Abstract
Recent unprecedented increases in mortality and morbidity during midlife are often ascribed to rising despair in the US population. An alternative and less often examined explanation is that these trends reflect, at least in part, the lagged effects of the obesity epidemic. Adults in midlife today are more likely to live with obesity and have a greater cumulative exposure to excess adiposity during their lifetime than any previous generation. Prior work has demonstrated a link between obesity and mortality risk at midlife, but the mechanisms remain unclear. Pain may represent one important pathway linking obesity to mortality trends. Pain is a debilitating condition that has increased significantly over recent decades and is associated with both morbidity and mortality, including suicide and opioid-related mortality. Evidence suggests obesity and pain may be linked, but there is little evidence of an association at the population level. In this paper, we examine to what extent increases in overweight and obesity explain the rising trends in chronic pain observed among middle-aged adults in the US from 1992 to 2016. We assess trends in both mild/moderate nonlimiting pain and severe and/or limiting pain. In doing so, we draw attention to one mechanism through which overweight/obesity may have contributed to recent population health trends. Our analysis found that increases in BMI from 1992 to 2016 may account for up to 20% of the upward trend in mild/moderate nonlimiting pain and 32% of the trend in severe and/or limiting pain for women, and 10% and 19% of the trends respectively for men.
Keywords: Pain, Obesity, Trends, Activity limitations
Highlights
-
•
We study the contribution of overweight and obesity to recent trends in pain among middle-aged adults.
-
•
Overweight and obesity accounted for 32.1% of increases in severe or limiting pain among women and 19.0% among men.
-
•
Overweight and obesity explained a larger share of the increase in severe than mild/moderate pain.
-
•
The study highlights the importance of obesity prevention to decrease the prevalence of pain.
Introduction
There has been considerable attention over recent years to the changing health of US adults during midlife (Case & Deaton, 2015; Woolf et al., 2018). Social scientists have observed several remarkable and potentially interrelated trends, including declines in self-reported mental and physical health along with increases in pain, morbidity, suicide, poisonings, and all-cause mortality (Case & Deaton, 2017; Goldman et al., 2018; Nahin et al., 2019; Woolf & Schoomaker, 2019). While these trends have been consistently documented, researchers remain divided about the underlying factors driving them.
In the context of the opioid epidemic and the discussion of its role in rising morbidity and mortality, scholarship has characterized both the supply-side factors that increased the availability of opioids to middle-aged adults and the demand-side factors that affected this population's economic, social, and health-related vulnerability (Clark & Schumacher, 2017; Dasgupta et al., 2018; Van Zee, 2009). Much of the debate over the role of demand-side factors has focused on a controversial “deaths of despair” argument put forward by Case and Deaton, which suggests that stagnating wages and declines in economic opportunity have driven changes in health during midlife (Case & Deaton, 2017). While the “deaths of despair” narrative has received significant popular and scholarly attention (Masters et al., 2017; Ruhm, 2018; Siddiqi et al., 2019; Soelberg et al., 2017; Venkataramani et al., 2019), Case and Deaton have also suggested that several other demand-side factors could be implicated in these trends, such as increases in obesity (Case & Deaton, 2017), which have received less attention to-date.
In this paper, we posit that rising population-level trends in obesity are a critical and under-appreciated factor underlying changes in health occurring among adults in the US during midlife. While obesity has previously been linked to a slowdown in the rate of mortality improvement (Preston et al., 2018), the pathways through which obesity operates remain unclear.
One potential pathway that may link obesity to deaths of despair and the broader changes in Americans health during midlife is chronic pain. Obesity is strongly associated with chronic pain, and chronic pain is in turn associated with functional limitations, disability, and many psychological conditions such as depression and anxiety (Gureje et al., 1998) and has been suggested as an important contributor to population-level trends in quality of life, suicide, and opioid-related mortality (Olfson et al., 2017; Petrosky et al., 2018; Stokes et al., 2019). The prevalence of chronic pain has grown steeply over recent decades (Case & Deaton, 2017; Freburger et al., 2009; Grol-Prokopczyk, 2017; Zimmer & Zajacova, 2018), imposing a substantial population health burden in the US. (Institute of Medicine (US) Committee on Advancing Pain Research et al., 2011) The total annual economic costs of chronic pain exceed 600 billion dollars in health care expenses and lost worker productivity (Gaskin & Richard, 2012).
In 2011, the Institute of Medicine (IOM) published a blueprint calling for coordinated action among researchers, policymakers, and health professionals to address the increasing levels of chronic pain in the US. (Institute of Medicine (US) Committee on Advancing Pain Research et al., 2011) The report recommended an increased focus on chronic pain prevention, in particular on stopping progression of acute pain to chronic pain and chronic pain to pain that poses activity limitations. In subsequent years, a comprehensive U.S. National Pain Strategy was developed (Interagency Pain Research Coordinating Committee, 2019). According to this strategy, identifying modifiable risk factors for chronic pain and pain that causes activity limitations remains a national priority for population health research.
Scholars have speculated that obesity may be an underlying factor in rising pain although studies have not systematically examined its role (Zimmer & Zajacova, 2018; Institute of Medicine (US) Committee on Advancing Pain Research et al., 2011). Preliminary evidence suggesting the importance of rising obesity in the increasing prevalence of chronic pain over time is substantial. First, obesity rates have increased over the past several decades, rising from 23% of the adult US population in the early 1990s to 40% in 2015–2016 (Fryar et al., 1960; Hales et al., 2018). Recent birth cohorts are also becoming obese younger and thus are having a greater lifetime exposure to adiposity than prior generations, which could have multiple delayed public health consequences (Lee et al., 2010). Second, obesity is closely associated with numerous painful conditions including osteoarthritis (Grotle et al., 2008), lower back pain (Shiri et al., 2010), and diabetic neuropathy (Shiri et al., 2010). Such associations may relate to the biomechanical effects of excess weight gain as well as obesity's role in creating a pro-inflammatory state (Okifuji & Hare, 2015). A causal association between obesity and chronic pain is also supported by clinical trials and observational studies showing that weight loss can lead to substantial reductions in pain (Christensen et al., 2007; Khoueir et al., 2009; Larsson, 2004; Lim et al., 2010).
