Significance
Liquid electrolytes with thermophysical properties analogous to solid polymers, but with exceptional liquidlike ionic conductivities, are formed spontaneously when moderate amounts (≤1 M) of inorganic salts coordinate strongly with small molecules in a conventional aprotic solvent. Specifically, we report that electrolytes composed of the cyclic liquid ether, dioxolane (DOL), and containing the simple salt LiNO3 are able to completely bypass the liquid → crystalline solid thermal transition, and to exhibit abnormally high bulk and interfacial ionic conductivities down to temperatures as low as −50 °C. Through physical, spectroscopic, and ion-transport measurements it is shown that strong interactions between LiNO3 and DOL distort bonds in DOL, couple motions of individual solvent molecules, and lower the thermodynamic activity of the electrolyte.
Keywords: electrolytes, ion transport, lithium batteries, thermal transition, coupled dynamics
Abstract
In the presence of Lewis acid salts, the cyclic ether, dioxolane (DOL), is known to undergo ring-opening polymerization inside electrochemical cells to form solid-state polymer batteries with good interfacial charge-transport properties. Here we report that LiNO3, which is unable to ring-open DOL, possesses a previously unknown ability to coordinate with and strain DOL molecules in bulk liquids, completely arresting their crystallization. The strained DOL electrolytes exhibit physical properties analogous to amorphous polymers, including a prominent glass transition, elevated moduli, and low activation entropy for ion transport, but manifest unusually high, liquidlike ionic conductivities (e.g., 1 mS/cm) at temperatures as low as −50 °C. Systematic electrochemical studies reveal that the electrolytes also promote reversible cycling of Li metal anodes with high Coulombic efficiency (CE) on both conventional planar substrates (1 mAh/cm2 over 1,000 cycles with 99.1% CE; 3 mAh/cm2 over 300 cycles with 99.2% CE) and unconventional, nonplanar/three-dimensional (3D) substrates (10 mAh/cm2 over 100 cycles with 99.3% CE). Our finding that LiNO3 promotes reversibility of Li metal electrodes in liquid DOL electrolytes by a physical mechanism provides a possible solution to a long-standing puzzle in the field about the versatility of LiNO3 salt additives for enhancing reversibility of Li metal electrodes in essentially any aprotic liquid electrolyte solvent. As a first step toward understanding practical benefits of these findings, we create functional Li||lithium iron phosphate (LFP) batteries in which LFP cathodes with high capacity (5 to 10 mAh/cm2) are paired with thin (50 μm) lithium metal anodes, and investigate their galvanostatic electrochemical cycling behaviors.
Isolating and understanding the various roles played by the electrolyte solvent in regulating ion transport in bulk liquid phases and at emergent solid-state phases (solid electrolyte interphases, SEI) formed by electrochemical reactions and polymerization of the electrolyte solvent are important in all rechargeable batteries that utilize aprotic liquid electrolytes (1–4). They are considered crucial steps toward practically relevant batteries that use alkali metals as anode to achieve high-energy and/or lower-cost portable storage of electrical energy (5–12). It is known for instance that with minimal assistance from other electrolyte components (e.g., salts, organic molecular additives, particulate fillers, etc.), some cyclic ether solvents exhibit such high stability in extended contact with working Li and Na anodes that these solvents are often used as benchmark systems for evaluating other battery components (e.g., separator, artificial SEI, and current collector architectures) that improve reversibility of the Li anode (13–15).
Electrolytes based on the cyclic ether 1,3-dioxalane (DOL) are promising for Li metal anodes because DOL is known to facilitate reversible lithium deposition. It is believed that the electrochemical reduction products (oligomeric/low molecular weight Li+ conducting polymer molecules) formed by DOL passivate the Li anode, preventing continuous degradation of the electrolyte (16–20). DOL also has a low room-temperature viscosity (0.59 mPa⋅s) (21), wide liquidus range (from −95 to 78 °C) at atmospheric pressure, and higher lowest unoccupied molecular orbital energy (1.5 eV) than carbonate solvent (e.g., ethylene carbonate: −0.28 eV), all of which suggest that it is of potential interest in batteries able to operate stably under extreme weather conditions (22, 23). DOL is, however, conventionally not used as a stand-alone electrolyte solvent in Li-ion batteries due to its relatively poor oxidative stability (18) and instability with the most commonly used salt (LiPF6) (24). In Li-S batteries, DOL is nonetheless widely used in combination with linear ether and small quantities of LiNO3 salt, because the dimethoxyethane (DME) offers high solubility of electronically insulating Li-polysulfides (Li-PS) formed at the cathode, while the DOL, LiNO3, and dissolved Li-PS are thought to form a stable, protective film on the lithium metal, enabling extended cycling of a Li-S batteries with high reversibility (14, 19, 25, 26).
Recently, we reported that addition of even small concentrations of Lewis acidic salts (e.g., aluminum triflate [Al(CF3SO3)3]) (18), or halides [e.g., AlI3 or AlF3] (27) to a liquid DOL electrolyte improves the thermal, mechanical, and electrochemical stability of DOL by initiating ring-opening polymerization of DOL inside a battery cell. Because the formed solid-state polymer electrolytes originate from liquids able to wet all components of the cell, the high interfacial resistances typical of solid-state electrolytes are avoided, and the high-oxidation stability is improved. Motivated by this discovery we herein evaluate DOL as a stand-alone electrolyte solvent for Li metal batteries. Comparing the physical, thermal, and electrochemical characteristics of DOL-based electrolytes with those of other chemistries of contemporary interest, we find that DOL enables faster electrode kinetics, including high exchange current density and low charge-transfer resistance. Additionally, we find that the salt additive, LiNO3, which cannot initiate ring-opening polymerization of DOL, is able to drive the liquid electrolyte into a glassy thermal state in which liquidlike bulk and interfacial ion-transport properties coexist with glassy thermal and rheological properties. Optimization of the LiNO3 content in the DOL electrolytes yields systems that enable stable lithium stripping/plating over thousands of cycles with CEs exceeding 99%, even at high lithium-plating capacities (10 mAh/cm2)—among the highest reported in the literature. We evaluate the DOL electrolytes in Li||LiFePO4 full cells in which a thin Li foil (thickness: 50 μm) is paired with lithium iron phosphate (LFP) cathodes with high areal capacity (5 to 10 mAh/cm2) to yield cells with negative:positive (N:P) electrode capacity ratios of 2:1 and 1:1, respectively.
