Highlights
-
•
Serrapeptidase has immense applications in therapeutic areas.
-
•
Serrapeptidase is anti-inflammatory, anti-biofilm, analgesic, and anti-edemic.
-
•
Serrapeptidase need more scientific understanding for further exploration.
Keywords: Serratiopeptidase, Therapeutic application, Anti-inflammatory, Anti-biofilm, Clinical study
Abstract
Therapeutic applications of enzymes have been widely accepted in clinical practices for decades. Proteolytic enzymes in particular, have been used for the treatment of diseases and disorders. Serratiopeptidase is a proteolytic enzyme having immense applications in therapeutic areas which have been validated by several in vitro, in vivo, and clinical studies as well as through anecdotal evidences. These applications are attributable to its versatile properties including anti-inflammatory, anti-biofilm, analgesic, anti-edemic, and fibrinolytic effects. The significant impact of serratiopeptidase reported needs to be backed by more scientific data. This review encompasses the details of therapeutic applications of serratiopeptidase based on available in vitro, in vivo, and clinical studies. We found some strong evidences regarding the efficacy of serratiopeptidase. However data on safety, tolerability, and its mechanism of action need detailing. This review aims to further explore the available literature on serratiopeptidase as well as provide scientific details for existing applications.
1. Introduction
Enzymes are an essential part of most metabolic processes and are directly or indirectly important for the normal functioning of the human body. They control many physiological functions such as digestion, metabolism, immune function, reproduction, and respiration. Enzymes are obtained from plant, animal, and microbial sources and currently used in clinical practices for the treatment and management of various diseases and disorders. Enzyme-based therapeutics is recently gaining more attention due to its selectivity, efficiency, and safety profile. The therapeutic efficacy of various enzymes including trypsin, chymotrypsin, papain, and bromelain has been proven [1]. Serratiopeptidase (serralysin/ serratia-protease/serrapeptidase) is a widely used proteolytic enzyme in therapeutic applications. It has shown significant anti-inflammatory, anti-edemic, and analgesic effects in various areas including surgery, orthopaedics, otorhinolaryngology, gynaecology, and dentistry [2]. It is well known amongst researchers for its caseinolytic (proteolytic) and fibrinolytic properties.
Serratiopeptidase is a zinc containing metalloprotease of molecular weight 45–60 kDa. The enzyme has an EC number 3.4.24.40 and belongs to the group Serralysin. It is originally obtained from Serratia marcescens isolated from the intestine of the silkworm Bombyx mori L. Extensive review on analytical techniques used in the qualitative and quantitative analysis of serratiopeptidase has been published by [3], where the authors pointed out a need for selective and specific techniques for the quantification of serratiopeptidase. A detailed review of existing evidences for serratiopeptidase has been reported by [4]. The major role of this enzyme against inflammation is well mapped in the review written by [1]. Other applications of serratiopeptidase in clinical practices majorly include breast disease, atherosclerosis, Alzheimer’s disease, sinusitis, hepatitis, lung disorders, and uterine fibroids [5].
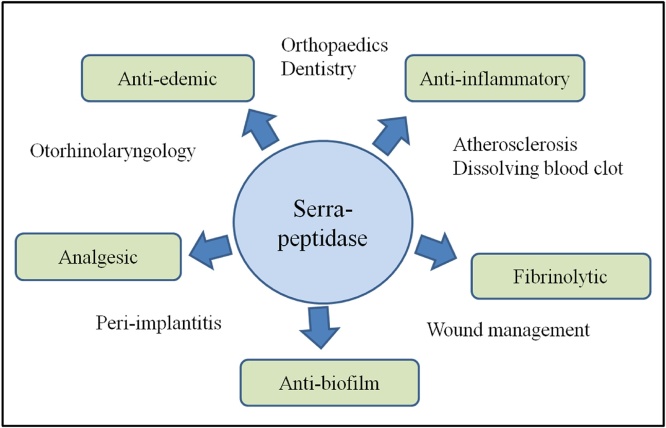
This review is a comprehensive study of the therapeutic potential of serratiopeptidase as well as its regulatory status. We have included in vitro, in vivo, and clinical study reports as well as some unconcluded studies from different therapeutic areas to our best capacity. This review helps to elucidate the potential of serratiopeptidase as well as identify lacunas in using it to its full extent and hence, identify areas of future research (Fig. 1).
Fig. 1.
Properties of serratiopeptidase contributing to its wide applications in therapeutics.
2. Role of enzymes in therapeutics
In 1964, de Duve first suggested use of enzymes as replacement therapy for genetic disorders [6]. With the emergence of novel diseases and failure of conventional treatments in certain conditions, enzyme-based therapeutics is playing an important role in the current century. Target specificity and multiple quick substrate conversion are two main properties which have made enzymes successful and popular over non-enzymatic drugs in therapeutic areas. Enzymes are widely used to treat cancer, cardiovascular diseases, digestive disorders, wound debridement, lysosomal storage disease, inflammatory reaction, genetic disorders, and bleeding disorders [7,8]. Several enzymes have been cited in literature for their therapeutic potential including collagenase, proteases, streptokinase, lysostaphin, laccase, glutaminase, and lipase [9]. Specifically, serine proteases have been used to treat blood vascular disorders; l-asparaginase and l,l-glutaminase have shown efficacy in the treatment of acute lymphoblastic leukemia; and caspases have been used for treating cancer and many classes of viruses [1].
Some obstacles in using enzymes in therapeutic applications include their large size making it difficult for distribution in the body, immunogenicity, short half-life, and impurities. Due to these factors, very few enzymes have been approved by the FDA despite their proven efficacy. Enzymes including altephase, reteplase, tenecteplase, urokinase, streptokinase, and anistreplase are FDA approved for the treatment of cardiovascular diseases [8]. Few enzymes with notable divergent properties include serratiopeptidase, superoxide dismutase, adenosine deaminase, phenylalanine ammonia lyase, dornase, and rasburicase [8]. Our review highlights the therapeutic molecule serratiopeptidase and its wide clinical applications.
3. Serratiopeptidase
Conventionally, serratiopeptidase is produced from Serratia marcescens, a Gram negative opportunistic pathogen in nutrient rich growth medium. The details of production process and media optimization were explained in earlier review [5]. It has been shown to have maximum activity at pH 9.0 and temperature 40 °C and is inactivated at 55 °C in 15 min [2]. It is stable in a wide range of pH (pH 3–10) as revealed in the circular dichroism study, where it showed stable secondary structure [10]. The gene encoding serratiopeptidase is made up of 470 amino acids, devoid of sulfur containing amino acids. The enzyme is produced, purified, characterized and modeled using SWISS-MODEL by [11] where authors have authenticated the structure by assessing the Ramachandran plot using PROCHECK server.
Presently, the demand of serratiopeptidase for the industry and pharmaceuticals is being satisfied by wild or mutant strains of Serratia marcescens. However, the pathogenic nature of the organism and hazard associated with the bulk biomass released after fermentation necessitates the research on the development of recombinant molecule. Attempts have been carried out to express serratiopeptidase genes in Escherichia coli using suitable vectors [5]. The failure of many of the attempts attributed to the unregulated intracellular expression of proteases causing cell lysis, growth inhibition, instability of the expression plasmids, lack of protein expression, or deposition of the proteins into non-functional misfolded aggregates [12]. Recently, [12] have demonstrated a production of recombinant serratiopeptidase in Escherichia coli successfully. Further, [13] have elucidated the optimized growth media and process conditions for the large scale production of the recombinant serratiopeptidase.