Thus, in the present study, we examine the role of increasing obesity in explaining rising trends in chronic pain observed among middle-aged adults in the US from 1992 to 2016. We assess trends in both mild/moderate nonlimiting pain and severe and/or limiting pain. In doing so, we draw attention to one pathway through which obesity may have contributed to the recent population health trends of adults during midlife.
Materials and methods
Data source
We used data from 13 consecutive biennial waves of the Health and Retirement Study (HRS) from 1992 to 2016. The HRS is a nationally representative longitudinal survey of non-institutionalized adults over the age of 50 years in the US. The survey was administered every 2 years with periodic recruitment to refresh samples in 1998, 2004, 2010, and 2016 (Supplementary Fig. 1). At each wave, we restricted our analysis to adults aged 55 to 61. We chose this specific group of middle-aged adults because HRS has followed this age group since 1992, allowing us to examine the rise of pain over a longer period than for other groups. Since item nonresponse was low (0.1%–1.6%), we excluded those with missing data on pain, BMI, or covariates. Participants with a BMI less than 20 kg per meters squared (kg/m2) were excluded due to potential confounding by diagnosed or preclinical diseases, and individuals with BMI over 70 were also excluded to guard against extreme measurement error. Lastly, adults who reported ever receiving a cancer diagnosis were excluded (Supplementary Fig. 2). The exclusion jointly removed 7595 observations from 60,879 adults aged 55 to 61, yielding the final analytic sample with 53,284 observations (21,356 unique respondents, with each respondent observed for an average of 2.5 waves).
Study design
To estimate and model time trends, we treated sample members in each wave as if they were representative cross-sections of the population. While the validity of this approach could be compromised by selective panel attrition and/or panel conditioning, a previous study (Grol-Prokopczyk, 2017) using the same data found little evidence of pain-related survey attrition. We also evaluated cohort-period curve (Supplementary Fig. 3) for panel conditioning, and found no evidence of such bias. The prevalence of chronic pain in older cohorts, which had undergone multiple waves of survey, was not systematically higher than in newer cohorts.
Exposure and outcome measures
Our exposure variable was time in years since 1992. To be consistent with a prior study using HRS data(Zimmer and Zajacova, 2018), we created a 3-level outcome variable for pain that also considered activity limitations. Participants were asked, “Are you often troubled with pain?” Those who answered yes were asked, “How bad is the pain most of the time: mild, moderate, or severe?” and “Does the pain make it difficult for you to do your usual activities such as household chores or work?” At each wave, participants were then classified as no pain (not troubled by pain), mild/moderate nonlimiting pain (often troubled by pain that was mild or moderate and did not limit respondents’ ability to perform usual activities), and severe and/or limiting pain (often troubled by pain that was severe and/or made it difficult to perform activities).
Covariates
BMI was calculated from self-reported weight and height. We transformed BMI to reflect BMI above 25 kg/m2 by subtracting 25 kg/m2 (the beginning of the overweight range as defined by the World Health Organization (WHO, 2019)), with values between 20 and 25 kg/m2 assigned to zero (Preston et al., 2018). Other covariates that might explain the rising trend in chronic pain included the following demographic characteristics: age (Dahlhamer et al., 2018), sex (Fillingim et al., 2009) (male, female), race/ethnicity (Janevic et al., 2017) (non-Hispanic white, non-Hispanic black, Hispanic, other), and nativity (Janevic et al., 2017) (born in US, born outside US). We also measured socioeconomic status (Grol-Prokopczyk, 2017) according to educational attainment (some college or lower, BA or more) and smoking status (Goesling et al., 2015) (never, former, current). We did not examine depression, mood disorders, arthritis, or other physical conditions as covariates because we hypothesized these conditions may develop on the causal pathway between overweight/obesity and chronic pain (Jantaratnotai et al., 2017; Zis et al., 2147).
Statistical analyses
First, we examined the association between years since 1992 and pain, using multinomial logit models appropriate for our 3-level operationalization of pain (none, mild/moderate nonlimiting, and severe and/or limiting pain). The pain trends were first estimated by controlling only for age to provide a crude estimate of trends from 1992 to 2016. Next, we introduced race/ethnicity and nativity to the base model. In subsequent models, we added education and smoking individually. The final model included all covariates.
Since we were particularly interested in how the trend coefficient changed with the addition of BMI, we assessed each model with and without BMI included. The degree of attenuation in the coefficient for years since 1992 indicates the extent to which the trend in BMI can explain the pain trend. Since our models were non-linear, the Karlson-Holm-Breen (KHB) method was applied to allow accurate decomposition of total effects into direct and indirect effects (Kohler et al., 2011).
We stratified all models by sex. Sex differences in pain and obesity have been routinely observed (Mogil, 2012), and thus we hypothesized that obesity may influence pain trends differently by sex (Flegal, Carroll, Ogden, & Curtin, 2010; Kanter & Caballero, 2012; Zimmer & Zajacova, 2018). Finally, in an additional set of models, we examined if the impact of overweight/obesity on pain trends was consistent across sociodemographic characteristics and smoking status. We again estimated fully adjusted models except that we omitted one of the three covariates (race/ethnicity, education, and smoking status) and instead stratified by this covariate.
Stata statistical software version 15 (StataCorp) was used for all analyses. HRS sampling weights were applied to account for oversampling of demographic groups and attrition, enabling generalizability to the US population aged 55 to 61. Standard errors were also adjusted for intra-individual clustering. As the analyses relied on publicly available, de-identified data, Institutional Review Board approval was not required.
Sensitivity checks
Extensive sensitivity analyses were conducted to verify the robustness of our findings to alternative specifications and procedures. We first tested for non-linear time trends by including higher-order terms for years since 1992 in the full model. To relax the assumption that the impact of BMI on pain was constant over time, we included an interaction term between BMI and time since 1992.
We also examined the sensitivity of results through a number of alternative specifications of BMI. First, we tested a quadratic term for BMI. Second, instead of assigning zero to BMI values in the normal range, we used the original BMI values equal to 20 or higher and its quadratic term. Third, we treated BMI as a categorical variable using the WHO recommended BMI categories of 18.5–24.9 (normal), 25–29.9 (overweight), 30–34.9 (obese I), 35–39.9 (obese II), and 40 (obese III).
