Abstract
Background
The purpose of this pilot trial was to determine the feasibility of a self‐managed lymphedema randomized control trial to test the effectiveness of a head and neck‐specific exercise protocol.
Methods
Nine participants were randomized to receive usual treatment provided by an Australian metropolitan teaching hospital (n = 4) or usual treatment with an added head and neck exercise regime (n = 5). Feasibility was assessed through ease of recruitment, adherence, and safety. Lymphedema reduction and quality of life (QOL) data were assessed at baseline (0 week) and follow‐up (6 weeks).
Results
The study was feasible in terms of safety and participant retention. However, a slow recruitment rate and low adherence may impact future trials. There were no significant differences in lymphedema reduction or QOL between groups.
Conclusion
This pilot feasibility study demonstrated that a self‐management trial can be implemented, however, modifications will be required due to the slow recruitment and poor adherence rates.
Level of evidence
1b: Individualized randomized control trial.
Keywords: head, lymphedema, neck, self‐management, treatment
1. INTRODUCTION
Head and neck lymphedema is a significant issue for patients following head and neck cancer treatment 1 with a prevalence rate between 12% 2 and 90%. 3 Head and neck lymphedema may involve both external anatomical sites (eg, skin, face, neck, head) and internal structures (eg, oral cavity, pharynx), 4 , 5 , 6 resulting in symptom burden, functional impairments (speech 5 swallowing, 6 , 7 breathing 5 ), and decreased quality of life (QOL). 1 , 4 , 5 , 6 , 8 Due to cosmetic changes, this population experiences body image and psychological sequelae, such as anxiety and depression. 1
Head and neck lymphedema treatment guidelines are focused on a combination of complete decongestive therapy modalities including external compression, exercise, skin care, manual‐lymphatic drainage, and patient education for self‐management. 2 There is a lack of consensus on the combination of these therapy modalities, in particular, head and neck specific exercises and external compression. 9 , 10 , 11 Other treatment modalities, such as manual‐lymphatic drainage, may be more applicable for head and neck lymphedema due to the low adherence and difficulty in applying compression to this body region. 10 Despite the suggested benefit, an added exercise protocol may increase treatment burden to this vulnerable population. 11 There is a need to test the effectiveness of individual components for improved outcomes. 11
Patients often feel overwhelmed and exhausted by their cancer journey, including attendance at numerous appointments. 12 As nearly one third of Australians reside in regional or remote areas, 13 many patients have to travel significant distances to access cancer treatment. 13 Despite this, the majority of head and neck lymphedema treatment is focused on therapist‐directed treatment followed by self‐management. 2 , 14 There is a clear need to develop an effective self‐management protocol from the onset of treatment to reduce treatment burden. 8 , 10 , 13
The lymphedema treatment program at Sir Charles Gardiner Hospital (SCGH), Perth, Western Australia, currently promotes a daily self‐management protocol from the onset of treatment for head and neck lymphedema patients. The program is comprised of a combination of self‐lymphatic drainage, compression, skin care, and education on functional movement within meaningful activities.
To date, no studies have examined the effectiveness of a self‐managed program on lymphedema reduction and patient QOL using formal evaluation assessments with appropriate psychometric properties, such as the ALOHA protocol. 14 Therefore, the primary aim of this study is to investigate the feasibility of a randomized control trial (RCT) to determine the effectiveness of a head and neck‐specific exercise protocol in patients with head and neck lymphedema. The secondary aims were to (a) explore the adherence of compression garments over a 6‐week home therapy protocol; (b) explore the impact of a head and neck specific exercise protocol on lymphedema; and (c) investigate the impact of self‐management on symptomology and health and well‐being for patients with head and neck lymphedema.
2. MATERIALS AND METHODS
2.1. Design
This study was a pilot randomized pre‐test (baseline [T0], 0 week) and post‐test (follow‐up [T1], 6 weeks) trial. There were two arms to the study (active control [CON] and intervention protocol [INT]). The study was conducted at SCGH, Perth, Australia, a public Australian metropolitan teaching hospital. This study was conducted in collaboration with the School of Occupational Therapy, Social Work, and Speech Pathology at Curtin University, Perth, Australia. Ethical approval was received from both organizations' Human Research Ethics Committee. The trial was registered on the Australian New Zealand Clinical Trials Registry (ANZCTR12617001215314). All participants provided written and informed consent prior to data collection.
2.2. Participants
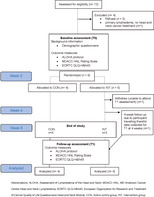
Participants met the following inclusion criteria: >18 years of age; experiencing external head and neck lymphedema, had a previous diagnosis of head and neck cancer; treated with radiotherapy, and/or chemotherapy, and/or surgery; were willing to undergo T0 and T1 assessment at SCGH facilities; and able to provide written and informed consent. A flow chart for eligible participants is shown in Figure 1.
FIGURE 1.
Study flow chart of participants
2.3. Recruitment
Participants (n = 9) were recruited via referrals to outpatient occupational therapy from the radiation oncology, speech pathology, and lymphedema services within SCGH. Participants were enrolled at different time points within their treatment course. Referrals were accepted over a 7‐month period. Due to low numbers of referrals, no eligibility criteria regarding time post‐treatment was placed upon recruitment.
