Key Points
Question
Among critically ill patients with acute kidney injury, what is the effect of a strategy using regional citrate anticoagulation, which consists of administration of citrate to the extracorporeal dialysis circuit, vs systemic heparin anticoagulation, which consists of intravenous heparin application, during continuous kidney replacement therapy on dialysis filter life span and mortality?
Findings
In this randomized clinical trial that included 596 patients and that was stopped early, anticoagulation with regional citrate administration, compared with systemic heparin anticoagulation, resulted in significantly longer median filter life span (47 hours vs 27 hours, respectively), and 90-day mortality was 51.2% vs 53.6%, respectively.
Meaning
Among critically ill patients with acute kidney injury receiving continuous kidney replacement therapy, anticoagulation with regional citrate, compared with systemic heparin anticoagulation, increased filter life span, but the trial was underpowered to reach conclusions regarding mortality.
Abstract
Importance
Although current guidelines suggest the use of regional citrate anticoagulation (which involves the addition of a citrate solution to the blood before the filter of the extracorporeal dialysis circuit) as first-line treatment for continuous kidney replacement therapy in critically ill patients, the evidence for this recommendation is based on few clinical trials and meta-analyses.
Objective
To determine the effect of regional citrate anticoagulation, compared with systemic heparin anticoagulation, on filter life span and mortality.
Design, Setting, and Participants
A parallel-group, randomized multicenter clinical trial in 26 centers across Germany was conducted between March 2016 and December 2018 (final date of follow-up, January 21, 2020). The trial was terminated early after 596 critically ill patients with severe acute kidney injury or clinical indications for initiation of kidney replacement therapy had been enrolled.
Interventions
Patients were randomized to receive either regional citrate anticoagulation (n = 300), which consisted of a target ionized calcium level of 1.0 to 1.40 mg/dL, or systemic heparin anticoagulation (n = 296), which consisted of a target activated partial thromboplastin time of 45 to 60 seconds, for continuous kidney replacement therapy.
Main Outcomes and Measures
Coprimary outcomes were filter life span and 90-day mortality. Secondary end points included bleeding complications and new infections.
Results
Among 638 patients randomized, 596 (93.4%) (mean age, 67.5 years; 183 [30.7%] women) completed the trial. In the regional citrate group vs systemic heparin group, median filter life span was 47 hours (interquartile range [IQR], 19-70 hours) vs 26 hours (IQR, 12-51 hours) (difference, 15 hours [95% CI, 11 to 20 hours]; P < .001). Ninety-day all-cause mortality occurred in 150 of 300 patients vs 156 of 296 patients (Kaplan-Meier estimator percentages, 51.2% vs 53.6%; unadjusted difference, –2.4% [95% CI, –10.5% to 5.8%]; unadjusted hazard ratio, 0.91 [95% CI, 0.72 to 1.13]; unadjusted P = .38; adjusted difference, –6.1% [95% CI, –12.6% to 0.4%]; primary adjusted hazard ratio, 0.79 [95% CI, 0.63 to 1.004]; primary adjusted P = .054). Of 38 prespecified secondary end points, 34 showed no significant difference. Compared with the systemic heparin group, the regional citrate group had significantly fewer bleeding complications (15/300 [5.1%] vs 49/296 [16.9%]; difference, –11.8% [95% CI, –16.8% to –6.8%]; P < .001) and significantly more new infections (204/300 [68.0%] vs 164/296 [55.4%]; difference, 12.6% [95% CI, 4.9% to 20.3%]; P = .002).
Conclusions and Relevance
Among critically ill patients with acute kidney injury receiving continuous kidney replacement therapy, anticoagulation with regional citrate, compared with systemic heparin anticoagulation, resulted in significantly longer filter life span. The trial was terminated early and was therefore underpowered to reach conclusions about the effect of anticoagulation strategy on mortality.
Trial Registration
ClinicalTrials.gov Identifier: NCT02669589
This randomized clinical trial compares the effect of regional citrate vs systemic heparin anticoagulation on dialysis filter life span and 90-day mortality among critically ill adults with acute kidney injury (AKI) receiving continuous kidney replacement therapy.
Introduction
Kidney replacement therapy remains the only therapeutic option to support kidney function among critically ill patients with severe acute kidney injury. In these patients, continuous kidney replacement therapy is often preferred over intermittent techniques to ensure hemodynamic stability, tight volume control, and acid-base balance1; however, effectiveness is dependent on the time receiving therapy. Premature dialysis filter loss due to clotting can reduce solute clearance and ultrafiltration, contribute to blood loss, and prompt need for transfusion.
Systemic heparin and regional citrate anticoagulation are the 2 main anticoagulation strategies used in daily practice. The Kidney Disease: Improving Global Outcomes (KDIGO) guidelines suggest the use of regional citrate anticoagulation for continuous kidney replacement therapy,1 but the evidence level was considered low.
Systemic heparin anticoagulation has been the first choice for decades.2 However, heparin may cause heparin-induced thrombocytopenia and bleeding complications. Regional citrate anticoagulation consists of citrate infusion to the blood before the filter of the extracorporeal circuit, thereby chelating ionized calcium, a key cofactor of many steps of the clotting cascade; the calcium-citrate complexes are then mainly removed by filtration and dialysis. Regional citrate anticoagulation has been associated with longer filter life span, a quality indicator of the procedure.3,4 However, there is uncertainty whether it has robust effects on patient-centered outcomes such as death. In addition, citrate protocols are very complex and associated with adverse metabolic complications. In contrast, bleeding complications may be significantly lower compared with those associated with systemic heparin anticoagulation.3,5,6,7 Current evidence is derived from few (underpowered) trials,4,5,8 and the clinical benefit of regional citrate anticoagulation on patient-centered outcomes remains unclear.
The Regional Citrate vs Systemic Heparin Anticoagulation for Continuous Kidney Replacement Therapy in Critically Ill Patients With Acute Kidney Injury (RICH) trial was conducted to test whether regional citrate anticoagulation prolongs dialysis filter life span and reduces 90-day-all-cause mortality in critically ill patients with acute kidney injury.
Methods
Study Design and Ethics
The RICH trial was a randomized, multicenter, parallel-group clinical trial of different anticoagulation strategies for critically ill patients. A detailed description of the study design has been published.9 The detailed protocol and the statistical analysis plan are available in Supplement 1 and Supplement 2, respectively. Institutional review board approval was obtained from the Research Ethics Committee of the Chamber of Physicians Westfalen–Lippe and the Westfalian Wilhelms University Muenster and the corresponding institutional review boards of all participating centers. The trial was approved by the Federal Institute for Drugs and Medical Devices and registered at ClinicalTrials.gov. The study was conducted in accordance with the Declaration of Helsinki.10 The trial coordinators obtained consent for participation from all patients prior to randomization. A data and safety monitoring board oversaw the study and reviewed blinded safety data. Onsite monitoring for the accuracy of the consent procedure was performed at all sites. All participating centers used both anticoagulation strategies prior to conducting the trial and used published protocols for regional citrate anticoagulation. An investigator at each center was responsible for enrolling patients, ensuring adherence to the trial protocol, and ensuring the accuracy of the data entry.
