Highlights
-
•
Stroke patients are capable of learning to use Brain-Computer interface based on TMS.
-
•
Sub-acute stroke patients used TMS neurofeedback to increase MEP amplitude.
-
•
TMS neurofeedback increased corticospinal excitability in paretic muscles.
Keywords: Stroke, TMS, Neurofeedback, Upper limb, Motor imagery, Brain-computer interface
Abstract
Upper limb weakness following a stroke affects 80% of survivors and is a key factor in preventing their return to independence. State-of-the art approaches to rehabilitation often require that the patient can generate some activity in the paretic limb, which is not possible for many patients in the early period following stroke. Approaches that enable more patients to engage with upper limb therapy earlier are urgently needed.
Motor imagery has shown promise as a potential means to maintain activity in the brain’s motor network, when the patient is incapable of generating functional movement. However, as imagery is a hidden mental process, it is impossible for individuals to gauge what impact this is having upon their neural activity.
Here we used a novel brain-computer interface (BCI) approach allowing patients to gain an insight into the effect of motor imagery on their brain-muscle pathways, in real-time. Seven patients 2–26 weeks post stroke were provided with neurofeedback (NF) of their corticospinal excitability measured by the size of motor evoked potentials (MEP) in response to transcranial magnetic stimulation (TMS). The aim was to train patients to use motor imagery to increase the size of MEPs, using the BCI with a computer game displaying neurofeedback.
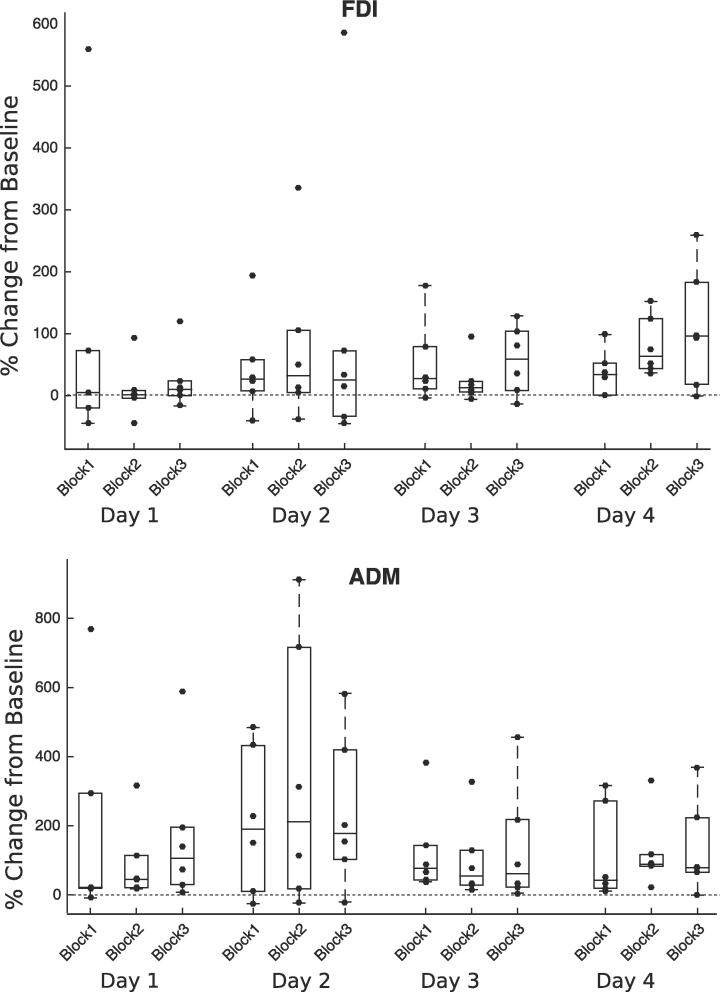
Patients training finger muscles learned to elevate MEP amplitudes above their resting baseline values for the first dorsal interosseous (FDI) and abductor digiti minimi (ADM) muscles. By day 3 for ADM and day 4 for FDI, MEP amplitudes were sustained above baseline in all three NF blocks.
Here we have described the first clinical implementation of TMS NF in a population of sub-acute stroke patients. The results show that in the context of severe upper limb paralysis, patients are capable of using neurofeedback to elevate corticospinal excitability in the affected muscles. This may provide a new training modality for early intervention following stroke.
1. Introduction
The incidence of stroke is expected to almost double between 2010 and 2030 (Feigin et al., 2010). Advances in acute stroke care over the last decade have resulted in a substantial increase in the number of survivors (Shah et al., 2019, Johnson et al., 2019, Stroke Association, 2017, NCPRM, 2017). Rehabilitation of these survivors to enable them to return to independent daily living is becoming an increasing research priority. Among the survivors, 8 out of 10 experience some degree of hemiparesis in the upper limb contralateral to the lesion site (Lawrence et al., 2001, Wade, 1992), ranging from mild to completely disabling. Many of the current gold standard approaches for improving upper limb function after stroke require the patient to be capable of generating some voluntary movement with the paretic limb. Constraint-induced movement therapy (CIMT), for example, has been shown to have beneficial effects when applied in the acute or chronic stages (Boake et al., 2016, Miltner et al., 1999, Taub et al., 1998) but can not be used for those with very poor function. Accordingly, CIMT is not suitable for many patients early after stroke who have not yet regained some residual movement capacity. Getting patients to a stage where they can generate voluntary muscle activity sooner would allow more intensive physical therapy to commence earlier during the critical period for neural reorganisation within movement pathways in the brain (Dromerick et al., 2015, Murphy and Corbett, 2009).
