Abstract
Patients with COVID-19 may present a hypercoagulable state, with an important impact on morbidity and mortality. Because of this situation pulmonary embolism is a frequent complication during the course of infection. We present the case of a patient recently discharged, after admission with confirmed COVID-19, who developed a pulmonary embolism and thrombosis of a biological mitral valve prosthesis, producing valve obstruction and stenosis. After 15 days of anticoagulant treatment, resolution of the thrombus and normalisation of prosthetic valve function was observed. This case supports current recommendations of administering full-dose anticoagulation therapy to COVID-19 patients with biological heart valve prosthesis, even after the acute phase of infection.
Résumé
Les patients atteints de COVID-19 présentent parfois une hypercoagulabilité, ce qui a un retentissement important sur la morbidité et la mortalité. C’est pour cette raison que l’embolie pulmonaire est une complication fréquemment observée chez les patients infectés. Nous présentons le cas d’un patient ayant récemment obtenu son congé de l’hôpital après avoir contracté la COVID-19, qui a présenté une embolie pulmonaire et une thrombose de prothèse mitrale biologique provoquant l’obstruction et la sténose de la valve. Après 15 jours d’anticoagulothérapie, la thrombose s’est résorbée et la prothèse valvulaire fonctionne de nouveau normalement. Ce cas milite en faveur des recommandations actuelles concernant l’administration d’une anticoagulothérapie à dose complète aux patients atteints de COVID-19 ayant une prothèse valvulaire biologique, même après la phase aiguë de l’infection.
Case
We present the case of a 77-year-old man who was admitted to the hospital during recovery from COVID-19 infection, with the diagnosis of pulmonary embolism and severe prosthetic mitral valve stenosis.
The patient had undergone mitral valve replacement with a biological prosthesis in 2017 during treatment for Staphylococcus aureus endocarditis with severe valve regurgitation. Follow-up transthoracic echocardiography (TTE) performed in November 2019 showed normal prosthesis function (transmitral gradient of 4 mm Hg). He had been recently admitted to our centre for COVID-19 pneumonia, without requiring intensive care, and was discharged 3 weeks before the reported clinical episode. During the 12 days of his admission for COVID-19, he received anticoagulation at prophylactic doses (3500 IU bemiparin every 24 h), following the current guidelines used in our centre.
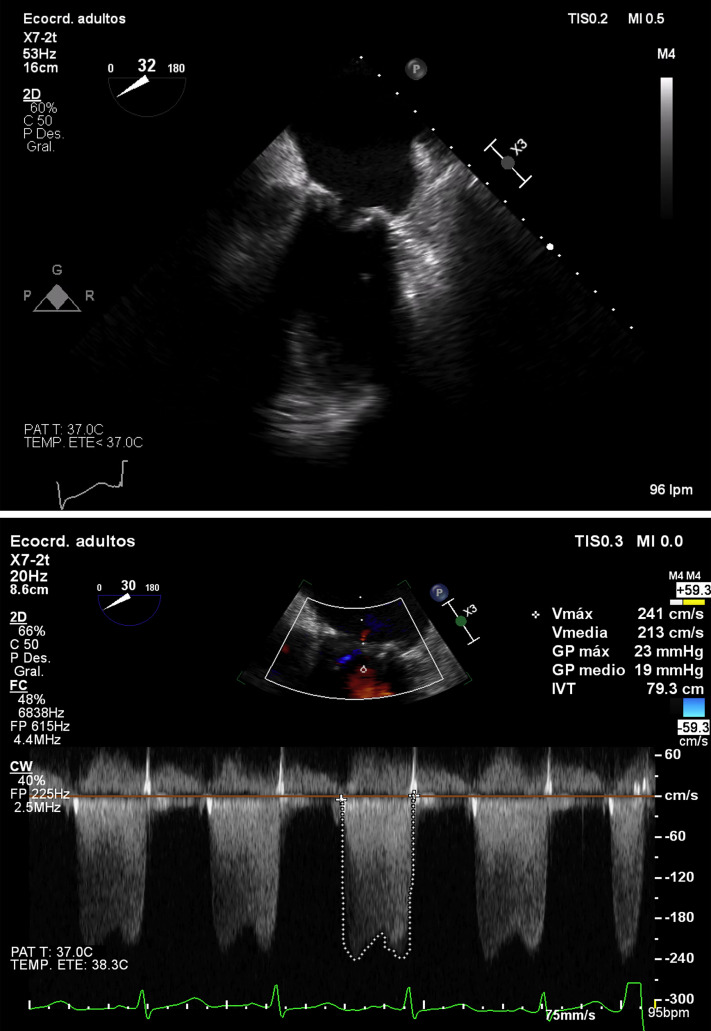
The patient presented 2 weeks later to the emergency department reporting dyspnea on light physical exertion. Physical examination showed a blood pressure of 115/80 mm Hg and oxygen saturation of 86%, crackles in both lung bases, and bilateral lower limb edema. We performed 12-lead electrocardiography, observing the presence of sinus tachycardia at 105 beats per minute. Chest radiography showed signs of pulmonary congestion with bilateral pleural effusion. Blood tests showed normal platelet count and high d-dimer (see Supplemental Table S1 for laboratory findings). We requested a contrast-enhanced thoracic and lungs computed tomography scan, which showed filling defects in segmental arteries suggesting the diagnosis of pulmonary embolism (PE). As part of the work-up, we performed TTE, revealing severe prosthetic mitral stenosis (Fig. 1
; Videos 1 and 2
 , view videos online). The mitral valve area estimated by pressure half-time was 0.5-0.6 cm2, and the mean transprosthetic gradient was 20 mm Hg, with pulmonary artery systolic pressure estimated to be 85 mm Hg and a dilated right ventricle with systolic dysfunction (TAPSE 11 mm, S′ 6.2 cm/s).
