Abstract
Objectives
To provide a state of the art review on accruing studies focused on defining the middle ear microbiome, highlighting the relationship of the microbiome to disease pathophysiology.
Data sources
Pubmed indexed peer‐reviewed articles and published textbooks.
Review methods
Comprehensive review of the literature using the following search terms: “microbiome” “bacterial pathogens” with the term “otitis media,” and “middle ear.”
Results
A multitude of microbiome studies have been published in the recent past. In general findings from these studies underscore distinct profiles based on disease category. The adenoidal reservoir theory may not explain all etiologies of middle ear effusion production. The host immune system appears to be associated to the bacterial population identified in the middle ear space. Atopic respiratory diseases correlate to the middle ear microbiome. Some novel middle ear bacterial genera may be protective in terms of disease.
Conclusion
The understanding of otitis media disease progression pathophysiology is evolving, informed by accruing middle ear microbiomic data. The functional implications of middle ear microbiome findings need to be studied further. This may help counterbalance probiotic vs antibiotic approaches to disease mitigation.
Keywords: adenoidal reservoir, bacteria, microbiome, middle ear effusion, otitis media
This is an invited state‐of‐the‐art review on Otitis Media Microbiology in consideration for publication in LIO. In this manuscript, we report on how the accruing literature on the middle ear microbiome may inform the pathophysiology of OM.

1. INTRODUCTION
Otitis media (OM) and its full spectrum of disorders (Table 1) continues to be one of the most prevalent childhood diseases. The spectrum encompasses acute otitis media (AOM), recurrent acute otitis media (RAOM), otitis media with effusion (EOM), chronic otitis media with effusion (COME), and chronic suppurative otitis media (CSOM). 1 Whereas the spectrum of disease differs in symptom duration, severity and long‐term complications, all are characterized by middle ear effusion (MEE) which contains a varied microbiome that has been widely studied in an attempt to better understand its pathogenesis and etiology. Historically the most commonly isolated bacteria from the MEE of children with OM were Streptococcus pneumonia (SP), Haemophilus influenza (Hi), and Moraxella catarrhalis (MC). 2
TABLE 1.
Definitions of the major spectrum of diseases encompassing OM
| Term | Definition |
|---|---|
| Otitis media (OM) | Umbrella term describing middle ear inflammation without reference to duration or etiology |
| Acute otitis media (AOM) | Rapid onset of inflammation of the middle ear. Characterized by bulging of the tympanic membrane (TM) with concurrent erythema of the TM or ear pain or acute ear discharge. |
| Recurrent acute otitis media (RAOM) | Four or more episodes of AOM in 1 year or Three or more episodes in 6 months |
| Otitis media with effusion (OME) | Fluid in the middle ear without signs of infection or TM perforation |
| Chronic otitis media with effusion (COME) | OME persisting for three or more months |
| Chronic suppurative otitis media (CSOM) | Chronic inflammation of the middle ear and mastoid mucosa with a TM perforation or ventilation tube and persistent ear discharge |
Note: Definitions adapted from Schilder et al. 1
Using traditional culture methods, the bacterial population across different stages of OM has been studied and compared. Holder et al 3 showed the presence of bacterial DNA in 87% of AOM samples detecting mostly only one pathogen, whereas only 51% of COME samples were positive, with the presence of multiple bacteria species. Homoe et al 4 showed the absence of bacterial biofilms in middle ear fluid smears from patients with COME compared with chronic suppurative OM CSOM fluid smears mostly showing the presence of bacterial biofilms. Interestingly, Calhoun et al 5 showed that the nature of the effusion also influences its bacterial content, with purulent COME samples more likely to be culture positive than mucoid samples which in turn are more likely to be positive than serous samples. Other groups did not find differences in bacterial content for effusions samples coming from patients with recurrent or nonrecurrent OM 6 or recurrent OM and COME. 7 , 8 These studies indicate bacterial findings may result from variability in sample collection, the population studied, specific disease stage, and/or the techniques used to assay bacteria presence in the middle ear mucosa (MEM) or effusions (MEE).