Results and Discussion
Regulating Kinetics of Electrolytes by the Solvent.
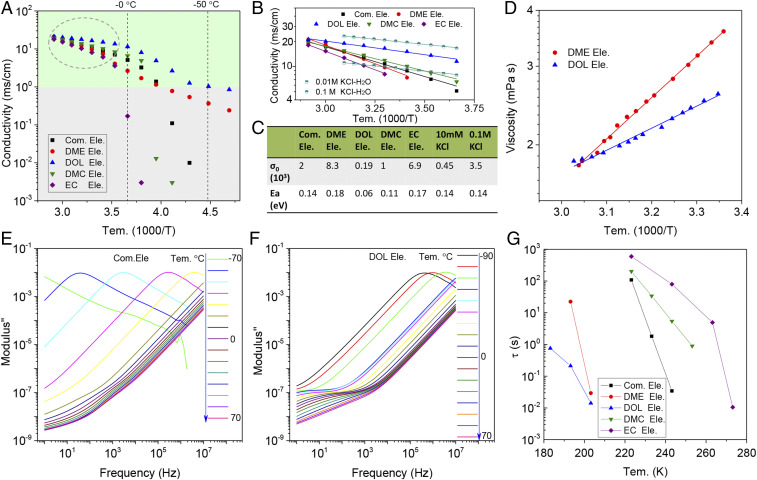
Fig. 1A compares the ionic conductivities of carbonate- and ether-based electrolytes composed of four solvents [ethylene carbonate (EC), dimethyl carbonate (DMC), DOL, DME] of contemporary interest. The same salt LiFSI is used in all cases because it has been reported to offer high solubility, high ionic conductivity, and forms favorable interphases on lithium metal (28–30). The conductivities of carbonate-based electrolytes, including typical commercial electrolytes (1 M LiPF6 in EC/DMC), are generally lower than 0.1 mS cm−1 at temperatures below −20 °C. Standard conductivities of aqueous KCl solutions are also listed for comparison (31). The conductivity of the DOL electrolyte is observed to exceed 1 mS cm−1 at −50 °C. To our knowledge the low-temperature ionic conductivity values for the DOL electrolyte are among the highest reported for lithium batteries (32, 33). In addition, the increasing trends of conductivity at temperatures well above the melting point are clearly distinguishing. We fitted the experimental results using the Arrhenius equation (Fig. 1B). The results reported in Fig. 1C show that ion transport in the DOL-based electrolytes involves a much lower activation energy barrier (Ea = 0.06 eV) than for any of the other electrolytes studied, including aqueous KCl. Taken together with the high ionic conductivity, the results mean that DOL is not only effective in producing large numbers of dissociated ion pairs, but that the mobility of the dissociated ions remains high over a wide temperature range. The Arrhenius preexponential factor () for the electrolytes are also reported in Fig. 1C. The value for the DOL electrolyte is substantially smaller than any of the other organic electrolytes, is related to the activation entropy () in Eyring theory for liquids; the much smaller value for the DOL indicates that ionic motions are less random (i.e., more correlated) than in the other electrolytes.
Fig. 1.
Ion-transport characteristics in electrolytes comprising different solvents. (A) DC conductivity versus temperature. The commercial electrolyte (Com. Ele.) is composed of 1 M LiPF6 dissolved in a symmetric EC/DMC (1:1 by volume) solvent blend. The electrolytes designated DME, DOL, DMC, and EC are each composed of a 2 M LiFSI salt dissolved in the respective solvents. (B) Arrhenius fitting of the measured ionic conductivity versus temperature at relatively high temperatures. Results for aqueous KCl electrolytes are also listed to facilitate comparisons. (C) Arrhenius preexponential factor (σ0) and Ea diffusion calculated from the fits in B for all electrolytes used in the study. (D) Arrhenius fits of the shear viscosity versus temperature data for DOL and DME-based electrolytes. Dielectric loss modulus spectra as a function of temperature for electrolytes composed of (E) 1 M LiPF6 in EC/DMC and (F) 2 M LiFSI in DOL. (G) Variation in characteristic relaxation times (τ) as a function of temperature. τ was obtained by fitting the frequency-dependent dielectric modul measured at each temperature using the H-N function.
It is conventionally assumed that dissociated ions in a simple molecular fluid move in tandem with the molecules of the fluid. Comparison of the activation energy deduced from solvent fluidity (1/viscosity) data provides a simple means of testing this hypothesis. We plot the shear viscosity of all electrolyte solvents used in the study in SI Appendix, Fig. S1A and report the activation energies obtained by fitting the data using the Arrhenius expression in SI Appendix, Fig. S1B. The results show that while the barrier for relative motion of DOL molecules is approximately half of that for H2O, the activation energy for pure DOL is unremarkable relative to the other ether and carbonate liquids. This conclusion is evidently quite different from the one reached by comparing the temperature-dependent viscosity for DOL and DME containing the LiFSI salt (Fig. 1D). At the same LiFSI concentration, the viscosity of the DOL electrolyte is not only substantially lower, but the temperature dependence is markedly weaker. This is in accordance with the trends seen in the conductivity.