While biofeedback of affected muscles is also beneficial (Crow et al., 2009), this too comes with the caveat that the patient must already be capable of generating some voluntary motor activity. One approach that does not require muscle activation is Brain Computer Interface (BCI), which has been used in a stroke rehabilitation context to detect movement intentions directly from the brains of patients with hemiparesis. The BCI readout is used to drive computerised or motorised effectors (Soekadar et al., 2016, Ushiba and Soekadar, 2016, Van Dokkum et al., 2015) or promote ‘healthy’ patterns of brain activity (Coscia et al., 2019, Laffont et al., 2014). It has been suggested that this form of feedback-based learning serves to close the dysfunctional central-peripheral loop following stroke, promoting Hebbian-like plasticity in the damaged brain circuits (Coscia et al., 2019). BCIs typically require the patient to engage in motor imagery in order to generate detectable electrical brain signals on the scalp over motor regions.
It is known that motor imagery activates many of the same neural circuits as actual movement (Ehrsson et al., 2003, Hanakawa et al., 2003, Lotze et al., 2006). This logic has been applied to neurological rehabilitation in stroke patients (Jackson et al., 2001) as when they are unable to execute movements with a paretic limb, imagination of movement may reactivate damaged motor pathways and encourage reorganisation to occur. However, the quality and type of motor imagery has a large impact upon the neural circuits that are activated, and the resulting effects on corticospinal excitability (Stinear et al., 2005). As imagery is a hidden mental process, it is impossible for individuals to gauge what impact it has upon their neural activity. Having overt feedback on their imagery-related brain activity may assist patients to learn how to best exploit this mechanism to augment stroke recovery. In the current investigation, we propose a novel BCI allowing patients to gain an insight into the effect of motor imagery on their brain-muscle pathways. This is achieved by providing real-time feedback of their corticospinal excitability measured by the size of motor evoked potentials (MEP) in response to transcranial magnetic stimulation (TMS).
Despite having no functional movement in the paretic limb, a majority of stroke patients exhibit MEPs when stimulated over the damaged motor areas of the brain. The presence of MEPs in the paretic limb indicates intact pathways from the brain to the muscles and predicts better subsequent functional recovery (Byblow et al., 2015, Stinear et al., 2012). In the early days following stroke, the excitability of neuronal pathways from the brain to the muscles is substantially reduced. This most likely reflects the demyelination of axons, leading to conduction failure. Within the first 12 weeks, a degree of spontaneous proportional recovery of motor function typically occurs, up to 70% of the individual’s maximum possible improvement. Interestingly, this time course of recovery coincides with the resolution of corticomotor excitability, which also resolves to 70% of normal levels within the same 12 week period (Byblow et al., 2015, Cicinelli et al., 2003, Thickbroom et al., 2004). The interconnectedness of the functional recovery process and resolution of corticomotor excitability provides some insight into the neurobiology of spontaneous motor recovery and raises the intriguing possibility that perhaps interventions directly targeting the neural mechanisms of corticomotor excitability in this sensitive period would causally impact upon and accelerate recovery.
In a recent investigation, we designed an operant conditioning protocol to train healthy individuals to optimise their motor imagery performance using real-time neurofeedback of their corticospinal excitability in order to reach two distinct ‘states’ (Ruddy et al., 2018). Over the course of 300 trials receiving feedback of their MEP amplitudes, participants learned to generate distinct patterns of brain activity to optimally excite (upregulate) or inhibit (downregulate) their motor pathways. By the end of two days of ‘up’ training and two days of ‘down’ training, they had converged upon effective mental strategies to volitionally increase or decrease excitability, which were associated with distinct oscillatory signatures in concurrent EEG recordings. Importantly, a control group executing motor imagery without feedback did not learn to modulate their excitability, implicating that the feedback was essential in order to reach the optimal state.
In the current investigation we applied the protocol described by Ruddy et al. (2018) in a clinical environment, with sub-acute in-patient stroke survivors. Additionally, we introduced an action observation component to the protocol, giving patients the opportunity to view wrist extension or hand opening movements while simultaneously imagining the same. Action observation alone has been shown to improve motor outcomes in stroke (Ertelt et al., 2007), but more recent evidence suggests that the strongest reactivation of the motor network occurs when action observation is combined with simultaneous motor imagery (Taube et al., 2015, Taube et al., 2014). We hypothesised that by providing feedback rewarding large MEP amplitudes to patients while they viewed and imagined movements, they would learn to effectively tailor their motor imagery strategies to optimally increase corticospinal excitability in the paretic limb. While we do not expect significant functional improvements over the course of this initial 4 day trial, we expect that the results will inform the development of a large scale randomised controlled trial testing the utility of TMS NF over a more extended recovery period.
