Abstract
One of the most refractory breast cancer types is triple negative (TN) breast cancer, in which cells are resistant to both hormone and Herceptin treatments and, thus, often cause recurrence and metastasis. Effective treatments are needed to treat TN breast cancer. We previously demonstrated that rMV-SLAMblind, a recombinant measles virus, showed anti-tumor activity against breast cancer cells. Here, we examined whether rMV-SLAMblind is effective for treating TN breast cancer. Nectin-4, a receptor for rMV-SLAMblind, was expressed on the surface of 75% of the analyzed TN breast cancer cell lines. rMV-SLAMblind infected the nectin-4-expressing TN breast cancer cell lines, and significantly decreased the viability in half of the analyzed cell lines in vitro. Additionally, intratumoral injection of rMV-SLAMblind suppressed tumor growth in xenografts of MDA-MB-468 and HCC70 cells. To assess treatment for metastatic breast cancer, we performed intravenous administration of the luciferase-expressing-rMV-SLAMblind to MDA xenografted mice. Virus replicated in the tumor and resulted in significant suppression of the tumor growth. The safety of the virus was tested by its intravenous injection into healthy cynomolgus monkeys, which did not cause any measles-like symptoms. These results suggest that rMV-SLAMblind is a promising candidate as a therapeutic agent for treating metastatic and/or TN type breast cancer.
Keywords: measles virus, oncovirotherapy, triple negative breast cancer, nectin-4, oncolytic virus, safety, non-human primate, intravenous, systemic
Graphical Abstract
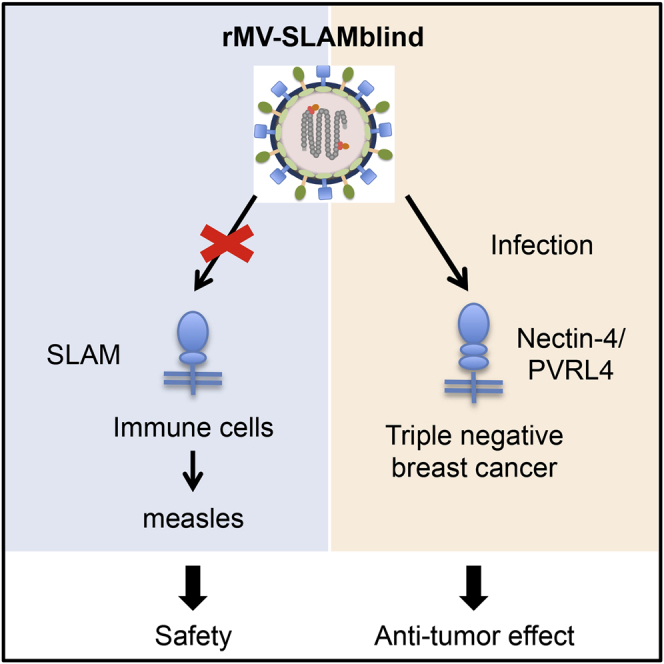
Triple negative breast cancer (TNBC) is one of the most refractory breast cancer types. A recombinant measles virus, rMV-SLAMblind, infected nectin-4-positive TNBC cells and significantly suppressed the tumor growth in xenograft models. Thus, rMV-SLAMblind is a promising candidate as a therapeutic agent for TNBC.
Introduction
Various therapies have been developed for cancer. However, there are still many refractory cancer cases. Therefore, novel therapies based on other mechanisms than existing therapies are required. Oncolytic virotherapy is expected as one of the novel therapies for cancer. Most of the oncolytic viruses developed for cancer therapies such as herpes simplex virus and adenovirus are able to infect non-cancer cells, as well as cancer cells, but they were genetically modified to replicate only in cancer cells. Unlike these viruses, we develop an oncolytic measles virus (MV), which can only infect cancer cells using a tumor cell marker as a receptor.
Oncovirotherapy with MV has been preceded using a MV vaccine strain (Edmonston B). Antitumor effects by MV vaccine strains were reported against various types of cancer and clinical trials are advancing.1, 2, 3, 4, 5, 6, 7, 8, 9, 10 The dominant receptor molecule of MV vaccine strains is CD46, which is ubiquitously expressed except erythrocyte but is upregulated in cancer cells. On the other hand, we developed a wild-type MV-derived therapy, because the major receptor molecule is different from CD46. Wild-type MV strains infect host cells via two different receptor molecules, SLAM and nectin-4.11, 12, 13 SLAM, which is selectively expressed in immune cells, is a principal receptor of MV, and MV infection of immune cells is the cause of severe immunosuppression, as well as severe pathogenicity. Arginine at 533 of the MV H protein is important for binding to SLAM,14 and its mutation leads to attenuation of MV.15 We previously reported that a wild-type MV (HL strain) showed oncolytic activity against human breast cancer cells and that a recombinant measles virus with an R533A mutation in the H protein (rMV-SLAMblind) uses nectin-4 as its receptor and thus attenuated in monkeys.16 Thus, rMV-SLAMblind targets nectin-4, differently from MV vaccine strains.
Nectin-4 is mainly expressed in the placenta of human females.17 In this decade, it was found that nectin-4 is upregulated in several types of tumor cells, including breast, lung, and ovarian cancers.18, 19, 20, 21 We expected that rMV-SLAMblind selectively targets and kills nectin-4 positive tumor cells and demonstrated that rMV-SLAMblind shows anti-tumor effects against various cell lines expressing nectin-4 derived from breast, lung, pancreas, and colorectal cancer.16,22, 23, 24
Breast cancer includes several subtypes that have different expression patterns of marker molecules.25 Luminal or human epidermal growth factor receptor 2 (HER2)-positive breast cancer is characteristics in its expression of the estrogen receptor, progesterone receptor, or HER2. Thus, hormone therapy or HER2-targeted therapy is the most commonly used treatment for these cancers. In contrast, triple negative (TN) breast cancer, which is negative for all of three markers, is resistant to those treatments, and a specific therapeutic target for this type of breast cancer has not yet been identified. TN breast cancer accounts for approximately 17% of breast cancer26 and is recognized as the most refractory type among breast cancers, because it tends to have a higher risk of recurrence and death.27, 28, 29, 30 Thus it is necessary to develop a novel effective therapy for TN breast cancer. In our previous study of breast cancer cell lines, rMV-SLAMblind suppressed significantly the tumor growth of nectin-4-positive ones. One of the cell lines included a TN breast cancer cell line (MDA-MB-453), which implies the possibility that rMV-SLAMblind is also efficient for other TN breast cancer cell lines.