The movements of molecules in liquids, especially at low temperature, can be inferred from the dipole relaxation time (), determined from dielectric spectroscopy. We measured the dielectric spectra of the electrolytes used in the study and compare the dielectric loss moduli (M″) in Fig. 1 E and F and SI Appendix, Fig. S2. At low temperature, clear and distinct loss maxima emerge in many of the electrolytes; the maxima shift to lower frequencies as temperature decreases. This behavior is well-known in soft materials and is associated with the slowdown of molecular relaxation dynamics. Comparison of M″ spectra for the commercial carbonate (Fig. 1E) and DOL electrolyte (Fig. 1F), as well as with the full set of electrolytes (SI Appendix, Fig. S2) reveals that at temperatures above −60 °C, the DOL electrolyte shows no obvious dielectric loss maxima, indicating that the selected frequencies (1 to 107 Hz) are too low to capture relaxations in the DOL electrolyte. The Havriliak−Negami (H-N) equation can be used to fit the dielectric loss spectra and to extract characteristic relaxation times, (Fig. 1G) for the various electrolytes (34). It is conventionally thought that when is over 0.1 s in an electrolyte, motions are too slow to support battery operations. It is remarkable that for the DOL electrolyte only crosses this threshold at −80 °C, and extraordinarily low temperature! In order to ascertain that these observations are electrochemically meaningful, we studied lithium stripping and plating in the DOL cells down to −50 °C. The results reported in SI Appendix, Fig. S3 clearly show that whereas electrochemical cells containing the DOL electrolytes can undergo fast lithium stripping and plating at −50 °C, the Li electrodes are completely inactivated in the commercial carbonate electrolyte at −50 °C. Electrochemical impedance spectroscopy (EIS) measurements (SI Appendix, Fig. S4) explain the observed behaviors—whereas the charge-transfer resistance at −50 °C increased to levels on the order of tens of thousands for the commercial electrolyte, the value is less than 100 Ω for the DOL electrolyte.
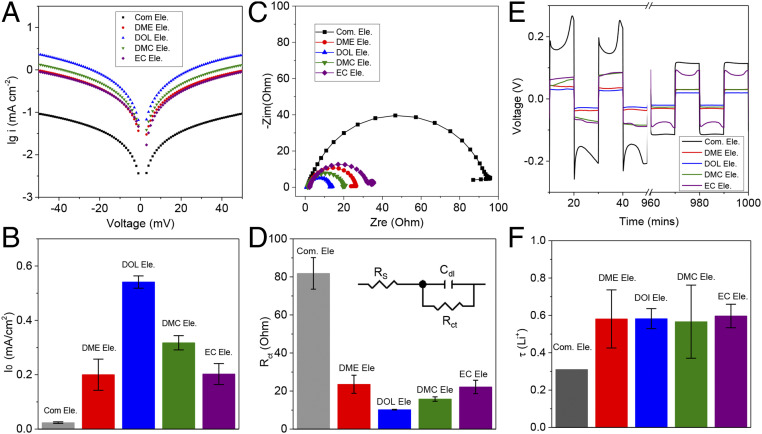
The large differences in ion transport and thermal properties observed in the DOL electrolytes motivated us to analyze other kinetic properties of the materials. Exchange current densities (I0) of the electrolytes at a lithium metal electrode were obtained from the respective Tafel plots (Fig. 2A and SI Appendix, Fig. S5). The results reported in Fig. 2B show that the I0 values for the DOL electrolytes are much larger than any of the other electrolytes studied. These results are consistent with those obtained from EIS analysis of symmetric lithium cells (Fig. 2C). All Nyquist plots show a single semicircle shape, which can be easily fitted a bulk resistance in series with a charge-transfer resistance that is in parallel with a double-layer capacitance. It is shown in Fig. 2D that the DOL electrolyte exhibits the smallest charge-transfer resistance (∼10 Ω), indicating the DOL electrolyte facilitates fast interfacial Li+ transport. Measurements of the overpotential required to galvanostatically strip and plate Li (Fig. 2E and SI Appendix, Fig. S6) in the various electrolytes provide a more straightforward method to assess differences in their ion-transport characteristics aggregated over the electrolyte bulk and interphases formed on the Li electrode. It is again apparent that cells that employ the DOL-based electrolytes show noticeably lower polarizations (<30 mV) than those based on the other electrolytes. The Li+ transport number for the electrolytes was quantified using direct current (DC) polymerization measurements. The results reported in Fig. 2F show that all electrolytes based on the LiFSI salt have nearly the same transference number (∼0.6), which is higher than the corresponding values for the commercial LiPF6-based electrolytes (35).
Fig. 2.
Electrochemical kinetics of electrolyte comprising different solvents. (A) Tafel plots derived from cyclic voltammetry measurements. (B) Exchange-current densities (I0) calculated from Tafel plots. The exchange-current density was calculated by fitting the linear region of Tafel plots (from 50 to 30 mV, and −50 to −30 mV). (C) EIS analysis. (D) Charge-transfer resistance (Rct) calculating by fitting the data in C with an equivalent circuit model (D, Inset). (E) Voltage response obtained from 10-min discharge and 10-min charge cycles at 1 mA cm−2. (F) Comparisons of Li+ transport number (τLi+) computed from DC polarization measurements at 5 mV using the Bruce–Vincent method. All of the data are obtained from symmetrical lithium batteries, in which the glass fiber (grade A) is used as separator to guarantee wetting by all electrolyte solvents used in the study.
We investigated the reversibility of Li stripping and plating processes using Li||Cu electrochemical cells (SI Appendix, Fig. S7). In these experiments, a certain capacity of lithium (typically 1 mAh cm−2) was deposited on Cu first and subsequently stripped from the Cu electrode. The CE is calculated as the percentage of the original Li electrodeposit plated on Cu that is removed in the subsequent Li stripping cycle. Electrolytes based on DOL exhibit both low polarization (∼40 mV) and very high CE values (∼99%) over 300 cycles (SI Appendix, Fig. S7E). The values compare favorably with those obtained for the EC electrolyte (∼99% for 100 cycles), DME electrolyte, and DMC electrolyte (CEs are reduced to <50% before 100 cycles). Remarkably, even at high current density (10 mA cm−2) and high Li plating capacity (3 mAh cm−2) (SI Appendix, Fig. S8), the DOL-based electrolytes still exhibit high CEs over 95%.
Regulating Stabilities of Electrolytes by Salt Addition.