Furthermore, a concern with our cross-sectional study design was that it could overestimate the effects of overweight/obesity on pain because of reverse causality. To assess this potential bias, we first compared results by re-estimating models replacing contemporary BMI measures with BMI lagged 2-years and lagged 4-years. Second, we determined whether baseline pain predicted subsequent BMI trajectories in a longitudinal framework. Third, we examined whether baseline BMI predicted incident pain status (i.e. transitioning from pain-free status at baseline to pain). These latter two investigations are described in the Supplementary Materials.
Results
Between 1992 and 2016, the proportion of US adults aged 55 to 61 with severe and/or limiting pain increased substantially, from 17.6% to 28.3% for females and 13.5% to 22.4% for males. Over the same period, the proportion of middle-aged adults with obesity/overweight increased from 61.4% to 74.9% for women, and 70.9% to 83.2% for men. (Table 1).
Table 1.
Descriptive statistics of the target population of adults aged 55–61 in HRS, 1992 and 2016, N = 53,284a.
| Characteristics | Female |
Male |
||||||
|---|---|---|---|---|---|---|---|---|
| 1992 | 2016 | 1992 | 2016 | |||||
| Chronic noncancer pain, % | ||||||||
| No pain | 74.0 | 58.3 | 79.2 | 65.2 | ||||
| Mild/moderate nonlimiting pain | 8.4 | 13.4 | 7.3 | 12.4 | ||||
| Severe and/or limiting pain | 17.6 | 28.3 | 13.5 | 22.4 | ||||
| Overweight/obesity | ||||||||
| Obesity/overweight, % | 61.4 | 74.9 | 70.9 | 83.2 | ||||
| BMI units above 25 kg/m2, mean (sd) | 3.1 | (4.4) | 5.7 | (6.3) | 2.8 | (3.4) | 4.8 | (5.1) |
| Age, mean (sd) | 57.8 | (1.9) | 57.8 | (2.0) | 57.8 | (2.0) | 57.9 | (1.9) |
| Race/ethnicity, % | ||||||||
| Non-Hispanic white | 79.5 | 70.1 | 82.7 | 70.2 | ||||
| Non-Hispanic black | 11.5 | 13.2 | 9.6 | 10.5 | ||||
| Hispanic | 6.6 | 10.8 | 5.7 | 11.4 | ||||
| Other | 2.3 | 5.8 | 2.0 | 7.9 | ||||
| Nativity | ||||||||
| Born in the US, % | 89.7 | 88.3 | 91.2 | 86.4 | ||||
| Education, % | ||||||||
| Some college or lower | 86.2 | 66.2 | 77.7 | 69.6 | ||||
| BA or more | 13.8 | 33.8 | 22.3 | 30.4 | ||||
| Smoking status, % | ||||||||
| Never | 46.3 | 46.5 | 26.3 | 46.0 | ||||
| Former | 30.8 | 36.9 | 48.5 | 34.7 | ||||
| Current | 22.9 | 16.7 | 25.2 | 19.3 | ||||
| N | 2653 | 2394 | 2564 | 2122 | ||||
Weighted percent and mean (sd) and unweighted sample size were presented.
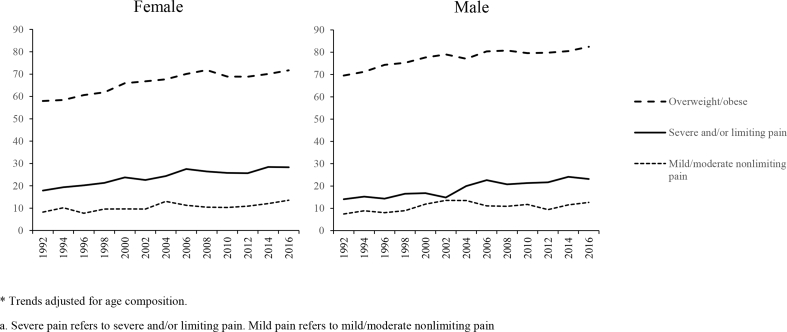
Fig. 1 plots the prevalence of overweight/obesity and chronic pain from 1992 to 2016 stratified by sex. Increases in overweight/obesity, mild/moderate nonlimiting pain, and severe and/or limiting pain were all observed. Severe and/or limiting pain was more prevalent than mild/moderate nonlimiting pain and rose more rapidly.
Fig. 1.
Trends in the prevalence of overweight/obesity and pain among US adult aged 55–61: HRS 1992 to 2016 *
* Trends adjusted for age composition.
Severe pain refers to severe and/or limiting pain. Mild pain refers to mild/moderate nonlimiting pain.
Table 2 presents nested multinomial logit models examining the association of years since 1992 with the prevalence of mild/moderate nonlimiting pain and severe and/or limiting pain versus no pain. For each sex, we estimated 5 models. In each case, we first fit the model without BMI (Col. a) and then added BMI (Col. b). Controlling only for age, the log-odds of reporting mild and severe and/or limiting pain among women increased by 0.024 and 0.027 units per year (Model 1a), respectively, while the log-odds of reporting mild and severe and/or limiting pain among men increased by 0.018 and 0.029 units per year, respectively. In the fully adjusted model, after decomposing total effects by the KHB method, BMI was estimated to account for 20.3% (95% CI 15.8%, 24.9%) of the trend in mild/moderate nonlimiting pain and 32.1% (95% CI 28.2%, 36.0%) of the trend in severe and/or limiting pain for women, and 10.4% (95% CI 5.6%, 15.1%) of the trend in mild/moderate nonlimiting pain and 19.0% (95% CI 15.8%, 22.1%) of the trend in severe and/or limiting pain for men (Model 5). Full models with all coefficients can be found in Supplementary Tables 1 and 2
Table 2.
