Abstract
Purpose
Anemia and sarcopenia associated with renal dysfunction caused by cytokine imbalance can contribute to decreased quality of life for older individuals. Growth differentiation factor-15 (GDF-15) is associated with renal dysfunction, although whether it is related to anemia or sarcopenia is unclear. In this study we examined the association of GDF-15 with renal function, hemoglobin and sarcopenia in healthy community-dwelling older females in Japan.
Methods
A total of 66 healthy older community-dwelling females (age: 75.8 ± 6.2 years) were enrolled for this study. Skeletal muscle mass index was determined by bioelectrical impedance analysis. Hand-grip strength and walking speed were also assessed. Serum GDF-15 concentration was determined by enzyme-linked immunosorbent assay and both hemoglobin (Hb) level and estimated glomerular filtration rate (eGFR) were measured.
Results
Serum GDF-15 levels positively correlated with age but negatively correlated with eGFR and walking speed. In multiple regression analysis, eGFR and hemoglobin (Hb) were independent variables to predict serum GDF-15 levels, even after adjusting for age and body mass index (eGFR: β = −0.423, p < 0.001; Hb: β = −0.363, p = 0.004). Serum GDF-15 level was an independent variable to predict eGFR and Hb.
Conclusions
Both Hb and eGFR are predictors for serum GDF-15 concentration in healthy older females. In these community-dwelling older females, renal dysfunction via GDF-15 may be accompanied by anemia, but not sarcopenia.
Keywords: Community-dwelling older females, GDF-15, Hemoglobin, Renal function
1. Introduction
In an aging society, the number of individuals with renal dysfunction, which affects healthy life expectancy, increases [1], [2]. Renal dysfunction is associated with comorbid conditions, including frailty, sarcopenia and anemia. Physical performance-based measures can predict mortality [3], and sarcopenia is a significant problem in chronic kidney disease (CKD). Frailty is more prevalent among older subjects with CKD than those having normal renal function [4]. Anemia is highly prevalent among older individuals, and is associated with physical and cognitive function [5]. Moreover, the prevalence of anemia is substantially higher among patients with an estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2 [6], [7]. Anemia and sarcopenia as well as renal dysfunction decrease the quality of life for older individuals. However, whether anemia or sarcopenia that accompany renal dysfunction are associated with cytokine dysregulation in older subjects is unclear.
Growth differentiation factor-15 (GDF-15) is a member of the transforming growth factor-β (TGF-β) cytokine superfamily and plays multiple roles in various pathological conditions, including cardiovascular disease [8], inflammation [9], cancer [10] and kidney disease [11]. Cardiomyocytes [12] and vascular smooth muscle cells [13] produce and secrete GDF-15 in response to oxidative stress or proinflammatory cytokines. A limited number of studies have investigated the association between serum GDF-15 and renal function in older subjects [14], [15]. In these studies, multiple regression analysis showed that eGFR is an independent variable to predict GDF-15 [14], [15], and that hemoglobin (Hb) is an independent determinant of GDF-15 [15]. In addition, anterior thigh muscle thickness measured by echography as well as eGFR levels is independent determinants of GDF-15 levels in patients with cardiovascular disease [16]. However, to our knowledge, no previous studies have investigated independent variables to predict GDF-15 using eGFR, Hb and skeletal muscle mass as independent variables in healthy community-dwelling older subjects.
The present study was performed to clarify the association of GDF-15 with renal dysfunction, anemia and sarcopenia in older community-dwelling subjects.
2. Methods
2.1. Participants
A total of 68 healthy older females (age: 75.7 ± 6.1 years) were enrolled. Two female subjects were excluded because body composition testing was not performed in one subject and serum GDF-15 measurements was not performed in another subject. All subjects lived in Yokohama and Yokosuka city and regularly attended the exercise class offered at Kanagawa University of Human Services. All subjects had no severe diseases, but some were receiving medical treatment including antihypertensive agent (n = 9), cholesterol-lowering agent (n = 2), stomach medication (n = 2), analgesics (n = 2), vitamin D (n = 2), bisphosphonate (n = 1) and aspirin (n = 1). The study was approved by the Ethics Committee of the Kanagawa University of Human Services (No. 7-7), and the Regional Ethics Committee of Dokkyo Medical University Hospital (No. R-30-2J). Informed consent was obtained from all participants.