2. Materials and methods
2.1. Participants
Eight sub-acute stroke patients (4-26wks post stroke) were recruited from the stroke rehabilitation unit of China Medical University, Shengjing Hospital in Shenyang, China over a one month period in June 2018. Patients were eligible to participate if this was their first stroke, they had a single-hemisphere lesion, exceeded a score of 24 on the Mini-Mental State Examination, exhibited no visuospatial neglect and presented with an upper limb deficit (see Supplementary Fig. 5). Patients provided written informed consent, and procedures were approved by the Medical Ethics Committee of Shengjing Hospital, China Medical University, approval No. 2018PS371K, in accordance with the Declaration of Helsinki. Following a screening procedure using TMS (described in detail below), 1 patient exhibited no MEPs in the arm and/or hand when stimulated over the motor cortex contralateral to the paretic limb, and thus was deemed ineligible. Of the 7 who participated in the study 2 were female, mean age 59.0 ± 6.5 years. All lesions were ischemic (locations reported in Table 1). Mean upper limb function on the Medical Research Council (MRC) stroke scale was 1.8 ± 1.5 (out of a possible 5), and 29.3 ± 18.5 on the Fugl-Meyer upper extremity scale (out of a possible 66). All patients scored 2 or less on the Modified Ashworth Scale for spasticity (mean 1.1 ± 0.8). For all patient scores and other functional measures see Table 1 and Supplementary Table 1. Patients continued to engage in their usual standard upper limb rehabilitation activities throughout the course of this trial.
Table 1.
Patient Characteristics. Patient demographic, stroke information and Fugl-Meyer upper extremity scores pre and post TMS NF training. Other functional measures are reported in Supplementary Table 1.
| Patient code | Age | Lesion location | Lesion side | Lesion type | Time since stroke (wks) | FM-UE(66 max) |
FM wrist (10max) |
FM finger (14max) |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| pre | post | pre | post | pre | post | ||||||
| NF1 | 66 | unclear | Left | Ischemic | 9 | 56 | 57 | 7 | 7 | 14 | 14 |
| NF2 | 65 | Basal Ganglia | Right | Ischemic | 21 | 15 | 23 | 0 | 4 | 1 | 1 |
| NF3 | 66 | semiovale, radiation crown area | Right | Ischemic | 7 | 49 | 54 | 7 | 7 | 9 | 13 |
| NF4 | 55 | Basal Ganglia | Left | Ischemic | 26 | 14 | 17 | 0 | 0 | 3 | 2 |
| NF5 | 57 | Basal Ganglia | Left | Ischemic | 9 | 10 | 13 | 0 | 2 | 1 | 1 |
| NF6 | 51 | Basal Ganglia | Right | Ischemic | 17 | 39 | 38 | 4 | 4 | 6 | 6 |
| NF7 | 53 | Basal Ganglia | Left | Ischemic | 14 | 22 | 21 | 0 | 0 | 0 | 1 |
| Mean | 59.0 | 14.7 | 29.3 | 31.9 | 2.6 | 3.4 | 4.9 | 5.4 | |||
| SD | 6.5 | 7.0 | 18.5 | 17.9 | 3.4 | 2.9 | 5.1 | 5.8 | |||
2.2. MEP status screening
The Abductor Digiti Minimi (ADM) of the stroke affected hand and wrist Extensor Carpi Radialis (ECR) were prepared for electromyography (EMG) recordings using an abrasive gel and alcohol wipe to ensure good conductance with the skin. A fitted swimming cap was placed onto the patients head to provide a smooth surface for drawing a grid of 1 cm squares with a coordinate system. The head was measured and the approximate scalp location overlaying the cortical motor representation of finger muscles in the lesioned hemisphere was marked on the head (5 cm lateral and 1 cm anterior from the vertex). This marking at the coordinate [5,1] served as the starting point for a search procedure to locate the motor representation of the finger muscles. TMS was applied with a figure of eight coil (internal coil diameter 70 mm) connected to a Magstim Rapid2 stimulator (Magstim, Whitland, UK). The coil was held over the starting coordinate marking with the optimal orientation for evoking a descending volley in the corticospinal tract (approximately 45 degrees from the sagittal plane in order to induce posterior-anterior current flow). Magnetic pulses were first delivered at a low intensity (~30% of Maximum stimulator output, MSO), to familiarise the patient with the sensation of TMS. This was gradually incremented in 5% steps until an MEP > 50 µV was detected in the ADM muscle. If no MEP was detected even at 100% MSO, the coil was placed on each of the surrounding coordinates and 10 pulses were delivered at each, until an MEP was detected. If no MEP was detected at the coordinates within a 1 cm radius of the starting coordinate, the locations within a 2 cm radius were tested, followed by those within a 3 cm and 4 cm radius. The presence of MEPs in the wrist ECR was monitored concurrently. If no MEPs were detected in the ADM muscle, but were detected for the wrist ECR, the participant was eligible to participate in the wrist feedback variant of the task (MEP feedback from ECR). If MEPs in the stroke affected limb were smaller than the 50 µV criterion but clearly discernible from background EMG, the patient was still deemed MEP positive. If an MEP was detected at any time during this procedure, the patient was eligible to participate. Also, if a patient had no MEPs at rest but did during mild contraction with resistance applied by an experimenter, they were eligible to participate. If no MEP was detected at any of the tested locations for finger or wrist muscles, the patient was deemed to have a negative MEP status and was not eligible to participate.