Multinomial logit model predicting chronic pain with and without control for BMI in HRS, 1992–2016, N = 53,284.
| Mild/moderate nonlimiting pain |
Severe and/or limiting pain |
|||||
|---|---|---|---|---|---|---|
| Modelsa | Without BMIb (a) | With BMIb (b) | % Explained by BMIc [95% CI] | Without BMIb (a) | With BMIb (b) | % Explained by BMIc [95% CI] |
| Panel A: Female | ||||||
| 1) Age | 0.024 | 0.020 | 20.1 [15.6–24.6] | 0.027 | 0.017 | 38.4 [33.7–43.2] |
| 2) Age + Race + Nativity | 0.023 | 0.019 | 20.2 [15.7–24.7] | 0.025 | 0.016 | 38.6 [33.6–43.6] |
| 3) Age + Race + Nativity + Education | 0.025 | 0.021 | 20.6 [16.1–25.2] | 0.033 | 0.023 | 31.8 [27.9–35.7] |
| 4) Age + Race + Nativity + Smoking | 0.023 | 0.020 | 19.6 [15.2–24.0] | 0.027 | 0.018 | 36.1 [31.4–40.9] |
| 5) Age + Race + Nativity + Education + Smokingd | 0.025 | 0.021 | 20.3 [15.8–24.9] | 0.034 | 0.023 | 32.1 [28.2–36.0] |
| Panel B: Male | ||||||
| 1) Age | 0.018 | 0.016 | 13.9 [8.4–19.5] | 0.029 | 0.022 | 23.1 [19.3–26.9] |
| 2) Age + Race + Nativity | 0.019 | 0.017 | 12.9 [7.6–18.2] | 0.028 | 0.021 | 23.5 [19.6–27.5] |
| 3) Age + Race + Nativity + Education | 0.021 | 0.019 | 11.1 [6.1–16.0] | 0.032 | 0.026 | 19.6 [16.2–23.0] |
| 4) Age + Race + Nativity + Smoking | 0.021 | 0.019 | 12.0 [7.1–16.9] | 0.034 | 0.027 | 20.9 [17.5–24.3] |
| 5) Age + Race + Nativity + Education + Smoking | 0.022 | 0.020 | 10.4 [5.6–16.1] | 0.036 | 0.029 | 19.0 [15.8–22.1] |
Abbreviations: BMI, body mass index; CI, confidence interval.
Column (a) and (b) show multinomial logit regression coefficients for a linear time trend. See Supplementary Tables 1 and 2 for the complete regression results.
BMI was transformed to reflect the number of BMI units above 25 kg/m2, with values between 20 and 25 assigned to zero.
% explained was calculated by KHB method.
After adjusting for age, race, nativity, education, and smoking, the coefficient for the mild/moderate nonlimiting pain trend over time was 0.025 among females (i.e. 2.5% annual increase in the odds of reporting mild/moderate nonlimiting pain). After additionally adjustment for BMI, the coefficient dropped to 0.021. Using the KHB method, we then calculated that 20.3% (15.8–24.9%) of the female mild/moderate nonlimiting pain trend was explained by BMI.
To assess heterogeneity in the role of BMI in rising pain trends, we stratified the regressions by sociodemographic characteristics and smoking status (Table 3). Chronic pain increased in all subgroups. However, the rates of increase varied substantially. While a formal test for differences in the context of the KHB decomposition was not possible, the method does provide estimates with 95% confidence intervals that allow informal comparisons of group differences. Overall, BMI explained a larger proportion of trends in severe and/or limiting pain relative to mild/moderate nonlimiting pain and trends among women than among men. There were no consistent differences in contributions of BMI to pain trends across race/ethnicity groups or by educational attainment, except for severe and/or limiting pain among women. BMI accounted for 34.8% (95% CI 30.2%, 39.3%) of rising severe and/or limiting pain among women with less than a BA education but only 22.6% (95% CI 15.4%, 29.7%) among those with a BA or higher education. Differences by smoking status for severe and/or limiting pain were more substantial. BMI explained 20.7% (95% CI 15.8%, 25.7%) of increases in severe and/or limiting pain among male former smokers and 34.7% (95% CI 27.5%, 41.8%) for female former smokers in comparison to 7.4% (95% CI 4.2%, 10.6%) and 19.7% (95% CI 14.5%, 24.9%) for current smokers.
Table 3.
Multinomial logit model predicting chronic noncancer pain with and without control for BMI in HRS, 1992–2016, stratified by characteristics, N = 53,284.
| Mild/moderate nonlimiting pain |
Severe and/or limiting pain |
|||||
|---|---|---|---|---|---|---|
| Modela | Without BMIb (a) | With BMIb (b) | % Explained by BMIc [95% CI] | Without BMIb (a) | With BMIb (b) | % Explained by BMIc [95% CI] |
| Panel A: Female | ||||||
| By race/ethnicity | ||||||
| White | 0.023 | 0.019 | 22.6 [16.2–29.1] | 0.037 | 0.025 | 32.2 [27.6–36.7] |
| Black | 0.046 | 0.039 | 16.3 [10.3–22.2] | 0.031 | 0.022 | 34.0 [25.4–42.7] |
| Hispanic | 0.018 | 0.016 | 15.7 [3.2–28.1] | 0.013 | 0.007 | 44.7 [25.7–63.8] |
| By education | ||||||
| High school or less | 0.023 | 0.019 | 21.6 [15.9–27.3] | 0.032 | 0.022 | 34.8 [30.2–39.3] |
| BA or above | 0.033 | 0.029 | 15.8 [8.9–22.7] | 0.040 | 0.031 | 22.6 [15.4–29.7] |
| By smoking status | ||||||
| Never smokerd | 0.025 | 0.021 | 19.0 [12.8–25.3] | 0.023 | 0.013 | 45.2 [37.0–53.5] |
| Former | 0.021 | 0.016 | 26.6 [16.8–36.3] | 0.034 | 0.023 | 34.7 [27.5–41.8] |
| Current |
0.034 |
0.029 |
17.7 [8.1–27.4] |
0.051 |
0.042 |
19.7 [14.5–24.9] |
| Panel B: Male | ||||||
| By race/ethnicity | ||||||
| White | 0.019 | 0.017 | 12.5 [5.6–15.1] | 0.037 | 0.031 | 18.8 [15.1–22.5] |
| Black | 0.047 | 0.046 | 2.9 [-2.9–8.8] | 0.033 | 0.029 | 13.9 [7.3–20.5] |
| Hispanic | 0.023 | 0.021 | – | 0.037 | 0.030 | 17.9 [5.7–30.2] |
| By education | ||||||
| High school or less | 0.021 | 0.018 | 14.0 [8.1–19.8] | 0.035 | 0.028 | 19.5 [15.8–23.2] |
| BA or above | 0.026 | 0.025 | 1.4 [-6.9-9.6] | 0.042 | 0.034 | 17.5 [11.4–23.6] |
| By smoking status | ||||||
| Never smoker | 0.019 | 0.017 | 10.5 [1.0–20.0] | 0.011 | 0.003 | – |
| Former | 0.024 | 0.022 | 11.5 [4.3–18.7] | 0.040 | 0.032 | 20.7 [15.8–25.7] |
| Current | 0.022 | 0.021 | 9.2 [0.8–17.6] | 0.054 | 0.050 | 7.4 [4.2–10.6] |
-- The % explained was not estimated because the 95% CI for the underlying pain trend was overlapping with 0.
