Abstract
Background
The objective was a large-scale analysis of the relation between hypertension, memory problems, and brain function.
Methods
The study design was to measure the association between a history of hypertension, and the functional connectivity between 94 brain regions, and prospective and numeric memory, in 19,507 participants from the UK Biobank, with cross-validation in 1,002 participants in the Human Connectome Project, and 13,441 individuals in the second release of the UK Biobank. A history of hypertension was measured by whether individuals were admitted to hospital for the treatment of hypertension, with the control group admissions for other reasons.
Findings
A history of hypertension was associated with reduced functional connectivity of the hippocampus, and with reduced prospective memory score (FDR correction p<0.01). The reduced functional connectivity mediated the association between the hypertension history and the prospective memory score. A graded linear relation between both the hippocampal functional connectivity and memory impairment, was found across a wide range of blood pressure (r=-0.04). In 502,537 participants from the UK Biobank, a history of hypertension was associated with impaired prospective memory (p = 9.1 × 10−41, Cohen's d=-0.08) and numeric memory (p = 4.7 × 10−24, Cohen's d=-0.10). The association between hypertension, functional connectivity, and impaired memory was cross-validated with 1,002 participants from the Human Connectome Project; and for functional connectivity in 13,441 individuals in the second release of the UK Biobank imaging dataset.
Interpretation
The reduced functional connectivity of the hippocampus, and the memory impairments, both related to hypertension across a wide range of blood pressure, are important for clinical practice.
Keywords: Hypertension, Hippocampus, Prospective memory, Functional connectivity, Cognitive decline, Memory
Research in context.
Evidence before this study
Hypertension has been associated with memory and cognitive problems. It has been reported previously that structural changes of some brain regions, including the prefrontal cortex, hippocampus, inferior temporal cortex, and inferior parietal lobule, occur in hypertension. No previous large-scale analysis of how functional connectivity of the brain, a useful indicator of brain function, has been found in the literature, yet this is very important for establishing robust findings, and showing whether the brain and memory changes relate to the whole range of blood pressure, or are found mainly at the very high end of the distribution.
Added value of this study
This neuroimaging study with 19,507 participants from the UK Biobank established that a history of hypertension was associated with reduced functional connectivity of the hippocampus, and to a smaller extent the inferior temporal cortex, posterior cingulate cortex and dorsolateral prefrontal cortex. The reduced hippocampal functional connectivity mediated a reduction in the prospective memory score. Of clinical relevance, a graded linear relation between the reduced hippocampal functional connectivity, and the memory impairment, was found across a wide range of systolic blood pressure. The results are of value because they are robust: the association between hypertension, functional connectivity, and impaired memory was cross-validated with 1002 participants from the Human Connectome Project; and for functional connectivity in 13,441 individuals in the second release of the UK Biobank neuroimaging dataset. Further added value is that in 502,537 participants from the UK Biobank, it was further established that a history of hypertension was associated with impaired prospective memory (p = 9.1 × 10−41, Cohen's d=−0.08) and numeric memory (p = 4.7 × 10−24, Cohen's d=−0.10).
Implications of all the available evidence
Taken together, the evidence now shows that increasing impairments of memory, which are associated with reduced functional connectivity of a key brain region involved in memory, the hippocampus, are associated in a graded way with increased systolic blood pressure throughout the range of <=120 to >= 160 mm Hg. This has implications for clinical advice and treatment of high blood pressure.
Alt-text: Unlabelled box
1. Introduction
Hypertension affects approximately one third of the population, especially those older than 60 years, of whom 63% have hypertension [1]. Hypertension is associated with many aspects of health, including cognitive function [2], [3], [4], and antihypertensive treatment may prevent cognitive decline [5]. One type of cognitive function, prospective memory, has been associated with a history of hypertension [6], and the association between hypertension and cognition can be independent of age [3].
One aim of the present investigation was to test in a large sample the relation between hypertension and memory, and the brain regions that are involved. We measured the functional connectivity between 94 brain regions, where functional connectivity was defined by the correlation between the BOLD signal in each pair of the 94 regions. Another aim was to assess the relation between different extents of hypertension, and brain functional connectivity, and impaired memory, to discover whether the alterations in functional connectivity and impaired memory were graded throughout the range of blood pressure, or mainly evident with high blood pressure. The hypotheses to be tested were whether hypertension is associated with differences in the functional connectivity between some brain areas (such as the hippocampus), and with differences in memory in a large-scale study; and whether any association between hypertension and functional connectivity is related to altered memory function in hypertension. The first part of the study design was to measure the association between a history of hypertension, and the functional connectivity between 94 brain regions, and prospective and numeric memory, in 19,507 participants from the UK Biobank, with cross-validation in 1002 participants in the Human Connectome Project, and 13,441 individuals in the second release of the UK Biobank. The second part of the study design was to measure whether across very large populations in the UK Biobank, there was an association between blood pressure and memory.
Previous investigations have shown that hippocampal functional connectivity can be associated with measures of memory [7,8], but we know of no previous investigation of the relation between hypertension and functional connectivity.
The relationship between hypertension, the brain, and cognitive function may be partially mediated by reduced blood flow associated with a reduced number of capillaries, and thickened and fibrotic basement membranes [3]. Hypertension is known to affect the structure of some brain regions, including the prefrontal cortex, hippocampus, inferior temporal cortex, and inferior parietal lobule [9], [10], [11], [12], [13]. Understanding how hypertension affects brain function may lead to better treatments for the cognitive decline that is associated with hypertension, and may also emphasize the importance of treating hypertension.
2. Methods
2.1. Participants
The dataset used for this investigation was selected from the September 2019 public data release from the UK Biobank which includes a wide range of phenotypic information, as well as biological samples, for more than 500,000 participants. The UK Biobank sample used in these analyses included 502,537 participants (of whom 273,395 [54.4%] were female; age range, 37–73 years). The UK Biobank received ethical approval from the research ethics committee (REC reference 11/NW/0382). The present analyses were conducted under UK Biobank application number 1954. Written informed consent was obtained from each subject. The demographic characteristics of participants including their hypertension history and prospective memory scores, are summarized in Table 1.
Table 1.
