Highlights
-
•
In young adult women, slower epigenetic clock predicted less symptoms of anxiety.
-
•
In young adult women, slower epigenetic clock predicted greater cortical GM volume.
-
•
This effect of epigenetic clock in young adult women was largest in frontal lobe.
-
•
The link of epigenetic clock and anxiety was mediated by GM volume in frontal lobe.
-
•
No similar relationships were found in young adult men or adolescents.
Keywords: DNA methylation age, Anxiety, Gray matter volume, Frontal lobe, Sex differences
Abstract
DNA methylation changes consistently throughout life and age-dependent alterations in DNA methylation can be used to estimate one’s epigenetic age. Post-mortem studies revealed higher epigenetic age in brains of patients with major depressive disorder, as compared with controls. Since MDD is highly correlated with anxiety, we hypothesized that symptoms of anxiety, as well as lower volume of grey matter (GM) in depression-related cortical regions, will be associated with faster epigenetic clock in a community-based sample of young adults. Participants included 88 young adults (53% men; 23–24 years of age) from the European Longitudinal Study of Pregnancy and Childhood (ELSPAC) who participated in its neuroimaging follow-up and provided saliva samples for epigenetic analysis. Epigenetic age was calculated according to Horvath (Horvath, 2013). Women had slower epigenetic clock than men (Cohen’s d = 0.48). In women (but not men), slower epigenetic clock was associated with less symptoms of anxiety. In the brain, women (but not men) with slower epigenetic clock had greater GM volume in the cerebral cortex (brain size-corrected; R2 = 0.07). Lobe-specific analyses showed that in women (but not men), slower epigenetic clock was associated with greater GM volume in frontal lobe (R2 = 0.16), and that GM volume in frontal lobe mediated the relationship between the speed of epigenetic clock and anxiety trait (ab = 0.15, SE = 0.15, 95% CI [0.007; 0.369]). These findings were not replicated, however, in a community-based sample of adolescents (n = 129; 49% men; 12–19 years of age), possibly due to the different method of tissue collection (blood vs. saliva) or additional sources of variability in the cohort of adolescents (puberty stages, socioeconomic status, prenatal exposure to maternal smoking during pregnancy).
1. Introduction
Major depressive disorder (MDD) is the leading cause of years lost due to disability worldwide (Ustun et al., 2004). The lifetime prevalence of MDD is 16% (Kessler et al., 2005). It can develop at any age but the median age at onset is 32.5 years (America AaDAo, 2018). Diagnosis of MDD is highly correlated with anxiety symptoms and diagnosis (Jacobson and Newman, 2017). Still, the underlying mechanisms are not fully understood.
One of the mechanistic theories of depression is the accelerated molecular aging (Sibille, 2013, Rozycka and Liguz-Lecznar, 2017). An unbiased investigation of genetic architecture with genome-wide association studies (GWAS) and gene expression in the human brain (transcriptome) provided evidence for common biological pathways between depression and brain aging (Ding et al., 2015). Further evidence for accelerated aging in depression comes from research on telomere length reporting shorter telomeres in MDD patients than healthy controls (Schutte and Malouff, 2015, Ridout et al., 2016). Recently, additional evidence for the hypothesis of accelerated aging in depression came from epigenetic research. Using a novel index of epigenetic age corrected for chronological age, post mortem studies of brain tissue as well as in vivo studies using blood tissue demonstrated higher epigenetic age in MDD patients compared to controls, particularly those who experienced higher level of trauma in childhood (Han et al., 2018).
The current study focuses on epigenetic aging and tests the possibility that it varies as a function of symptoms of anxiety in community-based cohorts of adolescents and young adults (with no clinical diagnosis of any major psychiatric disorders, as determined by self-report). This work is framed in the context of a DNA methylation-based “epigenetic clock”. In this framework, DNA methylation changes consistently throughout life and age-dependent alterations in DNA methylation, particularly the methylation status of 5’-cytosine-phosphate-guanine-3’ (CpG) sites, can be used to estimate one’s “methylation age”. There are two frequently used DNA methylation-based predictors of age: the Hannum’s (Hannum et al., 2013) and the Horvath’s (Horvath, 2013) epigenetic clocks. The Hannum’s epigenetic clock is based on 71 CpG sites that estimate age based on blood-based DNA methylation (Hannum et al., 2013). The Horvath’s epigenetic clock is based on 353 CpG sites and can estimate age irrespective of the DNA source within the organism (Horvath, 2013). Given the two sources of DNA in this report (epithelial cells and blood cells), as well as the higher number of CpG sites, we have opted for using the Horvath method. The gap between DNA-methylation age and chronological age, often referred to as accelerated/de-accelerated aging (delta age), may reflect deviations from trajectories of “typical” (molecular) development. Positive delta age (wherein DNA methylation age > chronological age) is largely believed to reflect latent pathological influences and could serve as a biomarker of deviations from normal developmental trajectories, age-associated health problems or psychiatric disease.
At the level of the brain, normal developmental trajectory is characterized by age-related decreases in gray matter (GM) volume, which start during childhood (Ducharme et al., 2016, Walhovd et al., 2017) and are thought to reflect a variety of neurobiological processes, such as age-related changes in spine density (Petanjek et al., 2011) and intra-cortical myelination (Miller et al., 2012). While the most significant remodeling of the cortex occurs by the late 20 s and early 30 s (Shaw et al., 2008, Giedd and Rapoport, 2010), cortical thinning and volume loss continue at slower pace also through middle age (Fjell et al., 2015) and accelerate again in later life (Pfefferbaum et al., 2013), possibly reflecting neurodegenerative processes (Fletcher et al., 2018). Although the early studies suggested that these developmental trajectories might be non-linear and lobe-specific (Giedd et al., 1999), larger and more recent studies demonstrated that cortical thickness follows a simple linear decline starting in most cortical regions by 5 years of age, and in all regions by 8 years of age (Ducharme et al., 2016). These linear trajectories have been confirmed in other datasets and re-analyses (Walhovd et al., 2017).
These structural changes are paralleled by changes in cognitive control, emotion regulation and executive function, which are known to be impaired in depression and anxiety (Gotlib and Joormann, 2010, Joormann and Quinn, 2014, Joormann and Tanovic, 2015, Salthouse, 2012). Further research focusing on GM structure suggested that depression, or vulnerability to its development, may be associated with a developmental trajectory reminiscent of premature aging. In particular, a longitudinal study by Luby et al demonstrated that early childhood depression was associated with faster age-dependent GM loss in prefrontal regions during adolescence (Frodl et al., 2008). A prospective longitudinal study of MDD patients and healthy controls revealed that patients who remitted during the 3-year period of the study had less decline in cortical as well as hippocampal volume than the non-remitted MDD patients (Frodl et al., 2008). A meta-analysis of voxel-based morphometry studies reported lower GM volumes in adults with MDD vs. healthy controls (Schmaal et al., 2017). A recent ENIGMA-based meta-analysis of structural alterations in MDD vs. healthy controls found lower cortical thickness across multiple regions in adult MDD patients but pointed out that, in adolescence, the MDD-related differences in thickness had a different spatial pattern (Schmaal et al., 2017).