Most of the studies of the microbiome of OM were initially conducted using traditional culture methods and later genomic PCR which are now being replaced by 16 seconds rRNA amplicon sequencing that can reliably identify unculturable and novel bacteria in the MEE. 9 These new identification methods are now calling into question long held hypothesis of OM pathogenesis, such as the “adenoidal reservoir” theory. New data is also emerging that highlights the involvement of host defense mechanisms such as epithelial adaptation and mucin production in the development of chronic OM. In this review we contrast the previously reported microbiome of OM with the most recent data and its implications on the pathogenesis and etiology of OM.
2. MICROBIOME IDENTIFICATION
A systematic review of MEE from patients with OM from 1970 to 2014 found that the most commonly isolated bacteria in MEE of children with OM were Streptococcus pneumonia (SP), Haemophilus influenza (Hi), Moraxella catarrhalis (MC). Studies using the traditional culture methods to identify bacteria in MEE have profound limitations, frequently showing decreased detection rates and sensitivity to well described species in MEE. 2 With the introduction of PCR, increased sensitivity and detection was possible for non‐culturable bacteria, however, metagenomic DNA sequencing captures all available DNA non‐discriminately. 10 The advent of 16 seconds rRNA sequencing has allowed researchers the ability to take advantage of nine hypervariable regions (V1‐V9) in the 16 seconds rRNA to reliably identify prokaryotic DNA without being confounded by eukaryotic genetic material. 10 When first implemented to study OM, 16 seconds rRNA sequencing used short read sequences to provided family and genus level classification using operational taxonomic units (OTU). 9 , 10 More recent studies have shown that the same data can be classified using amplicon sequence variants (ASV) yielding greater genus and species level classification rates. 11 With the eventual implementation of full length 16 seconds RNA sequencing, subspecies level classification will be also possible. 12
Despite improved detection and reliability with 16 seconds rRNA sequencing, a metanalysis of recent publications has highlighted sampling and methodological inconsistencies which precludes deriving overarching conclusions about the OM microbiome. 13 What is evident is that novel species such as Turicella and Alloicoccus are being detected at significant relative abundances in OM, and a more diverse group are being reported as the dominant taxa in MEE samples (Figure 1). 13 New evidence is also identifying several species including some lactic acid bacilli and Dolorsigranulum, that may be protective to the middle ear and may play a role in probiotic development as a novel treatment modality. 14
FIGURE 1.
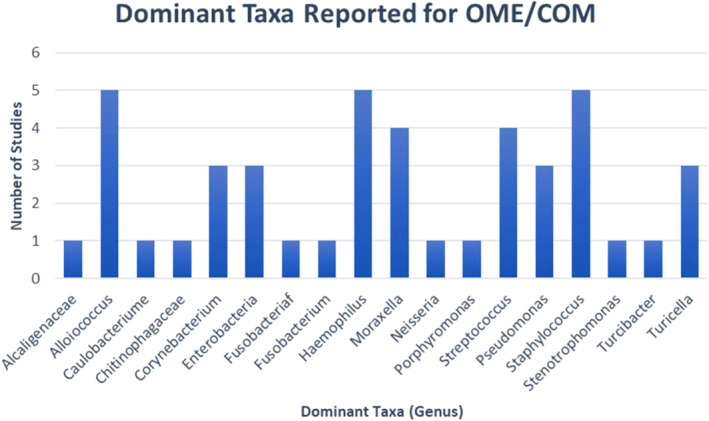
Dominant taxa in MEE of OME/COM reported by five studies 9 , 15 , 16 , 17 , 33 without tympanic membrane rupture or tympanostomy tube. Data were adapted from Marsh et al 13
3. NASOPHARYNGEAL MICROBIOME AND ITS EFFECTS ON OM
Eustachian tubes (ET) of children are shorter and enter the nasopharynx at a shallower angle than in adults. The proximity of the torus tubarius to the adenoidal and tubal lymphoid tissue has led to the hypothesis that the nasopharyngeal lymphoid tissue serves as a reservoir for otopathogens to reside and travel up the ETs, leading to OM. 15 Most of the research supporting this hypothesis was conducted using traditional culture methods that isolated the most commonly studied otopathogens SP, Hi, and MC from the adenoids. 15 , 16 However, more recent studies using 16 seconds rRNA sequencing have found that the MEE microbiome was dissimilar from the adenoidal microbiome in children with COME. 15 , 16 Interestingly, the adenoid microbiome was found to be similar between patients without ear disease and those with COME. 17 However, it has also been reported that adenoids associated with OM are less diverse than those of healthy individuals. 18 Additionally, the adenoidal microbiome was found to be more closely related with the microbiome of the palatine tonsils than to the middle ear. 15