Demographic Characteristics of the 502,537 UK Biobank Participants.
| Characteristics | No. (%) |
|---|---|
| Age, mean (SD), year | 56.53 (8.10) |
| Female | 273,395 (54.4%) |
| Townsend deprivation index, mean (SD), points | −1.30 (3.10) |
| Drinking Frequency | |
| Prefer not to answer | 594 (0.12%) |
| Daily or almost daily | 97,749 (19.45%) |
| Three or four times a week | 114,509 (22.79%) |
| Once or twice a week | 131,437 (26.15%) |
| One to three times a month | 56,829 (11.31%) |
| Special occasions only | 59,648 (11.87%) |
| Never | 41,771 (8.31%) |
| Smoking Status | |
| Prefer not to answer | 0 (0.0%) |
| Never | 275,843 (54.89%) |
| Previous | 173,388 (34.50%) |
| Current | 53,306 (10.61%) |
| Education Qualifications | |
| College or University degree | 54,532 (10.85%) |
| A levels/AS levels or equivalent | 14,145 (2.81%) |
| O levels/GCSEs or equivalent | 91,517 (18.21%) |
| CSEs or equivalent | 33,873 (6.74%) |
| NVQ or HND or HNC or equivalent | 71,479 (14.22%) |
| Other professional qualifications e.g.: nursing, teaching | 236,991 (47.16%) |
| Hypertension | 110,758 (22.04%) |
| Prospective Memory Score | |
| Instruction not recalled, either skipped or incorrect | 9683 (1.93%) |
| correct recall on first attempt | 156,875 (31.22%) |
| correct recall on second attempt | 34,726 (6.91%) |
| no records | 301,253 (59.95%) |
2.2. Imaging data collection and pre-processing
The multi-modal imaging was collected using a standard Siemens Skyra 3T running VD13A SP4, with a standard Siemens 32-channel RF receive head coil. The resting-state functional brain imaging data used in this study were from 22,331 participants and were obtained and processed by the UK Biobank. After quality controls and removing some participants without behavioral data, 19,507 participants remained in the neuroimaging analysis. The details of the image acquisition are provided at the UK Biobank website in the form of a protocol (http://biobank.ctsu.ox.ac.uk/crystal/refer.cgi?id=2367). All the quality checking and data preprocessing procedures were conducted by the UK Biobank and the details of the preprocessing are available on the UK Biobank website (http://biobank.ctsu.ox.ac.uk/crystal/refer.cgi?id=1977) and elsewhere [14]. Briefly, data pre-processing was carried out using FSL (FMRIB Software Library). All the data preprocessing procedures were performed by the UK Biobank team as described in [14]. The data preprocessing included correction for spatial and gradient distortions and head motion, intensity normalization and bias field removal, registration to the T1 weighted structural image, transformation to 2 mm Montreal Neurological Institute (MNI) space, and the FIX artefact removal procedure [15,16]. Finally, the head motion parameters were regressed out and structured artefacts were removed by ICA+FIX processing (Independent Component Analysis followed by FMRIB's ICA-based X-noiseifier [17,18]). The data preprocessing pipeline developed by FMRIB (Oxford University centre for Functional MRI of the Brain) used here has been widely used in resting state fMRI studies [15,[19], [20], [21]].
2.3. Construction of the whole-brain functional network
After the pre-processing, the gray matter of the whole brain was divided into 94 regions using the AAL2 (Automated Anatomical Labelling) atlas [22]. Based on the AAL2 atlas, the time series were extracted by determining the mean of the signals of all voxels within each region across 490 time points. The whole-brain functional network (94 × 94 regions with 4371 links) was established by calculating the Pearson correlation between the BOLD (blood oxygen level-dependent) signal for all pairs of brain regions for each individual participant, followed by z transformation to improve normality [23,24]. The anatomical regions in the AAL2 atlas are shown in Table S1.
2.4. Hypertension history and prospective memory phenotypes
The definition of hypertension history applied in this study is a hospital discharge diagnosis of essential hypertension without dementia or stroke. For a hospital discharge diagnosis of a history of hypertension, individuals were included in the history of hypertension group (N = 2720) if they had a hospital admission with a primary or secondary International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10), code for essential hypertension (Field id: 41,202 and 41,204), and individuals were included in the control group (N = 12,366) if they had a hospital admission record that showed that they did not have a diagnosis of hypertension. Four cognitive measures with continuous test scores available in the UK Biobank for the individuals studied here were prospective memory, numeric memory, fluid intelligence, and reaction time, and we measured whether these measures were correlated with hypertension. Prospective memory is the memory for tasks that need to be completed in the future, and is an example of episodic memory [25,26]. The measure of prospective memory (Field id: 20,018) in the UK Biobank, provides three scores: 0 - Instruction not recalled, either skipped or incorrect; 1 - correct recall on first attempt; 2 - correct recall on second attempt. These values were recoded so that a larger number reflects better performance. Numeric memory (the digit span, which is a measure of short term memory) was available in Field id: 4282).
2.5. Statistical analysis
2.5.1. Association analysis
A general linear model (GLM) was used to test the associations of the history of hypertension with the functional connectivity, and with the cognitive measures available in the UK Biobank including prospective memory, numeric memory and fluid intelligence. The independent variable was the history of hypertension (a binary variable), the dependent variables were functional connectivity links or a cognitive measure (all non-binary), and the covariates to be regressed out of the analysis were gender, age, Townsend deprivation index (which includes information for example about the loss of parents), frequency of drinking alcohol, smoking status, educational qualifications, and head motion (mean Framewise Displacement). A t-statistic and Cohen's d were obtained for each GLM to reflect the association between the history of hypertension and the dependent variable. False discovery rate (FDR) correction was used to correct for multiple comparisons across all functional connectivities and cognitive measures.
2.5.2. Mediation analysis
A standard mediation analysis was performed using the Mediation Toolbox developed by Tor Wager's group (https://github.com/canlab/MediationToolbox), which has been widely used in many neuroimaging studies [27], [28], [29]. A standard 3-variable path model was used here [30], with the detailed methodology description in the supplementary material of Wager et al. [27]. Briefly, mediation analysis tests whether the association between two variables can be explained by a third variable (the mediator). The hypothesis tested here was whether the functional connectivity was a mediator between the history of hypertension and prospective memory. Confounding variables as in the association analysis were regressed out in the mediation model. The significance of the mediation was estimated by the bias-corrected bootstrap approach (with 10,000 random samplings). FDR correction was used to correct for multiple comparisons across functional connectivities.
2.6. Cross-Validation using two independent datasets
2.6.1. The second release of the UK Biobank imaging dataset
Another imaging dataset with 17,779 participants was released in January 2020 by the UK Biobank, and this was used for cross-validation in this study. The pre-processing with the second release of the UK Biobank imaging dataset was the same as described above for the first release. The group with a history of hypertension was defined in the same way as for the first release, by hospital discharge with a diagnosis of essential hypertension without dementia or stroke. In this second release, the data needed for the analysis were available from 2200 participants with a history of hypertension, and 11,241 participants without a hospital record of hypertension as the control group.