Within the current research, we aim to test whether the volume of cortical GM might mediate the relationship between faster epigenetic clock and symptoms of anxiety in young adults. To date, there has been only one study assessing an association between faster epigenetic clock and GM volume in two regions of interest – hippocampus and amygdala (Davis et al., 2017). In this report, faster epigenetic clock was associated with lower volumes of the hippocampus but not amygdala in 46 adolescent girls, and faster epigenetic clock mediated the association between diurnal cortisol and left hippocampal volume (Davis et al., 2017). Based on these findings, the authors concluded that faster epigenetic clock is associated with GM volume in region with glucocorticoid-induced atrophy but not other hypothalamo-pituitary-adrenal (HPA) axis subcortical regions responsive to stress (Davis et al., 2017).
The current study investigated the relationships between the Horvath’s epigenetic clock, volume of cortical GM and symptoms of anxiety in young adults participating in the European Longitudinal Study of Pregnancy and Childhood (ELSPAC), and its neuroimaging follow-up (VULDE). We also attempted to replicate our findings in an independent cohort of adolescents participating in Saguenay Youth Study (SYS Pausova et al., 2017). We hypothesized that faster epigenetic clock will be associated with more symptoms of anxiety as well as less cortical GM in regions associated with anxiety and depression (Frodl et al., 2008, Schmaal et al., 2017, Davis et al., 2017, Pausova et al., 2017), in these two community-based samples, and that GM volume will be the mediating factor linking the DNA methylation age and symptoms of anxiety. Given the sex-specific trajectories of brain development (Giedd et al., 1999, Tanaka et al., 2012) and the higher prevalence of anxiety in women vs. men (Organization WH, 2002, Organization WH, 2016), we also hypothesized that these relationships might differ by sex.
2. Materials and methods
2.1. Study 1 – Young adults from ELSPAC/VULDE cohort
2.1.1. Participants
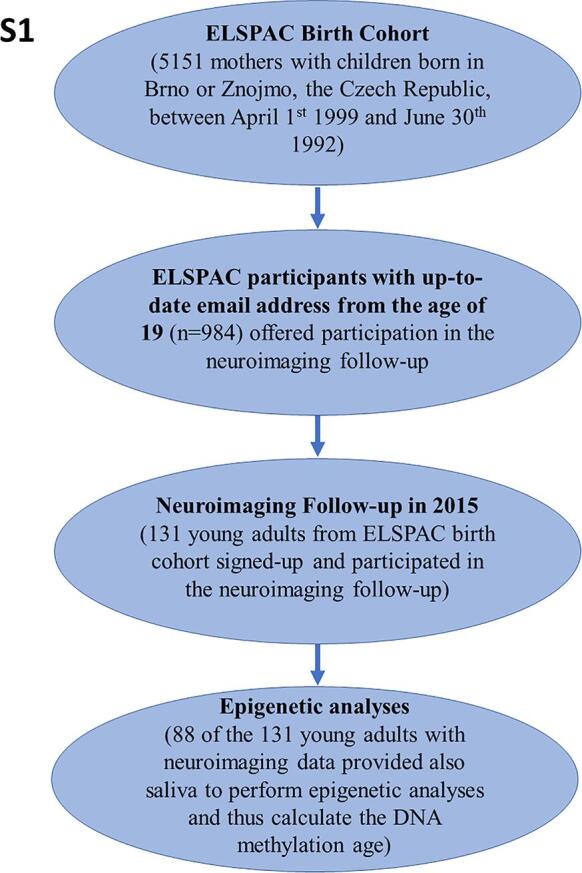
Participants included 88 young adults (53% men; 23–24 years of age; all of European ancestry) from the European Longitudinal Study of Pregnancy and Childhood (ELSPAC-CZ) (Prokhorskas et al., 1989, Piler et al., 2017), a birth cohort born in the South Moravian Region of the Czech Republic between 1991 and 1992, who also participated in its neuroimaging follow-up Biomarkers and Underlying Mechanisms of Vulnerability to Depression (VULDE) at the Central European Institute of Technology, Masaryk University (CEITEC MU), and provided saliva sample for epigenetic analysis. The recruitment flow diagram is provided in Fig. S1.. Further characteristics of the sample are provided in Table S1. All participants provided written informed consent with participation in the VULDE study, including the agreement to merge data from VULDE with their historic data from ELSPAC-CZ. Ethical approval for the VULDE study was obtained from the ELSPAC Ethics Committee.
2.1.2. DNA methylation
DNA methylation was assessed with the Illumina EPIC platform. “Methylation age” was estimated as follows. First, R package ChAMP (Tian et al., 2017) was used to process the raw Illumina microarray data. Raw data were trimmed of: (i) probes with <3 beads in at least 5% of samples per probe, (ii) SNP-related probes, (iii) multi-hit probes, (iv) probes located in chromosomes X and Y. Beta mixture quantile normalization (BMIQ (Teschendorff et al., 2013) method was used to adjust the beta-values of type II design probes into a statistical distribution characteristic of type I probes. Next, DNA methylation age was calculated using an epigenetic clock developed by Horvath (2013), which uses 353 CpG sites to estimate DNA methylation age. Since 20 of the 353 CpGs on the Horvath’s list were either not available on the EPIC microarray or did not pass quality control criteria, the DNA methylation age was calculated using 323 CpG probes. These calculations used the R software tutorial published in the original Horvath’s paper (Horvath, 2013).
Next, we residualized the DNA methylation age estimates on the proportion of epithelial cells in each participant (the average proportion was 80% of epithelial and 20% of immune cells; SD = 13% in each group) and on the batch and saved the residuals from the analysis as the cell-corrected DNA methylation age. Finally, the delta age was defined as the difference between this cell-corrected DNA-methylation age and chronological age with positive values reflecting accelerated aging/faster maturation and negative values reflecting decelerated aging/slower maturation.
2.1.3. MRI imaging
T1-weighted images (TR = 2300 ms, TE = 2.34 ms, flip angle = 8°, 240 slices, resolution 1 × 1 × 1 mm) were acquired on a 3T Siemens Prisma MRI scanner on the same day as the saliva collection and processed using Toronto Volumetric Pipeline to calculate the total volume of cortical gray matter (GM) and the volumes of GM in the four cerebral lobes. See Supplementary Methods for the exact procedures.
