neumann I. D., Vasopressin and oxytocin release within the brain: A dynamic concept of multiple and variable modes of neuropeptide communication. Front. Neuroendocrinol. 25, 150–176 (2004). [DOI] [PubMed] [Google Scholar]
- 68.Asher M., Asnaani A., Aderka I. M., Gender differences in social anxiety disorder: A review. Clin. Psychol. Rev. 56, 1–12 (2017). [DOI] [PubMed] [Google Scholar]
- 69.Kessler R. C., et al. , Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch. Gen. Psychiatry 62, 593–602 (2005).Correction in: Arch. Gen. Psychiatry62, 768 (2005). [DOI] [PubMed] [Google Scholar]
- 70.Bosch O. J. et al., Oxytocin in the nucleus accumbens shell reverses CRFR2-evoked passive stress-coping after partner loss in monogamous male prairie voles. Psychoneuroendocrinology 64, 66–78 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dong H. W., Swanson L. W., Projections from bed nuclei of the stria terminalis, anteromedial area: Cerebral hemisphere integration of neuroendocrine, autonomic, and behavioral aspects of energy balance. J. Comp. Neurol. 494, 142–178 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data have been deposited in Figshare (10.6084/m9.figshare.12811745, 10.6084/m9.figshare.12811760, 10.6084/m9.figshare.12811787, and 10.6084/m9.figshare.12811763).