Column (a) and (b) show multinomial logit regression coefficients for a linear time trend. Models controlled for age, sex, race/ethnicity, nativity, education and smoking status.
BMI was transformed to reflect the number of BMI units above 25 kg/m2, with values between 20 and 25 assigned to zero.
% explained was calculated by KHB method.
Sample interpretation: “After adjusting for age, race, nativity, and education, the coefficient for the mild/moderate nonlimiting pain trend over time was 0.025 among female never smokers. After adjusting for BMI, the coefficient dropped to 0.021. Using the KHB method, we then calculated that 19.0% (12.8–25.3%) of the mild/moderate nonlimiting pain trend among female never smokers was explained by individuals having a BMI over 25 kg/m2
Auxiliary analyses were conducted to examine to what extent estimates were potentially affected by reverse causality. When contemporary BMI measures were replaced with BMI lagged 2-year and lagged 4-year measures, no meaningful differences were observed (Supplementary Table 3). While pain status at baseline was associated with a higher initial BMI, it was not associated with a faster rate of increase in BMI. In fact, severe and/or limiting pain at baseline was associated with a slower increase in BMI compared to those who have no pain at baseline (Supplementary Table 4). Supplementary Tables 5 and 6 present associations between baseline BMI status and subsequent incident pain for those who were initially pain-free. For females, each unit increase in BMI above 25 was associated with a 0.07 and 0.09 unit increase in the odds of reporting any pain and severe and/or limiting pain, respectively. The corresponding numbers were 0.03 and 0.04 for men. Taken together, these results did not support a systematic overestimation of the association between overweight/obesity and chronic pain owing to reverse causality.
We tested for non-linear time trends by including higher-order terms for time since 1992 in the full model. Other than a negative second-degree term for time in the model for men on the outcome of mild/moderate pain (indicating a slight deceleration of the increase in mild/moderate pain over time), no other deviation from linearity was detected. To relax the assumption that the impact of BMI on chronic pain was constant over time, we included an interaction term between BMI and year post-baseline. We found an interaction only for mild/moderate pain among males, which did not alter results materially. In addition, the alternative BMI measures induced little change in the estimated pain trend or reduction in the period trend when accounting for BMI.
Discussion
The rapidly rising prevalence of chronic pain (Case & Deaton, 2017; Freburger et al., 2009; Grol-Prokopczyk, 2017; Nahin et al., 2019; Zimmer & Zajacova, 2018) has contributed substantially to morbidity and mortality in midlife across the US (Case & Deaton, 2017). It has been posited that obesity is an underlying driver of such trends. In this study, we found that rising trends in overweight and obesity were associated with population-level increases in pain from 1992 to 2016, especially increases in severe and/or limiting pain.
Our analysis showed that increases in BMI from 1992 to 2016 may account for up to 20% of the upward trend in mild/moderate nonlimiting pain and 32% of the trend in severe and/or limiting pain for women, and 10% and 19% of the trends respectively for men. The increase in BMI contributed more to trends in severe and/or limiting pain than mild/moderate nonlimiting pain and was a more important factor among women than men. A broad set of biological and psychosocial explanations may account for the sex differences observed in associations of BMI and pain (Fillingim, 2000; Fillingim et al., 2009).
Coupled with existing evidence showing that weight loss can lead to substantial reductions in pain (Larsson, 2004; Christensen et al., 2007), our findings lend support to a causal interpretation of the relationship between BMI and pain. Though much remains to be clarified, an increasing body of evidence illuminates the mechanisms underlying the obesity-pain link such as increased loading on joints and spine (Okifuji & Hare, 2015). Higher levels of BMI are also associated with greater wear-and-tear on knee cartilage (Ding et al., 2005), greater disk compression force while lifting (Singh et al., 2015), higher risk for degenerative disk disorders (Samartzis et al., 2012), and altered body mechanics and postures (Fabris de Souza et al., 2005). In particular, overloading on lower back, hip, and knee joints may contribute to structural damage and lead to osteroarthritis (McVinnie, 2013).
In addition to the mechanical and structural changes brought on by obesity, studies have identified biochemical mediators as well. As an endocrine organ, adipose tissue produces and releases proinflammatory cytokines and adipokins, which may increase pain (Ronti et al., 2006). Obesity is also associated with leptin, a hormone which may facilitate inflammation and joint damage (Considine, 2005; Vuolteenaho et al., 2014). Depression also acts as an important link between obesity and pain (McVinnie, 2013). Depression and obesity have a bidirectional relationship, meaning that the presence of obesity increases the risk of depression and vice versa (Jantaratnotai et al., 2017). Seretonin depletion, which is involved in the pathophysiology of depression, is known to amplify pain symptoms (Shelton & Miller, 2010). Depression can also contribute to disrupted sleep and activity limitations, which increase pain severity (Harrison et al., 2016). Thus, depression may serve as an additional mechanism through which obesity increases the amount of pain in the US population.
Given the substantial economic and health-related burden of chronic pain, our findings highlight the importance of primary and secondary obesity prevention. Obesity has been increasing in the United States and globally over the past several decades (Finucane et al., 2011; Flegal et al., 2010), driven largely by environmental effects that undermine individual capacity to regulate personal diet and physical activity (Gortmaker et al., 2011). There is emerging consensus on core policy actions to reduce obesity at the population level by reversing systemic and environmental drivers of the obesogenic environment (Hawkes et al., 2013). Examples include nutrition labeling, food taxes or targeted subsidies, restriction of food advertising, regulation of food nutritional quality and availability in schools, and public awareness campaigns (Roberto et al., 2015). An integrated approach that involves regulatory actions from government, increased efforts from industry, health professionals, civil society and individuals is necessary to address the epidemic of obesity effectively, which will in turn reduce the burden of chronic pain in the US population (Gortmaker et al., 2011; Roberto et al., 2015). While obesity appears to be an important factor in the rising prevalence of pain, other factors such as changing social acceptance of pain as a symptom and increases in depression, mood disorders, psychosocial stress, trauma, occupational injury, smoking, and physical inactivity during youth and adulthood may also each play important roles in pain trends and warrant further research (Linton & Shaw, 2011).