Blood samples were collected after a 12-hour fast. Hb, serum iron (Fe), serum albumin (Alb) levels were measured, and high-sensitive C reactive protein (hsCRP) level was measured via an immunonephelometric assay (BML Inc., Tokyo, Japan). Estimated glomerular filtration rate (eGFR) was evaluated for female subjects only using the following equation:
2.2. Enzyme linked immunosorbent assay (ELISA)
To measure fasting serum GDF-15 concentration, peripheral venous blood was collected in pyrogen-free tubes with and without EDTA in the morning. Plasma and serum for all enzyme linked immunosorbent assays (ELISA) were stored at −80 °C. Serum GDF-15 level was measured using a Human Quantikine ELISA Kit (DGD150 for GDF-15, R&D Systems, Minneapolis, MN, USA) as previously reported [16]. The mean intra-assay coefficient of variation (CV) was 2.3%, and the inter-assay CV was 5.4%. Samples, reagents and buffers were prepared according to the manufacturer’s recommendations. The detection threshold for GDF-15 was 0.002 ng/mL.
2.3. Measurement of walking speed and hand-grip strength
Hand-grip strength for the right hand was measured twice and the higher value was used. Walking speed was measured as the time needed to walk 10 m. Two measurements were taken and the higher value was adopted.
2.4. Bioelectrical impedance analyzer (BIA) measurements
Body composition was measured as previously described with a multi-frequency bioelectrical impedance analyzer (BIA; InBody S10 Biospace, Biospace Co., Ltd., Korea/Model JMW140) while the patient was in a supine position [17]. Body fat volume, body fat percentage and skeletal muscle mass were calculated. Skeletal muscle mass index (SMI) was calculated as (Skeletal muscle mass, kg)/(Body height, m)2. Sarcopenia was evaluated only in females and defined according to Asian Working Group for Sarcopenia (AWGS) criteria (hand-grip < 18 kg or walking speed ≦0.8 m/s, and SMI < 5.7 kg/m2) [18].
2.5. Statistical analysis
Data are shown as mean values ± SD. After testing normality using Kolmogorov-Smirnov methods, associations among parameters were evaluated by Pearson methods for normally distributed parameters and by Spearman methods for non-normally distributed parameters. Multiple linear regression analyses with log (serum GDF-15 concentration), log (eGFR) and Hb as the dependent variable were performed to identify influencing independent factors (e.g., clinical laboratory or physical data). Age and body mass index (BMI) were used as covariates. All analyses were performed using SPSS version 26 for Windows (IBM Corp., New York, NY, USA). A p value < 0.05 was regarded as significant.
3. Results
3.1. Study participant characteristics
A total of 66 healthy older females were enrolled. The mean age of the patient population was 75.8 ± 6.2 years and the mean BMI was 22.8 ± 2.8 kg/m2. Among the study subjects, 4 (6%) had sarcopenia. Body composition testing and serum GDF-15 measurements were performed for all subjects. Values for physical and clinical laboratory tests are summarized in Table 1.
Table 1.
Mean values for participant parameters.
| Total (n = 66) | |
|---|---|
| Age, years | 75.8 ± 6.2 |
| BMI, kg/m2 | 22.8 ± 2.8 |
| Walking speed, m/s | 1.42 ± 0.31 |
| Hand-grip strength, kgf | 22.9 ± 4.3 |
| Body fat percentage, % | 33.5 ± 6.1 |
| Body fat volume, kg | 17.8 ± 5.2 |
| SMI, kg/m2 | 6.04 ± 0.60 |
| eGFR, mL/min/1.73 m2 | 67.0 ± 13.4 |
| Hb, g/dL | 13.1 ± 1.1 |
| Fe, μg/dL | 99.2 ± 3.7 |
| hsCRP, mg/L | 0.11 ± 0.26 |
| Alb, g/dL | 4.4 ± 0.2 |
| GDF-15, ng/mL | 1.01 ± 0.50 |
Among the total of 66 subjects, 2 had normal eGFR (≧90 mL/min/1.73 m2), 44 had mildly decreased eGFR (60–89 mL/min/1.73 m2) and 20 had moderately decreased eGFR (30–59 mL/min/1.73 m2). No subjects exhibited severely decreased eGFR (15–29 mL/min/1.73 m2) or kidney failure (eGFR < 15 mL/min/1.73 m2). The mean Hb value was 13.1 ± 1.1 g/dL and mean serum levels of Fe, hsCRP and Alb were 99.2 ± 3.7 μg/dL, 0.11 ± 0.26 mg/L, and 4.4 ± 0.2 g/dL, respectively.