In this study, we examined the efficacy of rMV-SLAMblind for the treatment of TN breast cancer by using twelve cell lines. In the application of rMV-SLAMblind to treating advanced breast cancer patients, particularly those with metastatic cancer, systemic administration may be necessary. Therefore, we also examined the efficacy of rMV-SLAMblind via the intravenous route and evaluated the safety of repeated intravenous administrations of this virus in monkeys.
Results
Susceptibility of Nectin-4-Expressing TN Breast Cancer Cells to rMV-SLAMblind Infection
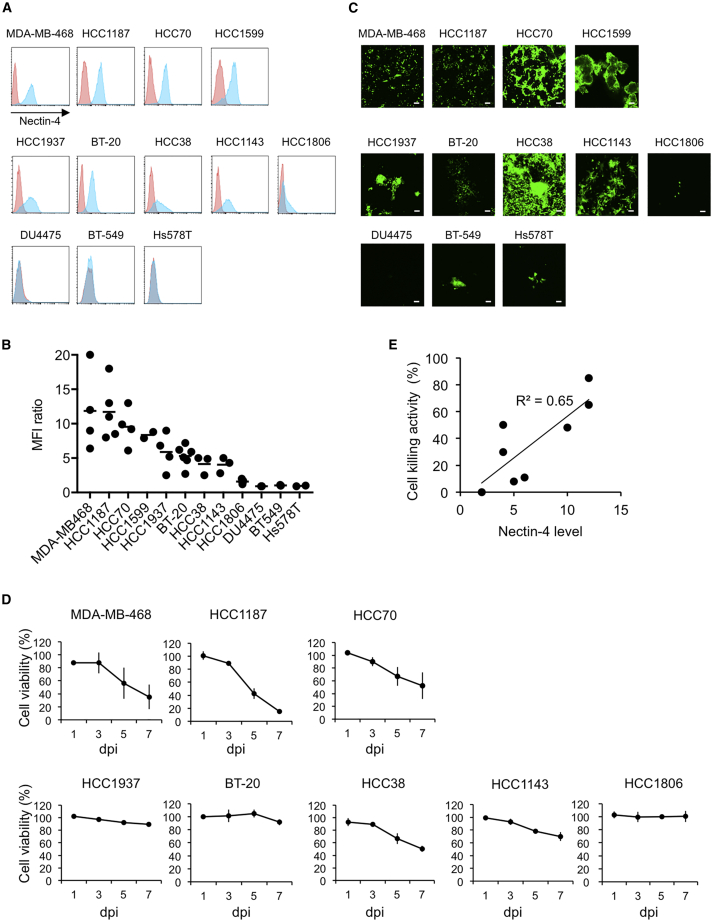
In our previous study to show that rMV-SLAMblind infected and killed breast cancer cell line, we used three cell lines including one derived from TN breast cancer (MDA-MB-453).16 However, there is no further information on rMV-SLAMblind efficacy to TN breast cancer cells. We collected TN breast cancer cell lines to examine whether infection of the cell lines with rMV-SLAMblind causes cytotoxicity in vitro. We examined the nectin-4 expression level on the surface of 12 TN breast cancer cell lines by flow cytometry. Nectin-4 was expressed on the surfaces of nine out of 12 TN breast cancer cell lines (MDA-MB-468, HCC1187, HCC70, HCC1599, HCC1937, BT-20, HCC38, HCC1143, and HCC1806; Figures 1A and 1B). The expression level of nectin-4 was relatively high in MDA-MB-468, HCC1187, HCC70, and HCC1599, and low in HCC1937, BT-20, HCC38, HCC1143, and HCC1806 cells. No expression was observed in DU4475, BT-549, and Hs578T.
Figure 1.
Susceptibility of TN Breast Cancer Cell Lines to rMV-SLAMblind
(A) Cell surface expressions of nectin-4 were analyzed by flow cytometry (nectin-4, blue histogram; isotype control, red histogram). (B) Mean fluorescence intensity (MFI) of nectin-4 was divided by that of isotype control to calculate relative nectin-4 expression level. (C) Cells were inoculated with rMV-EGFP-SLAMblind at a MOI of 2. Representative photos at 5 dpi are shown. Scale bar, 100 μm. (D) Cells were inoculated with rMV-EGFP-SLAMblind at a MOI of 1. The cell viability was measured at 1, 3, 5, and 7 dpi by WST-1 assays. Data are shown as the average ± SEM of three experiments. (E) Relationship between nectin-4 expression levels on cultured cells and cell killing activity by rMV-SLAMblind.
When we inoculated the cells with rMV-SLAMblind expressing enhanced green fluorescent protein (EGFP), rMV-EGFP-SLAMblind, at a multiplicity of infection (MOI) of 2, most of the nectin-4-expressing cells had observed fluorescence and grew syncytia except HCC1806, whereas the nectin-4 negative cell lines were minimally infected with the virus (Figure 1C).
To examine whether rMV-SLAMblind infection leads to cell death in nectin-4-expressing-cell lines, the cell viability of the eight TN breast cancer cell lines (MDA-MB-468, HCC1187, HCC70, HCC38, HCC1143, HCC1937, BT-20, and HCC1806) were kinetically examined after inoculation with rMV-EGFP-SLAMblind. The viabilities of MDA-MB-468, HCC1187, HCC70, and HCC38 cells were remarkably decreased within 7 dpi (Figure 1D). Infection of these cell lines by rMV-EGFP-SLAMblind led to the death of over 40% of the cells. In contrast, rMV-EGFP-SLAMblind infection killed 30% of the HCC1143 cells and less than 20% of HCC1937, BT-20, or HCC1806 cells. These results suggest that infection with rMV-SLAMblind caused cell death in TN breast cancer cells and that the cytotoxicity of this virus tends to correlate with the expression level of nectin-4 (Figure 1E).