2.1.4. Anxiety trait in young adulthood
Anxiety trait in young adulthood was assessed using the Spielberger’s State-Trait Anxiety Inventory (STAI) (Spielberger et al., 1983), a self-report questionnaire assessing anxiety trait (20questions) using a 4-point Likert scale.
2.1.5. Statistical analyses
All statistical analyses were done in JMP version 10.0.0 (SAS Institute Inc., Cary, NC). First, we used t-test to assess possible sex differences in the delta age (defined as the difference between cell-corrected DNA-methylation age and chronological age). Second, a full factorial general linear model (GLM) assessed the relationships between delta age, sex, and behavioral outcomes (anxiety trait). Current body mass index (BMI), smoking and alcohol use in the past 30 days were added as covariates. Next, we assessed the relationship between delta age, sex and absolute GM volume (corrected for brain size) and followed these findings with a full factorial GLM assessing the relationships between delta age, sex, GM volume and lobe. Again, BMI, smoking and alcohol use were added as covariates. Finally, a mediation analysis using bootstrapping evaluated whether GM volume might mediate the relationship between delta age and the behavioral outcomes (anxiety trait). This analysis set-up reflects a framework in which the brain is considered as the intermediate phenotype that mediates the relationship between molecular processes and behavior.
2.2. Study 2 – Adolescents from SYS
2.2.1. Participants
Participants included 129 adolescents (49% men; 12–19 years of age) from the Saguenay Youth Study (Pausova et al., 2017), a cohort recruited from population of the Saguenay Lac St Jean region of Quebec, Canada, who underwent structural MRI and provided blood sample for the epigenetic analyses. Further characteristics of the sample are provided in Table S2. All adolescents and their parents provided written informed assent and consent, respectively, with participation in the SYS study and ethical approval for the SYS study was obtained from the Research Ethics Committee of the Chicoutimi Hospital.
2.2.2. DNA methylation
DNA methylation was assessed using the Infinium Human Methylation 450 K Bead Chip (>485 000 CpGs) from blood, which included all the 353 CpGs present on the Horvath’s list. Remaining steps regarding the calculation of DNA methylation age were done as described in Study 1 above.
In order to correct for the cell proportions in blood, and other possible confounders known to be associated with DNA methylation (Suarez et al., 2018, Binder et al., 2018, Simpkin et al., 2016, Javed et al., 2016, Lee et al., 2015), we identified 5 principal components of the estimated cell type proportions (Houseman et al., 2012) and calculated the cell-corrected DNA methylation age by residualizing the DNA methylation age estimates on the 5 principal components of the estimated cell type proportions and on batch. Finally, the delta age was defined as the difference between this cell-corrected DNA methylation age and chronological age with positive values reflecting accelerated aging/faster maturation and negative values reflecting decelerated aging/slower maturation.
2.2.3. MRI imaging