These findings cast doubt over the adenoidal reservoir theory and lend themselves to suggest that the pathogenesis of OM is dependent on a more complex interaction between commensal organisms and ever‐present otopathogens in the middle ear and adenoids. This is further corroborated by the findings of Marsh et al's metanalysis (Figure 1), which demonstrated that the bacteria reported with the highest relative abundance was Alloicoccus, followed by Hi, Staphylococcus, Corynebacteria, SP, and MC. They found an inverse correlation with Alloicoccus and Hi in MEE which supports the theory that Alloicoccus otitidis, is a commensal organism of the middle ear, found in low relative abundances in the adenoids.
Most studies examining the adenoidal microbiome used surface level swabs to sample the tissue and little was known about the spatial organization of bacterium within the adenoidal tissue, especially the crypts. Swidsinski et al 19 examined the bacterial organization within adenoids and found foci of purulent infection in otherwise asymptomatic individuals which were shielded by adenoidal tissue, precluding their sampling via swabbing methods. Additionally, intracellular bacteria hidden within cells of the innate immune system such as macrophages would be difficult to sample and quantify in meaningful relative abundance measurements. 15 , 19 This casts further uncertainty over the adenoidal reservoir theory, highlighting that more extensive classification of the adenoidal microbiome is necessary before the theory can be either validated or disproven.
Interestingly, a recent study suggested that the general respiratory disease status of patients does influence the middle ear microbiome. The MEE microbiome was found to be less diverse in participants with concurrent lower airway disease (asthma or bronchiolitis) than in patients without, and phylogenetic β‐diversity (weighted UniFrac) was significantly different based on lower airway disease status. 11 Differential abundance in patients with lower airway disease was observed for the genera Haemophilus, Moraxella, Staphylococcus, Alloiococcus, and Turicella. These findings suggest a link between COME and respiratory illnesses, perhaps reflecting a postulated association of COME with atopic disease. 20 , 21
A significant limitation of most of the microbiome studies of middle ear and adenoidal samples is that it is difficult to control for recent or repeated usage of antibiotics, particularly in the disease samples relative to the “healthy” control samples. This limitation should direct caution when interpreting some of these findings.
4. THE INFLUENCE OF HOST RESPONSE ON OM
Although pervasive, AOM most often resolves without complications or long term sequalae either via spontaneous resolution or with antibiotic treatment. 22 The detrimental effects of OM arise when it becomes chronic or recurs persistently. This can lead to conductive hearing loss, myringosclerosis, retraction pockets, cholesteatomas, and a host of other complications via extension into the surrounding bony and soft tissue. Because most cases resolve without complication, new studies have begun to examine the innate immune system and what role it plays in the pathogenesis of OM.