, view videos online). The mitral valve area estimated by pressure half-time was 0.5-0.6 cm2, and the mean transprosthetic gradient was 20 mm Hg, with pulmonary artery systolic pressure estimated to be 85 mm Hg and a dilated right ventricle with systolic dysfunction (TAPSE 11 mm, S′ 6.2 cm/s).
Figure 1.
Transesophageal echocardiography (2-dimensional) on admission: mesoesophageal 2-chamber view showing thickened leaflets compatible with laminar thrombus with limited mitral valve opening (transmitral gradient of 19 mm Hg).
Management
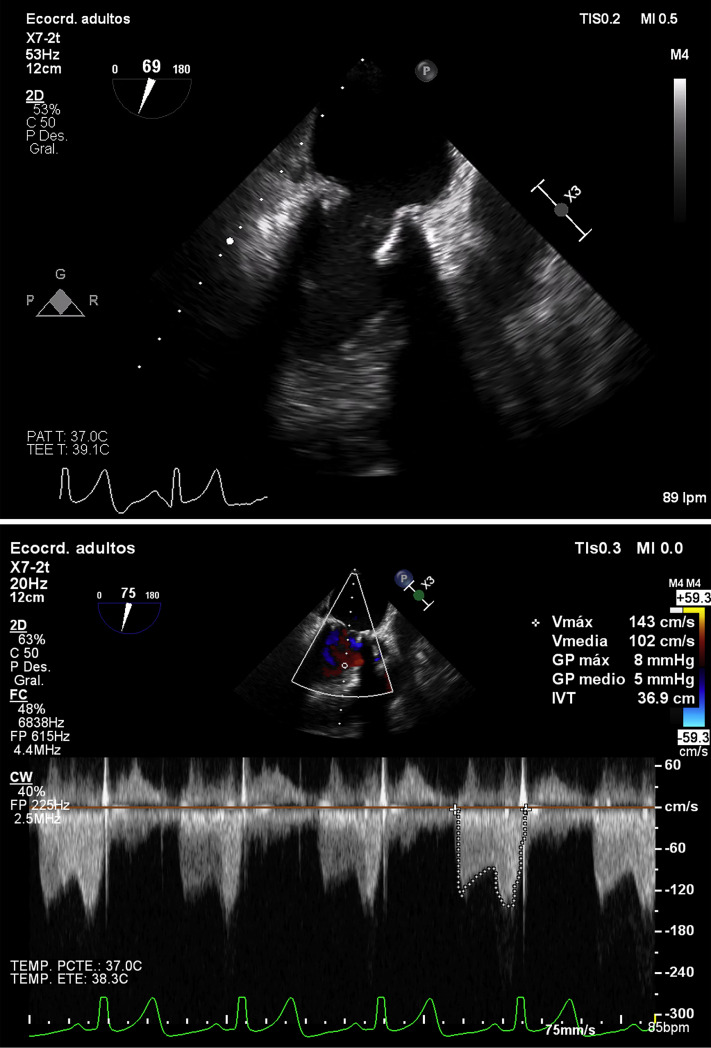
On admission, we performed transesophageal echocardiography (TEE), confirming the findings of the TTE, observing thickened leaflets suggesting the presence of a laminar thrombus, with limited opening (valve orifice area of 0.6 cm2 as determined by means of 3-dimensional planimetry) and a high mean gradient (19 mm Hg). Blood cultures were negative, and Doppler ultrasound of the lower limbs showed no signs of deep vein thrombosis. We started standard treatment for heart failure with intravenous diuretics and weight-adjusted anticoagulation with low-molecular-weight heparin as part of the treatment for PE. The patient presented a satisfactory clinical response, and follow-up TEE was performed 15 days later, confirming the resolution of the prosthetic thrombus and stenosis (Fig. 2
; Videos 3 and 4
 , view videos online) with thin leaflets, normal valve opening (planimetry of 2.1 cm2), and normalisation of the transprosthetic mean gradient (5 mm Hg), and only mild anterior leaflet restriction persisting with complete telediastolic opening.
, view videos online) with thin leaflets, normal valve opening (planimetry of 2.1 cm2), and normalisation of the transprosthetic mean gradient (5 mm Hg), and only mild anterior leaflet restriction persisting with complete telediastolic opening.
Figure 2.