T1-weighted images (TR = 25 ms, TE = 5 ms, flip angle = 30°, 140–160 slices, resolution 1x1x1mm) were acquired on 1T Philips MRI scanner approximately 19 days before the blood collection (M = 19 days, SD = 37.6). Toronto Volumetric Pipeline was used to calculate the total volume of cortical GM and the volumes of GM in the four cerebral lobes. The processing steps in the Toronto Volumetric Pipeline were the same as described in the Methods for Study 1. In addition, the total (lobar) GM volume as well as the individual lobar volumes were corrected for chronological age.
2.2.4. Anxiety in adolescence
Anxiety in adolescence were assessed using the Diagnostic Interview Schedule for Children (DISC) Predictive Scales (DPS Lucas et al., 2001).
2.2.5. Statistical analyses
The statistical analyses were done in JMP version 10.0.0 (SAS Institute Inc., Cary, NC) and mirrored those described in Study 1. In addition to the covariates used also in the Study 1 (BMI, smoking in the past 30 days) the full factorial GLMs in Study 2 used also puberty stage and prenatal exposure to maternal cigarette smoking as covariates.
3. Results
3.1. Study 1 – Young adults from ELSPAC/VULDE cohort
3.1.1. Sex differences in the delta age
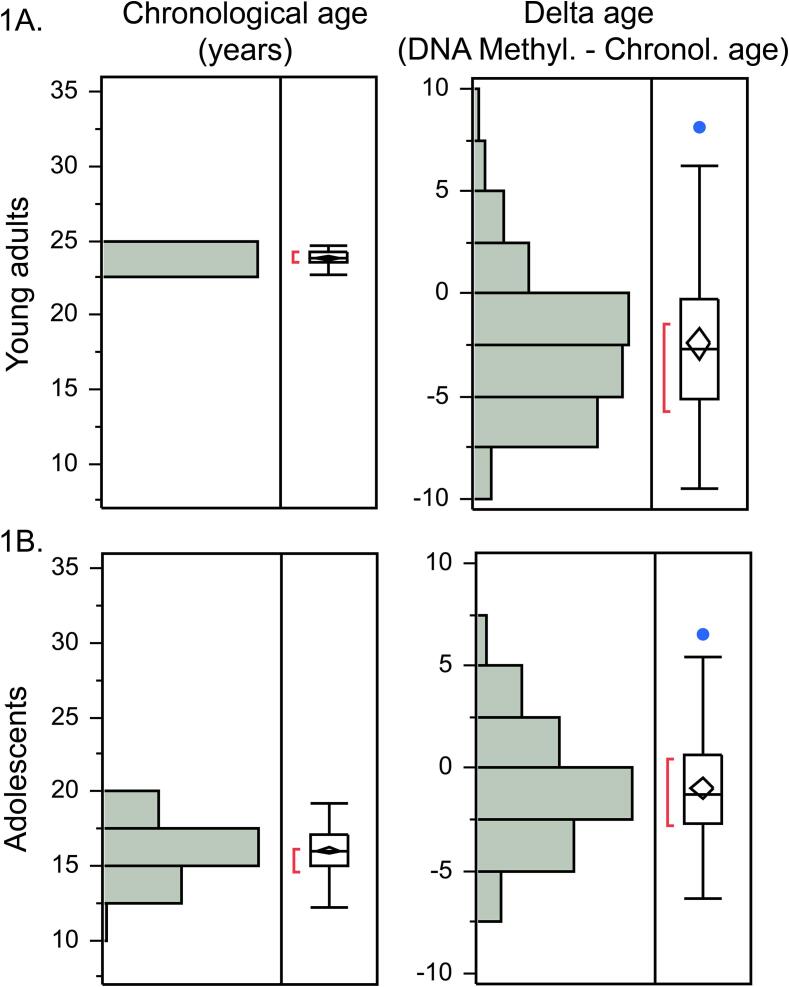
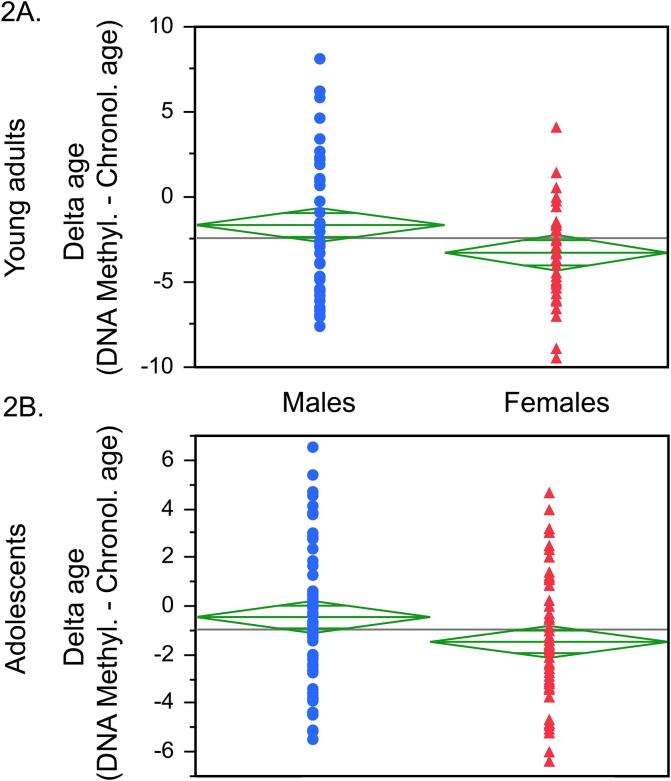
While the chronological age of all participants was 23–24 years, their delta age ranged from −9 to +8 years (Fig. 1A). We observed sex differences in the delta age (t(87) = −2.24, p = 0.03; Cohen’s d = 0.48; Fig. 2A), with men having significantly higher delta age than women. These sex differences remained significant after the correction for covariates (BMI, smoking and alcohol use in the past 30 days): beta = 0.23, p = 0.05, Adj R2 = 0.07.
Fig. 1.
Delta age in young adulthood and adolescence. While the chronological age of all young adults from Study 1 was 23–24 years, their delta age ranged from −9 to +8 years (1A). The chronological age of all adolescents from Study 2 was 12–19 years and their delta age ranged from −6 to +6 years (1B).
Fig. 2.
Sex differences delta age. In young adulthood, delta age was higher in men than women (t(87) = −2.24, p = 0.03; Cohen’s d = 0.48; 2A) and this effect survived also the correction for BMI, smoking and alcohol use in the past 30 days. The delta age was also higher in the male vs. female adolescents (t(127) = −2.27, p = 0.034, Cohen’s d = 0.40; 2B) and this effect also survived the correction for all covariates (BMI, puberty stage, smoking and prenatal exposure to maternal cigarette smoking (beta = 0.21, p = 0.03, AdjR2 = 0.06).
3.1.2. Delta age and its relationship with anxiety trait
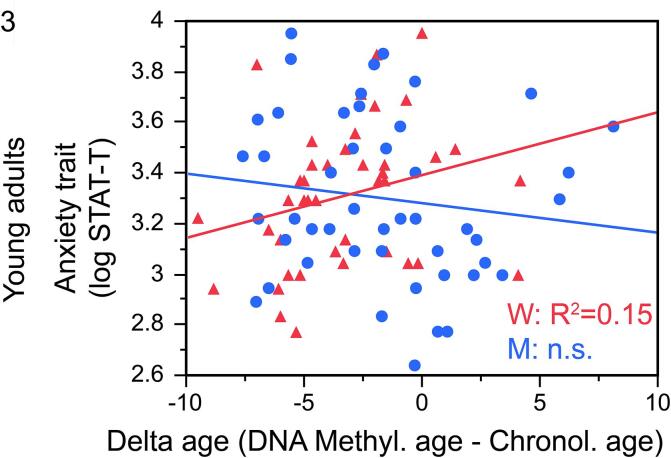
Sex moderated the associations between delta age and anxiety trait (beta = −0.30, p = 0.009; Fig. 3); this sex-by-delta age interaction was independent of all the covariates (BMI, smoking, alcohol use). Posthoc analyses revealed that, in women, delta age was positively associated with anxiety trait (beta = 0.63, p = 0.04, R2 = 0.15). This was not the case in men (p > 0.12).
Fig. 3.
In young adult women, higher delta age was associated with more anxiety trait (beta = 0.63, p = 0.04, R2 = 0.15).
3.1.3. Delta age and its relationship with volume of cortical GM
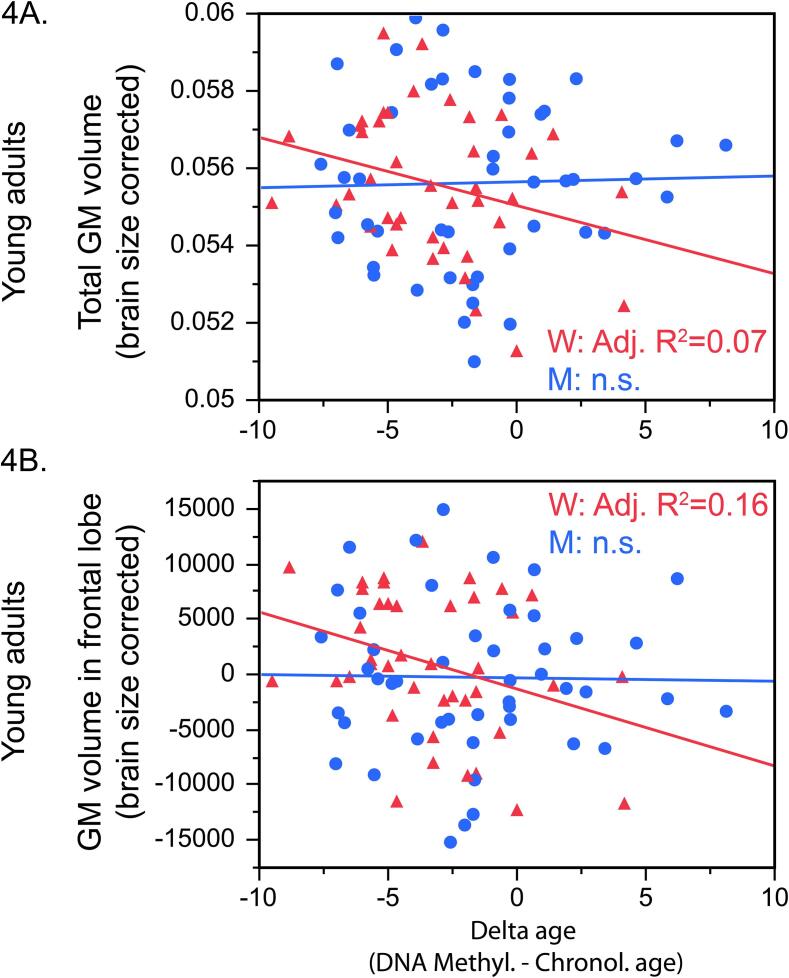
In the brain, sex moderated the association between delta age and brain-size corrected volume of cortical GM (beta = 0.27, p = 0.02; Fig. 4A); this sex-by = delta age interaction was independent of all the covariates (BMI, smoking, alcohol use). Posthoc analyses revealed that, in women, delta age was negatively associated with volume of cortical GM (beta = −0.35, p = 0.04, AdjR2 = 0.07). This was not the case in men (p > 0.30).