Our study had several limitations. First, the estimated role of BMI in rising chronic pain was predicated on a causal relation between BMI and chronic pain. While we found little evidence that the association between overweight/obesity and pain was biased by reverse causality, the relationship between BMI and chronic pain was still subject to the influence of confounding factors such as genes, childhood and early adulthood socioeconomic environment, and behavioral choices at younger age (Hu, 2008). The absence of unobserved and unmeasured factors from our models is likely to bias the estimates of association upwards. On the other hand, residual confounding by smoking could have led us to under-estimate the population-level association of BMI and pain trends. Consistent with this possibility, our findings showed that BMI had less explanatory power among current smokers than among other smoking groups. Second, BMI values were based on self-reported height and weight, which tend to overestimate measured BMI values at the low end of the BMI scale and underestimate BMI values at the high end (Stommel & Schoenborn, 2009). However, correlations between self-reported and measured BMI values are high and similarly predictive of health risks (Stommel & Schoenborn, 2009). Third, the HRS does not probe the duration of chronic pain and therefore the measures used in the present study may not be comparable to other studies, which generally define chronic pain as pain lasting for 3 or 6 months. Nevertheless, given that the measures are consistent across HRS waves, analyses of trends are unlikely to be affected. HRS also does not link chronic pain to particular conditions or site-specific types of chronic pain. Finally, the present study was restricted to US adults aged 55–61 years. Future studies examining the role of BMI in rising pain trend at other ages are needed.
In conclusion, the present study indicated a strong association between the rising prevalence of overweight/obesity and chronic pain among middle-aged adults in the US from 1992 to 2016. The magnitude of the association was stronger for severe and/or limiting pain than for mild/moderate nonlimiting pain and stronger in women as compared to men. Our findings proved consistent across alternative specifications of obesity and did not appear to be a consequence of reverse causality between obesity and pain. The results of the present study highlight the need for integrating obesity prevention and treatment into public health efforts to reduce the burden of chronic pain in the US population. They also call attention to the complex and under-appreciated role of obesity in driving population health trends among US adults during midlife. Further research is needed to examine obesity's contribution to other underlying factors that are commonly associated with deaths of despair and other recent trends in morbidity and mortality.
Ethics statement
This secondary data analysis relied on publicly available, de-identified data from the Health and Retirement Study. Thus, there was no need to obtain ethics approval. To download the data, see instructions in https://hrs.isr.umich.edu/data-products. The computer code used for these analyses is available from the authors upon request.
CRediT authorship contribution statement
Andrew C. Stokes: Conceptualization, Methodology, Software, Formal analysis, Writing - original draft, Writing - review & editing, Supervision. Wubin Xie: Conceptualization, Methodology, Software, Formal analysis, Data curation, Writing - original draft, Writing - review & editing. Dielle J. Lundberg: Conceptualization, Writing - original draft, Writing - review & editing. Katherine Hempstead: Conceptualization, Writing - original draft, Writing - review & editing. Anna Zajacova: Methodology, Writing - original draft, Writing - review & editing. Zachary Zimmer: Writing - original draft, Writing - review & editing. Dana A. Glei: Writing - original draft, Writing - review & editing. Ellen Meara: Writing - review & editing. Samuel H. Preston: Conceptualization, Methodology, Writing - original draft, Writing - review & editing.
Disclosure and acknowledgments
Research reported in this publication was supported by the Robert Wood Johnson Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funder. Publication of the study results was not contingent on the sponsor's approval of the manuscript. A. Stokes has reported receiving research funding from Ethcon Inc outside of the submitted work. The other authors declare no conflicts of interest.
The authors would like to thank Dr. Maxine Weinstein for her contributions in reviewing the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ssmph.2020.100644.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Case A., Deaton A. Rising morbidity and mortality in midlife among white non-Hispanic Americans in the 21st century. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(49):15078. doi: 10.1073/pnas.1518393112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case A., Deaton A. Mortality and morbidity in the 21st century. Brookings Papers on Economic Activity. 2017;2017:397–476. doi: 10.1353/eca.2017.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen R., Bartels E.M., Astrup A., Bliddal H. Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: A systematic review and meta-analysis. Annals of the Rheumatic Diseases. 2007;66(4):433–439. doi: 10.1136/ard.2006.065904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D.J., Schumacher M.A. America's opioid epidemic: Supply and demand considerations. Anesthesia & Analgesia. 2017;125(5) doi: 10.1213/ANE.0000000000002388. https://journals.lww.com/anesthesia-analgesia/Fulltext/2017/11000/America_s_Opioid_Epidemic__Supply_and_Demand.35.aspx [DOI] [PubMed] [Google Scholar]
- Considine R.V. Human leptin: An adipocyte hormone with weight-regulatory and endocrine functions. Seminars in Vascular Medicine. 2005;5(1):15–24. doi: 10.1055/s-2005-871738. [DOI] [PubMed] [Google Scholar]
- Dahlhamer J., Lucas J., Zelaya C. Prevalence of chronic pain and high-impact chronic pain among adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001–1006. doi: 10.15585/mmwr.mm6736a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta N., Beletsky L., Ciccarone D. Opioid crisis: No easy fix to its social and economic determinants. American Journal of Public Health. 2018;108(2):182–186. doi: 10.2105/AJPH.2017.304187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding C., Cicuttini F., Scott F., Cooley H., Jones G. Knee structural alteration and BMI: A cross-sectional study. Obesity Research. 2005;13(2):350–361. doi: 10.1038/oby.2005.47. [DOI] [PubMed] [Google Scholar]
- Fabris de Souza S.A., Faintuch J., Valezi A.C. Postural changes in morbidly obese patients. Obesity Surgery. 2005;15(7):1013–1016. doi: 10.1381/0960892054621224. [DOI] [PubMed] [Google Scholar]
- Fillingim R.B. Sex, gender, and pain: Women and men really are different. Current Review of Pain. 2000;4(1):24–30. doi: 10.1007/s11916-000-0006-6. [DOI] [PubMed] [Google Scholar]
- Fillingim R.B., King C.D., Ribeiro-Dasilva M.C., Rahim-Williams B., Riley J.L. Sex, gender, and pain: A review of recent clinical and experimental findings. The Journal of Pain. 2009;10(5):447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finucane M.M., Stevens G.A., Cowan M.J. National, regional, and global trends in body-mass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. The Lancet. 2011;377(9765):557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal K.M., Carroll M.D., Ogden C.L., Curtin L.R. Prevalence and trends in obesity among US adults, 1999-2008. Journal of the American Medical Association. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Freburger J.K., Holmes G.M., Agans R.P. The rising prevalence of chronic low back pain. Archives of Internal Medicine. 2009;169(3):251–258. doi: 10.1001/archinternmed.2008.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryar CD, Carroll MD, Ogden CL. Prevalence of overweight, obesity, and extreme obesity among adults: United States, trends 1960–1962 through 2009–2010. Hyattsville, MD: National Center for Health Statistics. Published online 2012.