2.3. Familiarisation session
Immediately following screening with TMS, eligible patients (who exhibited MEPs when stimulated- ie. MEP positive) underwent a familiarisation procedure, where they were introduced to the techniques that would be used. They were shown videos of wrist extension/flexion and whole hand finger spreading, and asked to perform these movements with their healthy limbs, attempt the same with the paretic limb, and then imagine the feeling of performing the same movements (kinaesthetic imagery) with the paretic limb. This was to prepare them for the subsequent training sessions, and allow time for full explanation and answering of patient questions and concerns. During this session we also established which muscles would be the most appropriate targets for TMS-NF (Finger muscles or wrist extensor), based upon the patient’s functional needs and bearing in mind which muscles exhibited MEPs when stimulated. Participants were instructed that during the training sessions they would see the videos again and should try to imagine the feeling of executing the same movement with their paretic limb. They were told that a TMS pulse would be delivered during each video, followed by a feedback bar on screen showing the size of their muscle response.
3. TMS neurofeedback training sessions
3.1. TMS setup and EMG recording
Patients were seated in a comfortable chair in front of a computer monitor with arms supported with foam pillows (Fig. 1A). Six muscles were prepared for EMG recordings (Fig. 1B), using an abrasive gel and alcohol wipe to ensure good conductance with the skin. For all patients we recorded First Dorsal Interosseous (FDI), Abductor Digiti Minimi (ADM), Flexor Carpi Radialis (FCR) and Extensor Carpi Radialis (ECR) in the paretic limb. If TMS NF was to be provided from finger muscles, EMG was additionally recorded from FDI and ADM on the healthy side, or healthy ECR and FCR for the wrist feedback variant. Thus, healthy side recordings were taken from the muscles homologous to the target muscles to ensure that they remained quiescent throughout training. TMS delivery and neurofeedback visual display were controlled via custom MATLAB software.
Fig. 1.
Experimental setup, neurofeedback display and EMG traces. Panel A shows the general experimental setup with patient seated in front of a monitor viewing feedback of MEP amplitude (adapted from Cretu et al., 2019). Panel B shows the visual sequence of events for one trial. Each trial commenced with a ‘traffic lights’ display (i) indicating background muscle activity in non-involved muscles, followed by a video (ii) showing a wrist or finger movement, during which the TMS pulse was delivered. Following TMS, a rectangular bar representing the MEP amplitude from the paretic muscles was displayed (iii). The colour of the bar (green or red) and a positive or negative soundbyte indicated to the patient whether their MEP had exceeded (or not) the amplitude of their baseline values recorded prior to training. Panel C shows EMG traces from one patient (NF6) during their final session (Day 4), with exemplar trials shown at i) baseline, ii) during the final block of TMS neurofeedback and iii) at rest post neurofeedback training. TMS delivery is denoted by a black dashed line. A period of 105 ms background EMG prior to TMS is shown. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
TMS was applied with a figure of eight coil (internal coil diameter 70 mm) connected to a Magstim Rapid2 stimulator (Magstim, Whitland, UK). The coil was held on the healthy hemisphere over the ‘hotspot’ of the target muscle(s), at the location with the largest and most consistent MEPs, and with the optimal orientation for evoking a descending volley in the corticospinal tract (approximately 45 degrees from the sagittal plane in order to induce posterior-anterior current flow). Once the hotspot was established, the lowest stimulation intensity at which MEPs > 50 µV on 5/10 consecutive trials was taken as Resting Motor Threshold. The same procedure was repeated on the stroke affected hemisphere, using the mirrored location of the healthy hotspot (and knowledge of healthy hemisphere RMT) as an initial guide for searching for MEPs. If MEPs in the stroke affected limb were smaller than the 50 µV criterion but clearly discernible from background EMG, the patient was still deemed MEP positive and proceeded with the protocol. The intensity chosen for evoking MEPs throughout the remainder of the experiment was 125% of stroke hemisphere RMT, or 100% maximum stimulator output, whichever was lowest.
3.2. Format of TMS neurofeedback training
On each of the 4 training days, following hotspotting/finding RMT, 20 MEPs were collected at rest serving as a baseline measurement for calibration of the subsequent feedback display. This was followed by three blocks of TMS neurofeedback, each containing 20 trials.
Each trial began with a ‘traffic lights’ display showing background muscle activity in each of the muscles that were being recorded that were NOT the targets of feedback (Fig. 1C). The colour of each circle represented (in real-time) the root mean squared EMG in one of the muscles in the previous 100 ms epoch, with values below 7 µV represented by green and above this by red. Patients were instructed to try to keep all traffic lights green at all times. The trial could not progress until all muscles remained relaxed for 5 consecutive 100 ms epochs. If the 7 µV criterion was not appropriate for the patient due to spasticity, this threshold could be adjusted. The traffic lights display was intended to deter the patient from developing inappropriate compensatory strategies to bias MEP amplitude in the target muscles by tensing or moving non-involved muscles. Background EMG was not discouraged in the paretic muscles as the ultimate goal was to improve their motor output.