2.6.2. Human Connectome Project (HCP) dataset
The dataset used for cross-validation was selected from the Mar 2017 public data release from the Human Connectome Project (HCP, N = 1200), WU-Minn Consortium. Our sample included 1002 subjects (ages 22–37 years, 534 females) scanned on a 3-T Siemens connectome-Skyra scanner. The WU-Minn HCP Consortium obtained full informed consent from all participants, and research procedures and ethical guidelines were followed in accordance with the Institutional Review Boards (IRB). The demographic characteristics of participants are summarized in Table S2. The participants with systolic/diastolic blood pressure (SBP/DBP) higher than 130/85 [31] were defined as the high blood pressure group. There were 144 participants with high blood pressure as the hypertension group, and 582 participants with normal blood pressure as the healthy control group. The collection and preprocessing of the data are provided at the HCP website (http://www.humanconnectome.org/). The parameters and data preprocessing procedures are very consistent in both datasets which makes the HCP dataset useful for cross-validating the findings described here based on the UK Biobank dataset
2.6.2.1. Data availability and ethics statement
Data for the UK Biobank are available at https://www.ukbiobank.ac.uk/, and for the Human Connectome Project at https://www.humanconnectome.org/, together with details of the ethical permissions obtained for those investigations. The funders had no role in the design or implementation of the research.
3. Results
3.1. Functional connectivity links different between the history of hypertension group and the control group
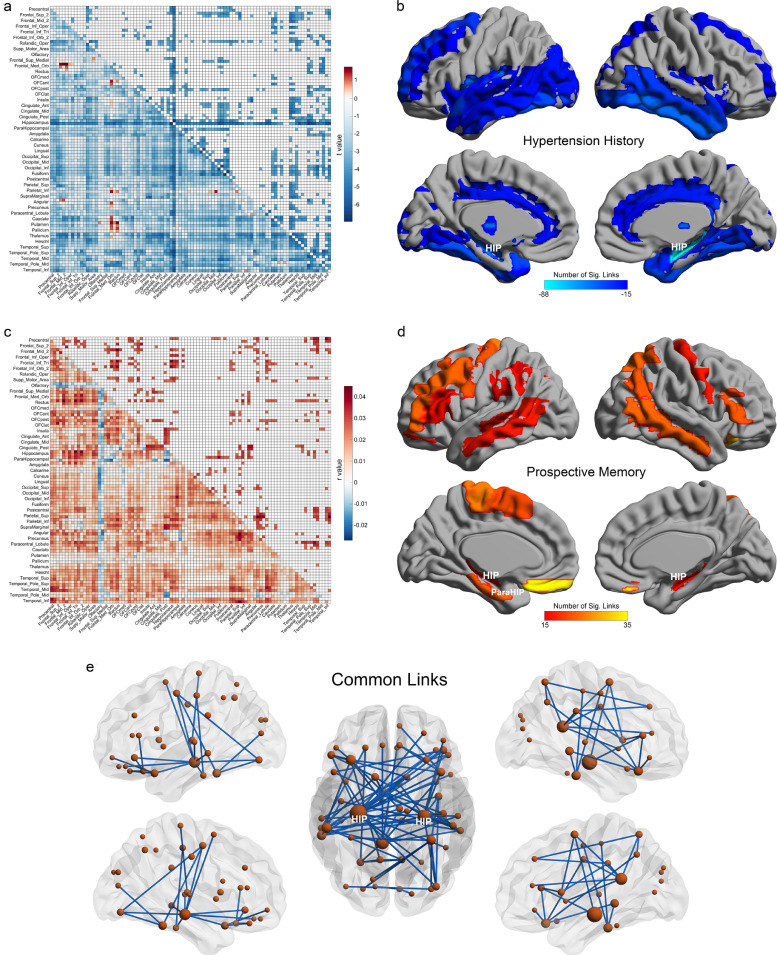
Functional connectivity was measured in 19,507 participants from the UK Biobank release 1. We found that 847/4371 links between brain areas using the automatic anatomical labeling atlas 2 (See Table S1 and [22]) had a lower functional connectivity in a group of 2720 individuals with a history of hospitalization for hypertension (FDR corrected, p<0.005, corresponding to an uncorrected threshold p value =9.7 × 10−4), as shown in Fig. 1a. The brain region with the most significantly reduced functional connectivity links was the hippocampus, with 139 of the 186 links that the hippocampus has with other AAL2 regions significantly reduced. Other brain regions with reduced functional connectivity links included the inferior temporal cortex and temporal pole; the thalamus; the posterior cingulate cortex; prefrontal cortical areas (Frontal_Sup and Frontal_Mid); and the orbitofrontal cortex (Fig. 1a and b). Table 3 shows the 20 most significantly different functional connectivity links between the history of hypertension and the control groups, with large t and very significant p values, and with Cohen's d indicating a reasonable effect size. 12 of the 20 links involved the hippocampus.
Fig. 1.
a) The differences of functional connectivity between the group with a history of hypertension and the control group, with 19,507 participants. The lower triangle matrix shows t values for the functional connectivity differences for the whole brain. Negative t values (blue) indicated reduced functional connectivity. The upper triangle matrix shows the significant links after FDR correction (p<0.005, threshold=9.7 × 10−4). The regions are the AAL2 regions in the order shown in Table S1. b) The AAL2 areas with significantly different functional connectivity between the history of hypertension and control groups. The measure shown is the number of significant links FDR corrected, p<0.005, threshold=9.7 × 10−4 for each AAL2 region, using a threshold of 15 links per region. c) The association between the prospective memory and the functional connectivity. The lower triangle matrix shows r values for the association. The upper triangle matrix shows the significant links after FDR correction (p<0.01, threshold=9.8 × 10−4). d) The AAL2 areas with significant associations of functional connectivity with prospective memory. The measure shown is the number of significant links FDR corrected, p<0.01, threshold=9.8 × 10−4 for each AAL2 region, using a threshold of 15 links per region. e) The common links associated with both hypertension history and prospective memory. The size of the nodes indicates the number of common links, with the largest node the hippocampus.
Table 3.
The most significant 20 Functional Connectivity Links associated with Hypertension history from the UK Biobank analysis using 15,086 participants. A t-test was performed for different functional connectivities between the group categorized as having a history of hypertension (n = 2720) and the participants without a history of hypertension (n = 12,366). The significance value for the links is p<0.005 corrected by the FDR method. Each link is between two areas defined in the AAL2 atlas. A link negatively correlated with hypertension indicates that the functional connectivity of that link is lower in the hypertension group than the control group. The brain area names used are those for the corresponding AAL2 area shown in Table S1.