A full schedule of group allocations using a permuted random blocking strategy was developed prior to the commencement of the study. As participants were recruited, they were allocated to the next treatment regime on the list (CON or INT). The blocking strategy ensured that participants were allocated to both arms of the study at approximately equal rates. Investigators (Jane J. Broadhurst and Tess M. McLaughlin) were blinded to the group allocation list and were responsible for participant recruitment and assessment.
2.4. Procedure
An outpatient lymphedema occupational therapist employed by SCGH (Jane J. Broadhurst) screened each new referral for eligibility. Eligible patients were approached regarding participation and provided written and informed consent. On completion of T0 assessments, participants were randomly allocated to CON or INT group, ensuring therapist was blinded to recruitment and allocation at T0. The participant allocation list was kept offsite at Curtin University by a person not involved in participant recruitment (Courtenay J. Harris). Lymphedema treatment commenced immediately following T0 assessment for both arms of the study and was undertaken by Tess M. McLaughlin, with supervision from research team (Jane J. Broadhurst, Sharon L. Keesing, and Courtenay J. Harris).
2.5. Active control protocol group (CON)
The CON group received treatment as usual for head and neck lymphedema, a 45‐minute session delivered by a SCGH occupational therapist. This treatment protocol included:
Education using a diagram explaining the role of the lymphatic system and the impact of cancer treatment.
Instruction and demonstration in self‐lymphatic drainage. Participants were instructed to perform self‐lymphatic drainage daily for 30 minutes. The pattern of drainage was customized to the individual, based on scar severity. Participants were asked to demonstrate self‐lymphatic drainage in front of the occupational therapist to ensure full comprehension. Written information including detailed pictures were provided to participants.
Instruction of functional movement. Participants were directed to move their head and neck when performing functional tasks within pain and range of motion limitations. Education on limiting typical compensatory strategies was provided and participants were encouraged to continue engaging in activities most enjoyable to them.
Provision of compression (Marena Recovery Minimal Coverage Face Mask‐Mid Neck Support FM100‐B). Participants were instructed to wear the compression garment for a minimum of 4 hours per day with progression of increasing wear including at night if tolerated. Verbal, written, and visual information were provided to participants ensuring correct don and doffing of compression garment.
Education regarding skin care to minimize the risk of infection included keeping the skin clean, using moisturizer daily, and avoiding insect bites and sunburn. Written information was provided and each participant was treated based on individual need. If scars were present, the use of contact media such as silicon was applied in conjunction with scar massage techniques.
2.6. Intervention protocol group (INT)
The INT group received usual treatment as per the protocol for the CON group. In addition, participants' T0 appointment durations were extended to 60 minutes and they received a head and neck‐specific exercise protocol as demonstrated by the occupational therapist. This exercise protocol has been previously published. 11 The set exercise protocol takes 10 minutes to complete, and includes 15 exercises involving activation of chest wall, shoulder, neck, and facial muscles. The program aligns to the theoretical basis, type and principles of exercise, being frequency, intensity, timing and type (FITT principles), which, according to the oncology literature, are to be considered as part of rehabilitation protocols. 15
Participants demonstrated each exercise to ensure full comprehension of the treatment and comfort. Participants were instructed to perform the exercise protocol three to five times per day while wearing their compression garment with best results found when preforming five times per day. 11 Written information including detailed pictures of each exercise was provided to participants. Participants were asked to record (in a daily diary) the number of times per day that they completed the exercise protocol.
2.7. Outcome measures
All outcomes, excluding demographic data, were assessed at T0 and T1.
2.7.1. Demographic and feasibility data
Patient demographic data were collected at T0. A demographic questionnaire collected key characteristics which may influence participant treatment outcomes. Data obtained included age, sex, cancer type, cancer location, cancer treatment received (chemotherapy, radiotherapy, surgery), time since cancer treatment, and living distance from SCGH.
The primary aim of this study was to determine feasibility. Criteria for feasibility were as follows 16 :
Ease of recruitment: Ease of recruitment included referrals, exclusion, participant acceptance to be randomized, and withdrawal rates.
Safety: Safety and ease of the home program were evaluated through a weekly phone call aimed at identifying the presence of any adverse effects. Telephone calls were conducted by researcher (Tess M. McLaughlin) and allowed participants or a significant other to ask questions and relay any adverse side effects. Adverse effects were those that are noted clinically including increase in pain, visible increase in edema, color, and sensory changes to the skin. If adverse side effects were noted, referral to the cancer nurse was completed, and lymphedema treatment discontinued until medical clearance was gained.
Adherence: Adherence to self‐management protocols were documented through a participant diary that was completed daily over the 6‐week intervention period and returned at the T1 assessment. Participants in both groups documented time use for wearing of the compression garment. Participants allocated to the INT group documented amount of times head and neck exercise protocol was performed per day. Adherence to self‐lymphatic drainage was not documented by any participants.
These measures were based on previous feasibility studies conducted within a similar hospital setting. 16
2.7.2. Assessment of lymphedema
External head and neck lymphedema were assessed using the ALOHA tape measurement system, 14 , 17 with the ALOHA standardized positioning protocol used for all assessments. 17 The ALOHA protocol includes a combination of tape measurements and tissue dielectric constant (TDC) measurements and has strong interrater reliability allowing accurate head and neck lymphedema comparison of within person change over time. 17 Due to the high cost and lack of availability, a TDC meter was not accessed or used in this study.