When the traffic lights criterion was met, the trial proceeded with a brief video clip showing an actor’s arm performing a movement. Depending on the target muscle, the video depicted wrist extension and flexion (6 s), or whole hand finger spreading (2 s, Fig. 1C). A TMS pulse was delivered during the video, at the point when the viewed target muscle(s) reached peak extension (±200 ms jitter to avoid anticipation effects). Participants were instructed to attend to the video and imagine the feeling of performing the same movements with the target muscles. Immediately following the video a feedback screen appeared showing the size of the MEP in the target muscle(s) represented by a coloured bar. If the MEP was larger than the patient’s baseline (indicated as a horizontal white line) the bar was green, a green tick was displayed and a positive sound was played. Otherwise, the bar was red, a red cross was shown and a negative sound was played. Participants were told that their goal on every trial was to get the bar high enough to cross the horizontal line. In the wrist variant of the task, feedback was given from the ECR muscle, and in the finger variant from the average of FDI and ADM MEPs. The feedback remained on screen for 4 s, before being replaced by the traffic lights display preceding the next trial. Twenty such trials comprised one block of training. Patients undertook three blocks per day, and were allowed to rest between blocks for as long as required. Following the three blocks, 20 MEPs were collected at rest.
The same TMS NF procedure was repeated over 4 consecutive days. After the last day of training, the patient’s upper limb function was assessed again using the same measurement instruments as pre-training (see Supplementary Table 1 for all measures).
4. Results
4.1. Patient characteristics
Following the screening procedure with 8 volunteer sub-acute stroke patients, 7 were deemed MEP positive and eligible to participate in the study. Six of the 7 patients engaged in TMS NF of finger muscles (averaged FDI/ADM with finger spreading video). One patient with no finger function and very poor wrist function engaged in TMS NF of the ECR, with wrist extension video. All had MMSE scores > 28 indicating good cognitive function. No adverse events were recorded relating to the experimental procedures, and all patients who took part completed the full 4 day protocol with three blocks of neurofeedback. All reported that they did not deviate from the recommended mental strategy throughout, which was to imagine the feeling of performing the same movement that was shown to them in the videos.
4.2. Training related changes in MEP amplitudes
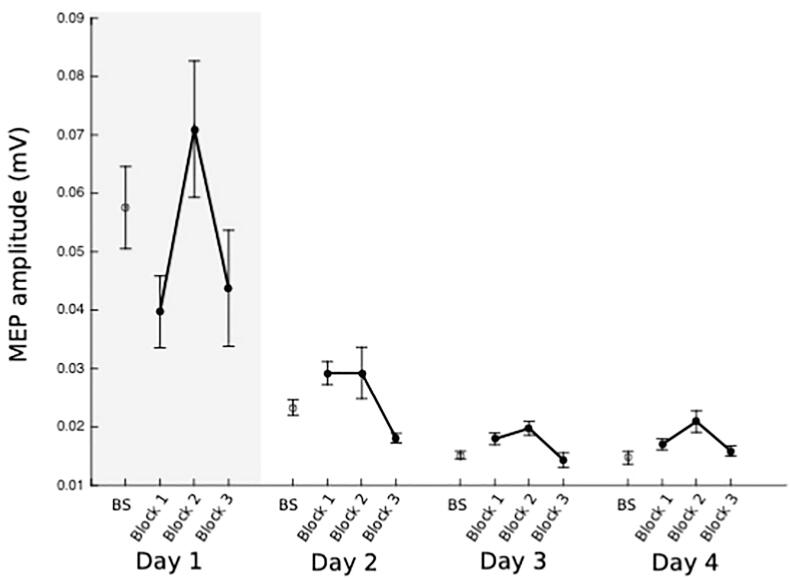
Using TMS NF, all 6 patients training finger muscles learned to elevate MEP amplitudes above their resting baseline values for FDI and ADM (Fig. 2). By day 3 for ADM and day 4 for FDI, MEP amplitudes were sustained above baseline in all three NF blocks. (See Supplementary Fig. 1, Fig. 2 for averaged FDI/ADM plots). For FDI, combining data from all 3 blocks on each of the 4 training days shows a linear progression towards increasing MEP amplitudes from day 1–4 (Fig. 3A). The same trend is not evident for ADM (Fig. 3B). In the case study of one patient who trained with TMS NF of the wrist ECR (patient NF4), although the patient required resistance in order to evoke MEPs on day 1, for days 2,3 and 4 small but clearly discernible MEPs were present (Fig. 4). The patient was able to elevate MEP amplitudes in blocks 1 and 2 of each day from day 2 onwards, but reported fatigue each day in block 3 which is reflected in poorer performance.
Fig. 2.
Percentage change in MEP amplitudes from resting baseline in stroke affected limb. Boxplots with superimposed individual datapoints show MEP amplitudes from stroke affected finger muscles expressed as percentage of resting baseline for 6 patients over 4 days of TMS NF training of averaged FDI/ADM. Data for all 3 blocks of neurofeedback are shown separately. Values above 0 (dotted line) indicate increased MEP amplitudes.
Fig. 3.
Percentage change in MEP amplitudes from baseline collapsed across training days. Data for each day of training were averaged across the 3 training blocks. Individual patients’ data are plotted as separate data points. Black trendline shows median values across the 6 patients for FDI (Panel A) and ADM (Panel B).
Fig. 4.
Case study of patient engaging in TMS NF of wrist extensor. Data shown are raw MEP amplitudes recorded from Extensor Carpi Radialis (ECR) from one patient with very poor residual finger or wrist function. Red points indicate baseline measurements on each separate day, followed by three blocks of TMS NF from ECR. The shaded area in Day 1 indicates where resistance was applied by a therapist in order to evoke MEPs, as none were present at rest. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4.3. Upper limb function
On average (n = 7) upper extremity Fugl-Meyer (FM-UE) scores changed by 2.6 (±3.3) points (See Table 1). The patient training with wrist TMS NF (NF4) increased UE-FM by 3 points. Spasticity scores measured by the modified Ashworth scale were unaffected (0.1 point change). Wrist extension force measured by the Medical Research Council stroke scale (MRC) remained the same for 3 patients, increased for 3 and reduced for 1. In the Box and Block test, patients were able to move on average 3.3(±1.3) more blocks with their paretic hand at the end of training (Supplementary Table 1).