3.2. Correlation between clinical parameters and serum concentrations of GDF-15, High-sensitive CRP and Hb
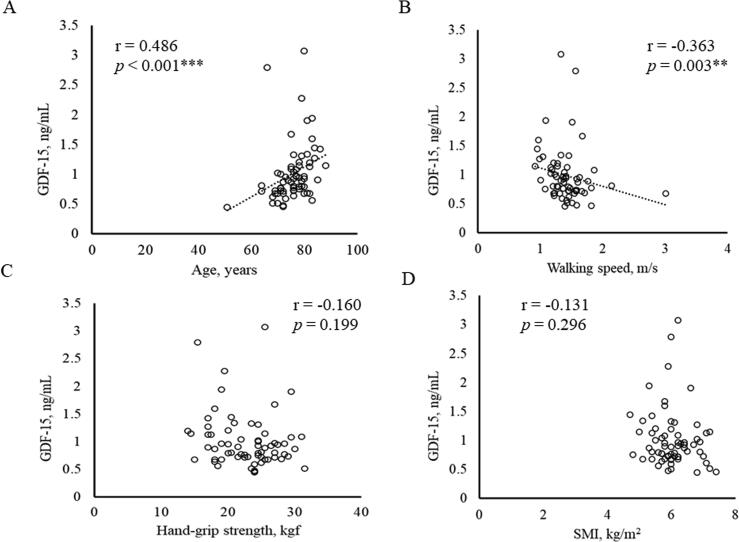
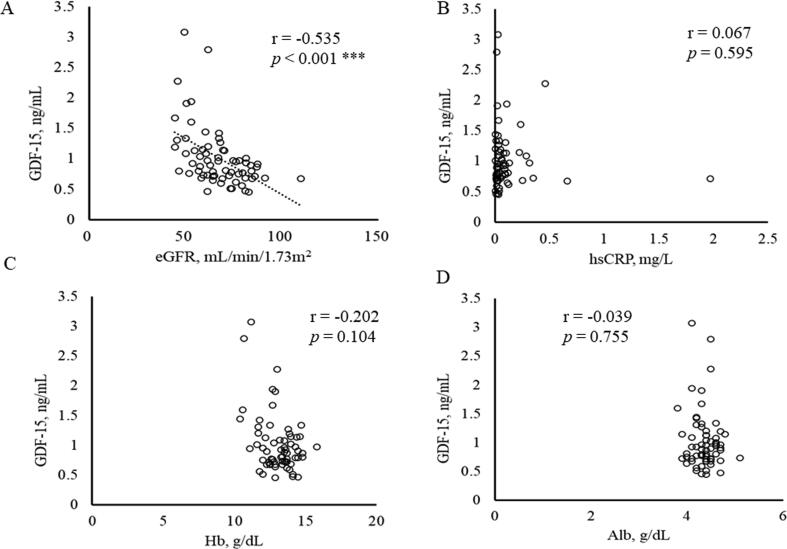
Relationships between serum GDF-15 level and hsCRP and Hb, as well as with physical and clinical data were next assessed (Table 2, Fig. 1, Fig. 2). The serum GDF-15 level was positively correlated with age (Fig. 1A) and negatively correlated with walking speed (Fig. 1B). There was no correlation between GDF-15 levels and hand-grip strength (Fig. 1C) or SMI (Fig. 1D). Serum GDF-15 levels were also negatively correlated with eGFR (Fig. 2A) and serum Fe levels, but had no correlation with hsCRP, Hb or Alb levels (Fig. 2C–D).