Patient Recruitment
Prior to randomizing patients into the study, the trial coordinators obtained consent for participation in the study. Assuming all inclusion criteria were fulfilled and no exclusion criteria were met, each patient received a study identification number and treatment allocation at enrollment. Inclusion criteria were (1) KDIGO stage 3 acute kidney injury classification (urine output <0.3 mL/kg/h for ≥24 hours, and/or >3-fold increase in serum creatinine level compared with baseline, and/or serum creatinine level of ≥4 mg/dL [353.6 μmol/L] with an acute increase of at least 0.5 mg/dL [44.2 μmol/L] within 48 hours) OR an absolute indication for continuous kidney replacement therapy (serum urea levels >150 mg/dL, serum potassium levels >6 mmol/L, serum magnesium levels >9.7 mg/dL [4 mmol/L], blood pH <7.15, urine production <200 mL/12 h or anuria, or fluid overload with edema in the presence of acute kidney injury resistant to diuretic treatment); (2) at least 1 additional condition (severe sepsis or septic shock, use of vasopressor, refractory fluid overload); (3) age between 18 and 90 years; (4) intention to provide full intensive care treatment for at least 3 days; and (5) provision of written informed consent.
Patients were excluded if they had an increased bleeding risk, had diseases with hemorrhagic diathesis, needed therapeutic anticoagulation, had previous allergic reactions to one of the anticoagulants, had known heparin-induced thrombocytopenia, or had persistent and severe lactic acidosis (pH <7.2 in 2 consecutive measurements for >2 hours and lactate level >72.1 mg/dL [8 mmol/L]) in the context of acute liver failure, shock, or both. Patients with severe lactic acidosis in the context of liver failure or shock were excluded based on the risk of citrate accumulation and toxicity1,11,12,13 as well as the recommendations of the Federal Institute for Drugs and Medical Devices. Additional exclusion criteria included dialysis-dependent chronic kidney disease; acute kidney injury caused by permanent occlusion or surgical lesion of both kidney arteries; acute kidney injury caused by glomerulonephritis, interstitial nephritis, vasculitis, or urinary tract obstruction; kidney transplant within the last 12 months; hemolytic-uremic syndrome/thrombotic thrombocytopenic purpura; no machine for continuous kidney replacement therapy available at the moment of inclusion; participation in another clinical intervention trial in the last 3 months; any kind of dependency on the investigator or employed by the sponsor or investigator; pregnancy and nursing period; and impending miscarriage.9
Randomization
Randomization was performed centrally by the Clinical Trials Centre Leipzig in a 1:1 proportion. A computerized minimization method with random component was used that provided treatment assignment to be balanced by the factors study center, sex, cardiovascular Sequential Organ Failure Assessment (SOFA) score (0-2 vs 3-4), and presence or absence of oliguria (Supplement 3).14
Procedures
Before starting continuous kidney replacement therapy (in patients with KDIGO stage 3, continuous kidney replacement therapy was started within 24 hours after meeting KDIGO stage 3 criteria; in patients with an absolute indication, continuous kidney replacement therapy was initiated as soon as possible), patients were randomized to receive either regional anticoagulation with citrate (target ionized calcium level 0.25-0.35 mmol/L, after hemofilter) or systemic anticoagulation with heparin (target activated partial thromboplastin time 45-60 seconds). In the regional citrate anticoagulation group, citrate was added continuously to the blood before the filter of the extracorporeal circuit; based on the ionized calcium levels, citrate rates were adjusted according to published protocols.9 In the heparin group, heparin was administered systemically through intravenous lines. The prescribed dose was 30 mL/kg/h according to the recommendation of the KDIGO guidelines to achieve a delivered dose of 20 to 25 mL/kg/h.1 Blood flow was kept above 100 mL/min. Filters should be changed every 72 hours (according to the recommendations of the manufacturer). Other reasons for filter changes were not prespecified but were documented. Depending on the patient’s condition, continuous kidney replacement therapy could be replaced by an intermittent technique 5 days after randomization.
Outcomes
The coprimary end points were dialysis filter life span and 90-day all-cause mortality. Filter life span was defined as the time from the beginning of kidney replacement therapy with the corresponding filter until filter replacement (because of the upper limited time for filter of 72 hours) or termination of kidney replacement therapy because of one of the following reasons: extracorporeal coagulation due to clotting, nonclotting events (eg, extended interventions exceeding the allowed circling time, changes of anticoagulation method) or termination of kidney replacement therapy (achievement of treatment goal, death). Filter changes were performed by the treating personnel, who were not blinded to treatment allocation but who were independent of the study personnel.
Secondary outcomes included intensive care unit (ICU) and hospital length of stay, duration and complications of kidney replacement therapy, downtime, bleeding complications (defined as major bleeding with transfusion requirement and/or the need of reoperation and/or new onset of intracranial bleeding without traumatic event) and number of transfused patients, rate of infection during ICU stay (new infection since start of dialysis was defined as new infection in patients with preexisting infection but with a pathogen other than baseline or without baseline infection at baseline after initiation of kidney replacement therapy up to the end of ICU stay or day 28 [whichever occurred first]; all infections were culture proven), Sequential Organ Failure Assessment (SOFA) scores at days 1 to 14, day 21, and 28; recovery of kidney function (complete recovery defined as serum creatinine level ≤0.5 mg/dL [44.2 μmol/L] higher than baseline; partial recovery, as serum creatinine level >0.5 mg/dL higher than baseline but no dialysis dependence; nonrecovery, as patients who remained dialysis-dependent at days 28, 60, 90, and 365); need for kidney replacement therapy at days 28, 60, 90, and 365; all-cause mortality at days 28, 60, and 365; and major adverse kidney events (defined as the composite of death, use of kidney replacement therapy, and persistent kidney dysfunction [defined by serum creatinine level ≥200% of reference without dialysis dependency]15 at days 28, 60, 90, and 365). Because cost and laboratory parameters were not the focus of this manuscript, those end points will be analyzed separately.
Statistical Analysis
An adaptive study design with 1 interim analysis based on a group sequential plan according to O’Brien-Fleming was established that allowed for early proof of superiority of 1 of the 2 treatments, stopping for futility, and sample size recalculation.
The required sample size was calculated, setting the multiple (2-sided) significance level to α = .05 and the required power regarding the first and second coprimary end point to 90% and 80%, respectively. A mean difference of filter life span between the regional citrate and systemic heparin anticoagulation group was expected to be at least 5 hours (SD, 27 hours within each group).6 The 90-day mortality rate in the systemic heparin anticoagulation treatment group was expected to be 48%.16,17 The treatment effect on mortality was considered to be clinically relevant if the 90-day mortality rate in the regional citrate anticoagulation group was 40% or lower. The 8% minimal clinically important difference was set in accordance with the Intensity of Continuous Renal-Replacement Therapy in Critically Ill Patients (RENAL) Study.17 Ten percent of living patients were expected to be lost to follow-up during the 90-day follow-up period. Because of these considerations, the planned total numbers of patients with available primary outcome data included in the interim and final statistical analysis were 400 and 1260 patients, respectively. Sample size recalculation was planned to be limited to a maximal total number of 1450 patients. Power calculations were performed using ADDPLAN (Icon).
A nonbinding stopping rule for futility was specified for the second coprimary outcome of 90-day mortality. In the interim analysis, futility was determined if the (unadjusted) P value of favorable survival in the citrate group is .50 or larger and if stochastic curtailment shows a conditional power of the final statistical analysis with the prespecified maximal total number of 1450 patients that is lower than 50%. Beyond the above 2 stopping criteria, the final decision to stop or continue recruitment was made by the principal investigator based on the recommendation of the data and safety monitoring board.
Statistical analyses were performed using the full analysis set, which includes all randomized and evaluable patients. Patients were analyzed according to the group to which they were randomized.
The treatment effect on filter life span was evaluated using a 2-sided inverse normal likelihood ratio test based on a multivariable linear mixed model that included a patient-specific random effect. The treatment effect on overall survival was evaluated using a 2-sided inverse normal likelihood ratio test based on a multivariable Cox regression model. Both likelihood ratio tests were performed within multivariable models that adjusted for the factors study center, baseline cardiovascular SOFA score, presence or absence of oliguria, sex, and an additional factor that indicated if a patient was recruited before or after implementation of amendment 1 of the study protocol (eTable 1 in Supplement 3). The additional factor of recruitment before vs after protocol amendment 1 accounts for minor changes of inclusion criteria and was prespecified in the amended trial protocol and in the statistical analysis plan.
