Abstract
To determine the incidence, spectrum, timing, and clinical features of CD19 Chimeric antigen receptor (CAR-T) cell therapy-associated fatal toxic effects. We initiated a comprehensive analysis. First, we retrospectively queried the real-world data from a World Health Organization (WHO) pharmacovigilance database (Vigilyze). Furthermore, we performed a meta-analysis of published trials of CD19 CAR-T cell therapy. From screening the WHO database, we identified 1200 patients: 499 received Kymriah therapy, and 701 received Yescarta therapy. We compared the adverse reactions of the two drugs. We evaluated all published clinical trials, comprising 19 trials and 890 patients. Our meta-analysis showed that the incidence of fatal toxic effects associated with death was 5.4%. Infections and infestations appeared to present the highest risk of death. The toxic effect specific median time to death was 30, 30, and 68 days for total, cytokine release syndrome (CRS), and nervous system disorders (NSD), respectively. We observed a high mortality rate for some toxic effects and an early onset of death with varied causes, indicating the need for clinicians to pay more attention to the monitoring and treatment of these fatal toxic effects when using CD19 CAR-T cell therapy, especially for infections and infestations.
Keywords: CD19 CAR-T cell therapy, immunotherapy, fatal toxic effects, hematological malignancies
INTRODUCTION
Chimeric antigen receptor T cell (CAR-T) therapy was first proposed in 1989 [1]. After several decades of innovation, with the approval of Kymriah and Yescarta by the FDA in 2017, CAR-T cell therapy has become one of the important treatments for tumors, especially hematological malignancies [2–5].
With the approval of CAR-T products and the frequent use of CAR-T cell therapy, the toxic effects of treatment have begun to manifest. CAR-T cell therapy can result in the rapid death of cancer cells, which will cause a variety of immune factors to be released, including IL-6, IL-1, IL-12, TNF-α, IFN-α, GM-CSF and so on. The excessive activation of immune factors will trigger severe toxic reactions, such as CRS and nervous system disorders (NSD), and potential death. [6–8].
However, due to the dispersibility of patients using CAR-T cell therapy and the lack of large-scale clinical studies, the types and rate of fatal toxic effects are not yet clear. With increasing uptake in clinical application, the number of deaths caused by the fatal toxicities associated with CD19 CAR-T cell therapy is gradually increasing. Therefore, the analysis of the landscape of fatal toxicity associated with CD19 CAR-T cell therapy is extremely significant.
Currently, the approved CAR-T products are mainly in anti-CD19. Therefore, we selected CD19 CAR-T cell therapy as the study subject and summarized the toxicity reported by all published CD19 CAR-T related clinical trials. We also analyzed the toxicity data of Kymriah and Yescarta in the WHO database (VigiBase-Vigilyze) [9].
We systematically analyzed the probability, timing, and types of fatal toxic effects, and we hope that this study will provide a guideline for clinicians and reduce patients’ mortality caused by the fatal toxic effects associated with CD19 CAR-T cell therapy.
RESULTS
Global pharmacovigilance analysis
To evaluate the spectrum of CD19 CAR-T cell-related toxic effects, we reviewed the global adverse drug reaction database (Vigilyze-VigiBase). From screening the database, we identified 1200 patients: 499 received Kymriah therapy, and 701 received Yescarta therapy (Table 1).
Table 1. Spectrum of adverse events in vigilyze.