| Functional Connectivity | t value | Cohens's d | p value | |
|---|---|---|---|---|
| Temporal_Pole_Sup_L | Temporal_Pole_Mid_L | −6.94 | −0.113 | 4.20E-12 |
| Hippocampus_L | Hippocampus_R | −6.82 | −0.111 | 9.72E-12 |
| Fusiform_L | Fusiform_R | −6.56 | −0.107 | 5.39E-11 |
| Hippocampus_R | Temporal_Pole_Sup_L | −6.49 | −0.106 | 8.62E-11 |
| Occipital_Inf_L | Occipital_Inf_R | −6.27 | −0.102 | 3.65E-10 |
| Lingual_L | Lingual_R | −6.21 | −0.101 | 5.57E-10 |
| Hippocampus_R | Temporal_Inf_R | −6.19 | −0.101 | 6.12E-10 |
| Hippocampus_R | Temporal_Pole_Sup_R | −6.17 | −0.100 | 7.04E-10 |
| Hippocampus_R | Occipital_Inf_L | −6.11 | −0.099 | 1.03E-09 |
| Hippocampus_R | Temporal_Mid_L | −5.99 | −0.098 | 2.16E-09 |
| Hippocampus_R | Heschl_R | −5.96 | −0.097 | 2.56E-09 |
| Hippocampus_R | Temporal_Inf_L | −5.92 | −0.096 | 3.31E-09 |
| Temporal_Pole_Sup_L | Temporal_Mid_L | −5.92 | −0.096 | 3.39E-09 |
| Hippocampus_R | Occipital_Inf_R | −5.89 | −0.096 | 3.89E-09 |
| Temporal_Pole_Sup_L | Temporal_Pole_Mid_R | −5.86 | −0.095 | 4.73E-09 |
| Hippocampus_R | Paracentral_Lobule_L | −5.83 | −0.095 | 5.57E-09 |
| Insula_R | SupraMarginal_R | −5.82 | −0.095 | 6.06E-09 |
| Thalamus_L | Thalamus_R | −5.82 | −0.095 | 6.17E-09 |
| Hippocampus_L | Temporal_Pole_Sup_L | −5.77 | −0.094 | 8.30E-09 |
| Frontal_Sup_2_L | Hippocampus_R | −5.74 | −0.093 | 9.67E-09 |
Given this discovery, we next analysed whether hypertension was associated with memory and other cognitive problems, as the hippocampus is implicated in memory [32], [33], [34], [35].
3.2. Association between hypertension history and cognitive function
Significant associations were found between a history of hypertension and prospective memory, numeric memory, and fluid intelligence, as shown in Table 2. This analysis was based on 502,537 participants, 22.04% of whom had been diagnosed with a history of an essential hypertension episode without dementia or stroke. For example, a history of hypertension was associated with impaired prospective memory (N = 138,512, Cohen's d=−0.08, β=−0.145, p = 9.1 × 10−41). Numeric memory (the short term memory for a set of numbers) was also significantly impaired in the history of hypertension group (N = 133,439, Cohen's d=−0.10, β=−0.062, p = 4.7 × 10−24). In addition, fluid intelligence was significantly negatively associated with hypertension history (N = 133,439, Cohen's d=−0.11, β=−0.058, p = 3.97 × 10−76).
Table 2.
A History of Hypertension is associated with reduced prospective memory, numeric memory, and fluid intelligence. The association was measured by the regression in the GLM (which included regressing out any possible effects of the following 6 variables: gender, age, education qualifications, Townsend deprivation index, frequency of drinking alcohol, and smoking status).
| Cohen's d | Regression coefficient | P value | |
|---|---|---|---|
| Prospective memory (N = 138,512) | −0.08 | −0.184 | 9.05 × 10−41 |
| Numeric memory (N = 42,392) | −0.10 | −0.255 | 4.71 × 10−24 |
| Fluid intelligence (N = 133,439) | −0.11 | −0.281 | 3.97 × 10−76 |
3.3. Functional connectivity links correlated with prospective memory
Because the hippocampus is involved in memory [34,36,37], we analysed whether the reduced functional connectivity associated with hypertension is associated with memory impairments. First, we measured the association between prospective memory (an episodic memory measure available for the UK Biobank dataset) and functional connectivity, and found significant associations as shown in Fig. 1c (429 significant links after FDR correction p<0.01, threshold=9.8 × 10−4, N = 19,507). Significant associations were found for the hippocampus, gyrus rectus, temporal lobe, and posterior cingulate cortex, and Frontal_Mid and Frontal_Sup: better prospective memory scores were associated with higher functional connectivities of these regions (Fig. 1c and d; Table S4).
In addition, the mean strength of functional connectivities associated with hypertension history (as shown in Fig. 1a) was also associated with prospective memory (p = 2.6 × 10−3, N = 19,507). The common links related to both high blood pressure and impaired prospective memory are shown in Fig. 1e.
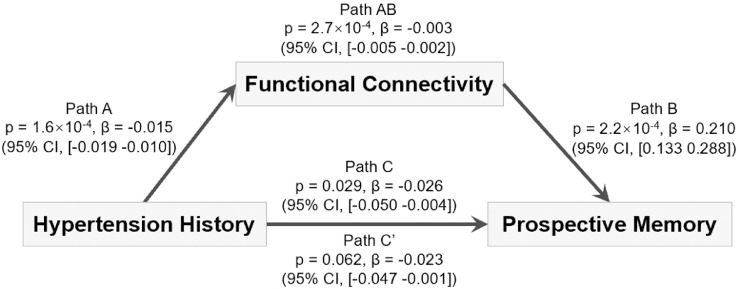
3.3.1. Functional connectivity mediates the association between a history of hypertension and prospective memory
Given the association between hypertension and both prospective memory and functional connectivity, we investigated whether the functional connectivity differences associated with hypertension are related to the impairments in prospective memory associated with a history of hypertension. We performed this analysis for the links that were significantly associated with a history of hypertension and prospective memory. The mediation analysis shown in Fig. 2 shows that these differences in functional connectivity explain 11.5% of the association between a history of hypertension and prospective memory (β=−0.003; p = 2.7 × 10−4; 95% CI, −0.005 to −0.002, N = 19,507), in that the association was reduced when the mediating effect of functional connectivity was removed. It was also found that most (84/88) of these individual common links also significantly (FDR corrected, p<0.05, threshold=0.03) mediated the association between a history of hypertension and prospective memory, including 26/88 links involving the hippocampus.
Fig. 2.
Mediation by functional connectivity of the association between a history of hypertension and reduced prospective memory. Path A: the association between the hypertension history and the mediator (the mean strength of common functional connectivities as shown in Fig. 1E); Path B: the association between the mediator and the outcome (prospective memory); Path C shows that the regression coefficient (beta value) of hypertension history on the prospective memory was high when the functional connectivity was not taken into account. The beta values show the regression coefficient of the effect of the independent variable (hypertension history) on the outcome (prospective memory). Path C’ indicates the direct association between hypertension history and the outcome (prospective memory) controlling for the mediator. Path C’ (when compared to C) shows a significant reduction in the regression coefficient when the association with the functional connectivity was taken into account. Path AB shows that taking functional connectivity into account explains 11.5% of the association between a history of hypertension and prospective memory (p = 2.7 × 10−4).