Antitumor Effect of rMV-SLAMblind In Vivo
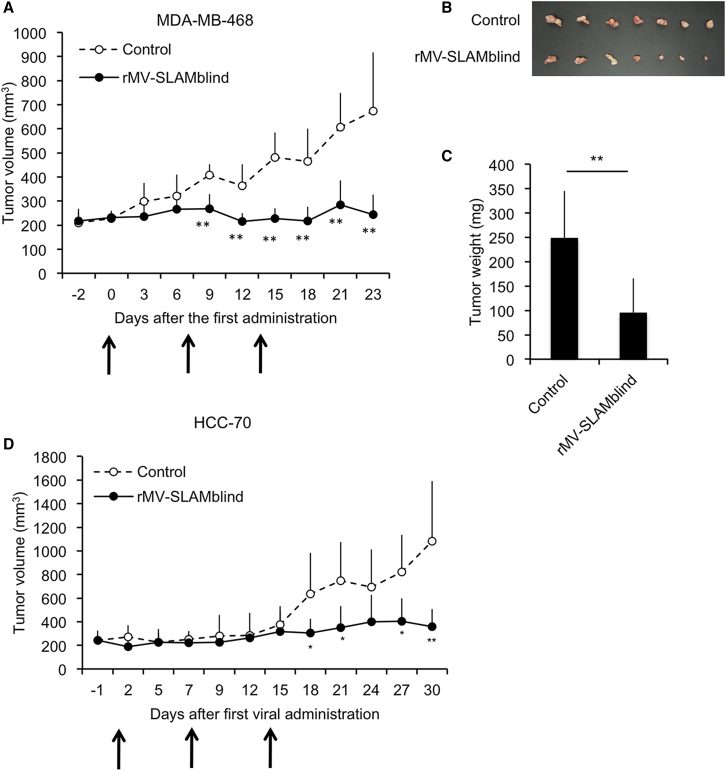
We next examined the antitumor effect of rMV-SLAMblind in vivo by using mouse xenograft models. HCC70 and MDA-MB-468 were selected for this experiment because of their higher expression levels of nectin-4, permissibility to rMV-EGFP-SLAMblind infection, and transplantability into severe combined immune deficiency (SCID) mice.31,32 We transplanted the cells to SCID mice subcutaneously. After the tumor volume reached approximately 200 mm3, we intratumorally administered 1 × 106 TCID50 of rMV-EGFP-SLAMblind three times. The growths of tumors resulting from either HCC70 or MDA-MB-468 cells were almost totally suppressed following administration of rMV-EGFP-SLAMblind compared with those of control (Figures 2A and 2D). Tumors excised at 26 dpi of MDA-MB-468 xenografts were smaller than those of control mice (Figures 2B and 2C). These results suggest that rMV-SLAMblind has a strong anti-tumor effect in vivo against TN breast cancer cells expressing nectin-4.
Figure 2.
Anti-Tumor Effect of rMV-SLAMblind in Xenograft Models of TN Breast Cancer Cell Lines
(A and D) MDA-MB-468 (A) or HCC70 (D) cells were transplanted into SCID mice. rMV-EGFP-SLAMblind (filled circle) or medium (open circle) was administered three times at 0, 7, and 14 days after the first inoculation (arrows). Tumor volumes of individual mice (n = 7) for each group as the average + SD. (B and C) Tumors of MDA-MB-468 xenografts were excised at 26 dpi (B), and their weights were measured. (C) Tumor weights are shown as the average + SD. ∗p < 0.05, ∗∗p < 0.01, Welch’s t test.
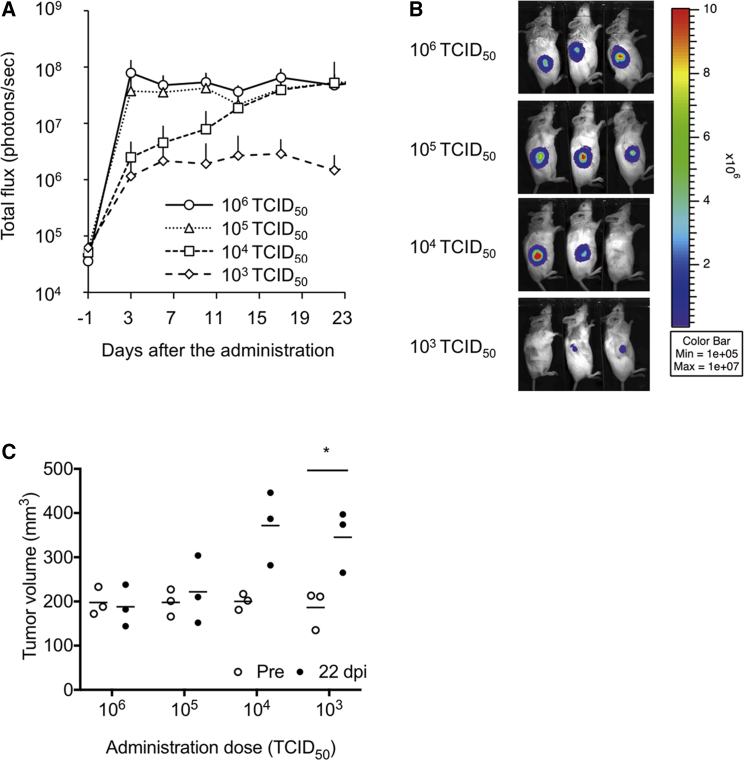
To analyze the antitumor effect of rMV-SLAMblind in vivo kinetically, we administered different dose of rMV-SLAMblind to MDA-MB-468 xenograft. In this experiment, we used luciferase-expressing-rMV-SLAMblind (rMV-LUC-SLAMblind) to follow the virus replication. With the administration of 106 and 105 TCID50 of the virus, virus replication observed by In Vivo Imaging System (IVIS) rapidly increased at 3 dpi and maintained during the observation period (Figure 3A). Virus replication gradually increased in two of three treated mice with 104 TCID50. In mice treated with 103 TCID50, the virus did not replicate efficiently (Figure 3B). Consistently, tumor volumes did not increase with the administration of 106 and 105 TCID50, whereas the tumor volumes with 104 and 103 TCID50 increased (Figure 3C). These results strongly suggest that replication of rMV-SLAMblind suppressed the tumor growth.
Figure 3.
Dose-Dependent Anti-Tumor Effect of rMV-SLAMblind in a Xenograft Model
rMV-LUC-SLAMblind was once administered to the tumor of MDA-MB-468 xenografts. (A) Luciferase activity was shown as the average + SD. (B) Bioluminescent images of the mice at 23 dpi are shown. (C) Tumor volume of individual mice (n = 3) was plotted with the average. ∗p < 0.05, Welch’s t test.
We next examined whether the observed anti-tumor effect continues over a longer period. We performed another in vivo experiment using the MDA-MB-468 cells and continued observations for 3 months. The tumor growth was entirely suppressed even 2 months after the last treatment (Figure 4A). The virus RNA was detected by RT-PCR analysis in all of virus treated tumors (data not shown). In addition, we observed the virus-derived EGFP signals from the tumor sections in one of two mice analyzed (Figure 4B). These results suggest a lasting effect of rMV-SLAMblind against these tumor cells.
Figure 4.
Lasting Anti-Tumor Effect of rMV-SLAMblind in a Xenograft Model
(A) MDA-MB-468 cells were transplanted into SCID mice, and rMV-EGFP-SLAMblind (filled circle, n = 6) or medium (open circle, n = 8) was administered three times at 0, 16, and 30 days after the first inoculation (arrows). Tumor volumes are shown as the average + SD. ∗p < 0.05, ∗∗p < 0.01, Welch’s t test. (B) Observation of a tumor tissue section under a fluorescence microscope. Original magnification, 40× objective lens. Scale bar, 100 μm.