All measurements were recorded with each participant positioned in supine, therapist positioned on participants' right side using a Jobst retractable measurement tape held flat against participants' skin (skin tension). 17 Measurement points included upper neck circumference, lower neck circumference, and ear to ear length intersecting a point 8 cm inferior to the lower lip edge. 17 A detailed administration of the ALOHA protocol has previously been published. 17
External head and neck lymphedema were further assessed through palpation and graded using the MD Anderson Cancer Centre Head and Neck Lymphedema (MDACC HNL) Rating Scale. 9 The MDACC HNL Rating Scale is a 4‐point scale and is used to describe the characteristics of lymphedema. 18 The MDACC HNL Rating Scale was used to measure the minimally clinically important difference (MCID) of lymphedema. 17 , 18 A change of one level, for example, 1b to 1a, was understood to be a clinically relevant change. 14 The MDACC HNL Rating Scale has been previously used in literature as the MCID for head and neck lymphedema. 14
2.7.3. Quality of life
Patients' perception concerning QOL, swallowing, and body image data was collected using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Head and Neck Module (EORTC QLQ‐H&N43). 19 The EORTC QLQ‐H&N43 contains the core 30 questions from the EORTC QLQ‐C30 version three and an additional 43 questions measuring QOL in patients with head and neck cancer. 19 Despite the EORTC QLQ‐H&N43 containing multiple system scales, researchers only analyzed scales concerned with QOL, body image, and swallowing. Other system scales were excluded to align with study aims and reduce participant burden.
All symptomology items use a Likert‐like response format ranging from 1 to 4 (not at all = 1; a little = 2; quite a bit = 3; and very much = 4). 19 The global health related QOL measures use a Likert‐like response format ranging from 1 to 7 (very poor = 1 to excellent = 7). The EORTC QLQ‐ H&N43 demonstrates acceptable psychometric properties to successfully measure QOL, swallowing, and body image within this population. 19 , 20 Participants documented responses using a standardized pro forma.
2.8. Data analysis
The Statistical Package for the Social Sciences (IBM SPSS Statistics, version 22) software was used for data analysis. Descriptive statistics were used to summarize the characteristics of the participants and any changes recorded. Feasibility data including recruitment rate, exclusion rate, participant adherence, and safety are described in the results.
Normality was not tested due to the small sample size thus nonparametric tests were used to compare the groups (CON and INT) at T0 to ensure the groups were initially similar with respect to demographic data as well as lymphedema severity. A Fisher's exact test was used for categorical variables including, gender, cancer type, cancer location, treatment received, time since cancer treatment, and MD Anderson Cancer Center Rating Scale. A Mann‐Whitney U test was used for continuous variables including age and living distance from SCGH.
Changes in lymphedema from T0 to T1 were analyzed using a Mann‐Whitney U test for each person. This test was used for the following outcomes: upper neck circumference, lower neck circumferences, ear to ear measurements, and the MDACC HNL Rating Scale. Within group, differences from T0 to T1 were analyzed using the Wilcoxon signed rank test. All outcome measures were included.
Changes in participants' perception of QOL, swallowing, and body image from T0 to T1 were analyzed using a Mann‐Whitney U test for each person. Within group, differences from T0 to T1 for QOL, swallowing, and body image were analyzed using the Wilcoxon signed rank test.
3. RESULTS
3.1. Feasibility
3.1.1. Ease of recruitment
Figure 1 summarizes the flow of participants through the study. Thirteen patients were eligible for the study over a time frame of 7 months. Three potential participants did not accept inclusion into the study, due to feeling overwhelmed with appointments and lack of time. One participant was excluded due to experiencing primary lymphedema with no previous head and neck cancer diagnosis or treatment. A total of nine participants were recruited into the study (n = 5 INT; n = 4 CON). One participant allocated to INT completed T1 appointment at week 4 due to travelling overseas. One participant allocated to INT withdrew from the study. This participant withdrew due to feeling overwhelmed with travel and unable to attend T1 appointment. Therefore, a total of nine participants' demographic and baseline data were analyzed from T0 appointment (n = 5 INT; n = 4 CON) and a total of eight participants follow‐up data were analyzed from T1 appointment (n = 4 INT; n = 4 CON). The majority of participants were male (67%), had a diagnosis of squamous cell carcinoma (89%), and were treated with combination chemotherapy and radiotherapy (56%). The participants' residential location from SCGH ranged from 16 to 766 km with the median being 38.5 km. At T0, there were no significant differences between the participants allocated to INT or CON (P ≥ .05). Demographic characteristics are summarized in Tables 1 and 2.
TABLE 1.