The basic model assumptions of the linear mixed model are the normality and constant variance of the random effect and the random error term. Model diagnostics were performed using the studentized conditional residuals. Histograms, normal q-q plots, and residual vs predicted plots revealed that the basic model assumptions were met. The model was fit using the maximum likelihood method with the residual variance profiled out of the likelihood. The Cox regression model was fit using the partial likelihood with Breslow estimate of the baseline hazard function. The proportionality assumption was tested and confirmed based on the Schoenfeld residuals using the Grambsch-Therneau method. Detailed results of model diagnostics are reported in Supplement 3.
The primary effectiveness analysis provided confirmatory statistical evidence, using a multiple 2-sided significance level of α = .05. To account for multiplicity, a multiple testing procedure with fixed a priori ordered hypotheses was applied. First, the null hypothesis of equal filter life span in both treatment groups was tested and subsequently the null hypothesis of equal overall survival.
Two scenarios were possible that represent an overall “positive” result of this trial. First, statistically significant treatment differences with respect to both coprimary outcomes: citrate anticoagulation as compared with heparin prolongs filter life span and overall survival. Second, statistically significant treatment differences with respect to the first coprimary outcome but not with respect to the second coprimary outcome: citrate anticoagulation as compared with heparin prolongs filter life span, but the treatment effect on overall survival is not statistically significant.
Statistical analyses of secondary end points were not adjusted for multiplicity. Therefore, because of the potential for type I error, findings should be interpreted as exploratory.
According to the statistical analysis plan, missing values for effectiveness or safety parameters were not replaced by any kind of statistical imputation. Statistical methods of post hoc analyses, and a preplanned subgroup analysis of surgical and nonsurgical patients, are described in Supplement 3.
Statistical analyses were performed using SAS version 9.4 for Windows (SAS Institute Inc).
Results
Patients
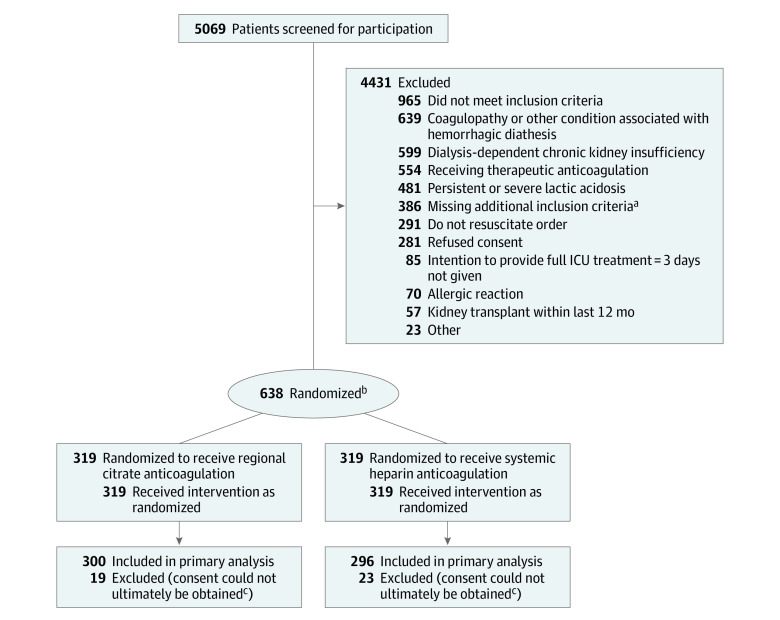
The trial was conducted from March 2016 to December 2018 in 26 intensive care units in Germany (end of follow-up, January 21, 2020). A total of 5069 patients were screened for inclusion; of these, 638 patients were enrolled and randomized to receive either regional citrate anticoagulation (n = 319) or systemic heparin anticoagulation (n = 319) (Figure 1). The interim analysis showed that the preplanned end of the trial according to the protocol was reached before the complete number of patients was recruited. Recruitment was stopped on January 4, 2019, for significant effectiveness in terms of the first coprimary outcome of filter life span and for futility in terms of the second coprimary outcome of 90-day mortality. Further details of the results of the interim analysis are reported in Supplement 3.
Figure 1. Participant Flow in the RICH Trial.
Abbreviations: ICU, intensive care unit; RICH, Regional Citrate vs Systemic Heparin Anticoagulation for Continuous Kidney Replacement Therapy in Critically Ill Patients with Acute Kidney Injury.
aSevere sepsis or septic shock, use of vasopressor, refractory fluid overload.
bRandomization was performed centrally in a 1:1 proportion using the Pocock minimization method of stratified randomization, accounting for the factors study center, sex, cardiovascular Sequential Organ Failure Assessment (SOFA) score (0-2 vs 3-4), and presence or absence of oliguria.9,14
cReasons for including but not analyzing patients were refusal of the guardianship procedure by the local court, no written consent of guardian prior to the death of the patient, no written consent of the guardian at all, or timeline issues (details reported in Supplement 3)
Forty-two patients had to be excluded from the analysis because of incomplete consent process according to the European regulations. Reasons for including but not analyzing patients were refusal of the guardianship procedure by the local court, no written consent of guardian prior to the death of the patient, no written consent of the guardian at all, or timeline issues (details reported in Supplement 3). Five hundred ninety-six patients were included in the primary analysis. Nineteen patients were lost to follow-up for the end point 90-day mortality (17 patients because of withdrawal of consent and 2 for other reasons).
Baseline characteristics are reported in Table 1. eTable 2 in Supplement 3 demonstrates the additional criteria for study inclusion. The median time from randomization to initiation of kidney replacement therapy was similar in both groups (2.4 hours [interquartile range {IQR}, 1.2-4.6 hours] vs 2.5 hours [IQR, 1.0-4.5 hours]). Nine hundred sixty-five filters were used in the regional citrate group and 1104 in the systemic heparin anticoagulation group (Table 2). Characteristics of kidney replacement therapy, details on catheters used for kidney replacement therapy, and fluid balance characteristics at different days are reported in eTables 3-5, respectively (Supplement 3).
Table 1. Baseline Characteristics in the RICH Trial.