Therapeutic Efficacy of rMV-SLAMblind via Intravenous Administration
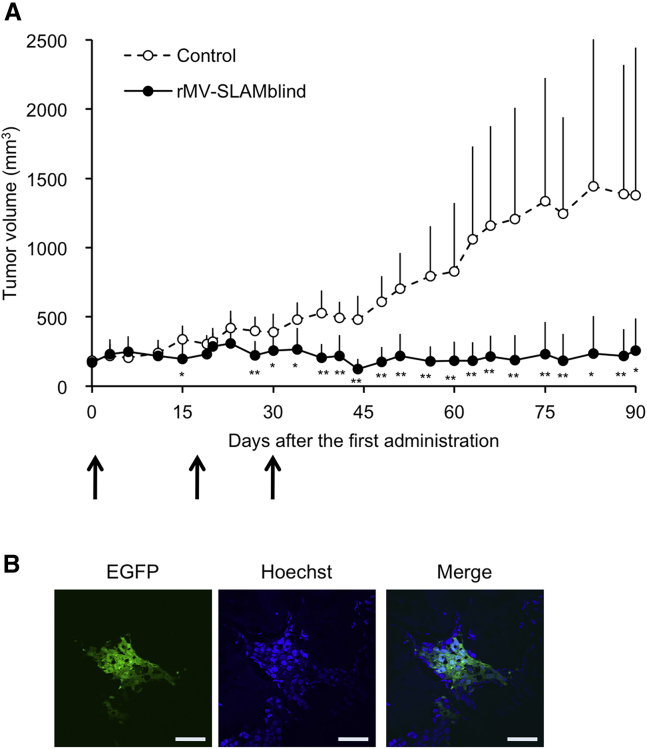
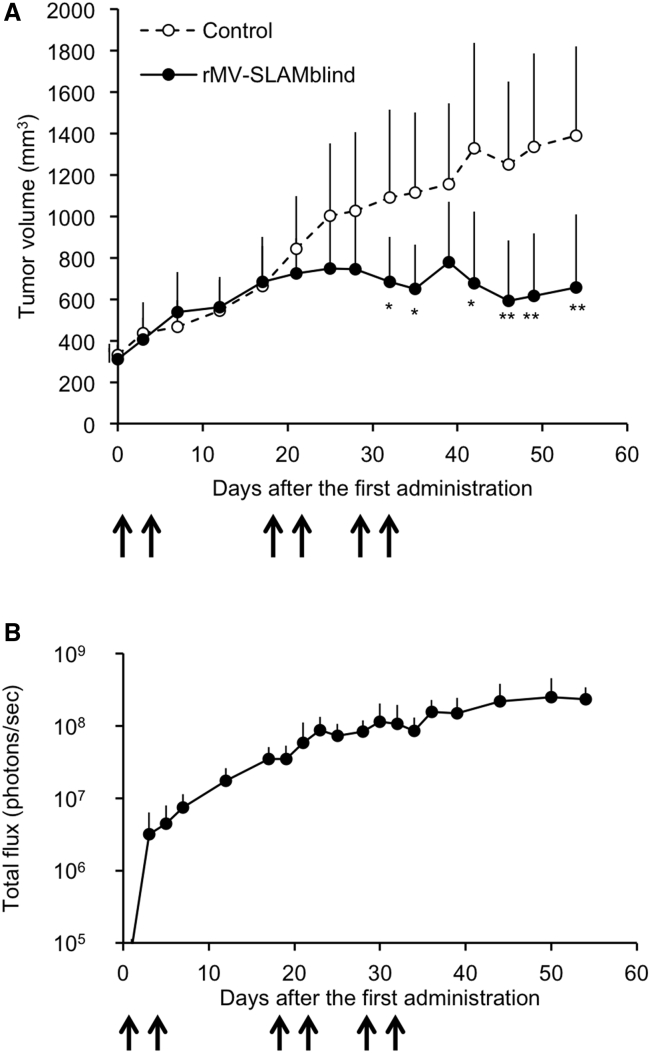
TN breast cancer often causes metastases and, thus, has a poor prognosis. To apply the rMV-SLAMblind therapy to the treatment of metastatic cancers, systemic administration is desirable. Previously, we reported that intravenously administered rMV-SLAMblind in a mouse xenograft model could reach scattered tumors of lung cancer cells in the lungs.22 However, the anti-tumor effect of the virus was not tested in that experiment. To examine the antitumor effect of rMV-SLAMblind following systemic administration, we intravenously administered the virus repeatedly to a xenograft model in which MDA-MB-468 cells were transplanted subcutaneously. The administration of rMV-SLAMblind resulted in the virus replication in the tumors and successfully suppressed tumor growth (Figures 5A and 5B). These results suggest that the systemic administration of rMV-SLAMblind led virus replication in the tumor and efficiently suppressed tumor growth.
Figure 5.
Effect of Systemic Administration of rMV-SLAMblind on TN Breast Cancer Cells in a Xenograft Model
(A) MDA-MB-468 cells were transplanted into SCID mice (n = 6 for each group). rMV-LUC-SLAMblind (1 × 107 TCID50) or medium (open triangle) was administered intravenously six times at 0, 4, 18, 22, 29, and 33 days after the first administration (arrows). Tumor volumes are shown as the average + SD. ∗p < 0.05, ∗∗p < 0.01, Welch’s t test. (B) Luminescent signals of the tumors of the same mice as shown in (A) were monitored after the virus administration. Luminescent levels are shown as the average + SD.
Safety of Intravenously Administered rMV-SLAMblind in Monkeys
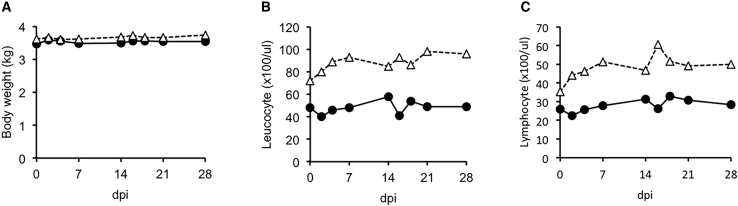
Previously, we demonstrated the safety of subcutaneously administered 1 × 105 TCID50 of rMV-SLAMblind in monkeys.16 In this study, to assess the safety of the virus when administered via the route most likely needed for its use in future clinical therapy, we intravenously administered rMV-SLAMblind to cynomolgus monkeys. Additionally, for the practical use of this virus, a higher dose and/or repeated administrations would be needed. Therefore, we tested a dose of 2 × 107 TCID50, and the same dose of virus was administered again at 14 dpi. During the 28 days following the first administration of rMV-SLAMblind, none of the treated monkeys showed any clinical signs of measles, such as rash, anorexia, diarrhea, and body weight loss (Figure 6A), though the parent MV-HL strain of rMV-SLAMblind had induced rash with a much lower dose (3 × 105 TCID50).33 One of the major symptoms of measles in humans is severe leukocytopenia, and inoculation of monkeys with wild-type MV also causes this symptom.33 Notably, the number of leucocytes and lymphocytes in the monkeys in this study were not decreased following administration with rMV-SLAMblind (Figures 6B and 6C). These results suggest that repeated intravenous administration of a higher dose of rMV-SLAMblind is not harmful for the host animals. To examine whether the administered virus was excreted, we performed RT-PCR analyses on samples from feces, urine, blood, and swabs from the eye, nose, and trachea that were collected at 2, 4, 7, 14, 16, 18, 21, and 28 dpi. The virus RNA was not detected in any samples (data not shown), suggesting that the administered virus had been excreted at very low level below the detection limit if any.