Table 2.
Correlation matrix of relationships of patient parameters with serum GDF-15, hsCRP and Hb.
| GDF-15 | hsCRP | Hb | |
|---|---|---|---|
| Age | 0.486 (<0.001)*** | 0.028 (0.826) | −0.042 (0.737) |
| BMI | 0.043 (0.732) | 0.356 (0.003)** | 0.462 (<0.001)*** |
| Walking speed | −0.363 (0.003)** | −0.035 (0.786) | 0.103 (0.419) |
| Hand-grip strength | −0.160 (0.199) | −0.143 (0.251) | 0.257 (0.037)* |
| Body fat percentage | 0.186 (0.135) | 0.446 (<0.001)*** | 0.331 (0.007)** |
| Body fat volume | 0.093 (0.459) | 0.459 (<0.001)*** | 0.415 (0.001)** |
| SMI | −0.131 (0.296) | 0.120 (0.336) | 0.481 (<0.001)*** |
| eGFR | −0.535 (<0.001)*** | 0.001 (0.996) | 0.055 (0.659) |
| Hb | −0.202 (0.104) | 0.244 (0.048)* | – |
| Fe | −0.316 (0.010)* | −0.280 (0.023)* | 0.496 (<0.001)*** |
| hsCRP | 0.067 (0.595) | – | 0.244 (0.048)* |
| Alb | −0.039 (0.755) | −0.287 (0.019)* | 0.261 (0.034)* |
| GDF-15 | – | 0.067 (0.595) | −0.202 (0.104) |
Fig. 1.
Correlation between physical data (age, walking speed, grip strength and SMI) and serum concentration of GDF-15. Relationships between serum concentration of GDF15 and (A) age, (B) walking speed, (C) hand grip strength and (D) SMI. ** p < 0.01, *** p < 0.001.
Fig. 2.
Correlation between serum concentration of GDF-15 and (A) eGFR, (B) hsCRP, (C) Hb and (D) Alb. *** p < 0.001.
Serum hsCRP levels were positively correlated with BMI, body fat percentage, body fat volume and Hb, and decreased with decreases in serum Fe and Alb levels. Hb levels were positively correlated with BMI, hand-grip strength, body fat percentage, body fat volume, and SMI, as well as with serum Fe and Alb levels.
3.3. Multiple regression analysis of serum GDF-15 levels and clinical parameters
Table 3 shows standardized partial regression coefficients (β) and p values in multiple regression analysis of serum GDF-15 levels and clinical parameters (corresponding adjusted R2 values and unstandardized B coefficients are presented in Supplementary Tables 1 and 2, respectively). A linear regression analysis with serum GDF-15 level as the dependent variable and clinical data (eGFR, SMI, walking speed, hand-grip strength, Hb and serum Alb) as the independent variable was conducted for all study subjects (Table 3A). This analysis showed that eGFR (β = −0.423, p < 0.001) and Hb (β = −0.363, p = 0.004) were independent variables to predict serum GDF-15 levels after adjusting for age and BMI.
Table 3.
Multiple linear regression analysis of relationship between serum GDF-15 level and clinical parameters in healthy older females.