Demographic characteristics of participants at baseline
| Demographic characteristics | Total sample (n = 9) | Control group (n = 4) | Intervention group (n = 5) | Significance (P value) |
|---|---|---|---|---|
| Age, years | .712 | |||
| Mean | 62 | 61 | 63 | |
| Median | 66 | 66.5 | 63 | |
| Range | 35‐76 | 35‐76 | 58‐69 | |
| SD | 11.5 | 17.9 | 4.6 | |
| Sex | .167 | |||
| Male, number (%) | 6 (67) | 4 (100) | 2 (33) | |
| Female, number (%) | 3 (33) | 0 (0) | 3 (100) | |
| Cancer type, number (%) | .444 | |||
| Squamous cell carcinoma | 8 (89) | 3 (75) | 5 (100) | |
| Adenoid cystic carcinoma | 1 (11) | 1 (25) | 0 (0) | |
| Cancer location, number (%) | .524 | |||
| Oropharyngeal | 4 (44) | 1 (25) | 3 (60) | |
| Oral cavity | 1 (11) | 0 (0) | 1 (20) | |
| Salivary | 1 (11) | 1 (25) | 0 (0) | |
| Laryngeal | 1 (11) | 0 (0) | 1 (20) | |
| Infraorbital | 1 (11) | 1 (25) | 0 (0) | |
| Hypopharyngeal | 1 (11) | 1 (25) | 0 (0) | |
| Lymph node removal | ||||
| No | 6 (67) | 3 (50) | 3 (50) | 1.0 |
| Yes | 3 (33) | 1 (33) | 2 (67) | |
| Unilateral | 1 (33) | 1 (100) | 0 (0) | .333 |
| Bilateral | 2 (67) | 0 (0) | 2 (100) | |
| Radiotherapy | ||||
| External beam | 9 (100) | 4 (100) | 5 (100) | 1.0 |
| Mean total dose | 2 Gy | 2 Gy | 2 Gy | 1.0 |
| Dose range | 60‐70 Gy | 60‐70 Gy | 66‐70 Gy | .314 |
| Unilateral | 2 (22) | 2 (50) | 0 (0) | .167 |
| Bilateral | 7 (78) | 2 (50) | 5 (100) | |
| Cancer treatment received, number (%) | 1.0 | |||
| Radiotherapy | 1 (11) | 1 (25) | 0 (0) | |
| Chemotherapy and radiotherapy | 5 (56) | 2 (50) | 3 (60) | |
| Radiotherapy and surgery | 2 (22) | 1 (25) | 1 (20) | |
| Radiotherapy, chemotherapy, and surgery | 1 (11) | 0 (0) | 1 (20) | |
| Time since cancer treatment, number (%) | .333 | |||
| 1‐4 weeks | 1 (11) | 0 (0) | 1 (20) | |
| 1‐3 months | 4 (44) | 3 (75) | 1 (20) | |
| 3‐6 months | 4 (44) | 1 (25) | 3 (60) | |
| MD Anderson Cancer Center Rating Scale, number (%) | 1.0 | |||
| 2 (firm pitting edema, not reversible with no tissue changes) | 4 (44) | 2 (50) | 2 (40) | |
| 1b (soft pitting edema, reversible) | 5 (55.6) | 2 (50) | 3 (60) | |
| Living distance from hospital (km) | .624 | |||
| Mean | 167.6 | 100.65 | 220.82 | |
| Median | 38.5 | 29.8 | 65 | |
| Range | 16‐766 | 16‐324 | 16.1‐766 | |
| SD | 250.4 | 317.3 | 149.2 |
TABLE 2.
Time from radiation treatment
| Participant | Surgical lymph node dissection | No. of radiotherapy treatments | Time from cancer treatment, months |
|---|---|---|---|
| 1 | No | 35 (70 Gy) | 6 months |
| 2 | No | 35 (70 Gy) | 3 months |
| 3 | Unilateral | 33 (66 Gy) | 3 months |
| 4 | Bilateral | 33 (66 Gy) | Still undergoing radiation |
| 5 | No | 35 (70 Gy) | 6 months |
| 6 | Bilateral | 33 (66 Gy) | 5 months |
| 7 | No | 33 (66 Gy) | 3 months |
| 8 | No | 30 (60 Gy) | 7 months |
| 9 | No | 35 (70 Gy) | 3 months |
Abbreviation: Gy, Gray.
3.1.2. Safety
No adverse events, symptoms, or safety concerns were noted by or reported to the occupational therapist, treating medical team, or participants throughout the duration.
3.1.3. Adherence
Adherence was measured through participant completion of diaries collected as T1 from all participants. Compression use throughout the study period is presented in Figures 2 and 3. As seen, seven of the eight participants (87.5%) began wearing compression at home. At week 3, 100% of participants allocated to INT group and 50% of participant allocated to CON group had ceased compression use. Overall, one participant (12.5%, CON) documented using the compression garment for the entire 6‐week study period.
FIGURE 2.
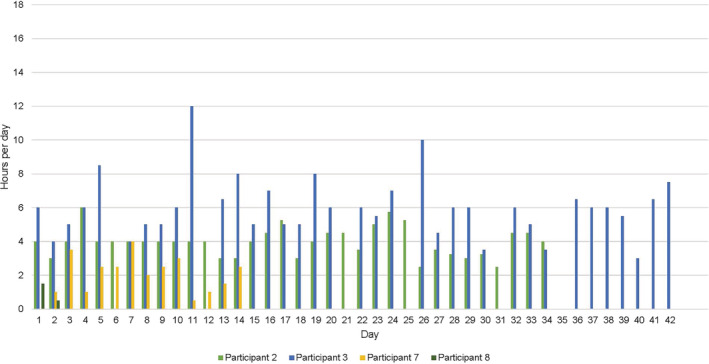
Control group compression use from baseline to follow‐up
FIGURE 3.