Figure 6.
Safety Evaluation of Intravenous Injection of rMV-SLAMblind in Cynomolgus Monkeys
rMV-SLAMblind (2 × 107 TCID50) was intravenously administered to two cynomolgus monkeys (closed circle and open triangle) at 0 and 14 dpi. (A–C) Body weight (A), leucocyte number (B), and lymphocyte number (C) were measured at 0, 2, 4, 7, 14, 16, 18, 21, and 28 days after the first administration.
Discussion
The prognosis for patients with TN breast cancer in metastatic state is generally very poor, even though various therapies are currently available for treating breast cancers. In this study, we demonstrated that rMV-SLAMblind has a strong antitumor effect against nectin-4-expressing TN breast cancer cell lines, in both in vitro and in xenograft models. We also demonstrated the efficacy of rMV-SLAMblind via intravenous administration. These results suggest that rMV-SLAMblind is a promising candidate as a novel therapeutic agent for the treatment of TN breast cancer expressing nectin-4.
It has been suggested that nectin-4 is correlated with cancer progression in breast, lung, and pancreatic cancer.18,19,34 More detailed analyses suggested importance of nectin-4 as a prognostic biomarker for breast cancer.35, 36, 37 In addition, it is suggested that nectin-4 is a breast cancer stem cell marker.38 Therefore, targeting nectin-4 is a promising strategy to treat breast cancer. The cytotoxicity of rMV-SLAMblind tended to correlate with the nectin-4 expression level, except HCC1937 and BT-20. Therefore, to apply rMV-SLAMblind to therapy, the selection of patients with higher levels of nectin-4 may raise the possibility of therapeutic potency. Because it has been reported that nectin-4 is shed into the serum of patients with breast cancer or ovarian cancer,19,21 measurement of the nectin-4 level in serum will be an effective way to select such patients for a future clinical trial. Cytotoxicity to HCC1937 and BT-20 were lower than others, whereas they expressed nectin-4. The mutation status of TNBC cell lines was well understood, but no specific feature was found commonly to HCC1937 and BT-20 is revealed.39 Several less-responsive cell lines to rMV-SLAMblind were also found among lung and pancreatic cancer.22,24 Molecular mechanism underlying different responsiveness to the virus will be clarified in future.
Antitumor efficacy of rMV-SLAMblind was exerted in xenograft models of two different cell lines, MDA-MB-468 and HCC70. Especially, the efficacy was observed in a dose-dependent manner, strongly suggesting that the virus did cause the tumor suppression. In addition, the tumor suppression was maintained for at least 2 months after the last treatment. When we observed frozen sections of the excised tumors from two mice, we could detect the virus derived EGFP signals from one of them (Figure 4B). The virus may continue to grow in the tumor cells and spread through the tumor until the virus kills all the susceptible tumor cells. Whereas nectin-4-targeting therapy using anti-nectin-4 monoclonal antibody has been also developed,40,41 oncovirotherapy is expected to be more economic, because as long as virus replication continues the effectiveness will be exerted.
For clinical use, it is desirable that a therapeutic agent be effective and safe, even when administered systemically. Our previous study demonstrated that rMV-SLAMblind reaches scattered tumors in the lung of a xenograft mouse model of a human lung cancer cell line.22 In the present study, the intravenous administration of rMV-SLAMblind showed an anti-tumor effect in a xenograft model of a breast cancer cell line, though higher dose was required than that with an intratumoral route. MV can interact with mouse nectin-4,13 but the rMV-SLAMblind could reach and replicate in the tumor in the mouse model, which suggests that nectin-4 in non-cancer cells may not interfere the delivery of rMV-SLAMblind to tumors also in human. Additionally, the intravenous administration of this virus did not induce any measles symptoms in healthy monkeys, suggesting that rMV-SLAMblind administration is safe in primates, even when administered via an intravenous route. The virus RNA was not detected in urine, feces, or swab samples, suggesting that the administered virus is not excreted. Therefore, rMV-SLAMblind therapy is promising to be safe and effective for nectin-4-positive cancer therapy.
Materials and Methods
Cells
BT-20 (ATCC HTB-19), BT-549 (ATCC HTB-122), DU4475 (ATCC HTB-123), HCC1143 (ATCC CRL-2321), HCC1187 (ATCC CRL-2322), HCC1599 (ATCC CRL-2331), HCC1806 (ATCC CRL-2335), HCC1937 (ATCC CRL-2336), HCC38 (ATCC CRL-2314), HCC70 (ATCC CRL-2315), and MDA-MB-468 (ATCC HTB-132) cells were purchased from American Type Culture Collection (Rockville, MD, USA). Hs578T-Luc was purchased from National Institute of Biomedical Innovation (Osaka, Japan). Cells were maintained according to each manufacturer’s protocol. MCF7 was maintained RPMI or DMEM supplemented with 5% FCS.
Viruses
rMV-SLAMblind possessing a green fluorescent protein gene (rMV-EGFP-SLAMblind) or a luciferase gene (rMV-LUC-SLAMbind) had been constructed in the previous study.16 The virus was harvested and titrated using MCF7 cells as previously described.16,22
Virus Infection of TN Breast Cancer Cells
Cells were cultured in 24-well plate and inoculated with rMV-EGFP-SLAMblind at a MOI of 2. Fluorescent signals were observed at days post inoculation (dpi) under a confocal microscope (FV1000, Olympus, Tokyo, Japan)
Flow Cytometry
Cells were washed with phosphate buffered saline (PBS) and stripped with 0.025% trypsin and 0.24 mM EDTA. Cells (1 × 106 cells) were pelleted and then were resuspended in Hank’s Balanced Salt Solution (HBSS, Life Technologies, Carlsbad, CA, USA) or PBS containing 2% fetal calf serum (FCS) and incubated with 0.25 μg of anti-human nectin-4 monoclonal antibody (Clone 337516, R&D Systems, Minneapolis, MN, USA) on ice for at least 30 min. Next, the cells were washed in PBS containing 2% FCS and incubated with anti-mouse immunoglobulin G (IgG)-Alexa 488 diluted 1:2,000 (Life Technologies) on ice for at least 30 min. Finally, the cells were washed with PBS containing 2% FCS, and the fluorescence intensity was measured by a FACSCalibur (BD Biosciences, San Jose, CA, USA). Data were analyzed by FlowJo software.