Fig. 4.
In young adult women, higher delta age was associated with less brain size-corrected gray matter volume. These effects were significant for the total (lobar) gray matter volume (beta = −0.35, p = 0.04, AdjR2 = 0.07; 4A) and particularly in the frontal lobe (beta = −0.40, p = 0.02, AdjR2 = 0.16; 4B).
Further, a full-factorial GLM showed that delta age was associated with GM volume in sex- and lobe-specific manner (F(3,216) = 3.09, p = 0.03); post-hoc analyses revealed that higher delta age was associated with lower GM volume in the frontal lobe of women (beta = −0.40, p = 0.02, AdjR2 = 0.16; Fig. 4B) but not men (beta = −0.03, p = 0.89) and this association was independent of all the covariates (BMI, smoking, alcohol use). There were no associations between delta age and GM volume in the other lobes.
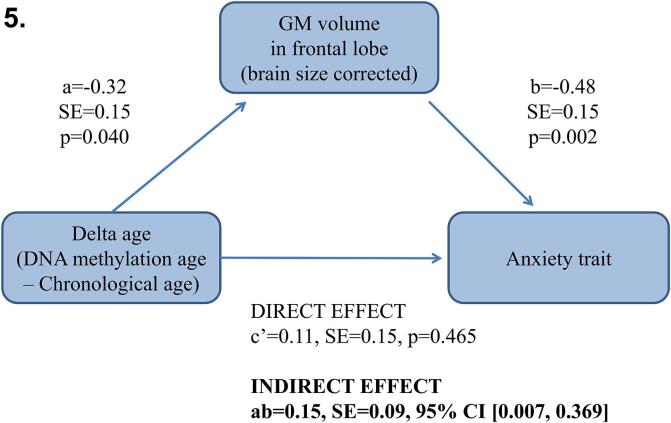
3.1.4. Does GM volume in the frontal lobe mediate the relationship between delta age and anxiety in women?
A mediation analysis revealed that, in women GM volume in frontal lobe mediated the relationship between delta age and anxiety trait (ab = 0.15, SE = 0.15, 95% CI [0.007; 0.369]; Fig. 5).
Fig. 5.
In young adult women, GM volume in frontal lobe mediated the relationship between delta age and anxiety trait (ab = 0.15, SE = 0.15, 95% CI [0.007; 0.369]). Standardized coefficients are provided.
3.2. Study 2 – Adolescents from SYS
3.2.1. Sex differences in the delta age
While the chronological age of all participants was 12–19 years, their delta age ranged from −6 to +6 years (Fig. 1B). Similarly to the ELSPAC/VULDE cohort, we observed significant sex differences (men > women) in the delta age (t(127) = −2.27, p = 0.034, Cohen’s d = 0.40; Fig. 2B), which remained significant also after the correction for puberty stage, BMI, smoking and prenatal exposure to maternal cigarette smoking (beta = 0.21, p = 0.03, AdjR2 = 0.06).
3.2.2. Delta age and its relationship with anxiety
We did not find any relationship between delta age and anxiety (beta = 0.05, p = 0.56) as measured by DISC (Lucas et al., 2001) or any interactions between delta age and sex (beta = 0.03, p = 0.70). Correcting for the covariates (puberty stage, BMI, smoking, exposure to maternal cigarette smoking) did not change these results (delta age on anxiety: beta = 0.04, p = 0.64; delta age * sex on anxiety: beta = 0.06, p = 0.51).
3.2.3. Delta age and its relationship with volume of cortical GM
There was no association between delta age and the age- and brain-size corrected cortical GM volume (beta = 0.02, p = 0.80) or the age- and brain-size corrected GM volume in the frontal lobe (beta = 0.02, p = 0.82); there was no interaction between delta age and sex vis-à-vis age- and brain-size corrected GM volume (beta = −0.06, p = 0.53) or GM volume in the frontal lobe (beta = 0.004, p = 0.97). These results did not change when correcting for puberty stage, BMI, smoking and prenatal exposure to maternal cigarette smoking (all p > 0.38).
4. Discussion
This study used structural MRI and epigenetics data from young adults participating in the European Longitudinal Study of Pregnancy and Childhood (ELSPAC), and its neuroimaging follow-up (VULDE) to assess the relationships between epigenetic clock (Horvath, 2013); gray-matter volume of the cerebral cortex, and symptoms of anxiety. While all young adult participants were 23–24 years old at the time of saliva sampling, MRI and behavioral assessments, their delta age ranged from −9 to +8 years. Young women had lower DNA-methylation age and slower epigenetic clock than young men. In young women (but not men), the speed of epigenetic clock was positively associated withsymptoms of anxiety. In the brain, the speed of epigenetic clock in young women (but not men) was negatively associated with total volume of cortical GM (corrected for brain size). Lobe-specific analyses showed that in young women (but not men), the speed of epigenetic clock was negatively associated with GM volume in the frontal lobe and that the GM volume in the frontal lobe mediated the relationship between the epigenetic clock (delta age, defined as the difference between epigenetic and chronological age) and anxiety. We were not able to replicate these findings in a sample of adolescents from Saguenay Youth Study.
The relatively wide range of delta age (−9 to +8 years) in our sample of young adults (all 23–24 years old, all of European ancestry, born in the same region of Czech Republic) is most likely related to both genetic and environmental factors. Horvath (Horvath, 2013) demonstrated that the heritability of delta age is 100% in newborns and 39% in older subjects, which suggests that non-genetic factors become more relevant with age. In our sample, the mean delta age was negative, thus reflecting rather slower speed of the biological clock and slower (molecular) maturation. Since genes co-located with the 353 CpGs defining the DNA methylation age are enriched for cell death/survival, cellular growth/proliferation, organismal/tissue development and cancer (Horvath, 2013), we conclude that individuals with greater discrepancy between their chronological and epigenetic age experienced altered developmental/aging processes, leading to slower or faster maturation of the brain.