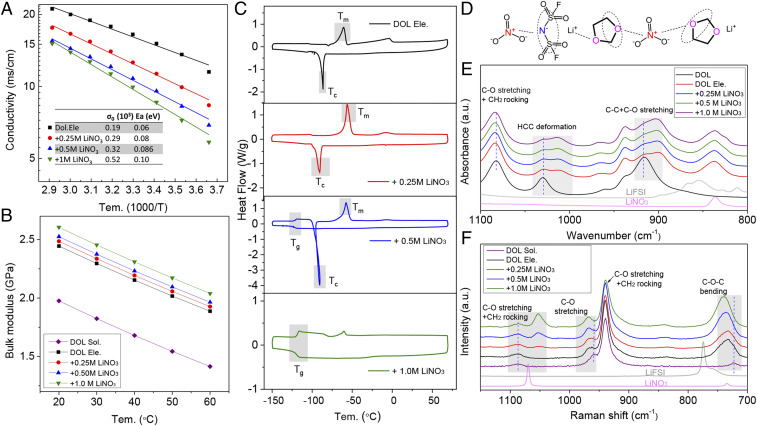
LiNO3 has been reported in numerous studies to be an effective salt additive in improving the reversibility of Li metal electrodes in liquid electrolytes (14, 19). It is commonly assumed that the beneficial effects of LiNO3 come mostly from its role in regulating chemistry and transport properties of interphases formed on the Li electrode. Various literature has also explained the beneficial effects of LiNO3 in terms of its influence on Li surface chemistry and solvation of intermediates formed at Li electrodes in liquid electrolytes (14, 29, 36–38). Because Li already exhibits exceptional reversibility in the DOL electrolyte, we hypothesized that these electrolytes would provide a good testbed for understanding how LiNO3 works. Our results reported in Fig. 3 suggest that in addition to the widely held view that LiNO3 functions as an interphase agent, LiNO3 also markedly alters the solvation environment and bulk electrolyte properties, particularly at higher concentration. The bulk, DC ionic conductivity of the LiFSI/DOL electrolytes containing LiNO3 decreases progressively as the LiNO3 content rises. However, even in electrolytes containing 1 M LiNO3, the conductivity remains very high (∼1 mS cm−1) at −50 °C (SI Appendix, Fig. S9). The activation energy (Ea) increases with the addition of LiNO3 and the preexponential factors () are also larger. To understand the source of these behaviors we determined the bulk mechanical modulus of the DOL electrolytes with/without LiNO3 by measuring the speed of sound in the respective liquids. The results shown in Fig. 3B clearly show that LiNO3 increases the bulk modulus of electrolytes, meaning that the compressibility of the liquid DOL is lowered by LiNO3, but the modulus does not show any obvious change in its temperature dependence. The results suggest that LiNO3 reinforces the electrolyte bulk perhaps through its ability to strongly interact with and electrostatically cross-link DOL molecules. This sort of cross-linking would not only slow down bulk ionic motions, as is already evidenced in the DC conductivity, but should also impact thermal properties of the electrolytes. We performed differential scanning calorimetry (DSC) to investigate the latter effect and the results are reported in Fig. 3C. The LiFSI/DOL electrolyte crystallizes at −87 °C, which is close to the melting point of DOL (−95 °C). Addition of 0.25 M LiNO3 causes the peak to shift to −91 °C and to disappear entirely in electrolytes LiNO3 to 0.5 M; in these systems, the crystallization transition is replaced by a glass transition region at −121 °C (Tg). However, in the subsequent heating process, a massive, sharp reentrant freezing peak located at −90 °C is observed, which is followed by a normal melting peaking located at −58 °C. The latter sequence of observations is well-known in glassy materials that undergo a cold crystallization process when quenched too quickly into the glassy zone (39, 40). We note finally that when the LiNO3 content increases to 1 M, only a reversible glass transition with Tg of −119 °C is observed. The observation of cold crystallization and vitrification of DOL electrolytes in the presence of LiNO3 is well-known in studies of supercooled semicrystalline polymers, but is not expected for a simple molecular fluid such as DOL. The stability of electrolytes against polymerization was assessed using a combination of visual interrogation of their flowability (SI Appendix, Fig. S10) and NMR analysis (SI Appendix, Fig. S11). The results show that the electrolytes do not polymerize to any meaningful extent on the timescales of our experiments. Therefore, it is thought to provide additional evidence that the LiNO3 is strongly coordinated with DOL molecules, constraining their motions and producing solid, polymerlike thermal characteristics in a liquid electrolyte.
Fig. 3.
Transport, thermal, and spectra properties of DOL-based electrolytes containing LiNO3 as additive. (A) Arrhenius plots of ionic conductivity versus temperature for electrolytes with different concentrations of LiNO3. (Inset) The values of σ0 and Ea provided were obtained by fitting the experimental data (symbols) using the Arrhenius ion-transport model (lines). (B) Effect of LiFSI and LiNO3 salt content on the temperature-dependent bulk modulus of DOL-based liquid electrolytes. (C) Heat flow as a function of temperature obtained from DSC analysis of the same electrolytes in B. (D) Proposed interactions of the electrolyte components. (E) ATR-FTIR and (F) Raman spectra of designed electrolytes. The electrolyte designated as DOL Ele. is a 2 M solution of LiFSI in the electrolyte solvent DOL Sol. We added different concentrations of LiNO3 (0.25, 0.5, 1 M) to the DOL Ele. to explore the effect of LiNO3 on ion transport, compressibility, and thermal properties of the electrolytes.
The interactions of different ingredients in the electrolytes are considered to generate because of electron-lacking N-atom in LiNO3, which will attack both DOL and LiFSI in the electrolytes (Fig. 3D). This hypothesis is further proved by vibrational spectroscopy. Attenuated total reflection–Fourier-transform infrared spectroscopy (ATR-FTIR) measurements (Fig. 3E) show that addition of LiFSI to DOL produces multiple changes. First, the vibration associated with the H-C-C deformation splits into two peaks (41, 42), indicating that cyclic DOL molecules are highly strained, and undergo a shape change as a result of their interactions with LiFSI. This interaction increases with addition of LiNO3. Second, the IR peak located at about 920 cm−1, which can be assigned to the C-C + C-O ring stretching vibration, can still be observed when LiFSI is present in the electrolyte, but gradually disappears and shifts to lower wavenumber as the concentrations of LiNO3 is increased. These changes are amplified in the corresponding Raman spectra (Fig. 3F). The C-O-C bending peaking associated with DOL and the S-N-S (775 cm−1) vibration of LiFSI combine into one broad peak after adding LiFSI into DOl (43). Upon addition of LiNO3, this peak progressively shifts to higher energy, which indicates the combined interactions of three components. The phenomenon is also observed in highly concentrated LiFSI/DMC electrolytes, when DMC solvent is coordinated with salt (44). Finally, the Raman band at 1,070 cm−1 associated with NO3− in anhydrous LiNO3 shifts to 1,053 cm−1 in the presence of DOL, particularly when the concentration of NO3− reaches 0.5 M. This change has been previously observed in phases of LiNO3⋅xH2O (45), indicating that DOL molecules are also highly coordinated with LiNO3. The cumulative results from our vibrational spectroscopy therefore appear to conclusively demonstrate that both the structure and dynamics of the DOL molecule are altered by LiNO3.