| Variable | No. (%) | P | |
| Kymriah (n=499) | Yescarta (n=701) | ||
| Type of ADR | |||
| Immune system disorders | 261 (52) | 433 (62) | 0.001 |
| Cytokine release syndrome (CRS) | 237 (47) | 426 (61) | < 0.001 |
| Nervous system disorders | 153 (31) | 422 (60) | |
| General disorders and administration site conditions | 259 (52) | 286 (41) | |
| Blood and lymphatic system disorders | 150 (30) | 137 (20) | |
| Cardiac disorders | 58 (12) | 119 (17) | 0.1 |
| Psychiatric disorders | 52 (10) | 109 (16) | 0.01 |
| Vascular disorders | 99 (20) | 104 (15) | 0.024 |
| Infections and infestations | 112 (22) | 99 (14) | < 0.001 |
| Investigations | 173 (35) | 92 (13) | |
| Respiratory, thoracic and mediastinal disorders | 89 (18) | 83 (12) | 0.004 |
| Gastrointestinal disorders | 64 (13) | 72 (10) | 0.196 |
| Benign Neoplasms, Malignant Neoplasm and unspecified (incl cysts and polyps) | 120 (24) | 49 (7) | < 0.001 |
| Renal and urinary disorders | 39 (8) | 39 (6) | 0.124 |
| Metabolism and nutrition disorders | 49 (10) | 29 (4) | < 0.001 |
| Injury, poisoning and procedural complications | 21 (4) | 26 (4) | 0.654 |
| Musculoskeletal and connective tissue disorders | 26 (5) | 25 (4) | 0.191 |
| Skin and subcutaneous tissue disorders | 23 (5) | 15 (2) | 0.019 |
| Surgical and medical procedures | 0 | 15 (2) | 0.031 |
| Hepatobiliary disorders | 15 (3) | 11 (2) | 0.108 |
| Eye disorders | 17 (3.4) | 8 (1) | 0.12 |
| Endocrine disorders | 3 (0.6) | 7 (0.9) | 0.536 |
| Social circumstances | 1 (0.2) | 5 (0.7) | 0.41 |
| Congenital, familial and genetic disorders | 1 (0.2) | 3 (0.4) | 0.645 |
| Product issues | 14 (3) | 3 (0.4) | 0.001 |
| Reproductive system and breast disorders | 2 (0.4) | 1 (0.1) | 0.574 |
| Ear and labyrinth disorders | 3 (0.6) | 0 | 0.072 |
| Age group | |||
| 28 days to 23 months | 7 | — | |
| 2 - 11 years | 106 | — | |
| 12 - 17 years | 85 | 1 | |
| 18 - 44 years | 95 | 80 | |
| 45 - 64 years | 43 | 245 | |
| 65 - 74 years | 29 | 141 | |
| ≥ 75 years | 7 | 23 | |
| Unknown | 127 | 211 | |
| ADR reports per year | |||
| 2019 (up to August 2019) | 322 | 585 | |
| 2018 | 162 | 116 | |
| 2017 | 8 | — | |
| 2016 | 5 | — | |
| 2015 | 2 | — | |
| Geographical distribution | |||
| Americas | 407 | 623 | |
| Europe | 82 | 78 | |
| Oceania | 10 | — | |
The types of fatal toxic effects associated with CD19 CAR-T cell therapy depend on the regimens (Table 1, Supplementary Table 1 in the Supplement). With Kymriah therapy, immune system disorders, general and administration site conditions, and cytokine release syndrome (CRS) was highly predominant (261 [52%], 259 [52%], and 237 [47%], respectively). For Yescarta, patients had a wide variety of fatal toxic effects, including immune system disorders (433 [62%]), cytokine release syndrome (CRS) (426 [61%]), nervous system disorders (422 [60%]), general and administration site conditions (286 [41%]), and blood and lymphatic system disorders (137 [20%]).
In addition, we compared the adverse reactions of the two drugs. The incidence of general and administration site conditions; blood and lymphatic system disorders; vascular disorders; infections and infestations; investigations; respiratory, thoracic and mediastinal disorders; neoplasms, including benign, malignant and unspecified (including cysts and polyps); metabolism and nutrition disorders; skin and subcutaneous tissue disorders; and product-related issues were higher in Kymriah therapy than in Yescarta (P < 0.05). Conversely, immune system disorders, CRS, NSD, psychiatric disorders, and surgical and medical procedures were lower in Kymriah therapy than in Yescarta (P < 0.05). There was no significant difference in cardiac disorders; congenital, familial and genetic disorders; endocrine disorders; or other disorders (P > 0.05) (Table 1).
Meta-analysis
Although the WHO databases are useful for defining the clinical characteristics and spectrum of toxic effects, these databases are unable to conclusively establish their frequency. To determine the frequency of fatal toxic effects, we evaluated all published clinical trials of CD19 CAR-T cell therapy, comprising 19 trials and 890 patients.
To provide additional validation regarding the spectrum of CD19 CAR-T cell-related fatal toxic effects, we assessed the types of fatal toxic effects among the 505 published events (Supplementary Table 2 in the Supplement). CRS was the most frequent cause of death with CD19 CAR-T cell therapy (10 of 33 CD19 CAR-T-related deaths). Nervous system disorders occurred in 6 (18.18%) patients, infections, and infestations in 4 (12.12%), and blood and lymphatic system disorders in 3 (9.09%). Of 7 Kymriah-associated deaths, 2 resulted from blood and lymphatic system disorders, 1 from a nervous system disorder, and 1 from infection and infestation. Of 9 Yescarta-associated deaths, 2 resulted from CRS; 2 from respiratory, thoracic, and mediastinal disorders; and 2 from unknown causes (Table 2). The incidence of fatal toxic effects mortality was not different between Kymriah and Yescarta in all types (P > 0.05, Table 2).