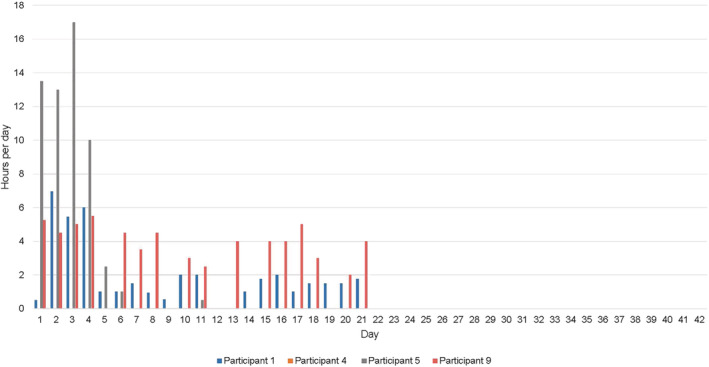
Intervention group compression use from baseline to follow‐up
With respect to adherence with the head and neck exercise protocol for INT group, no participant performed the protocol at the minimum intensity. On average, through review of all participants diaries, the exercise protocol was performed on average 1.29 to 2.29 times per day, see Figure 4 for the number of hours of exercise protocol for each participant. Patient's adherence to self‐lymphatic drainage was not recorded in this study. However, clinical observation at T1 appointment was that majority of participants were not correctly performing self‐lymphatic drainage. This required redemonstration and correction of massage technique during T1 appointment.
FIGURE 4.
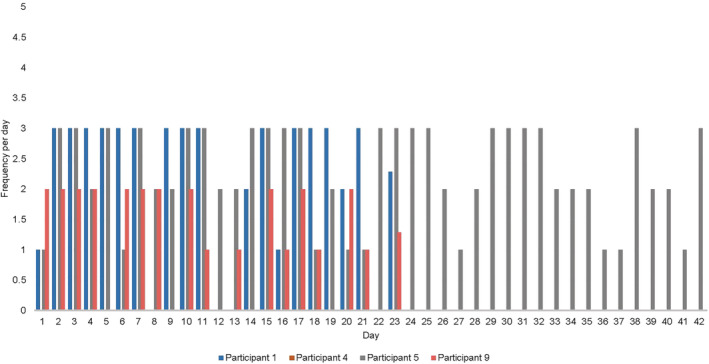
Intervention group frequency of exercise protocol from baseline to follow‐up
3.2. Secondary outcomes
3.2.1. ALOHA
Baseline to T1 data for the ALOHA measures are presented in Table 3. Due the small sample size of this study, individual scores are given (Table 3). There were no statistically significant between group differences for the ALOHA measures (change in upper neck circumference P = .486; change in lower neck circumference P = .686; change in ear to ear measurement P = 1.000). There were no significant within group differences from T0 to T1 (P > .05) (Table 4).
TABLE 3.
Individual participant scores for the control and intervention groups on lymphedema measures at baseline (T0) and follow‐up (T1)
| Recruitment order | Group allocation | ALOHA measurements | MDACC HNL Rating Scale | ||
|---|---|---|---|---|---|
| Upper neck circumference, cm | Lower neck circumference, cm | Ear to ear measurement, cm | MDACC HNL Rating Scale | ||
| T0, T1 (diff) | T0, T1 (diff) | T0, T1 (diff) | T0, T1 (diff) | ||
| 1 | INT | 46.0, 46.5 (+0.50) a | 42.5, 43.1 (+0.60) a | 28.0, 27.1 (−0.90) | 1b, 1b (0) |
| 2 | CON | 44.5, 44.5 (0) | 42.3, 43.0 (+0.70) a | 26.0, 27.0* (+1.00) a | 1b, 1b (0) |
| 3 | CON | 43.5, 43.5 (0) | 44.0, 43.3 (−0.70) | 29.0, 27.0 (−2.00) | 2, 1b (−1) |
| 4 | INT | 41.0, 44.6 (+3.60) a | 39.4, 42.1 (+2.70) a | 26.3, 27.0 (+0.70) a | 2, 2 (0) |
| 5 | INT | 38.3, 37.5 (−0.80) | 38.1, 37.1 (−1.00) | 24.6, 24.5 (−0.10) | 1b, 1a (−1) |
| 6 | INT | 35.0, NR | 34.1, NR | 23.5, NR | 2, NR |
| 7 | CON | 53.0, 48.0 (−5.00) | 46.0, 43.6 (−2.40) | 26.0, 25.8 (−0.20) | 1b, 1b (0) |
| 8 | CON | 45.1, 42.3 (−2.80) | 37.4, 36.5 (−0.90) | 25.3, 25.1 (−0.20) | 2, 2 (0) |
| 9 | INT | 45.2, 41.8 (−3.40) | 43.0, 42.2 (−0.80) | 29.0, 28.6 (−0.40) | 1b, 1b (0) |
Abbreviations: ALOHA, assessment of lymphedema of the head and neck; (diff), difference; MDACC HNL, MD Anderson Cancer Center Head and Neck Lymphedema; NR, not recorded; T0, baseline; T1, follow‐up.
Participant demonstrated a negative change from baseline to follow‐up.
TABLE 4.
Within group differences on lymphedema and patient reported measures: P‐value
| ALOHA measurements | MDACC HNL Rating Scale | EORTC QLQ‐H&N43 | |||||
|---|---|---|---|---|---|---|---|
| Group allocation | Upper neck circumference | Lower neck circumference | Ear to ear measurement | MDACC HNL Rating Scale | QOL | Swallowing | Body image |
| CON | 0.317 | 0.180 | 0.197 | 0.461 | 0.102 | 0.109 | 0.317 |
| INT | 1.000 | 1.000 | 0.465 | 0.317 | 0.109 | 0.414 | 0.414 |
Abbreviations: ALOHA, assessment of lymphedema of the head and neck; CON, active control group; EORTC QLQ‐H&N43, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Head and Neck Module; INT, intervention group; MDACC HNL, MD Anderson Cancer Centre Head and Neck Lymphedema; QOL, quality of life.