| A: Multiple linear regression analysis of GDF-15 and clinical data | |||
|---|---|---|---|
| Dependent variable: log (GDF-15) | |||
| Model 1 | Model 2 | Model 3 | |
| Independent variable | β-value (p) | β-value (p) | β-value (p) |
| eGFR (log) | −0.518 (<0.001)*** | −0.453 (<0.001)*** | −0.423 (<0.001)*** |
| SMI | −0.023 (0.858) | 0.101 (0.435) | 0.005 (0.972) |
| Walking speed | −0.060 (0.589) | 0.070 (0.553) | 0.087 (0.463) |
| Hand-grip strength | −0.167 (0.160) | −0.123 (0.279) | −0.092 (0.431) |
| Hb | −0.245 (0.044)* | −0.323 (0.007)** | −0.363 (0.004)** |
| Alb (log) | −0.012 (0.910) | −0.008 (0.941) | −0.002 (0.987) |
| B: Multiple linear regression analysis of eGFR and clinical data | |||
| Dependent variable: log (eGFR) | |||
| Model 1 | Model 2 | Model 3 | |
| Independent variable | β-value (p) | β-value (p) | β-value (p) |
| GDF-15 (log) | −0.523 (<0.001)*** | −0.515 (<0.001)*** | −0.479 (<0.001)*** |
| SMI | −0.113 (0.373) | −0.121 (0.379) | −0.007 (0.968) |
| Walking speed | 0.229 (0.039)* | 0.220 (0.075) | 0.192 (0.124) |
| Hand-grip strength | −0.227 (0.056) | −0.228 (0.057) | −0.256 (0.036)* |
| Hb | −0.031 (0.804) | −0.024 (0.860) | 0.032 (0.820) |
| Alb (log) | 0.019 (0.865) | 0.018 (0.868) | 0.011 (0.920) |
| C: Multiple linear regression analysis of Hb and clinical data | |||
| Dependent variable: Hb | |||
| Model 1 | Model 2 | Model 3 | |
| Independent variable | β-value (p) | β-value (p) | β-value (p) |
| GDF-15 (log) | −0.284 (0.044)* | −0.374 (0.007)** | −0.380 (0.004)** |
| SMI | 0.386 (0.003)** | 0.474 (<0.001)*** | 0.194 (0.226) |
| Walking speed | −0.035 (0.772) | −0.103 (0.412) | −0.137 (0.256) |
| Hand-grip strength | −0.008 (0.952) | 0.020 (0.868) | 0.087 (0.468) |
| eGFR (log) | −0.035 (0.804) | −0.024 (0.860) | 0.030 (0.820) |
| Alb (log) | 0.144 (0.217) | 0.131 (0.237) | 0.130 (0.217) |
Model 1, unadjusted; Model 2, adjusted by age; Model 3, adjusted by age and BMI.
A regression analysis between eGFR and clinical data (GDF-15, SMI, walking speed, hand-grip strength, Hb and serum Alb) was also performed (Table 3B). Multiple regression analysis showed that serum GDF-15 level (β = −0.479, p < 0.001), and hand-grip strength (β = −0.256, p = 0.036) were independent variables to predict eGFR after adjusting for age and BMI.
Multiple regression analysis between Hb and clinical data (GDF-15, SMI, walking speed, hand-grip strength, eGFR and serum Alb) showed that serum GDF-15 level (β = −0.380, p = 0.004) was an independent variable to predict Hb after adjusting for age and BMI (Table 3C).
4. Discussion
In the present study, serum GDF-15 levels were positively correlated with age and negatively correlated with eGFR and walking speed. In multiple regression analyses, eGFR and Hb were independent variables to predict serum GDF-15 levels even after adjusting for age and BMI. Meanwhile, multiple regression analyses showed that serum GDF-15 level was an independent predictor of eGFR and Hb. To our knowledge, this is the first study to demonstrate a robust association between serum GDF-15, and eGFR and Hb using parameters including skeletal muscle mass as independent variables in healthy community-dwelling older subjects.
GDF-15 is a member of the TGF-β cytokine superfamily and its levels increase under various pathological conditions [8], [9], [10], [11]. Here we showed that serum GDF-15 levels were negatively correlated with eGFR in healthy older females. These findings are in agreement with consistent reports of association between GDF-15 and numerous risk factors and biomarkers for cardiovascular diseases [16], [15], [19]. Nakajima et al. [16] showed that eGFR and anterior thigh muscle thickness were independent determinants of GDF-15 after adjusting for age, sex and BMI in patients with cardiovascular disease. Other studies revealed that eGFR, diabetes mellitus and Hb were predictors for GDF-15 in community-dwelling older subjects [15].