4. Application of serratiopeptidase in therapeutics
Serratiopeptidase has been used by healthcare professionals in Japan and Europe for therapeutic applications for decades. Recently the clinical use of serratiopeptidase alone or in combination with other drugs is increasing worldwide.
4.1. Serratiopeptidase as an anti-inflammatory agent
Inflammation serves as a defense mechanism against injury and infection. The immune system responds rapidly to any foreign substances as well as tissue injury by recruiting immune cells and inflammatory mediators to the target site. Hence, inflammation is considered to be the cleaning process of the body leading to maintenance of homeostasis [14]. Based on the pathologic conditions of the tissue and intensity of the trigger, inflammation can be acute or chronic. Though acute inflammation is a protective measure against injury or infection, failure of its resolution leads to chronic inflammation. Inflammatory disorders such as arthritis, sinusitis, bronchitis, fibrocystic breast disease, and carpal tunnel syndrome etc. are common worldwide. Conventional nonsteroidal anti-inflammatory drugs (NSAIDs) alone or in combination with other drugs are prescribed to combat acute inflammation, whereas steroidal drugs are combined with NSAIDs to treat chronic inflammation [1]. The enormous limitations of these drugs have necessitated research on other possible treatments, including natural molecules. Hence, enzyme-based drugs are now popular in many therapeutic areas including inflammation.
Serine proteases are commonly used in therapeutic areas including inflammation. They were found to have high affinity for cyclooxygenases (COX-I and COX-II), key enzymes in the production of different inflammatory mediators. Serratiopeptidase was first used for its anti-inflammatory effects in Japan in 1957 [1]. Further, many researchers have evaluated the potential of serratiopeptidase against inflammation in different therapeutic areas. It has also been used along with other NSAIDs to achieve a combined effect. Though serratiopeptidase has been proven to be an effective anti-inflammatory molecule in many studies, efforts are needed to optimize its dose based on the application. The concentration of serratiopeptidase in the plasma was found to vary with body mass. Hence, validated cross over studies and optimization are necessary steps to be taken before recommending and prescribing serratiopeptidase [15].
4.1.1. Pre-clinical and clinical studies
[16] compared the anti-inflammatory effect of serratiopeptidase with aspirin and other proteolytic enzymes trypsin and chymotrypsin in albino rats against carrageenan induced paw edema. Serratiopeptidase showed better anti-inflammatory activity alone as well as showed a synergistic effect with aspirin in both acute and subacute models of inflammation in rats. Another small pre-clinical study by [17] on 16 Charles Foster albino rats evaluated the anti-inflammatory effect of serratiopeptidase against diclofenac sodium. Serratiopeptidase at (10−20 mg/kg of body weight) showed comparable results to that of diclofenac sodium (0.5 mg/kg) in inhibiting acute as well as chronic inflammation in paw edema. The dosage of serratiopeptidase required was higher as compared to diclofenac. This may be due to the low bioavailability of serratiopeptidase, which can be improved using a suitable delivery mode (explained in appropriate section of this review).
Ulcerative colitis is an inflammatory bowel disease caused by overstimulation or inadequate regulation of the mucosal immune system, affecting rectal and colonic mucosa. [18] conducted an impactful study to test the potential of serratiopeptidase on acetic acid-induced ulcerative colitis in mice. Several inflammatory markers including C-reactive protein, myeloperoxidase, glutathione, and nitric oxide were examined, along with histopathological examination. Serratiopeptidase reduced the disease activity index and prevented colonic shortening, spleen enlargement, glutathione depletion, lipid peroxidation, and nitric oxide production as compared to the control group. Further, there was significant reduction in C-reactive protein level in serratiopeptidase treated mice as compared to control. Moreover, myeloperoxidase, an important enzyme marker of inflammation was reduced by serratiopeptidase treatment. These results confirm the anti-inflammatory potential of serratiopeptidase.
There is much clinical data available on the anti-inflammatory effects of serratiopeptidase on pain, swelling, and trismus, details of which are shown in Table 1.
Table 1.
Clinical studies in various therapeutic areas to evaluate efficiency of serratiopeptidase as an anti-inflammatory agent.
| Type of Inflammation | Treatment method (Drug(s) taken along with oral administration of serratiopeptidase) | Duration of treatment | Result | Adverse effect reported (Yes/No) | Reference |
|---|---|---|---|---|---|
| Knee osteoarthritis | Metformin | 5 months | -Reduced pain score -Low level of Tumor necrosis factor alpha (TNF-α), interleukin 1 beta (IL-1ß) and interleukin 8 (IL-8) |
No | Ateia et al. [19] |
| Acne vulgaris | Doxycycline with retin A cream application and panoxyl gel | 5 months | Combined therapy showed significantly rapid improvement in acne appearance | – | Mikhael and Mohammed [20] |
| Postoperative pain, swelling and trismus after surgical removal of mandibular third molars | Methylprednisolone | 5 days | Serratiopeptidase showed moderate analgesic activity but effective control of swelling and trismus compared to methylprednisolone | – | Chappi et al. [21] |
| Postoperative pain, swelling and trismus after surgical extraction of mandibular third molars | – | 7 days | Significant reduction in the occurrence of post-surgical swelling and pain, but no effect on postoperative trismus | No | Ai-Khateeb and Nusair [15] |
| Acute and chronic inflammation of otorhinolaryngology | – | 7−8 days | Rapid effect with a significant improvement in symptoms | No | Mazzone et al. [22] |
| Swelling of the ankle produced by supination trauma | – | 5 months | -Swelling decreased by 50 % on the third post-operative day, which was faster than the control group -Serratiopeptidase treated group was rapidly pain free compared to the control group |
– | Esch et al. [23] |
| Postoperative buccal swelling (Caldwell-luc antrotomy) |
– | 5 days | Serratiopeptidase treated patients showed lesser degree of swelling than placebo-treated patients at every point of observation | No | Tachibana et al. (1984) |
| Postoperative pain, swelling and trismus after surgical removal of mandibular third molars | – | 3 days | Serratiopeptidase showed good effect on trismus but not on swelling and pain | – | Murugesan et al. [24] |
However, some reports on hand showed comparatively lesser effect of serratiopeptidase. In a study by [25] the efficacy and safety of serratiopeptidase and seaprose S in the treatment of venous inflammatory disease were compared. Seaprose S had a higher efficacy as compared to serratiopeptidase (85 % vs 65 %) with no adverse effects. Further, in this study, one out of 20 patients that received serratiopeptidase showed mild gastrointestinal disturbance. [26] found comparatively lesser analgesic and anti-inflammatory effect of serratiopeptidase as compared to Betamethasone and Ibuprofen. Further, a combination of trypsin and chymotrypsin had a higher efficacy in wound management as compared to serratiopeptidase in a 75 patient study [27]. Contradictory results were observed in two independent studies done on a rat paw edema model. In a study by [28] comparing serratiopeptidase with diclofenac, serratiopeptidase treatment (5.4 mg/kg of body weight) did not result in significant improvement in the inflammation of paw edema. These results are not in agreement with those of [17] where serratiopeptidase (10 mg/kg of body weight) showed promising anti-inflammatory activity. The different dosages used in these two studies make it difficult to draw conclusion.
Overall, serratiopeptidase can be seen as a promising candidate in modern medicine when used either alone or in combination with other agents, particularly in situations where NSAIDs do not show satisfactory results (Table 2, Table 3).
Table 2.