Table 2. Incidence and types of CD19 CAR-T cell therapy-related fatalities from systematic review and meta-analysis.
| Variable | CD-19 Car-T (n=890) | Kymriah (n=201) | Yescarta (n=209) | P* |
| Deaths, No. (%) | 33 (3.71%) | 7 (3.48%) | 9 (4.31%) | 0.667 |
| Type of fatal toxic effect | ||||
| CRS | 10 (30.30%) | 0 | 2 (22.22%) | 0.499 |
| Nervous system disorders | 6 (18.18%) | 1 (14.29%) | 1 (11.11%) | 1 |
| Infections and infestations | 4 (12.12%) | 1 (14.29%) | 0 | 0.49 |
| Blood and lymphatic system disorders | 3 (9.09%) | 2 (28.57%) | 1 (11.11%) | 0.617 |
| Cardiac disorders | 2 (6.06%) | 1 (14.29%) | 1 (11.11%) | 1 |
| Respiratory, thoracic and mediastinal disorders | 2 (6.06%) | 0 | 2 (22.22%) | 0.499 |
| Gastrointestinal disorders | 2 (6.06%) | 0 | 0 | NA |
| Hepatobiliary disorders | 1 (3.03%) | 1 (14.29%) | 0 | 1 |
| unknown cause | 3 (9.09%) | 1 (14.29%) | 2 (22.22%) | 1 |
* Fatal toxic effects rates of Kymriah and Yescarta were compared using χ2 testing.
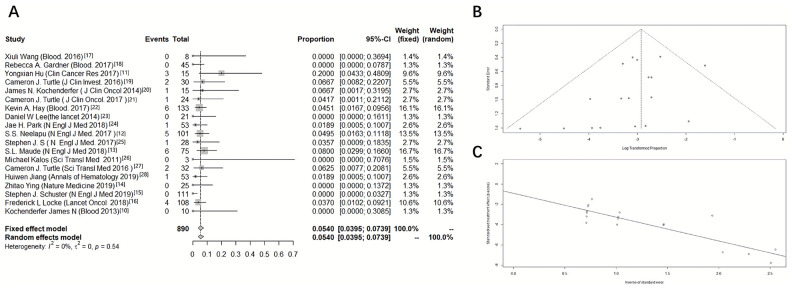
To describe the analytical results simply and intuitively, we created a forest plot, and the results showed that of the 890 participants, 33 had fatal toxic effects. Our meta-analysis showed that the incidence of fatal toxic effects related to death was 5.4%, and the 95% confidence interval of fatal toxic effects was between 3.95% and 7.39% (I2 = 0%, P = 0.54, Figure 1A).
Figure 1.
Forest plot of the incidence of death due to fatal toxic effects associated with CD19 CAR-T cell therapy. A meta-analysis was performed using R statistical software. Event rates and their corresponding 95% confidence intervals were estimated using both a fixed-effects model and a random-effects model. (A) Forest plot, (B) funnel plot, and (C) Egger test.
To determine the presence of publication bias, a funnel plot and the Egger linear regression method were used. The funnel plot presented a general symmetry, and the P-value of the Egger test was 0.0622. The results of the above two methods indicated that there was no publication bias (Figure 1B, 1C).
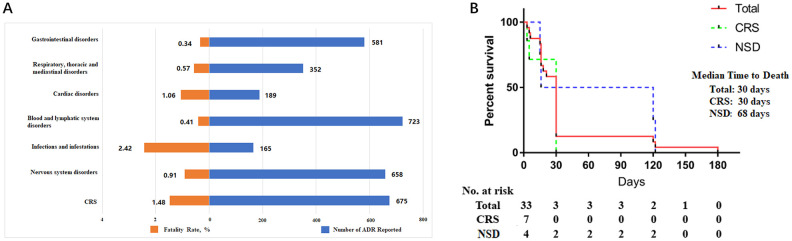
To determine the risk of fatality associated with particular toxic effects, we assessed fatality rates for different classes of toxic effects (Figure 2A). Infections and infestations appeared to present the highest risk of death, with 4 (2.41%) deaths among 165 cases. Toxic effects related to CRS, cardiac disorders, nervous system disorders, and respiratory disorders were associated with fatalities in 0.5% to 1.5% of the reported cases. Gastrointestinal and blood and lymphatic system disorders had the lowest reported fatality rates (0.34% and 0.41%, respectively). According to our meta-analysis, the toxic effect specific median time to death was 30, 30, and 68 days for total, CRS, and NSD (nervous system disorders), respectively (Figure 2B).
Figure 2.
Clinical characteristics of fatal toxic effects and time to death onset of fatal toxic effects. (A) The number of cases (blue) and fatality rate (orange) for each class of toxic effect. (B) The time to death onset due to fatal toxic effects. Total (red), CRS: cytokine release syndrome (green), NSD: nervous system disorders (blue).