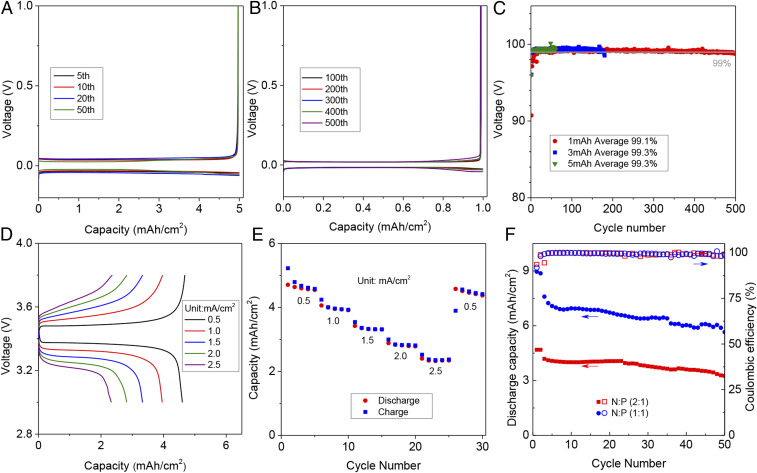
Electrochemical Performances of Polymerlike Electrolytes.
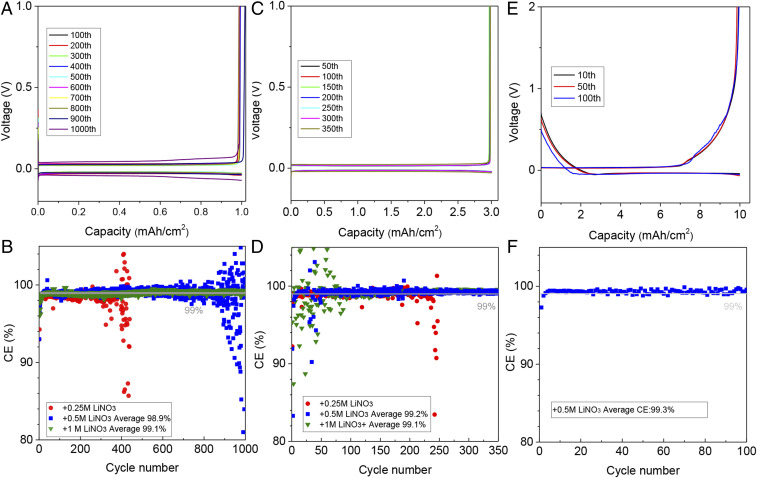
Li||Cu electrochemical cells were assembled to study the efficiency of lithium plating/stripping in LiFSI/DOL electrolytes containing LiNO3. Fig. 4B reports the CE values in electrolytes with different amounts of LiNO3. The CEs increase from 98.9 to 99.1% in electrolytes containing 0.5 and 1 M LiNO3, respectively. The corresponding galvanostatic lithium plating/stripping profiles for the electrolytes with 0.5 M LiNO3 are provided in Fig. 4A. The results show that small levels of polarization are maintained for over 900 cycles. Increasing the capacity of lithium platting to 3 mAh cm−2, the LiFSI/DOL electrolytes containing 0.5 and 1 M LiNO3 exhibit high CEs (>99%) for over 350 cycles (Fig. 4 C and D). In order to determine how the reversibility of Li changes at high lithium deposition capacity, we deposited 10 mAh cm−2 of Li at a current density of 5 mA cm−2 on a commercial three-dimensional carbon cloth current collector and studied its cycling behavior (Fig. 4 E and F). A high CE of 99.3% is retained for at least 100 cycles. The uniform deposition of lithium contributes to the high CEs (SI Appendix, Fig. S12). We progressively increased the Li plating rate from 1 to 9 mA cm−2, with the capacity maintained at 3 mAh cm−2, and measured the CE values. Again, high CE values (>99%; SI Appendix, Fig. S13) are observed. When the concentration of LiFSI is reduced to 0.5 or 1 M, the Li||Cu electrochemical cells still show high CE of ∼99% after 200 cycles when LiNO3 is added (0.5 to 1 M) (SI Appendix, Fig. S14). We note further that similar benefits of LiNO3 are observed in the EC, DMC, and DME electrolytes (SI Appendix, Fig. S15), but again the most sustained benefits are for the ether-based electrolyte. Meanwhile, when other salts [LiCF3SO3 and LiN(CF3SO2)2-LiTFSI] are dissolved in DOL to study lithium deposition, the CEs are also largely improved with the addition of LiNO3 (SI Appendix, Fig. S16).
Fig. 4.
Electrochemical stability of electrolytes containing LiNO3 as an additive. (A) Li stripping and plating profiles for Li//Cu electrochemical cells and (B) the corresponding CEs as a function of cycle number at a fixed current density of 1 mA cm−2 and for a Li plating capacity of 1 mAh cm−2 per cycle. (C and D) Results from analogous experiments to A and B) except a higher Li plating capacity per cycles (3 mAh cm−2 at 1 mA cm−2) was employed in the measurements. (E and F) Galvanostatic cycling characteristics of high-capacity/high-rate Li half-cells composed of Li//nonplanar (carbon cloth) current collector. The Li plating capacity per cycle is 10 mAh cm−2 and the current density is 5 mA cm−2. The profiles for A, C, and E are for the electrolyte previously designated DOL Ele. fortified with 0.5 M LiNO3.