Increased levels of GDF-15 in renal dysfunction may reflect several underlying conditions including ischemia [12], or merely indicate decreased renal excretion of GDF-15 due to renal dysfunction. Higher levels of GDF-15 were reported to be associated with mortality [14], [19], and may also be associated with decreased quality of life due to renal dysfunction.
Here we showed that Hb was an independent variable to predict serum GDF-15 levels, and that serum GDF-15 levels were an independent predictor of Hb. The increase in GDF-15 is proposed to reflect ineffective erythropoiesis and induces anemia in patients with thalassemia syndrome [20]. Bone-marrow derived GDF-15 can down-regulate expression of the peptide hormone hepatic hepcidin [21], which inhibits Fe absorption through enterocytes and Fe release from macrophages by inactivating the Fe export pump ferroportin [22]. Consequently, downregulation of hepcidin expression via increased GDF-15 induces Fe absorption and leads to Fe overload and in turn anemia due to ineffective erythropoiesis. On the other hand, Fe deficiency is reported to be associated with elevated serum GDF-15 concentration, and Fe-mediated regulation of GDF-15 concentrations occurs in the presence of decreased levels of Fe [23]. The aging process likely has an inflammatory component, and some biomarkers of inflammation-associated anemia overlap with those of Fe deficiency. Thus, increased GDF-15 levels might be induced by Fe deficiency. Here we showed that serum Fe concentration was negatively correlated with GDF-15 level, which was in accordance with Fe regulation of GDF-15. Therefore, anemia mediated by GDF-15 may be due to Fe deficiency in healthy older females, but additional studies are needed to confirm this possibility.
Only 6% of subjects enrolled in this study had sarcopenia. A study by Tanimoto et al. [24] that investigated walking speed, hand-grip strength and SMI by bioelectrical impedance analysis in community-dwelling older subjects in Japan showed that 10.2% of female study subjects had sarcopenia. This difference in incidence may be because subjects enrolled in the present study were attending exercise class regularly.
The present study has several limitations. First, we considered only females and thus whether the results apply to males is unclear. Second, the study population was small and further studies involving larger numbers of subjects are needed to clarify the pathophysiological roles of GDF-15 in healthy older females.
In conclusion, both renal function and hemoglobin are independent variables to predict serum GDF-15 level in community-dwelling older females in Japan. This finding suggests that renal dysfunction mediated by GDF-15 is accompanied by anemia, but not sarcopenia, in healthy older females.
5. Financial disclosure
This study was supported in part by JSPS KAKENHI Grant Number 19H03981 (to T.N.) and 20K11259 (to T.F.), and research grant from Kanagawa University of Human Services.
Declaration of Competing Interest
The authors report no relationships that could be construed as a conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2020.100651.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Anderson S., Halter J.B., Hazzard W.R., Himmelfarb J., Horne F.M., Kaysen G.A., Kusek J.W., Nayfield S.G., Schmader K., Tian Y., Ashworth J.R., Clayton C.P., Parker R.P., Tarver E.D., Woolard N.F., High K.P. Prediction, progression, and outcomes of chronic kidney disease in older adults. J. Am. Soc. Nephrol.: JASN. 2009;20(6):1199–1209. doi: 10.1681/ASN.2008080860. [DOI] [PubMed] [Google Scholar]
- 2.Xue J.L., Daniels F., Star R.A., Kimmel P.L., Eggers P.W., Molitoris B.A., Himmelfarb J., Collins A.J. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J. Am. Soc. Nephrol.: JASN. 2006;17(4):1135–1142. doi: 10.1681/ASN.2005060668. [DOI] [PubMed] [Google Scholar]