The sex differences in delta age found in young adults are consistent with the relatively higher epigenetic aging rate found in saliva as well as blood and brain tissue of men vs. women in previous research (Horvath et al., 2016). They are also consistent with the larger lifespan in women vs. men (Case and Paxson, 2005, Oksuzyan et al., 2008). Since Suarez et al (Suarez et al., 2018) reported a relationship between higher delta age and higher pubertal stage of breast/genital development, Binder et al (Binder et al., 2018) reported a relationship between higher delta age and earlier puberty, Levine et al (Levine et al., 2016) demonstrated that menopause accelerates the epigenetic clock and Horvath (Horvath, 2013) demonstrated an association between higher delta age and endocrine system disorders and reproductive system disease, it might be that sex hormones and sex-hormone related genes might contribute to the sex differences in delta age.
The positive relationship between the speed of epigenetic clock and symptoms of anxiety in young adult women is consistent with Suarez et al. (2018) who found a relationship between accelerated aging and internalizing and affective problems in adolescents. The fact that we observed these relationships between the delta age and anxiety only in women fits with the generally higher risk of anxiety in adult women than men (Organization WH, 2002, Organization WH, 2016), and is also consistent with Davis et al. (2017)) who found a relationship between higher accelerated aging and higher diurnal cortisol and lower hippocampal volume in adolescent females.
The negative relationship between the speed of epigenetic clock and GM is consistent with the normal age-related trajectories of cortical volume (Giedd et al., 1999, Wierenga et al., 2014). The fact that these effects reached largest effects in the frontal lobe of young adult women, and that it mediated the relationship between delta age and anxiety symptoms, might reflect the sex-specific trajectories of brain development (Giedd et al., 1999, Tanaka et al., 2012).
Since prior work suggests that higher levels of trait anxiety are associated with future vulnerability to depression (Wang et al., 2019), particularly in the context of stress (Weger and Sandi, 2018), we interpret these effects as reflecting probable risk for subsequent psychopathology. Further prospective longitudinal studies are planned in order to test this conjecture in the current cohort. Longitudinal data would also allow us to explore the directionality of the effects reported by our mediation model.
Given the relatively small size of our discovery (ELSPAC/VULDE) sample (n = 88), we attempted to replicate these findings in an independent cohort of adolescents (n = 129; 12–19 years old). While the delta age in adolescents ranged from −6 to +6 years similarly to the ELSPAC/VULDE cohort (ranging from −9 to +8 years), and while the mean delta age was also slightly negative, suggesting a rather slower maturation, particularly in adolescent females whose delta age was more negative than in males (Cohen’s d = 0.40), we did not find any relationship between adolescents’ delta age and anxiety (measured by DISC (Lucas et al., 2001) or the GM volume (age and brain size-corrected). This lack of replication in the adolescents might be related to the different method of tissue collection for epigenetic analysis (blood in adolescents vs. saliva in young adults) or additional sources of variability in the cohort of adolescents (e.g., puberty stages (Suarez et al., 2018), socioeconomic status (Simons et al., 2016, Hughes et al., 2018), prenatal exposure to maternal smoking during pregnancy (Lee et al., 2015) known to be associated with epigenetic aging). It is also possible, that the effects of delta age on the GM volume and symptoms of anxiety might fully manifest only in young adulthood. In fact, Schmaal et al. (2017) pointed out that MDD-related structural brain abnormalities differ between adults and adolescents and suggested that greater cortical thinning might be more pronounced in adult-onset MDD patients. In addition, previous research in MDD adolescents reported lower (Boes et al., 2008, Fallucca et al., 2011, Shad et al., 2012), higher (Fallucca et al., 2011, Reynolds et al., 2014), or no differences (Whittle et al., 2014) in cortical thickness. It is also possible that the result in the ELSPAC/VULDE cohort is due to chance and not replicable. Thus, future research should try to replicate the ELSPAC/VULDE findings in a larger sample of young adults and use saliva for the epigenetic analyses. Future research might also use larger datasets to explore the impact of epigenetic clock on subcortical volumes previously associated with anxiety and depression.
5. Conclusions
Overall, we conclude that in young adult women, the speed of epigenetic clock is negatively associated with total volume of cortical GM (corrected for brain size), particularly in the frontal lobe, and that GM volume in the frontal lobe mediates the relationship between the speed of epigenetic clock and anxiety trait. Since our imaging studies focused on healthy and relatively highly functioning young adults, where the variability of anxiety symptoms does not span the full range of severity, future studies should aim to recruit individuals with clinically diagnosed mental illness to confirm the generalizability and potential clinical relevance of the current findings. Further, while the ELSPAC/VULDE cohort was followed-up prospectively, our DNA-methylation age as well as the imaging data were cross-sectional. Future research on DNA methylation age should use longitudinal data to determine the stability and behavioral relevance of the effects of epigenetic age in time. Such future research should also determine whether individuals with higher delta age experience an earlier onset but then the same speed (and thus the same slope) of aging-related processes or whether they experience faster (and thus a steeper slope) aging-related processes throughout their lives.
Funding
This work was supported by the European Union (Marie Curie Intra-European Fellowship for Career Development, FP7-PEOPLE-IEF-2013, grant #6485124), CETOCOEN EXCELLENCE Teaming 2 project supported by Horizon2020 (857560), the Czech Health Research Council (NU20J-04-00022), and the Czech Ministry of Education, Youth and Sports/MEYS CR (CZ.02.1.01/0.0/0.0/18_046/0015975), and the projects by MEYS CR (CEITEC 2020, LQ1601, LM2018121). We also acknowledge the core facility MAFIL of CEITEC MU supported by the Czech-BioImaging large RI projects (LM2015062, LM2018129 funded by MEYS CR) for their support with obtaining scientific data presented in this paper. The Canadian Institutes of Health Research and the Heart and Stroke Foundation of Canada fund the SYS. Computations were performed on the GPC supercomputer at the SciNet HPC Consortium. SciNet is funded by the Canada Foundation for Innovation under the auspices of Compute Canada; the Government of Ontario; Ontario Research Fund - Research Excellence; and the University of Toronto.
CRediT authorship contribution statement
Klára Marečková: Conceptualization, Investigation, Methodology, Formal analysis, Visualization, Writing - original draft. Anna Pačínková: Formal analysis, Software. Anja Klasnja: Formal analysis, Software. Jean Shin: Formal analysis, Software. Lenka Andrýsková: Investigation, Resources, Funding acquisition. Kateřina Stano-Kozubík: Investigation, Resources. Zdenka Pausová: Funding acquisition. Milan Brázdil: Funding acquisition. Tomáš Paus: Validation, Resources, Writing - review & editing, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2020.102458.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary figure 1.