In vitro studies of a combination of serratiopeptidase and antibiotics for biofilm treatment.
| Serratiopeptidase combinations with the antibiotics | Biofilms | Results | Reference |
|---|---|---|---|
| Ofloxacin |
Staphylococcus epidermidis Pseudomonas aeruginosa |
-Serratiopeptidase enhanced the activity of ofloxacin and inhibited biofilm formation | Selan et al. [29] |
| Azithromycin | Bacterial biofilms growing on vascular graft surface | - Combination of serratiopeptidase and azithromycin was found to be very effective against Stayphalococcus strains by showing less minimum inhibitory concentration (MIC) compared to other antibiotic treatments | Thaller et al. [30] |
| Ciprofloxacin | Staphylococcus aureus | - Combination of serratiopeptidase (50 μg/mL) and ciprofloxacin at sub-MIC concentration cleared biofilm on catheter. | Gupta and Nagarsenke (2015) |
| Vancomycin and rifampicin | Methicillin resistant and methicillin susceptible strains of S. aureus | - Effective in dispersing biofilm independent of the strain of organism. | Hogan et al. [31] |
| Levofloxacin | Staphylococcus aureus | - Eradicated >90 % of the preformed biofilm - Combination showed synergistic activity |
Gupta et al. [32] |
Table 3.
Mode of delivery of serratiopeptidase for different applications.
| Mode of delivery (Entrapement/carrier) | Results | Reference |
|---|---|---|
| Serratiopeptidase loaded albumin nanoparticles | Entrapment efficiency and percentage drug release was found to be 85 % and 79.3 % respectively | Kaur and Singh [33] |
| Encapsulation in liposome | Serratiopeptidase encapsulated in liposome along with antibiotic levofloxacin helps in biofilm irradiation in S. aureus infected rat | Gupta et al. [32] |
| Clove oil emulsified buccal patch of serratiopeptidase | Significant entrapment efficiency was obtained along with controlled released for 24h | Shende et al. [34] |
| Poly(D,L-lactic-co-glycolic acid) microspheres of serratiopeptidase and gentamicin entrapped into polyvinyl alcohol-gelatin hydrogel | -The developed delivery system is effective in promoting natural debridement by hydrating necrotic tissue -It provides direct sustained release of antibiotics and serratiopeptidase for better and faster wound healing |
Singh and Singh [35] |
| Serratiopeptidase loaded chitosan nanoparticles | -Sustained release upto 24h -Prolonged anti-inflammatory activity upto 32h |
Mali et al. [36] |
| Microsphere of serratiopeptidase in polymer Eudragit RS100 | Prolonged release of serratiopeptidase for sustained therapeutic effect | Hire et al. [37] |
| Liposomal formulations of serratiopeptidase | -A maximum entrapment efficiency of 86 % was found -Liposomal formulation of serratiopeptidase improved its permeability as revealed by an in vitro study using PAMPA and caco-2 model |
Sandhya et al. [38] |
| Serratiopeptidase transdermal patch by lipid-based transfersomes | -Entrapment efficiency was 96.76 % -In vitro and in vivo release was controlled and steady |
Shende et al. [39] |
| Serratiopeptidase niosomal gel | -Maximum entrapment efficiency was 54.82 % with a consistent release pattern -Anti-inflammatory activity was comparable to that of diclofenac gel as revealed by in vivo efficacy study |
Shinde and Kanojiya [40] |
| Serratiopeptidase and metronidazole loaded on alginate microspheres by emulsification | -Good loading efficiency -Improved wound healing as observed in in vivo testing in rabbits |
Rath et al. [41] |
| Serratiopeptidase immobilized on amino-functionalized magnetic nanoparticles |
-Immobilization increased permeation through the membrane as observed in in vitro studies -Immobilization reduced the dose of serratiopeptidase for anti-inflammatory effect as studied in a rat edema model |
Kumar et al. [42] |
4.2. Serratiopeptidase as an anti-biofilm agent
Bacterial biofilms are multicellular structures of dense and highly hydrated communities of microorganisms embedded within a matrix of self-synthesised polymeric or proteinaceous material [43,44]. They can be attached to biotic or abiotic surfaces. One of the characteristic properties of biofilms is high resistance to the adaptive and innate immune systems as well as tolerance to high concentrations of antibiotics/antimicrobial agents. This leads to persistent infection, making these entities a medical and economic nuisance [31]. They are associated with a variety of infections including urinary tract infections, chronic lung infections, endocarditis, osteomyelitis, chronic otitis media etc. [44]. Biofilms commonly develop on implants and medical devices like catheters, pacemakers, prosthetic joints, tooth surfaces, and various host tissue surfaces leading to chronic infections [44]. They are nearly 100 times more resistant to antimicrobial agents as compared to individual bacterial colonies, thus leading to antibiotic treatment failure.
Various strategies to provide a suitable solution to biofilm associated health problems including inhibition, dispersal, and use of biofilm eradicating agents (antibiotics) have been attempted. Surface proteins and secreted proteins have found to play an important role in biofilm formation, stability, and regulation [44]. Hence, proteases were hypothesized to be a potential treatment of biofilms, which was further supported by scientific studies. Proteases purified from different organisms have tested successfully against biofilm, with metalloproteases in particular, playing an important role [45]. Moreover, commercial proteases have also been successful in the eradication of biofilms [46,47]. Serratiopeptidase, a commercially available bacterial metalloprotease has proven to be effective against a variety of biofilm-associated medical conditions due to the following reasons:
-
1
It can modify the virulent phenotype of bacteria in biofilms [48,49]
-
2
It is effective against mature biofilms [49]
-
3
It enhances the bactericidal effect of antibiotics against bacterial biofilms [50].
4.2.1. In vitro studies
Various in vitro studies show a positive impact of serratiopeptidase against biofilms. It affects a discrete number of proteins involved in fundamental mechanisms associated with bacterial virulence, such as adhesion, invasion, and biofilm formation [51]. Serratiopeptidase reduces cell surface proteins Ami4b, autolysin, internalinB, and ActA and hence reduces the ability of Listeria monocytogenes to form biofilms and to invade host cells. This leads to the prevention of initial adhesion of Listeria monocytogenes to the human gut [52]. [48] tested the role of three serine proteases (proteinase K, trypsin, and chymotrypsin) and two metalloproteases (serratiopeptidase and carboxypeptidase) against biofilm formation and in human cell invasion processes using different strains of Staphylococcus aureus and Staphylococcus epidermidis. Among all the proteases tested, only serratiopeptidase was found to inhibit the activity of all the tested strains. It slightly affected the adhesion efficiency (20 %) but drastically reduced the invasion efficiency (200-fold) of Staphylococcus aureus. Serratiopeptidase neither affected bacterial viability nor showed any cytotoxic effects on the eukaryotic cell lines, alluding to its safety.
A study of the anti-infective capability of serratiopeptidase against Staphylococcus aureus revealed its effects on a discrete number of surface proteins [49]. One such protein is At1 which helps in internalization of staphylococcus in host cells, confirming that serratiopeptidase modulates adhesins and autolysins in Staphylococcus aureus. Further, the action of serratiopeptidase is not only restricted to initial bacterial attachment on abiotic surface but is also effective on mature biofilms. The author emphasised that serratiopeptidase hinders the entry of pathogens in human tissue as well as impairs adhesion of pathogens to prostheses, catheters, and medical devices.