- Gaskin D.J., Richard P. The economic costs of pain in the United States. The Journal of Pain. 2012;13(8):715–724. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Goesling J., Brummett C.M., Meraj T.S., Moser S.E., Hassett A.L., Ditre J.W. Associations between pain, current tobacco smoking, depression, and fibromyalgia status among treatment-seeking chronic pain patients. Pain Medicine. 2015;16(7):1433–1442. doi: 10.1111/pme.12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman N., Glei D.A., Weinstein M. Declining mental health among disadvantaged Americans. Proceedings of the National Academy of Sciences of the United States of America. 2018;115(28):7290. doi: 10.1073/pnas.1722023115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gortmaker S.L., Swinburn B.A., Levy D. Changing the future of obesity: Science, policy, and action. The Lancet. 2011;378(9793):838–847. doi: 10.1016/S0140-6736(11)60815-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grol-Prokopczyk H. Sociodemographic disparities in chronic pain, based on 12-year longitudinal data. Pain. 2017;158(2):313–322. doi: 10.1097/j.pain.0000000000000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotle M., Hagen K.B., Natvig B., Dahl F.A., Kvien T.K. Obesity and osteoarthritis in knee, hip and/or hand: An epidemiological study in the general population with 10 years follow-up. BMC Musculoskeletal Disorders. 2008;9(1):132. doi: 10.1186/1471-2474-9-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gureje O., Von Korff M., Simon G.E., Gater R. Persistent pain and well-being: A World health organization study in primary care. Journal of the American Medical Association. 1998;280(2):147–151. doi: 10.1001/jama.280.2.147. [DOI] [PubMed] [Google Scholar]
- Hales C.M., Fryar C.D., Carroll M.D., Freedman D.S., Ogden C.L. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007-2008 to 2015-2016. Journal of the American Medical Association. 2018;319(16):1723–1725. doi: 10.1001/jama.2018.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison L., Wilson S., Heron J., Stannard C., Munafò M.R. Exploring the associations shared by mood, pain-related attention and pain outcomes related to sleep disturbance in a chronic pain sample. Psychology and Health. 2016;31(5):565–577. doi: 10.1080/08870446.2015.1124106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes C., Jewell J., Allen K. A food policy package for healthy diets and the prevention of obesity and diet-related non-communicable diseases: The NOURISHING framework. Obesity Reviews. 2013;14(S2):159–168. doi: 10.1111/obr.12098. [DOI] [PubMed] [Google Scholar]
- Hu F. Oxford University Press; USA: 2008. Obesity epidemiology. [Google Scholar]
- Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education . National Academies Press (US); 2011. Relieving pain in America: A blueprint for transforming prevention, care, education, and research.http://www.ncbi.nlm.nih.gov/books/NBK91497/ Accessed June 10, 2019. [PubMed] [Google Scholar]
- Interagency Pain Research Coordinating Committee National pain strategy: A comprehensive high-level strategy for pain. https://www.iprcc.nih.gov/National-Pain-Strategy/Overview Accessed November 25, 2019.
- Janevic M.R., McLaughlin S.J., Heapy A.A., Thacker C., Piette J.D. Racial and socioeconomic disparities in disabling chronic pain: Findings from the health and retirement study. The Journal of Pain. 2017;18(12):1459–1467. doi: 10.1016/j.jpain.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantaratnotai N., Mosikanon K., Lee Y., McIntyre R.S. The interface of depression and obesity. Obesity Research & Clinical Practice. 2017;11(1):1–10. doi: 10.1016/j.orcp.2016.07.003. [DOI] [PubMed] [Google Scholar]
- Kanter R., Caballero B. Global gender disparities in obesity: A review. Adv Nutr. 2012;3(4):491–498. doi: 10.3945/an.112.002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoueir P., Black M.H., Crookes P.F., Kaufman H.S., Katkhouda N., Wang M.Y. Prospective assessment of axial back pain symptoms before and after bariatric weight reduction surgery. The Spine Journal. 2009;9(6):454–463. doi: 10.1016/j.spinee.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Kohler U., Karlson K.B., Holm A. Comparing coefficients of nested nonlinear probability models. STATA Journal. 2011;11(3):420–438. [Google Scholar]
- Larsson U.E. Influence of weight loss on pain, perceived disability and observed functional limitations in obese women. International Journal of Obesity. 2004;28(2):269. doi: 10.1038/sj.ijo.0802534. [DOI] [PubMed] [Google Scholar]
- Lee J.M., Pilli S., Gebremariam A. Getting heavier, younger: Trajectories of obesity over the life course. International Journal of Obesity. 2010;34(4):614–623. doi: 10.1038/ijo.2009.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J.-Y., Tchai E., Jang S.-N. Effectiveness of aquatic exercise for obese patients with knee osteoarthritis: A randomized controlled trial. PM&R. 2010;2(8):723–731. doi: 10.1016/j.pmrj.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Linton S.J., Shaw W.S. Impact of psychological factors in the experience of pain. Physical Therapy. 2011;91(5):700–711. doi: 10.2522/ptj.20100330. [DOI] [PubMed] [Google Scholar]
- Masters R.K., Tilstra A.M., Simon D.H. Explaining recent mortality trends among younger and middle-aged White Americans. International Journal of Epidemiology. 2017;47(1):81–88. doi: 10.1093/ije/dyx127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVinnie D.S. Obesity and pain. Br J Pain. 2013;7(4):163–170. doi: 10.1177/2049463713484296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil J.S. Sex differences in pain and pain inhibition: Multiple explanations of a controversial phenomenon. Nature Reviews Neuroscience. 2012;13(12):859–866. doi: 10.1038/nrn3360. [DOI] [PubMed] [Google Scholar]