References
- Ustun T.B., Ayuso-Mateos J.L., Chatterji S., Mathers C., Murray C.J. Global burden of depressive disorders in the year 2000. Br. J. Psychiatry. 2004;184:386–392. doi: 10.1192/bjp.184.5.386. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Berglund P., Demler O., Jin R., Merikangas K.R., Walters E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- America AaDAo (2018): Facts and Statistics.
- Jacobson N.C., Newman M.G. Anxiety and depression as bidirectional risk factors for one another: a meta-analysis of longitudinal studies. Psychol. Bull. 2017;143:1155–1200. doi: 10.1037/bul0000111. [DOI] [PubMed] [Google Scholar]
- Sibille E. Molecular aging of the brain, neuroplasticity, and vulnerability to depression and other brain-related disorders. Dialogues Clin. Neurosci. 2013;15:53–65. doi: 10.31887/DCNS.2013.15.1/esibille. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozycka A., Liguz-Lecznar M. The space where aging acts: focus on the GABAergic synapse. Aging Cell. 2017;16:634–643. doi: 10.1111/acel.12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., Chang L.C., Wang X., Guilloux J.P., Parrish J., Oh H. Molecular and genetic characterization of depression: overlap with other psychiatric disorders and aging. Mol. Neuropsychiatry. 2015;1:1–12. doi: 10.1159/000369974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutte N.S., Malouff J.M. The association between depression and leukocyte telomere length: a meta-analysis. Depress Anxiety. 2015;32:229–238. doi: 10.1002/da.22351. [DOI] [PubMed] [Google Scholar]
- Ridout K.K., Ridout S.J., Price L.H., Sen S., Tyrka A.R. Depression and telomere length: a meta-analysis. J. Affect. Disord. 2016;191:237–247. doi: 10.1016/j.jad.2015.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L.K.M., Aghajani M., Clark S.L., Chan R.F., Hattab M.W., Shabalin A.A. Epigenetic aging in major depressive disorder. Am. J. Psychiatry. 2018;175:774–782. doi: 10.1176/appi.ajp.2018.17060595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannum G., Guinney J., Zhao L., Zhang L., Hughes G., Sadda S. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol. Cell. 2013;49:359–367. doi: 10.1016/j.molcel.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14:R115. doi: 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme S., Albaugh M.D., Nguyen T.V., Hudziak J.J., Mateos-Perez J.M., Labbe A. Trajectories of cortical thickness maturation in normal brain development–The importance of quality control procedures. Neuroimage. 2016;125:267–279. doi: 10.1016/j.neuroimage.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd K.B., Fjell A.M., Giedd J., Dale A.M., Brown T.T. Through Thick and thin: a need to reconcile contradictory results on trajectories in human cortical development. Cereb. Cortex. 2017;27:1472–1481. doi: 10.1093/cercor/bhv301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petanjek Z., Judas M., Simic G., Rasin M.R., Uylings H.B., Rakic P. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc. Natl. Acad. Sci. USA. 2011;108:13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D.J., Duka T., Stimpson C.D., Schapiro S.J., Baze W.B., McArthur M.J. Prolonged myelination in human neocortical evolution. Proc. Natl. Acad. Sci. USA. 2012;109:16480–16485. doi: 10.1073/pnas.1117943109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P., Kabani N.J., Lerch J.P., Eckstrand K., Lenroot R., Gogtay N. Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J.N., Rapoport J.L. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67:728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell A.M., Grydeland H., Krogsrud S.K., Amlien I., Rohani D.A., Ferschmann L. Development and aging of cortical thickness correspond to genetic organization patterns. Proc. Natl. Acad. Sci. USA. 2015;112:15462–15467. doi: 10.1073/pnas.1508831112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A., Rohlfing T., Rosenbloom M.J., Chu W., Colrain I.M., Sullivan E.V. Variation in longitudinal trajectories of regional brain volumes of healthy men and women (ages 10 to 85 years) measured with atlas-based parcellation of MRI. Neuroimage. 2013;65:176–193. doi: 10.1016/j.neuroimage.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher E., Gavett B., Harvey D., Farias S.T., Olichney J., Beckett L. Brain volume change and cognitive trajectories in aging. Neuropsychology. 2018;32:436–449. doi: 10.1037/neu0000447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., Castellanos F.X., Liu H., Zijdenbos A. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gotlib I.H., Joormann J. Cognition and depression: current status and future directions. Annu. Rev. Clin. Psychol. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J., Quinn M.E. Cognitive processes and emotion regulation in depression. Depress Anxiety. 2014;31:308–315. doi: 10.1002/da.22264. [DOI] [PubMed] [Google Scholar]
- Joormann J., Tanovic E. Cognitive vulnerability to depression: examining cognitive control and emotion regulation. Curr. Opin. Psychol. 2015;4:86–92. [Google Scholar]
- Salthouse T.A. How general are the effects of trait anxiety and depressive symptoms on cognitive functioning? Emotion. 2012;12:1075–1084. doi: 10.1037/a0025615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T.S., Koutsouleris N., Bottlender R., Born C., Jager M., Scupin I. Depression-related variation in brain morphology over 3 years: effects of stress? Arch. Gen. Psychiatry. 2008;65:1156–1165. doi: 10.1001/archpsyc.65.10.1156. [DOI] [PubMed] [Google Scholar]
- Schmaal L., Hibar D.P., Samann P.G., Hall G.B., Baune B.T., Jahanshad N. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol. Psychiatry. 2017;22:900–909. doi: 10.1038/mp.2016.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis E.G., Humphreys K.L., McEwen L.M., Sacchet M.D., Camacho M.C., MacIsaac J.L. Accelerated DNA methylation age in adolescent girls: associations with elevated diurnal cortisol and reduced hippocampal volume. Transl. Psychiatry. 2017;7 doi: 10.1038/tp.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pausova Z., Paus T., Abrahamowicz M., Bernard M., Gaudet D., Leonard G. Cohort Profile: The Saguenay Youth Study (SYS) Int. J. Epidemiol. 2017;46 doi: 10.1093/ije/dyw023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka C., Matsui M., Uematsu A., Noguchi K., Miyawaki T. Developmental trajectories of the fronto-temporal lobes from infancy to early adulthood in healthy individuals. Dev. Neurosci. 2012;34:477–487. doi: 10.1159/000345152. [DOI] [PubMed] [Google Scholar]
- Organization WH (2002): Gender Disparities in Mental Health.
- Organization WH (2016): Global Health Estimates 2015: Disease burden by Cause, Age, Sex, by Country and by Region, 2000-2015.