[53] developed a mutant form of serratiopeptidase with no proteolytic activity. The developed mutant was found to maintain anti-biofilm property, suggesting that this property is independent of the proteolytic activity of serratiopeptidase. They concluded that serratiopeptidase is a potential antipathogenic agent with or without proteolytic activity and prevents the formation of biofilms on medical devices [53]. Further, authors suggested the need for research to identify the mechanism of action of serratiopeptidase in biofilm regulation that is unrelated to proteolytic activity. Recently, [54] evaluated the anti-biofilm ability of serratiopeptidase against Staphalococcus aureus infection on osteoblastic MG-63 cells. The pro-inflammatory chemokine MCP-1 was used as an immunological marker. Serratiopeptidase impaired the invasion capability of Staphylococcus aureus in the osteoblastic cells and lowered the secretion of MCP-1. It is worthwhile to note that serratiopeptidase did not affect the viability or proliferation of the osteoblastic cells, indicating its safety in the treatment of bone infection.
Further, combined treatment of serratiopeptidase with antibiotics is a novel approach in the treatment against bacterial biofilm infection. The combination has been shown to have synergistic effects. Serratiopeptidase is thought to work like a biological “nanodrill” and disrupt the bacterial biofilm membrane, thus paving the way for antibiotics to act [32]. Serratiopeptidase was also shown to be more effective as compared to other proteases [29].
4.2.2. Pre-clinical and clinical studies
In an in vivo study on 60 Sprague-Dawley rats, serratiopeptidase eradicated periprosthetic infection caused by Staphylococcus epidermis [55]. Histological and microbiological testing revealed that 5.6 % of rats showed infection in the serratiopeptidase + antibiotic treated group vs 37.5 % in the antibiotic alone treated group. The authors concluded that the anti-biofilm property of serratiopeptidase enhances the efficacy of antibiotics. The study reported adverse effects on joint cartilage and synovial tissue attributable to serratiopeptidase. However, the authors believe that serratiopeptidase use is safe as shown in previous studies and commented that a lower dose may not show this adverse effect. Hence, enzyme dosage and application sites should be carefully considered before using an enzyme-antibiotic combination treatment.
Clinical studies of peri-implantitis are important, given the large number of implant surgeries being performed worldwide. Early diagnosis of peri-implantitis is difficult due to initial mild or no symptoms as well as lack of proper diagnostic tests. Antibiotic resistance is another obstacle in its treatment. Colonization of bacteria occurs at the surface of the implant, forming a biofim which causes severe inflammation of the peri-implant tissue and damages the implant supporting bone. In a controlled study of 64 adults, [50] evaluated the combined effect of serratiopeptidase with antibiotics in peri-implantitis. The combined treatment significantly improved clinical, microbiological, and inflammatory parameters as compared to the control group. The authors concluded that serratiopeptidase enhanced the efficacy of antibiotics by increasing tissue concentration of the antibiotics [56,57]. A retrospective study in 544 patients was conducted by [58] on partially edentulous patients treated for peri-implantitis by evaluating clinical charts. The study indicated that serratiopeptidase helps in the repair of bone lesions and speeds up the clinical healing process. The authors strongly believe that the co-administration of serratiopeptidase with antibiotics to be a preferred option in peri-implantitis treatment.
4.3. Other applications
The analgesic effect of serratiopeptidase is widely known and has been reported in clinical studies. The ability of serratiopeptidase to hydrolyse bradykinin, histamine, and serotonin contributes to its analgesic activity. Serratiopeptidase was found to relieve pain in patients with root canal treatment [59] and control toothache when emulsified with clove oil [34]. The analgesic activity of serratiopeptidase has also been proven in cases of surgical extraction of mandibular third molars reported by Al-Khateeb and Nusair, 2008.
A clinical trial by [60] on 70 patients with breast engorgement demonstrated that serratiopeptidase treatment resulted in moderate to marked improvement in breast pain, swelling, and induration with no adverse events reported. A prospective trial to explore the application of serratiopeptidase in the treatment of carpel tunnel syndrome showed clinical improvement in 65 % of the patients [61]. Serratiopeptidase has been widely used in Japan as an anti-inflammatory and mucolytic agent. Airway mucus is a viscoelastic gel important for the airway defense system. However, in chronic airway disease, increased secretion of mucus and decreased clearance causes its accumulation. [62] evaluated the effect of serratiopeptidase on viscosity and elasticity of nasal mucus in patients with chronic sinusitis. Serratiopeptidase was found to reduce viscosity but not elasticity of the mucus. Further, study done by [63] demonstrated that the Serrapeptidase could enhance mucus clearance in patients with chronic airway disease. This effect was attributed to the ability of serratiopeptidase to reduce neutrophil count and modify viscoelasticity of the mucus.
Another innovative and interesting application is in the treatment of Alzheimer’s disease via reduction of amyloidosis. Serratiopeptidase was found to be as effective as nattokinase (an enzyme shown to degrade amyloid fibroid) in relieving Alzheimer’s disease pathophysiology in a rat model [64]. Oral administration of an enzyme decreased brain acetylcholinesterase activity, as well as levels of transforming growth factor ß, Fas, and interleukin-6, all of which were significantly increased in patients with Alzheimer’s disease. These results were confirmed by histological examination of brain tissue. This study demonstrates that serratiopeptidase can down-regulate the amyloidogenic pathway due to its proteolytic, anti-oxidant, and anti-amyloidogenic effects. The study was further supported by a recent reports [65,66] showing dissociation of insulin amyloids by serratiopeptidase both in vitro and in vivo. Amyloidosis is a result of misfolding of normal cellular protein to protease resistant β-sheets making insoluble aggregates. These aggregates build up in the body and their clearance is highly difficult. The amyloid dissociation ability of serratiopeptidase was better than that of the standard amyloid dissociating agent, nattokinase. This novel approach paves the way to explore the therapeutic potential of serratiopeptidase in different amyloid related disorders.
A combination therapy consisting of enteric coated Vitamin C, extract of Withaferania somnia, and serratiopeptidase has been used to treat thyroid cancer [67]. Complete remission of thyroid tumor was found after 18 months of combination therapy. Serratiopeptidase plays an important role in cleaning the dead cells from the target site leading to an increased rate of killing of tumor cells. This study further highlights the scope for injecting enzyme directly to the target site of a tumor to enhance efficiency.
5. Mechanism of action
Despite its wide therapeutic applications, the mechanism of serratiopeptidase action has not been well elucidated.
Wound healing: Serratiopeptidase helps to thin the fluids in inflamed areas, thus facilitating drainage. This results in reduction of swelling, pain, and enhances tissue repair. Serratiopeptidase also accelerates the healing process due to its unique property of dissolving dead tissue surrounding the injured area without harming living tissue. Further, it hydrolyses bradykinin, histamine, and serotonin which helps to decrease pain and swelling and improve microcirculation, which in turn supports the wound healing process [68].
Anti-inflammatory: Serratiopeptidase acts as an anti-inflammatory agent by regulating inflammatory cytokines and hence the onset of chronic inflammation. It significantly modifies cell adhesion molecules that guide inflammatory cells to the sites of inflammation. It promotes wound healing, repair, and restores the skin temperature at the target inflammation site. It should be noted that serratiopeptidase is more stable and has higher efficacy when used in combination with metal ions like zinc and manganese [1].
Antibiofilm: The anti-biofilm ability of serratiopeptidase is accredited to its capability of modulating the expression of adhesion molecules and reduces cell surface proteins of bacteria [51]. It prevents biofilm formation as well as helps to disperse preformed biofilm. Its anti-biofilm ability helps to enhance the penetration of antibiotics through the resistant biofilm and hence increases susceptibility of biofilms to antibiotics.
Fibrinolytic: Serratiopeptidase is known to dissolve blood clots and artherosclerotic plaques by breaking down fibrin and other dead or damaged tissue [2]. It can also remove deposits of fatty substances, cholesterol, and cellular waste inside the arteries. The fibrinolytic property of serratiopeptidase may also help with the problems of thick blood, risk of stroke, and thrombophlebitis [1].