We noticed that the incidence of CRS and nervous system disorders was higher than that of other adverse reactions, so we carried a meta-analysis on the incidence of CRS and NSD over grade 3. For CRS, the incidence was more than 23% (I2 = 80%, P <0.01, Supplementary Figure 1A); for NSD, the incidence was more than 20% (I2 = 77%, P < 0.01, Supplementary Figure 1E). Since there was high heterogeneity, we performed a subgroup analysis, which indicated that age might be one of the reasons for the heterogeneity (Supplementary Figure 1B, 1F).
DISCUSSION
Our work is the largest sample study on the toxicity of CD19 CAR-T treatment. Using the WHO database, we analyzed the toxic effects of Kymriah and Yescarta in real-world clinical applications. It showed that the proportion of immune system disorders, CRS, and NSD of Yescarta are higher than that of Kymriah. We systematically analyzed the data of published clinical trials. We found that the mortality caused by CD19 CAR-T-related lethal toxicity was 5.4%, which was higher than that of other immunotherapies (immune checkpoint therapy 0.36% - 1.08%) [10]. There is no significant difference between Kymriah and Yescarta in terms of fatal toxic effects.
We also found that although the incidence of toxic effects of the three groups was high, comparing all toxic effects, mortality was mostly related to infections and infestation, which is related to immunosuppression resulting from hematological malignancies. Although CRS and NSD are the most serious toxic effects, there are drugs to address CRS and NSD [6]; therefore, their associated mortality rate was low.
Both IL-6 and C-reactive protein (CRP) levels can predict CRS, and the level of IL-6 is positively correlated with the grade of CRS [11, 12]. We mainly use anti-IL-6 monoclonal antibodies to treat CRS, such as tocilizumab and siltuximab. Some studies have shown that therapeutic plasma exchange (TPE) may be the other option [13]. The use of glucocorticoids is also one of the main therapeutic methods, which are generally used to supplement anti-IL-6 therapy [14, 15].
However, studies have shown that glucocorticoid treatment of CRS may reduce the efficacy of CAR-T treatment, which is a controversial finding that needs further elucidation [16, 17].
NSD, also known as CAR-T cell-related encephalopathy syndrome (CRES), is related to the diffusion of cytokines into the nervous system [18]. Therefore, clinical studies have shown that anti-IL-6 drugs can also be used to treat CRES [6, 19]. However, there are also disputes regarding the choice of drugs. Some studies have shown that tocilizumab can alleviate neurotoxic effects while others have pointed out that it cannot cross the blood-brain barrier (BBB) [20, 21]. The mechanism of siltuximab is different and can effectively reduce the level of IL-6 and reduce neurotoxicity [22]. In addition, the Memorial Sloan Kettering Cancer Center MSKCC team found that anakinra, an anti-IL-1 drug, can be an option for reducing neurotoxicity [23]. To our knowledge, there is no official guideline on the treatment of nervous system toxicity. In clinical practice, it is necessary to have a high index of suspicion for fatal toxic effects during and after CD19 CAR-T cell therapy.
Contrary to our understanding of CAR-T therapy, our study found that infections and infestations are fatal toxic effects and should, therefore, receive more attention. When monitoring CRS and CRES, we should also be vigilant on the occurrence of infections and infestations, use antibiotics promptly, and reduce the infection risks of patients as much as possible during the treatment.
Also, with more patients receiving CAR-T treatment, we are increasingly learning some rare fatal toxic effects—for example, Lee DW et al. [19] found a case in which the CAR that should have been integrated into T cells was integrated into tumor cells, forming CAR-tumor cells, further increasing tumor drug resistance, and the patient died from the progression of the tumor. In addition to the report of CAR integration error causing an off-target effect or gene mutation, the JUNE team [24], the discoverer of CAR-T cell therapy, found CAR insertion into the TER2 gene of one patient, causing gene dysfunction, but this insertion brought a better therapeutic effect and indicated that CAR integration technology needs to be further improved. Currently, there are two types of transposition subsystems that have a good foreground, “Sleeping Beauty” transposition subsystems [25] and “piggy BAC” systems [26], which are expected to reduce the incidence of CAR integration errors.
Furthermore, as drugs are used to treat toxic effects, many researchers are also developing new ways to reduce the occurrence of toxic effects, such as integrating corresponding regulatory elements, inserting genes into CAR and so on, which can effectively prevent off-target and toxic effects while enhancing the therapeutic efficacy [27–31].
Limitations
Our research has some limitations. Since the WHO database only recorded the number of toxic effects and did not include the number of deaths, we were unable to provide a real-world data analysis of the fatality rate. We hope that there will be a study with large sample size and more detailed real-world data to verify and analyze the fatal toxic effects associated with CAR-T cell therapy.