5. Discussion
In the current pilot investigation we tested the efficacy of using TMS neurofeedback to increase MEP amplitudes in sub-acute stroke survivors. This is the first application of the protocol described in Ruddy et al. (2018) in a clinical setting, with a population of in-patient first-time stroke survivors. Despite substantial upper limb disability and hemiparesis, patients learned over a 4 day period to use feedback of their MEP amplitudes to increase corticospinal excitability in the paretic muscles.
5.1. TMS NF to enhance functional upper limb recovery
While we did not observe clinically meaningful changes in scores on functional movement assessments over the 4 day period (which would require a change of 5.25 or more on the Fugl-Meyer assessment), the study constitutes the first step towards development of a more extended therapeutic version optimally geared towards boosting function. Previous work using brain-computer interfaces in a stroke rehabilitation context has suggested that a minimum of 18 sessions may be required in order to produce clinically significant improvements in function (Ang et al., 2015). Further testing of TMS NF over an extended recovery period will be required in order to provide recommendations on dosage and optimal spacing of sessions.
5.2. Methodological considerations
As stroke is a characteristically heterogenous condition, each patient presented with unique challenges precluding the application of a blanket ‘one size fits all’ approach to the protocol. Thus, we found it necessary to edit experimental parameters throughout in order to accommodate varying levels of disability and spasticity. One example was the ‘traffic lights’ display of background muscle EMG that preceded every trial. In the previous study with healthy adults (Ruddy et al., 2018) we demonstrated that it was possible to learn to modulate MEP amplitude using a mental-only strategy, strictly discouraging background muscle activation using the traffic lights display combined with a software-controlled monitor that stopped the trial at any moment if EMG in the target or related non-target muscles exceeded 7 µV. In the current study this was often an unrealistic target for the paretic muscles due to spasticity. Also, early in training patients showed attempts to use inappropriate compensatory strategies to ‘move’ the paretic limb to influence MEP amplitude (eg. shoulder or torso movements). Thus, we learned during the trial that it was more beneficial to position EMG electrodes on non-target muscles that we wished to discourage activity in, to present as the traffic lights display in order to enable patients to visualise how to relax those muscles prior to each trial. Thus, each trial would only commence when the non-involved muscles were relaxed. The target (paretic) muscles did not constitute this display, as EMG activity in those was to be encouraged rather than prevented. This leaves open the possibility that elevations in MEP amplitude that emerged throughout the training were due to the patient being able to generate small amounts of background muscle activity, ruling out a brain-only strategy. In a clinical context where the goal is to maximise output to the appropriate muscles (keeping other muscles relaxed), this only serves to boost the rehabilitation potential of the therapy.
In other cases, due to complete paralysis and low muscle tone the background rms EMG was much lower than 7 µV, and we found it necessary to reconsider what would be an acceptable MEP amplitude. In the context of this very low background activation clearly distinct MEPs with a peak-peak around 30 µV were visible, but traditionally would not be counted as this is below the usual 50 µV criterion. Therefore, a lesson learned from this pilot study is that strict parameters used in an experimental lab-based setting may not be appropriate for the clinical context, and a more patient specific tailored approach is required.
Another practical consideration arising from the study was the need for some patients to have gentle resistance applied against the limb in order to evoke MEPs. In the case of the one patient who trained with the wrist, MEPs were present during the familiarisation session and first training session only when the therapist applied gentle support to the back of the hand requiring the patient to attempt to extend the wrist to push against them. While this patient could not generate appropriate voluntary wrist extension, they were capable of this mild contraction against resistance, which enabled MEPs to be produced. On the first day of training MEPs were evoked in this way, but by the second day it was clear that the resistance was no longer necessary as MEPs were evident at rest. This patient also reported improved sensation in the paretic hand on the second day of training, something which was not formally quantified in the current study but should be for future investigations.
6. Conclusions
Here we have described the first clinical implementation of TMS NF in a population of sub-acute stroke patients, demonstrating that in the context of severe upper limb paralysis, patients are capable of using feedback to elevate corticospinal excitability in the affected muscles. As the focus of the trial was to assess whether the same acute training-related modulation of MEP amplitude observed in healthy controls was present in stroke patients over a short (4 day) intervention, substantial improvements in upper limb function were not anticipated and indeed not revealed. Also, it should be noted that there was no control group. However, the results of this pilot investigation have informed the design of a larger scale randomized controlled trial assessing functional recovery in a large sample of patients in the first six months following the injury. This will ultimately allow more sophisticated analyses to be conducted to establish whether there is a direct contingency between training-induced elevations in MEP amplitude and upper limb functional recovery. Further, this will allow investigation into how changes in MEP amplitude may (or may not) be a useful read-out of the state of brain-muscle pathways following stroke, and whether manipulation of this process using feedback and training can augment recovery outcomes.
7. Data availability
Data for this study are openly available on the Open Science Framework https://osf.io/f3jwq/?view_only=1bf2d44ebef94faf97c8f5b3e01ac19b.