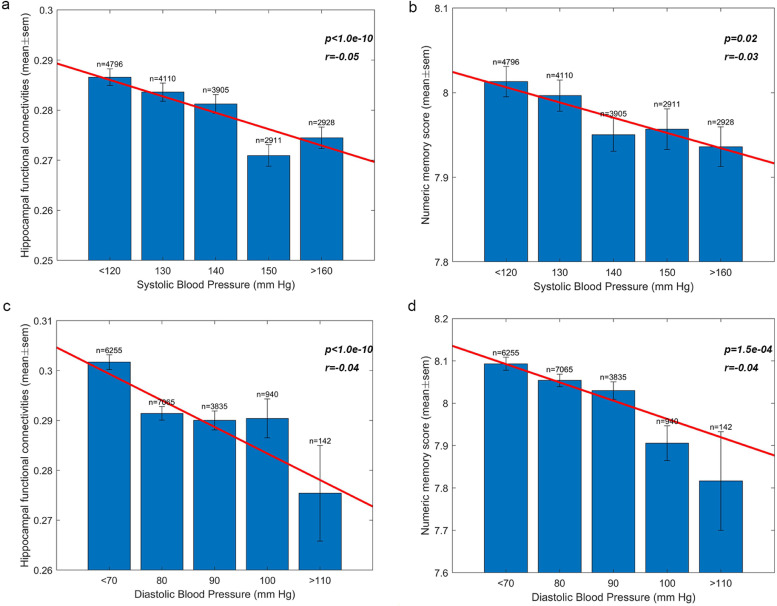
3.3.2. Higher blood pressure is associated with reduced hippocampal functional connectivity and impaired memory
The analyses described above were for a group with a history of hypertension compared to a control group. We also investigated for the 19,507 participants with neuroimaging data the relation between the blood pressure, hippocampal functional connectivity, and memory. This analysis was thus independent of whether an individual had been treated in hospital for hypertension, and age was regressed out of the analysis. Fig. 3a shows that there is a graded reduction between the functional connectivity of all 186 hippocampal links in AAL2 and systolic blood pressure (p<10−10, r= −0.05), with Fig. 3c showing a similar reduction related to diastolic blood pressure (p<10−10, r=−0.04). This analysis shows that the reduction of hippocampal connectivity is not associated just with extreme values of blood pressure, but is graded throughout the range, with the linear regression coefficient significant at p<10−10. Fig. 3b shows that in the same participants numeric memory has a very similar graded reduction with higher values of systolic blood pressure with the linear regression coefficient significant at p<0.05, and Fig. 3d shows a reduction in numeric memory with higher values of diastolic blood pressure with the linear regression coefficient significant at p = 1.5 × 10−4.
Fig. 3.
Higher blood pressure is associated with reduced hippocampal functional connectivity and impaired memory. a. For the 19,507 participants with neuroimaging data, there was a graded reduction in the functional connectivity of the 88 hippocampal functional connectivity links and systolic blood pressure. The mean functional connectivity ± the sem is shown, together with the number of participants. The line shows the linear regression computed over all 19,507 participants, with age regressed out, and was significant at p<10−10. b. For the same participants there was a graded reduction in the numeric memory score with higher values of systolic blood pressure, with the linear regression coefficient significant at p = 0.02 and age regressed out. c. For the 19,507 participants with neuroimaging data, there was a graded reduction in the functional connectivity of the 88 hippocampal functional connectivity links and diastolic blood pressure. The mean functional connectivity ± the sem is shown, together with the number of participants. The line shows the linear regression computed over all 19,507 participants, with age regressed out, and was significant at p<10−10. d. For the same participants there was a graded reduction in the numeric memory score with higher values of diastolic blood pressure, with the linear regression coefficient significant at p = 1.5 × 10−4 and age regressed out.
These results show that there is a clear and graded relation between high blood pressure and reduced hippocampal functional connectivity and impaired numeric memory when the measure is blood pressure in the whole group of participants, independently of whether they had received treatment for hypertension.
We were also able to investigate whether there were gender and age-related differences in the association between hypertension, and hippocampal functional connectivity effects and prospective memory and numeric memory. Two-way ANOVAs, with one factor hypertension, and the second factor age or gender, were performed, with the dependent variables hippocampal functional connectivity effects or prospective memory or numeric memory. A significant interaction would indicate that some of the associations were age- or gender-dependent. No significant age-related interaction effects were found for the relation between any measure of hypertension, and age, on hippocampal functional connectivity or prospective memory or numeric memory. For gender, there was some tendency for the effects of a history of hypertension on functional connectivity, prospective memory, and numeric memory, to be greater in males, but the effects were only just significant, given that many different tests were performed. The details are provided in the Supplementary Material.
3.4. Cross-Validation using the second release of the UK Biobank imaging dataset
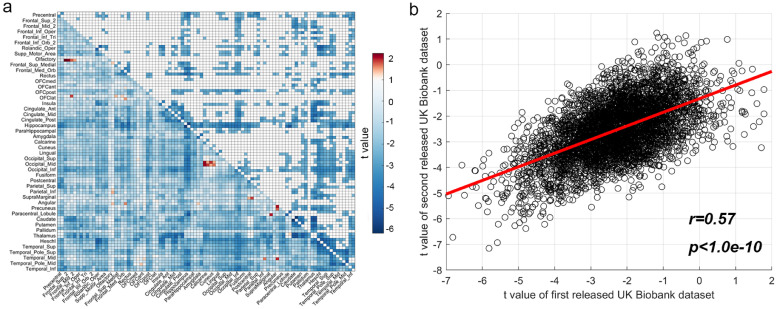
We cross-validated our imaging findings by using the second release of the UK Biobank image data. 1228/4371 links had a lower functional connectivity in the history of hypertension group (FDR corrected, p<0.005, N = 13,441) and 544/1228 significant links overlapped with the results based on the first release of the UK Biobank dataset. The brain region with most significantly reduced functional connectivity links was the hippocampus, with 126 of the 186 links that the hippocampus has with other AAL2 regions significantly reduced, cross-validating a key finding within the first release of the UK Biobank data. Further, the differences in the 4371 whole brain functional connectivity links between the hypertension and control groups were also similar for these two UK Biobank imaging datasets released at different times (r = 0.57, p<10−10, Fig. 4b). Thus the second release of the UK Biobank neuroimaging data provided clear cross-validation for the association of a history of hypertension with reduced functional connectivity links that included the hippocampus. (Memory data are not available for the second release.)
Fig. 4.
Cross-validation with the second release of the UK Biobank dataset. a. The differences of functional connectivity between the group with a history of hypertension and the control group, released in March 2020. The lower triangle matrix shows t values for the functional connectivity differences for the whole brain. Negative t values (blue) indicate reduced functional connectivity. The upper triangle matrix shows the significant links after FDR correction (p<0.005, threshold= p = 1.4 ×). The regions are the AAL2 regions in the order shown in Table S1. b. Correlation between the t values for the differences related to hypertension of the 4371 whole brain functional connectivity links for the two UK Biobank data sets.