One component of innate immunity is the epithelial cell barriers that line the middle ear. The middle ear is normally lined by a single layer of cubical squamous epithelium, but after repeated bouts of OM the epithelium exhibits metaplastic changes and the squamous epithelium converts to pseudostratified columnar with increased goblet cell concentrations. 23 These cells can then form invaginations giving rise to mucus glands that are not found in healthy middle ears. 23 These cells produce an increased amount of mucins, leading to MEEs that are more difficult to clear, 24 which may result in the persistence of MEEs in OME. These mucins appear to play a key role in the innate response to infection, with MUC5B and MUC5AC being the predominantly secreted mucins. 25 Recent work by Kruger et al found that MUC5B was present in 94.5% MEEs while MUC5AC was only detected in 65.5%. 9 Their study also found that the microbiome of middle ear fluid from children with COME differs according to specific clinical features, such as mucin content, age and presence of hearing loss. Samples where MUC5AC was present showed a predominance of Haemophilus species, whereas that predominance was absent in samples with only MUC5B. This corroborates previous reports that Hi propagates an immune response to induce production of MUC5AC 23 and highlights the fact that the immune response is deeply interconnected with the microbiome of the middle ear space. More research into the role of mucins in host defense and adaptation are still needed to uncover their interplay in the pathogenesis of OM. Specifically, more work is needed to determine whether MUC5B is protective against otopathens and what role different bacteria may play in its expression. Notably, MUC5B null mice are susceptible to severe and fulminant infectious disease in the middle ear cavity, underscoring the essential role for this specific mucin in upper airway immune defense. 26
5. LIMITATIONS AND CHALLENGES OF MICROBIOME STUDIES IN OTITIS MEDIA
The rapid technological advances on NGS has stimulated the study of human microbiomes at both DNA (amplicon and shotgun sequencing) and RNA level (metatranscriptomics). As reviewed above, most of the middle ear microbiome studies to date have used 16S rRNA amplicon sequencing followed by metagen dataset analysis as a methodology to characterize the ear bacteriome. Less is known, however, about the fungal and viral components of the middle ear and its functional diversity—as inferred by shotgun metagenomics and metatranscriptomics. Similarly, studies assessing host‐microbe interactions (systems biology) during otitis media are practically inexistent. Regarding the characterization of microbial communities, new bioinformatics methods have been recently published discussing how low biomass issues can often be a problem in 16S rRNA sequencing/metagen data set analysis. For OM studies specifically, low bacterial biomass is often a feature of nasopharyngeal and middle ear fluid specimens, and this may in fact confer bacterial detection more difficult to ascertain with contaminants posing a risk to data interpretation. 27 , 28 But even when optimal cellular biomass conditions are met, the best analytical pipeline to infer community composition from short amplicon sequences (via Operative Taxonomic Units or Amplicon Sequence Variants) is still under debate. 29 Ear microbiome research also suffers from the other methodological issues common to omic data generation (eg, PCR chimeras and sequencing errors), which is still missing best practice protocols for standard molecular procedures in the field (eg, DNA extraction, PCR, library preparation or high‐throughput sequencing). Additionally, other bioinformatic challenges in metagenomics come from the need to continuously update taxonomic and genomic databases as more data are generated, and renew your analytical toolkit with the latest (not always better) methods and software packages for genome assembly and annotation, taxonomic classification, biodiversity estimation, and functional analysis. Metagenomic and metratranscriptomic analyses can be so laborious and computationally intense that many biotech companies already offer automated on‐demand computing services (cloud computing), so scientists can more easily handle the wealth of data from their metagenomic projects. Concomitantly, given the continual increase in sequencing capacity and decline of costs, new data management issues (big data) also arise to store, curate and share omic information, detect and visualize meaningful patterns (ie, potential biological mechanisms) and efficiently (fast and easy) integrate multi‐omic data with clinical and demographic information. Ironically, at the same time, much of the freely available omic data lie in databases and repositories underutilized or not used at all. 30 The metagenomic field is also seeded of statistical challenges due to the high‐dimensionality of the data under study; points of concern are normalization and quantification of relative taxa and gene abundances, dimensionality reduction, multiple hypothesis testing or characterization of random effects. 31 Taken all together, these issues seem to suggest that middle ear microbiome research is still in its infancy and we have a winding road ahead; but large strides have been already made in tackling the issues above in other related areas (eg, see the iHGP). 32 Therefore, as new technologies and omic insights from these projects percolate into the “ears” of OM researchers, new exciting and groundbreaking discoveries are likely to pop up in this field too.