Both salts (LiFSI and LiNO3) that facilitate high Li reversibility in DOL-based electrolytes have low oxidation stability, which would normally be thought of as disadvantageous because they would exacerbate the already poor stability of DOL in contact with a high-voltage cathode (18). Surprisingly, we observe that the strong coordination of DOL with LiNO3 enhances both the reductive and oxidative stability of DOL in an electrochemical cell (SI Appendix, Fig. S17). The results reported in SI Appendix, Fig. S17 A and B show that the LiNO3 significantly lowers the initial discharge capacity in Li||Cu cells, which is related to SEI formation/parasitic degradation of the electrolyte prior to the onset of Li metal plating. On the basis of our earlier findings, we hypothesize that LiNO3 coordination lowers the reactivity of DOL at all potentials.
We evaluated the electrochemical performance of lithium batteries containing the 2 M LiFSI-0.5 M LiNO3-DOL electrolyte. These assessments were deliberately performed under strict conditions (e.g., thin lithium with thickness of 50 μm paired with high-loading commercial cathodes). The CE of lithium plating/stripping measured in thin-Li||Cu electrochemical cells is similar to those observed in cells in which the typical large excess (thick lithium) are used (Fig. 5 A–C). We assembled Li||LFP batteries containing cathodes with different active material mass loadings to initiate and understand failure modes of the DOL electrolytes in practical electrochemical cells. In low mass-loading LFP cathodes, a capacity retention of ∼80% is observed after 500 cycles (SI Appendix, Fig. S18). At a higher LFP loading of 32 mg cm−2, the Li||LFP cells exhibit capacities of 4.6, 4.0, 3.3, 2.8, and 2.3 mAh cm2 at current density 0.5, 1.0, 1.5, 2.0, and 2.5 mA cm−2, respectively (Fig. 5D). Fig. 5E shows that when the current density decreases to 0.5 mA cm−2, the discharge capacity increases to 4.6 mAh cm2, indicating the high current tolerance of optimized electrolytes. This battery can also operate at a temperature of −30 °C (SI Appendix, Fig. S19). Fig. 5F shows preliminary cycling performance of Li//LFP battery at different anode-to-cathode capacity ratio (N:P = 1:1 or 2:1). The capacity retentions are close to (N:P = 1:1) or higher than 80% (N:P = 2:1) after 50 cycles compared to the third cycle. The performance can be improved by further optimizing the structures of cathodes.
Fig. 5.
Electrochemical characterization of Li||Cu and Li||LFP cells employing thin (50-μm) lithium foil as the negative electrode and thick LFP as the cathode. (A and B) Galvanostatic Li stripping and plating profiles as a function of cycle index for Li||Cu cells at a current density of 1 mA cm−2 and Li capacity per cycle of 5 mAh cm−2 (A) and 1 mAh cm−2 (B). (C) CEs deduced from the cycling profiles at 1 mA cm−2, as a function of cycle index. (D) Discharge/charge profiles for Li||LFP electrochemical cells at current densities ranging from 0.5 to 2.5 mA cm−2. (E) Cycling performance of Li||LFP cells at different current density. (F) Cycling performance and corresponding CEs of Li||LFP cells with different N:P ratio. The N:P ratio is varied by changing the mass loading of LFP in the cathode. The results denoted in red correspond to Li//LFP cells in which the 50-µm Li foil is paired with a commercial LFP cathode with mass loading ∼32 mg cm−2, for an N:P ratio of ∼2. Cells were subjected to a brief formation process in which they were discharged/charged at a relatively low current density of 0.5 mA cm−2 for the first two cycles, whereafter they were galvanostatically cycled at 1.5 mA cm−2. The results denoted with blue symbols utilized LFP with double the standard active material mass loading (i.e., an LFP mass loading of approximately 64 mg cm−2), corresponding to the theoretical N:P ratio of unity. For these measurements, the formation process consisted of discharging and charging the cells at a current density of 1 mA cm−2 for the first two cycles, followed by extended cycling at a fixed rate of 2 mA cm−2.
Conclusion
In summary, we report that electrolytes composed of the cyclic ether, DOL, exhibit exceptional physical, thermal, and electrochemical characteristics, including high bulk and interfacial ionic conductivities down to −50 °C and low Ea barriers for ion transport. Above a threshold concentration of ∼0.5 M, addition of LiNO3 to a DOL-based electrolyte causes the electrolyte to transition to a highly correlated but amorphous state in which crystallization is completely arrested, molecular relaxation slows down, but the high ionic conductivities are preserved. By means of physical, spectroscopic, and ion-transport measurements we find that strong interactions between LiNO3 and DOL distort bonds in DOL, couple motions of individual molecules, but do not produce ring opening. The resultant structured liquid electrolytes facilitate highly reversible lithium stripping/plating, over thousands of cycles with CE exceeding 99%, even at Li throughputs as high as 10 mAh cm−2. An initial assessment of the DOL electrolytes in Li||LiFePO4 full cells with N:P ratios of 2:1 and 1:1 demonstrates the potential of DOL electrolytes in Li batteries with wide temperature- and voltage-stability windows.
Methods
Materials Preparation and Characterizations.
All electrolytes are prepared in argon glove box (Inert Inc), in which the content of O2 and H2O content are lower than 0.5 ppm. The company and purity of raw materials: lithium bis(fluorosulfonyl)amide [LiFSI, 99% purity, (Oakwood Products Inc. [OAK])], lithium nitrate (LiNO3, 99.99% metal basis, Chem-Impex Int’l. Inc.), 1,3-Dioxolane (DOL, 99.8%, Sigma-Aldrich), DME (99.5%, Sigma-Aldrich), DMC (≥ 99%, Sigma-Aldrich), EC (99%, Sigma-Aldrich), 1.0 M LiPF6 in EC/DMC = 50/50 (vol/vol) (battery grade, Sigma-Aldrich). DC conductivity, dielectric loss modulus, and EIS test are performed using a dielectric/impedance spectrometer (Novocontrol Broad band). For conductivity and dielectric loss modulus tests, the temperature increases from −90 to 70 °C and the test is operated every 10 °C. The viscosity is tested using Vibro SV-10 Viscometer. DSC is tested using TA Instruments DSC Q2000. The sound speeds of electrolytes are measured by DMA density meter. Renishaw inVia confocal Raman microscope is used for Raman tests of electrolytes (excitation wavelength: 785 nm). ATR-FTIR spectra were conducted using a Thermo Scientific spectrometer. Spectra (1H NMR and 13C NMR) are performed by dissolving electrolytes in dimethyl sulfoxide-d6. All of the experiments are conducted less than 5 d after the preparation of electrolytes.