| Characteristic | Regional citrate anticoagulation (n = 300) | Systemic heparin anticoagulation (n = 296) |
|---|---|---|
| Demographics | ||
| Age, mean (SD), y | 67.5 (12.3) | 67.6 (12.5) |
| Weight, mean (SD), kg | 88.2 (26.8) | 87.4 (23.2) |
| Height, mean (SD), cm | 173.0 (8.6) | 173.7 (9.2) |
| Sex, No. (%) | ||
| Men | 206 (68.7) | 207 (69.9) |
| Women | 94 (31.3) | 89 (30.1) |
| Baseline characteristics | ||
| Baseline creatinine, mean (SD), mg/dL | 1.1 (0.3) | 1.2 (0.4) |
| eGFR, mean (SD), mL/min/1.73 m2 | 69.7 (26.1) | 64.8 (21.8) |
| Comorbidities, No. (%) | ||
| Hypertension | 201 (67.4) | 213 (72.2) |
| Chronic kidney disease (eGFR <60 mL/min/1.73 m2) | 113 (37.7) | 135 (46.1) |
| Cardiac arrhythmia | 105 (35.1) | 109 (36.8) |
| Congestive heart failure | 85 (30.4) | 85 (31.4) |
| Diabetes | 79 (26.3) | 100 (33.8) |
| COPD | 43 (14.3) | 53 (17.9) |
| Cirrhosis | 4 (1.3) | 5 (1.7) |
| Source of hospital admission, No. (%) | ||
| Surgical | 225 (75.0) | 228 (77.0) |
| Nonsurgical | 75 (25.0) | 68 (23.0) |
| Reason for admission to ICU, No. (%) | ||
| Cardiac surgery | 75 (25.0) | 80 (27.0) |
| Sepsisa | 56 (18.7) | 53 (17.9) |
| General surgery | 37 (12.3) | 37 (12.5) |
| Vascular surgery | 28 (9.3) | 26 (8.8) |
| Pneumonia/ARDS | 25 (8.3) | 26 (8.8) |
| Trauma | 17 (5.7) | 12 (4.1) |
| Other | 62 (20.7) | 62 (20.9) |
| Clinical measures at randomization, No. (%) | ||
| Mechanical ventilation | 242 (80.7) | 249 (84.1) |
| Receiving vasopressors | 277 (92.6) | 267 (90.5) |
| Creatinine, mean (SD), mg/dL | 2.4 (1.0) | 2.4 (1.2) |
| Oliguriab | 254 (84.7) | 251 (84.8) |
| No oliguria b | 46 (15.3) | 45 (15.2) |
| SOFA score, mean (SD)c | 11.5 (3.0) | 11.5 (3.0) |
| SOFA cardiovascular score, No. (%) | ||
| 0-2b,d | 43 (14.3) | 39 (13.2) |
| 3-4b,d | 257 (85.7) | 257 (86.8) |
| APACHE II, mean (SD)e | 28.4 (6.9) | 28.5 (7.1) |
| Fluid balance, median (IQR), mLf | 4725.0 (1750.0-8400.0) | 4044.0 (1458.0-8142.0) |
| Culture-proven infection | 145 (48.3) | 134 (45.3) |
| Gram positive | 76 (25.3) | 80 (27.0) |
| Gram negative | 89 (29.7) | 73 (24.7) |
| Gram positive and negative | 36 (12.0) | 31 (10.5) |
| Multidrug resistant | 22 (7.3) | 15 (5.1) |
| Fungal infection | 50 (16.7) | 51 (17.2) |
| Indication for CKRT, No./total No. patients with nonmissing data (%) | ||
| KDIGO stage 3g | 189/300 (63.0) | 173/296 (58.4) |
| Clinical indication | 261/300 (87.0) | 260/296 (87.8) |
| Urine output <200 mL/12 h or anuria | 109/173 (63.0) | 101/172 (58.7) |
| Fluid overload with edema | 89/170 (52.4) | 74/168 (44.0) |
| Serum urea >150 mg/dL | 36/174 (20.7) | 45/176 (25.6) |
| Potassium >6 mmol/L | 21/174 (12.1) | 33/176 (18.8) |
| Blood pH <7.15 | 11/173 (6.4) | 11/176 (6.3) |
| Magnesium >9.7 mg/dL | 0/152 (0) | 0/152 (0) |
| Time-based measures, median (IQR) | ||
| Time from randomization to initiation of CKRT | 2.4 (1.2-4.6) | 2.5 (1.0-4.5) |
| Time from ICU admission to CKRT initiation | ||
| All patients | 44.3 (22.3-91.9) | 35.5 (19.6-86.3) |
| Surgical patients | 47.5 (24.8-95.4) | 40.2 (22.4-86.9) |
| Nonsurgical patients | 28.5 (15.8-76.8) | 22.2 (10.7-78.1) |
| Time from operation to CKRT initiation (surgical patients) | 46.7 (24.8-95.6) | 43.0 (25.5-86.9) |
Abbreviations: ARDS, acute respiratory distress syndrome; APACHE II, Acute Physiology and Chronic Health Evaluation Score; CKRT, continuous kidney replacement therapy; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; ICU, intensive care unit; IQR, interquartile range; KDIGO, Kidney Disease Improving Global Outcomes; RICH, Regional Citrate vs Systemic Heparin Anticoagulation for Continuous Kidney Replacement Therapy in Critically Ill Patients with Acute Kidney Injury; SOFA, Sequential Organ Failure Assessment.
SI conversion factors: To convert creatinine values to μmol/L, multiply by 88.4; magnesium values to mmol/L, multiply by 0.4114.
According to the Sepsis-3 guidelines.
Stratification variable.
Score that evaluates the degree of organ dysfunction and helps determine the mortality risk of a patient. SOFA score values range from 0 to 24, with higher values indicating highest risk of mortality (SOFA 10-11 indicates 50% mortality).
Cardiovascular SOFA score is the subscore of the SOFA score related to the hemodynamic situation of the patient. Values range from 0 to 4, with higher values indicating greater hemodynamic instability and vasoactive support.
An ICU mortality prediction score with values ranging from 0 to 71. Higher values indicate higher probability of mortality (score 25-29 indicates 55% mortality).
Fluid balance from ICU admission to randomization, irrespective of the underlying condition.
KDIGO stages acute kidney injury in 3 categories, with KDIGO stage 3 being acute kidney injury with the greatest severity.
Table 2. Clinical Outcomes in the RICH Trial.
| Regional citrate anticoagulation (n = 300) | Systemic heparin anticoagulation (n = 296) | Absolute difference (95% CI) | OR or HR (95% CI) | P value | |
|---|---|---|---|---|---|
| Primary outcome | |||||
| Filter life span, h | |||||
| Mean (SD) | 44.9 (26.9) | 33.3 (25.1) | 11.2 (8.2 to 14.3) [adjusted]a | <.001 | |
| Median (IQR) | 46.5 (18.8-70.3) | 26.0 (12.0-50.6) | 15.4 (11.3 to 19.5)b | ||
| 90-d all-cause mortality | |||||
| Unadjusted, No. (%) | 150 (51.2)c | 156 (53.6)c | −2.4 (−10.5 to 5.8)d | HR, 0.91 (0.72 to 1.13) | .38 |
| Adjusteda | −6.1 (−12.6 to 0.4)d,e | HR, 0.79 (0.63 to 1.004) | .054 | ||
| Secondary outcomes | |||||
| Dialysis- and kidney-associated outcomes, median (IQR) | |||||
| Total filter downtime, min | 120 (0-720) | 300 (0-930) | −30 (−120 to 0)b | .011 | |
| Duration of CKRT, df | 9.6 (3.9-57.0) | 8.4 (3.6-45.0) | 1.2 (−5.8 to 4.7) | HR, 0.89 (0.72 to 1.09) | .24 |
| Bleeding and transfusion, No./total No. patients who started CKRT (%) | |||||
| Bleedingg | 15/293 (5.1) | 49/290 (16.9) | −11.8 (−16.8 to 6.8)d | OR, 0.27 (0.15 to 0.49) | <.001 |
| Received RBC transfusion | 197/293 (67.2) | 184/290 (63.4) | 3.8 (−3.9 to 11.5)d | OR, 1.18 (0.84 to 1.66) | .34 |
| RBC transfusion volume, median (IQR), mL | 500 (0-1085) [n = 293] | 560 (0-1200) [n = 290] | 0 (0 to 0)b | .85 | |
| Infection, No. (%) | |||||
| New culture-proven infection since start of dialysis | 204 (68.0) | 164 (55.4) | 12.6 (4.9 to 20.3)d | OR, 1.71 (1.23 to 2.39) | .002 |
| Length of stay, median (IQR), d | |||||
| ICU (primary) | |||||
| Naiveh | 16.0 (8.0-29.0) | 13.5 (7.0-25.0) | 1.0 (−1.0 to 3.0)b | .19 | |
| Censoredf | 25.0 (13.0-43.0) | 25.0 (12.0-52.0) | −1.0 (−7.0 to 7.0) | HR, 0.97 (0.78 to 1.21) | .80 |
| Hospital | |||||
| Naiveh | 27.0 (13.0-51.0) | 27.0 (14.0-49.5) | 0.0 (−3.0 to 4.0)b | .83 | |
| Censoredf | 46.0 (28.0-99.0) | 55.0 (32.0-95.0) | −9.0 (−18.0 to 5.0) | HR, 1.04 (0.83 to 1.30) | .75 |
| All-cause mortality, No. (%)i | |||||
| 28 d | 114 (38.7) | 128 (43.8) | −5.1 (−13.0 to 2.9)d | HR, 0.84 (0.66 to 1.09) | .19 |
| 60 d | 137 (46.7) | 147 (50.4) | −3.7 (−11.9 to 4.4)d | HR, 0.88 (0.70 to 1.11) | .27 |
| 365 d | 175 (60.1) | 174 (60.0) | 0.1 (−7.9 to 8.1)d | HR, 0.95 (0.77 to 1.17) | .63 |
Abbreviations: CKRT, continuous kidney replacement therapy; HR, hazard ratio; ICU, intensive care unit; IQR, interquartile range; OR, odds ratio; RBC, red blood cell; RICH, Regional Citrate vs Systemic Heparin Anticoagulation for Continuous Kidney Replacement Therapy in Critically Ill Patients with Acute Kidney Injury.