CONCLUSIONS
Our study showed that the incidence of fatal toxic effects associated with CD19 CAR-T cell therapy is high. Infections and infestation, CRS, and nervous system disorders are the main causes of death. Whether it is clinical trials or WHO real-world data, the incidences of CRS and nervous system disorders were both higher than 50%. The overall mortality rate was 5.4% of all patients. However, the highest mortality rate, at 2.42% of patients, was related to infections and infestations. Most of the patients died within 30 days after treatment, indicating the need for clinicians to pay more attention to the monitoring and timely treatment of these fatal toxic effects, especially for infections and infestations.
MATERIALS AND METHODS
Vigilyze database
The study is based on adverse drug reactions reported in VigiBase (http://www.vigiaccess.org/), the WHO global Individual Safety Case Report (ISCR) database, originating from >130 countries. VigiBase is managed by the Uppsala Monitoring Center (UMC) and contains >16,000,000 ISCRs submitted by national pharmacovigilance centers since 1968. These reports originate from different sources, such as healthcare professionals, patients, and pharmaceutical companies, and are generally filed post-marketing [9].
We accessed Vigilyze on 30th July 2019 and queried for Kymriah and Yescarta. We included all adverse drug reaction reports in which known CD19 CAR-T cell-related toxic effects occurred (Supplementary Table 1 in the Supplement). Patients with resolved toxic effects, unknown outcomes, or known/presumed cancer-related deaths were excluded. All the categories of toxic effects associated with CD19 CAR-T cell therapy are according to the rules of the Vigilyze Database.
Meta-analysis
Inclusion criteria
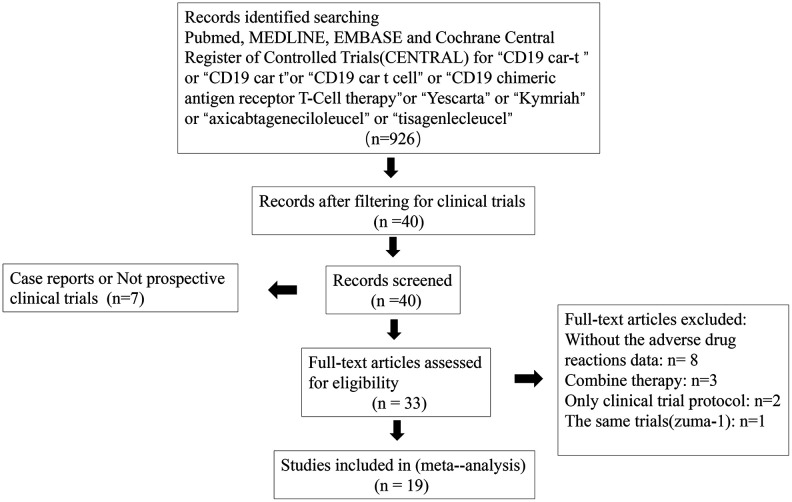
The study was registered in the PROSPERO (CRD42018112366). We identified records by searching PubMed, Medline, Embase and the Cochrane Central Register of Controlled Trials (CENTRAL) for “CD19 CAR-T” or “CD19 CAR T” or “CD19 CAR T cell” or “CD19 chimeric antigen receptor T-cell therapy” or “Yescarta” or “Kymriah” or “axicabtagene ciloleucel” or “tisagenlecleucel” on 30th July 2019 (Figure 3). English-language clinical trials were included and spanned from 2013 to 2019.
Figure 3.
PRISMA diagram for articles selected for meta-analysis.
Exclusion criteria
All 926 studies were screened, and those that were clinical trials were included (n = 40), non-prospective clinical trials or case reports were all excluded (n = 7). Trials without adverse drug reaction data, those with combination therapy, those with only the clinical trial protocol provided, and those that were duplicates were excluded (n = 13). When two publications reported the same trial, the article with the longer follow-up time was selected (a phase II study that was initially reported and then subsequently reported with a prolonged follow-up, n=1).
The remaining trials (n = 19) were assessed individually for CD19 CAR-T cell therapy, Kymriah and Yescarta, the total number of patients treated, and the number and type of fatal toxic effects. The incidences of fatal toxic effects associated with CD19 CAR-T cell therapy were compared. Similarly, the types of toxic effects were compared (Supplementary Table 2, 3) [17, 19, 32–48]. All the categories of toxic effects associated with CD19 CAR-T cell therapy are according to the rules of the Vigilyze Database. The mortality data included in our cohort were from the patients who died from CD19 CAR-T cell therapy-related toxic effects.