6. CONCLUSION
In conclusion, the knowledge regarding bacterial composition across OM stages has increased exponentially over the past decade. New laboratory techniques for MEE analysis, proliferation of DNA sequencing methods along with increasing computational power of bioinformatics have led to a somewhat redefined descriptive render of OM pathophysiology based in the middle ear microbiome. These studies underscore how critical it is to not lump all cases of MEE into one single disease. The adenoidal reservoir theory may not explain all etiologies of MEE formation. The host immune system is clearly interconnected to the bacterial population in ways yet to be determined. Given the indiscriminate over‐usage of antibiotics for OM, efforts to clarify what happens in the different stages of the disease are critical. This includes the study of MUC5B, the predominant mucin in middle ear effusions, as there is a general lack of understanding of its regulation in OM. Counterweighing probiotic vs antibiotic approaches could help in maintaining homeostasis in a healthy middle ear.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Nogues JC, Pérez‐Losada M, Preciado D. Review of otitis media microbiome studies: What do they tell us? Laryngoscope Investigative Otolaryngology. 2020;5:936–940. 10.1002/lio2.460
REFERENCES
- 1. Schilder AG, Chonmaitree T, Cripps AW, et al. Otitis media. Nat Rev Dis Primers. 2016;2:16063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ngo CC, Massa HM, Thornton RB, Cripps AW. Predominant bacteria detected from the middle ear fluid of children experiencing otitis media: a systematic review. PLoS ONE. 2016;11(3):e0150949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Holder RC, Kirse DJ, Evans AK , et al. One third of middle ear effusions from children undergoing tympanostomy tube placement had multiple bacterial pathogens. BMC Pediatr. 2012;12:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Homoe P, Bjarnsholt T, Wessman M , et al. Morphological evidence of biofilm formation in Greenlanders with chronic suppurative otitis media. Eur Arch Otorhinolaryngol. 2009;266(10):1533‐1538. [DOI] [PubMed] [Google Scholar]
- 5. Calhoun KH, Norris WB, Hokanson JA , et al. Bacteriology of middle ear effusions. South Med J. 1988;81(3):332‐336. [DOI] [PubMed] [Google Scholar]
- 6. Kim SJ, Chung JH, Kang HM, Yeo SG. Clinical bacteriology of recurrent otitis media with effusion. Acta Otolaryngol. 2013;133(11):1133‐1141. [DOI] [PubMed] [Google Scholar]
- 7. Stol K, Verhaegh SJC, Graamans K, et al. Microbial profiling does not differentiate between childhood recurrent acute otitis media and chronic otitis media with effusion. Int J Pediatr Otorhinolaryngol. 2013;77(4):488‐493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thornton RB, Rigby PJ, Wiertsema SP, et al. Multi‐species bacterial biofilm and intracellular infection in otitis media. BMC Pediatr. 2011;11:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Krueger A, Val S, Pérez‐Losada M, et al. Relationship of the middle ear effusion microbiome to secretory Mucin production in pediatric patients with chronic otitis media. Pediatr Infect Dis J. 2017;36(7):635‐640. [DOI] [PubMed] [Google Scholar]
- 10. Davidson RM, Epperson LE. Microbiome sequencing methods for studying human diseases. Methods Mol Biol. 2018;1706:77‐90. [DOI] [PubMed] [Google Scholar]
- 11. Kolbe AR, Castro‐Nallar E, Preciado D, Pérez‐Losada M. Altered middle ear microbiome in children with chronic otitis media with effusion and respiratory illnesses. Front Cell Infect Microbiol. 2019;9:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Callahan BJ, Wong J, Heiner C, et al. High‐throughput amplicon sequencing of the full‐length 16S rRNA gene with single‐nucleotide resolution. Nucleic Acids Res. 2019;47(18):e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marsh RL, Aho C, Beissbarth J, et al. Panel 4: recent advances in understanding the natural history of the otitis media microbiome and its response to environmental pressures. Int J Pediatr Otorhinolaryngol. 2020;130(Suppl 1):109836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van den Broek MFL, De Boeck I, Kiekens F , et al. Translating recent microbiome insights in otitis media into probiotic strategies. Clin Microbiol Rev. 2019;32(4):e00010‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johnston J, Hoggard M, Biswas K, et al. Pathogen reservoir hypothesis investigated by analyses of the adenotonsillar and middle ear microbiota. Int J Pediatr Otorhinolaryngol. 2019;118:103‐109. [DOI] [PubMed] [Google Scholar]