Results adjusted by study center, baseline cardiovascular SOFA (Sequential Organ Failure Assessment) score, presence or absence of oliguria, sex, and an additional factor that indicates if a patient was recruited before or after implementation of amendment 1 of the study protocol. Further adjustment accounts for the adaptive study design with 1 interim analysis.
Hodges-Lehmann estimator of location shift.
Kaplan-Meier estimator (19 patients were censored before day 90).
Absolute difference in percentages.
Reported absolute difference refers to a male patient with cardiovascular SOFA score 3-4 and oliguria, who was recruited after implementation of amendment 1 of the study protocol.
Censored at day of death or end of follow-up, whichever occurred first.
Major bleeding with transfusion requirement, and/or need for reoperation, and/or new onset of intracranial bleeding without traumatic event.
Stay was considered to be finished regardless of the reason for the end of stay, handling scheduled discharges of living patients, deaths, and end of follow-up in the same way.
Kaplan-Meier estimator.
Primary Outcomes
Filter life span was significantly longer in the regional citrate anticoagulation group (unadjusted median, 46.5 hours [IQR, 18.8-70.3 hours] vs 26.0 hours [IQR, 12.0-50.6 hours]; unadjusted absolute difference, 11.6 hours [95% CI, 8.5 to 14.7 hours]; adjusted absolute difference, 11.2 hours [95% CI, 8.2 to 14.3 hours]; P < .001) (Table 2). Results were confirmed when filter life span was censored at 72 hours to account for the mandatory filter change at 72 hours (unadjusted hazard ratio [HR], 0.64 [95% CI, 0.59 to 0.71]). There was no significant difference regarding the coprimary end point 90-day all-cause mortality (150/300 vs 156/296 [Kaplan-Meier estimator percentages, 51.2% vs 53.6%]; unadjusted absolute difference, –2.4% [95% CI, –10.5% to 5.8%); unadjusted HR, 0.91 [95% CI, 0.72 to 1.13]; unadjusted P = .38; adjusted absolute difference, –6.1% [95% CI, –12.6% to 0.4%]; primary adjusted HR, 0.79 [95% CI, 0.63 to 1.004]; primary adjusted P = .054) (Table 2, Figure 2).
Figure 2. Kaplan-Meier Curves With Hall-Wellner Confidence Bands.
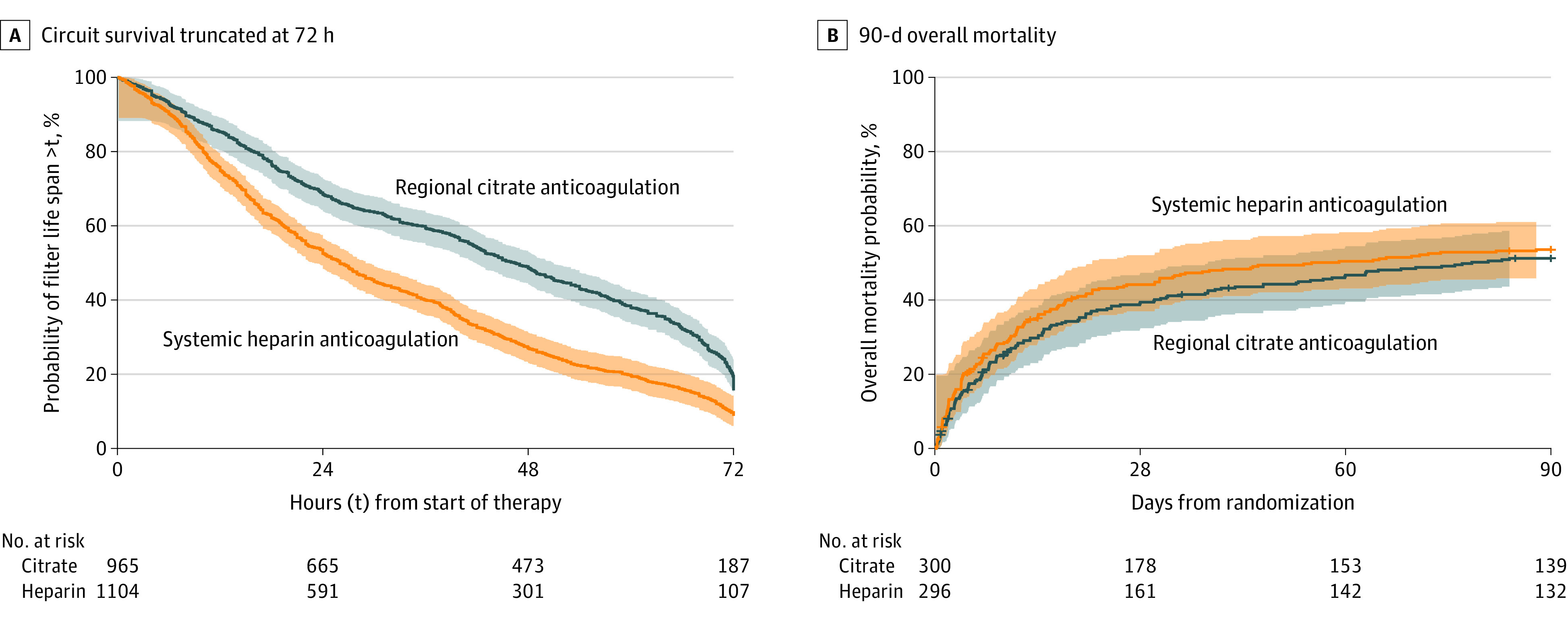
A, Time (hours) from start of kidney replacement therapy to filter replacement. All circuits were observed to failure or 72 hours. B, 90-day overall mortality, with median (interquartile range) observation time 90 days. Ticks perpendicular to curves indicate censored patients (n = 19).
Secondary Outcomes
Patients randomized to the regional citrate anticoagulation group showed a significantly reduced total treatment downtime (120 minutes [IQR, 0-720 minutes] vs 300 minutes [IQR, 0-930 minutes]; P = .01), significantly higher rates of new infections since start of dialysis (68.0% vs 55.4%; P = .002; odds ratio [OR], 1.71 [95% CI, 1.23 to 2.39]), and persistent kidney dysfunction after 90 days (28.4% vs 15.0%; P = .02; OR, 2.25 [95% CI, 1.11 to 4.56]) (Table 2; eTable 6 in Supplement 3). Details on infections are reported in eTable 7 in Supplement 3.