Fitting of DC Ionic Conductivity.
Arrhenius equation (Fig. 1B) was used to fit the conductivity of electrolytes, where is preexponential factor, is Boltzmann constant, Ea is the activation energy, T is the absolute temperature. Further studies on preexponential factor are based on the Eyring model, in which the transport is dominated by molecular contacts. The ionic conductivity follows the Nernst–Einstein equation,
| [1] |
in which the diffusivity follows the Stokes–Einstein equation,
| [2] |
and can be calculated by the Eyring model,
| [3] |
In Eqs. 1–3, is Boltzmann’s constant, is absolute temperature, is Faraday constant, is valence of ions, is the concentration of ions, is the radius of spherical ions, is the viscosity of electrolyte, and is the distance between ions, is the volume of the spherical ions, is Avogadro constant, is Planck constant, is the gas constant, and Gibbs free energy . Thus, as most of the parameters are constant for various electrolytes, the ionic conductivity can be roughly simplified as
Therefore the preexponential factor is related with entropy; the low preexponential factor indicates the low entropy.
Calculation of Relaxation Times.
The experimentally measured spectra were fitted with the generalized H-N model near the maximum region as follows:
where
and are the electric storage modulus at and , respectively; is the relaxation time, and and are exponents that describe the symmetric and asymmetric distribution of the relaxation times. The fitting was performed with lsqcurvefit function using MATLAB.
Calculation of Bulk Modulus.
The bulk modulus of electrolytes is calculated by Newton–Laplace equations.
where c is the speed of sound and is the density of electrolytes.
Electrochemical Cells Test.
Coin 2032 cells model were assembled for electrochemical tests. Lithium foil acts as anode and Al2O3 coated Celgard acts as separator. (In cells using LiFSI/EC electrolyte and symmetrical Li//Li cells, glass fiber acts as separator to guarantee the full wetness.) Galvanostatic lithium stripping/plating tests (Li//Cu [or carbon cloth] electrochemical cells and Li//Li symmetrical electrochemical cells) and galvanostatic discharge/charge tests (Li//LFP battery) were operated at room temperature using Neware battery tester. The transference number of Li+ is tested by a DC polarization according to the reports using solartron battery tester (35). Cyclic voltammetry profiles are obtained from A CH 600E electrochemical workstation. Low-loading LFP cathodes were prepared by mixing LFP powder, super P conductivities, and polyvinylidene fluoride binder with weight ratio of 80:10:10. High-loading LFP cathode on carbon cloth is prepared according to our previous report (46).
Supplementary Material
Acknowledgments
This work was supported by the Department of Energy Basic Energy Sciences Program through Award DE-SC0016082 and the NSF through Award DMR-1609125. The characterizations of electron images are supported by the Cornell Center for Materials Research with funding from the NSF Materials Research Science and Engineering Centers (MRSEC) program (Grant DMR-1719875).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2004576117/-/DCSupplemental.
Data Availability.
All study data are included in the article and SI Appendix.
References
- 1.Xu K., Electrolytes and interphases in Li-ion batteries and beyond. Chem. Rev. 114, 11503–11618 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Goodenough J. B., Park K. S., The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 135, 1167–1176 (2013). [DOI] [PubMed] [Google Scholar]
- 3.Armand M., Tarascon J. M., Building better batteries. Nature 451, 652–657 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Verma P., Maire P., Novák P., A review of the features and analyses of the solid electrolyte interphase in Li-ion batteries. Electrochim. Acta 55, 6332–6341 (2010). [Google Scholar]
- 5.Lu Y., Tu Z., Archer L. A., Stable lithium electrodeposition in liquid and nanoporous solid electrolytes. Nat. Mater. 13, 961–969 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Lin D., Liu Y., Cui Y., Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 12, 194–206 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Tikekar M. D., Choudhury S., Tu Z., Archer L. A., Design principles for electrolytes and interfaces for stable lithium-metal batteries. Nat. Energy 1, 16114 (2016). [Google Scholar]
- 8.Cheng X. B., Zhang R., Zhao C. Z., Zhang Q., Toward safe lithium metal anode in rechargeable batteries: A review. Chem. Rev. 117, 10403–10473 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Seh Z. W., Sun J., Sun Y., Cui Y., A highly reversible room-temperature sodium metal anode. ACS Cent. Sci. 1, 449–455 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J. et al., Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 4, 180–186 (2019). [Google Scholar]
- 11.Kasemchainan J. et al., Critical stripping current leads to dendrite formation on plating in lithium anode solid electrolyte cells. Nat. Mater. 18, 1105–1111 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Bruce P. G., Freunberger S. A., Hardwick L. J., Tarascon J. M., Li-O2 and Li-S batteries with high energy storage. Nat. Mater. 11, 19–29 (2011). [DOI] [PubMed] [Google Scholar]
- 13.Pang Q., Liang X., Kwok C. Y., Nazar L. F., Advances in lithium–sulfur batteries based on multifunctional cathodes and electrolytes. Nat. Energy 1, 16132 (2016). [Google Scholar]
- 14.Aurbach D. et al., On the surface chemical aspects of very high energy density, rechargeable Li–sulfur batteries. J. Electrochem. Soc. 156, A694 (2009). [Google Scholar]
- 15.Gao Y. et al., Polymer-inorganic solid-electrolyte interphase for stable lithium metal batteries under lean electrolyte conditions. Nat. Mater. 18, 384–389 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Aurbach D., Youngman O., Dan P., The electrochemical behavior of 1,3-dioxolane—LiClO4 solutions—II. Contaminated solutions. Electrochim. Acta 35, 639–655 (1990). [Google Scholar]