3.5. Cross-Validation using the Human Connectome Project dataset
A further cross-validation analysis was performed using the HCP dataset, for which high (SBP/DBP, 130/85) vs control (SBP=[85–130], DBP=[60–85]) blood pressure measures were available (N = 144 vs 582 respectively). The mean strengths of the whole brain resting state functional connectivity links for the AAL2 atlas for 1002 participants within the HCP dataset were very similar to those in the UK Biobank dataset (r = 0.94, p<10−10), providing an indication that the HCP dataset is suitable to utilize for cross validation in this study. In the high blood pressure group, the 220 functional connectivity links with lower values than in the control group (FDR corrected, p<0.01, uncorrected threshold p = 4.6 × 10−4) are shown in Fig. S1a and b. 88 of these 220 links in the HCP involved the hippocampus and parahippocampus, cross-validating a key finding within the UK Biobank data. Further, the differences in the functional connectivities between the hypertension and control groups were similar for the HCP and UK Biobank data (r = 0.23, p<10−10) (Fig. 1a and b). The brain regions included the hippocampus, parahippocampal gyrus, inferior temporal gyrus, and prefrontal cortex.
The data shown in Fig. 1 are from participants in the UK Biobank hospitalized for hypertension, and the data shown in Fig. S1 are for HCP participants with a measured high blood pressure. We therefore performed an additional comparison. Using the same blood pressure criterion for both the HCP and UK Biobank datasets, we show in Fig. S2a that across all functional connectivity links, there is a high correlation (r = 0.34, p<10−10) between the differences in functional connectivity associated with high blood pressure vs controls in the UK Biobank and the HCP datasets. The links with reduced functional connectivity in both datasets for the hypertension groups are shown in Fig. S2b, with 21 of the 81 common links involving the hippocampus, the largest node in Fig. S2b. Permutation tests revealed that across these two datasets, the functional connectivities involving the hippocampus were more likely to be present in both datasets than would be expected by chance (p = 3 × 10−4). Thus, the cross-validation with data from the HCP shows that hypertension is associated with reductions in functional connectivity of the hippocampal formation as in the UK Biobank.
Further, the relationship between high blood pressure and cognitive function was also cross-validated in the HCP data set, in that the Episodic memory test score in the HCP dataset was negatively correlated with hypertension (N = 1002, Cohen's d=−0.215, β=−0.02, p = 8.2 × 10−3).
Thus, the HCP dataset provided clear cross-validation for the association of hypertension both with reduced functional connectivity links that included the hippocampus, and with the memory impairments found in the UK Biobank dataset.
4. Discussion
This investigation provides evidence in a very large population that impairments in cognitive function including prospective memory, numeric memory, and fluid intelligence are associated with hypertension; that functional connectivity reductions especially involving the hippocampus and some related areas such as the parahippocampal gyrus, temporal cortex, prefrontal cortex, posterior cingulate cortex, and gyrus rectus (part of the ventromedial prefrontal cortex [38]) are associated with a history of hypertension; and that these reduced functional connectivities mediate the impairments in prospective memory. The participants were drawn from the general population (mainly 37–73 years old). Given that we showed for the first time that brain regions involved in episodic / prospective memory such as the hippocampus are related to hypertension and mediate the association between hypertension and memory problems, there is a further highlight on the potential utility of paying attention to and treating high blood pressure.
Prospective memory is the memory for tasks that need to be completed in the future, and is an example of episodic memory [25,26]. Another type of memory, numeric memory (the digit span, which is a measure of short term memory, but the primacy part of which utilizes a longer term form of memory), was also associated with a history of hypertension (Table 2); so the association of hypertension with impaired memory is extensive. As these participants were drawn from a general population not selected to have memory problems, an implication is that hypertension may be associated with memory problems that have not been detected in individuals.
The results just described are for a group with a history of hypertension compared to a control group. But in an important extension of the research, we showed that for the 19,507 participants with neuroimaging data there was a graded relation between increasing blood pressure and reduced hippocampal functional connectivity (Fig. 3a) and impaired numeric memory (Fig. 3b). This analysis was thus independent of whether an individual had been treated in hospital for hypertension, and age was regressed out of the analysis. Significant reductions were also found for prospective memory (p<10−10) and for fluid intelligence (p<10−10) as a function of systolic blood pressure. These results show that there is a clear and graded relation between high blood pressure and reduced hippocampal functional connectivity and impaired memory when the measure is blood pressure in the whole group of participants, independently of whether they have received treatment for hypertension. An implication is that the association between high blood pressure, reduced hippocampal functional connectivity, and impaired memory is present throughout the population, in individuals who may not have been diagnosed with high blood pressure or impaired memory. This has implications for the treatment of high blood pressure.
Most of the brain regions identified in this study as associated with memory impairments in hypertension are involved in memory. The hippocampus, gyrus rectus, and posterior cingulate cortex are all implicated in episodic memory including autobiographical memory [25,26,35,39]. The prefrontal cortex is implicated in short term and working memory [40], [41], [42], of which the numeric memory test is an example.
The functional connectivity results described here are very robust, in that they were replicated in data from 1002 participants from the Human Connectome Project (HCP) dataset, and in 13,441 individuals in the second release of the UK Biobank imaging dataset. The sizes of these populations in which functional connectivity was measured and related to biological measures such as blood pressure and to cognitive problems is relatively unprecedented, and paves the way for similarly robust investigations in the future.
This is an association study showing a correlation between hypertension, reduced hippocampal function connectivity, and impaired memory, with the role of the hippocampus in the memory effects supported by a mediation analysis. Association studies enable discoveries of the type described here to be made, and are part of the rationale for the huge investment in large-scale data sets such as the UK Biobank and Human Connectome Project. Of course in terms of limitations such association studies do not necessarily address all the causal factors that may underlie the association, but once the association has been discovered, hypotheses about the causal factors, and tests of treatments suggested by the discovery, can be tested. We note that any general change in the haemodynamic response function associated with hypertension is unlikely to account for the results described here, for as shown in Fig. 1, the reduced functional connectivity was prominent for especially the hippocampus and a few related brain areas.
In this context, it will be of interest in future research to understand better the mechanisms by which high blood pressure may impair the functional connectivity of the brain regions such as the hippocampus identified in this investigation. Perhaps in some ways these brain areas have metabolism that is especially sensitive to disturbances in microperfusion [3]. Central nervous system small vessel disease is associated with hypertension, and may be associated with white matter hyperintensities, so further investigations along these lines would be of interest [43]. The most widely accepted approach to treatment of central nervous system small vessel disease is to intensively control well-established vascular risk factors, of which hypertension is the most important [43].