6. Absorption and safety of serratiopeptidase
Meagre literature is available on the absorption and safety of serratiopeptidase in the human body. It is known that orally taken serratiopeptidase gets absorbed through the intestine and transported into the blood. The intestinal absorption of serratiopeptidase has been tested in rats by evaluating its concentration in plasma, lymph, and inflammatory tissue extract using the sandwich enzyme immunoassay technique. The study showed that the concentration of serratiopeptidase in plasma and lymph is dose dependent. Peak plasma concentration was reached at 0.25−0.5 h after intake and the enzyme was measurable upto 6 h [69]. Further, [70] demonstrated that the concentration of serratiopeptidase was higher in inflammatory tissue that that of in plasma. The authors proposed that serratiopeptidase is absorbed from the intestine and distributed to inflammatory sites via blood or lymph. Serratiopeptidase forms a complex with plasma protease inhibitor alpha-1 macroglobulin in the ratio of 1:1 as observed in a rat study [71]. This binding masks its antigenicity with 20 % retention of its original caseinolytic activity. This complex helps to transport serratiopeptidase via blood to the target sites. The dosage of serratiopeptidase generally ranges from 10−60 mg per day (2000/mg Unit activity). It is highly recommended to get a prescription for serratiopeptidase from health experts because its dosage requirement varies depending on the application and disease state.
Serratiopeptidase is a natural molecule that is being used for decades, hence commonly considered as safe. The safety of this enzyme in different areas of therapeutics is supported by several studies [15,19,22,48,54,72] in which no side effects or adverse events were reported. However, some studies have reported adverse effects of this molecule, but at a rare frequency. The explanation of the same has been provided above in the respective section of this review. Further, Stevens-Johnson syndrome [73] and buccal space abscess [74] have also been reported as side effects of this molecule. These side effects may be dose dependent or possibly due to a combination effect when used with other drugs. Detailed, scientifically designed controlled clinical studies need to be conducted to further examine the safety profile.
7. Mode of delivery of serratiopeptidase
Common problems associated with drug delivery are poor solubility, toxicity, instability, incompatibility, and poor penetration [75]. Each drug needs a suitable delivery system depending on its characteristics. Serratiopeptidase suffers high risk of enzymatic degradation in the gastrointestinal tract due to its proteinacious nature. Further, its hydrophilic nature causes low permeability through the intestinal membrane. These factors impose the use of a very high dosage for significant effects. Controlled and sustained released of serratiopeptidase is vital approach to decrease the frequency of dosing and to improve patient compliance. Hence, different delivery modes have been studied including magnetic nanoparticles, microspheres, encapsulation in liposomes, and emulsification. In vitro release profiles and in vivo efficacy are important parameters need to be studied to develop suitable delivery modes.
New modes of delivery have been evaluated in cases of dentistry and wound healing. Novel effective biocompatible moist system for complete wound management was studied by [35]. Poly(D,L-lactic-co-glycolic acid) microspheres of serratiopeptidase and gentamicin were entrapped into polyvinyl alcohol-gelatin hydrogel. In vitro and in vivo studies showed the direct sustained release of serratiopeptidase along with antibiotics at the wound site leading to a better and faster healing process. [76] developed a topical formulation of serratiopeptidase in the form of an ointment and a gel and evaluated its anti-inflammatory effects. The authors highlighted that topical application circumvents the drawbacks of oral delivery including anorexia, nausea, and GI disturbance, if any. An optimized formulation was evaluated for in vitro release profile and in vivo anti-inflammatory action where it showed satisfactory inhibition of ear edema in a rat study. The absence of any allergic reaction in rats supports the safety profile of the serratiopeptidase formulation. Enteric dispersion of serratiopeptidase with the polymer Eudragit has shown promising results for controlled release of the drug [37,77].
8. Regulatory aspects
Though serratiopeptidase is widely used in clinical practice around the globe, its regulatory status varies in different countries. The marketing and use of this molecule in any intended country needs approval by designated regulatory bodies. Background administrative, chemistry, pre-clinical and clinical data are needed for approval where quality, efficacy, and safety are important parameters to be considered. Serratiopeptidase has been approved in Canada for use as an ingredient in dietary supplements to reduce pain and swelling [78]. In India, it is approved as a pharmaceutical ingredient for the treatment of acute pain in combination with other drugs [80]. Regulatory authorities provide approval status for specific applications. However, a few regulatory authorities have published approvals for serratiopeptidase to be used as a dietary supplement or as an active pharmaceutical ingredient as listed in Table 4.
Table 4.
Regulatory status of serratiopeptidase in major global markets.
| Country | Regulatory Agency | Approval status | Details | References |
|---|---|---|---|---|
| USA | U.S. Food & Drug Administration | New Dietary Ingredient Notification (NDIN) | NDIN# 6 was filed by Specialty Enzymes & Probiotics | Dietary supplement health and education act (DSHEA) [81] |
| Drug Master file (DMF) | DMF# 23,557 filed by Specialty Enzymes & Probiotics and DMF# 27,172 filed by Newgen Biotech | 21 Code of federal regulations (CFR) part- 314 | ||
| Canada | Health Canada | Natural health product (NHP) | -Enteric coated version is allowed. Can be used for reduction in pain and swelling and to combat throat infections | NHP Ingredients Database: Serrapeptase [78] |
| Europe | European commission (EC) | Novel Food | Serratiopeptidase is considered as Novel Food. Safety evaluation is needed under 2015/2283 regulation | Regulation (EU) No. 2015/2283 |
| India | Central Drug Standard Control Organization (CDSO) | Approved as active pharmaceutical ingredient (API) | Serratiopeptidase is widely available. It is generally sold in combination with other APIs. | The Drug and Cosmetic Act [80] |
Besides, in Japan, proprietor, Takeda has voluntarily withdrew serratiopeptidase in 2011 then after, Singapore government has taken decision to phase out serratiopeptidase containing preparations as medicinal products due to some controversial results of the trials and lack of substantive scientific evidences [79].
Label/health claim guidelines need to be followed for products containing serratiopeptidase as a dietary ingredient or pharmaceutical ingredient. The big challenge for manufactures and distributors is to understand the array of label claims that are allowed to be used for serratiopeptidase containing products. In the United States, dietary supplements can make structure/function statements [81]. However, regulatory agencies in Europe and Canada have special provisions to allow broader health claims. Those are approved under regulation (EC) no. 1924/2006 in Europe and Food and Drugs act (section 30(j)) in Canada. Manufacturers need detailed case by case evaluation of the approved claims in each country before marketing serratiopeptidase.
9. Conclusion
The proteolytic enzyme serratiopeptidase is being widely used in the treatment of many diseases and disorders for decades across the world. In vitro studies, controlled, uncontrolled pre-clinical and clinical studies, and some anecdotal reports highlight its potential in the therapeutics. However, negative and controversial results obtained as well as some reports of mild to moderate side effects cannot be overlooked. Hence, more research is needed to confirm its therapeutic potential, elucidate the mechanism(s) of action as well as safety of different doses and formulations. Well designed in vitro, in vivo, and clinical studies will help to establish the use of serratiopeptidase in various therapeutic areas.
Author contribution statement
The conception and design of the study: S. J and Abhijit R; acquisition of data, analysis, and interpretation of data: S. J, Ankit R, and N. S; Drafting the article: S. J, N. S, and Ankit R; revising the article critically for important intellectual content: N. S, S. J and V. R; final approval of the version to be submitted: V. R and Abhijit R.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
Authors are grateful to Advanced Enzymes Technologies Ltd., India for support.