Statistics
Fatal toxic effect rates were compared using χ2 testing. Other clinical variables of interest were evaluated descriptively. Statistical analyses were performed in GraphPad Prism (version 7, GraphPad Software); the meta-analysis was performed using R statistical software (packages metafor and meta, R Foundation). Event rates and their corresponding 95% confidence intervals were estimated using both a fixed-effects model and a random-effects model. Forest plots were constructed to summarize the data for each analysis group with the incidence rate and to provide a visual analysis of studies evaluating fatal drug-related events.
Supplementary Material
Footnotes
AUTHOR CONTRIBUTIONS: C.C., D.T., H.S., and S.Z. designed the study. C.C., D.T., and Y.H. collected the data and performed the major analysis. S.Z. And H.S. supervised the study. C.C. and D.T. analyzed and interpreted the data. E.S. and Y.F. did the statistical analysis. C.C., D.T., C.G., and O.A. drafted the manuscript. All authors read and approved the final manuscript.
CONFLICTS OF INTEREST: The authors declare no conflicts of interests.
FUNDING: This study was supported by grants from the National Natural Science Foundation of China (No. 81070362, 81172470, 81372629, 81772627, 81874073 and 81974384 to S.Z and H.S), the National Key R & D Program of China (No. 2018YFC1313303 to S.Z and H.S), two key projects from the Nature Science Foundation of Hunan Province (No. 2015JC3021 and 2016JC2037 to S.Z and H.S), the Fundamental Research Funds for the Central Universities of Central South University (No.2020 to C.C) and a project from the China Cancer Elite Team Innovative Grant (No. 201606 to S.Z and H.S).
REFERENCES
- 1.Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci USA. 1989; 86:10024–28. 10.1073/pnas.86.24.10024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bach PB, Giralt SA, Saltz LB. FDA approval of tisagenlecleucel: promise and complexities of a $475 000 cancer drug. JAMA. 2017; 318:1861–62. 10.1001/jama.2017.15218 [DOI] [PubMed] [Google Scholar]
- 3.Zheng PP, Kros JM, Li J. Approved CAR T cell therapies: ice bucket challenges on glaring safety risks and long-term impacts. Drug Discov Today. 2018; 23:1175–82. 10.1016/j.drudis.2018.02.012 [DOI] [PubMed] [Google Scholar]
- 4.Liu B, Yan L, Zhou M. Target selection of CAR T cell therapy in accordance with the TME for solid tumors. Am J Cancer Res. 2019; 9:228–41. [PMC free article] [PubMed] [Google Scholar]
- 5.Fu Y, Liu S, Zeng S, Shen H. From bench to bed: the tumor immune microenvironment and current immunotherapeutic strategies for hepatocellular carcinoma. J Exp Clin Cancer Res. 2019; 38:396. 10.1186/s13046-019-1396-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, Komanduri KV, Lin Y, Jain N, Daver N, Westin J, Gulbis AM, Loghin ME, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2018; 15:47–62. 10.1038/nrclinonc.2017.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahadeo KM, Khazal SJ, Abdel-Azim H, Fitzgerald JC, Taraseviciute A, Bollard CM, Tewari P, Duncan C, Traube C, McCall D, Steiner ME, Cheifetz IM, Lehmann LE, et al. , and Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network. Management guidelines for paediatric patients receiving chimeric antigen receptor T cell therapy. Nat Rev Clin Oncol. 2019; 16:45–63. 10.1038/s41571-018-0075-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abu-Sbeih H, Tang T, Ali FS, Luo W, Neelapu SS, Westin JR, Okhuysen PC, Foo WC, Curry JL, Richards DM, Ge PS, Wang Y. Gastrointestinal adverse events observed after chimeric antigen receptor T-cell therapy. Am J Clin Oncol. 2019; 42:789–96. 10.1097/COC.0000000000000596 [DOI] [PubMed] [Google Scholar]
- 9.Lindquist M. VigiBase, the WHO Global ICSR Database System: Basic Facts. Drug Information Journal. 2008; 42:409–419. 10.1177/009286150804200501 [DOI] [Google Scholar]
- 10.Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, Rathmell WK, Ancell KK, Balko JM, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018; 4:1721–28. 10.1001/jamaoncol.2018.3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norelli M, Camisa B, Barbiera G, Falcone L, Purevdorj A, Genua M, Sanvito F, Ponzoni M, Doglioni C, Cristofori P, Traversari C, Bordignon C, Ciceri F, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018; 24:739–48. 10.1038/s41591-018-0036-4 [DOI] [PubMed] [Google Scholar]