- 16. Chan CL, Wabnitz D, Bassiouni A, Wormald PJ, Vreugde S, Psaltis AJ. Identification of the bacterial reservoirs for the middle ear using phylogenic analysis. JAMA Otolaryngol Head Neck Surg. 2017;143(2):155‐161. [DOI] [PubMed] [Google Scholar]
- 17. Chan CL, Wabnitz D, Bardy JJ, et al. The microbiome of otitis media with effusion. Laryngoscope. 2016;126(12):2844‐2851. [DOI] [PubMed] [Google Scholar]
- 18. Kim SK, Hong SJ, Pak KH , et al. Analysis of the microbiome in the adenoids of Korean children with otitis media with effusion. J Int Adv Otol. 2019;15(3):379‐385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Swidsinski A, Göktas O, Bessler C, et al. Spatial organisation of microbiota in quiescent adenoiditis and tonsillitis. J Clin Pathol. 2007;60(3):253‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gamble JE, Bizal JA, Daetwyler EP. Otitis media and chronic middle ear effusion in the asthmatic pediatric patient. Ear Nose Throat J. 1992;71(9):397‐399. [PubMed] [Google Scholar]
- 21. Bjur KA, Lynch RL, Fenta YA, et al. Assessment of the association between atopic conditions and tympanostomy tube placement in children. Allergy Asthma Proc. 2012;33(3):289‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Venekamp RP, Sanders SL, Glasziou PP , et al. Antibiotics for acute otitis media in children. Cochrane Database Syst Rev. 2015;2015(6):CD000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Val S. Basic Science Concepts in Otitis Media Pathophysiology and Immunity: Role of Mucins and Inflammation, in Otitis Media: State of the Art Concepts and Treatment. Springer International Switzerland AG Gewerbestrasse. Springer; 2015:53‐79. [Google Scholar]
- 24. Dodson KM, Cohen RS, Rubin BK. Middle ear fluid characteristics in pediatric otitis media with effusion. Int J Pediatr Otorhinolaryngol. 2012;76(12):1806‐1809. [DOI] [PubMed] [Google Scholar]
- 25. Preciado D, Goyal S, Rahimi M, et al. MUC5B is the predominant Mucin glycoprotein in chronic otitis media fluid. Pediatr Res. 2010;68(3):231‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roy MG, Livraghi‐Butrico A, Fletcher AA, et al. Muc5b is required for airway defence. Nature. 2014;505(7483):412‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bender JM, Li F, Adisetiyo H, et al. Quantification of variation and the impact of biomass in targeted 16S rRNA gene sequencing studies. Microbiome. 2018;6(1):155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eisenhofer R, Minich JJ, Marotz C, Cooper A, Knight R, Weyrich LS. Contamination in low microbial biomass microbiome studies: issues and recommendations. Trends Microbiol. 2019;27(2):105‐117. [DOI] [PubMed] [Google Scholar]
- 29. Caruso V, Song X, Asquith M, Karstens L. Performance of microbiome sequence inference methods in environments with varying biomass. mSystems. 2019;4(1):e00163‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nardini C, Dent J, Tieri P. Editorial: Multi‐omic data integration. Front Cell Dev Biol. 2015;3:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gloor GB, Macklaim JM, Pawlowsky‐Glahn V, Egozcue JJ. Microbiome datasets are compositional: and this is not optional. Front Microbiol. 2017;8:2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Integrative, H.M.P.R.N.C . The integrative human microbiome project. Nature. 2019;569(7758):641‐648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jervis‐Bardy J, Rogers GB, Morris PS, et al. The microbiome of otitis media with effusion in indigenous Australian children. Int J Pediatr Otorhinolaryngol. 2015;79(9):1548‐1555. [DOI] [PubMed] [Google Scholar]