References
- 1.Tiwari M. The role of serratiopeptidase in the resolution of inflammation. Asian J. Pharm. Sci. 2017;12:209–215. doi: 10.1016/j.ajps.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santhosh K. The emerging role of serratiopeptidase in oral surgery: literature update. Asian J. Clin. Pharm. Res. 2018;11(3):19–23. [Google Scholar]
- 3.Gupte V., Luthra U. Analytical techniques for serratiopeptidase: a review. J. Pharm. Anal. 2017;7(4):203–207. doi: 10.1016/j.jpha.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhagat S., Agarwal M., Roy V. Serratiopeptidase: a systematic review of the existing evidence. Int. J. Sur. 2013;11:209–217. doi: 10.1016/j.ijsu.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Ethiraj S., Gopinath S. Production, purification, characterization, immobilization, and application of Serrapeptase: a review. Front. Biol. 2017;12(5):333–348. [Google Scholar]
- 6.De Duve C. The significance of lysosome in pathology and medicine. Proc. Inst. Med. Chic. 1966;26:73–76. [PubMed] [Google Scholar]
- 7.Tasaka K., Meshi T., Akagi M., Kakimoto M., Saito R., Okada I., Maki K. Anti-inflammatory activity of a proteolytic enzyme, Prozime-10. Pharmacology. 1980;21(1):43–52. doi: 10.1159/000137414. [DOI] [PubMed] [Google Scholar]
- 8.Kumar S.S., Abdulhammed S. Therapeutic enzymes. In: Sugathan S., Pradeep N.S., Adbulhammed S., editors. Bioresources and Bioprocess in Biotechnology. Springer; Singapore: 2017. pp. 45–73. [Google Scholar]
- 9.Reshma C.V. Microbial enzymes: therapeutic applications. Microbiol. Res. J. Int. 2019;27(2):1–8. [Google Scholar]
- 10.Gupte V., Luthra U., Desai N. Circular dichroism as a process analytical tool to monitor the quality of serratiopeptidase. Int. J. Eng. Tech. Sci. Res. 2017;4(11):590–595. [Google Scholar]
- 11.Nageswara S., Guntuku G., Yakkali B. Purification, characterization, and structural elucidation of serralysin-like alkaline metalloprotease from a novel source. J. Gent. Eng. Biotechnol. 2019;17:1–15. doi: 10.1186/s43141-019-0002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srivastava V., Mishra S., Chaudhuri T.K. Enhanced production of recombinant serratiopeptidase in Escherichia coli and its characterization as a potential biosimilar to native biotherapeutic counterpart. Microb. Cell Fact. 2019;18:215–230. doi: 10.1186/s12934-019-1267-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doshi P., Bhargava P., Singh V., Pathak C., Joshi C., Joshi M. Escherichia coli strain engineering for enhanced production of serratiopeptidase for therapeutic applications. Int. J. Biol. Macromol. 2020;160:1050–1060. doi: 10.1016/j.ijbiomac.2020.05.256. [DOI] [PubMed] [Google Scholar]
- 14.Li H., Manwani B., Leng S.X. Frailty, inflammation, and immunity. Aging Dis. 2011;2(6):466–473. [PMC free article] [PubMed] [Google Scholar]
- 15.Ai-Khateeb T.H., Nusair Y. Effect of proteolytic enzyme serrapeptase on swelling, pain and trismus after surgical extraction of mandibular third molars. Int. J. Oral Maxillofac. Surg. 2008;37:264–268. doi: 10.1016/j.ijom.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Viswanatha Swamy A.H.M., Patil P.A. Effect of some clinically used proteolytic enzymes on inflammation in rats. Indian J. Pharm. Sci. 2008;70:114–117. doi: 10.4103/0250-474X.40347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jadav S.P., Patel N.H., Shah T.G., Gajera M.V., Trivedi H.R., Shah B.K. Comparison of anti-inflammatory activity of serratiopeptidase and diclofenac in albino rats. J. Pharmacol. Pharmacother. 2010;1(2):116–117. doi: 10.4103/0976-500X.72362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajinikanth B., Venkatachalam V.V., Manavalan R. Investigations on the potential of serratiopeptidase- a proteolytic enzyme, on acetic acid induced ulcerative colitis in mice. Int. J. Pharm. Pharm. Sci. 2014;6(5):525–531. [Google Scholar]
- 19.Ateia Y.A., Al-Edanni M.S.H., Al-Qurtas M.I. Impact of metformin and serratiopeptidase in obese patients with knee osteoarthritis. Int. J. Pharm. Pharm. Sci. 2018;10(2):37–41. [Google Scholar]
- 20.Mikhael E.M., Mohammed M.Y. Serratiopeptidase a hope in a rapid and better improvement of inflammatory acne vulgaris. Iraqi. J. Pharm. Sci. 2012;21(1):78–81. [Google Scholar]
- 21.Chappi D.M., Suresh K.V., Patil M.R., Desai R., Tauro D.P., Shiva Bharani K.N.S., Parkar M.I., Babaji H.V. Comparison of clinical efficacy of methylprednisolone and serratiopeptidase for reduction of postoperative sequelae after lower third molar surgery. J. Clin. Exp. Dent. 2015;7:217–222. doi: 10.4317/jced.51868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazzone A., Catalani M., Costanzo M., Drusian A., Mandoli A., Russo S., Guarini E., Vesperini G. Evaluation of serratia peptidase in acute or chronic inflammation of otorhinolaryngology pathology: a multicentre, double-blind, randomized trial versus placebo. J. Int. Med. Res. 1990;18:379–388. doi: 10.1177/030006059001800506. [DOI] [PubMed] [Google Scholar]
- 23.Esch P.M., Gerngross H., Fabian A. Reduction of postoperative swelling. Objective measurement of swelling of the upper ankle joint in treatment with serrapeptase - A prospective study. Fortschr. Med. 1989;10(4):67–72. 107. [PubMed] [Google Scholar]
- 24.Murugesan K., Sreekumar K., Sabapathy B. Comparison of the roles of Serration peptidase and dexamethasone in the control of inflammation and trismus following impacted third molar surgery. Indian J. Dent. Res. 2012;23:709–713. doi: 10.4103/0970-9290.111243. [DOI] [PubMed] [Google Scholar]
- 25.Bracale G., Selvetella L. Clinical study of the efficacy of and tolerance to seaprose S in inflammatory venous disease. Controlled study versus serratio-peptidase. Minerva. Cardioangiol. 1996;44:515–524. [PubMed] [Google Scholar]
- 26.Chopra D., Rehan H.S., Mehra P., Kakkar A.K. A randomized, double-blind, placebo- controlled study comparing the efficacy and safety of paracetamol, serratiopeptidase, ibuprofen and betamethasone using the dental impaction pain model. Int. J. Oral Maxillofac. Surg. 2009;38:350–355. doi: 10.1016/j.ijom.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 27.Chandanwale A., Langade D., Sonawane D., Gavai P. A randomized, clinical trial to evaluate efficacy and tolerability of trypsin:chymotrypsin as compared to serratiopeptidase and trypsin:bromelain:rutoside in wound management. Adv. Ther. 2017;34(1):180–198. doi: 10.1007/s12325-016-0444-0. [DOI] [PubMed] [Google Scholar]
- 28.Joshi K.K., Nerurkar R.P. Anti-inflammatory effect of the serratiopeptidase- rationale or fashionable: a study in rat paw oedema model induced by the carrageenan. Indian J. Physiol. Pharmacol. 2012;56(4):367–374. [PubMed] [Google Scholar]