- 12.Porter D, Frey N, Wood PA, Weng Y, Grupp SA. Grading of cytokine release syndrome associated with the CAR T cell therapy tisagenlecleucel. J Hematol Oncol. 2018; 11:35. 10.1186/s13045-018-0571-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Chen X, Wang D, Li H, Huang J, Zhang Z, Qiao Y, Zhang H, Zeng Y, Tang C, Yang S, Wan X, Chen YH, et al. Hemofiltration Successfully Eliminates Severe Cytokine Release Syndrome Following CD19 CAR-T-Cell Therapy. J Immunother. 2018; 41:406–410. 10.1097/CJI.0000000000000243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: mechanisms, manifestations and management. Blood Rev. 2019; 34:45–55. 10.1016/j.blre.2018.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frey N, Porter D. Cytokine release syndrome with chimeric antigen receptor T cell therapy. Biol Blood Marrow Transplant. 2019; 25:e123–27. 10.1016/j.bbmt.2018.12.756 [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Hu Y, Huang H. Acute lymphoblastic leukemia relapse after CD19-targeted chimeric antigen receptor T cell therapy. J Leukoc Biol. 2017; 102:1347–56. 10.1189/jlb.5RU0817-315R [DOI] [PubMed] [Google Scholar]
- 17.Hu Y, Wu Z, Luo Y, Shi J, Yu J, Pu C, Liang Z, Wei G, Cui Q, Sun J, Jiang J, Xie J, Tan Y, et al. Potent anti-leukemia activities of chimeric antigen receptor-modified T cells against CD19 in Chinese patients with relapsed/refractory acute lymphocytic leukemia. Clin Cancer Res. 2017; 23:3297–306. 10.1158/1078-0432.CCR-16-1799 [DOI] [PubMed] [Google Scholar]
- 18.Hay KA. Cytokine release syndrome and neurotoxicity after CD19 chimeric antigen receptor-modified (CAR-) T cell therapy. Br J Haematol. 2018; 183:364–74. 10.1111/bjh.15644 [DOI] [PubMed] [Google Scholar]
- 19.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M, Shah NN, Steinberg SM, Stroncek D, Tschernia N, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015; 385:517–28. 10.1016/S0140-6736(14)61403-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, Mahnke YD, Melenhorst JJ, Rheingold SR, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014; 371:1507–17. 10.1056/NEJMoa1407222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, Grupp SA, Mackall CL. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014; 124:188–95. 10.1182/blood-2014-05-552729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gust J, Hay KA, Hanafi LA, Li D, Myerson D, Gonzalez-Cuyar LF, Yeung C, Liles WC, Wurfel M, Lopez JA, Chen J, Chung D, Harju-Baker S, et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017; 7:1404–19. 10.1158/2159-8290.CD-17-0698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Z, Han W. Biomarkers of cytokine release syndrome and neurotoxicity related to CAR-T cell therapy. Biomark Res. 2018; 6:4. 10.1186/s40364-018-0116-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Busque L, Patel JP, Figueroa ME, Vasanthakumar A, Provost S, Hamilou Z, Mollica L, Li J, Viale A, Heguy A, Hassimi M, Socci N, Bhatt PK, et al. Recurrent somatic TET2 mutations in normal elderly individuals with clonal hematopoiesis. Nat Genet. 2012; 44:1179–81. 10.1038/ng.2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chicaybam L, Abdo L, Carneiro M, Peixoto B, Viegas M, de Sousa P, Fornazin MC, Spago MC, Albertoni Laranjeira AB, de Campos-Lima PO, Nowill A, Barros LR, Bonamino MH. CAR T cells generated using sleeping beauty transposon vectors and expanded with an EBV-transformed lymphoblastoid cell line display antitumor activity in vitro and in vivo. Hum Gene Ther. 2019; 30:511–22. 10.1089/hum.2018.218 [DOI] [PubMed] [Google Scholar]
- 26.Wang P, Qin W, Liu T, Jiang D, Cui L, Liu X, Fang Y, Tang X, Jin H, Qian Q. PiggyBac-engineered T cells expressing a glypican-3-specific chimeric antigen receptor show potent activities against hepatocellular carcinoma. Immunobiology. 2020; 225:151850. 10.1016/j.imbio.2019.09.009 [DOI] [PubMed] [Google Scholar]
- 27.Li J, Li W, Huang K, Zhang Y, Kupfer G, Zhao Q. Chimeric antigen receptor T cell (CAR-T) immunotherapy for solid tumors: lessons learned and strategies for moving forward. J Hematol Oncol. 2018; 11:22. 10.1186/s13045-018-0568-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russo V, Bondanza A, Ciceri F, Bregni M, Bordignon C, Traversari C, Bonini C. A dual role for genetically modified lymphocytes in cancer immunotherapy. Trends Mol Med. 2012; 18:193–200. 10.1016/j.molmed.2011.12.003 [DOI] [PubMed] [Google Scholar]