Funding
KR would like to acknowledge funding from the Health Research Board, Ireland grant number HRB-EIA-2019-003 and Irish Research Council GOIPD/2017/798.
CRediT authorship contribution statement
W. Liang: Project administration, Data curation. Y. Xu: Project administration, Data curation. J. Schmidt: Project administration, Data curation. L. Zhang: Supervision, Resources, Project administration. K.L. Ruddy: Conceptualization, Methodology, Writing - original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors would like to thank Nicole Wenderoth, Daniel Woolley, Xue Zhang and Ernest Mihelj for helpful discussions that contributed to this finished work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2020.102465.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Ang K.K., Chua K.S.G., Phua K.S., Wang C., Chin Z.Y., Kuah C.W.K. A randomized controlled trial of EEG-based motor imagery brain- computer interface robotic rehabilitation for stroke. Clin. EEG Neurosci. 2015;46:310–320. doi: 10.1177/1550059414522229. [DOI] [PubMed] [Google Scholar]
- Boake C., Noser E., Ro T., Baraniuk S., Gaber M., Johnson R., Salmeron E.T., Tran T.M., Lai J.M., Taub E., Moye L.A., Grotta J.C., Levin H.S. Constraint-induced movement therapy during early stroke rehabilitation. Neurorehabil. Neural Repair. 2016;21(1):14–24. doi: 10.1177/1545968306291858. [DOI] [PubMed] [Google Scholar]
- Byblow W.D., Stinear C.M., Barber P.A., Petoe M.A., Ackerley S.J. Proportional recovery after stroke depends on corticomotor integrity. Ann. Neurol. 2015;78(6):848–859. doi: 10.1002/ana.24472. [DOI] [PubMed] [Google Scholar]
- Cicinelli P., Pasqualetti P., Zaccagnini M., Traversa R., Oliveri M., Rossini P.M. Interhemispheric Asymmetries of Motor Cortex Excitability in the Postacute Stroke Stage: A Paired-Pulse Transcranial Magnetic Stimulation Study. Stroke. J. Cerebr. Circul. 2003;34(11):2653–2658. doi: 10.1161/01.STR.0000092122.96722.72. [DOI] [PubMed] [Google Scholar]
- Coscia M., Wessel M.J., Chaudary U., Millian J.D.R., Micera S., Guggisberg A., Vuadens P., Donoghue J., Burbaumer N., Hummel F.C. Nuerotechnology-aided interventions for upper limb motor rehabilitation in severe chronic stroke. Brain. 2019;142:2182–2197. doi: 10.1093/brain/awz181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cretu A., Germann M., Ruddy K., Wenderoth N. Motor resonance in primary motor cortex reflects the integration of kinematic and contextual information in accordance to a Bayesian predictive coding framework. J. Neurophysiol. 2019;121(4):1451–1464. doi: 10.1152/jn.00655.2018. [DOI] [PubMed] [Google Scholar]
- Crow, J.L., Lincoln, N.N., Nouri, F.M., De. Weerdt, W. 2009. The effectiveness of EMG biofeedback in the treatment of arm function after stroke. Int. Disabil. Stud. 11(4), 155-160. [DOI] [PubMed]
- Dromerick A.W., Edwardson M.A., Edwards D.F., Giannetti M.L., Barth J., Brady K.P., Chan E., Tan M.T., Tamboli I., Chia R., Orquizza M., Padilla R.M., Cheema A.K., Mapstone M.E., Fiandaca M.S., Federoff H.J., Newport E.L. Critical Periods after stroke study: translating animal stroke recovery experiments into a clinical trial. Front. Hum. Neurosci. 2015;9(231) doi: 10.3389/fnhum.2015.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrsson H.H., Geyer S., Naito E. Imagery of Voluntary Movement of Fingers, Toes, and Tongue Activates Corresponding Body-Part-Specific Motor Representations. J. Neurophysiol. 2003;90(5):3304–3316. doi: 10.1152/jn.01113.2002. [DOI] [PubMed] [Google Scholar]
- Ertelt D., Small S., Solodkin A., Dettmers C., McNamara A., Binkofski F., Buccino G. Action observation has a positive impact on rehabilitation of motor deficits after stroke. NeuroImage. 2007;36:T164–T173. doi: 10.1016/j.neuroimage.2007.03.043. [DOI] [PubMed] [Google Scholar]
- Feigin V.L., Forouzanfar M.H., Krishnamurthi R., Mensah G.A., Connor M., Bennett D.A. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. 2010;2014(383):245–255. doi: 10.1016/s0140-6736(13)61953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanakawa T., Immisch I., Toma K., Dimyan M.A., van Gelderen P., Hallett M. Functional Properties of Brain Areas Associated With Motor Execution and Imagery. J. Neurophysiol. 2003;89(2):989–1002. doi: 10.1152/jn.00132.2002. [DOI] [PubMed] [Google Scholar]
- Jackson P.L., Lafleur M.F., Malouin F., Richards C., Doyon J. Potential role of mental practice using motor imagery in neurologic rehabilitation. Arch. Phys. Med. Rehabil. 2001;82(8):1133–1141. doi: 10.1053/apmr.2001.24286. [DOI] [PubMed] [Google Scholar]