- 29.Selan L., Berlutti F., Passariello C., Comodi-Ballanti M.R., Thaller M.C. Proteolytic enzymes: a new treatment strategy for prosthetic infections? Antimicrob. Agents Chemother. 1993;37:2618–2621. doi: 10.1128/aac.37.12.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thaller M.C., Selan L., Fiorani P., Passariello C., Rizzo L., Speziale F. A comparative in vitro evaluation of different therapeutic protocols for vascular graft infections. Eur. J. Vasc. Endovasc. Surg. 1997;14:35–37. doi: 10.1016/s1078-5884(97)80151-7. [DOI] [PubMed] [Google Scholar]
- 31.Hogan S., Zapotoczna M., Stevens N.T., Humphreys H., O’Gara J.P., O’Neill E. Potential use of targeted enzymatic agents in the treatment of Staphylococcus aureus biofilm-related infections. J. Hosp. Infect. 2017;96(2):177–182. doi: 10.1016/j.jhin.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Gupta P.V., Nirwane A.M., Bellubi T., Nagarsenker M.S. Pulmonary delivery of synergistic combination of fluoroquinolone antibiotic complemented with proteolytic enzyme: a novel antimicrobial and antibiofilm strategy. Nanomedicine. 2017;13(7):2371–2384. doi: 10.1016/j.nano.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 33.Kaur H., Singh A. Design, development and characterization of serratiopeptidase loaded albumin nanoparticles. J. App. Pharm. Sci. 2015;5(2):103–109. [Google Scholar]
- 34.Shende P.K., Gaud R.S., Bakal R., Yeole Y. Clove oil emulsified buccal patch of serratiopeptidase for controlled release in toothache. J. Bioequiv. 2016;8:134–139. [Google Scholar]
- 35.Singh D., Singh M.R. Development of antibiotic and debriding enzyme-loaded PLGA microspheres entrapped in PVA-gelatin hydrogel for complete wound management. Artif. Cell. Blood Sub. Biotechnol. 2012;40(5):345–353. doi: 10.3109/10731199.2012.675337. [DOI] [PubMed] [Google Scholar]
- 36.Mali N., Wavikar P., Vavia P. Serratiopeptidase loaded chitosan nanoparticles by polyelectrolyte complexation: in vitro and in vivo evaluation. AAPS Pharm. Sci. Tech. 2015;16(1):59–66. doi: 10.1208/s12249-014-0201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hire N.N., Deore A.B., Derle D.V., Nathe R. Formulation and evaluation of serratiopeptidase microspheres using eudragit rs100 polymer. World J. Pharm. Res. 2014;3(2):3207–3218. [Google Scholar]
- 38.Sandhya K.V., Devi G.S., Mathew S.T. Liposomal formulations of serratiopeptidase: in vitro studies using PAMPA and Caco-2 models. Mol. Pharm. 2008;5(1):92–97. doi: 10.1021/mp700090r. [DOI] [PubMed] [Google Scholar]
- 39.Shende P.K., Bakal R.L., Gaud R.S., Batheja K.N., Kawadiwale M.S. Modulation of serratiopeptidase transdermal patch by lipid-based transfersomes. J. Adhes. Sci. Technol. 2015;29(23):2622–2633. [Google Scholar]
- 40.Shinde U.A., Kanojiya S.S. Serratiopeptidase niosomal gel with potential in topical delivery. J. Pharm. 2014:1–10. doi: 10.1155/2014/382959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rath G., Johal E.S., Goyal A.K. Development of serratiopeptidase and metronidazole based alginate microspheres for wound healing. Artif. Cells Blood Substit. Immobil. Biotechnol. 2011;39(1):44–50. doi: 10.3109/10731199.2010.494580. [DOI] [PubMed] [Google Scholar]
- 42.Kumar S., Jana A.K., Dhamija I., Singla Y., Maiti M. Preparation, characterization and targeted delivery of serratiopeptidase immobilized on amino-functionalized magnetic nanoparticles. Eur. J. Pharm. Biopharm. 2013;85:413–426. doi: 10.1016/j.ejpb.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 43.Gupta P.V., Nagarsenker M.S. Antimicrobial and antibiofilm activity of enzybiotic against Staphylococcus aureus. In: Mendez-Vilas A., editor. The Battle Against Microbial Pathogens: Basic Science, Technological Advances and Educational Programs. Formatex Research Center; 2015. pp. 364–372. [Google Scholar]
- 44.Mukherji R., Patil A., Prabhune A. Role of extracellular proteases in biofilm disruption of gram positive bacteria with special emphasis on Staphylococcus aureus biofilms. Enz. Eng. 2015;4(1) [Google Scholar]
- 45.Saggu S.K., Jha G., Mishra P.C. Enzymatic degradation of biofilm by metalloprotease from Microbacterium sp. SKS10. Front. Bioeng. Biotech. 2019;7:192. doi: 10.3389/fbioe.2019.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elchinger P.H., Delattre C., Faure S., Roy O., Badel S., Bernardi T., Taillefumier T., Michaud P. Effect of proteases against biofilms of Staphylococcus aureus and Staphylococcus epidermidis. Lett. Appl. Microbiol. 2014;59:507–513. doi: 10.1111/lam.12305. [DOI] [PubMed] [Google Scholar]
- 47.Molobela I.P., Cloete T.E., Beukes M. Protease and amylase enzymes for biofilm removal and degradation of extracellular polymeric substances (EPS) produced by Pseudomonas fluorescens bacteria. Afr. J. Microbiol. Res. 2010;4(14):1515–1524. [Google Scholar]
- 48.Artini M., Papa R., Scoarughi G.L., Galano E., Barbato G., Pucci P., Selan L. Comparison of the action of different proteases on virulence properties related to the staphylococcal surface. J. Appl. Microbiol. 2013;114(1):266–277. doi: 10.1111/jam.12038. [DOI] [PubMed] [Google Scholar]
- 49.Papa R., Artini M., Cellini A., Tilotta M., Galano E., Pucci P., Amoresano A., Selan L. A new anti-infective strategy to reduce the spreading of antibiotic resistance by the action on adhesion-mediated virulence factors in Staphylococcus aureus. Microb. Pathog. 2013;63:44–53. doi: 10.1016/j.micpath.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 50.Passariello C., Lucchese A., Pera F., Gigola P. Clinical, microbiological and inflammatory evidence of the efficacy of combination therapy including serratiopeptidase in the treatment of periimplantitis. Eur. J. Inflam. 2012;10(3):463–472. [Google Scholar]
- 51.Selan L., Artini M., Papa R. Compounds from natural sources for new diagnostics and drugs against biofilm infections. In: Dhanasekaran D., editor. Microbial Biofilms - Importance and Applications. 2016. pp. 487–509. [Google Scholar]