In terms of possible limitations at least of the implications of this study, we note that the effect size for the relation between both hippocampal functional connectivity and numeric memory, and diastolic blood pressure across the whole UK Biobank population with neuroimaging data, was reasonable if not large (with r values around −0.04 and p in the range 10−4 to 10−10). In this context, the more major and primary findings reported here, that hypertension requiring hospitalization was associated with both reduced hippocampal functional connectivity and impaired memory, had larger effect sizes (with Cohen's d in the order of −0.1), and were highly statistically significant (with p values in the order of 10−24 to 10−41). This helps to place the implications of the findings described here in a quantitative perspective.
In conclusion, an important novel finding is that the hypertension history in a general population is correlated with reduced functional connectivities of a number of brain areas including the hippocampus and regions connected to it. This finding is based on 19,507 participants from the UK Biobank dataset, and cross-validated in the USA Human Connectome Project. Another new finding is that the decreased functional connectivity involving brain regions such as the hippocampus provides a neural basis for the association between a history of hypertension and memory impairments. Another new finding is that a graded linear relation between both the functional connectivity of the hippocampal links, and memory impairment, was found across a wide range of systolic blood pressure (Fig. 3). Another new finding is that in 502,537 participants from the UK Biobank, it was further established that a history of hypertension was associated with impaired prospective memory (p = 9.1 × 10−41, Cohen's d=−0.08) and numeric memory (p = 4.7 × 10−24, Cohen's d=−0.10). The findings have implications for the treatment of hypertension and memory decline, which is further associated with decreases in cognitive function, as shown here by the impairment in fluid intelligence. The reduced functional connectivity of the hippocampus, and the memory impairments related to hypertension, across a wide range of systolic blood pressure, need to be taken into account in clinical practice.
5. Contributors
Ruiqing Feng, MSc. University of Warwick, Coventry, UK. Performed the statistical analyses and made the figures.
Edmund Rolls DPhil, DSc. University of Warwick, Coventry, UK. Led the project, contributed to the analyses, and wrote the paper.
Wei Cheng PhD. Fudan University, Shanghai, China. Accessed and prepared the data, supervised the statistical analyses, and contributed to writing the paper.
Jianfeng Feng PhD. University of Warwick, Coventry, UK and Fudan University, Shanghai. Provided support for the research
All authors read and approved the final version of the manuscript.
Declaration of interests
The authors report no disclosures and competing interests relevant to the manuscript.
Acknowledgements and Funding
Use of the UK Biobank (https://www.ukbiobank.ac.uk) and HCP (http://www.humanconnectome.org/) dataset is acknowledged. J.Feng is supported by National Key R&D Program of China (No.2019YFA0709502 and 2018YFC1312904), the 111 Project (No.B18015), Shanghai Municipal Science and Technology Major Project (No.2018SHZDZX01), ZJLab, and Shanghai Center for Brain Science and Brain-Inspired Technology. W.Cheng is supported by grants from the National Natural Sciences Foundation of China (No. 82071997 and 81701773) and Natural Science Foundation of Shanghai (No. 18ZR1404400). The funders took no part in the design or implementation of this research.
Data availability statement. Data for the UK Biobank are available at https://www.ukbiobank.ac.uk/, and for the Human Connectome Project at https://www.humanconnectome.org/.
Footnotes
Funding: National Natural Science Foundation of China (No. 82071997 and81701773) and Shanghai Municipal Science and Technology Major Project (No.2018SHZDZX01).
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2020.103082.
Contributor Information
Edmund T Rolls, Email: Edmund.Rolls@oxcns.org, https://www.oxcns.org.
Wei Cheng, Email: wcheng@fudan.edu.cn.
Appendix. Supplementary materials
References
- 1.Fryar C.D., Ostchega Y., Hales C.M., Zhang G., Kruszon-Moran D. Hypertension Prevalence and Control Among Adults: united States, 2015-2016. NCHS Data Brief. 2017;(289):1–8. [PubMed] [Google Scholar]
- 2.Iadecola C., Yaffe K., Biller J. Impact of Hypertension on Cognitive Function: a Scientific Statement From the American Heart Association. Hypertension. 2016;68(6):e67–e94. doi: 10.1161/HYP.0000000000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gasecki D., Kwarciany M., Nyka W., Narkiewicz K. Hypertension, brain damage and cognitive decline. Curr Hypertens Rep. 2013;15(6):547–558. doi: 10.1007/s11906-013-0398-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iadecola C., Gottesman R.F. Neurovascular and cognitive dysfunction in hypertension. Circ Res. 2019;124(7):1025–1044. doi: 10.1161/CIRCRESAHA.118.313260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soros P., Whitehead S., Spence J.D., Hachinski V. Antihypertensive treatment can prevent stroke and cognitive decline. Nat Rev Neurol. 2013;9(3):174–178. doi: 10.1038/nrneurol.2012.255. [DOI] [PubMed] [Google Scholar]
- 6.Scullin M.K., Gordon B.A., Shelton J.T., Lee J.H., Head D., McDaniel M.A. Evidence for a detrimental relationship between hypertension history, prospective memory, and prefrontal cortex white matter in cognitively normal older adults. Cogn Affect Behav Neurosci. 2013;13(2):405–416. doi: 10.3758/s13415-013-0152-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang L., Negreira A., LaViolette P., Bakkour A., Sperling R.A., Dickerson B.C. Intrinsic interhemispheric hippocampal functional connectivity predicts individual differences in memory performance ability. Hippocampus. 2010;20(3):345–351. doi: 10.1002/hipo.20771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bai F., Zhang Z., Watson D.R. Abnormal functional connectivity of hippocampus during episodic memory retrieval processing network in amnestic mild cognitive impairment. Biol Psychiatry. 2009;65(11):951–958. doi: 10.1016/j.biopsych.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 9.Raz N., Lindenberger U., Ghisletta P., Rodrigue K.M., Kennedy K.M., Acker J.D. Neuroanatomical correlates of fluid intelligence in healthy adults and persons with vascular risk factors. Cereb Cortex. 2008;18(3):718–726. doi: 10.1093/cercor/bhm108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raz N., Rodrigue K.M., Kennedy K.M., Acker J.D. Vascular health and longitudinal changes in brain and cognition in middle-aged and older adults. Neuropsychology. 2007;21(2):149–157. doi: 10.1037/0894-4105.21.2.149. [DOI] [PubMed] [Google Scholar]
- 11.Romanowski C.A., Wilkinson I.D. Atrophy: when too much atrophy is too little brain. Neuroradiology. 2011;53(Suppl 1):S133–S139. doi: 10.1007/s00234-011-0929-0. [DOI] [PubMed] [Google Scholar]