WST-1 Assay
The cells were washed with PBS and stripped with 0.025% trypsin and 0.24 mM EDTA. Next, the cells (1.25 × 105) were pelleted by centrifugation at 220× g for 3 min. The pelleted cells were resuspended in 300 μL of culture medium or virus inoculum with rMV-EGFP-SLAMblind at a MOI of 1. The cells were incubated at 37°C for 1 h, pelleted by centrifugation at 220× g for 3 min to remove the inoculum, and resuspended in 5 mL of culture medium containing 2% FCS. 200 μL (5 × 103 cells) was added to each well of a 96-well plate, and the cells were cultured at 37°C. Cell viability was determined using a WST-1 Cell Proliferation kit (Takara Bio, Shiga, Japan) at 1, 3, 5, and 7 dpi, according to the manufacturer’s protocol. The viability of the infected cells was calculated as the mean of quadruplicate absorbance values divided by that of uninfected cells and was expressed as a percentage. HCC1599 could not be analyzed because the cells could not be maintained until 7 dpi in this condition.
Xenograft Model
Animal experiments were approved by the Animal Experiment Committee of the Institute of Medical Science, The University of Tokyo. 5- or 6-week-old female SCID mice were purchased from Clea Japan (Tokyo, Japan). MDA-MB-468 and HCC70 cells were suspended in HBSS containing 2% FCS to a concentration of 2 × 108 cells/mL and mixed with an equal volume of Matrigel (BD GF Reduced, BD Biosciences, San Diego, CA, USA). 100 μL of the cell suspension (1 × 108 cells/mL) was injected subcutaneously (1 × 107 cells/mouse). Tumor volume was calculated as the (width × width × length)/2. After the tumor started to grow (5 days for HCC70, Figure 2D; 26 days for MDA-MB-468, Figure 2A; 22 days for MDA-MB-468, Figure 4), 106 TCID50 of rMV-EGFP-SLAMblind was intratumorally administered to the mice. Fluorescent microscopy was performed as described previously.42
To examine dose-dependent anti-tumor efficacy (Figure 3), at 44 days post-implantation of MDA-MB-468 cells, we administered 103, 104, 105, or 106 TCID50 of rMV-LUC-SLAMblind in 100 μL once intratumorally. To examine the efficacy of rMV-SLAMblind following intravenous administration (Figure 5), at 36 days post-implantation of MDA-MB-468 cells, we intravenously administered rMV-LUC-SLAMblind (107 TCID50/mouse) or medium control to the mice via the tail vein. Virus administration was repeated at 4, 18, 22, 29, and 33 dpi. Luciferase activity was measured by IVIS as previously described.22
Statistical Analysis
Welch’s t test was performed to analyze the differences in tumor volumes and sizes by using GraphPad PRISM 7.
Safety Examination using Monkeys
Animal experiments were approved by the Animal Experiment Committee of the Institute of Medical Science, The University of Tokyo. Two cynomolgus monkeys (Macaca fascicularis, 5–6 years old, seronegative for MV) were imported from the Philippines (LSG, Tokyo, Japan). Before import of the monkeys, serum samples from several monkeys were imported, and their antibody titers against MV were tested using an indirect enzyme-linked immunosorbent assay (ELISA) as described previously to screen for individuals who did not possess anti-MV antibodies.43 The imported monkeys were maintained in Amami Laboratory of Injurious Animals of the Institute of Medical Science, The University of Tokyo, as described previously.44 Two cynomolgus monkeys were intravenously administered with rMV-SLAMblind (2 × 107 TCID50/monkey). At 2 weeks post-administration, the monkeys received a second inoculation of the same dose as the first administration. Animals were observed daily for clinical signs of measles. At 2, 4, 7, 14, 16, 18, 21, and 28 dpi, their body weight was recorded under anesthesia. Urine, feces, blood, and swabs from the eye, nose, and trachea were also collected. The total numbers of white blood cells were determined as described previously,44 and the number of lymphocytes was determined by blood smear examination.
Author Contributions
T.F., M.Y., and C.K. designed the study. T.F., Y.A., K.S., T.K., A.S., M.A., H.S., S.H., and M.Y. performed experiments. T.F., Y.A., S.K., and T.K. analyzed the data. T.F. and C.K. wrote the manuscript. C.K. supervised the study.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This study was supported by Health and Labour Sciences Research Grants (15ck0106001h0003), AMED (19ck0106281h0003), and KAKENHI (16K20991).