- 3.Guralnik J.M., Ferrucci L., Pieper C.F., Leveille S.G., Markides K.S., Ostir G.V., Studenski S., Berkman L.F., Wallace R.B. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J. Gerontol. Series A, Biol. Sci. Med. Sci. 2000;55(4):M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shlipak M.G., Stehman-Breen C., Fried L.F., Song X., Siscovick D., Fried L.P., Psaty B.M., Newman A.B. The presence of frailty in elderly persons with chronic renal insufficiency. Am. J. Kidney Dis.: Off. J. Natl. Kidney Foundation. 2004;43(5):861–867. doi: 10.1053/j.ajkd.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 5.Chaves P.H., Xue Q.L., Guralnik J.M., Ferrucci L., Volpato S., Fried L.P. What constitutes normal hemoglobin concentration in community-dwelling disabled older women? J. Am. Geriatr. Soc. 2004;52(11):1811–1816. doi: 10.1111/j.1532-5415.2004.52502.x. [DOI] [PubMed] [Google Scholar]
- 6.Astor B.C., Muntner P., Levin A., Eustace J.A., Coresh J. Association of kidney function with anemia: the Third National Health and Nutrition Examination Survey (1988–1994) Arch. Int. Med. 2002;162(12):1401–1408. doi: 10.1001/archinte.162.12.1401. [DOI] [PubMed] [Google Scholar]
- 7.Ble A., Fink J.C., Woodman R.C., Klausner M.A., Windham B.G., Guralnik J.M., Ferrucci L. Renal function, erythropoietin, and anemia of older persons: the InCHIANTI study. Arch. Int. Med. 2005;165(19):2222–2227. doi: 10.1001/archinte.165.19.2222. [DOI] [PubMed] [Google Scholar]
- 8.Bloch S.A., Lee J.Y., Wort S.J., Polkey M.I., Kemp P.R., Griffiths M.J. Sustained elevation of circulating growth and differentiation factor-15 and a dynamic imbalance in mediators of muscle homeostasis are associated with the development of acute muscle wasting following cardiac surgery. Crit. Care Med. 2013;41(4):982–989. doi: 10.1097/CCM.0b013e318274671b. [DOI] [PubMed] [Google Scholar]
- 9.Bootcov M.R., Bauskin A.R., Valenzuela S.M., Moore A.G., Bansal M., He X.Y., Zhang H.P., Donnellan M., Mahler S., Pryor K., Walsh B.J., Nicholson R.C., Fairlie W.D., Por S.B., Robbins J.M., Breit S.N. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc. Natl. Acad. Sci. USA. 1997;94(21):11514–11519. doi: 10.1073/pnas.94.21.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lerner L., Hayes T.G., Tao N., Krieger B., Feng B., Wu Z., Nicoletti R., Chiu M.I., Gyuris J., Garcia J.M. Plasma growth differentiation factor 15 is associated with weight loss and mortality in cancer patients. J. Cachexia, Sarcopenia Muscle. 2015;6(4):317–324. doi: 10.1002/jcsm.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nair V., Robinson-Cohen C., Smith M.R., Bellovich K.A., Bhat Z.Y., Bobadilla M., Brosius F., de Boer I.H., Essioux L., Formentini I., Gadegbeku C.A., Gipson D., Hawkins J., Himmelfarb J., Kestenbaum B., Kretzler M., Magnone M.C., Perumal K., Steigerwalt S., Ju W., Bansal N. Growth Differentiation Factor-15 and Risk of CKD Progression. J. Am. Soc. Nephrol.: JASN. 2017;28(7):2233–2240. doi: 10.1681/ASN.2016080919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kempf T., Eden M., Strelau J., Naguib M., Willenbockel C., Tongers J., Heineke J., Kotlarz D., Xu J., Molkentin J.D., Niessen H.W., Drexler H., Wollert K.C. The transforming growth factor-beta superfamily member growth-differentiation factor-15 protects the heart from ischemia/reperfusion injury. Circ. Res. 2006;98(3):351–360. doi: 10.1161/01.RES.0000202805.73038.48. [DOI] [PubMed] [Google Scholar]