Bleeding complications were significantly lower in the regional citrate as compared with the systemic heparin anticoagulation group (5.1% vs 16.9%; P < .001; OR, 0.27 [95% CI, 0.15 to 0.49]) (Table 2). However, the number of transfused patients was not significantly different in the 2 groups (67.2% in the regional citrate group vs 63.4% in the systemic heparin group; P = .34; OR, 1.18 [95% CI, 0.84 to 1.66]) (Table 2; eFigure 1 in Supplement 3).
There were no significant differences between the groups in other secondary outcomes, namely, duration of kidney replacement therapy; ICU and hospital length of stay; SOFA scores; mortality at days 28, 60, and 365; requirement of kidney replacement therapy at days 28, 60, 90, and 365; persistent kidney dysfunction at days 28, 60, and 365; and major adverse kidney events at days 28, 60, 90, and 365 (Table 2; eFigure 2 and eTable 6 in Supplement 3).
Adverse Events
Patients in the regional citrate anticoagulation group more frequently developed severe alkalosis (2.4% vs 0.3%) and hypophosphatemia (15.4% vs 6.2%) (Table 3). Hyperkalemia was more frequent in the heparin group (0.0% in citrate group vs 1.4% in heparin anticoagulation group). Gastrointestinal complications were lower in the regional citrate as compared with the systemic heparin anticoagulation group (0.7% vs 3.4%). All other adverse events did not differ between the groups (Table 3; eFigure 3 in Supplement 3).
Table 3. Adverse Events.
| No. (%) | ||
|---|---|---|
| Regional citrate anticoagulation (n = 300) | Systemic heparin anticoagulation (n = 296) | |
| Hypophosphatemiaa | 45 (15.4) | 18 (6.2) |
| Severe cardiac rhythm disorders | 10 (3.4) | 9 (3.1) |
| Heparin-induced thrombocytopeniab | 8 (2.7) | 9 (3.1) |
| Severe alkalosisc | 7 (2.4) | 1 (0.3) |
| Neurologic complicationsd | 4 (1.4) | 4 (1.4) |
| Severe hypocalcemiae | 4 (1.4) | 1 (0.3) |
| Respiratory complicationsf | 3 (1.0) | 6 (2.1) |
| Citrate accumulationg | 2 (0.7) | 0 |
| Gastrointestinal complicationsh | 2 (0.7) | 10 (3.4) |
| Other cardiovascular complicationsi | 2 (0.7) | 5 (1.7) |
| Metabolic acidosisj | 1 (0.3) | 2 (0.7) |
| Hyperkalemia | 0 | 4 (1.4) |
| Thrombotic, thromboembolic complications | 0 | 3 (1.0) |
Phosphate level less than 0.5 mmol/L.
Positive antibody test result.
pH greater than 7.50 and bicarbonate concentration greater than 30 mmol/L.
Includes complications such as seizures, delirium, and hypoxic brain damage.
Ionized calcium level less than 0.9 mmol/L.
Includes complications such as pneumonia, acute respiratory distress syndrome, Horowitz index less than 200 for at least 1 hour, and respiratory complications with the need of reintubation or recannulation.
Ratio of Ca2+ total to Ca2+ion, 2.5 or greater.
Includes all gastrointestinal bleeding events requiring at least 1 unit of packed red blood cells.
Includes complications such as ischemia, cardiogenic shock, cardiac decompensation.
pH less than 7.2 and bicarbonate concentration less than 20 mmol/L (excluding lactic acidosis).
A preplanned subgroup analysis of surgical and nonsurgical patients revealed the same results in terms of primary and secondary outcomes and adverse events (eTables 8-10 in Supplement 3). In nonsurgical patients, bleeding and gastrointestinal complications were not different in the 2 groups (eTable 10 in Supplement 3).
Post Hoc Analyses
After including baseline fluid balance as variable for adjustment, results regarding the treatment effect on the coprimary end point 90-day all-cause mortality did not differ significantly (HR, 0.89 [95% CI, 0.71 to 1.12]; P = .33).
In post hoc multivariable analyses adjusted for the factors anticoagulation strategy, study center, cardiovascular SOFA score, presence or absence of oliguria, sex, and recruitment before vs after protocol amendment 1, the modality of kidney replacement therapy was significantly associated with filter life span, with continuous venovenous hemodialysis (CVVHD) having the longest filter life span (CVVHD vs continuous venovenous hemofiltration [CVVH]: mean difference, 14.68 hours [95% CI, 5.71 to 23.65 hours]; P = .001; CVVHD vs continuous venovenous hemodiafiltration [CVVHDF]: mean difference, 5.14 hours [95% CI, 0.23 to 10.05 hours]; P = .04), followed by CVVHDF (CVVHDF vs CVVH: mean difference, 9.54 hours [95% CI, 0.23 to 18.85 hours]; P = .045) and CVVH. There was no effect on 90-day mortality.
A multivariable model with the additional time-dependent factor new infections since start of dialysis showed that after a new infection occurred, mortality was increased significantly (HR, 1.64 [95% CI, 1.26 to 2.13]; P = .002). The difference in mortality between regional citrate anticoagulation and systemic heparin anticoagulation remained nonsignificant, regardless of whether the corresponding HR was adjusted for new infection since start of dialysis (HR, 0.84 [95% CI, 0.67 to 1.06]; P = .14).
A further multivariable logistic regression analysis demonstrated that infection rate increased with longer filter life (OR, 1.08 [95% CI, 1.05 to 1.11] per additional 12 hours; P < .001).
Discussion
In this randomized clinical trial of critically ill patients with acute kidney injury who were receiving continuous kidney replacement therapy, anticoagulation with regional citrate, compared with systemic heparin anticoagulation, resulted in significantly longer dialysis filter life span but no statistically significant difference in 90-day all-cause mortality. However, the trial was terminated early and was therefore underpowered to reach definitive conclusions about the comparative effect of these anticoagulation strategies on mortality. There were fewer bleeding and gastrointestinal complications in the regional citrate anticoagulation group but more adverse events in terms of metabolic complications and a significantly higher infection rate.
Continuous kidney replacement therapy often requires the use of anticoagulation to ensure filter function and thus adequate hemofiltration. The KDIGO guidelines for acute kidney injury recommend the use of regional citrate anticoagulation in this setting. However, the evidence is based on small randomized clinical trials3,5,18 and is therefore only a weak suggestion.1 This multicenter trial confirmed the results of these previous trials and demonstrated longer filter life span in the regional citrate anticoagulation group, which was associated with a reduced total downtime. It is possible that filter life span may be even longer than demonstrated, as in the regional citrate anticoagulation group more filters were interrupted for scheduled or planned interventions or procedures, thereby possibly affecting life span. However, this remains only theoretical, as these data reflect daily clinical practice. Moreover, blood flow may also affect filter life span through clotting, and the blood flows in this trial were lower than recommended by the KDIGO guideline.1 However, filtration fraction, which has been described as associated with filter clotting, was below the described cutoff value of 25%, making it unlikely to have affected clotting and consequently filter life span in this trial.19
It is possible that the short-term benefit of longer filter life span resulted in an improved outcome through higher effectiveness of the therapy, namely, reduced occurrence of adverse events and complications (besides reduced workload and costs). Most trials comparing regional citrate with systemic heparin anticoagulation in continuous kidney replacement therapy had a low number of patients and mainly focused on filter life span.3,18,20,21 Only 3 trials reported mortality as an outcome measure.5,8,22 The findings of this trial are in concordance with those of 2 recent trials comparing systemic heparin to regional citrate anticoagulation,5,8 in which mortality was similar for both groups. However, the results are in contrast with a trial comparing citrate anticoagulation with the low-molecular-weight heparin nadroparin,22 in which significantly lower mortality was observed in the citrate anticoagulation group (90-day mortality, 62% for nadroparin and 45% for citrate; P = .03). However, this was a single-center trial, and as with many single-center trials, treatment effects might be inflated.23
The potential advantage of citrate anticoagulation for continuous kidney replacement therapy is that it may reduce the occurrence of adverse events as systemic effects on anticoagulation are avoided. The results demonstrated that patients in the regional citrate anticoagulation group had lower rates of bleeding and gastrointestinal complications (mainly resulting from bleeding). These results were also confirmed in the prespecified subgroup analysis of surgical patients and agree with those from recent reports.3,18,21 However, no significant difference in transfusion was detected between the 2 groups. Even in the prespecified subgroup analyses, transfusion of packed red blood cells was not different. One explanation for this result is that bleeding complications in the heparin group were not significantly different for either the surgical or the nonsurgical patients. Another explanation might be that heparin anticoagulation was directly stopped if bleeding complications occurred. In addition, the transfusion trigger was not standardized and transfusion also reflects blood loss through sampling for routine analyses, frequently performed in ICUs.