3.2.2. Lymphedema
Baseline to T1 data for the MDACC HNL rating scale is presented in Table 3. A reduction in the MDACC HNL rating scale was recorded for two of the eight participants (25%) (n = 1 INT, n = 1 CON). No worsening in lymphedema was recorded.
3.2.3. Body image, swallowing, and QOL
There were no significant between group differences for body image (P = .343), swallowing (P = .200), and QOL (P = .686). Similarly, there were no significant differences within group for body image, swallowing, and QOL (P > .05) (Table 4). Two participants reported a reduction in body image symptomology, overall feeling more satisfied with their body image (n = 1 INT, n = 1 CON); four remained the same (n = 1 INT, n = 3 CON); and two recorded higher symptomology of body image from T0 to T1 (n = 2 INT). Five participants noted a reduction in swallowing difficulties’ (n = 2 INT, n = 3 CON); two participants scores remained the same (n = 1 INT, n = 1 CON); and one reported higher symptomology during swallowing (INT). The QOL item scores for six participants increased from T0 to T1, reporting greater QOL (n = 3 INT, n = 3 CON); two participants remained the same (n = 1 INT, n = 1 CON). No worsening in QOL was recorded for any participant.
4. DISCUSSION
This pilot study completed with head and neck cancer related lymphedema patients was feasible in terms of safety and participant retention. However, the feasibility of conducting a larger RCT may be problematic due to the slow recruitment and adherence issues evident in this study. Future studies may remediate this if participants are recruited from multiple sites and outcome assessment time frames are reconsidered.
Results from the diary entries demonstrated a low adherence with compression garments for the majority of participants. The INT group may have had less adherence due to increase burden of the additional exercise protocol. This finding supports previous research with patients acknowledging compression is not well accepted, 21 reporting low adherence due to the garment's aesthetic look. 12 Previous research further suggests that cost related burden may impact compression adherence, however, at SCGH there was no cost to patients for compression garments. Due to the low adherence with compression and the lack of evidence supporting its benefit, 12 the question is raised regarding the necessity of compression for this population. Although no conclusions can be made due to the underpowered nature of this study, this is an important area for further research. If compression provides significant benefit to this population, new methods to encourage adherence are required for patients to achieve long term outcomes.
Within this trial, care was taken to monitor the adherence of self‐management through weekly telephone calls to participants and/or significant others. However, the low adherence results in both groups suggest that more frequent contact may be necessary for self‐management protocols. Participants who lived a considerable distance from SCGH created a unique challenge to the provision of frequent contact. Literature suggests that in‐person interaction and guidance increases the patient's skill and motivation for head and neck lymphedema self‐care. 21 Future trials may consider earlier outcome time frames at 3 weeks or incorporating technology (Skype, FaceTime) to provide feasible, real‐time guidance from the occupational therapist. This will allow the occupational therapist to repeat demonstrations of techniques and evaluate self‐management between appointments increasing patient motivation, engagement, and adherence. Additionally, as majority of patients returned at T1 appointment demonstrating incorrect self‐management methods this may suggest that one face to face session with written and pictured handouts is not sufficient to properly train patients for a self‐management protocol. Incorrect technique may contribute to lack of success in the treatment outcomes. This further highlights the potential for incorporating technology to increase patient outcomes and success of treatment.
Findings from this study indicate that following 6 weeks of self‐managed lymphedema treatment there is no significant change to head and neck lymphedema between CON or INT groups. This may suggest that the head and neck‐specific exercise protocol has no additional effect on lymphedema reduction. These results differ from previous research emphasizing the importance of head and neck specific exercises. 11 Based on the underlying anatomy of the head and neck, exercises may enhance lymphatic flow through muscle pump action. 2 It is clinically accepted that exercises may compensate for a reduction in natural muscle movements occurring as a result of cancer treatment including eating softer foods and reduced activity. 22 Although the current study's findings are contradictory, due to the small sample size, this study may have been insufficiently powered to detect small changes between groups. Future studies should be adequately powered to test the effectiveness of a head and neck lymphedema specific exercise protocol for this population.