- 29.Springer CJ, Niculescu-Duvaz I. Prodrug-activating systems in suicide gene therapy. J Clin Invest. 2000; 105:1161–67. 10.1172/JCI10001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiong W, Chen Y, Kang X, Chen Z, Zheng P, Hsu YH, Jang JH, Qin L, Liu H, Dotti G, Liu D. Immunological synapse predicts effectiveness of chimeric antigen receptor cells. Mol Ther. 2018; 26:963–75. 10.1016/j.ymthe.2018.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ye B, Stary CM, Li X, Gao Q, Kang C, Xiong X. Engineering chimeric antigen receptor-T cells for cancer treatment. Mol Cancer. 2018; 17:32. 10.1186/s12943-018-0814-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kochenderfer JN, Dudley ME, Carpenter RO, Kassim SH, Rose JJ, Telford WG, Hakim FT, Halverson DC, Fowler DH, Hardy NM, Mato AR, Hickstein DD, Gea-Banacloche JC, et al. Donor-derived CD19-targeted T cells cause regression of Malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood. 2013; 122:4129–39. 10.1182/blood-2013-08-519413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, Timmerman JM, Stiff PJ, Friedberg JW, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017; 377:2531–44. 10.1056/NEJMoa1707447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD, Qayed M, De Moerloose B, Hiramatsu H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018; 378:439–48. 10.1056/NEJMoa1709866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ying Z, Huang XF, Xiang X, Liu Y, Kang X, Song Y, Guo X, Liu H, Ding N, Zhang T, Duan P, Lin Y, Zheng W, et al. A safe and potent anti-CD19 CAR T cell therapy. Nat Med. 2019; 25:947–53. 10.1038/s41591-019-0421-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, Jäger U, Jaglowski S, Andreadis C, Westin JR, Fleury I, Bachanova V, Foley SR, et al. , and JULIET Investigators. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019; 380:45–56. 10.1056/NEJMoa1804980 [DOI] [PubMed] [Google Scholar]
- 37.Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, Lin Y, Braunschweig I, Hill BT, Timmerman JM, Deol A, Reagan PM, Stiff P, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019; 20:31–42. 10.1016/S1470-2045(18)30864-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X, Popplewell LL, Wagner JR, Naranjo A, Blanchard MS, Mott MR, Norris AP, Wong CW, Urak RZ, Chang WC, Khaled SK, Siddiqi T, Budde LE, et al. Phase 1 studies of central memory-derived CD19 CAR T-cell therapy following autologous HSCT in patients with B-cell NHL. Blood. 2016; 127:2980–90. 10.1182/blood-2015-12-686725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gardner RA, Finney O, Annesley C, Brakke H, Summers C, Leger K, Bleakley M, Brown C, Mgebroff S, Kelly-Spratt KS, Hoglund V, Lindgren C, Oron AP, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017; 129:3322–31. 10.1182/blood-2017-02-769208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, Sommermeyer D, Melville K, Pender B, Budiarto TM, Robinson E, Steevens NN, Chaney C, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016; 126:2123–38. 10.1172/JCI85309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, Yang JC, Phan GQ, Hughes MS, Sherry RM, Raffeld M, Feldman S, Lu L, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell Malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015; 33:540–49. 10.1200/JCO.2014.56.2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turtle CJ, Hay KA, Hanafi LA, Li D, Cherian S, Chen X, Wood B, Lozanski A, Byrd JC, Heimfeld S, Riddell SR, Maloney DG. Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor-modified T cells after failure of ibrutinib. J Clin Oncol. 2017; 35:3010–20. 10.1200/JCO.2017.72.8519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hay KA, Hanafi LA, Li D, Gust J, Liles WC, Wurfel MM, López JA, Chen J, Chung D, Harju-Baker S, Cherian S, Chen X, Riddell SR, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017; 130:2295–306. 10.1182/blood-2017-06-793141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ, Sauter C, Wang Y, Santomasso B, Mead E, Roshal M, Maslak P, Davila M, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018; 378:449–59. 10.1056/NEJMoa1709919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak Ö, Brogdon JL, Pruteanu-Malinici I, Bhoj V, Landsburg D, Wasik M, Levine BL, Lacey SF, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017; 377:2545–54. 10.1056/NEJMoa1708566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011; 3:95ra73. 10.1126/scitranslmed.3002842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turtle CJ, Hanafi LA, Berger C, Hudecek M, Pender B, Robinson E, Hawkins R, Chaney C, Cherian S, Chen X, Soma L, Wood B, Li D, et al. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med. 2016; 8:355ra116. 10.1126/scitranslmed.aaf8621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang H, Liu L, Guo T, Wu Y, Ai L, Deng J, Dong J, Mei H, Hu Y. Improving the safety of CAR-T cell therapy by controlling CRS-related coagulopathy. Ann Hematol. 2019; 98:1721–32. 10.1007/s00277-019-03685-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.