- 12.Raz N., Lindenberger U., Rodrigue K.M. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15(11):1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- 13.Bender A.R., Daugherty A.M., Raz N. Vascular risk moderates associations between hippocampal subfield volumes and memory. J Cogn Neurosci. 2013;25(11):1851–1862. doi: 10.1162/jocn_a_00435. [DOI] [PubMed] [Google Scholar]
- 14.Miller K.L., Alfaro-Almagro F., Bangerter N.K. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat Neurosci. 2016;19(11):1523–1536. doi: 10.1038/nn.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navarro Schroder T., Haak K.V., Zaragoza Jimenez N.I., Beckmann C.F., Doeller C.F. Functional topography of the human entorhinal cortex. Elife. 2015;4 doi: 10.7554/eLife.06738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith S.M., Beckmann C.F., Andersson J. Resting-state fMRI in the Human Connectome Project. Neuroimage. 2013;80:144–168. doi: 10.1016/j.neuroimage.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salimi-Khorshidi G., Douaud G., Beckmann C.F., Glasser M.F., Griffanti L., Smith S.M. Automatic denoising of functional MRI data: combining independent component analysis and hierarchical fusion of classifiers. Neuroimage. 2014;90:449–468. doi: 10.1016/j.neuroimage.2013.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffanti L., Salimi-Khorshidi G., Beckmann C.F. ICA-based artefact removal and accelerated fMRI acquisition for improved resting state network imaging. Neuroimage. 2014;95:232–247. doi: 10.1016/j.neuroimage.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colclough G.L., Smith S.M., Nichols T.E. The heritability of multi-modal connectivity in human brain activity. Elife. 2017;6 doi: 10.7554/eLife.20178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vidaurre D., Abeysuriya R., Becker R. Discovering dynamic brain networks from big data in rest and task. Neuroimage. 2018;180(Pt B):646–656. doi: 10.1016/j.neuroimage.2017.06.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith S.M., Nichols T.E., Vidaurre D. A positive-negative mode of population covariation links brain connectivity, demographics and behavior. Nat Neurosci. 2015;18(11):1565–1567. doi: 10.1038/nn.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rolls E.T., Joliot M., Tzourio-Mazoyer N. Implementation of a new parcellation of the orbitofrontal cortex in the automated anatomical labeling atlas. Neuroimage. 2015;122:1–5. doi: 10.1016/j.neuroimage.2015.07.075. [DOI] [PubMed] [Google Scholar]
- 23.Finn E.S., Shen X., Scheinost D. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat Neurosci. 2015;18(11):1664–1671. doi: 10.1038/nn.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenberg M.D., Finn E.S., Scheinost D. A neuromarker of sustained attention from whole-brain functional connectivity. Nat Neurosci. 2016;19(1):165–171. doi: 10.1038/nn.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCormick C., Ciaramelli E., De Luca F., Maguire E.A. Comparing and contrasting the cognitive effects of hippocampal and ventromedial prefrontal cortex damage: a review of human lesion studies. Neuroscience. 2018;374:295–318. doi: 10.1016/j.neuroscience.2017.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonnici H.M., Maguire E.A. Two years later - Revisiting autobiographical memory representations in vmPFC and hippocampus. Neuropsychologia. 2018;110:159–169. doi: 10.1016/j.neuropsychologia.2017.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wager T.D., Davidson M.L., Hughes B.L., Lindquist M.A., Ochsner K.N. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59(6):1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wager T.D., Waugh C.E., Lindquist M., Noll D.C., Fredrickson B.L., Taylor S.F. Brain mediators of cardiovascular responses to social threat: part I: reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. Neuroimage. 2009;47(3):821–835. doi: 10.1016/j.neuroimage.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim S.L., Padmala S., Pessoa L. Segregating the significant from the mundane on a moment-to-moment basis via direct and indirect amygdala contributions. Proc Natl Acad Sci U S A. 2009;106(39):16841–16846. doi: 10.1073/pnas.0904551106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baron R.M., Kenny D.A. The moderator–mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 31.Neutel J.M., Smith D.H., Silfani T.N., Lee Y., Weber M.A. Effects of a structured treatment algorithm on blood pressure goal rates in both stage 1 and stage 2 hypertension. J Hum Hypertens. 2006;20(4):255–262. doi: 10.1038/sj.jhh.1001974. [DOI] [PubMed] [Google Scholar]
- 32.Morris R.G., Moser E.I., Riedel G. Elements of a neurobiological theory of the hippocampus: the role of activity-dependent synaptic plasticity in memory. Philos Trans R Soc Lond B Biol Sci. 2003;358:773–786. doi: 10.1098/rstb.2002.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Squire L.R., Genzel L., Wixted J.T., Morris R.G. Memory consolidation. Cold Spring Harb Persp. Biol. 2015;7(8) doi: 10.1101/cshperspect.a021766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kesner R.P., Rolls E.T. A computational theory of hippocampal function, and tests of the theory: new developments. Neurosci Biobehav Rev. 2015;48:92–147. doi: 10.1016/j.neubiorev.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 35.Rolls E.T. The storage and recall of memories in the hippocampo-cortical system. Cell Tissue Res. 2018;373:577–604. doi: 10.1007/s00441-017-2744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andersen P., Morris R.G.M., Amaral D.G., Bliss T.V.P., O'Keefe J. Oxford University Press; London: 2007. The hippocampus book. [Google Scholar]
- 37.Rolls E.T., Wirth S. Spatial representations in the primate hippocampus, and their functions in memory and navigation. Prog Neurobiol. 2018;171:90–113. doi: 10.1016/j.pneurobio.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 38.Du J., Rolls E.T., Cheng W. Functional connectivity of the orbitofrontal cortex, anterior cingulate cortex, and inferior frontal gyrus in humans. Cortex. 2020;123:185–199. doi: 10.1016/j.cortex.2019.10.012. [DOI] [PubMed] [Google Scholar]
- 39.Rolls E.T. The cingulate cortex and limbic systems for emotion, action, and memory. Brain Struct Funct. 2019;224(9):3001–3018. doi: 10.1007/s00429-019-01945-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fuster J. 5th ed. Academic Press; London: 2015. The prefrontal cortex. [Google Scholar]
- 41.Rolls E.T. Oxford University Press; Oxford: 2016. Cerebral cortex: principles of operation. [Google Scholar]
- 42.Nee D.E., D'Esposito M. The Representational Basis of Working Memory. Curr Top Behav Neurosci. 2018;37:213–230. doi: 10.1007/7854_2016_456. [DOI] [PubMed] [Google Scholar]
- 43.Cannistraro R.J., Badi M., Eidelman B.H., Dickson D.W., Middlebrooks E.H., Meschia J.F. CNS small vessel disease: a clinical review. Neurology. 2019;92(24):1146–1156. doi: 10.1212/WNL.0000000000007654. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data for the UK Biobank are available at https://www.ukbiobank.ac.uk/, and for the Human Connectome Project at https://www.humanconnectome.org/, together with details of the ethical permissions obtained for those investigations. The funders had no role in the design or implementation of the research.