- Nahin R.L., Sayer B., Stussman B.J., Feinberg T.M. Eighteen-year trends in the prevalence of, and health care use for, noncancer pain in the United States: Data from the medical expenditure panel survey. The Journal of Pain. 2019 doi: 10.1016/j.jpain.2019.01.003. Published online January 15. [DOI] [PubMed] [Google Scholar]
- Okifuji A., Hare B.D. The association between chronic pain and obesity. Journal of Pain Research. 2015;8:399–408. doi: 10.2147/JPR.S55598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfson M., Wall M., Wang S., Crystal S., Blanco C. Service use preceding opioid-related fatality. The Australian Journal of Pharmacy. 2017;175(6):538–544. doi: 10.1176/appi.ajp.2017.17070808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrosky E., Harpaz R., Fowler K.A. Chronic pain among suicide decedents, 2003 to 2014: Findings from the national violent death reporting system. Annals of Internal Medicine. 2018;169(7):448. doi: 10.7326/M18-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston S.H., Vierboom Y.C., Stokes A. The role of obesity in exceptionally slow US mortality improvement. Proceedings of the National Academy of Sciences. 2018;115(5):957–961. doi: 10.1073/pnas.1716802115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto C.A., Swinburn B., Hawkes C. Patchy progress on obesity prevention: Emerging examples, entrenched barriers, and new thinking. The Lancet. 2015;385(9985):2400–2409. doi: 10.1016/S0140-6736(14)61744-X. [DOI] [PubMed] [Google Scholar]
- Ronti T., Lupattelli G., Mannarino E. The endocrine function of adipose tissue: An update. Clinical Endocrinology. 2006;64(4):355–365. doi: 10.1111/j.1365-2265.2006.02474.x. [DOI] [PubMed] [Google Scholar]
- Ruhm C.J. 2018. Deaths of despair or drug problems? National bureau of economic research working paper series. No. 24188. [DOI] [Google Scholar]
- Samartzis D., Karppinen J., Chan D., Luk K.D.K., Cheung K.M.C. The association of lumbar intervertebral disc degeneration on magnetic resonance imaging with body mass index in overweight and obese adults: A population-based study. Arthritis & Rheumatism. 2012;64(5):1488–1496. doi: 10.1002/art.33462. [DOI] [PubMed] [Google Scholar]
- Shelton R.C., Miller A.H. Eating ourselves to death (and despair): The contribution of adiposity and inflammation to depression. Progress in Neurobiology. 2010;91(4):275–299. doi: 10.1016/j.pneurobio.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiri R., Karppinen J., Leino-Arjas P., Solovieva S., Viikari-Juntura E. The association between obesity and low back pain: A meta-analysis. American Journal of Epidemiology. 2010;171(2):135–154. doi: 10.1093/aje/kwp356. [DOI] [PubMed] [Google Scholar]
- Siddiqi A., Sod-Erdene O., Hamilton D., Cottom T.M., William Darity J. Growing sense of social status threat and concomitant deaths of despair among whites. SSM: Population Health. 2019;9 doi: 10.1016/j.ssmph.2019.100449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D., Park W., Hwang D., Levy M. Severe obesity effect on low back biomechanical stress of manual load lifting. Work. 2015;51(2):337–348. doi: 10.3233/WOR-141945. [DOI] [PubMed] [Google Scholar]
- Soelberg C.D., Brown R.E.J., Du Vivier D., Meyer J.E., Ramachandran B.K. The US opioid crisis: Current federal and state legal issues. Anesthesia & Analgesia. 2017;125(5):1675–1681. doi: 10.1213/ANE.0000000000002403. [DOI] [PubMed] [Google Scholar]
- Stokes A., Berry K.M., Collins J.M. The contribution of obesity to prescription opioid use in the United States. Pain. 2019 doi: 10.1097/j.pain.0000000000001612. Published online May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stommel M., Schoenborn C.A. Accuracy and usefulness of BMI measures based on self-reported weight and height: Findings from the NHANES & NHIS 2001-2006. BMC Public Health. 2009;9(1):421. doi: 10.1186/1471-2458-9-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zee A. The promotion and marketing of oxycontin: Commercial triumph, public health tragedy. American Journal of Public Health. 2009;99(2):221–227. doi: 10.2105/AJPH.2007.131714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataramani A.S., Bair E.F., O'Brien R.L., Tsai A.C. Association between automotive assembly plant closures and opioid overdose mortality in the United States: A difference-in-differences analysis. JAMA Internal Medicine. 2019 doi: 10.1001/jamainternmed.2019.5686. Published online December 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuolteenaho K., Koskinen A., Moilanen E. Leptin – a link between obesity and osteoarthritis. Applications for prevention and treatment. Basic and Clinical Pharmacology and Toxicology. 2014;114(1):103–108. doi: 10.1111/bcpt.12160. [DOI] [PubMed] [Google Scholar]
- WHO Obesity, WHO. https://www.who.int/topics/obesity/en/ Accessed November 25, 2019.
- Woolf S.H., Chapman D.A., Buchanich J.M., Bobby K.J., Zimmerman E.B., Blackburn S.M. Changes in midlife death rates across racial and ethnic groups in the United States: Systematic analysis of vital statistics. BMJ. 2018;362:k3096. doi: 10.1136/bmj.k3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf S.H., Schoomaker H. Life expectancy and mortality rates in the United States, 1959-2017. Journal of the American Medical Association. 2019;322(20):1996–2016. doi: 10.1001/jama.2019.16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer Z, Zajacova A. Persistent, consistent, and extensive: The trend of increasing pain prevalence in older Americans. Journal of Gerontology: Series B. Published online 2018:gbx162–gbx162. [DOI] [PMC free article] [PubMed]
- Zis P, Daskalaki A, Bountouni I, Sykioti P, Varrassi G, Paladini A. Depression and chronic pain in the elderly: Links and management challenges. Clinical Interventions in Aging. doi:10.2147/CIA.S113576. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.