Despite no between group differences, a reduction in lymphedema did occur from T0 to T1 for the majority of participants. A recent study evaluated a 22‐week intervention involving therapist directed manual lymphatic drainage for 4 weeks followed by participant self‐management. 14 Results at 6 weeks indicated a greater reduction in lymphedema to the current study. 14 Of interest, some participants in the current study demonstrated a similar reduction to those receiving therapist‐directed treatment. 14 This may suggest that self‐managed lymphedema treatment may be as beneficial as therapist‐directed lymphedema treatment for some individuals. This finding reflects recent literature challenging the necessity of therapist‐directed treatment for this population due to the high appointment burden and improved outcomes of self‐management. 7 , 11 With many patients living in rural and regional areas, not in close vicinity to hospitals, this finding has broad applicability. It is important to note that lymphedema increased in some participants over the 6‐week study period. The reason for this is not clear, however, this may suggest that self‐management may not be suited for all patients with head and neck lymphedema. This concurs with previous literature stating that therapist‐directed treatment is still necessary if patients are noncompliant or have unsatisfactory results with self‐management. 11 Future research is required to identify patients best suited for self‐management. 7
The researchers found the ALOHA protocol feasible to use in current practice due to the low cost, however, found identifying anatomical landmarks difficult, where severe edema is present. Recent literature speculates that the ALOHA protocol may not be reliable in detecting small changes in lymphedema. 14 This may explain the limited change detected in this current study. Consistent and improved data collection techniques will be essential for future trials testing the effectiveness of individual treatment modalities. 12 Future trials examining the effectiveness of treatment modalities should consider including a measurement of TDC, assessed using the Moisture Meter D (MMD) in addition to the ALOHA protocol. The MMD is a novel objective measurement demonstrating potential in both the diagnosis and the evaluation of change over time for head and neck lymphedema. 17 Using both the MMD and the ALOHA protocol in future trials may further assist in comparing lymphedema outcomes and guide management strategies. 17
The MCID reference for this study was the use of the MDACCHNL Rating Scale. 14 The authors found this measure highly subjective and difficult to categorize as many participants presented with both fibrotic and soft edema in different regions. A recent study using the MDACCHNL rating scale as the MCID stated that it was useful in supporting the reduction in lymphedema, however, is a generous measure for the use of a MCID. 14 Adaptions of various lymphedema grading scales have been used for head and neck lymphedema, however, further work is required to truly capture an objective measurement tool. 11 Using visual diagrams to map specific anatomical locations is a necessary step forward in increasing the objectivity of the measurement as well as inter‐rater reliability.
In addition to lymphedema reduction, this study looked at the impact of self‐managed treatment on QOL, body image, and swallowing ability. At T0 all participants reported a low QOL and high symptom burden associated with body image and swallowing. This finding is consistent with previous literature stating the high symptom burden associated with head and neck lymphedema impacts on health related QOL. 6 Within the current study, it is unclear if change in QOL, body image, or swallowing ability were attributed to the self‐management protocols. Future studies should incorporate a qualitative measure together with home programs to explore the direct effect and impact of these phenomena.
Although not formally assessed, the weekly telephone calls to participants allowed the researches to maintain a client‐centered approach and discuss concerns during the cancer treatment journey. During the telephone calls, majority of the time was spent checking in with patient's emotional state, providing psychosocial support and education on cancer support groups including Solaris Cancer Care, Cancer Council WA, and lymphedema forums. Researchers believed that this regular contact provided emotional benefit to participants and linkage to community services. Future studies should consider formally assessing the benefit of follow ups for this cohort.
This study has important limitations that need to be acknowledged. Although this research is a pilot study, the small sample size is a limitation. Although some positive effects were seen with both self‐management protocols, the study was insufficiently powered to detect any significant within or between group differences. Future studies would benefit from larger sample sizes to determine the effectiveness of individual components and to enhance the generalizability of the results. However, the slow recruitment and low adherence with protocols over the study period is a limitation likely to affect the feasibility of larger scale trials. Participants' adherence to self‐lymphatic drainage was not monitored in this study. Self‐lymphatic drainage is a large contributor of the overall effectiveness of the home program. 14 To determine adherence with self‐management techniques and the subsequent effect on head and neck lymphedema, it is important to record all aspects of the home program. 14 Future studies should monitor adherence of each individual management technique to allow analysis of the entire self‐management protocol. Although two researchers (Jane J. Broadhurst and Tess M. McLaughlin) were present at both T0 and T1 appointments and received clarification on the ALOHA protocol from the authors, internal validity may be weakened due to limited time using this protocol. Additionally, researchers did not have access to the TDC, therefore only the ALOHA tape measurement system was used. Furthermore, all females recruited into this study were randomly allocated to INT group. Despite these limitations, this study has important findings for the recommendation of future trials.
5. CONCLUSION
This pilot study demonstrated that a self‐management trial is feasible with patients who have head and neck cancer related lymphedema. The slow recruitment experienced within this study may impact the feasibility of a larger trial unless multiple sites are included. High quality, well planned, and adequately powered studies are required to demonstrate effectiveness of individual self‐management modalities including compression, exercise, and self‐lymphatic drainage on lymphedema reduction and QOL outcomes. In doing so, the burden of lymphedema treatment would be minimized for patients with head and neck lymphedema.
CONFLICT OF INTEREST
The authors declared no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
All authors contributed significantly to the development and critique of this study. Sarah McGarry, Courtenay J. Harris, Jane J. Broadhurst, and Tess M. McLaughlin were responsible for the development of the project question and literature review. Tess M. McLaughlin was responsible for data collection, statistical analysis, and writing the main draft. Jane J. Broadhurst was responsible for data collection and critiquing. Sharon L. Keesing, Courtenay J. Harris, and Sarah McGarry were responsible for assisting in data analysis, critiquing writing style, and overall completion of the article.
ACKNOWLEDGMENT
The authors would like to thank all the participants who gave their time and commitment to this study.
McLaughlin TM, Broadhurst JJ, Harris CJ, McGarry S, Keesing SL. A randomized pilot study on self‐management in head and neck lymphedema. Laryngoscope Investigative Otolaryngology. 2020;5:879–889. 10.1002/lio2.455
Contributor Information
Tess M. McLaughlin, Email: tess.mclaughlin@health.wa.gov.au.