Transesophageal echocardiography (2-dimensional) 15 days later: mesoesophageal 2-chamber view showing resolution of prosthetic stenosis with thin leaflets and normal valve opening, only mild restriction of anterior mitral leaflet persisting with complete telediastolic opening (transmitral gradient of 5 mm Hg).
At hospital discharge, we switched to oral anticoagulation with vitamin K antagonist (acenocoumarol) for an international normalized ratio goal of 3-3.5, bridging with heparin. We decided on a 3-month duration of anticoagulation treatment, with further echocardiographic reevaluation.
Discussion
During the pandemic, SARS-CoV-2 infection has been associated with arterial (acute limb ischemia) and venous thromboembolic phenomena (especially at pulmonary level) as well as, less frequently, consumption coagulopathies.1 This seems to be due to a hypercoagulable state promoted by COVID-19, whose mechanisms have not yet been explained. As far as we know, this may happen as a consequence of endothelial damage secondary to direct viral injury as well as to systemic inflammatory response, where the complement system plays an important role.2 Recently, some reported cases have been published, in which PE occurred despite the administration of anticoagulation treatment at prophylactic doses.3 Furthermore, in most cases the thromboembolic event occurred after the acute phase of infection, namely, in the recovery phase.4 Therefore, the use of anticoagulation as primary prevention has been proposed despite limited evidence. However, some scientific societies have already made some recommendations about the anticoagulation management,5 including the administration of anticoagulant therapy up to 45 days after hospital discharge if there are thrombotic risk factors (eg, reduced mobility, active cancer, elevated d-dimer > 2 times the upper limit of normal) and low risk of bleeding.
We performed a literature review on the topic of COVID-19–associated heart valve prosthesis thrombosis and did not find any cases reporting mechanical or biological prosthesis thrombosis. Typically, bioprosthetic valve thrombosis is a possible but infrequent finding. In this case, the patient had a biological mitral prosthesis thrombosis simultaneously with PE. Making the correct diagnosis was required to provide the patient with the optimal anticoagulation strategy, after which we verified the resolution of the thrombus and consequently of the prosthetic valve stenosis.
We conclude that patients with COVID-19 may present hypercoagulability, causing the occurrence of pulmonary thromboembolism and in some cases also thrombosis of heart valve prostheses, as was the case for this patient 2 weeks after he was discharged from the hospital for COVID-19 pneumonia. With these findings, we support the recommendation of a prolonged anticoagulation strategy at full therapeutic doses after discharge of selected COVID-19 patients, particularly those with valve prostheses, including biological ones. Further studies are needed to shed light on the role of SARS-CoV-2 in thromboembolic phenomena and to confirm the most appropriate duration of anticoagulation therapy in selected patients.
Funding Sources
The authors have no funding sources to declare.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
See page 938.e6 for disclosure information.
To access the supplementary material accompanying this article, visit the online version of the Canadian Journal of Cardiology at www.onlinecjc.ca and at https://doi.org/10.1016/j.cjca.2020.10.008.
Supplementary Material
Transesophageal echocardiography (2-dimensional) on admission: mesoesophageal 2-chamber view showing thickened leaflets compatible with laminar thrombus with limited mitral valve opening.
Transesophageal echocardiography (3-dimensional) on admission: ventricular view showing limited mitral valve opening.
Transesophageal echocardiography (2-dimensiona) 15 days later: mesoesophageal 2-chamber view showing resolution of prosthetic stenosis with thin leaflets and normal valve opening, and only mild restriction of anterior mitral leaflet persisting with complete telediastolic opening.
Transesophageal echocardiography (3-dimensional) 15 days later: ventricular view showing resolution of the prosthetic thrombus and normal valve opening.
References
- 1.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chauhan A.J., Wiffen L.J., Brown T.P. COVID-19: a collision of complement, coagulation and inflammatory pathways. J Thromb Haemost. 2020;1:2110–2117. doi: 10.1111/jth.14981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mestre-Gómez B., Lorente-Ramos R.M., Rogado J., et al. Incidence of pulmonary embolism in noncritically ill COVID-19 patients. Predicting factors for a challenging diagnosis. J Thromb Thrombolysis. 2021;51:40–46. doi: 10.1007/s11239-020-02190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langer F., Kluge S., Klamroth R., et al. Coagulopathy in COVID-19 and its implication for safe and efficacious thromboprophylaxis. Hamostaseologie. 2020;40:264–269. doi: 10.1055/a-1178-3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bikdeli B., Madhavan M.V., Jimenez D., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transesophageal echocardiography (2-dimensional) on admission: mesoesophageal 2-chamber view showing thickened leaflets compatible with laminar thrombus with limited mitral valve opening.
Transesophageal echocardiography (3-dimensional) on admission: ventricular view showing limited mitral valve opening.
Transesophageal echocardiography (2-dimensiona) 15 days later: mesoesophageal 2-chamber view showing resolution of prosthetic stenosis with thin leaflets and normal valve opening, and only mild restriction of anterior mitral leaflet persisting with complete telediastolic opening.
Transesophageal echocardiography (3-dimensional) 15 days later: ventricular view showing resolution of the prosthetic thrombus and normal valve opening.