References
- 1.Grote D., Russell S.J., Cornu T.I., Cattaneo R., Vile R., Poland G.A., Fielding A.K. Live attenuated measles virus induces regression of human lymphoma xenografts in immunodeficient mice. Blood. 2001;97:3746–3754. doi: 10.1182/blood.v97.12.3746. [DOI] [PubMed] [Google Scholar]
- 2.Peng K.W., TenEyck C.J., Galanis E., Kalli K.R., Hartmann L.C., Russell S.J. Intraperitoneal therapy of ovarian cancer using an engineered measles virus. Cancer Res. 2002;62:4656–4662. [PubMed] [Google Scholar]
- 3.McDonald C.J., Erlichman C., Ingle J.N., Rosales G.A., Allen C., Greiner S.M., Harvey M.E., Zollman P.J., Russell S.J., Galanis E. A measles virus vaccine strain derivative as a novel oncolytic agent against breast cancer. Breast Cancer Res. Treat. 2006;99:177–184. doi: 10.1007/s10549-006-9200-5. [DOI] [PubMed] [Google Scholar]
- 4.Iankov I.D., Msaouel P., Allen C., Federspiel M.J., Bulur P.A., Dietz A.B., Gastineau D., Ikeda Y., Ingle J.N., Russell S.J., Galanis E. Demonstration of anti-tumor activity of oncolytic measles virus strains in a malignant pleural effusion breast cancer model. Breast Cancer Res. Treat. 2010;122:745–754. doi: 10.1007/s10549-009-0602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jing Y., Tong C., Zhang J., Nakamura T., Iankov I., Russell S.J., Merchan J.R. Tumor and vascular targeting of a novel oncolytic measles virus retargeted against the urokinase receptor. Cancer Res. 2009;69:1459–1468. doi: 10.1158/0008-5472.CAN-08-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jing Y., Bejarano M.T., Zaias J., Merchan J.R. In vivo anti-metastatic effects of uPAR retargeted measles virus in syngeneic and xenograft models of mammary cancer. Breast Cancer Res. Treat. 2015;149:99–108. doi: 10.1007/s10549-014-3236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galanis E., Hartmann L.C., Cliby W.A., Long H.J., Peethambaram P.P., Barrette B.A., Kaur J.S., Haluska P.J., Jr., Aderca I., Zollman P.J. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res. 2010;70:875–882. doi: 10.1158/0008-5472.CAN-09-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russell S.J., Federspiel M.J., Peng K.-W., Tong C., Dingli D., Morice W.G., Lowe V., O’Connor M.K., Kyle R.A., Leung N. Remission of disseminated cancer after systemic oncolytic virotherapy. Mayo Clin. Proc. 2014;89:926–933. doi: 10.1016/j.mayocp.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galanis E., Atherton P.J., Maurer M.J., Knutson K.L., Dowdy S.C., Cliby W.A., Haluska P., Jr., Long H.J., Oberg A., Aderca I. Oncolytic measles virus expressing the sodium iodide symporter to treat drug-resistant ovarian cancer. Cancer Res. 2015;75:22–30. doi: 10.1158/0008-5472.CAN-14-2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dispenzieri A., Tong C., LaPlant B., Lacy M.Q., Laumann K., Dingli D., Zhou Y., Federspiel M.J., Gertz M.A., Hayman S. Phase I trial of systemic administration of Edmonston strain of measles virus genetically engineered to express the sodium iodide symporter in patients with recurrent or refractory multiple myeloma. Leukemia. 2017;31:2791–2798. doi: 10.1038/leu.2017.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tatsuo H., Ono N., Tanaka K., Yanagi Y. SLAM (CDw150) is a cellular receptor for measles virus. Nature. 2000;406:893–897. doi: 10.1038/35022579. [DOI] [PubMed] [Google Scholar]
- 12.Mühlebach M.D., Mateo M., Sinn P.L., Prüfer S., Uhlig K.M., Leonard V.H., Navaratnarajah C.K., Frenzke M., Wong X.X., Sawatsky B. Adherens junction protein nectin-4 is the epithelial receptor for measles virus. Nature. 2011;480:530–533. doi: 10.1038/nature10639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noyce R.S., Bondre D.G., Ha M.N., Lin L.T., Sisson G., Tsao M.S., Richardson C.D. Tumor cell marker PVRL4 (nectin 4) is an epithelial cell receptor for measles virus. PLoS Pathog. 2011;7:e1002240. doi: 10.1371/journal.ppat.1002240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delpeut S., Noyce R.S., Siu R.W., Richardson C.D. Host factors and measles virus replication. Curr. Opin. Virol. 2012;2:773–783. doi: 10.1016/j.coviro.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Leonard V.H., Hodge G., Reyes-Del Valle J., McChesney M.B., Cattaneo R. Measles virus selectively blind to signaling lymphocytic activation molecule (SLAM; CD150) is attenuated and induces strong adaptive immune responses in rhesus monkeys. J. Virol. 2010;84:3413–3420. doi: 10.1128/JVI.02304-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugiyama T., Yoneda M., Kuraishi T., Hattori S., Inoue Y., Sato H., Kai C. Measles virus selectively blind to signaling lymphocyte activation molecule as a novel oncolytic virus for breast cancer treatment. Gene Ther. 2013;20:338–347. doi: 10.1038/gt.2012.44. [DOI] [PubMed] [Google Scholar]
- 17.Reymond N., Fabre S., Lecocq E., Adelaïde J., Dubreuil P., Lopez M. Nectin4/PRR4, a new afadin-associated member of the nectin family that trans-interacts with nectin1/PRR1 through V domain interaction. J. Biol. Chem. 2001;276:43205–43215. doi: 10.1074/jbc.M103810200. [DOI] [PubMed] [Google Scholar]
- 18.Takano A., Ishikawa N., Nishino R., Masuda K., Yasui W., Inai K., Nishimura H., Ito H., Nakayama H., Miyagi Y. Identification of nectin-4 oncoprotein as a diagnostic and therapeutic target for lung cancer. Cancer Res. 2009;69:6694–6703. doi: 10.1158/0008-5472.CAN-09-0016. [DOI] [PubMed] [Google Scholar]
- 19.Fabre-Lafay S., Monville F., Garrido-Urbani S., Berruyer-Pouyet C., Ginestier C., Reymond N., Finetti P., Sauvan R., Adélaïde J., Geneix J. Nectin-4 is a new histological and serological tumor associated marker for breast cancer. BMC Cancer. 2007;7:73. doi: 10.1186/1471-2407-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fabre-Lafay S., Garrido-Urbani S., Reymond N., Gonçalves A., Dubreuil P., Lopez M. Nectin-4, a new serological breast cancer marker, is a substrate for tumor necrosis factor-alpha-converting enzyme (TACE)/ADAM-17. J. Biol. Chem. 2005;280:19543–19550. doi: 10.1074/jbc.M410943200. [DOI] [PubMed] [Google Scholar]