Jane J. Broadhurst, Email: jane.broadhurst@health.wa.gov.au.
BIBLIOGRAPHY
- 1. McGarvey AC, Osmotherly PG, Hoffman GR, Chiarelli PE. Lymphoedema following treatment for head and neck cancer: impact on patients, and beliefs of health professionals. Eur J Cancer Care (Engl). 2014;23(3):317‐327. [DOI] [PubMed] [Google Scholar]
- 2. Tacani PM, Franceschini JP, Tacani RE, et al. Retrospective study of the physical therapy modalities applied in head and neck l ymphedema treatment. Head Neck. 2016;38(2):301‐308. [DOI] [PubMed] [Google Scholar]
- 3. Ridner S, Dietrich MS, Niermann K, Cmelak A, Mannion K, Murphy B. A prospective study of the lymphedema and fibrosis continuum in patients with head and neck cancer. Lymphat Res Biol. 2016;14(4):198‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Deng J, Murphy BA, Dietrich MS, Sinard RJ, Mannion K, Ridner SH. Differences of symptoms in head and neck cancer patients with and without lymphedema. Support Care Cancer. 2016;24(3):1305‐1316. [DOI] [PubMed] [Google Scholar]
- 5. Deng J, Ridner SH, Murphy BA. Lymphedema in patients with head and neck cancer. Oncol Nurs Forum. 2011;38(1):E1‐E10. [DOI] [PubMed] [Google Scholar]
- 6. Deng J, Murphy BA, Dietrich MS, et al. Impact of secondary lymphedema after head and neck cancer treatment on symptoms, functional status, and quality of life. Head Neck. 2013;35(7):1026‐1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smith BG, Hutcheson KA, Little LG, et al. Lymphedema outcomes in patients with head and neck cancer. Otolaryngol Head Neck Surg. 2015;152(2):284‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deng J, Ridner SH, Aulino JM, Murphy BA. Assessment and measurement of head and neck lymphedema: state‐of‐the‐science and future directions. Oral Oncol. 2015;51(5):431‐437. [DOI] [PubMed] [Google Scholar]
- 9. Smith BG, Lewin JS. Lymphedema management in head and neck cancer. Curr Opin Otolaryngol Head Neck Surg. 2010;18(3):153‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lasinski BB, Thrift KM, Squire D, et al. A systematic review of the evidence for complete decongestive therapy in the treatment of lymphedema from 2004 to 2011. PM&R. 2012;4(8):580‐601. [DOI] [PubMed] [Google Scholar]
- 11. Jeffs E, Huit M. Treatment and outcomes of head and neck oedema referrals to a hospital‐based lymphoedema service. Br J Community Nurs. 2015;S6‐S13. 10.12968/bjcn.2015.20.Sup4.S6. [DOI] [PubMed] [Google Scholar]
- 12. Huit M. Lymphoedema in patients treated for head and neck cancer. J Lymphoedema. 2011;6(1):50‐57. [Google Scholar]
- 13. Butow P, Phillips F, Schweder J, White K, Underhill C, Goldstein D. Psychosocial well‐being and supportive care needs of cancer patients living in urban and rural/regional areas: a systematic review. Support Care Cancer. 2012;20(1):1‐22. [DOI] [PubMed] [Google Scholar]
- 14. Pigott A, Nixon J, Fleming J, Porceddu S. Head and neck lymphedema management: evaluation of a therapy program. Head Neck. 2018;40:1131‐1137. [DOI] [PubMed] [Google Scholar]
- 15. Feldman E. Exercising after cancer: newest evidence‐based guidelines. Integr Med Alert. 2020;23(6). [Google Scholar]
- 16. Lannin NA, Cusick A, Hills C, et al. Upper limb motor training using a Saebo orthosis is feasible for increasing task‐specific practice in hospital after stroke. Aust Occup Ther J. 2016;63:364‐372. [DOI] [PubMed] [Google Scholar]
- 17. Purcell A, Nixon J, Fleming J, McCann A, Porceddu S. Measuring head and neck lymphedema: the "ALOHA" trial. Head Neck. 2016;38(1):79‐84. [DOI] [PubMed] [Google Scholar]
- 18. Nixon J, Purcell A, Fleming J, McCann A, Porceddu S. Pilot study of an assessment tool for measuring head and neck lymphoedema. Br J Community Nurs. 2014;S6:S8‐S11. [PubMed] [Google Scholar]
- 19. Singer S, Araújo C, Arraras JI, et al. Measuring quality of life in patients with head and neck cancer: update of the EORTC QLQ‐H&N Module, phase III. Head Neck. 2015;37(9):1358‐1367. [DOI] [PubMed] [Google Scholar]
- 20. Trivic SK, Trivic A, Singer S, et al. European Organization for Research and Treatment of Cancer Quality of Life Questionnaire‐Head and Neck Module, updated version: preliminary psychometric data from Serbian laryngectomized patients. Head Neck. 2016;38(Suppl 1):E916‐E924. [DOI] [PubMed] [Google Scholar]
- 21. Deng J, Murphy BA. Lymphedema self‐care in patients with head and neck cancer: a qualitative study. Support Care Cancer. 2016;24(12):4961‐4970. [DOI] [PubMed] [Google Scholar]
- 22. Purcell A. Head and neck lymphoedema management practices. J Lymphoedema. 2013;8(2):8‐15. [Google Scholar]