- Prokhorskas R., Ignatyeva R., Dragonas T., Golding J. European longitudinal study of pregnancy and childhood (ELSPAC) Paediatr. Perinat. Epidemiol. 1989;3:460–469. doi: 10.1111/j.1365-3016.1989.tb00533.x. [DOI] [PubMed] [Google Scholar]
- Piler P., Kandrnal V., Kukla L., Andryskova L., Svancara J., Jarkovsky J. Cohort Profile: The European Longitudinal Study of Pregnancy and Childhood (ELSPAC) in the Czech Republic. Int. J. Epidemiol. 2017;46 doi: 10.1093/ije/dyw091. 1379–1379f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y., Morris T.J., Webster A.P., Yang Z., Beck S., Feber A. ChAMP: updated methylation analysis pipeline for Illumina BeadChips. Bioinformatics. 2017;33:3982–3984. doi: 10.1093/bioinformatics/btx513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teschendorff A.E., Marabita F., Lechner M., Bartlett T., Tegner J., Gomez-Cabrero D. A beta-mixture quantile normalization method for correcting probe design bias in Illumina Infinium 450 k DNA methylation data. Bioinformatics. 2013;29:189–196. doi: 10.1093/bioinformatics/bts680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Lushene R., Vagg P.R., Jacobs G.A. Consulting Psychological Press; Palo Alto, CA: 1983. Manual for the State-Trait Anxiety Inventory. [Google Scholar]
- Suarez A., Lahti J., Czamara D., Lahti-Pulkkinen M., Girchenko P., Andersson S. The epigenetic clock and pubertal, neuroendocrine, psychiatric, and cognitive outcomes in adolescents. Clin. Epigenetics. 2018;10:96. doi: 10.1186/s13148-018-0528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder A.M., Corvalan C., Mericq V., Pereira A., Santos J.L., Horvath S. Faster ticking rate of the epigenetic clock is associated with faster pubertal development in girls. Epigenetics. 2018;13:85–94. doi: 10.1080/15592294.2017.1414127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkin A.J., Hemani G., Suderman M., Gaunt T.R., Lyttleton O., McArdle W.L. Prenatal and early life influences on epigenetic age in children: a study of mother-offspring pairs from two cohort studies. Hum. Mol. Genet. 2016;25:191–201. doi: 10.1093/hmg/ddv456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javed R., Chen W., Lin F., Liang H. Infant's DNA methylation age at birth and epigenetic aging accelerators. Biomed Res. Int. 2016;2016:4515928. doi: 10.1155/2016/4515928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.W., Richmond R., Hu P., French L., Shin J., Bourdon C. Prenatal exposure to maternal cigarette smoking and DNA methylation: epigenome-wide association in a discovery sample of adolescents and replication in an independent cohort at birth through 17 years of age. Environ. Health Perspect. 2015;123:193–199. doi: 10.1289/ehp.1408614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houseman E.A., Accomando W.P., Koestler D.C., Christensen B.C., Marsit C.J., Nelson H.H. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinf. 2012;13:86. doi: 10.1186/1471-2105-13-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas C.P., Zhang H., Fisher P.W., Shaffer D., Regier D.A., Narrow W.E. The DISC Predictive Scales (DPS): efficiently screening for diagnoses. J. Am. Acad. Child Adolesc. Psychiatry. 2001;40:443–449. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- Horvath S., Gurven M., Levine M.E., Trumble B.C., Kaplan H., Allayee H. An epigenetic clock analysis of race/ethnicity, sex, and coronary heart disease. Genome Biol. 2016;17:171. doi: 10.1186/s13059-016-1030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case A., Paxson C. Sex differences in morbidity and mortality. Demography. 2005;42:189–214. doi: 10.1353/dem.2005.0011. [DOI] [PubMed] [Google Scholar]
- Oksuzyan A., Juel K., Vaupel J.W., Christensen K. Men: good health and high mortality. Sex differences in health and aging. Aging Clin. Exp. Res. 2008;20:91–102. doi: 10.1007/bf03324754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M.E., Lu A.T., Chen B.H., Hernandez D.G., Singleton A.B., Ferrucci L. Menopause accelerates biological aging. Proc. Natl. Acad. Sci. USA. 2016;113:9327–9332. doi: 10.1073/pnas.1604558113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga L.M., Langen M., Oranje B., Durston S. Unique developmental trajectories of cortical thickness and surface area. Neuroimage. 2014;87:120–126. doi: 10.1016/j.neuroimage.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Wang T., Li M., Xu S., Liu B., Wu T., Lu F. Relations between trait anxiety and depression: a mediated moderation model. J. Affect. Disord. 2019;244:217–222. doi: 10.1016/j.jad.2018.09.074. [DOI] [PubMed] [Google Scholar]
- Weger M., Sandi C. High anxiety trait: a vulnerable phenotype for stress-induced depression. Neurosci. Biobehav. Rev. 2018;87:27–37. doi: 10.1016/j.neubiorev.2018.01.012. [DOI] [PubMed] [Google Scholar]
- Simons R.L., Lei M.K., Beach S.R., Philibert R.A., Cutrona C.E., Gibbons F.X. Economic hardship and biological weathering: the epigenetics of aging in a U.S. sample of black women. Soc. Sci. Med. 2016;150:192–200. doi: 10.1016/j.socscimed.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A., Smart M., Gorrie-Stone T., Hannon E., Mill J., Bao Y. Socioeconomic position and DNA methylation age acceleration across the life course. Am. J. Epidemiol. 2018;187:2346–2354. doi: 10.1093/aje/kwy155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boes A.D., McCormick L.M., Coryell W.H., Nopoulos P. Rostral anterior cingulate cortex volume correlates with depressed mood in normal healthy children. Biol. Psychiatry. 2008;63:391–397. doi: 10.1016/j.biopsych.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallucca E., MacMaster F.P., Haddad J., Easter P., Dick R., May G. Distinguishing between major depressive disorder and obsessive-compulsive disorder in children by measuring regional cortical thickness. Arch. Gen. Psychiatry. 2011;68:527–533. doi: 10.1001/archgenpsychiatry.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shad M.U., Muddasani S., Rao U. Gray matter differences between healthy and depressed adolescents: a voxel-based morphometry study. J. Child Adolesc. Psychopharmacol. 2012;22:190–197. doi: 10.1089/cap.2011.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds S., Carrey N., Jaworska N., Langevin L.M., Yang X.R., Macmaster F.P. Cortical thickness in youth with major depressive disorder. BMC Psychiatry. 2014;14:83. doi: 10.1186/1471-244X-14-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S., Lichter R., Dennison M., Vijayakumar N., Schwartz O., Byrne M.L. Structural brain development and depression onset during adolescence: a prospective longitudinal study. Am. J. Psychiatry. 2014;171:564–571. doi: 10.1176/appi.ajp.2013.13070920. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.