- 13.Bermudez B., Lopez S., Pacheco Y.M., Villar J., Muriana F.J., Hoheisel J.D., Bauer A., Abia R. Influence of postprandial triglyceride-rich lipoproteins on lipid-mediated gene expression in smooth muscle cells of the human coronary artery. Cardiovasc. Res. 2008;79(2):294–303. doi: 10.1093/cvr/cvn082. [DOI] [PubMed] [Google Scholar]
- 14.Eggers K.M., Kempf T., Wallentin L., Wollert K.C., Lind L. Change in growth differentiation factor 15 concentrations over time independently predicts mortality in community-dwelling elderly individuals. Clin. Chem. 2013;59(7):1091–1098. doi: 10.1373/clinchem.2012.201210. [DOI] [PubMed] [Google Scholar]
- 15.Kim J.S., Kim S., Won C.W., Jeong K.H. Association between Plasma Levels of Growth Differentiation Factor-15 and Renal Function in the Elderly: Korean Frailty and Aging Cohort Study. Kidney Blood Press. Res. 2019;44(3):405–414. doi: 10.1159/000498959. [DOI] [PubMed] [Google Scholar]
- 16.Nakajima T., Shibasaki I., Sawaguchi T., Haruyama A., Kaneda H., Nakajima T., Hasegawa T., Arikawa T., Obi S., Sakuma M., Ogawa H., Toyoda S., Nakamura F., Abe S., Fukuda H., Inoue T. Growth Differentiation Factor-15 (GDF-15) is a Biomarker of Muscle Wasting and Renal Dysfunction in Preoperative Cardiovascular Surgery Patients. J. Clin. Med. 2019;8(10) doi: 10.3390/jcm8101576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yasuda T., Nakajima T., Sawaguchi T., Nozawa N., Arakawa T., Takahashi R., Mizushima Y., Katayanagi S., Matsumoto K., Toyoda S., Inoue T. Short Physical Performance Battery for cardiovascular disease inpatients: implications for critical factors and sarcopenia. Sci. Rep. 2017;7(1):17425. doi: 10.1038/s41598-017-17814-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L.K., Liu L.K., Woo J., Assantachai P., Auyeung T.W., Bahyah K.S., Chou M.Y., Chen L.Y., Hsu P.S., Krairit O., Lee J.S., Lee W.J., Lee Y., Liang C.K., Limpawattana P., Lin C.S., Peng L.N., Satake S., Suzuki T., Won C.W., Wu C.H., Wu S.N., Zhang T., Zeng P., Akishita M., Arai H. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Directors Assoc. 2014;15(2):95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 19.Lajer M., Jorsal A., Tarnow L., Parving H.H., Rossing P. Plasma growth differentiation factor-15 independently predicts all-cause and cardiovascular mortality as well as deterioration of kidney function in type 1 diabetic patients with nephropathy. Diabetes Care. 2010;33(7):1567–1572. doi: 10.2337/dc09-2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanno T., Noel P., Miller J.L. Growth differentiation factor 15 in erythroid health and disease. Curr. Opin. Hematol. 2010;17(3):184–190. doi: 10.1097/MOH.0b013e328337b52f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanno T., Bhanu N.V., Oneal P.A., Goh S.H., Staker P., Lee Y.T., Moroney J.W., Reed C.H., Luban N.L., Wang R.H., Eling T.E., Childs R., Ganz T., Leitman S.F., Fucharoen S., Miller J.L. High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nat. Med. 2007;13(9):1096–1101. doi: 10.1038/nm1629. [DOI] [PubMed] [Google Scholar]
- 22.E. Nemeth, M.S. Tuttle, J. Powelson, M.B. Vaughn, A. Donovan, D.M. Ward, T. Ganz, J. Kaplan, Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization, Science (New York, N.Y.) 306(5704) (2004) 2090–2093. [DOI] [PubMed]
- 23.Lakhal S., Talbot N.P., Crosby A., Stoepker C., Townsend A.R., Robbins P.A., Pugh C.W., Ratcliffe P.J., Mole D.R. Regulation of growth differentiation factor 15 expression by intracellular iron. Blood. 2009;113(7):1555–1563. doi: 10.1182/blood-2008-07-170431. [DOI] [PubMed] [Google Scholar]
- 24.Tanimoto Y., Watanabe M., Sun W., Tanimoto K., Shishikura K., Sugiura Y., Kusabiraki T., Kono K. Association of sarcopenia with functional decline in community-dwelling elderly subjects in Japan. Geriatrics Gerontol. Int. 2013;13(4):958–963. doi: 10.1111/ggi.12037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.