- 21.Derycke M.S., Pambuccian S.E., Gilks C.B., Kalloger S.E., Ghidouche A., Lopez M., Bliss R.L., Geller M.A., Argenta P.A., Harrington K.M., Skubitz A.P. Nectin 4 overexpression in ovarian cancer tissues and serum: potential role as a serum biomarker. Am. J. Clin. Pathol. 2010;134:835–845. doi: 10.1309/AJCPGXK0FR4MHIHB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujiyuki T., Yoneda M., Amagai Y., Obayashi K., Ikeda F., Shoji K., Murakami Y., Sato H., Kai C. A measles virus selectively blind to signaling lymphocytic activation molecule shows anti-tumor activity against lung cancer cells. Oncotarget. 2015;6:24895–24903. doi: 10.18632/oncotarget.4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amagai Y., Fujiyuki T., Yoneda M., Shoji K., Furukawa Y., Sato H., Kai C. Oncolytic Activity of a Recombinant Measles Virus, Blind to Signaling Lymphocyte Activation Molecule, Against Colorectal Cancer Cells. Sci. Rep. 2016;6:24572. doi: 10.1038/srep24572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Awano M., Fujiyuki T., Shoji K., Amagai Y., Murakami Y., Furukawa Y., Sato H., Yoneda M., Kai C. Measles virus selectively blind to signaling lymphocyte activity molecule has oncolytic efficacy against nectin-4-expressing pancreatic cancer cells. Cancer Sci. 2016;107:1647–1652. doi: 10.1111/cas.13064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irvin W.J., Jr., Carey L.A. What is triple-negative breast cancer? Eur. J. Cancer. 2008;44:2799–2805. doi: 10.1016/j.ejca.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 26.Anders C.K., Carey L.A. Biology, metastatic patterns, and treatment of patients with triple-negative breast cancer. Clin. Breast Cancer. 2009;9(Suppl 2):S73–S81. doi: 10.3816/CBC.2009.s.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dent R., Trudeau M., Pritchard K.I., Hanna W.M., Kahn H.K., Sawka C.A., Lickley L.A., Rawlinson E., Sun P., Narod S.A. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin. Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 28.Li J., Gonzalez-Angulo A.M., Allen P.K., Yu T.K., Woodward W.A., Ueno N.T., Lucci A., Krishnamurthy S., Gong Y., Bondy M.L. Triple-negative subtype predicts poor overall survival and high locoregional relapse in inflammatory breast cancer. Oncologist. 2011;16:1675–1683. doi: 10.1634/theoncologist.2011-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin N.U., Vanderplas A., Hughes M.E., Theriault R.L., Edge S.B., Wong Y.N., Blayney D.W., Niland J.C., Winer E.P., Weeks J.C. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer. 2012;118:5463–5472. doi: 10.1002/cncr.27581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malorni L., Shetty P.B., De Angelis C., Hilsenbeck S., Rimawi M.F., Elledge R., Osborne C.K., De Placido S., Arpino G. Clinical and biologic features of triple-negative breast cancers in a large cohort of patients with long-term follow-up. Breast Cancer Res. Treat. 2012;136:795–804. doi: 10.1007/s10549-012-2315-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Purcell J.W., Davis J., Reddy M., Martin S., Samayoa K., Vo H., Thomsen K., Bean P., Kuo W.L., Ziyad S. Activity of the kinesin spindle protein inhibitor ispinesib (SB-715992) in models of breast cancer. Clin. Cancer Res. 2010;16:566–576. doi: 10.1158/1078-0432.CCR-09-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chao D.T., Su M., Tanlimco S., Sho M., Choi D., Fox M., Ye S., Hsi E.D., Durkin L., Yin J. Expression of TweakR in breast cancer and preclinical activity of enavatuzumab, a humanized anti-TweakR mAb. J. Cancer Res. Clin. Oncol. 2013;139:315–325. doi: 10.1007/s00432-012-1332-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobune F., Takahashi H., Terao K., Ohkawa T., Ami Y., Suzaki Y., Nagata N., Sakata H., Yamanouchi K., Kai C. Nonhuman primate models of measles. Lab. Anim. Sci. 1996;46:315–320. [PubMed] [Google Scholar]
- 34.Nishiwada S., Sho M., Yasuda S., Shimada K., Yamato I., Akahori T., Kinoshita S., Nagai M., Konishi N., Nakajima Y. Nectin-4 expression contributes to tumor proliferation, angiogenesis and patient prognosis in human pancreatic cancer. J. Exp. Clin. Cancer Res. 2015;34:30. doi: 10.1186/s13046-015-0144-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lattanzio R., Ghasemi R., Brancati F., Sorda R.L., Tinari N., Perracchio L., Iacobelli S., Mottolese M., Natali P.G., Piantelli M. Membranous Nectin-4 expression is a risk factor for distant relapse of T1-T2, N0 luminal-A early breast cancer. Oncogenesis. 2014;3:e118. doi: 10.1038/oncsis.2014.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rajc J., Gugić D., Fröhlich I., Marjanović K., Dumenčić B. Prognostic role of Nectin-4 expression in luminal B (HER2 negative) breast cancer. Pathol. Res. Pract. 2017;213:1102–1108. doi: 10.1016/j.prp.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 37.M-Rabet M., Cabaud O., Josselin E., Finetti P., Castellano R., Farina A., Agavnian-Couquiaud E., Saviane G., Collette Y., Viens P. Nectin-4: a new prognostic biomarker for efficient therapeutic targeting of primary and metastatic triple-negative breast cancer. Ann. Oncol. 2017;28:769–776. doi: 10.1093/annonc/mdw678. [DOI] [PubMed] [Google Scholar]
- 38.Siddharth S., Goutam K., Das S., Nayak A., Nayak D., Sethy C., Wyatt M.D., Kundu C.N. Nectin-4 is a breast cancer stem cell marker that induces WNT/β-catenin signaling via Pi3k/Akt axis. Int. J. Biochem. Cell Biol. 2017;89:85–94. doi: 10.1016/j.biocel.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 39.Chavez K.J., Garimella S.V., Lipkowitz S. Triple negative breast cancer cell lines: one tool in the search for better treatment of triple negative breast cancer. Breast Dis. 2010;32:35–48. doi: 10.3233/BD-2010-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Challita-Eid P.M., Satpayev D., Yang P., An Z., Morrison K., Shostak Y., Raitano A., Nadell R., Liu W., Lortie D.R. Enfortumab Vedotin Antibody-Drug Conjugate Targeting Nectin-4 Is a Highly Potent Therapeutic Agent in Multiple Preclinical Cancer Models. Cancer Res. 2016;76:3003–3013. doi: 10.1158/0008-5472.CAN-15-1313. [DOI] [PubMed] [Google Scholar]
- 41.Pavlova N.N., Pallasch C., Elia A.E., Braun C.J., Westbrook T.F., Hemann M., Elledge S.J. A role for PVRL4-driven cell-cell interactions in tumorigenesis. eLife. 2013;2:e00358. [Google Scholar]
- 42.Shoji K., Yoneda M., Fujiyuki T., Amagai Y., Tanaka A., Matsuda A., Ogihara K., Naya Y., Ikeda F., Matsuda H. Development of new therapy for canine mammary cancer with recombinant measles virus. Mol. Ther. Oncolytics. 2016;3:15022. doi: 10.1038/mto.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fujiyuki T., Horie R., Yoneda M., Kuraishi T., Yasui F., Kwon H.J., Munekata K., Ikeda F., Hoshi M., Kiso Y. Efficacy of recombinant measles virus expressing highly pathogenic avian influenza virus (HPAIV) antigen against HPAIV infection in monkeys. Sci. Rep. 2017;7:12017. doi: 10.1038/s41598-017-08326-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujiyuki T., Yoneda M., Yasui F., Kuraishi T., Hattori S., Kwon H.J., Munekata K., Kiso Y., Kida H., Kohara M., Kai C. Experimental infection of macaques with a wild water bird-derived highly pathogenic avian influenza virus (H5N1) PLoS ONE. 2013;8:e83551. doi: 10.1371/journal.pone.0083551. [DOI] [PMC free article] [PubMed] [Google Scholar]