- 52.Longhi C., Scoarughi G.L., Poggiali F., Cellini A., Carpentieri A., Seganti L., Pucci P., Amoresano A., Cocconcelli P.S., Artini M., Costerton J.W., Selan L. Protease treatment affects both invasion ability and biofilm formation in Listeria monocytogenes. Microb. Pathog. 2008;45(1):45–52. doi: 10.1016/j.micpath.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 53.Selan L., Papa R., Tilotta M., Vrenna G., Carpentieri A., Amoresano A., Pucci P., Artini M. Serratiopeptidase: a well-known metalloprotease with a new non-proteolytic activity against S. aureus biofilm. BMC microbial. 2015;15:207. doi: 10.1186/s12866-015-0548-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Selan L., Papa R., Ermocida A., Cellini A., Ettorre E., Vrenna G., Campoccia D., Montanaro L., Arciola C.R., Artini M. Serratiopeptidase reduces the invasion of osteoblasts by Staphylococcus aureus. Int. J. Immunopath. Pharm. 2017;30(4):423–428. doi: 10.1177/0394632017745762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mecikoglu M., Saygi B., Yildirim Y., Karadag-Saygi E., Ramadan S.S., Esemenli T. The effect of proteolytic enzyme serratiopeptidase in the treatment of experimental implant-related infection. J. Bone Joint Surg. Am. 2006;88(6):1208–1214. doi: 10.2106/JBJS.E.00007. [DOI] [PubMed] [Google Scholar]
- 56.Aratani H., Tateishi H., Negita S. Studies on the distribution of antibiotics in the oral tissues: Experimental staphyloccal infection in rats, and effect of serratiopeptidase on the distribution of antibiotics. Jpn. J. Antibiot. 1980;33:623–635. [PubMed] [Google Scholar]
- 57.Ishihara Y., Kitamura S., Takaku F. Experimental studies on distribution of cefotiam, a new beta-lactam antibiotic, in the lung and trachea of rabbits. II. Combined effects with serratiopeptidase. Jpn. J. Antibiot. 1983;36:2665–2670. [PubMed] [Google Scholar]
- 58.Sannino G., Gigola P., Puttini M., Pera F., Passariello C. Combination therapy including serratiopeptidase improves outcomes of mechanical-antibiotic treatment of periimplantitis. Int. J. Immunopathol. Pharmacol. 2013;26(3):825–831. doi: 10.1177/039463201302600332. [DOI] [PubMed] [Google Scholar]
- 59.Mane P., Atre K., Mayee R. Comparison between the pain relieving action of serratiopeptidase, NSAIDs and combination of both in the root canal treatment patients. Int. J. Curr. Res. Rev. 2011;3:11–17. [Google Scholar]
- 60.Kee W.H., Tan S.L., Lee V., Salmon Y.M. The treatment of breast engorgement with Serrapeptase (Danzen); a randomized double-blind controlled trial. Singapore Med. J. 1989;30:48–54. [PubMed] [Google Scholar]
- 61.Panagariya A., Sharma A.K. A preliminary trial of serratiopeptidase in patients with carpal tunnel syndrome. J. Assoc. Physicians India. 1999;47:1170–1172. [PubMed] [Google Scholar]
- 62.Majima Y., Inagaki M., Hirata K., Takeuchi K., Morishita A., Sakakura Y. The effect of an orally administered proteolytic enzyme on the elasticity and viscosity of nasal mucus. Arch. Otorhinolaryngol. 1988;244:355–359. doi: 10.1007/BF00497464. [DOI] [PubMed] [Google Scholar]
- 63.Nakamura S., Hashimoto Y., Mikami M., Yamanaka E., Soma T., Hino M., Azuma A., Kudoh S. Effect of the proteolytic enzyme serrapeptase in patients with chronic airway disease. Respirology. 2003;8:316–320. doi: 10.1046/j.1440-1843.2003.00482.x. [DOI] [PubMed] [Google Scholar]
- 64.Fadl N.N., Ahmed H.H., Booles H.F., Sayed A.H. Serrapeptase and nattokinase intervention for relieving Alzheimer’s disease pathophysiology in rat model. Hum. Exp. Toxicol. 2013;32(7):721–735. doi: 10.1177/0960327112467040. [DOI] [PubMed] [Google Scholar]
- 65.Metkar S.K., Girigoswami A., Murugesan R., Girigoswami K. In vitro and in vivo insulin amyloid degradation mediated by Serratiopeptidase. Mater. Sci. Eng. C. 2017;70:728–735. doi: 10.1016/j.msec.2016.09.049. [DOI] [PubMed] [Google Scholar]
- 66.Metkar S.K., Girigoswami A., Vijayashree R., Girigoswami K. Attenuation of subcutaneous insulin induced amyloid mass in vivo using lumbrokinase and serratiopeptidase. Int. J. Biol. Macromol. 2020;163:128–134. doi: 10.1016/j.ijbiomac.2020.06.256. [DOI] [PubMed] [Google Scholar]
- 67.Jentschura U.D. Enzyme-supported immunotherapy: case study and possible generalizations. J. Cancer Ther. 2018;9:156–162. [Google Scholar]
- 68.Desser L., Rehberger A., Kokron E., Paukovits W. Cytokine synthesis in human peripheral blood mononuclear cells after oral administration of polyenzyme preparations. Oncology. 1993;50:403–407. doi: 10.1159/000227219. [DOI] [PubMed] [Google Scholar]
- 69.Moriya N., Nakata M., Nakamuma M., Takaoka M., Iwasa S., Kato K., Kakinuma A. Intestinal absorption of serrapeptase (TSP) in rats. Biotechnol. Appl. Biochem. 1994;20:101–108. [PubMed] [Google Scholar]
- 70.Moriya N., Shoichi A., Yoko H., Fumio H., Yoshiaki K. Intestinal absorption of serrapeptase and its distribution to the inflammation sites. Japan Pharmacol. Therap. 2003;31:659–666. [Google Scholar]
- 71.Kakinuma A., Moriya N., Kawahara K., Sugino H. Repression of fibrinolysis in scalded rats by administration of serratia protease. Biochem. Pharmaco. 1982;31:2861–2866. doi: 10.1016/0006-2952(82)90255-6. [DOI] [PubMed] [Google Scholar]
- 72.Tachibana M., Mizukoshi O., Harada Y., Kawamoto K., Nakai Y. A multi-centre, double-blind study of serrapeptase versus placebo in post-antrotomy buccal swelling. Pharmatherapeutica. 1984;3:526–530. [PubMed] [Google Scholar]
- 73.Moitra S., Sen S., Banerjee I., Das P., Tripathi S.K. Diclofenac- Serratio peptidase combination induced stevens–Johnson syndrome - A rare case report with review of literature. J. Clin. Diagn. Res. 2014;8:8–11. doi: 10.7860/JCDR/2014/9509.4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rajaram P., Bhattacharjee A., Ticku S. Serratio peptidase - A cause for spread of infection. J. Clin. Diagn. Res. 2016;10:31–32. doi: 10.7860/JCDR/2016/21388.8302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Patel V.R., Agarwal Y.K. Nanosuspension: an approach to enhance solubility of drug. J. Adv. Pharm. Technol. Res. 2011;2:81–87. doi: 10.4103/2231-4040.82950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nirale N.M., Menon M.D. Topical formulations of serratiopeptidase: development and pharmacodynamic evaluation. Indian J. Pharm. Sci. 2010;72(1):65–71. doi: 10.4103/0250-474X.62246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rani A.P., Uppala A. Enteric dispersion of serratiopeptidase with eudragit L100 and formulation of controlled release tablets of serratiopeptidase. Int. J. Pharm. Tech. Res. 2019;12(2):139–144. [Google Scholar]
- 78.Health Canada . 2018. Natural Health Product Ingredient Monograph: Serrapeptase.http://webprod.hc-sc.gc.ca/nhpid-bdipsn/atReq.do?atid=serrapeptase&lang=eng [Google Scholar]
- 79.HSA, Singapore, https://www.hsa.gov.sg/about-us.
- 80.List of approved drug combinations with CDSO, India. https://cdscoonline.gov.in/CDSCO/Drugs.
- 81.US Food and Drug Administration. The Dietary Supplement Health and Education Act of 1994 (DSHEA) Added Section 403(r)(6) to the Federal Food, Drug, and Cosmetic Act (FD&C Act) https://www.fda.gov/food/new-dietary-ingredients-ndi-notification-process/submitted-75-day-premarket-notifications-new-dietary-ingredients.