- Johnson C.O., Nguyen M., Roth G.A., Nichols E., Alam T., Abate D., Adebayo O.M. Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):439–458. doi: 10.1016/S1474-4422(19)30034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffont I., Bakhti K., Coroian F., van Dokkum L., Mottet D., Schweighofer N. Innovative technologies applied to sensori- motor rehabilitation after stroke. Ann. Phys. Rehabil. Med. 2014;57:543–551. doi: 10.1016/j.rehab.2014.08.007. [DOI] [PubMed] [Google Scholar]
- Lawrence E.S., Coshall C., Dundas R., Stewart J., Rudd A.G., Howard R. Estimates of the prevalence of acute stroke impairments and disability in a multiethnic population. Stroke. 2001;32:1279–1284. doi: 10.1161/01.str.32.6.1279. [DOI] [PubMed] [Google Scholar]
- Lotze M., Montoya P., Erb M., Hülsmann E., Flor H., Klose U. Activation of Cortical and Cerebellar Motor Areas during Executed and Imagined Hand Movements: An fMRI Study. J. Cogn. Neurosci. 2006;11(5):491–501. doi: 10.1162/089892999563553. [DOI] [PubMed] [Google Scholar]
- Miltner W.H.R., Bauder H., Sommer M., Dettmers C., Taubb R. Effects of constraint induced movement therapy on patients with chronic motor defecits after stroke; A replication. Stroke. 1999;30(3):586–592. doi: 10.1161/01.str.30.3.586. [DOI] [PubMed] [Google Scholar]
- Murphy T.H., Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat. Rev. Neurosci. 2009;10:861–872. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- National Clinical Programme for Rehabilitation Medicine (NCPRM). 2017. Rehabilitation Medicine. Model of Care for the provision of specialist rehabilitation services in Ireland. http://hdl.handle.net/10147/622907.
- Ruddy, K., Balsters, J., Mantini, D., Liu, Q., Kassraian-Fard, P., Enz, N., Mihelj, E., Chander, B., Soekadar, S., Wenderoth, N. 2018. Neural activity related to volitional regulation of cortical excitability. eLife, 7:e40843 DOI: 10.7554/eLife.40843. [DOI] [PMC free article] [PubMed]
- Shah R., Wilkins E., Nichols M., Kelly P., El-Sadi F., Wright L., Townsend N. Epidemiology report: trends in sex-specific cerebrovascular disease mortality in Europe based on WHO mortality data. Eur. Heart J. 2019;40(9):755–764. doi: 10.1093/eurheartj/ehy378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soekadar, S.R., Witkowski, M., Gomez, C., Opisso, E., Medina, J., Cortese, M., Cempini, M., Carozza, M.C., Cohen, L.G., Birbaumer, N., Vitiello. 2016. Sci. Robot. 1. Eaag3296. [DOI] [PubMed]
- Stinear C.M., Byblow W.D., Steyvers M., Levin O., Swinnen S.P. Kinesthetic, but not visual, motor imagery modulates corticomotor excitability. Exp. Brain Res. 2005;168(1–2):157–164. doi: 10.1007/s00221-005-0078-y. [DOI] [PubMed] [Google Scholar]
- Stinear C., Barber A., Petoe M., Anwar S., Byblow W. The PREP algorithm predicts potential for upper limb recovery after stroke. Brain. 2012;136(8):2527–2535. doi: 10.1093/brain/aws146. [DOI] [PubMed] [Google Scholar]
- Stroke Association. 2017 State of the Nation; Stroke Statistics. Stroke.org.uk.
- Taub E., Crago J.E., Uswatte G. Constraint-induced movement therapy: a new approach to treatment in physical rehabilitation. Rehabil. Psychol. 1998;43:152–170. [Google Scholar]
- Taube W., Lorch M., Zeiter S., Keller M. Non-physical practice improves task performance in an unstable, perturbed environment: motor imagery and observational balance training. Front. Hum. Neurosci. 2014;8(522):1048. doi: 10.3389/fnhum.2014.00972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube, W., Mouthon, M., Leukel, C., Cortex, H. H., 2015. Brain activity during observation and motor imagery of different balance tasks: an fMRI study. Cortex 64, 102-114.http://doi.org/10.1016/j.cortex.2014.09.022. [DOI] [PubMed]
- Thickbroom G.W., Byrnes M.L., Archer S.A., Mastaglia F.L. Motor outcome after subcortical stroke correlates with the degree of cortical reorganization. Clin. Neurophysiol. 2004;115(9):2144–2150. doi: 10.1016/j.clinph.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Ushiba J., Soekadar S.R. Brain-machine interfaces for rehabilitation of poststroke hemiplegia. Prog. Brain Res. 2016;228:163–183. doi: 10.1016/bs.pbr.2016.04.020. [DOI] [PubMed] [Google Scholar]
- Van Dokkum L.E.H., Ward T., Laffont I. Brain computer interfaces for neurorehabilitation—its current status as a rehabilitation strategy post-stroke. Ann. Phys. Rehabil. Med. 2015;58:3–8. doi: 10.1016/j.rehab.2014.09.016. [DOI] [PubMed] [Google Scholar]
- Wade D.T. Measurement in neurological rehabiltation. Curr. Opin. Neurol. Neurosurg. 1992;5:682–686. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data for this study are openly available on the Open Science Framework https://osf.io/f3jwq/?view_only=1bf2d44ebef94faf97c8f5b3e01ac19b.