- 17.Alamgir M., Moulton R. D., Abraham K. M., Li+-conductive polymer electrolytes derived from poly(1,3-dioxolane) and polytetrahydrofuran. Electrochim. Acta 36, 773–782 (1991). [Google Scholar]
- 18.Zhao Q., Liu X., Stalin S., Khan K., Archer L. A., Solid-state polymer electrolytes with in-built fast interfacial transport for secondary lithium batteries. Nat. Energy 4, 365–373 (2019). [Google Scholar]
- 19.Peled E., Lithium-sulfur battery: Evaluation of dioxolane-based electrolytes. J. Electrochem. Soc. 136, 1621–1625 (1989). [Google Scholar]
- 20.Ma Q. et al., Improved cycling stability of lithium-metal anode with concentrated electrolytes based on lithium (fluorosulfonyl) (trifluoromethanesulfonyl) imide. ChemElectroChem 3, 531–536 (2016). [Google Scholar]
- 21.Wohlfarth Ch., Lechner M. D., Eds., “Viscosity of 1,3-dioxolane” in Landolt-Börnstein - Group IV Physical Chemistry 25 (Supplement to IV/18), (Springer Materials Press, 2008). [Google Scholar]
- 22.Chen X. et al., Towards stable lithium-sulfur batteries: Mechanistic insights into electrolyte decomposition on lithium metal anode. Energy Storage Mater. 8, 194–201 (2017). [Google Scholar]
- 23.Cao X. et al., Monolithic solid-electrolyte interphases formed in fluorinated orthoformate-based electrolytes minimize Li depletion and pulverization. Nat. Energy 4, 796–805 (2019). [Google Scholar]
- 24.Liu F. Q. et al., Upgrading traditional liquid electrolyte via in situ gelation for future lithium metal batteries. Sci. Adv. 4, eaat5383 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H. et al., Electrolyte additives for lithium metal anodes and rechargeable lithium metal batteries: Progress and perspectives. Angew. Chem. Int. Ed. Engl. 57, 15002–15027 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Barchasz C., Leprêtre J.-C., Patoux S., Alloin F., Revisiting TEGDME/DIOX binary electrolytes for lithium/sulfur batteries: Importance of solvation ability and additives. J. Electrochem. Soc. 160, A430–A436 (2013). [Google Scholar]
- 27.Ma L., Kim M. S., Archer L. A., Stable artificial solid electrolyte interphases for lithium batteries. Chem. Mater. 29, 4181–4189 (2017). [Google Scholar]
- 28.Qian J. et al., High rate and stable cycling of lithium metal anode. Nat. Commun. 6, 6362 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen S. et al., High-voltage lithium-metal batteries enabled by localized high-concentration electrolytes. Adv. Mater. 30, e1706102 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Zhang X. Q. et al., Regulating anions in the solvation sheath of lithium ions for stable lithium metal batteries. ACS Energy Lett. 4, 411–416 (2019). [Google Scholar]
- 31.Lide D. R., “Standard KCl solutions for calibrating conductivity cells” in CRC Handbook of Chemistry and Physics, (CRC Press, Boca Raton, FL, 90th Ed., 2010), Vol. 5, p. 73. [Google Scholar]
- 32.Yang Y. et al., High-efficiency lithium-metal anode enabled by liquefied gas electrolytes. Joule 3, 1986–2000 (2019). [Google Scholar]
- 33.Fan X. L. et al., All-temperature batteries enabled by fluorinated electrolytes with non-polar solvents. Nat. Energy 4, 882–890 (2019). [Google Scholar]
- 34.Agrawal A., Wenning B. M., Choudhury S., Archer L. A., Interactions, structure, and dynamics of polymer-tethered nanoparticle blends. Langmuir 32, 8698–8708 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Dong T. T. et al., A multifunctional polymer electrolyte enables ultra-long cycle-life in a high-voltage lithium metal battery. Energy Environ. Sci. 11, 1197–1203 (2018). [Google Scholar]
- 36.Giordani V. et al., Rechargeable-battery chemistry based on lithium oxide growth through nitrate anion redox. Nat. Chem. 11, 1133–1138 (2019). [DOI] [PubMed] [Google Scholar]
- 37.Burke C. M., Pande V., Khetan A., Viswanathan V., McCloskey B. D., Enhancing electrochemical intermediate solvation through electrolyte anion selection to increase nonaqueous Li-O2 battery capacity. Proc. Natl. Acad. Sci. U.S.A. 112, 9293–9298 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi Q., Zhong Y., Wu M., Wang H., Wang H., High-capacity rechargeable batteries based on deeply cyclable lithium metal anodes. Proc. Natl. Acad. Sci. U.S.A. 115, 5676–5680 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schick C., Differential scanning calorimetry (DSC) of semicrystalline polymers. Anal. Bioanal. Chem. 395, 1589–1611 (2009). [DOI] [PubMed] [Google Scholar]
- 40.Hatakeyma T., Kasuga H., Tanaka M., Hatakeyama H., Cold crystallization of poly(ethylene glycol)–water systems. Thermochim. Acta 465, 59–66 (2007). [Google Scholar]
- 41.Barker S. A., Bourne E. J., Pinkard R. M., Whiffen D. H., 161. Spectra of acetals. Part I. The infrared and Raman spectra of 1: 3-dioxolan. J. Chem. Soc. (Resumed), 802–806 (1959). [Google Scholar]
- 42.Mohaček-Grošev V., Furić K., Ivanković H., Observed bands in Raman and infrared spectra of 1,3-dioxolane and their assignments. Vib. Spectrosc. 64, 101–107 (2013). [Google Scholar]
- 43.Kerner M., Plylahan N., Scheers J., Johansson P., Thermal stability and decomposition of lithium bis(fluorosulfonyl)imide (LiFSI) salts. RSC Adv. 6, 23327–23334 (2016). [Google Scholar]
- 44.Wang J. et al., Superconcentrated electrolytes for a high-voltage lithium-ion battery. Nat. Commun. 7, 12032 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Irish D. E., Nelson D. L., Brooker M. H., Quasilattice features of concentrated aqueous LiNO3 solutions. J. Chem. Phys. 54, 654–657 (1971). [Google Scholar]
- 46.Zheng J. et al., Physical orphaning versus chemical instability: Is dendritic electrodeposition of Li fatal? ACS Energy Lett. 4, 1349–1355 (2019). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and SI Appendix.