In terms of heparin-induced thrombocytopenia, the results demonstrated no significant difference in the 2 groups. Prophylactic anticoagulation is necessary in nearly all critically ill patients to prevent thromboembolic complications and is recommended by guidelines.24 Even patients receiving citrate anticoagulation are exposed to heparin; thus, the risk for heparin-induced thrombocytopenia is not diminished.
Metabolic derangements are a potential disadvantage of using citrate anticoagulation, and complication rates in this cohort are similar to those in previous reports.5,8,11,22 Metabolic derangements are closely linked to the use of certain solutions for kidney replacement therapy. For instance, citrate solutions of higher concentrations are associated with higher occurrence of metabolic alkalosis.25 Further, hypophosphatemia is a notable metabolic derangement, with higher rates in the citrate anticoagulation group. Higher doses of kidney replacement therapy have been shown to be associated with the occurrence of hypophosphatemia.16,17 Because the dose in this trial was lower compared with doses in the other 2 trials, it is unlikely that the dose alone was responsible for the occurrence of hypophosphatemia. However, the underlying cause remains unknown.
This study showed that regional citrate anticoagulation significantly affects the rate of infection. Because catheter details as well as the number of catheters inserted were similar in both groups, catheter infections may not be the underlying cause. While citrate has been shown to have antimicrobial properties,26,27,28 the increased rate of nosocomial infections found in this study may be related to factors such as greater monitoring (circuit sampling) occurring with time at risk through longer filter life span. Another explanation could be a reduced filter efficacy over time with filters in use longer. In addition, hypophosphatemia may affect infection rates, as hypophosphatemia has been shown to be associated with negative effects on leukocyte chemotaxis and phagocytosis, negatively affecting immune response, and it is further correlated with higher levels of inflammatory cytokines.29 However, this observation of a higher infection rate in the regional citrate group has to be investigated in greater detail in future trials.
Limitations
This study has several limitations. First, blinding of the investigators or the treating intensivists was not possible because of the complex nature of kidney replacement therapy. Second, the trial was stopped early, because the results of the interim analysis showed that the preplanned end of the trial according to the protocol was reached. The results of this analysis demonstrated a statistically significant longer duration of filter life span in the regional citrate anticoagulation group and no significant difference regarding mortality. However, the trial was underpowered to detect smaller differences in mortality rate. Third, a considerable amount of follow-up data were missing, which may result in an ascertainment bias. However, the results of this trial are comparable with results of other trials, including patients with severe acute kidney injury requiring kidney replacement therapy, and these data are in line with the known problems of missing data when performing a long-term follow-up of patients after severe acute kidney injury.30,31,32,33
Conclusions
Among critically ill patients with acute kidney injury receiving continuous kidney replacement therapy, anticoagulation with regional citrate, compared with systemic heparin anticoagulation, resulted in significantly longer filter life span. The trial was terminated early and was therefore underpowered to reach conclusions about the effect of anticoagulation strategy on mortality.
Trial Protocol
Statistical Analysis Plan
eMethods
eResults
eFigure 1. Hemoglobin Values at Different Time Points
eFigure 2. SOFA Scores at Different Time Points
eFigure 3. Magnesium Levels at Different Time Points
eTable 1. Protocol Changes After Amendment
eTable 2. Additional Inclusion Criteria for Dialysis Initiation
eTable 3.Characteristics of Kidney Replacement Therapy
eTable 4. Characteristics of the Catheters Used for Kidney Replacement Therapy
eTable 5. Fluid Balance at Different Days
eTable 6. Further Dialysis and Kidney Associated Outcomes
eTable 7. Details on Infections
eTable 8. Subgroup Analysis Surgical vs. Non-surgical Patients
eTable 9. Adverse Events in Surgical Events
eTable 10. Adverse Events in Non-surgical Events
eReferences
Data Sharing Statement
References
- 1.Kellum J A LN, Aspelin P, Barsoum RS, et al. KDIGO clinical practice guideline for acte kidney injury 2012. Kidney Int Suppl. 2012;2(1):1-138. https://kdigo.org/wp-content/uploads/2016/10/KDIGO-2012-AKI-Guideline-English.pdf [Google Scholar]
- 2.van de Wetering J, Westendorp RG, van der Hoeven JG, Stolk B, Feuth JD, Chang PC. Heparin use in continuous renal replacement procedures: the struggle between filter coagulation and patient hemorrhage. J Am Soc Nephrol. 1996;7(1):145-150. [DOI] [PubMed] [Google Scholar]
- 3.Kutsogiannis DJ, Gibney RT, Stollery D, Gao J. Regional citrate versus systemic heparin anticoagulation for continuous renal replacement in critically ill patients. Kidney Int. 2005;67(6):2361-2367. doi: 10.1111/j.1523-1755.2005.00342.x [DOI] [PubMed] [Google Scholar]
- 4.Gattas DJ, Rajbhandari D, Bradford C, Buhr H, Lo S, Bellomo R. A randomized controlled trial of regional citrate versus regional heparin anticoagulation for continuous renal replacement therapy in critically ill adults. Crit Care Med. 2015;43(8):1622-1629. doi: 10.1097/CCM.0000000000001004 [DOI] [PubMed] [Google Scholar]
- 5.Schilder L, Nurmohamed SA, Bosch FH, et al. ; CASH Study Group . Citrate anticoagulation versus systemic heparinisation in continuous venovenous hemofiltration in critically ill patients with acute kidney injury: a multi-center randomized clinical trial. Crit Care. 2014;18(4):472. doi: 10.1186/s13054-014-0472-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu MY, Hsu YH, Bai CH, Lin YF, Wu CH, Tam KW. Regional citrate versus heparin anticoagulation for continuous renal replacement therapy: a meta-analysis of randomized controlled trials. Am J Kidney Dis. 2012;59(6):810-818. doi: 10.1053/j.ajkd.2011.11.030 [DOI] [PubMed] [Google Scholar]
- 7.Zhang Z, Hongying N. Efficacy and safety of regional citrate anticoagulation in critically ill patients undergoing continuous renal replacement therapy. Intensive Care Med. 2012;38(1):20-28. doi: 10.1007/s00134-011-2438-3 [DOI] [PubMed] [Google Scholar]
- 8.Hetzel GR, Schmitz M, Wissing H, et al. Regional citrate versus systemic heparin for anticoagulation in critically ill patients on continuous venovenous haemofiltration: a prospective randomized multicentre trial. Nephrol Dial Transplant. 2011;26(1):232-239. doi: 10.1093/ndt/gfq575 [DOI] [PubMed] [Google Scholar]
- 9.Meersch M, Küllmar M, Wempe C, et al. ; SepNet Critical Care Trials Group . Regional Citrate versus Systemic Heparin Anticoagulation for Continuous Renal Replacement Therapy in Critically Ill Patients With Acute Kidney Injury (RICH) trial: study protocol for a multicentre, randomised controlled trial. BMJ Open. 2019;9(1):e024411. doi: 10.1136/bmjopen-2018-024411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 11.Morgera S, Schneider M, Slowinski T, et al. A safe citrate anticoagulation protocol with variable treatment efficacy and excellent control of the acid-base status. Crit Care Med. 2009;37(6):2018-2024. doi: 10.1097/CCM.0b013e3181a00a92 [DOI] [PubMed] [Google Scholar]
- 12.Durão MS, Monte JC, Batista MC, et al. The use of regional citrate anticoagulation for continuous venovenous hemodiafiltration in acute kidney injury. Crit Care Med. 2008;36(11):3024-3029. doi: 10.1097/CCM.0b013e31818b9100 [DOI] [PubMed] [Google Scholar]
- 13.Kramer L, Bauer E, Joukhadar C, et al. Citrate pharmacokinetics and metabolism in cirrhotic and noncirrhotic critically ill patients. Crit Care Med. 2003;31(10):2450-2455. doi: 10.1097/01.CCM.0000084871.76568.E6 [DOI] [PubMed] [Google Scholar]
- 14.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103-115. doi: 10.2307/2529712 [DOI] [PubMed] [Google Scholar]
- 15.Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17(1):R25. doi: 10.1186/cc12503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palevsky PM, Zhang JHY, O'Connor TZ, et al. ; VA/NIH Acute Renal Failure Trial Network . Intensity of renal support in critically ill patients with acute kidney injury [published correction appears in N Engl J Med. 2009;361(24):2391]. N Engl J Med. 2008;359(1):7-20. doi: 10.1056/NEJMoa0802639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellomo R, Cass A, Cole L, et al. ; RENAL Replacement Therapy Study Investigators . Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361(17):1627-1638. doi: 10.1056/NEJMoa0902413 [DOI] [PubMed] [Google Scholar]
- 18.Monchi M, Berghmans D, Ledoux D, Canivet JL, Dubois B, Damas P. Citrate vs. heparin for anticoagulation in continuous venovenous hemofiltration: a prospective randomized study. Intensive Care Med. 2004;30(2):260-265. doi: 10.1007/s00134-003-2047-x [DOI] [PubMed] [Google Scholar]
- 19.Joannidis M, Oudemans-van Straaten HM. Clinical review: patency of the circuit in continuous renal replacement therapy. Crit Care. 2007;11(4):218. doi: 10.1186/cc5937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bagshaw SM, Laupland KB, Boiteau PJ, Godinez-Luna T. Is regional citrate superior to systemic heparin anticoagulation for continuous renal replacement therapy? a prospective observational study in an adult regional critical care system. J Crit Care. 2005;20(2):155-161. doi: 10.1016/j.jcrc.2005.01.001 [DOI] [PubMed] [Google Scholar]
- 21.Betjes MG, van Oosterom D, van Agteren M, van de Wetering J. Regional citrate versus heparin anticoagulation during venovenous hemofiltration in patients at low risk for bleeding: similar hemofilter survival but significantly less bleeding. J Nephrol. 2007;20(5):602-608. [PubMed] [Google Scholar]
- 22.Oudemans-van Straaten HM, Bosman RJ, Koopmans M, et al. Citrate anticoagulation for continuous venovenous hemofiltration. Crit Care Med. 2009;37(2):545-552. doi: 10.1097/CCM.0b013e3181953c5e [DOI] [PubMed] [Google Scholar]
- 23.Unverzagt S, Prondzinsky R, Peinemann F. Single-center trials tend to provide larger treatment effects than multicenter trials: a systematic review. J Clin Epidemiol. 2013;66(11):1271-1280. doi: 10.1016/j.jclinepi.2013.05.016 [DOI] [PubMed] [Google Scholar]
- 24.Guyatt GH, Akl EA, Crowther M, Schunemann HJ, Gutterman DD, Lewis SZ. Introduction to the ninth edition: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2):48S-52S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davenport A, Tolwani A. Citrate anticoagulation for continuous renal replacement therapy (CRRT) in patients with acute kidney injury admitted to the intensive care unit. NDT Plus. 2009;2(6):439-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bos JC, Grooteman MP, van Houte AJ, Schoorl M, van Limbeek J, Nubé MJ. Low polymorphonuclear cell degranulation during citrate anticoagulation: a comparison between citrate and heparin dialysis. Nephrol Dial Transplant. 1997;12(7):1387-1393. doi: 10.1093/ndt/12.7.1387 [DOI] [PubMed] [Google Scholar]
- 27.Gritters M, Grooteman MP, Schoorl M, et al. Citrate anticoagulation abolishes degranulation of polymorphonuclear cells and platelets and reduces oxidative stress during haemodialysis. Nephrol Dial Transplant. 2006;21(1):153-159. doi: 10.1093/ndt/gfi069 [DOI] [PubMed] [Google Scholar]
- 28.Kottke-Marchant K, Anderson JM, Rabinovitch A, Huskey RA, Herzig R. The effect of heparin vs. citrate on the interaction of platelets with vascular graft materials. Thromb Haemost. 1985;54(4):842-848. doi: 10.1055/s-0038-1660145 [DOI] [PubMed] [Google Scholar]
- 29.Barak V, Schwartz A, Kalickman I, Nisman B, Gurman G, Shoenfeld Y. Prevalence of hypophosphatemia in sepsis and infection: the role of cytokines. Am J Med. 1998;104(1):40-47. doi: 10.1016/S0002-9343(97)00275-1 [DOI] [PubMed] [Google Scholar]
- 30.Barbar SD, Clere-Jehl R, Bourredjem A, et al. ; IDEAL-ICU Trial Investigators and the CRICS TRIGGERSEP Network . Timing of renal-replacement therapy in patients with acute kidney injury and sepsis. N Engl J Med. 2018;379(15):1431-1442. doi: 10.1056/NEJMoa1803213 [DOI] [PubMed] [Google Scholar]
- 31.Zarbock A, Kellum JA, Schmidt C, et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: the ELAIN randomized clinical trial. JAMA. 2016;315(20):2190-2199. doi: 10.1001/jama.2016.5828 [DOI] [PubMed] [Google Scholar]
- 32.Pannu N, Klarenbach S, Wiebe N, Manns B, Tonelli M; Alberta Kidney Disease Network . Renal replacement therapy in patients with acute renal failure: a systematic review. JAMA. 2008;299(7):793-805. doi: 10.1001/jama.299.7.793 [DOI] [PubMed] [Google Scholar]
- 33.Silver SA, Siew ED. Follow-up care in acute kidney injury: lost in transition. Adv Chronic Kidney Dis. 2017;24(4):246-252. doi: 10.1053/j.ackd.2017.05.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eMethods
eResults
eFigure 1. Hemoglobin Values at Different Time Points
eFigure 2. SOFA Scores at Different Time Points
eFigure 3. Magnesium Levels at Different Time Points
eTable 1. Protocol Changes After Amendment
eTable 2. Additional Inclusion Criteria for Dialysis Initiation
eTable 3.Characteristics of Kidney Replacement Therapy
eTable 4. Characteristics of the Catheters Used for Kidney Replacement Therapy
eTable 5. Fluid Balance at Different Days
eTable 6. Further Dialysis and Kidney Associated Outcomes
eTable 7. Details on Infections
eTable 8. Subgroup Analysis Surgical vs. Non-surgical Patients
eTable 9. Adverse Events in Surgical Events
eTable 10. Adverse Events in Non-surgical Events
eReferences
Data Sharing Statement